Abstract
Background & Aims
The pathogenesis of Wilson disease (WD) involves hepatic and brain copper accumulation resulting from pathogenic variants affecting the ATP7B gene and downstream epigenetic and metabolic mechanisms. Prior methylome investigations in human WD liver and blood and in the Jackson Laboratory (Bar Harbor, ME) C3He-Atp7btx-j/J (tx-j) WD mouse model revealed an epigenetic signature of WD, including changes in histone deacetylase (HDAC) 5. We tested the hypothesis that histone acetylation is altered with respect to copper overload and aberrant DNA methylation in WD.
Methods
We investigated class IIa HDAC4 and HDAC5 and H3K9/H3K27 histone acetylation in tx-j mouse livers compared with C3HeB/FeJ (C3H) control in response to 3 treatments: 60% kcal fat diet, D-penicillamine (copper chelator), and choline (methyl group donor). Experiments with copper-loaded hepatoma G2 cells were conducted to validate in vivo studies.
Results
In 9-week tx-j mice, HDAC5 levels increased significantly after 8 days of a 60% kcal fat diet compared with chow. In 24-week tx-j mice, HDAC4/5 levels were reduced 5- to 10-fold compared with C3H, likely through mechanisms involving HDAC phosphorylation. HDAC4/5 levels were affected by disease progression and accompanied by increased acetylation. D-penicillamine and choline partially restored HDAC4/5 and H3K9ac/H3K27ac to C3H levels. Integrated RNA and chromatin immunoprecipitation sequencing analyses revealed genes regulating energy metabolism and cellular stress/development, which, in turn, were regulated by histone acetylation in tx-j mice compared with C3H mice, with Pparα and Pparγ among the most relevant targets.
Conclusions
These results suggest dietary modulation of class IIa HDAC4/5, and subsequent H3K9/H3K27 acetylation/deacetylation can regulate gene expression in key metabolic pathways in the pathogenesis of WD.
Keywords: Copper, Liver, Histone Deacetylase, Metabolism
Abbreviations used in this paper: -ac suffix, acetylated; AICAR, 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside; AMPK, adenosine monophosphate-activated protein kinase; cAMP, cyclic AMP; C3H, C3HeB/FeJ; ChIP-seq, chromatin immunoprecipitation sequencing; DMR, differentially methylated region; ER, endoplasmic reticulum; HDAC, histone deacetylase; HepG2, hepatoma G2; HFD, 60% kcal fat diet; H3K, histone-3-lysine; -me3 suffix, trimethylated; p- prefix, phosphorylated; PBS, phosphate-buffered saline; PCA, D-penicillamine; PPAR, peroxisome proliferator-activated receptor; qPCR, quantitative polymerase chain reaction; RNA-seq, RNA sequencing; SAH, S-adenosylhomocysteine; TBST, tris-buffered saline with Tween-20; TF, transcription factor; tx-j, Jackson Laboratory toxic milk mouse C3He-Atp7btx-J/J; WD, Wilson disease
Graphical abstract
Summary.
Histone deacetylases 4 and 5 are involved in the epigenetic regulation of key Wilson disease (WD) metabolic pathways and are targets sensitive to dietary modulations. Understanding epigenetic mechanisms in WD contributes to better comprehension of WD phenotypic variability and other common liver conditions.
Wilson disease (WD) is a systemic disease caused by the inheritance of recessive mutations affecting the ATP7B copper transporter gene. As a result of transporter loss of function, copper accumulates predominantly in the liver and the brain. Although hepatic and neurologic signs and symptoms are the most common manifestations, any organ can be affected with various degrees of involvement. The growing knowledge about WD pathogenesis has also contributed to the understanding of more common conditions, including alcohol-associated liver disease, nonalcoholic fatty liver, and autoimmune hepatitis,1, 2, 3 as the involved pathogenic pathways partially overlap and the clinical and laboratory presentations have common features.4, 5, 6 Although the genetic deficiency in WD causes copper accumulation, gene variants themselves do not explain the whole array of clinical manifestations as there is no evidence of genotype–phenotype correlation.7 Given the similarity of systemic involvement and hepatic phenotypes with other metabolic liver diseases, WD is ideal for understanding the mechanisms leading to epigenetic and metabolic dysregulation during disease onset and progression as well as the extent of organ involvement. Moreover, genetic mouse models of hepatic copper accumulation replicate many of the metabolic derangements observed in human beings.8, 9, 10, 11
One-carbon metabolism and DNA methylation are among the processes identified as being aberrant in WD, with specific patterns distinguishing WD from other liver diseases.12,13 The mechanism underlying the connection between copper accumulation and methionine metabolism involves an inhibitory effect of copper on the enzyme S-adenosylhomocysteine (SAH) hydrolase with consequent accumulation of SAH; SAH can interfere with DNA methylation mechanisms by inhibiting DNA methyltransferases. DNA methylation alterations are observed in patients with WD and in the Jackson Laboratory toxic milk mouse C3He-Atp7btx-j/J (tx-j), a model of spontaneous hepatic copper accumulation.9 Hypermethylated differentially methylated regions (DMRs) were enriched in enhancer sites, suggesting functional relevance of methylation changes affecting gene expression regulation. DNA methylation changes partially overlapped between patients and mice; changes were evident in fetal and adult mouse livers10 as well as in human blood and liver, indicating a universal presence of DNA methylation alterations characterizing WD.
Furthermore, mechanistically meaningful pathways were identified from DNA methylation analyses that could lead to a deeper understanding of WD pathogenesis. Interestingly, among the identified regulatory genes, histone deacetylase 5 (HDAC5) was hypermethylated in the gene body both in blood and liver tissue from patients with WD compared with healthy subjects. HDACs, which are categorized into classes I–IV, catalyze histone deacetylations, resulting in epigenetic chromatin modifications that inhibit gene expression. HDAC5 is a class IIa enzyme shuttled between the nucleus and cytosol when phosphorylated in a rapid response to various environmental stimuli, including nutrients, inflammation, and oxidative stress.14 Therefore, in addition to DNA methylation, HDACs can be considered an interface between nutrition and gene expression regulation. Increasing evidence from cancer literature indicates a multi-layered interplay between chromatin structure, histone acetylation, and DNA methylation. Inhibition of DNA methyltransferases or HDACs is known to have synergistic effects on cellular replication and differentiation, as observed in in vitro cancer models.15,16 Histone post-translational modifications, including trimethylation of histone-3-lysine sites (H3K9me3 and H3K27me3), are commonly known to be associated with regulatory loci.
There is growing interest regarding the role of epigenetic mechanisms in metabolic conditions whose presentation and liver involvement can mimic WD. Hepatic class I and class II HDAC transcript levels and activities are affected by ethanol consumption in a mouse model of alcoholic liver disease and HDAC levels are dysregulated at the promoter of genes critical for liver damage progression.17 Alcohol-induced histone 3 acetylation has been studied extensively in alcoholic liver disease and it appears to play a role in the progression of fibrosis.18,19 HDAC5 changes have also been implicated in liver damage when C57Bl/6 mice were fed a high-fat diet.20 Acetylated H3K9 (H3K9ac) and 5-methylcytosine levels were altered at regulatory loci of aging-associated genes in mouse livers.21 There is, however, a lack of knowledge in WD about the network involving methyl group availability and its effects on histone acetylation. This is crucial information as in-depth understanding of epigenetic mechanisms and their cross-talk with metabolic factors and gene expression regulation will contribute to a better understanding of WD phenotypic variability as well as the metabolic underpinnings of more common liver conditions with similar hepatic presentations. In this study, we tested the hypothesis that hepatic copper levels and methyl group availability interact with HDAC5 and histone acetylation in regulating gene transcript levels in animal models of WD.
Results
Histone Deacetylase 5 Is Downregulated in Multiple Models of WD
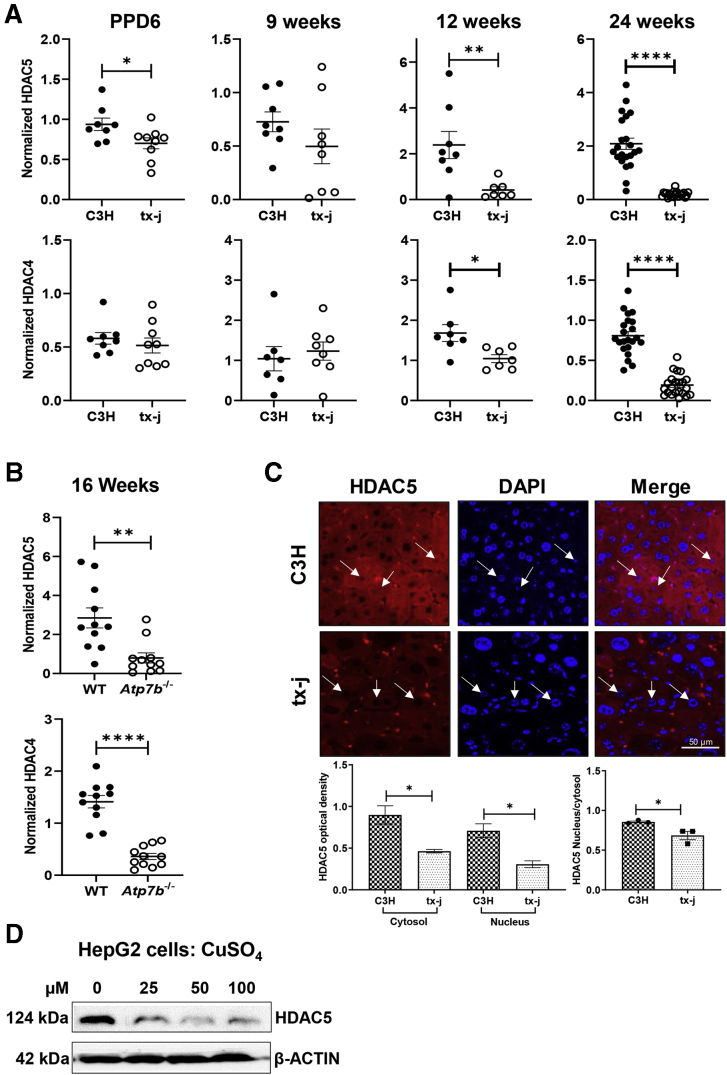
To test the hypothesis that epigenetic changes to HDAC5 resulted in changes to protein levels, Western blot analyses were performed in multiple in vivo and in vitro models of WD (Figure 1, animal study design). First, HDAC5 protein levels were reduced in tx-j livers compared with C3HeB/FeJ (C3H) control mice (Figure 2A, Supplementary Figure 1A). At 6 days postpartum, when hepatic copper levels are still normal, hepatic HDAC5 levels were approximately 1.3-fold lower in tx-j mice. The difference became progressively more significant, concomitantly with copper accumulation. Hepatic HDAC5 levels were approximately 5-fold lower in 12-week-old tx-j mice to 10-fold lower in 24-week-old tx-j mice compared with C3H. Similarly, class IIa histone deacetylase HDAC4 also was reduced in tx-j mouse livers, indicating a widespread effect on class II HDACs. HDAC4 showed reduced expression at the latest stages of disease progression from 12 to 24 weeks of age. In the Atp7b-/- mouse model of WD, HDAC4 and HDAC5 levels also were reduced significantly in 16-week-old Atp7b-/- compared with wild-type mice (Figure 2B, Supplementary Figure 1B).
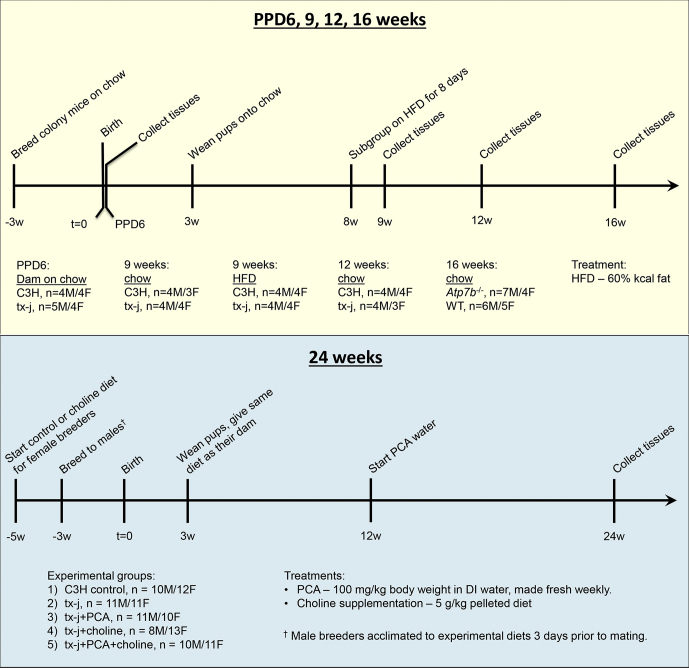
Figure 1.
Animal study design. C3H, C3HeB/FeJ; DI, deionized; HFD, high-fat diet; PCA, D-penicillamine; PPD6, postpartum day 6; tx-j, C3He-Atp7btx-j/J; WT, wild-type.
Figure 2.
Expression of HDAC4 and HDAC5 in mouse and HepG2 models of WD. Immunoblot densitometry analyses are normalized to β-actin; data are represented as means ± SEM and statistical significance was determined by Student's t-test (∗P < .05, ∗∗P < .01, and ∗∗∗∗P < .0001). (A) Total protein liver lysate densitometries for HDAC4 and HDAC5 protein expression in tx-j mice compared with C3H control at postpartum day 6 (PPD6; C3H, n = 4 males/4 females; tx-j, n = 5 males/4 females), 9 weeks (C3H, n = 4 males/3 females; tx-j, n = 4 males/4 females), 12 weeks (C3H, n = 4 males/4 females; tx-j, n = 4 males/3 females), and 24 weeks (C3H, n = 10 males/12 females; tx-j, n = 11 males/11 females). (B) Total protein liver lysate densitometry analyses for HDAC4 and HDAC5 protein expression in 16-week-old Atp7b-/- mice (n = 7 males/4 females) and wild-type (WT, n = 6 males/5 females). (C) Immunohistochemical analysis of 24-week-old C3H and tx-j mouse livers for HDAC5 (red) and 4′,6-diamidino-2-phenylindole (DAPI, blue). Images display cytosolic and nuclear HDAC5 localization. White arrows indicate nuclei. Scale bar: 50 μm. Bar graphs represent HDAC5 optical density in cytosol, nuclei, and the nucleus/cytosol ratio; n = 3 mice/group, 60 cells/mouse. (D) HDAC5 immunoblot of HepG2 cell lysates treated with CuSO4 (0–100 μmol/L; n = 3 per treatment) for 24 hours.
We then compared HDAC5 subcellular localization in liver tissue of C3H and tx-j mice by immunohistochemistry, observing reduced total HDAC5 signals in tx-j mice compared with C3H for both nuclear and cytoplasmic fractions (Figure 2C, Supplementary Figure 1C). The HDAC5 signal intensity of the nucleus/cytosol ratio was reduced significantly in tx-j mice.
In an in vitro WD model, copper-loaded hepatoma G2 (HepG2) cells also demonstrated reduced HDAC5 levels compared with control, indicating a direct effect of copper on reduced HDAC5 levels, independent from hepatic inflammation or fibrosis (Figure 2D). HDAC5-activator isoproterenol, previously shown to cause decreased hepatic and cardiac copper levels in male Wistar rats,22 increased HDAC5 levels in a dose-dependent manner when applied to copper-loaded HepG2 cells (Supplementary Figure 1D). Together, these results indicate Atp7b mutation-induced copper accumulation reduces class IIa histone deacetylases HDAC4 and HDAC5 in WD.
Activation of Adenosine Monophosphate -Activated Protein Kinase (AMPK) Signaling Is Associated With HDAC5 Phosphorylation in tx-j Mice
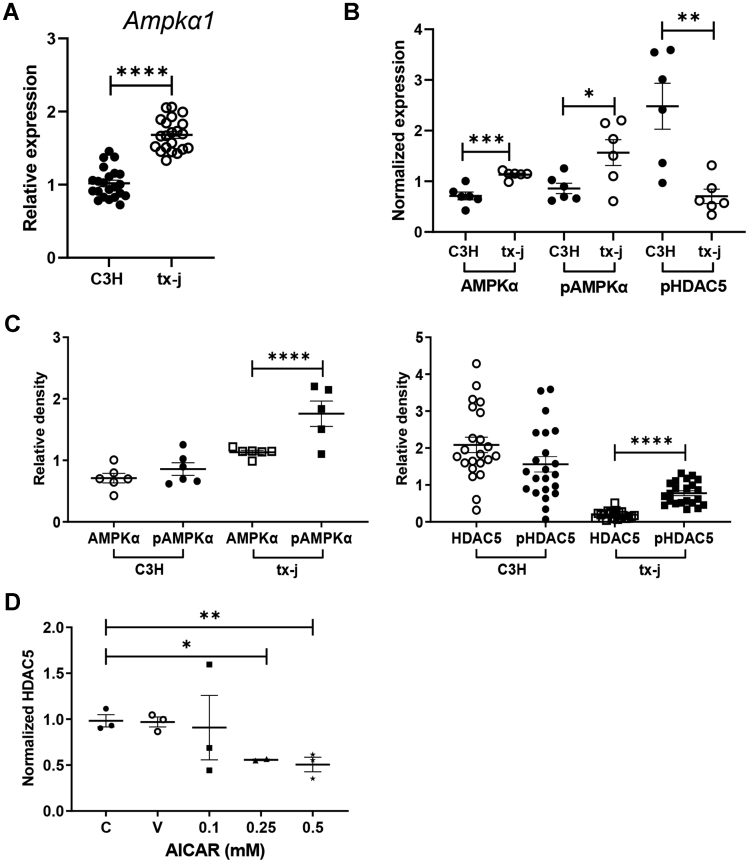
We hypothesized the AMPK-HDAC5 regulatory pathway, previously studied in diabetes and cardiovascular disease,23 may be involved with the reduction of HDAC5 levels in WD mice, since activated AMPK signaling has been reported in animal models of copper accumulation.24 When AMPK is phosphorylated (pAMPK), it becomes active and can phosphorylate HDAC5 (pHDAC5) in the nucleus, marking HDAC5 for cytosolic exportation and possible degradation. AMPK and pAMPK protein and transcript levels were examined in livers of 24-week-old tx-j mice compared with C3H (Figure 3A and B, Supplementary Figure 2A). AMPK and pAMPK protein and transcript levels were increased significantly in tx-j mice compared with C3H, consistent with the hypothesis. When compared with total protein levels, pAMPK and pHDAC5 ratios were increased significantly in tx-j mice relative to total AMPK and total HDAC5, respectively, whereas no significant difference was observed in C3H mice (Figure 3C).
Figure 3.
Activation of AMPKα signaling in 24-week-old tx-j mice and HepG2 cells. Data are represented as means ± SEM. Statistical significance was determined by Student's t-test (∗P < .05, ∗∗P < .01, and ∗∗∗∗P < .0001). (A) Transcript levels of Ampka1 normalized to Gapdh (C3H, n = 10 males/12 females; tx-j, n = 11 males/11 females). (B) Immunoblot densitometries of total AMPKα, pAMPKα, and pHDAC5 in total protein liver lysates obtained from 24-week-old C3H (n = 3 males/3 females) and tx-j (n = 3 males/2 females). Densitometry values were normalized to β-actin. (C) Relative density comparisons of total AMPKα with pAMPKα and total HDAC5 with pHDAC5, normalized to β-actin, in 24-week-old C3H and tx-j mice. (D) HDAC5 immunoblot densitometry analysis, normalized to β-actin, of HepG2 cell lysates treated with 50 μmol/L CuSO4 for 24 hours, followed by AICAR treatment (0–0.5 mmol/L; n = 3 per dose), an AMPK activator, for 24 hours. C, control; V, treated with vehicle (DMSO) only.
To determine whether activation of endogenous AMPK results in decreased HDAC5, HepG2 cells were loaded with CuSO4 followed by treatment with 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR), an AMPK activator, for 24 hours. AICAR treatment decreased HDAC5 protein levels in a dose-dependent manner (Figure 3D, Supplementary Figure 2B).
These results confirm activated AMPK is associated with increased pHDAC5 in tx-j mice compared with C3H and is the likely explanation for lower tx-j levels of HDAC5.
HDAC4/HDAC5 Deficiency in WD Models Is Restored Through Copper Chelation and Dietary Methyl Donors
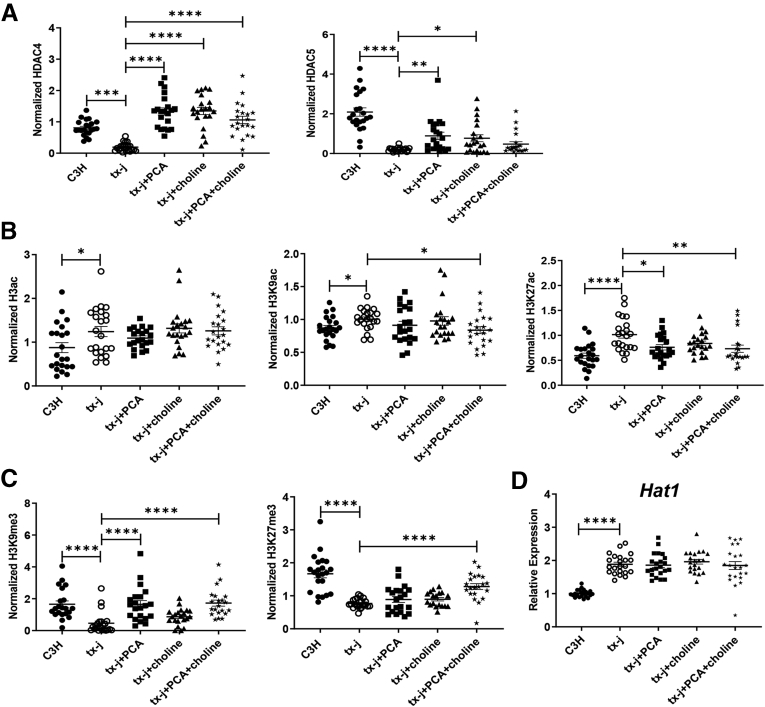
We next tested whether or not deficient HDAC5 levels in tx-j mice could be restored through interventions that reduce copper levels or increase methyl group donors. Twelve-week provision of D-penicillamine (PCA), a copper chelator, was associated with a significant increase in hepatic HDAC4 and HDAC5 levels, similar to choline supplementation (methyl group donor). However, combined treatment of PCA and choline only restored HDAC4 levels and not HDAC5 levels (Figure 4A). Acetylation and methylation status in response to PCA or choline treatments were examined by assessing total histone 3 acetylation as well as histone acetylation and methylation marks H3K9 and H3K27. Consistent with reduced HDAC4 and HDAC5 levels, H3ac, H3K9ac, and H3K27ac levels were increased in livers of tx-j mice compared with C3H, with H3K27ac changes the most prominent. PCA intervention counteracted histone acetylation and methylation mark effects, with restoration of H3K9ac and H3K27ac to C3H levels most apparent with combined treatments; however, total H3 acetylation remained unaffected (Figure 4B, Supplementary Figure 3). This implies the H3 acetylation response to PCA is specific to H3K9 and H3K27. H3K27me3 and H3K9me3 were reduced significantly in tx-j compared with C3H mouse livers, and PCA restored H3K9me3 and H3K27me3 to levels observed in C3H livers (Figure 4C). In contrast, choline supplementation alone was not associated with any changes in histone acetylation and methylation marks. The reduced levels of H3K9ac and H3K27ac and increased levels of H3K9me3 and H3K27me3 in the PCA+choline group compared with untreated tx-j, therefore, were likely an effect of PCA alone and not the combined effect of the 2 interventions. As histone acetylation is dynamically regulated by the balance between histone acetyltransferase and HDAC activities, it is worth noting Hat1 hepatic transcript levels were increased in tx-j mice at baseline with no significant effects induced by the interventions (Figure 4D).
Figure 4.
HDAC4 and HDAC5 downregulation is associated with increased histone acetylation and impaired methylation. Total protein liver lysate analyses of 24-week-old C3H (n = 10 males/12 females) and tx-j mice (n = 11 males/11 females), and tx-j mice treated with PCA (n = 11 males/10 females), choline (n = 8 males/13 females), and PCA+choline (n = 10 males/11 females). Immunoblot densitometries of HDAC4, HDAC5, H3ac, H3K9ac, H3K27ac, H3K9me3, and H3K27me3 were normalized to β-actin. Data are represented as means ± SEM and statistical analyses were performed with Kruskal–Wallis 1-way analysis of variance followed by an uncorrected Dunn's test (∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001). (D) Transcript levels of Hat1 normalized to Gapdh (C3H, n = 10 males/12 females; tx-j, n = 11 males/11 females).
Chromatin Immunoprecipitation Sequencing (ChIP-seq) for H3K9ac and H3K27ac Integrated With RNA Sequencing (RNA-seq) Reveals Altered Epigenetic Regulation and Transcript Levels of Genes Involved in Energy Metabolism and Liver Regeneration
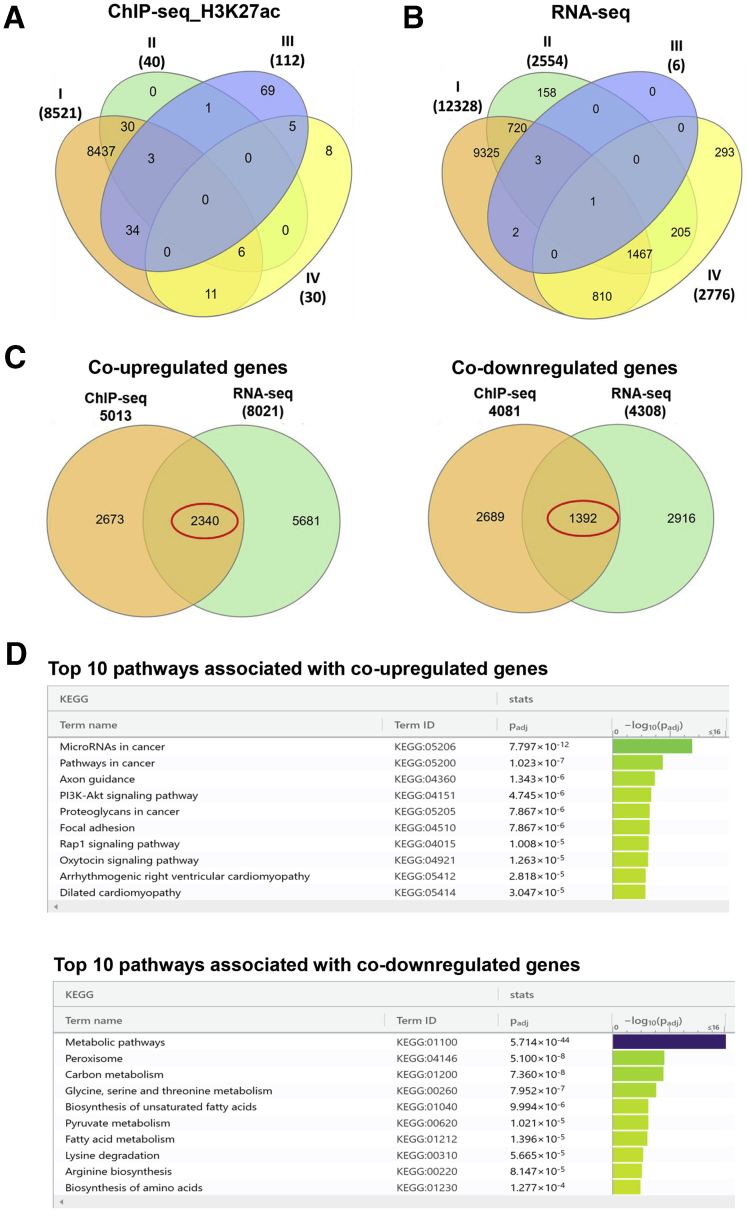
To identify genes regulated by the epigenetic alterations to histone acetylation in tx-j mice, we conducted and integrated analyses of H3K9ac and H3K27ac by ChIP-seq and gene expression by RNA-seq in the liver. Differential H3K27ac peaks in tx-j compared with C3H liver were mapped to more than 8000 genes (I) (Supplementary Table 1), but the number of significant differential H3K27ac-associated genes was reduced when tx-j mice were compared with those treated with PCA (II), choline (III), or the combination of the 2 interventions (IV) (Figure 5A). Differential H3K9ac peaks between tx-j and C3H liver were mapped to more than 500 genes (Supplementary Table 2). Similar to the H3K27ac analysis, transcriptome analysis revealed more than 10,000 genes differentially expressed in tx-j compared with C3H liver (I) (Supplementary Table 3), with fewer differentially expressed genes comparing groups of tx-j mice in response to interventions (II, III, and IV) (Figure 5B). When comparing untreated tx-j with PCA-treated tx-j liver, 40 genes were found to be differentially enriched for H3K27ac, whereas 2554 genes were differentially expressed (Supplementary Table 4 and 5). Increased methyl group availability through choline supplementation showed a small effect on histone acetylation-mediated gene transcription with 112 genes differentially acetylated and 6 genes differentially expressed when comparing untreated with choline-treated tx-j mice (Supplementary Tables 6 and 7). In PCA+choline-treated mice vs untreated, only 30 H3K27ac genes were enriched, whereas 2776 genes were differentially expressed (Supplementary Tables 8 and 9). As shown in volcano plots, there were significant differences between tx-j and C3H control mice for both H3K27ac ChIP-seq and RNA-seq, often in the same direction as either co–upregulated or co–downregulated genes (Supplementary Figure 4).
Figure 5.
H3K27ac-associated ChIP-seq and RNA-seq data analyses in the liver of tx-j mice. ChIP-seq and RNA-seq were performed in livers of 24-week-old C3H, tx-j, tx-j+PCA, tx-j+choline, and tx-j+PCA+choline groups (n = 3 males/3 females per group). (A and B) Venn diagrams showing numbers of H3K27ac-associated differentially enriched genes detected by ChIP-seq and differentially expressed genes by RNA-seq. I, C3H vs tx-j; II, tx-j vs tx-j+PCA; III, tx-j vs tx-j+choline; IV, tx-j vs tx-j+PCA+choline. (C) Numbers of common significantly co–upregulated and co–downregulated genes between ChIP-seq and RNA-seq analyses. (D) Pathway enrichment analysis showing the top 10 significantly associated pathways with differentially expressed co–upregulated and co–downregulated genes between ChIP-seq and RNA-seq by g:Profiler.
When ChIP-seq and RNA-seq analyses were overlapped, 2340 genes were co–upregulated and 1392 were co–downregulated between C3H and tx-j livers (Figure 5C, Supplementary Table 10). Pathway analysis of co–upregulated and co–downregulated genes from ChIP-seq and RNA-seq analyses using g:Profiler25 identified target genes related to the phosphatidylinositol-3-kinase and protein kinase B (PI3K–AKT) signaling pathway (55 genes), including Fgfr1, Bcl2l1, Ccnd2, Col4, Myb, and Lama5; 1-carbon metabolism (29 genes), including Gck, Aldh6a1, Cat, Eno, Adh5, Acat2, and Sdh; fatty acid metabolism (18 genes), including Scd1, Acaa2, Fasn, Scp2, and Acat2; lysine degradation (16 genes), including Kmt5a, Gcdh, Nsd1, Kmt2b, Setmar, Ehmt2, Acat2, and Setd1a; and biosynthesis of amino acids (20 genes), including Shmt2, Acy1, Eno1, Mat1a, and Pklr (Figure 5D, Supplementary Table 11).
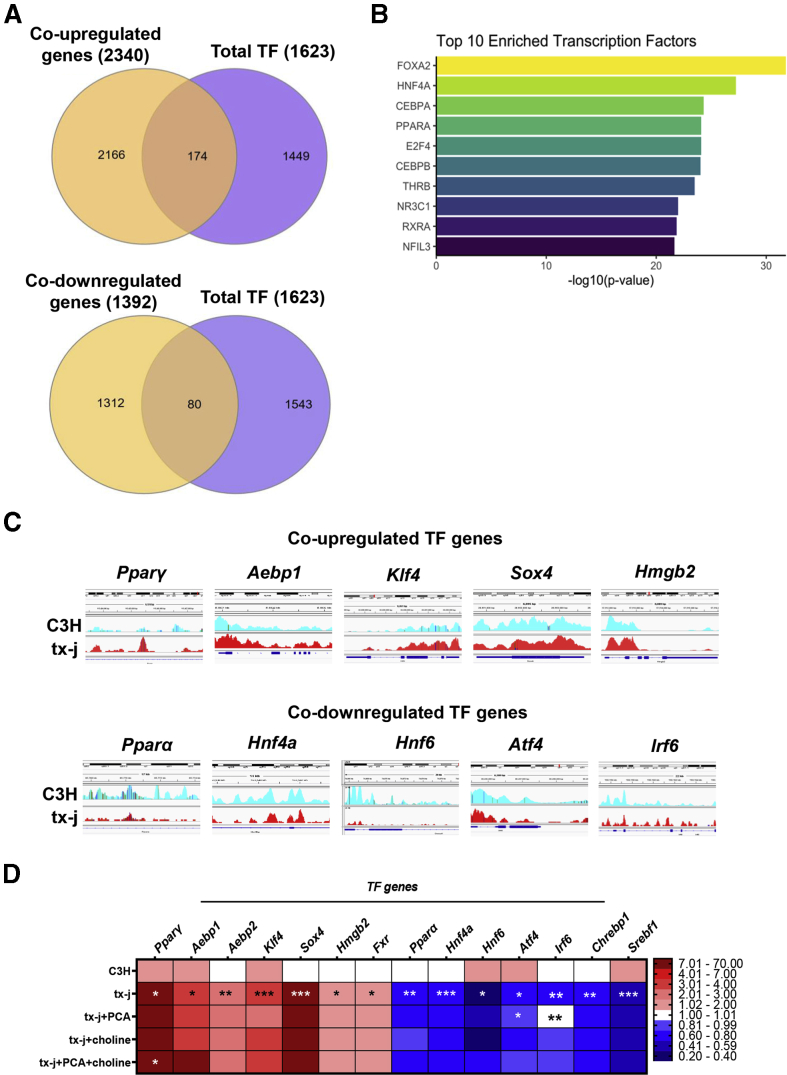
Next, we specifically examined differential expression of 1623 genes encoding transcription factors (TF) in tx-j compared with C3H mice. As shown in the Venn diagram, 174 TF genes were shared between tx-j co–upregulated genes and 80 TF genes were shared between tx-j co–downregulated genes when compared against total known TF genes in mice (Figure 6A, Supplementary Table 12). Epigenetic Landscape In Silico deletion Analysis (Lisa), which uses publicly available ChIP-seq data for transcription factors and chromatin regulators, was used to determine gene-enriched transcription factors. Top enrichments included transcription factors known to play a role in liver function/development (forkhead box A2 [FOXA2], hepatocyte nuclear factor 4 alpha [HNF4A], and retinoid x receptor alpha [RXRA]) and liver lipid metabolism (peroxisome proliferator-activated receptor α [PPARα]). (Figure 6B).
Figure 6.
Transcription factors within differentially expressed genes between C3H and tx-j mice. Using the ChIP-seq and RNA-seq data from livers of 24-week-old C3H and tx-j mice (3 males/3 females per group), differentially expressed genes in ChIP-seq and RNA-seq analyses were overlapped with total known mouse transcription factors. (A) Venn diagrams showing the number of common co–upregulated (174) and co–downregulated (80) transcription factor genes. (B) Bar graph showing the top 10 enriched TFs associated with differentially expressed co-regulated genes between ChIP-seq and RNA-seq by Lisa. (C) ChIP-seq peaks showing enrichment of H3K27ac for transcription factors, selected by their involvement in liver and metabolic diseases. (D) Heatmap displaying qPCR gene transcript levels of representative mouse transcription factors. Relative expression data are normalized to Gapdh (3 males/3 females per group). Data are represented as means ± SEM and statistical analyses were performed with Kruskal–Wallis 1-way analysis of variance followed by an uncorrected Dunn's test (∗P < .05, ∗∗P < .01, and ∗∗∗P < .001) for C3H vs tx-j and tx-j vs tx-j+PCA, choline, or combined treatments.
To gain mechanistic insight into histone deacetylation and acetylation-/methylation-associated TF regulation, we examined the transcript levels of key TF genes involved in liver development (Hnf4a and Hnf6), liver fibrosis (Aebp1 and 2, Smad2, and Hmgb2), steatosis and lipogenesis (Pparγ, Sox4, Fxr, Chrebp, Pparα, Irf6, and Srebf1), oxidative stress (Klf4 and Atf4), and apoptosis (Irf6). ChIP-seq peaks revealed notably different enrichment of H3K27 acetylation sites for several of these genes in tx-j mice compared with C3H (Figure 6C). Gene expression analysis revealed significantly upregulated TF genes, including, Pparγ, Aebp1 and Aebp2, Sox4, Klf4, Hmgb2, and Fxr, while significantly downregulated genes included Pparα, Hnf4a, Hnf6, Irf6, Atf4, Chrebp, and Srebf1 in tx-j compared with C3H mice (Figure 6D). Copper chelation with PCA significantly increased transcript levels of Irf6 and Atf4 while choline supplementation did not show any significant effect. PCA+choline only elicited a significant response from Pparγ.
HDAC5 Levels in tx-j Mice Are Modulated by High-Fat Dietary Intervention
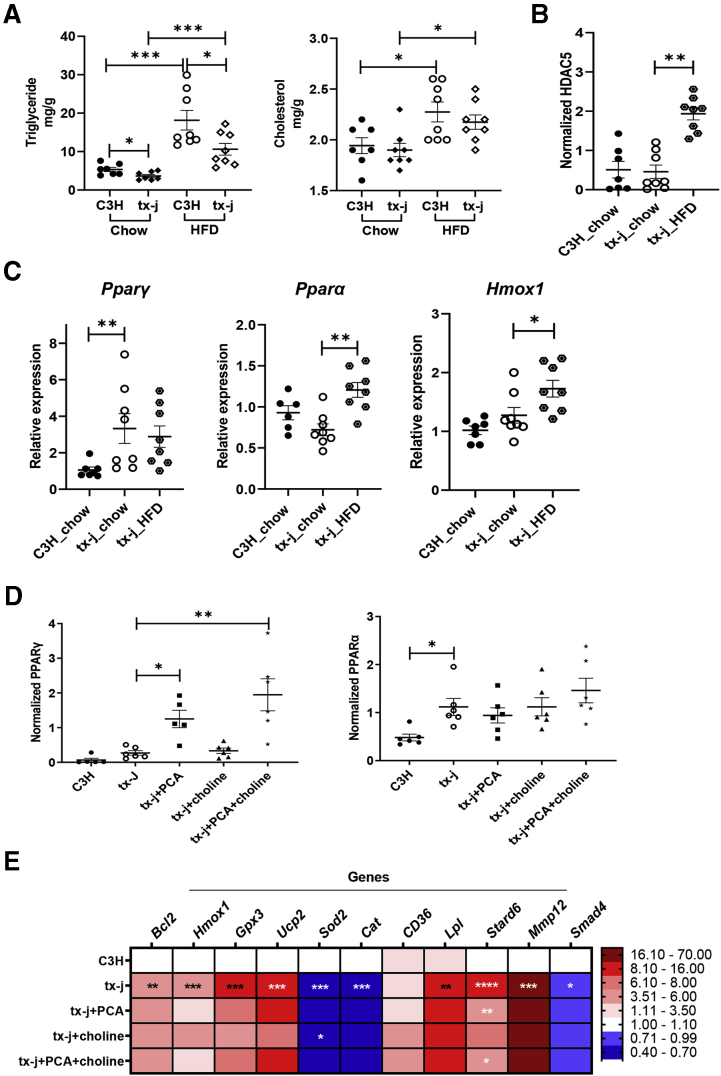
HDAC5 is known to be affected by nutrient availability and the metabolic state.26 Given this trait and the associated lipid-related metabolic pathways from the acetylome/transcriptome analyses, tx-j and C3H mice were challenged with a 60% kcal fat diet (HFD) for 8 days and livers were collected at 9 weeks of age. The 9-week time point was chosen as tx-j mice do not yet have significant liver disease at this early stage, therefore, changes seen in HDAC levels would be independent of liver damage. Tx-j mice on control chow demonstrated significantly lower levels of triglycerides compared with C3H (Figure 7A). Both tx-j and C3H mice on HFD had increased hepatic triglycerides and cholesterol compared with chow, confirming the HFD had a rapid and detectable effect in the liver. Following the same pattern, HDAC5 protein levels trended lower in tx-j mice on control chow compared with C3H; however, after 8 days of HFD, tx-j HDAC5 protein levels were significantly increased (Figure 7B, Supplementary Figure 5A).
Figure 7.
Dietary modulation (high fat, choline, PCA) of HDAC5 and metabolic regulators PPARα and PPARγ. (A–D) Data are represented as means ± SEM. Statistical significance for all analyses was determined by Student's t-test for comparison between 2 groups and Kruskal–Wallis 1-way analysis of variance followed by an uncorrected Dunn's test for multiple groups (∗P < .05, ∗∗P < .001, ∗∗∗P < .001, and ∗∗∗∗P < .001). (A) Liver triglyceride and total cholesterol of mice on chow (C3H, n = 4 males/3 females; tx-j, n = 4 males/4 females) compared with C3H and tx-j (n = 4 males/4 females each) fed a HFD. Mice were challenged with a HFD for 8 days and tissues were collected at 9 weeks of age. (B) HDAC5 protein expression in total protein liver lysate from mice on chow or HFD. Immunoblot densitometry analysis was normalized to β-actin. (C) Liver transcript levels of Pparγ, Pparα, and Hmox1 normalized to Gapdh in mice on chow or HFD. (D) Immunoblot densitometries of PPARγ and PPARα, normalized to β-actin, in total protein liver lysate of 24-week-old C3H (n = 3 males/3 females), tx-j (n = 3 males/2 females), tx-j+PCA (n = 3 males/3 females), tx-j+choline (n = 3 males/3 females), and tx-j+PCA+choline (n = 3 males/3 females). (E) Heatmap representing qPCR transcript levels of PPARα- and PPARγ-related genes measured in livers of 24-week-old C3H vs tx-j and tx-j vs tx-j+PCA, choline, or combined treatments (n = 3 males/3 females per group). Relative expression data are normalized to Gapdh.
PPARα and PPARγ were selected from the TF gene analysis for further examination as they are key regulators of lipid metabolism. PPARα and PPARγ were also shown to be associated with steatosis and the antioxidant response, and exhibited decreased and increased protein levels, respectively, concomitant with liver damage severity in patients with WD.27 During an early stage of disease progression (9 weeks of age), Pparγ transcript levels were significantly higher in tx-j than C3H mice and Pparα transcript levels were reduced, but 8 days of HFD were associated with significantly increased Pparα expression (Figure 7C). At a later stage of disease progression (24 weeks of age) in tx-j mice, PPARα and PPARγ (nonsignificant) protein levels were increased in tx-j compared with C3H mice (Figure 7D, Supplementary Figure 5B). In response to PCA, PPARγ levels were further increased compared with untreated tx-j, whereas PPARα was unaffected by PCA and/or choline. Moreover, transcript levels of PPARα- and PPARγ-associated genes Bcl2, Hmox1, Gpx3, Ucp2, Lpl, Stard6, and Mmp12 were significantly increased while Sod2, Cat, and Smad4 were decreased in tx-j mice compared with C3H. These findings indicate increases in fibrosis (Smad4), anti-apoptosis (Bcl2 and Mmp12), antioxidant responses (Hmox1, Ucp2, Gpx3, Cat, and Cd36), uptake of fatty acids and β-oxidation (Cat, Cd36, and Lpl), and lipogenesis (Stard6 and Sod2) in tx-j mice. PCA+choline treatment had a limited effect on gene expression, except on Stard6 (Figure 7E). Of note, Hmox1 was increased significantly in tx-j mice after 8 days of HFD (Figure 7C). Overall, these data suggest involvement of HDAC5 in the regulation of PPARα and PPARγ and their associated pathways in WD, and its potential to be modulated by dietary lipid intake.
Discussion
The clinical, metabolic, and liver pathology features of WD indicate this condition, although rare, presents characteristics of more common liver diseases, including nonalcoholic fatty liver disease. Even though copper accumulation is clearly the primary underlying mechanism of liver damage in WD, the intersection of dietary, epigenetic, bioenergetic, and metabolic defects are contributing, amplifying, and potentially acting as priming factors in the development of hepatic and extrahepatic manifestations. Therefore, it is of crucial importance to identify the modulating elements connecting genetic, epigenetic, and metabolic pathways in WD, and we propose HDACs as major players in this role. The main findings of our study are as follows: First, decreased HDAC4 and HDAC5 levels were identified in livers of tx-j mice starting at early stages of hepatic copper accumulation and progressing with age-related worsening of liver disease. Second, reduced levels of HDACs are associated with dysregulation of histone acetylation marks H3K9ac and H3K27ac, corresponding with effects on gene expression. Furthermore, HDAC and histone acetylation alterations were counteracted by dietary supplementation of fat or methyl groups, or copper chelation by PCA. Finally, the genes affected by H3K27 acetylation in WD are involved in metabolism regulation. In recent years, expanding data have shown HDACs are key regulators of glucose homeostasis, and cardiovascular and endothelial function.28,29 HDACs also are known to be regulated by dietary factors as well as fasting vs feeding status. In agreement with available research, we demonstrated class II HDAC levels are regulated by multiple factors including copper levels, availability of methyl groups, and dietary fat.
Several studies have shown class IIa HDACs are regulated by AMPK via phosphorylation.30 Class IIa HDACs are highly regulated by their phosphorylation/dephosphorylation status with consequent modulation of gene transcription. HDAC5 molecules are phosphorylated at a number of serine residues that provide binding sites for chaperone proteins to export HDAC from the nucleus to the cytosol.31 Sequence analysis indicated phosphorylation sites are largely preserved between HDAC isoforms. In mouse livers, class IIa HDACs are phosphorylated by AMPK family kinases.32 Hepatocyte-specific knockdown of class IIa HDACs via RNA interference-induced deacetylation of FOXO led to inhibition of FOXO target genes with consequent lower blood glucose levels and increased glycogen storage. Salt-inducible kinase–dependent dephosphorylation of HDAC4 increases lipolysis through FOXO1 inactivation that eventually leads to depletion of accumulated triglycerides. Conversely, HDAC4 downregulation restores lipid accumulation.33,34 In mouse primary hepatocytes, HDAC5 plays a role in integrating the fasting state and endoplasmic reticulum (ER) stress signals to regulate hepatic fatty acid oxidation. This suggests HDAC5 could be a target for the treatment of obesity-associated hepatic steatosis.20 Taken together, these studies indicate central roles for class II HDACs in metabolic processes, including glucose homeostasis, energy metabolism, and lipogenesis.
While the primary downstream effects of class II HDACs impact glucose and lipid metabolism, they also have detrimental systemic effects. A mouse model with a liver-specific knockdown of class IIa HDACs, including HDAC4, HDAC5, and HDAC7, exhibits not only carbohydrate and lipid alterations in hepatocytes, but also systemic manifestations involving kidney and spleen abnormalities.35 This suggests the combination of reduced class IIa HDAC levels and increased copper levels could explain WD systemic manifestations, which span from brain to heart and kidney involvement. Class II HDACs have also shown involvement in other conditions, including cardiovascular, neurologic, metabolic, and intestine-related disorders. HDAC4 and HDAC5 affect neuronal development and axon regeneration; a lack of HDAC4 leads to severe skeletal abnormalities, neuronal developmental defects, and impairment of memory function.36,37 Copper dysregulation occurs in neurologic disorders such as Parkinson’s disease and Alzheimer’s disease.38 Interestingly, Parkinson’s and Alzheimer’s diseases have been associated with both high and low copper levels in the brain.39,40 Corroborating our studies, increased histone acetylation was associated with reduced HDAC levels in mouse brains as well as midbrain tissues of patients with Parkinson’s disease.41 Previous studies revealed a role for class IIa HDACs in gene expression regulation of cardiac hypertrophy and contractile dysfunction.42 Cardiac hypertrophy is associated with copper deficiency.43 HDAC4 and HDAC5 regulate GLUT4 and LXR transcription in response to increased cyclic AMP (cAMP) signaling in cultured adipocytes of fasted mice.44 In 3T3-L1 adipocytes, hypoxia induced both HDAC5 and HDAC6 reduction with associated changes in the expression of adipokines and inducible cAMP early repressor and consequent development of metabolic abnormalities.45 It is also known that copper plays an important role in regulating lipid metabolism in various tissues, including skeletal muscle and adipose tissues.46,47 In the intestine, copper and lipid misbalances could be linked to gastrointestinal manifestations of WD,48 and HDAC effects on inflammation and barrier function in the intestinal epithelium are under investigation.49 Nevertheless, no studies have directly explored the connection between copper levels and HDAC regulation in brain, cardiovascular, or gastrointestinal diseases. We can speculate that copper may play an important role in gene expression through HDAC regulation in extrahepatic tissues of WD and other diseases.
A key element in interpreting our data is the time-course study on HDAC5 levels. Reduced hepatic HDAC5 levels were observed starting at day 6 postpartum, when hepatic copper levels are normal. The progression of hepatic copper accumulation and hepatic inflammation and fibrosis accompanied progressive reductions in tx-j HDAC levels, although it is evident that reduced HDAC levels are intrinsic to the tx-j mouse model and likely the result of impairments in development and regeneration mechanisms.10 These data are, therefore, more consistent with the reported proteasome-dependent degradation of phosphorylated class IIa HDACs in the cytosol50 than cytosolic protection by 14-3-3 binding.51
The regulation of HDAC5 and HDAC4 is complex. We propose the simplest mechanism explaining reduced HDAC5 in WD and its subsequent impact on transcription is phosphorylation by AMPK, leading to relocation from the nucleus to the cytosol and followed by degradation. Acute oxidative stress, ATP depletion, and transiently increased calcium are conditions thought to initiate phosphorylation of class IIa HDACs.52 Notably, AMPK is a kinase that is well known to be activated to various degrees by all these stimuli.53 The phosphorylation of threonine-172 activates AMPK and is associated with increased transcript levels of genes related to metabolism. As shown in our study and reported by previous in vitro studies, AMPK can phosphorylate HDAC5.23 Our current data showed increased ratios of phosphorylated to unphosphorylated AMPK and HDAC5 in WD mice (Figure 3). Moreover, hepatocyte treatment with an AMPK activator, AICAR, was associated with reduced expression of HDAC5, likely owing to a nucleus-to-cytosol pHDAC5 shift.
Our results reveal that HDAC5 levels are affected by dietary factors such as availability of methyl groups via choline supplementation and excess dietary fat. This likely has an effect on the risk of developing hepatic steatosis as well as systemic metabolic manifestations. Reduced class IIa HDAC4 and HDAC5 might play a central role in the regulation of H3K9 and H3K27 acetylation/methylation. Of note, HDAC5 knockdown was shown to increase H3K9 acetylation in mouse C3H10T1/2 cells,54 similar to tx-j vs C3H mice in our study. Altered HDAC4/5 regulation coincides with copper overload as shown in tx-j mice where acetylation and methylation are restored to control levels in response to copper chelation (Figure 4). H3K9ac and H3K27ac are associated with transcriptionally active chromatin, whereas H3K9me3 and H3K27me3 are associated with an inactive heterochromatin state. In our studies, this histone acetylation–methylation shift may lead to transcriptional reprogramming with consequent aberrant expression of numerous genes in WD.
ChIP-seq for H3K27 acetylation and RNA-seq analyses confirmed the regulation of hepatic metabolic pathways through histone acetylation mechanisms. In particular, the PI3K–AKT pathway was co-upregulated in tx-j compared with CH3 liver across both analyses. This pathway is of interest as it is extensively shown to be upregulated during the progression of nonalcoholic fatty liver disease with an associated risk of liver cancer.55,56 Other co-regulated pathways included one-carbon metabolism, which plays an important role in regulating energy metabolism and immune function in fatty liver disease and fibrosis.57 Our previous study also revealed copper-mediated inhibition of SAH hydrolase, and the consequent accumulation of SAH, leads to global DNA hypomethylation in tx-j mice.12 Our current findings corroborate and add to previous reports indicating an acetylation/methylation shift is involved in the regulation of metabolic pathways and related TF genes, including Pparα, Pparγ, Chrebp, Srebf1, Fxr, Lxr, Rxra, and Stard6 (Figure 6D, Supplementary Table 13). Some important differentially regulated genes involve the following pathways: cell regeneration (Hnf4a, Hnf6, and Myb); cell cycling, development, and differentiation (Fgfr1, Bcl2, Sox, Timp2, Gata2, and Klf4); fatty acid metabolism (Scd1, Fads, Fasn, and Acat2); and oxidative and ER stress (Hmox1 and Atf4). Our previous studies on DNA methylation in liver from WD patients showed hypermethylated WD liver DMRs were enriched in liver-specific enhancers, flanking active liver promoters and binding sites of liver developmental transcription factors, including HNF4A, RXRA, and FOXA2,9 which were recapitulated in the top 10 transcription factors enriched in this RNA-seq data set. This suggests convergence of DNA methylation and transcription factor regulation of gene expression in WD. Two of the most interesting targets are PPAR isoforms PPARα and PPARγ, known to regulate lipid and glucose homeostasis through their coordinated activities in liver, muscle, and adipose tissue.58 Our findings of increased PPARα and PPARγ in tx-j mice correspond to a study indicating changes in PPARα and PPARγ in WD patients.27 Previous studies have shown PPARα increases with mild liver damage and decreases with moderate or greater liver damage, while PPARγ was increased in WD patients concomitantly with the progression of liver damage. No previous studies have focused on PPAR signaling in WD. Many studies have reported the anti-inflammatory, antioxidant, and anti-apoptotic roles of PPARα and PPARγ,59, 60, 61, 62 which are supported further by our data of significantly increased PPARγ gene and protein expression after PCA treatment.
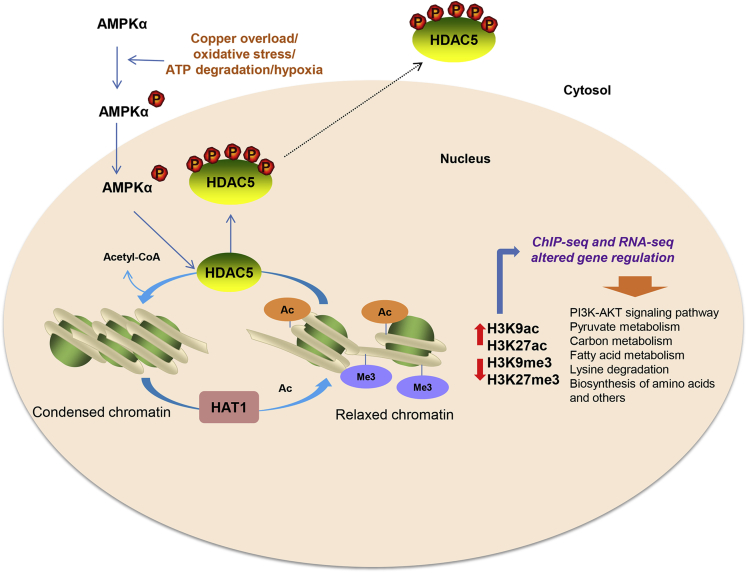
Activation of PPARα and PPARγ signaling is known for decreasing triglyceride and cholesterol levels in liver and plasma of mice and humans.63 Corroborating these effects, we also found liver (Figure 7A) and plasma (data not shown) triglyceride and total cholesterol levels reduced in tx-j mice on chow as well as HFD compared with control. Furthermore, the observed PPARγ effect on antioxidant gene expression of Hmox1, Ucp2, Gpx3, and Cd36, and anti-apoptotic gene Bcl2, could be part of a protective mechanism against copper-mediated oxidative stress in WD. A previous study showed mice fed a high-fat diet for 4 weeks exhibited increased HDAC5 levels in the medial hypothalamus64; we confirmed that even a short-term, 8-day, high-fat challenge induced a dramatic HDAC5 response. Interestingly, Hmox1 and Pparα (Figure 7C) showed significant upregulation after short-term HFD exposure in tx-j mice. Bringing together all of the studied elements, a working model of HDAC5 regulation and impact on histone acetylation and subsequent gene regulation is proposed in Figure 8.
Figure 8.
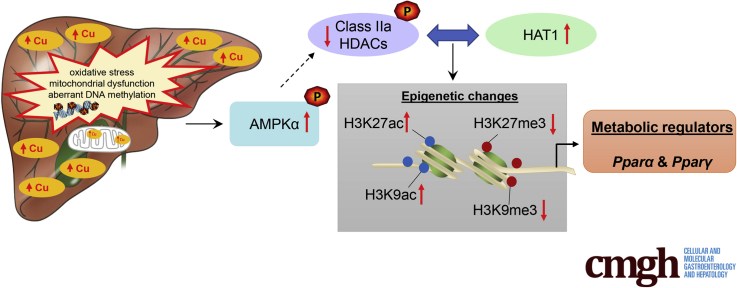
Proposed schematic of HDAC5-mediated H3K9ac and H3K27ac regulation of gene expression and affected biological pathways in WD. In WD, copper overload and oxidative stress might lead to phosphorylation of AMPK (active form). Increased phosphorylated AMPK could then phosphorylate HDAC5, which is subsequently exported to the cytosol. Lack of nuclear HDAC5 and increased histone acetyltransferase (HAT1) might cause an increase in acetylated histones (H3K9ac and H3K27ac) and decrease methylated histones (H3K9me3 and H3K27me3) with subsequent altered regulation of genes in WD. Our results show HDAC5 impacts the acetylation/methylation balance and serves as a critical regulator of genes central in metabolic regulation. ChIP-seq and RNA-seq revealed 3732 differentially expressed genes in the tx-j mouse model of WD, and the enrichment analysis of these genes included pathways related to lysine degradation, fatty acid metabolism, carbon metabolism, pyruvate metabolism, and signal transduction. PI3K-AKT, phosphatidylinositol-3-kinase and protein kinase B.
One disappointing but still insightful finding of our study is the limited effect of PCA, choline, and combined treatment on the number of genes regulated by histone acetylation. This could be interpreted as a potential explanation for the lack of a complete response to anti-copper treatment often observed in patients with WD. These findings may be due to the relatively short duration of intervention; copper chelation alone is not enough to reverse metabolic perturbations; and dietary choline can be shunted to other pathways in addition to DNA methylation. It remains unknown if metabolic aberrations in WD are independent or a direct result of copper accumulation. It is clear, however, that removal of excess copper alone cannot restore all epigenetically compromised pathways in WD. More interventions targeting HDAC5 and other identified dysregulated pathways are warranted and could serve as adjunct treatments. An appealing facet of HDAC5 is its ability to be modulated rapidly through diet, making it a potential target particularly for early intervention to prevent severe liver damage and associated metabolic complications. Among the identified TFs, PPARγ could emerge as a therapeutic target for fatty liver in WD, especially during the course of treatment with PCA or other copper chelators. Ultimately, class IIa HDAC4 and HDAC5, as key metabolic elements, represent a regulatory interface between genetic and metabolic factors affecting the varied phenotypic presentations and liver disease manifestations in WD.
Material and Methods
Animals
C3H control, tx-j, and Atp7b-/- colonies were maintained at 20°C–23°C, 40%–65% relative humidity, and a 14-hour light/10-hour dark light cycle. Mice were maintained ad libitum on deionized water and LabDiet 5001 chow (Purina Mills, Inc, St. Louis, MO). C3H and tx-j mice were each maintained as homozygous colonies. Atp7b-/- mice were maintained by a heterozygous breeding colony, which generated both homozygous mutant and wild-type controls. Tx-j mouse milk lacks sufficient copper for neonatal growth and development beyond day 10 on average, hence all tx-j pups were fostered to a lactating C3H dam by day 7 postpartum with the exception of postpartum day 6 pups, from which tissues were collected before fostering. Progeny were weaned between 21 and 28 days of age. A subgroup of the 9-week tx-j cohort was challenged with a 60% kcal fat diet (cat. D12492; Research Diets, New Brunswick, NJ), 8 days before tissue collection. At postpartum day 6, and 9, 12, 16, and 24 (C3H only) weeks of age, mice were euthanized and livers were flash-frozen in liquid nitrogen and then stored at -80°C until further analysis. All tissues were collected in the morning between 3 and 6 hours after lights went on in the animal room. All groups had relatively equal male/female representation.
For the 24-week tx-j cohort, tx-j females were fed either control Teklad 2020 chow (custom TD140163; Envigo, Madison, WI) or choline-supplemented Teklad 2020 chow (5 g/kg diet, custom TD160348; Envigo, Madison, WI) 2 weeks before mating. To circumvent the homozygous recessive mutation in tx-j dams that causes copper-deficient milk production, heterozygous tx-j breeders were used for this cohort to generate homozygous mutant tx-j progeny. Three days before mating, male breeders were fed whichever diet matched their prospective female to acclimate. Dams continued with their respective diets through mating, gestation, and lactation. DNA samples were obtained from pups between 8 and 12 days postpartum for genotyping. Genotyping was performed by the UC Davis Mouse Biology Program via TaqMan allelic discrimination. Progeny were weaned between 21 and 27 days of age onto the same diet as their dam. From 12 to 24 weeks of age, PCA (cat. P4875; Sigma-Aldrich, St. Louis, MO) was administered orally in the drinking water (100 mg/kg body weight/d) to a subgroup of tx-j control and choline-supplemented mice. Mice were euthanized at 24 weeks of age and livers were flash-frozen in liquid nitrogen and then stored at -80°C until further analysis. All tissues were collected in the morning between 3 and 6 hours after lights went on in the animal room. All groups had relatively equal male/female representation.
All mouse protocols were reviewed and approved annually by the UC Davis Institutional Animal Care and Use Committee and followed the guidelines of the American Association for Accreditation of Laboratory Animal Care. Humane care was provided according to criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (publication 86–23, revised 1985; Bethesda, MD).
HepG2 Cell Culture Experiments
HepG2 cells were obtained (HB-8065, lot: 70015966; American Type Culture Collection, Manassas, VA) and cultured in Eagle’s minimum essential medium with L-glutamate (cat. 50983283; Quality Biological, Gaithersburg, MD), 10% fetal bovine serum (cat. FB12999102; Fisher Scientific, Hanover Park, IL), and 1% penicillin/streptomycin (cat. 15140122; Gibco, Grand island, NY); cells were incubated at 37°C and 5% CO2. An equal number of cells were seeded in each well of 6-well or 10-cm collagen-treated cell culture plates per treatment group and incubated overnight to attach, with subsequent media changes twice per week. Upon 70%–75% confluency, cells were washed twice with phosphate-buffered saline (PBS) and treated with 100 mmol/L CuSO4 for 24 hours. Untreated HepG2 control cells were washed twice with PBS and provided with fresh medium. The following day, copper-treated cells were washed twice with PBS and subjected to treatment with PCA (1 mmol/L, cat. P4875; Sigma-Aldrich, St. Louis, MO), 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside in dimethyl sulfoxide (0.1–1 mmol/L, AICAR, cat. 501011663; Fisher Scientific, Hanover Park, IL), or isoproterenol (5–25 μmol/L, cat. I2760; Sigma-Aldrich, St. Louis, MO) for 24 hours.
RNA Isolation
Total RNA was extracted from frozen mouse liver (20–25 mg) and HepG2 cells (5–6 million) using the AllPrep DNA/RNA Mini Kit (cat. 80204; Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Sample purity and concentration were measured by a Thermo Scientific Nanodrop spectrophotometer (Carlsbad, CA) and RNA integrity was evaluated by agarose gel electrophoresis. Total RNA was stored at -80°C until further use.
Quantitative Polymerase Chain Reaction (qPCR)
Mouse liver total RNA (5 μg) and HepG2 total RNA (4 μg) was input to reverse-transcribe with the SuperScript III First-Strand cDNA Synthesis kit (cat. 18080051; Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Primers for mouse cDNA sequences were designed using the free online application Primer 3 (http://bioinfo.ut.ee/primer3-0.4.0) and blasted against the mouse genome using NCBI Nucleotide BLAST to check primer specificity (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The amplification efficiency (E) of all assays was calculated from the slope of a standard curve generated via 10-fold serial dilution of pooled control cDNA using the following formula: E = 10(-1/slope)-1. Melt curve analysis and agarose gel electrophoresis of the PCR product were checked to confirm amplicon specificity. All samples (no-template control, mouse cDNA 1/25 dilution, interplate QC were run in triplicate with SYBR Green Master Mix (cat. 4364344; Applied Biosystems, Foster City, CA) on an Applied Biosystems ViiA 7 Real-time PCR System. Primer sequence details are listed extensively in Supplementary Table 13.
Western Blot
Mouse liver samples (35–40 mg) were homogenized on ice with a hand-held OMNI International TH homogenizer with ice-cold RIPA lysis buffer containing Complete Mini Protease Inhibitor Cocktail and PhosSTOP Phosphatase Inhibitor Cocktail (cat. 11836170001 and 4906845001; Roche Diagnostics, Mannheim, Germany). In cell culture experiments, cells were lysed by adding ice-cold RIPA buffer directly to each well. Cell protein lysates were homogenized by sonication for 2–5 seconds. Both cell and tissue lysates were centrifuged for 20 minutes at 10,000 rpm and 4°C and the supernatant was collected. Protein concentration was determined with the Pierce BCA Protein Assay Kit (cat. 23225; Thermo Fisher Scientific, Rockford, IL) according to the manufacturer’s protocol. All samples and standards were plated in duplicate and read on a Bio Tek Synergy H1 microplate reader. Equal amounts of protein (25 μg) were denatured 1:1 in 2× Laemmli buffer (cat. 1610737; Bio-Rad, Hercules, CA) at 95°C for 10 minutes, and then held on ice. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto a pre-activated polyvinylidene difluoride membrane using the Bio-Rad Trans-Blot Turbo transfer system. Membranes were blocked with 5% nonfat milk in tris-buffered saline with Tween-20 (TBST) for 2 hours and then probed with the desired primary antibodies overnight at 4°C. Membranes were washed in TBST and then incubated with the appropriate horseradish-peroxidase–conjugated anti-rabbit or anti-mouse IgG secondary antibody for 1 hour at room temperature. Membranes were washed in TBST again and blots were developed with Clarity Western ECL substrate (cat. 1705061; Bio-Rad, Hercules, CA). Membranes were visualized using a FujiFilm LAS-4000 imaging system. Densitometry analyses were quantified using Multi Gauge software (Fujifilm, Cambridge, MA) and normalized to β-actin. Antibodies and dilutions are listed in Table 1.
Table 1.
Antibodies for Immunoblotting
| Antibody | MW | Vendor | Cat. number | Dilution |
|---|---|---|---|---|
| Primary antibodies | ||||
| AMPKα | 62 | Cell Signaling Technology, Danvers, MA | 23A3 | 1:1000 |
| H3ac | 17 | Millipore, Burlington, MA | 06-599 | 1:500 |
| H3K27ac | 15 | Abcam, Cambridge, MA | ab177178 | 1:1000 |
| H3K27me3 | 15 | Sigma-Aldrich, St. Louis, MO | 07-449 | 1:500 |
| H3K9ac | 17 | PMT BIOLABS, Chicago, IL | PMT-156 | 1:2000 |
| H3K9ac | 17 | Active Motif, Carlsbad, CA | 39918 | 1:500 |
| H3K9me3 | 17 | Abcam, Cambridge, MA | ab8898 | 1:1000 |
| HDAC5 | 124 | Cohesion Biosciences, London, UK | CPA2445 | 1:500 |
| HDAC4 | 119 | Boster Biological Technology, Pleasanton, CA | A00971-1 | 1:500 |
| PPARα | 52 | Abcam, Cambridge, MA | ab24509 | 1:500 |
| PPARγ | 53 | Cell Signaling Technology, Danvers, MA | 81B8 | 1:1000 |
| pAMPKα | 63 | Cell Signaling Technology, Danvers, MA | 2535 | 1:1000 |
| pHDAC5 | 124 | Affinity Biosciences, Pottstown, PA | ABIN6267700 | 1:500 |
| β-actin | 42 | Sigma-Aldrich, St. Louis, MO | A5441 | 1:5000 |
| Secondary antibodies | ||||
| Anti-mouse | Jackson Immunoresearch Laboratories, West Grove, PA | 115-035-003 | 1:10,000 | |
| Anti-rabbit | Jackson Immunoresearch Laboratories, West Grove, PA | 111-035003 | 1:10,000 | |
MW, molecular weight.
Immunohistochemistry
Immunohistochemical analysis was performed as previously described.65,66 Briefly, mouse liver tissues were fixed in 10% zinc formalin, embedded in paraffin, and sectioned. Tissue sections were stained with anti-HDAC5 antibody (cat. sc-133225; Santa Cruz Biotechnology, Dallas, TX) and cell nuclei were detected by 4′,6-diamidino-2-phenylindole. Parallel staining with nonspecific IgG was performed to detect background noise. Imaging was performed using a Keyence All-in-One Fluorescence Microscope BZ-X800 (Osaka, Japan), and optical density analysis was performed using ImageJ (version 1.53h, National Institutes of Health, Bethesda, MD). Tissues from 3 mice/group, 60 cells/mouse were analyzed.
Liver Triglycerides and Cholesterol
All samples were assayed by the UC Davis Mouse Metabolic Phenotyping Center. Briefly, 100–110 mg liver was homogenized using sodium sulfate and a pestle and mortar. Chloroform and methanol were added at a 2:1 ratio and samples were incubated overnight at 4°C. Sodium chloride (0.7% solution) was added the next day and samples were incubated overnight at 4°C to separate the chloroform layer. The next day, the supernatant was aspirated, and a sample was taken from the chloroform layer and evaporated using N2 gas. The sample was reconstituted with isopropanol and reagents from Fisher Diagnostics (Carlsbad, CA) were used to assay for total cholesterol (cat. TR13421) and triglycerides (cat. TR22421).
Chromatin Immunoprecipitation
ChIP was performed using the MAGnify Chromatin Immunoprecipitation System (cat. 492024; Thermo Fisher Scientific, Carlsbad, CA) according to the manufacturer’s instructions. Frozen liver tissue (50 mg) was minced in Dulbecco's PBS and homogenized with a dounce tissue grinder (cat. D8938-1SET, Sigma-Aldrich, St. Louis, MO). At room temperature, homogenized tissue was cross-linked with 1% formaldehyde for 10 minutes and then quenched with 0.125 mol/L glycine for 5 minutes, swirling gently approximately every 2 minutes during incubation. The cross-linked mixture was centrifuged at 200 × g for 10 minutes at 4°C and then washed twice with cold PBS buffer and resuspended in lysis buffer supplemented with proteinase inhibitors for 1 hour at 4°C. The lysates were sonicated with an E220 sonicator (Covaris, Woburn, MA; peak incident power, 140 W; duty factor, 5%; cycle/bust, 200; duration, 75 s) to yield 200–500 base pair DNA fragments. Samples were centrifuged at 12,000 × g for 15 minutes at 4°C to pellet debris and the chromatin-containing supernatant was transferred to new sterile tubes.
Next, the chromatin samples were diluted in cold dilution buffer with protease inhibitors to a final volume of 100 μL per reaction and 10 μL from each chromatin sample was reserved for input control. Coupled antibody–Dynabeads were prepared by adding Dynabeads protein A/G and 2 μg antibody (H3K9ac [cat. 39918; Active Motif, Carlsbad, CA] or H3K27ac [cat. ab177178; Abcam, Cambridge, MA]) to 100 μL ice-cold dilution buffer and rotating the tubes end-over-end at 4°C for 1 hour. ChIP was performed by incubating diluted chromatin with the appropriate antibody–Dynabeads complex and rotating the tubes end-over-end at 4°C for 2 hours. Using a Thermo Fisher Scientific DynaMag-PCR magnet, the beads were washed 3 times each with ice-cold IP buffer 1 and twice with ice-cold IP buffer 2. The supernatant was discarded, and reverse cross-linking buffer prepared with proteinase K was added into the IP sample tubes to reverse the cross-linking. The beads were resuspended fully by vortexing and incubated at 55°C for 15 minutes in a Bio-Rad C1000 Touch thermal cycler, and then repelleted with the magnet. The IP sample liquids were transferred to new sterile tubes, incubated at 65°C for 15 minutes, and cooled on ice for 5 minutes.
To purify the DNA, magnetic purification beads prepared with DNA purification buffer were added to each tube. After pipetting up and down 5 times to mix, samples were incubated at room temperature for 5 minutes, beads were pelleted with the magnet, and the supernatant was discarded. The beads were washed 2 times using DNA wash buffer following the same procedure. The DNA was eluted with DNA elution buffer by incubating at 55°C for 20 minutes in a thermal cycler. The purified DNA was analyzed by ChIP–qPCR data relative to input using the mouse negative control and mouse positive control primer set Gapdh in the ChIP-IT qPCR analysis kit (cat. 53029; Active Motif). Reaction conditions were programmed with initial denaturation at 50°C for 2 minutes and 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. The relative percentages of input enrichment and fold enrichment were calculated with the following formulas: [100∗2ˆ (Cq adjusted input-Cq IP)] and 2-ΔΔCq, respectively.
ChIP-Seq Analysis
KAPA Hyper Prep Kit (cat. KK8502; Kapa Biosystems, Wilmington MA) was used for end repair, A-tailing, and adapter ligation with the KAPA Unique Dual-Indexed Adapter Kit (cat. KK8727; Kapa Biosystems, Wilmington MA) according to the manufacturer’s instructions. Briefly, ChIP DNA was subjected to end repair and A-tailing by adding End Repair and A-Tailing buffer and enzyme mix, incubating at 20°C for 30 minutes, followed by 60°C for 30 minutes, and then cooling to room temperature. Adaptor ligation was performed by adding adapter stock, ligation buffer, DNA ligase, and PCR-grade water to the reaction product, and incubating at 20°C for 25 minutes. Post-ligation clean-up was performed with 0.8× KAPA Pure Beads (Kapa Biosystems, Wilmington MA); beads were mixed with the ligation product and incubated for 15 minutes to bind DNA to the beads. The bead/DNA complex was captured using the DynaMag-PCR magnet and washed twice with 80% ethanol. Purified DNA was eluted with DNA elution buffer at room temperature for 2 minutes. Library amplification was performed with KAPA HiFi HotStart ReadyMix and Library Amplification Primer Mix, and the following PCR program: initial denaturation at 98°C for 45 seconds, denaturation at 98°C for 15 seconds, annealing at 60°C for 30 seconds, extension at 72°C for 30 seconds, final extension at 72°C for 1 minute, and then hold at 4°C. Library amplification was performed for 7 (H3K27ac) and 13 cycles (H3K9ac), plus 1 full cycle on the PCR product to get rid of PCR bubbles. A 0.7×–0.9× KAPA Pure Beads double-sided size selection was performed after PCR amplification. An Agilent Bioanalyzer 2100 was used to profile the sizes, concentrations, and qualities of the libraries. Libraries were subjected to high-throughput sequencing by Novogene Corporation, Inc, on an Illumina NovaSeq S4 platform and 150-bp paired-end reads were generated.
ChIP-seq data analysis was performed by the UC Davis Bioinformatics Core. The raw read data were filtered using HTStream (version 1.2.0, Moscow, ID), which included screening for contaminants (such as PhiX), removing PCR duplicated reads, overlapping paired reads, quality-based trimming, and adapter trimming. BWA MEM (version 0.7.16a, Cambridge, UK) was used to align the processed data to the mouse genome (GRCm38). Calling peaks was performed using phantompeakqualtools (version 1.2.2, Stanford, CA). Bioconductor (version 3.11, Boston, MA) package DiffBind (version 2.12.0, Cambridge, UK) was used to combine raw count data for all peaks across replicates using R (version 3.6.3, Vienna, Austria). Finally, GREAT (version 4.0.4, Stanford, CA) was used to find the regulatory domains for the peaks and the genes within those domains.
RNA-Seq Analysis
RNA-seq library production and sequencing were performed by Novogene Corporation, Inc. (Sacramento, CA). RNA degradation and contamination were monitored on 1% agarose gels and purity was checked using the Implen NanoPhotometer spectrophotometer (Westlake Village, CA). RNA integrity and quantitation were assessed using the RNA Nano 6000 Assay Kit (cat. 5067-1511; Agilent, Santa Clara, CA) of the Agilent Bioanalyzer 2100 system. A total of 1 μg RNA per sample was used as input material. Sequencing libraries were generated using the NEBNext Ultra RNA Library Prep Kit for Illumina (cat. E7530L; New England Biolabs, Ipswich, MA) following the manufacturer’s recommendations, and index codes were added to attribute sequences to each sample. Briefly, mRNA was purified from total RNA using poly-T oligo-attached magnetic beads. Fragmentation was performed using divalent cations under elevated temperature in NEBNext First Strand Synthesis Reaction Buffer. First-strand cDNA was synthesized using random hexamer primers and M-MuLV Reverse Transcriptase (RNase H-). Second-strand cDNA synthesis was subsequently performed using DNA Polymerase I and RNase H. Remaining overhangs were converted into blunt ends via exonuclease/polymerase activities. After adenylation of the 3’ ends of DNA fragments, NEBNext adaptors (cat. E7600S; New England Biolabs, Ipswich, MA) with hairpin loop structure were ligated to prepare for hybridization. To preferentially select cDNA fragments of approximately 150–200 bp in length, the library fragments were purified with the AMPure XP system (cat. A63881; Beckman Coulter, Beverly, MA). USER enzyme (3 μL) was used with size-selected, adaptor-ligated cDNA at 37°C for 15 minutes followed by 5 minutes at 95°C before PCR. PCR was performed with Phusion High-Fidelity DNA polymerase, NEBNext Universal PCR primer for Illumina, and NEBNext Index primer. PCR products were purified again with the AMPure XP system and library quality was assessed on the Agilent Bioanalyzer 2100. The clustering of the index-coded samples was performed on a cBot Cluster Generation System using PE Cluster Kit cBot-HS (cat. PE-401-4001; Illumina, San Diego, CA) according to the manufacturer’s instructions. After cluster generation, the library preparations were sequenced on an Illumina NovaSeq S4 platform and 150-bp paired-end reads were generated.
Raw reads (FASTQ) were processed through fastp (version 0.20.0, Shenzhen, China), removing reads containing adapter and poly-N sequences and low-quality reads while simultaneously calculating Q20, Q30, and guanine-cytosine (GC) content. Paired-end clean reads were aligned to the mouse reference genome (GRCm38) using the Spliced Transcripts Alignment to a Reference software (v2.6.1d, Menlo Park, CA). FeatureCounts (v1.5.0-p3, Parkville, Australia) was used to count the reads mapped to each gene. Reads per kilobase of transcript, per million mapped reads of each gene was calculated based on the length of the gene and the reads mapped to the gene.
All sequencing data have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO series accession number GSE168972 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE168972).
Statistical Analysis
ChIP-seq
Bioconductor packages edgeR (version 3.26.8, Sydney, Australia) and limma (version 3.40.6, Parkville, Australia) were used to perform differential expression analysis across the called peaks. Data were prepared by first choosing to keep peaks that achieved at least 1 count per million in at least 1 sample; calculating normalization factors with the trimmed mean of M value; and applying the voom transformation. A completely randomized design was implemented, comparisons of interest were extracted using contrasts, and moderated statistics were computed using the empirical Bayes procedure, eBayes. Finally, each peak was corrected for multiple testing using the Benjamini–Hochberg false discovery rate correction. Differential expression was defined as an adjusted P < .05 or less.
RNA-seq
Differential expression analysis between 2 groups (3 biological replicates per group) was performed using the DESeq2 R package (v1.20.0, Heidelberg, Germany). The resulting P values were adjusted using the Benjamini–Hochberg approach for controlling the false discovery rate. Genes with an adjusted P < .05 were considered differentially expressed.
qPCR and Western blot
Student's t-test was used to compare differences between 2 groups. For multiple-group comparisons, Kruskal–Wallis 1-way analysis of variance followed by an uncorrected Dunn's test was performed with GraphPad Prism software (version 9.0, San Diego, CA). Data are presented as means ± SEM, and P < .05 was considered statistically significant.
Other
InteractiVenn (http://www.interactivenn.net/index.html), a web-based tool, was used for identifying differentially expressed co–upregulated and co–downregulated genes between RNA-seq and ChIP-seq analyses and creating Venn diagrams. Gene ontology enrichment analyses of differentially expressed genes were conducted with the web-based server g:Profiler (https://biit.cs.ut.ee/gprofiler/gost). Epigenetic Landscape In Silico deletion Analysis (lisa.cistrome.org) was used to determine functional relevance of differentially expressed TF genes.
All authors had access to the study data and have reviewed and approved the final manuscript.
CRediT Authorship Contributions
Gaurav V. Sarode, PhD (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Writing – original draft: Lead; Writing – review & editing: Lead)
Kari E. Neier, PhD (Formal analysis: Supporting; Writing – review & editing: Supporting)
Noreene M. Shibata (Data curation: Supporting; Investigation: Supporting; Writing – original draft: Supporting; Writing – review & editing: Lead)
Yuanjun Shen (Investigation: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Dmitry A. Goncharov (Investigation: Supporting; Writing – review & editing: Supporting)
Elena A. Goncharova, PhD (Funding acquisition: Supporting; Writing – review & editing: Supporting)
Tagreed A. Mazi (Writing – review & editing: Supporting)
Nikhil Joshi (Formal analysis: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Matthew Lee Settles (Formal analysis: Supporting; Writing – review & editing: Supporting)
Janine M. LaSalle, PhD (Conceptualization: Supporting; Funding acquisition: Supporting; Writing – review & editing: Lead)
Valentina Medici, Professor (Conceptualization: Lead; Funding acquisition: Lead; Methodology: Lead; Project administration: Lead; Supervision: Lead; Writing – original draft: Lead; Writing – review & editing: Lead)
Footnotes
Conflicts of interest This author discloses the following: Valentina Medici serves on the Advisory Board of Alexion Pharmaceuticals. The remaining authors disclose no conflicts.
Funding The research was supported by National Institutes of Health grants R01DK104770 (V.M.), R01ES029213 (J.M.L.), R01AA027075 (J.M.L.), R01HL113178 (E.A.G.), and DK092993 (University of California–Davis Mouse Metabolic Phenotyping Center).
Supplementary Material
References
- 1.Einer C., Leitzinger C., Lichtmannegger J., Eberhagen C., Rieder T., Borchard S., Wimmer R., Denk G., Popper B., Neff F., Polishchuk E.V., Polishchuk R.S., Hauck S.M., von Toerne C., Müller J.C., Karst U., Baral B.S., DiSpirito A.A., Kremer A.E., Semrau J., Weiss K.H., Hohenester S., Zischka H. A high-calorie diet aggravates mitochondrial dysfunction and triggers severe liver damage in Wilson disease rats. Cell Mol Gastroenterol Hepatol. 2019;7:571–596. doi: 10.1016/j.jcmgh.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basaranoglu M., Basaranoglu G. Pathophysiology of insulin resistance and steatosis in patients with chronic viral hepatitis. World J Gastroenterol. 2011;17:4055–4062. doi: 10.3748/wjg.v17.i36.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller T., Langner C., Fuchsbichler A., Heinz-Erian P., Ellemunter H., Schlenck B., Bavdekar A.R., Pradhan A.M., Pandit A., Müller-Höcker J., Melter M., Kobayashi K., Nagasaka H., Kikuta H., Müller W., Tanner M.S., Sternlieb I., Zatloukal K., Denk H. Immunohistochemical analysis of Mallory bodies in Wilsonian and non-Wilsonian hepatic copper toxicosis. Hepatology. 2004;39:963–969. doi: 10.1002/hep.20108. [DOI] [PubMed] [Google Scholar]
- 4.Mahmood S., Inada N., Izumi A., Kawanaka M., Kobashi H., Yamada G. Wilson's disease masquerading as nonalcoholic steatohepatitis. N Am J Med Sci. 2009;1:74–76. [PMC free article] [PubMed] [Google Scholar]
- 5.Patel R. Wilson disease: a diagnostic challenge in a patient with alcoholic liver disease. BMJ Case Rep. 2019;12 doi: 10.1136/bcr-2019-232449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santos B.C., Guedes L.R., Faria L.C., Couto C.A. Wilson’s disease presentation resembling autoimmune hepatitis. BMJ Case Rep. 2019;12 doi: 10.1136/bcr-2019-230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferenci P., Stremmel W., Członkowska A., Szalay F., Viveiros A., Stättermayer A.F., Bruha R., Houwen R., Pop T.L., Stauber R., Gschwantler M., Pfeiffenberger J., Yurdaydin C., Aigner E., Steindl-Munda P., Dienes H.P., Zoller H., Weiss K.H. Age and sex but not ATP7B genotype effectively influence the clinical phenotype of Wilson disease. Hepatology. 2019;69:1464–1476. doi: 10.1002/hep.30280. [DOI] [PubMed] [Google Scholar]
- 8.Mazi T.A., Sarode G.V., Czlonkowska A., Litwin T., Kim K., Shibata N.M., Medici V. Dysregulated choline, methionine, and aromatic amino acid metabolism in patients with Wilson disease: exploratory metabolomic profiling and implications for hepatic and neurologic phenotypes. Int J Mol Sci. 2019;20:5937. doi: 10.3390/ijms20235937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mordaunt C.E., Kieffer D.A., Shibata N.M., Członkowska A., Litwin T., Weiss K.H., Zhu Y., Bowlus C.L., Sarkar S., Cooper S., Wan Y.Y., Ali M.R., LaSalle J.M., Medici V. Epigenomic signatures in liver and blood of Wilson disease patients include hypermethylation of liver-specific enhancers. Epigenetics Chromatin. 2019;12:10. doi: 10.1186/s13072-019-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mordaunt C.E., Shibata N.M., Kieffer D.A., Czlonkowska A., Litwin T., Weiss K.H., Gotthardt D.N., Olson K., Wei D., Cooper S., Wan Y.Y., Ali M.R., LaSalle J.M., Medici V. Epigenetic changes of the thioredoxin system in the tx-j mouse model and in patients with Wilson disease. Hum Mol Genet. 2018;27:3854–3869. doi: 10.1093/hmg/ddy262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarode G.V., Kim K., Kieffer D.A., Shibata N.M., Litwin T., Czlonkowska A., Medici V. Correction to: Metabolomics profiles of patients with Wilson disease reveal a distinct metabolic signature. Metabolomics. 2019;16:3. doi: 10.1007/s11306-019-1623-1. [DOI] [PubMed] [Google Scholar]
- 12.Medici V., Shibata N.M., Kharbanda K.K., LaSalle J.M., Woods R., Liu S., Engelberg J.A., Devaraj S., Török N.J., Jiang J.X., Havel P.J., Lönnerdal B., Kim K., Halsted C.H. Wilson's disease: changes in methionine metabolism and inflammation affect global DNA methylation in early liver disease. Hepatology. 2013;57:555–565. doi: 10.1002/hep.26047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medici V., Kieffer D.A., Shibata N.M., Chima H., Kim K., Canovas A., Medrano J.F., Islas-Trejo A.D., Kharbanda K.K., Olson K., Su R.J., Islam M.S., Syed R., Keen C.L., Miller A.Y., Rutledge J.C., Halsted C.H., LaSalle J.M. Wilson disease: epigenetic effects of choline supplementation on phenotype and clinical course in a mouse model. Epigenetics. 2016;11:804–818. doi: 10.1080/15592294.2016.1231289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng Z.X., Gong J., Chen Z., Sun J., Xiao Y., Wang L., Li Y., Liu J., Xu X.Z.S., Lin J.D. Glucose sensing by skeletal myocytes couples nutrient signaling to systemic homeostasis. Mol Cell. 2017;66:332–344.e4. doi: 10.1016/j.molcel.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belinsky S.A., Klinge D.M., Stidley C.A., Issa J.P., Herman J.G., March T.H., Baylin S.B. Inhibition of DNA methylation and histone deacetylation prevents murine lung cancer. Cancer Res. 2003;63:7089–7093. [PubMed] [Google Scholar]
- 16.Zhu W.G., Otterson G.A. The interaction of histone deacetylase inhibitors and DNA methyltransferase inhibitors in the treatment of human cancer cells. Curr Med Chem Anticancer Agents. 2003;3:187–199. doi: 10.2174/1568011033482440. [DOI] [PubMed] [Google Scholar]
- 17.Kirpich I., Ghare S., Zhang J., Gobejishvili L., Kharebava G., Barve S.J., Barker D., Moghe A., McClain C.J., Barve S. Binge alcohol-induced microvesicular liver steatosis and injury are associated with down-regulation of hepatic Hdac 1, 7, 9, 10, 11 and up-regulation of Hdac 3. Alcohol Clin Exp Res. 2012;36:1578–1586. doi: 10.1111/j.1530-0277.2012.01751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park P.H., Miller R., Shukla S.D. Acetylation of histone H3 at lysine 9 by ethanol in rat hepatocytes. Biochem Biophys Res Commun. 2003;306:501–504. doi: 10.1016/s0006-291x(03)01040-4. [DOI] [PubMed] [Google Scholar]
- 19.Ghezzi A., Krishnan H.R., Lew L., Prado F.J., 3rd, Ong D.S., Atkinson N.S. Alcohol-induced histone acetylation reveals a gene network involved in alcohol tolerance. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu X., Li J., Lv S., Yu J., Jiang J., Yao J., Xiao Y., Xu B., He H., Guo F., Zhang Z.N., Zhang C., Luan B. HDAC5 integrates ER stress and fasting signals to regulate hepatic fatty acid oxidation. J Lipid Res. 2018;59:330–338. doi: 10.1194/jlr.M080382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kronfol M.M., Jahr F.M., Dozmorov M.G., Phansalkar P.S., Xie L.Y., Aberg K.A., McRae M., Price E.T., Slattum P.W., Gerk P.M., McClay J.L. DNA methylation and histone acetylation changes to cytochrome P450 2E1 regulation in normal aging and impact on rates of drug metabolism in the liver. Geroscience. 2020;42:819–832. doi: 10.1007/s11357-020-00181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zama N., Towns R.L. Effect of isoproterenol (ISO) on rat heart, liver, kidney, and muscle tissue levels of zinc, copper, and magnesium. Biol Trace Elem Res. 1986;10:189–199. doi: 10.1007/BF02795617. [DOI] [PubMed] [Google Scholar]
- 23.McGee S.L., van Denderen B.J.W., Howlett K.F., Mollica J., Schertzer J.D., Kemp B.E., Hargreaves M. AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes. 2008;57:860–867. doi: 10.2337/db07-0843. [DOI] [PubMed] [Google Scholar]
- 24.Wooton-Kee C.R., Robertson M., Zhou Y., Dong B., Sun Z., Kim K.H., Liu H., Xu Y., Putluri N., Saha P., Coarfa C., Moore D.D., Nuotio-Antar A.M. Metabolic dysregulation in the Atp7b−/− Wilson’s disease mouse model. Proc Natl Acad Sci U S A. 2020;117:2076–2083. doi: 10.1073/pnas.1914267117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raudvere U., Kolberg L., Kuzmin I., Arak T., Adler P., Peterson H., Vilo J. g. Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update) Nucleic Acids Res. 2019;47:W191–W198. doi: 10.1093/nar/gkz369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabra D.G., Pfuhlmann K., Garcia-Caceres C., Schriever S.C., Casquero Garcia V., Kebede A.F., Fuente-Martin E., Trivedi C., Heppner K., Uhlenhaut N.H., Legutko B., Kabra U.D., Gao Y., Yi C.X., Quarta C., Clemmensen C., Finan B., Muller T.D., Meyer C.W., Paez-Pereda M., Stemmer K., Woods S.C., Perez-Tilve D., Schneider R., Olson E.N., Tschop M.H., Pfluger P.T. Hypothalamic leptin action is mediated by histone deacetylase 5. Nat Commun. 2016;7:10782. doi: 10.1038/ncomms10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagasaka H., Miida T., Inui A., Inoue I., Tsukahara H., Komatsu H., Hiejima E., Fujisawa T., Yorifuji T., Hiranao K., Okajima H., Inomata Y. Fatty liver and anti-oxidant enzyme activities along with peroxisome proliferator-activated receptors γ and α expressions in the liver of Wilson's disease. Mol Genet Metab. 2012;107:542–547. doi: 10.1016/j.ymgme.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Yiew K.H., Chatterjee T.K., Hui D.Y., Weintraub N.L. Histone deacetylases and cardiometabolic diseases. Arterioscler Thromb Vasc Biol. 2015;35:1914–1919. doi: 10.1161/ATVBAHA.115.305046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crunkhorn S. Metabolic disease: new role for HDACs in glucose homeostasis. Nat Rev Drug Discov. 2011;10:492. doi: 10.1038/nrd3483. [DOI] [PubMed] [Google Scholar]
- 30.Mihaylova M.M., Shaw R.J. Metabolic reprogramming by class I and II histone deacetylases. Trends Endocrinol Metab. 2013;24:48–57. doi: 10.1016/j.tem.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haberland M., Montgomery R.L., Olson E.N. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giorgio E.D., Brancolini C. Regulation of class IIa HDAC activities: it is not only matter of subcellular localization. Epigenomics. 2016;8:251–269. doi: 10.2217/epi.15.106. [DOI] [PubMed] [Google Scholar]
- 33.Mihaylova M.M., Vasquez D.S., Ravnskjaer K., Denechaud P.D., Yu R.T., Alvarez J.G., Downes M., Evans R.M., Montminy M., Shaw R.J. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 2011;145:607–621. doi: 10.1016/j.cell.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang B., Moya N., Niessen S., Hoover H., Mihaylova M.M., Shaw R.J., Yates J.R., 3rd, Fischer W.H., Thomas J.B., Montminy M. A hormone-dependent module regulating energy balance. Cell. 2011;145:596–606. doi: 10.1016/j.cell.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziegler N., Raichur S., Brunner B., Hemmann U., Stolte M., Schwahn U., Prochnow H.-P., Metz-Weidmann C., Tennagels N., Margerie D., Wohlfart P., Bielohuby M. Liver-specific knockdown of class IIa HDACs has limited efficacy on glucose metabolism but entails severe organ side effects in mice. Front Endocrinol (Lausanne) 2020;11:598. doi: 10.3389/fendo.2020.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim M.S., Akhtar M.W., Adachi M., Mahgoub M., Bassel-Duby R., Kavalali E.T., Olson E.N., Monteggia L.M. An essential role for histone deacetylase 4 in synaptic plasticity and memory formation. J Neurosci. 2012;32:10879–10886. doi: 10.1523/JNEUROSCI.2089-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parra M. Class IIa HDACs - new insights into their functions in physiology and pathology. FEBS J. 2015;282:1736–1744. doi: 10.1111/febs.13061. [DOI] [PubMed] [Google Scholar]
- 38.Desai V., Kaler S.G. Role of copper in human neurological disorders. Am J Clin Nutr. 2008;88:855s–858s. doi: 10.1093/ajcn/88.3.855S. [DOI] [PubMed] [Google Scholar]
- 39.Bisaglia M., Bubacco L. Copper ions and Parkinson's disease: why is homeostasis so relevant? Biomolecules. 2020;10:195. doi: 10.3390/biom10020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ejaz H.W., Wang W., Lang M. Copper toxicity links to pathogenesis of Alzheimer's disease and therapeutics approaches. Int J Mol Sci. 2020;21:7660. doi: 10.3390/ijms21207660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park G., Tan J., Garcia G., Kang Y., Salvesen G., Zhang Z. Regulation of histone acetylation by autophagy in Parkinson disease. J Biol Chem. 2016;291:3531–3540. doi: 10.1074/jbc.M115.675488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang D., Hu X., Li J., Hoogstra-Berends F., Zhuang Q., Esteban M.A., de Groot N., Henning R.H., Brundel B.J.J.M. Converse role of class I and class IIa HDACs in the progression of atrial fibrillation. J Mol Cell Cardiol. 2018;125:39–49. doi: 10.1016/j.yjmcc.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Z., Johnson W.T., Kang Y.J. Regression of copper-deficient heart hypertrophy: reduction in the size of hypertrophic cardiomyocytes. J Nutr Biochem. 2009;20:621–628. doi: 10.1016/j.jnutbio.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weems J.C., Griesel B.A., Olson A.L. Class II histone deacetylases downregulate GLUT4 transcription in response to increased cAMP signaling in cultured adipocytes and fasting mice. Diabetes. 2012;61:1404–1414. doi: 10.2337/db11-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bricambert J., Favre D., Brajkovic S., Bonnefond A., Boutry R., Salvi R., Plaisance V., Chikri M., Chinetti-Gbaguidi G., Staels B., Giusti V., Caiazzo R., Pattou F., Waeber G., Froguel P., Abderrahmani A. Impaired histone deacetylases 5 and 6 expression mimics the effects of obesity and hypoxia on adipocyte function. Mol Metab. 2016;5:1200–1207. doi: 10.1016/j.molmet.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tao C., Wang Y., Zhao Y., Pan J., Fan Y., Liang X., Cao C., Zhao J., Petris M.J., Li K., Wang Y. Adipocyte-specific disruption of ATPase copper transporting α in mice accelerates lipoatrophy. Diabetologia. 2019;62:2340–2353. doi: 10.1007/s00125-019-4966-2. [DOI] [PubMed] [Google Scholar]
- 47.Lei L., Xiaoyi S., Fuchang L. Effect of dietary copper addition on lipid metabolism in rabbits. Food Nutr Res. 2017;61:1348866. doi: 10.1080/16546628.2017.1348866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pierson H., Muchenditsi A., Kim B.E., Ralle M., Zachos N., Huster D., Lutsenko S. The function of ATPase copper transporter ATP7B in intestine. Gastroenterology. 2018;154:168–180.e5. doi: 10.1053/j.gastro.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerbeth L., Glauben R. Histone deacetylases in the inflamed intestinal epithelium—promises of new therapeutic strategies. Front Med (Lausanne) 2021;8:655956. doi: 10.3389/fmed.2021.655956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin M., Kettmann R., Dequiedt F. Class IIa histone deacetylases: regulating the regulators. Oncogene. 2007;26:5450–5467. doi: 10.1038/sj.onc.1210613. [DOI] [PubMed] [Google Scholar]
- 51.Li X., Song S., Liu Y., Ko S.-H., Kao H.-Y. Phosphorylation of the histone deacetylase 7 modulates its stability and association with 14-3-3 proteins. J Biol Chem. 2004;279:34201–34208. doi: 10.1074/jbc.M405179200. [DOI] [PubMed] [Google Scholar]
- 52.McGee S.L., Hargreaves M. Histone modifications and skeletal muscle metabolic gene expression. Clin Exp Pharmacol Physiol. 2010;37:392–396. doi: 10.1111/j.1440-1681.2009.05311.x. [DOI] [PubMed] [Google Scholar]
- 53.Kemp B.E., Mitchelhill K.I., Stapleton D., Michell B.J., Chen Z.P., Witters L.A. Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem Sci. 1999;24:22–25. doi: 10.1016/s0968-0004(98)01340-1. [DOI] [PubMed] [Google Scholar]
- 54.Zhao J.-X., Yue W.-F., Zhu M.-J., Du M. AMP-activated protein kinase regulates β-catenin transcription via histone deacetylase 5. J Biol Chem. 2011;286:16426–16434. doi: 10.1074/jbc.M110.199372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen J., Chen J., Huang J., Li Z., Gong Y., Zou B., Liu X., Ding L., Li P., Zhu Z., Zhang B., Guo H., Cai C., Li J. HIF-2α upregulation mediated by hypoxia promotes NAFLD-HCC progression by activating lipid synthesis via the PI3K-AKT-mTOR pathway. Aging (Albany NY) 2019;11:10839–10860. doi: 10.18632/aging.102488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feng Z., Pang L., Chen S., Pang X., Huang Y., Qiao Q., Wang Y., Vonglorkham S., Huang Q., Lin X., Wei J. Didymin ameliorates dexamethasone-induced non-alcoholic fatty liver disease by inhibiting TLR4/NF-κB and PI3K/Akt pathways in C57BL/6J mice. Int Immunopharmacol. 2020;88:107003. doi: 10.1016/j.intimp.2020.107003. [DOI] [PubMed] [Google Scholar]
- 57.da Silva R.P., Eudy B.J., Deminice R. One-carbon metabolism in fatty liver disease and fibrosis: one-carbon to rule them all. J Nutr. 2020;150:994–1003. doi: 10.1093/jn/nxaa032. [DOI] [PubMed] [Google Scholar]
- 58.Evans R.M., Barish G.D., Wang Y.-X. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 59.Tailleux A., Wouters K., Staels B. Roles of PPARs in NAFLD: potential therapeutic targets. Biochim Biophys Acta. 2012;1821:809–818. doi: 10.1016/j.bbalip.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 60.Morán-Salvador E., López-Parra M., García-Alonso V., Titos E., Martínez-Clemente M., González-Périz A., López-Vicario C., Barak Y., Arroyo V., Clària J. Role for PPARγ in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J. 2011;25:2538–2550. doi: 10.1096/fj.10-173716. [DOI] [PubMed] [Google Scholar]
- 61.Abdelmegeed M.A., Yoo S.-H., Henderson L.E., Gonzalez F.J., Woodcroft K.J., Song B.-J. PPARα expression protects male mice from high fat–induced nonalcoholic fatty liver. J Nutr. 2011;141:603–610. doi: 10.3945/jn.110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Polvani S., Tarocchi M., Galli A. PPARgamma and oxidative stress: con(beta) catenating NRF2 and FOXO. PPAR Res. 2012;2012:641087. doi: 10.1155/2012/641087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kersten S. Peroxisome proliferator activated receptors and lipoprotein metabolism. PPAR Res. 2008;2008:132960. doi: 10.1155/2008/132960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Funato H., Oda S., Yokofujita J., Igarashi H., Kuroda M. Fasting and high-fat diet alter histone deacetylase expression in the medial hypothalamus. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen Y., Goncharov D.A., Avolio T., Ray A., Okorie E., DeLisser H., Mora A.L., Vanderpool R., Kudryashova T.V., Goncharova E.A. Differential effects of integrin-linked kinase inhibitor Cpd22 on severe pulmonary hypertension in male and female rats. Pulm Circ. 2020;10 doi: 10.1177/2045894019898593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kudryashova T.V., Goncharov D.A., Pena A., Kelly N., Vanderpool R., Baust J., Kobir A., Shufesky W., Mora A.L., Morelli A.E., Zhao J., Ihida-Stansbury K., Chang B., DeLisser H., Tuder R.M., Kawut S.M., Sillje H.H., Shapiro S., Zhao Y., Goncharova E.A. HIPPO-integrin-linked kinase cross-talk controls self-sustaining proliferation and survival in pulmonary hypertension. Am J Respir Crit Care Med. 2016;194:866–877. doi: 10.1164/rccm.201510-2003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.