Abstract
Background
Nasopharyngeal swab has long been considered the specimen of choice for the diagnosis of respiratory viral infections, including SARS-CoV-2 infection, but it suffers from several drawbacks: its discomfort limits screening acceptability, and it is vulnerable to shortages in both specialized materials and trained healthcare workers in the context of a pandemic.
Methods
We prospectively compared natural spring water gargle to combined oro-nasopharyngeal swab (ONPS) for the diagnosis of coronavirus disease 2019 (COVID-19) in paired clinical specimens (1005 ONPS and 1005 gargles) collected from 987 unique early symptomatic as well as asymptomatic individuals from the community.
Results
Using a direct RT-PCR method with the Allplex™ 2019-nCoV Assay (Seegene), the clinical sensitivity of the gargle was 95.3% (95% confidence interval [CI], 90.2 – 98.3%), similar to the sensitivity of the ONPS (93.8%; 95% CI, 88.2 – 97.3%), despite significantly lower viral RNA concentration in gargles, as reflected by higher cycle threshold values. No single specimen type detected all COVID-19 cases. SARS-CoV-2 RNA was stable in gargles at room temperature for at least 7 days.
Conclusion
The simplicity of this sampling method coupled with the accessibility of spring water are clear advantages in a pandemic situation where testing frequency, turnaround time and shortage of consumables and trained staff are critical elements.
Key words: COVID-19, SARS-CoV-2, Gargle, Saliva, PCR, Diagnosis
1. Introduction
Testing massively, rapidly and frequently has been considered one of the most important measure to control the COVID-19 pandemic [1 , 2]. Although nasopharyngeal swab has long been considered the specimen of choice for the diagnosis of respiratory viral infections, including SARS-CoV-2 infection, it suffers from several drawbacks: its discomfort limits screening acceptability, and it is vulnerable to shortages in both specialized materials and trained healthcare workers in the context of a pandemic [3].
Saliva has been well studied and is now considered an acceptable alternative specimen for the diagnosis of COVID-19 [4 , 5]. However, since direct SARS-CoV-2 RT-PCR (i.e. without prior RNA extraction) has been used to accelerate turn-around time and to circumvent the problem of extraction reagent shortages, saliva may be less suitable because it usually requires RNA extraction or additional sample preparation, such as proteinase K treatment and dilution, before PCR analysis [6]. Moreover, its viscosity can hamper laboratory automation [7 , 8].
Swish and gargle with a mouth rinse has been less studied as a sample for COVID-19 diagnosis. However, it is easier to obtain from individuals having difficulty producing saliva, and is also easier to manipulate in the laboratory since the sample is already diluted, which effectively reduces sample viscosity. One small study found gargle to be as sensitive as throat swab for the detection of respiratory pathogens but did not compare it to nasopharyngeal swab [9]. Some authors have used saline as a mouth rinse and gargle and found it to be at least as sensitive as nasopharyngeal swab for the diagnosis of COVID-19 [[10], [11], [12]], but they proceeded to RNA extraction as saline is less suitable for direct RT-PCR due to inhibition of some PCR assays by high concentration of sodium chloride [13]. Moreover, many medical grade supplies are prone to shortages during a pandemic. These shortcomings of actual studies justified our evaluation of the swish and gargle with natural spring water compared to combined oro-nasopharyngeal swab (ONPS) for the diagnosis of COVID-19 using a direct RT-PCR method without RNA extraction.
2. Methods
2.1. Study subjects and specimen collection
Individuals aged ≥ 6 years presenting to a drive-through test center were included either if they had symptoms suggestive of COVID-19 or if they were considered an asymptomatic close contact by public health services. Patients aged 5 years or less were excluded because there are usually unable to gargle adequately. After obtaining consent, an ONPS was first collected using a standardised method by a trained health care professional. The flocked swab was first used to swab the posterior oropharynx and the tonsillar arches, and then the same swab was inserted through one nostril parallel to palate until resistance was met or the distance was equivalent to the distance from the patient's ear to their nostril, rotated several times and left in place for 5 – 10 s prior to being removed as per Center for Disease Control instructions for collection [14]. The ONPS was placed in a conical 15 ml centrifuge tube containing 3 ml of molecular water (PCR grade water), which is a validated, standard-of-care specimen transport medium for SARS-CoV-2 testing [13 , 15]. Subjects were then provided with 5 ml of natural spring water (Eska water, St-Mathieu-d'Harricana, Québec, Canada or Naya water, Mirabel, Québec, Canada) in a disposable soft plastic cup (Plastic Medicine Cups, AMG Medical, Montreal, Quebec, Canada) and instructed to rinse their mouth for 5 s, tilt their head back and gargle for 5 s, repeat this cycle once, expel the water back in the plastic cup and empty it in a 15 ml conical polypropylene centrifuge tube. Both specimens were simultaneously submitted and processed for SARS-CoV-2 testing by a direct one-step reverse transcription-PCR (RT-PCR) method as described below. The specimens were refrigerated at the collection site and transported to the laboratory in insulated coolers with ice packs. Detailed instructions for the gargle procedure are included in the supplemental material. This project was considered by our research ethics committee at CISSS de Chaudière-Appalaches and deemed exempt in accordance with the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans – TCPS 2 (2018). The project was conducted in accordance with the Declaration of Helsinki.
2.2. SARS-CoV-2 detection
All samples were vortexed for 2–3 s, heat inactivated at 70 °C for 10 min in a water bath, and centrifuged at 3000 x g for 2 min. ONPS transport medium (molecular grade water) was diluted 1:3 in molecular grade water (500 µl in 1500 µl) in a 5 ml cryovial tube (Simport Scientific, Beloeil, Québec, Canada). This dilution was routinely used to lower the proportion of invalid RT-PCR results (data not shown). Gargles were directly transferred (1 ml) undiluted in a 5 ml cryovial tube. Cryovial tubes were loaded on a Seegene STARlet IVD (Seegene, Seoul, Republic of Korea and Hamilton Company, Reno, NV, USA) for preparation of PCR microplates for direct RT-PCR without RNA extraction. Direct RT-PCR was performed using the Allplex™ 2019-nCoV Assay kit on CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) as described by Merindol N et al., [13], and results interpreted using the Seegene Viewer software. Cycle thresholds (Ct) served as a relative indicator of viral load. Samples were considered to have low viral loads if less than 3 genes were detected or if the N gene Ct was equal to or greater than 35. This definition usually corresponds to a viral load of 720 copies/ml or less (data not shown). Results were considered invalid if neither the internal control nor any target gene were detected, which usually represents PCR inhibition. Discordant pairs and every pair containing at least one invalid result were also analyzed with the RealTime SARS-CoV-2 assay (Abbott, Chicago, IL, USA).
2.3. RNA stability study
A volunteer gargled with 5 ml of four different waters (Eska; Naya; Molecular water BP2819, Fisher Bioreagents; Sterile water for irrigation, Baxter), three times with each water. One SARS-CoV-2 PCR positive ONPS non-inactivated sample was used to spike the 12 gargle specimens (1:8 dilution). One spiked gargle from each brand of water was analyzed immediately and the two other spiked gargles from each brand of water were kept seven days at room temperature and at 4 °C, respectively, before being analyzed using the same method described above. Other waters, including tap water, were also tested to evaluate RT-PCR compatibility (see Table S1 in supplemental material).
3. Statistical methods
Data were described by percentage for categorical variables and median with interquartile range (IQR) for continuous variables. Both gargle and ONPS were compared to a composite reference standard, defined as positive if either the ONPS or the gargle was positive. Online MedCalc software (https://www.medcalc.org/calc/index.php) was used to calculate sensitivity, specificity, disease prevalence, predictive values, accuracy, likelihood ratios as well as comparison of proportions (chi-square test) and their respective confidence intervals. The level of agreement was also assessed using kappa statistics. By definition, Kappa values above 0.75 indicate excellent agreement, values between 0.40 and 0.75 indicate fair to good agreement, and values below 0.40 represent poor agreement beyond chance. P values for the comparison of means (t-test for paired samples) were calculated using the Statistica software (Statsoft Inc, OK, USA). P values for the comparison of standard deviations (F test for variances) were calculated using the online Statistics Kingdom calculator (https://www.statskingdom.com/220VarF2.html). Box-and-whisker graphs were generated using the online Good Calculators software (https://goodcalculators.com/box-plot-maker/).
4. Results
A total of 2010 paired clinical specimens (1005 ONPS and 1005 gargles) were collected from 987 unique participants between October 8th and 23rd 2020. The average age of study participants was 40 years (range 6 – 91 years, median 40 years), 633 (63%) were symptomatic (mean 3 days, median 2 days), 244 (24%) were asymptomatic but close contacts to confirmed COVID-19 cases, and 126 (13%) were asymptomatic but working in a unit or a workplace with a COVID-19 outbreak.
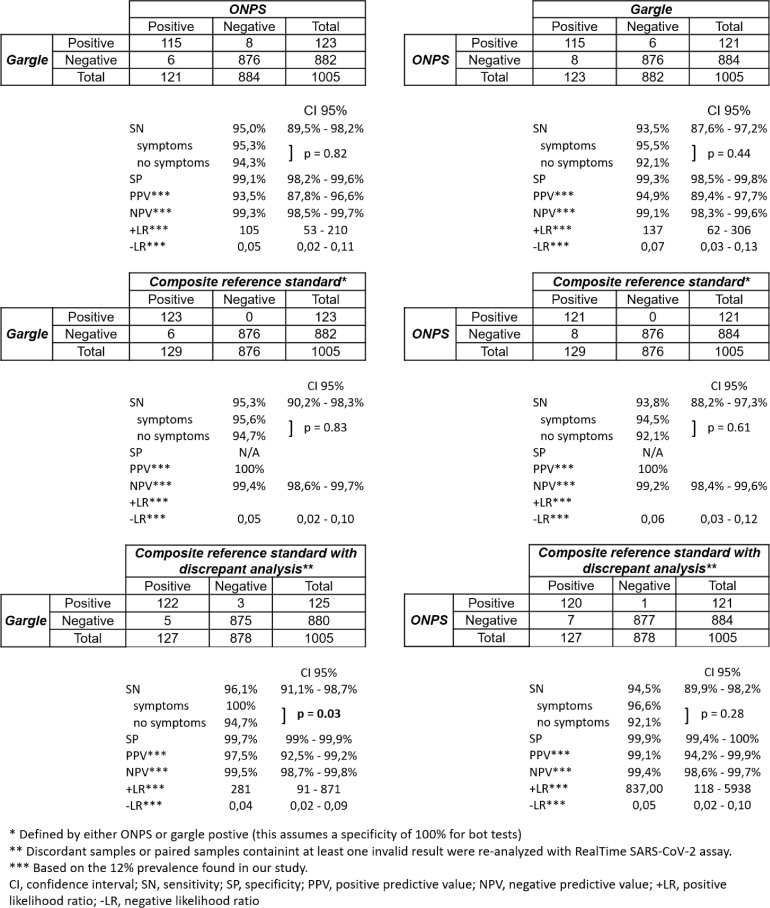
As shown in Fig. 1 , 129 (12.8%) individuals had SARS-CoV-2 detected in at least one sample: 115 (11.4%) in both samples, 6 (0.6%) only in ONPS, and 8 (0.8%) only in gargle. There was no difference in clinical sensitivity between gargle and ONPS, with a Cohen's kappa coefficient of 0.94 (95% CI 0.89 – 0.96). All 6 ONPS that were paired with a PCR-negative gargle contained low viral loads, and 3/6 gargles were found to be weakly PCR-positive when retested with the Abbott RealTime SARS-CoV-2 assay, which includes an RNA extraction step. Among the eight PCR-positive gargles paired with PCR-negative ONPS, six contained low viral loads, and 2/7 ONPS were found to be PCR-positive when retested with the Abbott RealTime SARS-CoV-2 assay (one ONPS was lost so could not be retested). Both types of samples showed a better clinical sensitivity with symptomatic participants, but the difference was only statistically significant when comparing gargle to the composite reference standard with discrepant analysis (Fig. 1). Among symptomatic participants, 94 out of 633 paired samples (14.8%) tested positive with gargle only (n = 6; 0.9%), ONPS only (n = 4; 0.6%) or both (n = 84; 13.3%), while 34 out 367 paired samples (9.3%) from asymptomatic participants tested positive with gargle only (n = 2; 0.5%), ONPS only (n = 2; 0.5%) or both (n = 30; 8.2%). Data regarding the presence or absence of symptoms was lacking for 5 participants.
Fig. 1.
2 × 2 tables comparing gargle and ONPS, with composite reference standard and discrepant analysis.
Results were invalid for 2.7% (27 of 1005) ONPS and for 0.9% (9 of 1005) gargles (difference 1.8%, 95% CI 0.64% – 3.07%, p = 0.002).
Cycle threshold (Ct) differences were observed for concordant positive paired samples (Fig. 2 ). Overall, gargles had higher Ct values with mean differences of 5.3, 5.6 and 5.2 for E, RdRp and N genes respectively (p < 0.0001). When analyzing Ct differences by different ONPS gene E Ct intervals, the differences were only significant when the ONPS had a Ct value lower than 25, representing higher viral loads (Fig. 2). Also, there was a statistically significant difference between standard deviations of gargles and ONPS Ct values for RdRp gene (4.18 vs 5.29, p = 0.02) and N gene (4.8 vs 6.0, p = 0.01), but not E gene (4.77 vs 5.57, p = 0.09).
Fig. 2.
Mean Ct difference between ONPS and gargle, overall and separated by different ONPS E gene Ct intervals for concordant positive sample pairs.
Symptomatic participants had lower mean ONPS Ct values compared to asymptomatic participants, but this difference was statistically significant only for RdRp gene [24.36 vs 26.90 (p = 0.02)]; for the E gene, mean Ct values were respectively 22.15 and 24.23 (p = 0.08), and for the N gene, they were respectively 24.99 and 26.52 (p = 0.23). As for the gargle Ct values, there was no difference between symptomatic and asymptomatic participants: RdRp gene 30.89 vs 30.12 (p = 0.45), E gene 27.94 vs 27.85 (p = 0.93), N gene 30.61 vs 30.75 (p = 0.89).
SARS-CoV-2 RNA was stable in natural spring waters and in gargle for at least 7 days, either at room temperature or refrigerated at 4 °C (Table 1 ).
Table 1.
SARS-CoV-2 RNA stability in different waters and gargles.
| Day 0 | Day 7 - 4 °Celsius | Day 7 - Room temperature | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E gene (Ct) | RdRp gene (Ct) | N gene (Ct) | IC (Ct) | E gene (Ct) | RdRp gene (Ct) | N gene (Ct) | IC (Ct) | E gene (Ct) | RdRp gene (Ct) | N gene (Ct) | IC (Ct) | |
| Naya water gargle | N/A | N/A | N/A | 26,62 | ||||||||
| Naya water gargle spiked | 26,38 | 29,26 | 28,62 | 26,27 | 26,90 | 29,47 | 28,44 | 26,41 | 27,76 | 29,37 | 29,67 | 27,04 |
| Ct value difference | 0,52 | 0,21 | −0,18 | 0,14 | 1,38 | 0,11 | 1,05 | 0,77 | ||||
| Molecular water gargle | N/A | N/A | N/A | 26,80 | ||||||||
| Molecular water gargle spiked | 26,40 | 27,80 | 27,29 | 25,88 | 26,56 | 29,39 | 28,67 | 26,06 | 26,56 | 29,29 | 28,74 | 26,21 |
| Ct value difference | 0,16 | 1,59 | 1,38 | 0,18 | 0,16 | 1,49 | 1,45 | 0,33 | ||||
| Eska water gargle | N/A | N/A | N/A | 26,26 | ||||||||
| Eska water gargle spiked | 26,93 | 30,19 | 29,11 | 26,06 | 27,33 | 30,74 | 29,75 | 26,56 | 27,28 | 29,70 | 29,74 | 27,23 |
| Ct value difference | 0,40 | 0,55 | 0,64 | 0,50 | 0,35 | −0,49 | 0,63 | 1,17 | ||||
| Baxter | medical water gargle | N/A | N/A | N/A | 27,81 | |||||||
| Baxter medical water gargle spiked | 27,28 | 31,26 | 29,57 | 27,29 | 27,20 | 32,35 | 29,53 | 26,97 | 27,40 | 30,97 | 30,09 | 27,23 |
| Ct value difference | −0,08 | 1,09 | −0,04 | −0,32 | 0,12 | −0,29 | 0,52 | −0,06 | ||||
5. Discussion
Testing frequency is considered important to control the COVID-19 pandemic, but this requires sampling methods with high acceptability in order to address testing hesitancy. Non-invasive samples like saliva or gargle clearly increase the acceptability of testing [11] and alleviate pressure on limited resources that are currently used to collect oro-nasopharyngeal swabs. We have gathered after this study many qualitative data confirming the impact of gargle on testing acceptability and we saw an important increase in healthcare workers adherence to systematic screening (data not shown). Gargle also widens the possibility of self-collection as it was shown to have similar sensitivity to a nasopharyngeal swab [11 , 12]. Self-collected non-invasive specimens can eliminate socio-economical barriers to testing hesitancy and increase patient satisfaction. Notwithstanding that some evidence suggests that testing frequency could be more important than analytical sensitivity [1 , 16], it is essential to demonstrate that novel sampling methods retain acceptable sensitivity. Like others, we were able to demonstrate that gargle can be as sensitive as ONPS [[10], [11], [12]], even without RNA extraction. Our data also demonstrates that it is important to use a composite reference standard when sampling methods offer similar analytical and clinical performance to avoid underestimation of the novel sampling method [5]. There was no difference in sensitivity between symptomatic and asymptomatic participants in our study, except when comparing gargle to composite reference standard combined with discrepant analysis, although we may have lacked power to demonstrate a difference.
Saliva generally requires dilution and/or enzymatic digestion to permit direct RT-PCR without significant rates of inhibition [17]. Since gargle is essentially a form of pre-diluted saliva, we obtained a low rate of PCR inhibition in this study. Preliminary tests showed that some natural spring waters, as well as local tap water, did not seem to affect direct RT-PCR efficiency when compared to molecular water (see supplemental material). We chose to use commercial natural spring waters that were bottled from unique sources, which offers homogeneity and high chemical stability across time. These water sources are highly regulated and human or agricultural activity are not allowed near them. In Canada, natural spring waters are readily available and represent a cheap alternative to more expensive medical grade products that are prone to shortages during a pandemic. Moreover, we were able to demonstrate stability of viral RNA for at least 7 days at room temperature, similarly to saliva [18 , 19]. This represents a significant advantage since getting rid of the cold-chain can help support surge capacity of SARS-CoV-2 diagnostic testing [20].
We observed a statistically significant difference between the viral RNA concentrations (extrapolated from PCR Ct) of both sample types, gargle having a lower relative viral load when considering all concordant pairs. Some studies have demonstrated that a lower quantity of viral RNA could be detected from saliva compared to nasopharyngeal swabs [[21], [22], [23]]. The lower relative viral loads (higher Ct values) we observed with gargle compared to EONPs could be related to the fact that gargle is a diluted saliva sample. However, the viral RNA concentrations difference was only significant in paired samples with higher ONPS viral loads (Ct < 25). This could be in part explained by the fact that viral RNA declines more rapidly in the nasopharynx, since some studies showed that SARS-CoV-2 RNA could be amplified from saliva for longer periods [[21], [22], [23]] and another study found that gargle was more often positive than ONPS even when symptoms were present for more than 7 days [11]. We could also hypothesize that the viral RNA concentration in the throat and saliva, and thus gargle, is possibly less variable from one day or from one sample to another compared to the nasopharyngeal swab. As a matter of fact, when comparing positive gargles and ONPS, our data shows statistically significant differences between the standard deviations of RdRp and N genes Ct values, gargles’ Ct values being less variable than those of ONPS. Moreover, some authors found greater variation in human RNase P Ct values in nasopharyngeal swab specimens than in saliva specimens [22], consistent with more variable nasopharyngeal swab specimens quality. The pharynx being at the junction between the upper and the lower respiratory tract, it could reflect the viral RNA content of both anatomical compartments as the virus progressively migrate from one site to another. The absence of significant Ct difference in paired samples containing lower viral loads in ONPS (Ct ≥ 25) is not explained by discordant samples, since the latter were well balanced between both sample types (6 gargle-/ONPS+ and 8 gargle+/ONPS-). One study comparing the performance of oropharyngeal swab with nasopharyngeal swab for the diagnosis of COVID-19 also found a similar sensitivity between both sample types, with discordant pairs being also well balanced between both sample types, and yet they found higher Ct values in oropharyngeal swabs [24].
Our study has some limitations. Since we were using a direct RT-PCR method, ONPS transport medium had to be diluted 1:3 to avoid PCR inhibition and hence decrease invalid results rate. This dilution could have decreased ONPS clinical sensitivity at the advantage of gargle. Also, symptomatic participants in this study having been recruited within the first seven days of symptom onset, we cannot extrapolate our results to individuals presenting later in the disease course. Moreover, we cannot either extrapolate our results to nursing home patients or infants/toddlers who were not included in this study, and who, in our experience, provide gargle samples with more difficulty.
6. Conclusion
Natural spring water gargle is as sensitive as ONPS for the diagnosis of SARS-CoV-2 in early symptomatic as well as asymptomatic individuals from the community using a direct RT-PCR method with the Allplex™ 2019-nCoV assay. Non-invasive sampling methods will increase acceptability of SARS-CoV-2 testing and reduce the need for precious medical grade products and trained healthcare workforce. More studies are needed and are underway to evaluate the clinical performance of natural spring water gargle with other PCR assays for the diagnosis of COVID-19.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors report no potential conflicts of interest relevant to this manuscript. This study was supported by the ministère de la Santé et des Service sociaux de la province de Québec (reagents and equipment) and the centre intégré de santé et de services sociaux de Chaudière-Appalaches (human and technical resources). We would particularly like to thank nurses and laboratory technologists from the hospital Hôtel-Dieu de Lévis, all the staff from the community assessment centers Archimède and Paul-Gilbert, as well as all the participants.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcv.2021.104995.
Appendix. Supplementary materials
References
- 1.Mina M.J., Parker R., Larremore D.B. Rethinking Covid-19 test sensitivity — a strategy for containment. N. Engl. J. Med. 2020;383:e120. doi: 10.1056/NEJMp2025631. [DOI] [PubMed] [Google Scholar]
- 2.E.T. Chin et al., Frequency of routine testing for coronavirus disease 2019 (COVID-19) in high-risk healthcare environments to reduce outbreaks. Clin. Infect. Dis., (2020). [DOI] [PMC free article] [PubMed]
- 3.Erdmann R., et al. Testing characteristic for novel specimens, populations, and platforms. Alberta Health Services, COVID-19 Scientific. Advisory Group. 2020 [Google Scholar]
- 4.Centers for Disease Control and Prevention. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html. Accessed February 8, 2021.
- 5.Butler-Laporte G., et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern. Med. 2021 doi: 10.1001/jamainternmed.2020.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.C.B.F. Vogels et al., (Cold Spring Harbor Laboratory, 2020).
- 7.Landry M.L., Criscuolo J., Peaper D.R. Challenges in use of saliva for detection of SARS CoV-2 RNA in symptomatic outpatients. J. Clin. Virol. 2020;130 doi: 10.1016/j.jcv.2020.104567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergevin M.A., et al. Validation of saliva sampling as an alternative to oro-nasopharyngeal swab for detection of SARS-CoV-2 using unextracted rRT-PCR with the Allplex 2019-nCoV assay. J. Med. Microbiol. 2021;70 doi: 10.1099/jmm.0.001404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett S., Davidson R.S., Gunson R.N. Comparison of gargle samples and throat swab samples for the detection of respiratory pathogens. J. Virol. Methods. 2017;248:83–86. doi: 10.1016/j.jviromet.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Mittal A., et al. Gargle lavage as a viable alternative to swab for detection of SARS-CoV-2. Indian J. Med. Res. 2020;152:77–81. doi: 10.4103/ijmr.IJMR_2987_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D.M. Goldfarb et al., Self-collected saline gargle samples as an alternative to healthcare worker collected nasopharyngeal swabs for COVID-19 diagnosis in outpatients. J. Clin. Microbiol., (2021). [DOI] [PMC free article] [PubMed]
- 12.Kandel C.E., et al. Detection of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) in outpatients: a multicenter comparison of self-collected saline gargle, oral swab, and combined oral–anterior nasal swab to a provider collected nasopharyngeal swab. Infect. Control Hosp. Epidemiol. 2021:1–5. doi: 10.1017/ice.2021.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merindol N., et al. SARS-CoV-2 detection by direct rRT-PCR without RNA extraction. J. Clin. Virol. 2020;128 doi: 10.1016/j.jcv.2020.104423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.K.E. Hanson et al., Infectious diseases society of america guidelines on the diagnosis of coronavirus disease 2019. Clin. Infect. Dis., (2020). [DOI] [PMC free article] [PubMed]
- 15.Freppel W., Merindol N., Rallu F., Bergevin M. Efficient SARS-CoV-2 detection in unextracted oro-nasopharyngeal specimens by rRT-PCR with the Seegene Allplex™ 2019-nCoV assay. Virol. J. 2020;17 doi: 10.1186/s12985-020-01468-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D.B. Larremore et al., (Cold Spring Harbor Laboratory, 2020).
- 17.C.B.F. Vogels et al., Salivadirect: a simplified and flexible platform to enhance SARS-CoV-2 testing capacity. Med, (2020). [DOI] [PMC free article] [PubMed]
- 18.I.M. Ott et al., (Cold Spring Harbor Laboratory, 2020).
- 19.Kandel C., et al. Detection of SARS-CoV-2 from saliva as compared to nasopharyngeal swabs in outpatients. Viruses. 2020;12:1314. doi: 10.3390/v12111314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.A. Bosworth, Whalley, C., Poxon, C., Wanigasooriya, K., Pickles, O., Aldera, E.L., Papakonstantinou, D., Morley, G.L., Walker, E.M., Zielinska, A.E., Mcloughlin, D., Webster, C., Plant, T., Ellis, A., Richter, A., Kidd, I.M., Beggs, A.D., Rapid implementation and validation of a cold-chain free SARS-CoV-2 diagnostic testing workflow to support surge capacity. J. Clin. Virol., (2020). [DOI] [PMC free article] [PubMed]
- 21.M. Miller et al., Cold Spring Harbor Laboratory. Validation of a Self-administrable, Saliva-based RT-qPCR Test Detecting SARS-CoV-2. medRxiv 2020.06.05.20122721; doi: 10.1101/2020.06.05.20122721, (2020). [DOI]
- 22.Wyllie A.L., et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N. Engl. J. Med. 2020;383:1283–1286. doi: 10.1056/NEJMc2016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.M. Rao et al., Comparing nasopharyngeal swab and early morning saliva for the identification of SARS-CoV-2. Clin. Infect. Dis., (2020). [DOI] [PMC free article] [PubMed]
- 24.Patel M.R., et al. Performance of oropharyngeal swab testing compared with nasopharyngeal swab testing for diagnosis of coronavirus disease 2019—United States, January 2020–February 2020. Clin. Infect. Dis. 2021;72:482–485. doi: 10.1093/cid/ciaa759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.