Abstract
Objectives:
To determine total postoperative opioid consumption by women ≥ 60 years during the first week after pelvic organ prolapse surgery. We secondarily aimed to describe opioid prescribing patterns in this cohort.
Methods:
This is a secondary analysis of a prospective cohort study assessing changes in cognition in women ≥ 60 years undergoing prolapse surgery. Postoperative opioid use at home during the first week was collected through daily self-reported diary entries. Total postoperative opioid consumption was calculated by adding opioid administration in the postoperative anesthesia recovery unit, inpatient setting, and home opioid use (as documented in diary). Regression models were used to identify demographic and clinical factors associated with total postoperative opioid consumption in the top quartile of this cohort and home opioid use.
Results:
Data from 80 women were analyzed. Mean age was 71.78±6.14 years (range 60–88). 62.5% (n=50) underwent vaginal surgery and 37.5% (n=30) underwent laparoscopic/robotic surgery, with concomitant hysterectomy in 58.8% (n=47).The median (interquartile range) total morphine milligram equivalents used during the first week after surgery was 30 (7.5–65.75). The median (interquartile range) total morphine milligram equivalents prescribed was 225 (150–225).
Conclusion:
Opioid consumption after prolapse surgery in older women is very modest and equates to a median (interquartile range) of four (1–9) oxycodone 5mg tablets. Opioid prescribing patterns should be adjusted accordingly.
Keywords: older adults, opioid use, postoperative opioid use, postoperative narcotic use, prolapse surgery
Single Sentence Summary:
Total postoperative opioid consumption in older women after prolapse surgery is modest (the equivalent of four oxycodone 5mg tablets) during the first week after surgery.
Introduction:
Chronic prescribing and overprescribing of opioids, especially after surgery, contributes to prolonged opioid use and misuse, which increases healthcare costs, morbidity, and mortality.1–5 In opioid-naïve patients, surgery is a risk factor for continued opioid use after surgery.6–9 Surgical patients of older age (> 50 years) tend to have persistent opioid use 90 days after surgery.7 Also concerning, 67–92% of surgical patients report having “leftover” opioid medication from their acute postoperative period, which may increase the likelihood of misuse.2
Few studies have investigated postoperative opioid use specifically in older gynecologic patients. One study by Swenson et al, which focused on opioid utilization after minimally invasive urogynecologic surgery, reported a median consumption of 13 opioid tablets in the 2-week postoperative period and that women used one-third of the opioids they were prescribed.10 Additionally, many institutions are incorporating multimodal pain management efforts (e.g. Enhanced Recovery After Surgery (ERAS) protocols) perioperatively to decrease opioid use and minimize pain. Implementation of these protocols may have a significant impact on patients’ pain experience after surgery, leading to less administration of opioids postoperatively. Given the growing population of older women seeking urogynecologic care and the ERAS protocol implementation for those undergoing surgery, we sought to gain further insight into postoperative opioid use in older women after urogynecologic surgery.
The primary aim of this study was to evaluate total postoperative opioid consumption in women 60 years and older during the first week after undergoing pelvic organ prolapse (POP) surgery. We hypothesized that this cohort would use less than 65 median morphine milligram equivalents (MMEs), the equivalent of nine oxycodone 5mg tablets. Our secondary aims were the following: 1) to describe opioid prescribing patterns in this specific cohort, 2) to identify factors associated with top quartile total postoperative opioid consumption, and 3) to identify factors associated with home opioid use after surgery.
Materials and Methods:
This study is a secondary analysis of a prospective cohort study of perioperative neurocognitive disorders in women aged 60 years and older undergoing POP surgery on the urogynecology service of 6 surgeons at an academic medical center from October 2016 to October 2018. Exclusion criteria included a known diagnosis of dementia or cognitive impairment, history of stroke or major neurologic condition, severe mental illness requiring antipsychotic medication, severe vision or hearing impairment despite corrective interventions, self-reported alcohol or drug abuse, or a positive cognitive impairment screen defined in the index study as a <84 score on the modified Mini Mental State (3MS) exam.11 Additionally for this analysis, women were excluded if they participated a separate intervention study of pain management. Baseline sociodemographic and medical history was obtained through electronic medical record (EMR) review and participant interview. Variables collected included age, race/ethnicity, body mass index (BMI), gravidity, parity, medical co-morbidities, surgical history, POP stage, depression/anxiety history, self-reported history of hearing and/or vision impairment, substance use history, current medications, education level, employment status, number of dependents, number of persons living with participant, home description, assistive ambulation device use, and fall history. Participants underwent frailty assessment using Fried Frailty Index (FFI)12 and completed validated questionnaires including the Pelvic Floor Distress Inventory (PFDI-20), Geriatric Depression Scale, Beck Anxiety Index, and Numerical Pain Rating Scale (scale 0 – 10) during the baseline assessment. Intraoperative and postoperative variables were abstracted from the EMR including procedures performed, anesthesia type and duration, quantities of administered opioid and non-opioid anesthetic medications, intraoperative complications, voiding mechanism at discharge, length of hospitalization, duration of catheter use for urinary retention, and home health nursing service utilization. Additionally, EMR was reviewed for postoperative adverse events, including emergency department encounters, unscheduled office visits, and hospital readmissions, that occurred within a 6-week period after undergoing surgery. These were categorized using Clavien-Dindo criteria.13
We measured opioid use at home after surgery using daily self-reported diaries. Participants were instructed to complete a diary, recording the time and the amount of opioid medication consumed, starting after hospital discharge and ending on postoperative day #7. Given the different types of opioid medications used for analgesia, all opioid dosing was standardized with conversion to MMEs using conversion calculations as per the Center for Disease Control and MD Anderson Cancer Center.14,15 Our primary outcome was total postoperative opioid consumption which was the sum of opioids administered in the postoperative anesthesia care unit (PACU), the inpatient setting, and at home. We confirmed the type, amount and date the opioid prescription was filled by review of the Pennsylvania Prescription Drug Monitoring Program (PDMP) database.
Data were managed using REDCap, a secure web-based application, through the University of Pittsburgh.16 Analyses were performed using summary statistics, such as chi-square tests and Student’s T tests to analyze categorical and continuous variables, to evaluate the association between variables of interest and opioid use. Fisher’s exact and Mann U Whitney tests were used to analyze nonparametric data. Exploratory logistic regression models were created to assess the association between sociodemographic/clinical factors with any home opioid use as well as with high postoperative opioid utilization. We defined high utilization as ≥75th percentile (top quartile) total postoperative opioid consumption. Exploratory multivariable logistic regression models were created using candidate variables (those with p-values <0.2).
Statistical analyses were performed using STATA SE (Release 15. College Station, TX: StataCorp LLC). Strengthening the reporting of the observational studies in epidemiology (STROBE) guidelines were followed.
Results:
Analyses were conducted on 80 out of 124 women enrolled in the primary study. Home opioid diaries were not returned by 25 women, and 19 women were enrolled in a randomized controlled trial assessing the role of pudendal nerve blocks in controlling postoperative pain.
Table 1 summarizes the sociodemographic and clinical factors for the cohort. The total study cohort was mostly white (95%) with a mean age of 71.8±6.1 years (range 60–88). Chronic opioid use before surgery was uncommon (2.5%). The median (IQR) baseline preoperative numerical pain scale score for the cohort was 0 (0–1.5). Most women underwent general anesthesia for surgery (92.5%). More women underwent vaginal surgery (62.5%) compared to laparoscopic/robotic surgery (37.5%). There were 28 (35%) obliterative procedures (colpectomy/colpocleisis), 28 (35%) laparoscopic/robotic sacrocolpopexies, 11 (13.8%) vaginal uterosacral ligament suspensions, nine (11.3%) sacrospinous ligament suspensions, two (2.5%) laparoscopic uterosacral ligament suspensions, and two (2.5%) transvaginal mesh augmented bilateral sacrospinous ligament suspensions (Uphold LITE® (Boston Scientific)). In the preoperative unit approximately 60 minutes prior to surgery, 55 women received 15mg of extended release morphine sulfate and 72 women received 1 gram of acetaminophen either as part of a service-specific ERAS protocol17 and/or attending surgeon preference. The majority of women (n=69, 86.3%) were discharged on the day of surgery. There were 63 women (78.8%) with adverse postoperative outcomes as per Clavien-Dindo classification within 6 weeks after surgery. Forty-one women experienced grade I adverse events including postoperative urinary retention, de novo urinary incontinence, diarrhea, constipation, persistent pelvic pain, and transient cardiac arrhythmia. Additionally, 21 women experience grade II adverse events inclusive of urinary tract infections, respiratory infections, vaginal/vulvar yeast infections, allergic reaction, and vestibulopathy. One woman experienced a serious adverse event (grade III) secondary to postoperative bleeding requiring return to the operating room on postoperative day 0 for evacuation of vaginal blood clots and resuturing of her posterior vaginal wall incision.
Table 1.
Demographic and Clinical Factors by Any Postoperative Opioid Use
| All Participants (n = 80) | Any Postoperative Opioid Use (n=66) | No Postoperative Opioid Use (n=14) | p-value | |
|---|---|---|---|---|
| Age, years | 71.78±6.14 | 71.77±614 | 71.79±6.35 | 0.99 |
| Race | ||||
| White | 76 (95) | 62 (93.9) | 14 (100) | 1.0 |
| Black | 2 (2.5) | 2 (3) | 0 (0) | |
| Other | 2 (2.5) | 2 (3) | 0 (0) | |
| Prolapse stage | ||||
| II | 15 (18.75) | 13 (19.7) | 2 (14.3) | 0.10 |
| III | 51 (63.75) | 39 (59.1) | 12 (85.7) | |
| IV | 14 (17.5) | 14 (21.2) | 0 (0) | |
| Body Mass Index, kg/m2 | 27.95 (24.95 −30.66) | 27.95 (25.39–30.80) | 26.40 (23.24 – 28.54) | 0.22 |
| Opioid Use (before surgery) | 2 (2.5) | 1 (1.5) | 1 (7.1) | 0.32 |
| History of anxiety or depression | 19 (23.75) | 17 (25.8) | 2 (14.3) | 0.50 |
| Lives with 1 or more persons | 66 (82.5) | 54 (81.8) | 12 (85.7) | 1.0 |
| Smoking Status | ||||
| Current | 4 (5) | 2 (3) | 2 (14.3) | 0.14 |
| Former or Never | 76 (95) | 64 (97) | 12 (85.7) | |
| Frailty Status | ||||
| Nonfrail | 16 (20) | 12 (18.2) | 4 (28.6) | 0.27 |
| Intermediate | 58 (72.5) | 50 (75.8) | 8 (57.1) | |
| Frail | 6 (7.5) | 4 (6.1) | 2 (14.3) | |
| ASA Classification (n, %) | ||||
| 1 | 2 (2.5) | 2 (3) | 0 (0) | 1.0 |
| 2 | 42 (52.5) | 34 (51.5) | 8 (57.1) | |
| 3 | 36 (45) | 30 (45.4) | 6 (42.9) | |
| Baseline Numerical Pain Rating Scale Score (scale: 0 – 10) | 0 (0–1.5) | 0 (0–2) | 0 (0–0) | 0.19 |
| Preoperative Extended Release Morphine Sulfate Administration | 55 (68.75) | 44 (66.7) | 11 (78.6) | 0.53 |
| General Anesthesia | 74 (92.5) | 61 (92.4) | 13 (92.9) | 1.0 |
| Estimated blood loss, mL | 50 (30–100) | 50 (30–100) | 50 (35–100) | 0.89 |
| Operating Time, minutes | 145.78±50.45 | 143.71±51.25 | 155.5±46.98 | 0.43 |
| Prolapse Surgery | ||||
| Vaginal | 50 (62.5) | 43 (65.2) | 7 (50) | 0.29 |
| Laparoscopic | 30 (37.5) | 23 (34.8) | 7 (50) | |
| Concomitant Hysterectomy | 47 (58.75) | 36 (54.5) | 11 (78.6) | 0.14 |
| Intraoperative Complication | 2 (2.5) | 2 (3) | 0 (0) | 1.0 |
| Same Day Discharge | 69 (86.3) | 57 (86.4) | 12 (85.7) | 1.0 |
| Length of Stay, days | 0.7 (0.6 – 0.8) | 0.7 (0.6 – 0.8) | 0.7 (0.6 – 0.8) | 0.35 |
| Unscheduled postoperative office visit | 11 (13.75) | 10 (15.2) | 1 (7.1) | 0.68 |
| Postoperative ER visit | 2 (2.5) | 2 (3) | 0 (0) | 1.0 |
| Hospital Readmissionb | 1 (1.25) | 1 (1.5) | 0 (0) | 1.0 |
| Adverse Eventc | 63 (78.75) | 51 (77.3) | 12 (85.7) | 0.72 |
| Serious Adverse Eventd | 1 (1.25) | 1 (1.5) | 0 (0) | 1.0 |
Data are presented as n(%), (median(IQR)), or mean±SD as appropriate.
Abbreviations: ASA, American Society of Anesthesiologists; ER, emergency room; IQR, interquartile range; SD, standard deviation
Statistically significant with p-value <0.05
Any hospital readmission within 30 days of surgery
As per Clavien-Dindo classification13
Defined as Grade III or higher per Clavien-Dindo classification13
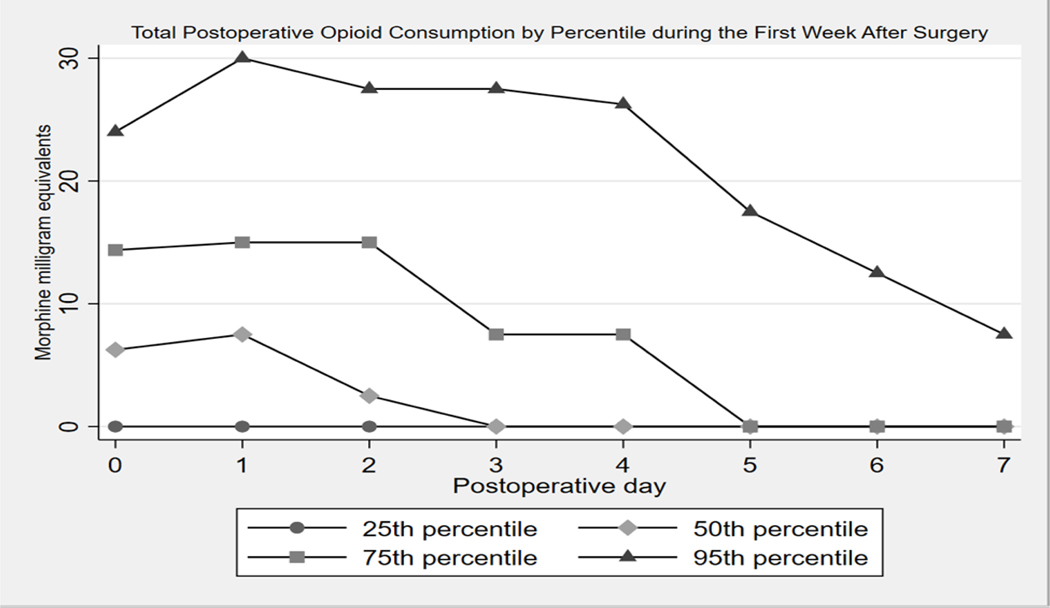
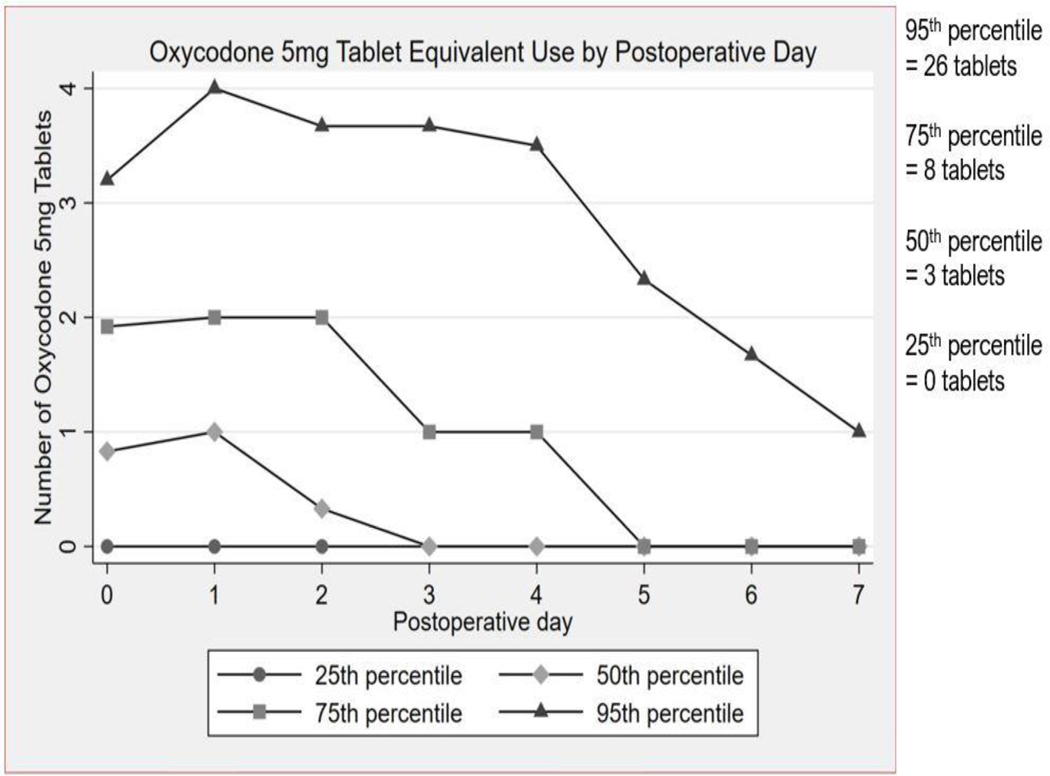
The median (IQR) total opioid consumption, inclusive of PACU/inpatient opioid administration and home opioid use, was 30 (7.5–65.75) MMEs during the first week after surgery which equates to four (1–9) oxycodone 5mg tablets. The range for total opioid consumption during the first week after surgery was 0–246 MMEs. When analyzed by postoperative day, the median (IQR) total postoperative opioid consumption was 6.25 (0–14.375) MMEs for postoperative day 0, 7.5 (0–15) MMEs for postoperative day 1, and 2.5 (0–15) MMEs for postoperative day 2. Notably, most of the opioid consumption occurred in the first 3 days after surgery. The median MMEs was 0 for postoperative days 3 through 7. Figure 1 summarizes postoperative opioid use by day for the 25th, 50th, 75th and 95th percentiles of total postoperative opioid consumption. Figure 2 shows the converted values of MMEs to equivalent number of oxycodone 5mg tablets. The total number of oxycodone 5mg tablets needed to cover the summed median MMEs consumed by percentile is 0 tablets for the 25thpercentile, 3 tablets for the 50th percentile, 8 tablets for the 75th percentile, and 26 tablets for the 95th percentile.
Figure 1.
Total Postoperative Opioid Consumption by Percentile and Postoperative Day during the First Week After Surgery
Figure 2.
Oxycodone 5 mg Tablet Equivalent Use by Percentile and Postoperative Day during the First Week After Surgery
Fifty-nine women were prescribed oxycodone, and 16 were prescribed hydrocodone. The median (IQR) number of tablets prescribed was 30 (24–30) with a range of 5–40. Five women in this cohort did not fill an opioid prescription provided by the surgeon. Of those women who did fill the prescribed opioid prescription, the median (IQR) MMEs prescribed was 225 (150–225).
In comparing women in the top quartile of total postoperative opioid consumption to the remaining cohort, the absence of intraoperative succinylcholine administration (p=0.01, unadjusted odds ratio 0.24) and a greater FFI exhaustion score (p=0.02, unadjusted odds ratio 4.71) were factors significantly associated with top quartile postoperative opioid consumption. With exploratory multivariable logistic regression, intraoperative succinylcholine use was the only variable significantly associated with a decreased odds of top quartile postoperative opioid consumption (p=0.02, adjusted odds ratio 0.27) (Table 2).
Table 2.
Univariable and Multivariable Logistic Regression Analyses for Factors Associated with Top Quartile (≥75th percentile) Opioid Use Postoperatively
| Unadjusted Odds Ratio | 95% Confidence Interval | Adjusted Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|---|
| Baseline Numerical Pain Scoreb | 1.17 | 0.92 – 1.48 | ||
| Anesthesia Approach (General vs. Regional)c | 0.30 | 0.06 – 1.61 | ||
| Surgical Approach (Vaginal vs. Abdominal) | 3.06 | 0.91 – 10.25 | ||
| Fried Frailty Index – Exhaustion Scored | 4.71a | 1.25 – 17.72a | ||
| Intraoperative Intravenous Propofole | 1.0 | 0.99 −1.00 | ||
| Intraoperative Intravenous Succinylcholine | 0.24a | 0.08 – 0.74a | 0.27a | 0.09 – 0.83a |
| Operative timef | 0.64 | 0.33 – 1.22 | ||
Statistically significant (p<0.05)
Baseline Numerical Pain Score fell out of final model due to insignificant p-value = 0.61
Anesthesia approach fell out of final model due to insignificant p-value = 0.66
Fried Frailty Index – Exhaustion Score fell out of final model due to insignificant p-value = 0.61
Intraoperative Intravenous Propofol fell out of final model due to insignificant p-value = 0.88
Operative time fell out of final model due to insignificant p-value = 0.59
Home postoperative opioid use (Table 3) was significantly associated with same day discharge (p=0.03, unadjusted odds ratio 4.61) and the absence of intraoperative hydromorphone administration (p=0.01, unadjusted odds ratio 0.10). Length of stay was not included in the multivariable models due to collinearity with same day discharge. With the exploratory multivariable model, intraoperative hydromorphone administration was the only factor that remained associated with a decreased odds of home opioid use (p=0.04).
Table 3.
Univariable and Multivariable Logistic Regression Analyses for Factors Associated with Home Opioid Use
| Unadjusted Odds Ratio | 95% Confidence Interval | Adjusted Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|---|
| Beck Anxiety Inventory Baseline Score | 1.07 | 0.97 – 1.18 | 1.10 | 0.98 – 1.24 |
| Concomitant Hysterectomy | 0.52 | 0.19 – 1.39 | 0.41 | 0.13 – 1.32 |
| Same Day Discharge | 4.61a | 1.21 – 17.54a | 3.68 | 0.75 – 18.11 |
| Any Intraoperative Intravenous Hydromorphone | 0.10a | 0.02 – 0.55a | 0.13a | 0.02 – 0.87a |
| Any Intraoperative Intravenous Ketamine | 1.03 | 0.99 – 1.06 | 2.34 | 0.68 – 8.06 |
| Any Intraoperative Intravenous Fentanylb | 0.48 | 0.17 – 1.37 | ||
Statistically significant (p<0.05)
Intraoperative Intravenous Fentanyl fell out of the final model due – p=0.89
Conclusions:
In this study of older women who underwent POP surgery, the median total opioid consumption in the first week after surgery was 30 MMEs, which is the equivalent of four oxycodone 5mg tablets. We found that most women in our study were prescribed 30 oxycodone 5mg tablets (an equivalent of 225 MMEs). At study inception, we hypothesized that total postoperative opioid consumption would be ≤ 65 MMEs given the findings from the Swenson, et al. study which assessed opioid use after minimally invasive urogynecologic surgery. Results from that study were based on home opioid consumption over the immediate two-week postoperative period.10 Notably, our study found that total opioid consumption was significantly less than 65 MMEs one week postoperatively with minimal additional opioid use after postoperative day 3. We hypothesize that total postoperative opioid consumption would not have increased significantly in the second week after surgery given our findings though we acknowledge this cannot be stated with certainty as our study diary did not extend into the second week after surgery.
Prescribers generated scripts for opioids in amounts disproportionately greater than average consumption. The majority of patients were prescribed 30 oxycodone 5mg tablets though the 75th percentile of total postoperative opioid consumption in the first week after surgery was 65.75 MMEs, an equivalent of nine oxycodone 5mg tablets. This finding is consistent with reports in published literature specific to opioid overprescribing in the postoperative setting across specialties.3–9, 10, 18–20
The basis for determination of opioid prescription amounts for most surgeons is multifaceted and likely related to experience, training, and patient access to care (e.g. ability to return to office in the setting of poor pain control). Currently, there are no specific guidelines for opioid prescribing after minimally invasive gynecologic surgery. Numerous studies have reported that opioid overprescribing frequently occurs in the acute postoperative setting.3–9,10,18–20 A systematic review examining perioperative opioid management for minimally invasive hysterectomy reported that studies collectively reported the misalignment of patient opioid consumption (range of 14 to 74 MMEs over a 2 weeks), with average physician prescriptions (25 to 300 MMEs), leading to an excess of 114 MMEs of opioid prescribed after minimally invasive hysterectomy.2 Additionally, a study by Ramaseshan, et al. reported a common opioid prescribing pattern of 50 opioid tablets (oxycodone or hydromorphone) after urogynecologic surgery and the finding of 33 unused opioid tablets per postsurgical opioid consumer 7 to 10 days after surgery.20 As exploratory aims, we identified factors associated with the highest quartile of total opioid consumption and home opioid use. The absence of intraoperative hydromorphone use was significantly associated with home opioid use. Top quartile opioid consumption was also associated the absence of intraoperative succinylcholine use. Generally, the ERAS protocol used at our institution discourages the use of opioids intraoperatively and reserves their use for poor pain control during anesthesia emergence.17 With this, only 9 women received intraoperative hydromorphone. Almost one quarter of the women in this cohort did not receive succinylcholine. We speculate that these findings may reflect bias introduced from the small sample size and lack of power for these exploratory aims. More robust studies or meta-analyses may better inform on these and other predictors of post-operative opioid demands.
A limitation of this study is that this cohort comes from a single large institution (6 attending surgeons) and was rather homogenous in its racial/ethnic patient sample, which limits generalizability. However, our findings of modest opioid consumption and excessive supply consistent with other surgical studies.3–6 Another limitation is that home opioid use is measured through self-reported diaries and not substantiated by pill counts. Although women are instructed to keep opioid medication diaries prospectively, it is still possible that some women may have documented inaccurately. Further, opioid use may have been impacted inadvertently through to process of women completing the diaries for the study. Additionally, women were not instructed to record other non-opioid analgesic medications used. This limits our ability to understand what was required to achieve pain control postoperatively in the home setting for these women. During this study, we did not recommend or prescribe neuropathic pain medications, like gabapentin, as a standard postoperative medication regimen. Generally, women were prescribed ibuprofen 600 to 800mg every 6 to 8 hours as needed for pain along with oral acetaminophen as needed postoperatively.
Our medical center introduced an ERAS protocol after the initiation of this study. This ERAS protocol includes preoperative optimization and multimodal pain management components designed to decrease postoperative nausea and minimize postoperative pain. Specifics of the protocol have been previously outlined by Carter-Brooks, et al.17 Due to the adjustments made to the protocol over time as it was incorporated into practice, we are unable to analyze ERAS as a variable in this dataset. However, we recognize that this multimodal approach of the ERAS protocol likely contributed to improved pain control postoperatively.
The role of Pennsylvania’s PDMP is to assist health care providers in safely prescribing controlled substances for patients as it provides insight on all filled prescriptions for controlled substances. A strength of this study is the use of the PDMP as an objective approach to obtain data regarding opioid prescribing and prescription filling patterns. Another strength is the prospective design with utilization of opioid diaries for women to record opioid use in real time minimizing recall bias.
This study contributes to the literature of very few studies that evaluate opioid consumption in older adults, an important population given their increased tendency to use opioids persistently after surgery as well as their increased risk of cognitive impairment and falls.7,21 Surgeons must keep in mind the age-related changes in pharmacokinetics, specific to renal and hepatic function, that alone or with comorbidities put this patient population at greater risk of adverse events such as fractures, falls, and delirium associated with opioid use.21 The American Geriatric Society Beers Criteria recommends reduction of other central nervous system-active medications, including antipsychotics, benzodiazepines, tricyclic antidepressants, and selective serotonin reuptake inhibitors, in the setting of taking opioids.22 Surgeons must also be aware of the potential for polypharmacy in the acute postoperative setting. As the geriatric population continues to grow, research should focus on attaining a greater understanding of the impact of postoperative opioid use in the recovery of older persons. Additionally, futureresearch should focus on development of opioid prescribing guidelines for postoperative pain management.
In summary, total postoperative opioid consumption in older women after POP surgery is modest and occurs primarily during the first three postoperative days. Opioid overprescribing continues to occur. Medical history, anticipated total opioid consumption, and geriatric physiology must be considered when prescribing opioids for older women after POP surgery.
Acknowledgments
Funding: This study was funded by the Audrey Hillman Fisher Foundation Grant, the American Urogynecologic Society Pelvic Floor Disorders Research Foundation Grant - Faculty Research Award, and the NIH NICHD Women’s Reproductive Health Research (WRHR) Career Development Program (5K12HD063087)
Conflict of Interest: Author MF Ackenbom has received research grant funding from the NIH NICHD Women’s Reproductive Health Research Career Development Program (5K12HD063087).
Footnotes
Otherwise, the authors report no relevant conflicts of interest.
References
- [1].Volkow N, Frieden T, Hyde P. and Cha S. Medication-Assisted Therapies - Tackling the Opioid-Overdose Epidemic. New England Journal of Medicine. (2014); 370(22): 2063–2066. [DOI] [PubMed] [Google Scholar]
- [2].Johnson CM, Makai GE. A Systematic Review of Perioperative Opioid Management for Minimally Invasive Hysterectomy. J Minim Invasive Gynecol. 2018September1. pii: S1553–4650(18)30443–6. doi: 10.1016/j.jmig.2018.08.024. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [3].Bicket M, Long J, Pronovost P, Alexander G. and Wu C. (2017). Prescription Opioid Analgesics Commonly Unused After Surgery. JAMA Surgery, 152(11), p.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hill MV, McMahon ML, Stucke RS, Barth RJ Jr., Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265(4):709–714. [DOI] [PubMed] [Google Scholar]
- [5].Bates C, Laciak R, Southwick A, Bishoff J. Overprescription of postoperative narcotics: a look at postoperative pain medication delivery, consumption and disposal in urological practice. J Urol. 2011;185(2):551–555. [DOI] [PubMed] [Google Scholar]
- [6].Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naïve patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018; 360: j5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sun EC, Darnali BD, Baker LC, Mackey S. Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naïve Patients in the Postoperative Period. JAMA Intern Med. 2016:176(9):1286–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017;152(6): e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hah JM, Bateman BT, Ratliff J, Curtin C, Sun E. Chronic Opioid Use After Surgery: Implications for Perioperative Management in the Face of the Opioid Epidemic. Anesth Analg 2017; 125:1733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Swenson CW, Kelley AS, Fenner DE, Berger MB. Outpatient Narcotic Use After Minimally Invasive Urogynecology Surgery. Female Pelvic Med Reconstr Surg 2016;22:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1978;.48(8), 314–8. [PubMed] [Google Scholar]
- [12].Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Amer Coll Surg 2010; 210: 901–908. [DOI] [PubMed] [Google Scholar]
- [13].Dindo D, Demartines N, Clavien PA Classification of Surgical Complications: A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann Surg. 2004August; 240(2): 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].U.S. Department of Health and Human Services Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage. Available at: https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf.Accessed September 1, 2018.
- [15].Postoperative Pain Management Workgroup. Postoperative Pain Management. 2018The University of Texas MD Anderson Cancer Center, Department of Clinical Effectiveness. Available at: https://www.mdanderson.org/documents/for-physicians/algorithms/clinical-management/clin-management-post-op-pain-web-algorithm.pdf.Accessed September 1, 2018. [Google Scholar]
- [16].Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform. 2009April;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Carter-Brooks CM, Du AL, Ruppert KM, et al. Implementation of a urogynecology-specific enhanced recovery after surgery (ERAS) pathway. Am J Obstet Gynecol 2018; 219:495. e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moulder JK, Boone JD, Buehler JM, Louie M. Opioid Use in the Postoperative Arena: Global Reduction in Opioids After Surgery Through Enhanced Recovery and Gynecologic Surgery. Clinical Obstetrics and Gynecology. 2018November6. doi: 10.1097/GRF.0000000000000410. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [19].Saini S, McDonald EL, Shakked R, et al. Prospective Evaluation of Utilization Patterns and Prescribing Guidelines of Opioid Consumption Following Orthopedic Foot and Ankle Surgery. Foot & Ankle International. 2018; 39(11): 1257–1265. [DOI] [PubMed] [Google Scholar]
- [20].Ramaseshan AS, Tunitsky-Bitton E, O’Sullivan DM, et al. Predictive Factors of Postdischarge Narcotic Use After Female Pelvic Reconstructive Surgery. Female Pelvic Med Reconstr Surg 2019;25: e18–e22. [DOI] [PubMed] [Google Scholar]
- [21].Naples JG, Gellad WF, Hanlon JT. Managing Pain in Older Adults: The Role of Opioid Analgesics. Clin Geriatr Med. 2016November; 32(4): 725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015, 63:2227–2246. [DOI] [PubMed] [Google Scholar]