Abstract
Introduction
Spinal cord injury (SCI) is a devastating condition with immediate impact on the individual’s health and quality of life. Major functional recovery reaches a plateau 3–4 months after injury despite intensive rehabilitative training. To enhance training efficacy and improve long-term outcomes, the combination of rehabilitation with electrical modulation of the spinal cord and brain has recently aroused scientific interest with encouraging results. The mesencephalic locomotor region (MLR), an evolutionarily conserved brainstem locomotor command and control centre, is considered a promising target for deep brain stimulation (DBS) in patients with SCI. Experiments showed that MLR-DBS can induce locomotion in rats with spinal white matter destructions of >85%.
Methods and analysis
In this prospective one-armed multi-centre study, we investigate the safety, feasibility, and therapeutic efficacy of MLR-DBS to enable and enhance locomotor training in severely affected, subchronic and chronic American Spinal Injury Association Impairment Scale C patients in order to improve functional recovery. Patients undergo an intensive training programme with MLR-DBS while being regularly followed up until 6 months post-implantation. The acquired data of each timepoint are compared with baseline while the primary endpoint is performance in the 6-minute walking test. The clinical trial protocol was written in accordance with the Standard Protocol Items: Recommendations for Interventional Trials checklist.
Ethics and dissemination
This first in-man study investigates the therapeutic potential of MLR-DBS in SCI patients. One patient has already been implanted with electrodes and underwent MLR stimulation during locomotion. Based on the preliminary results which promise safety and feasibility, recruitment of further patients is currently ongoing. Ethical approval has been obtained from the Ethical Committee of the Canton of Zurich (case number BASEC 2016-01104) and Swissmedic (10000316). Results will be published in peer-reviewed journals and presented at conferences.
Trial registration number
Keywords: rehabilitation medicine, neurological injury, spine, neurosurgery, clinical trials
Strengths and limitation of this study.
This prospective one-armed multi-centre proof-of-concept study investigates the safety, feasibility and therapeutic potential of mesencephalic locomotor region (MLR)-deep brain stimulation (DBS) to improve walking function after severe incomplete spinal cord injury.
Patients with completed in-patient rehabilitation with highly limited ambulatory capacity are screened and considered for study enrolment.
The study comprises a variety of clinical and electrophysiological assessments before, during and after electrode implantation.
Patients undergo intensive rehabilitative training with MLR-DBS and are followed up on a regular basis until 6 months post-implantation.
The primary endpoint is improvement of locomotion measured by the 6-minute walking test 6 months after electrode implantation compared with baseline performance.
Introduction
In the event of spinal cord injury (SCI) a person’s life turns upside down within a split second, and a multitude of body functions are either severely impaired or completely lost instantly. Reacquiring lost functions including locomotion is of high importance for affected patients.1 However, it remains a largely unmet medical need due to the lack of treatment options to sufficiently rewire interrupted fibre tracts and enhance repair of the damaged human spinal cord. Despite decades of basic research, neuro-rehabilitative training currently remains the only treatment option that increases the chances of long-term improvement of sensorimotor functions.2 3 Even though most SCIs spare some descending and ascending fibre tracts, leaving the sublesional spinal cord4 only incompletely disconnected from the brain, functional recovery remains limited in most cases.3 5 6 The number of spared descending fibres is often insufficient to convey appropriate control signals to sublesional locomotor circuits, for example, central pattern generators (CPGs), which are thus deprived of supraspinal input and modulation,7 and fail to induce rhythmic motor patterns.8 9 However, these local rhythm generators remain functional and can be reactivated, for example, by direct electrical stimulation in combination with training.10–12 To increase the efficiency and efficacy of neurorehabilitation, locomotor training has therefore been combined with electrical epidural and transcutaneous stimulation of the spinal cord in small cohorts of patients in recent years, yielding promising results.3 13–15 Another encouraging approach to recruit inactive, yet intact, sublesional motor circuits involves the electrical activation of spared descending reticulospinal tract fibres (figure 1).16 The majority of reticulospinal fibres arise from the medial medullary reticular formation, which relays the output of its upstream target, the mesencephalic locomotor region (MLR),17–19 to the spinal cord. The MLR is a phylogenetically conserved key locomotor control centre in the brainstem, and is comprised of two main nuclei, the pedunculopontine nucleus (PPN) and the cuneiform nucleus (CNF).20–22 The PPN is associated with exploratory behaviour,23 and deep brain stimulation (DBS) of the PPN in patients with Parkinson’s disease can result in a reversal of freezing of gait.24–27 On the other hand, the CNF is known to be a main control region for locomotion initiation, maintenance and speed regulation.23 28 29 Recently, the MLR has gained scientific and clinical interest as target for DBS to improve deficient gait after SCI16 and stroke30 with the CNF being proposed as main therapeutic target in recent rodent studies.23 28 29 Acute electrical activation of the rat MLR has been shown to enable close to physiological hindlimb movements during walking and swimming in a rodent model of chronic incomplete SCI resembling an American Spinal Injury Association (ASIA) Impairment Scale (AIS) D score in humans.16 In animals with severely paralysed hindlimbs (AIS A-C in humans) stroke movements re-appeared with gravity-support during swimming with MLR-DBS. In an acute rodent stroke model, MLR-DBS was able to improve walking speed and limb coordination.30 DBS in humans is considered safe, reversible and minimally invasive, and is being routinely and successfully applied in the treatment of various movement disorders31–36 with great technical progress in recent years.37–39 While DBS of the PPN in Parkinson’s disease has not only yielded clearly positive therapeutic effects,40 the CNF might be a promising target for locomotion initiation.
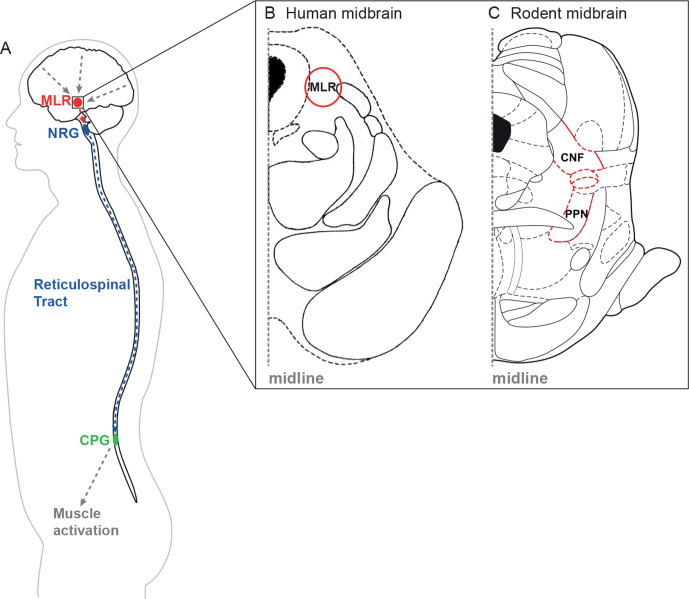
Figure 1.
Schematic illustration of the reticulospinal system. (A) Higher central nervous system centres of motion control send their signals to the mesencephalic locomotor region (MLR). The MLR is bilaterally linked to its downstream target, the gigantocellular reticular nucleus (NRG), which gives rise to the reticulospinal tract and drives the central pattern generators (CPG) for motoneuron activation and locomotion. (B and C) Horizontal section of the human (B) and cross section of the rat (C) midbrain at the level of the superior colliculi depicting the MLR (B: landmarks based on Afshar et al90; C: landmarks based on Paxinos and Watson91). CNF, cuneiform nucleus; PPN, pedunculopontine nucleus.
Function and anatomy of the brainstem motor systems are highly conserved across mammalian species.41 Due to their dispersed projection pattern throughout the spinal cord white matter,42 43 reticulospinal fibres are likely to be partially spared after incomplete SCI in humans,44 and are crucial for functional recovery after SCI.45 46
Encouraging results from animal studies16 30 47 have led to the initiation of a first in-man study that investigates MLR-DBS enabled intensive rehabilitative training and its potential to enhance locomotion in non-ambulatory, subchronic and chronic SCI patients. The study protocol is presented in this article.
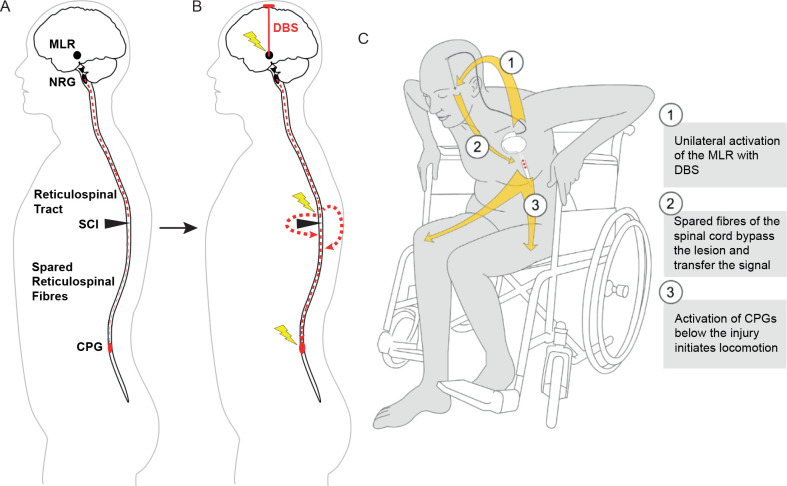
We hypothesise that MLR-DBS can modulate the activity of spared reticulospinal fibres that bypass the site of injury and reintegrate quiescent sublesional circuits into a functional network that supports walking (figure 2). We propose that enhancing excitability of sublesional spinal motor circuits increases training efficacy and promotes recovery of motor function in patients with incomplete, subchronic and chronic SCI.
Figure 2.
Schematic illustration of MLR-DBS. (A) After incomplete SCI, spared fibres of the reticulospinal tract are not sufficient to properly convey motor signals to sublesional locomotor circuits (CPG). The CPGs are thus deprived of their central input. However, these local rhythm generators remain intact. (B) MLR-DBS can recruit spared fibres of the reticulospinal tract system, enabling them to reactivate sublesional motor circuits. (C) Summary. CPG, central pattern generators; DBS, deep brain stimulation; MLR, mesencephalic locomotor region; NRG, gigantocellular reticular nucleus; SCI, spinal cord injury. (A) and (B) were modified from Hofer and Schwab3 with permission.
Methods and analysis
Study design
This prospective one-armed phase I/II multi-centre study is being conducted as cooperation of the University of Zurich, the University Hospital Zurich, and the Balgrist University Hospital Zurich. Patients are screened and selected by SCI specialists and physiotherapists at the Balgrist University Hospital. Incomplete SCI is confirmed based on clinical examinations, MRI and electrophysiological measurements, and each patient’s established drug therapy is recorded. After patient inclusion and baseline examinations, a DBS lead is stereotactically unilaterally implanted into the cuneiform part of the MLR, followed by infraclavicular or abdominal implantation of an impulse generator (IPG, figure 3). The side of lead placement is chosen based on the functional and anatomical lesion extent, with preference for the less severely affected side to transmit as much descending brainstem motor signal as possible beyond the lesion via the primarily uncrossed reticulospinal fibres. The patients are followed up on a regular basis until 6 months post-implantation, and the acquired data of each timepoint are compared with baseline findings. The primary outcome measure for improvement of ambulation in this study is the difference in covered distance in the 6-minute walking test (6MWT) at 6 months post-implantation compared with baseline level. The trial is considered successful if the patient’s performance in the 6MWT 6 months after treatment start is at least 30%48 higher compared with performance at baseline. For the design of the clinical trial protocol, we followed the Standard Protocol Items: Recommendations for Interventional Trials checklist.49
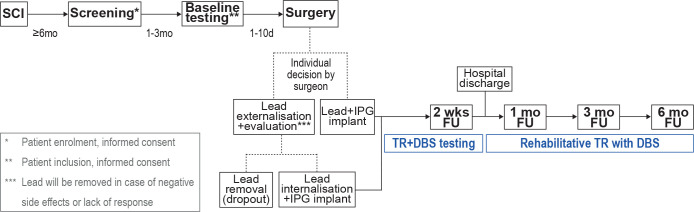
Figure 3.
Study timeline. Patients with a motor incomplete SCI at the level of T10 or above and at least 6 months of recovery after injury are eligible to undergo screening for study participation. Incomplete SCI is confirmed based on clinical examinations, MRI and electrophysiological measurements. One to three months after study enrolment, baseline testing is performed, followed by unilateral electrode implantation at the less severely affected side 1–10 days later. During surgery, the surgeon decides whether lead and impulse generator (IPG) will be implanted during one session, or whether the lead will be temporarily externalised, depending on intraoperative testing results. In case of lead externalisation, an evaluation period ensues where the patient’s responsiveness to MLR-DBS and potential negative side effects are assessed. In case of unsatisfactory results or withdrawal of consent, the lead is removed, and the patient is registered as a study dropout. In case of satisfactory testing, the lead is internalised and the IPG is implanted. After complete implantation, follow-up testing ensues at 2 weeks, 1 month, 3 months, and 6 months, respectively. Patients will be discharged from hospital after 2–3 weeks of training and testing. After hospital discharge, patients will undergo rehabilitative training with DBS at settings predefined during the first 2 weeks after implantation. d, day(s); DBS, deep brain stimulation; FU, follow-up; mo, month(s); SCI, spinal cord injury; TR, training; wks, weeks.
Study population
Female and male patients (18–75 years) with completed in-patient rehabilitation and at least 6 months of recovery after SCI are screened and considered for study enrolment. We aim at including five patients, who have to complete all preoperative and postoperative examinations until 6 months after electrode implantation, resulting in a total of 11 timepoints. In case of withdrawal of participation, dropouts and incomplete follow-up, we will include a maximum of two additional patients (replacement of dropouts/withdrawal). The study is open to national and international patients. Basic understanding of German or English is required. Patients who prematurely withdraw from the study will be offered complete removal of all implanted material, and will be followed up according to clinical standards. The patients’ study-related data will remain in the study.
Inclusion and exclusion criteria
To be eligible for the study, a participant must fulfil all inclusion criteria and none of the exclusion criteria (table 1).
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
| Informed consent | Enrolment of the investigator, her/his family members, employees and other dependent persons |
| Participation in two assessment sessions before enrolment (screening and baseline) | Limitation of standing and walking function based on accompanying (CNS) disorders |
| Willingness and ability to comply with the protocol and to attend required study training and visits | Cardiovascular disorders restricting physical training or peripheral nerve disorders |
| Female or male subject | Implanted technical devices (pacemaker, defibrillator, others) |
| Age 18–75 | History of significant autonomic dysreflexia |
| Motor incomplete SCI | Cognitive disorders/brain damage |
| Level of lesion at or above T10, based on AIS level, preservation of sacral function | Drug refractory epilepsy |
| Focal spinal cord disorder caused by either trauma or non-traumatic and non-progressive condition (like haemorrhage, benign tumour) | Severe joint contractures disabling or restricting lower limb movements |
| Minimum 6 months of recovery after SCI | Haematological disorders with increased risk of bleeding during surgical interventions |
| Completed in-patient rehabilitation programme | Participation in another study with investigational drug within 30 days preceding and during the present study |
| WISCI II, level >2 (0–20 items): assistance of one or more persons. Ability to walk at least 10 m | Congenital or acquired lower limb abnormalities (affection of joints and bone) |
| Stable medical and physical condition | Women who are pregnant or breast feeding or planning a pregnancy during the course of the study |
| Adequate caregiver support and access to appropriate medical care in patient’s home community | Lack of safe contraception |
| Inability of the participant to follow the procedures of the study, for example, due to language problems, psychological disorders, dementia, etc | |
| Known or suspected non-compliance, drug or alcohol abuse | |
| Current or prior malignancy |
AIS, ASIA (American Spinal Injury Association) Impairment Scale; CNS, central nervous system; PI, principal investigator; SCI, spinal cord injury; WISCI, Walking Index for Spinal Cord Injury.
Target area definition
While the rodent CNF and its microstructure are nowadays well characterised,23 28 29 the human CNF is poorly described, and presented only in a very limited number of stereotactic atlases. However, due to the high phylogenetic conservation,41 the CNF can be defined by surrounding landmarks and coordinates available from lead implantation into the PPN and rodent stereotactic atlases (figure 4).
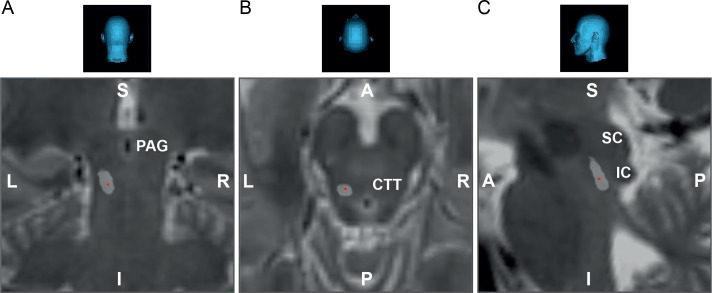
Figure 4.
Target area definition and electrode positioning. The mesencephalic locomotor region can be targeted by aiming anterior to the inferior colliculus (IC), lateral of the periaqueductal grey (PAG) and slightly posterior to the central tegmental tract (CTT).90 92 (A) Coronal, (B) axial and (C) sagittal view of the mesencephalon of the first patient successfully included in the deep brain stimulation-spinal cord injury trial, showing the localisation of the implanted lead (red dot in light grey area). A, anterior; I, inferior; L, left; P, posterior; R, right; S, superior; SC, superior colliculus.
Surgery
All individuals included in the study undergo unilateral stereotactic implantation of an intracranial lead (model 3389-28; Medtronic, Minneapolis, Minnesota, USA) via a unilateral burrhole under local anaesthesia. The distal end of the DBS lead features narrow (0.5 mm) spacing between each of the four stimulation contacts of 1.5 mm length each. After mounting of the stereotactic frame, high-resolution cranial CT scans are performed and fused with the individual’s MRI scan to retrieve stereotactic coordinates based on the preplanned trajectory. Depending on the patient’s preferences and the surgeon’s decision, patients either receive a full implant consisting of a DBS lead, an extension and an IPG within one surgical session, or receive a lead only, which is externalised for maximal 10 days for evaluation of side effects and responsiveness to stimulation. In the latter scenario, the patient undergoes a second surgery with either removal of the lead (dropout of the study participant) or completion of the DBS system. For completion, the lead is connected to a Medtronic Activa SC model 37 603 IPG using a Medtronic model 37 086-60 or 37 086-95 extension cable. The IPG is implanted subcutaneously in the pectoral or abdominal region, respectively, depending on the patient’s physiognomy and preference.
Intraoperatively, at first electrophysiological mapping of the CNF is performed. Microelectrodes are precisely inserted along a predefined trajectory aiming towards the CNF with the Neuro Omega neuromodulation system and manual drive (Alpha Omega Engineering, Nazareth, Israel) attached to the stereotactic device. During electrode insertion (0.5 mm steps), microelectrode recordings (30 s at each position) of single and multi-unit activity (local field potentials, LFPs) are performed during resting state, imagination of walking, passive and active lower limb movement within 10 mm prior and maximum 5 mm after the projected target point. Signals are band pass filtered (1–500 Hz). Depending on the patient’s anatomy up to five microelectrodes can be inserted simultaneously, in case of a presumed elevated risk of haemorrhage, the surgeon can decide to exclusively use macroelectrodes instead of microelectrodes. The centre of the region showing neuronal responsiveness to walking imagination, passive and active lower movement is subsequently stimulated while the patient performs a selection of motor tasks with the lower limbs hanging off the surgery table, accompanied by simultaneous electromyographic (EMG) recordings. Since this study is the first to investigate DBS of the CNF in human patients, no guidelines for optimal stimulation parameters are available. However, there is growing and comparable evidence from preclinical studies in various animal models suggesting low frequency stimulations (≤50 Hz) at medium to broad pulse widths (200–1000 µs)16 50 51 which is likely to be transferable to humans due to the evolutionarily conserved nature of the MLR across mammalian species. We thus initially stimulate with 20 Hz and 400 µs pulse width at increasing voltages, and frequency and pulse widths are then adjusted based on the individuals’ intraoperative behavioural response. Up to three different parameter settings of fixed frequency and pulse width with varying voltages are extensively tested intraoperatively. Stimulation amplitude is slowly increased, and changes in range of motion with and without stimulation are measured by goniometers attached to knee and ankle while the patient performs rhythmic knee and ankle flexion/extension movements. Furthermore, speech and cognition are tested with and without stimulation, and the appearance of side effects, in particular pain sensations and paraesthesia, is closely monitored and documented. Additional electrophysiological measurements, including motor evoked potentials (MEPs) and somatosensory evoked potentials (SSEPs), are performed for neuromonitoring, and event (ie, lower extremity motor response) related potentials are analysed. Ultimately, the coordinates resulting in best motor performance (eg, greatest range of motion of knee joint, highest frequency of rhythmic knee flexions/extensions) at the lowest stimulation parameters without provoking side effects are chosen, and the quadripolar DBS lead is implanted with contact 2 located within the centre of the identified area, fixed to the skull, and either temporarily externalised or connected to an extension and IPG. All subjects subsequently receive a postoperative cranial CT scan to verify correct lead position and exclude surgery-associated complications (eg, haemorrhages). Each patient recovers from surgery in the intermediate care unit overnight.
Clinical assessments
6-minute walking test
During the 6MWT,48 the patient is asked to cover a maximal distance within 6 min on even ground without any obstacles. The patient is accompanied by an experienced investigator (ie, physiotherapist) to prevent falling, and may rest at his own discretion and use a walking aid (consistent across all timepoints). The distance covered (m), time and number of rests (min, count) are documented. Each assessment is video recorded.
10-metre walking test
The 10-metre walking test (10MWT)52 is a widely used assessment tool to measure maximal walking speed (m/s). The patient is instructed to walk 10 m as quickly as possible, but safely, and is given 5 m for acceleration and deceleration. Patients may use assistive devices (consistent across all timepoints).
Timed-Up and Go test
The Timed-Up and Go (TUG) test is a basic evaluation tool of functional mobility. It measures the time (s) needed to rise from a chair, walk 3 m, turn around and return to a seated position. Participants are asked to perform the TUG at their self-selected normal speed, using their walking aid if required. The timer is started on the command ‘ready–set–go’ and stopped as the patient returns to a seated position.
Kinematic assessment
Kinematic assessments are performed during over-ground and treadmill walking. Individuals are secured using the FLOAT (‘Free Levitation for Overground Active Training’),53 54 a multidirectional overhead support system that allows patients to move in a large workspace that is equipped with a 3D motion capture system with infrared cameras (Vicon Motion Systems, Oxford, UK). The cameras are able to detect the position of reflective markers placed on patients’ anatomical landmarks, allowing the quantification of kinematic movement characteristics.55 56 Additionally, muscle activity is measured with an EMG setup (myon AG, Schwarzenberg, Switzerland). These measures allow the quantification of patients’ walking function with high precision and the comparison of gait patterns within (with and without DBS) and between different sessions. In addition to walking assessments, maximal knee and ankle range of motion is evaluated with and without stimulation with the motion capture system during rhythmic flexion/extension tasks performed by the patient in supine or sitting position. Besides quantitative assessment of locomotor function, the FLOAT allows patients to train diverse activities such as level walking, running, stair manoeuvres, chair interactions or walking on uneven terrain with and without stimulation at the limit of their abilities with tailored body weight support.
Long-term monitoring of physical activity
For constant monitoring of physical activity during training and daily life, wearable, wireless sensors (http://zurichmove.com/) are mounted to the patient’s wrists, ankles and wheelchair. Data are transferred via SSL-encrypted links (https) established between sites (eg, a patient’s home or rehab centre) and the Swiss Federal Institute of Technology Zurich (ETH).
ASIA Impairment Scale
The ASIA International Standards for Neurological Classification of SCI (ISNCSCI)57 is an internationally used gold standard method of assessing the neurological status of an individual with SCI. The AIS is carried out by trained medical staff using the ISNCSCI worksheet (https://asia-spinalinjury.org/international-standards-neurological-classification-sci-isncsci-worksheet/).
Modified Ashworth Scale
The Modified Ashworth Scale58 is a clinical scale used to assess muscle spasticity in patients with lesions of the central nervous system. It is the most commonly used tool to evaluate changes of muscle tone in response to therapeutic interventions, for example, anti-spasticity medication. Here, we aim to investigate the effects of MLR-DBS by itself on muscle tone and thus do not routinely modify each patient’s established antispasticity treatment unless medically indicated. However, potential drug-stimulation interactions are considered in data interpretation.
Spinal Cord Independence Measure III
The Spinal Cord Independence Measure (SCIM) is a reference tool for the assessment of overall functional ability after SCI. The last version (III) of SCIM contains 19 tasks organised into 3 subscales: self-care, respiration and sphincter management, and mobility.59 The combined scores on all 19 tasks result in an overall score ranging from 0 to 100, with higher scores reflecting greater functional ability.
Walking Index for Spinal Cord Injury II
The Walking Index for Spinal Cord Injury assesses walking function on an ordinal scale,60 and captures the extent and nature of assistance a person with SCI requires to walk. Rating is performed according to Ditunno et al.60
Assessment of lower urinary tract function
To address the burden of neurogenic lower urinary tract (LUT) dysfunction on patient’s quality of life (QoL) after SCI and to analyse the effect of MLR-DBS on recovery of LUT function, a combination of qualitative (bladder diary, QUALIVEEN questionnaire) and quantitative assessments (urodynamic measurements, renal ultrasound) of LUT function are applied in accordance to the European Association of Urology Guidelines on Neuro-Urology.61 62
Bladder diary: by completing the Three Day Bladder Chart63 information on daytime frequency, night-time frequency, voiding (eg, spontaneous), catheter use (transurethral, suprapubic, self-catheterisation), voided volume, post-void residual volume, incontinence episodes, pad use, fluid intake and amount of urine per 24 hours and pain (visual analogue scale 0–10) is acquired.
QUALIVEEN questionnaire: all patients fill in the QUALIVEEN questionnaire for self-judgement of LUT dysfunction according to Costa et al.64 Scores (0–4) are recorded for ‘limitations’, ‘constraints’, ‘fears’ and ‘feelings’, and the calculated arithmetic mean is transformed into values of 0–100.
Urodynamic assessments: cystometry, uroflowmetry, pressure-flow studies, electromyography and video-urodynamics provide objective information on functioning of the LUT and pelvic floor. Parameters retrieved are: cystometric capacity (mL), compliance (mL/cmH2O), detrusor overactivity (y/n), bladder volume at detrusor overactivity (mL), maximum detrusor pressure amplitude (cmH2O) during storage phase, urinary incontinence, maximum detrusor pressure (cmH2O) during voiding phase, detrusor pressure at maximum flow rate (cmH2O), maximum flow rate (mL/s), voided volume (mL), post-void residual (y/n and mL), pelvic floor EMG activity (normal/abnormal), vesico-uretero-renal reflux (y/n).
Renal and bladder ultrasound: indirect assessment of LUT function, for example, via post-void residual volume, detrusor thickness or distension of the renal pelvis or ureter.
Assessment of sexual function
The Female Sexual Function Index65 66 is gold standard for the evaluation of female sexual function in clinical trials. It is questionnaire-based and contains 19-items including sexual arousal, orgasm, satisfaction and pain (score 2–80). The International Index of Erectile Function67 is a standardised 15-item self-evaluation scale for male patients assessing erectile function, orgasmic function, sexual desire, satisfaction in sexual intercourse and in general.
Epworth Sleepiness Scale
The Epworth Sleepiness Scale68 measures a patient’s general level of daytime sleepiness. The patient rates the probability of falling asleep on a scale of increasing probability (0–3) for eight different situations.
Fatigue Severity Scale
The Fatigue Severity Scale69 evaluates the impact of fatigue based on a short questionnaire containing nine statements rating the severity of fatigue symptoms.
Pain assessment
The European Multicenter Study About Spinal Cord Injury pain assessment form70 71 and the Spinal Cord Injury Pain Instrument72–74 are standardised and validated tools to evaluate pain in individuals with SCI.
Graded Redefined Assessment of Strength, Sensation and Prehension
The Graded Redefined Assessment of Strength, Sensation and Prehension75 76 is a standardised upper-limb impairment measure specifically used to assess recovery of upper limb function (strength, sensation, prehension) in individuals with complete or incomplete tetraplegia.
Short Form Health Survey to Assess Quality of Life
Patients with SCI experience tremendous changes in several aspects of everyday life and thus QoL77 assessments are crucial in clinical trials. We employ the Short Form Health Survey to Assess Quality of Life,78 a multi-purpose, short-form health survey comprised of 36 questions that compares the relative burden of diseases and differentiates the health benefits produced by a wide range of different treatments. It yields an 8-scale profile of functional health and well-being scores, psychometrically based physical and mental health summary measures, and a preference-based health utility index. QoL is expressed as a score ranging from 0 to 100.
Electrophysiological assessments
Electrophysiological assessments are performed in addition to clinical examinations as they allow prediction of functional outcome and help objectify the extent of the spinal lesion, its stability and potentially recovery of specific functions after SCI.79 80 Intraoperative somatosensory and MEPs are recorded for neuromonitoring purposes due to the close relationship of the CNF with surrounding brainstem structures.
Short-latency somatosensory evoked potentials
SSEPs are performed to evaluate transmission of ascending signals within the dorsal column of the spinal cord and thus sensory function. The patient is in supine position, and stimulating electrodes are placed on the posterior tibial nerve (below the internal malleolus). Four subcutaneous recording electrodes are placed as follows: at L2 and L5, on the scalp (reference Fz and active Cz’, 2 cm behind Cz), and a ground around the ankle. Cortical recording electrodes are positioned in accordance with the International 10–20 system.81 Stimulation parameters are 200 µs, up to 100 mA at a frequency of 3.1 Hz. The signal is recorded between 30 and 300 Hz with 50 Hz notch filter. Waveforms are measured after 200–800 averages. Dorsal horn negativity (N24) is measured on the lumbar derivation (L5–L2) and represents peripheral conduction time. The post-Rolandic positivity (P45) is measured on the scalp derivation and represents the total conduction time. All measures are recorded before, during and after electrode implantation, and before and after first (week 1 after implantation) and last (6 months after implantation) 6MWT assessments. Response latency (ms) and amplitude (µV) are compared between timepoints and conditions (stim/no stim).
DBS evoked potentials
DBS-evoked potential testing is performed similar to SSEP measurements. However, instead of stimulating a peripheral nerve, the evoked cortical response is generated by repetitive low frequency stimulation of the target region (CNF/MLR). Outcome measures are response latency (ms) and amplitude (µV).
Motor evoked potentials
MEPs are tested to evaluate the ability of MLR-DBS enhanced training to induce remodelling of spinal pathways leading to amplification of descending signals. Surface recording electrodes are positioned on the tibialis anterior and the gastrocnemius medialis muscles. Transcranial magnetic stimulation is applied on the scalp close to Cz and on the lumbar spine in front of L5. After a test stimulus, the stimulation is increased stepwise up to 100% of the stimulator output and the response is recorded under 5%–10% voluntary muscle activation. Total conduction time is measured after scalp stimulation and peripheral conduction time after lumbar stimulation. All measures are recorded before, during and after electrode implantation, and before and after first (week 1 after implantation) and last (6 months after implantation) 6MWT assessments. Response latency (ms) and amplitude (µV) are compared between timepoints and conditions.
Local field potentials
LFPs are measured intraoperatively during probe insertion and postoperatively in case of temporary externalisation of the lead. Intraoperative LFPs are measured in the target region, starting 10 mm above the target and ending 5 mm below the target. Postoperative measurements are performed at the four contacts of the implanted lead. Signals are band pass filtered (1–500 Hz).
Electroencephalogram
To reconstruct patterns of specific neuronal activity and their change on MLR-DBS, non-invasive electroencephalogram (EEG) recordings are performed in the perioperative period and at the last assessment timepoint.
DBS during behavioural testing and rehabilitative training
In the first 2 weeks after lead implantation, different stimulation parameters (frequency, Hz; pulse width, µs; amplitudes, mV) are tested during rest and locomotor training in order to identify optimal stimulator settings including safety limits for each patient individually. The most promising monopolar stimulation settings identified intraoperatively (frequency, pulse width) are applied systematically first via lead contact 2 with varying voltages. In case of failure to induce motor responses or occurrence of side effects at already low voltages parameters will be adapted (frequency, pulse width, polarity, lead contact) sequentially depending on each patient’s efficacy and side effect profile. Subsequently, one set of parameters eliciting the best motor responses without side effects is chosen for rehabilitative training (eg, 20 Hz, 420 µs, suprathreshold intensity) and programmed to the patient programming device (up to three additional combinations could be additionally programmed to the device if needed). After 2 weeks, patients are discharged home or to a rehabilitation clinic located close to home. Training intensity is monitored and ensured by regular follow-ups by phone and by online activity monitoring via wearable sensors mounted to the patient’s wrists, ankles and wheelchair. Behavioural testing is performed with and without stimulation during each follow-up visit using the stimulation parameters applied during training.
Study endpoints
The primary endpoint of the DBS-SCI study is improvement of locomotor function, represented by an increased distance covered during the 6MWT when comparing performance at the 6 months timepoint with and without DBS with performance at baseline. Additionally, a variety of secondary endpoint assessments are performed (table 2). Table 3 summarises timing and schedule of the respective primary and secondary endpoint assessments.
Table 2.
Primary and secondary endpoint measures
| Primary endpoint measure | Secondary endpoint measures |
| 6-minute walking test (6MWT) at 6 months follow-up vs baseline | 6MWT at follow-up timepoints other than 6 months post-implantation |
| 10-metre walking test (10MWT) | |
| Timed Up and Go test (TUG) | |
| Kinematic assessments (FLOAT) | |
| Spinal Cord Independence Measure (SCIM III) | |
| Walking Index for Spinal Cord Injury (WISCI II) | |
| Activity counts (patient’s overall activity level) | |
| Electrophysiological measurements* | |
| Quality of life (SF-36) | |
| Lower urinary tract (LUT) function† | |
| Sexual function (FSFI/IIEF) | |
| Spasticity (MAS) | |
| Neurological classification of SCI (AIS) | |
| Upper limb function (GRASSP) | |
| Level of fatigue (FSS) | |
| Level of sleepiness (ESS) | |
| Pain (EPAF, SCIPI) |
*Local field potentials; somatosensory evoked potentials; motor evoked potentials; deep brain stimulation evoked potentials; electroencephalogram.
†Bladder diary, QUALIVEEN questionnaire, urodynamic measurements, bladder and renal ultrasound.
AIS, American Spinal Injury Association (ASIA) Impairment Scale; EPAF, EMSCI (European Multicenter Study About Spinal Cord Injury) Pain Assessment Form; ESS, Epworth Sleepiness Scale; FLOAT, Free Levitation for Overground Active Training; FSFI, Female Sexual Function Index; FSS, Fatigue Severity Scale; GRASSP, Graded Redefined Assessment of Strength, Sensation and Prehension; IIEF, International Index of Erectile Function; MAS, Modified Ashworth Scale; MLR, mesencephalic locomotor region; SCI, spinal cord injury; SCIPI, Spinal Cord Injury Pain Instrument; SF-36, Short Form Health Survey to Assess Quality of Life.
Table 3.
Flowchart summarising scheduling and timing of primary and secondary endpoint assessments
| Site | Study periods | Screening | Baseline | DBS surgery | Postimplantation phase | IPG implantation | Rehabilitation/follow-up phase | |||||||
| Visit | 1 | 2 | 3 | 4 | 5 | 6* | 7* | 8 | Discharge | Site | 9 | 10 | 11 | |
| Day (d)/month (mo) | −90 to −30 d | −10 to −1 d | 0 | 1 to 3 d | 4 to 7 d | 8 to 9 d | 6 to 10 d | 14 d | mo 1 | mo 3 | mo 6 | |||
| ±3 d | ±3 d | ±1 week | ||||||||||||
| University Hospital Zurich/Balgrist University Hospital | Study inclusion and consent | Balgrist University Hospital | ||||||||||||
| Consenting | X | |||||||||||||
| Enrolment | X | |||||||||||||
| Patient inclusion by PI | X | |||||||||||||
| Imaging | ||||||||||||||
| X-ray thorax | X | X | ||||||||||||
| X-ray skull, abdomen | X | |||||||||||||
| Stereotactic cranial CT | 2 X | |||||||||||||
| Diagnostic MRI (3T) | X | |||||||||||||
| Perisurgical examinations | ||||||||||||||
| Surgical examination (incl. wound check) | X | X | X | X | X | X | X | X | X | |||||
| Anaesthesiological examination | X | X | X | X | ||||||||||
| Neuropsychological assessment | X | X | ||||||||||||
| Psychiatric assessment | X | X | ||||||||||||
| Surgical procedures | ||||||||||||||
| DBS lead implantation | X | |||||||||||||
| Implantation of IPG or explantation of DBS lead | X (externalisation may be skipped and IPG implanted at visit 3) |
|||||||||||||
| Education in handling of patient programming device | X | |||||||||||||
| Electrophysiological assessments | ||||||||||||||
| EMG | X | X | X | X | X | |||||||||
| Microelectrode recording | X | |||||||||||||
| Nerve conduction | X | X | ||||||||||||
| Non-invasive EEG | X | X | X | X | X | |||||||||
| MEP, SSEP | X | X | X | X | X | |||||||||
| LFP, DBS-EP | X | X | X | |||||||||||
| Balgrist University Hospital | Clinical assessments | |||||||||||||
| AIS | X | X | X | X | X | X | X | |||||||
| WISCI II | X | X | X | X | X | X | X | |||||||
| SCIM III | X | X | X | X | X | X | X | |||||||
| TUG | X | X | X | X | X | X | X | |||||||
| Kinematic assessments | X | X | X | X | X | X | X | |||||||
| 6MWT | X | X | X | X | X | X | X† | |||||||
| 10MWT | X | X | X | X | X | X | X | |||||||
| AE assessment | X | X | X | X | X | X | X | X | X | X | ||||
| Questionnaires: QoL, FSFI, IIEF, ESS, FSS, EPAF, SCIPI | X | X | X | X | ||||||||||
| Questionnaire: MAS | X | X | X | X | X | X | ||||||||
| LUT assessments (Bladder diary, QUALIVEEN, urodynamics, bladder/renal ultrasound) | X | X | ||||||||||||
| GRASSP | X | X | ||||||||||||
*If impulse generator (IPG) is implanted at visit 3, visit 6 and visit 7 will be skipped.
†Primary endpoint.
AE, adverse event; AIS, American Spinal Injury Association (ASIA) Impairment Scale.; DBS, deep brain stimulation; DBS-EP, DBS-evoked potentials; EEG, electroencephalography; EMG, electromyography; ESS, Epworth Sleepiness Scale; FSFI, Female Sexual Function Index; FSS, Fatigue Severity Scale; GRASSP, Graded Redefined Assessment of Strength, Sensation and Prehension; IIEF, International Index of Erectile Function; IPG, impulse generator; LFP, local field potentials; LUT, lower urinary tract function; MAS, Modified Ashworth Scale; MEP, motor evoked potentials; 6MWT, 6-minute walking test; 10MWT, 10-metre walking test; QOL, quality of life; SCIM III, Spinal Cord Independence Measure; SCIPI, Spinal Cord Injury Pain Instrument; SSEP, somatosensory evoked potentials; 3T, 3 Tesla; TUG, Timed Up and Go test; WISCI II, Walking Index of Spinal Cord Injury.
Sample size
Based on data on the 6MWT48 82 83 published in the literature and our clinical experience we estimate a relative effect size of 30% improvement in the 6MWT 6 months after treatment start compared with performance at baseline to be clinically relevant. A sample size of five patients provides us with a power (1−β) of 80% (α=0.05). Founded on previous experience in DBS of the MLR,84 85 we judge that the selected sample size will provide acceptable clinical validity for the study objectives.
Statistical analysis
Considering the observational nature of this clinical trial, statistics will be restricted to descriptive statistics.
Trial status
The study has started recruiting patients in March 2017. To date, one patient has been successfully included on 26 November 2018. Another patient has been included on 15 March 2018, but withdrew consent prior to surgery (screening failure).
Patient and public involvement
Patients or the public were not and will not be involved in the design, conduct, reporting or dissemination plans of this research.
Ethics and dissemination
The study was approved by the local institutional review board (IRB) of the Ethical Committee of the Canton of Zurich (case number BASEC 2016-01104) and Swissmedic (10000316) in January and March 2017. Protocol modifications have to be approved by the local IRB and communicated to trial registries. Before inclusion of a patient, the potential participant is informed orally by the investigator, and all potential participants are additionally provided with a clear and comprehensive information sheet. Sufficient time is given to the potential participant to decide whether to participate or not. If potential participants agree to participate in the study, they are asked to sign a consent form at the moment of inclusion in the study. The data obtained in the course of the study is treated according to the local data protection law and is handled in strictest confidence. During the study, subjects are identified solely by an anonymised patient identifier. The findings of this trial will be submitted to a peer-reviewed journal and abstracts are presented at relevant national and international scientific conferences.
Discussion
Encouraging results on behavioural effects of MLR-DBS in preclinical models of neurotrauma16 30 have contributed to the initiation of this first in-man study, which is currently being carried out at the University Hospitals of Zurich. The primary aim of this study is to improve motor function and enable locomotion in wheelchair-bound, subchronic and chronic SCI patients with limited, non-functional ambulatory abilities with MLR-DBS, and to investigate the clinical feasibility and efficacy of MLR-DBS in humans. Ultimately, we aim at maximising the long-term restitution of lost motor functions in patients with severe motor incomplete SCI. A first patient has been included and implanted successfully, followed by intensive locomotor training with suprathreshold MLR-DBS.
The most important lesson learnt from our previous experience in the treatment of this patient is that MLR-DBS is safe, feasible and well-tolerated. No increase in pain, deterioration of residual motor or sensory functions, cognitive or emotional disturbances, increase in spasticity and no incontinence was observed. However, sufficient time has to be allocated to the identification of optimal stimulation parameters for efficient training to ensue as reference values from human patients are not yet available. Optimal stimulation parameters will have to be determined for each patient individually, however, based on the existing literature and our experience gained from one patient wider pulses (>400 µs) seem to be more effective for enhancement of locomotion and more convenient than shorter pulse widths. LFP measurements and preliminary results from behavioural testing suggest that lower stimulation frequencies (8–20 Hz) are appropriate, which is in line with preclinical data.86 Due to the heterogeneity and complexity of chronic SCI with individual therapeutic needs, standardisation of rehabilitative training is challenging. While assessments performed during each patient’s stay at the Balgrist University Hospital are standardised, rehabilitative training performed prior to study inclusion varies individually as we recruit patients internationally and include patients after completion of a rehabilitation programme as we require a stable neurological baseline condition prior to electrode implantation. After we discharge our patients, they train individually under our regular surveillance and constant activity monitoring to ensure a minimum training intensity of each patient. However, given that locomotion parameters like for example, speed, stepping frequency and body weight support are highly dependent on stimulation parameters chosen and since parameters for locomotion induction vary depending on for example, lesion size, training cannot be completely identical among study participants. This is therefore a limitation innate to this type of intervention. In addition, the patient’s symptoms, especially the individual severity degree of muscle spasticity, have an influence on the feasible training intensity and potentially also on the effect of the stimulation. In this study, medications of each patient are recorded but modified only if required for medical reasons as we first need to investigate the effect of stimulation as a single-therapy before being able to test combination therapies in follow-up studies.
Given that this proof-of-concept study is the first to investigate effects of DBS of the cuneiform nucleus, the sample size of this study was intentionally chosen to be small. However, our patients undergo a variety of clinically relevant assessments generating important knowledge for follow-up studies of a greater scale. With the 6MWT as primary outcome we have chosen a simple, internationally standardised and comparable test that can be performed anywhere without requiring sophisticated equipment. It measures the maximal distance covered while walking overground independently with a chosen walking aid for 6 min. This test is highly clinically relevant as one can also record the patient’s functionality in everyday life and analyse its changes over time. We expect a significant increase in the distance walked within 6 min and a reduction in the need for assistance when walking 6 months post-implantation compared with baseline. Based on preclinical studies that have shown a positive effect of MLR-DBS on temporal execution of stepping movements we additionally expect an increase in maximal walking speed (10MWT), improved overall functional mobility (TUG test), more efficient step cycle initiation and implementation (kinematic assessments), and increased overall physical activity (activity counts). As reports on improvements of lower urinary tract function in response to locomotor training are increasing,87 88 we are additionally measuring a variety of indicators for LUT function, where we expect changes in efficiency of bladder emptying. The variety of clinical scores generate non-parametric data and are obtained to identify and monitor side effects (eg, pain) rather than to statistically analyse therapeutic effects. All assessments performed in this study comprise standard tests applied internationally in SCI research that enable us to capture the variety of consequences of an injury to the spinal cord, for example, sensorimotor disturbances, autonomic nervous system dysfunction, and decreased QoL.
A particular challenge remains trajectory planning and lead implantation. Many regions of the brainstem, including the MLR subnuclei, are small and poorly described in humans when compared with the rodent PPN and CNF.23 28 29 Coordinates known from DBS of the PPN with successful reduction of freezing of gait symptoms in patients with Parkinson’s disease24–27 can be adapted based on landmarks in human and rodent stereotactic atlases in order to localise the CNF in relation to the PPN. However, to increase the accuracy of planned trajectories and intraoperative targeting, a more detailed description of the macroanatomy and microanatomy of the human MLR is urgently needed.
Another important step in trial design and treatment development is patient selection. In both rodents16 46 89 and humans,45 the reticulospinal system is crucial for functional recovery after SCI, and at least a small number of reticulospinal fibres needs to be preserved in order to reactivate lumbar CPGs via MLR-DBS. Thus, patients who have suffered an anatomically complete SCI are not envisioned eligible for MLR-DBS. Fortunately, the majority of SCIs are anatomically incomplete,4 and reticulospinal fibres are likely to be at least partially spared after SCI in humans44 due to their scattered projection pattern in the spinal cord white matter.42 43 Based on preclinical data and experience gained from the first study participant we suggest that patients with an incomplete SCI and residual proprioceptive function, who are able to stand, but suffer from deficient stepping initiation and walking function are most likely to benefit from MLR-DBS-enabled and -enhanced training. To allow for an integration of the effects of MLR-DBS into the anatomically still plastic spinal system during early phases after SCI, we are currently adapting the original study protocol so that patients can be included as early as 3 months after injury provided a stable neurological condition for the detection of stimulation-induced effects. Stratification of patients will be based on the expected outcome of walking function predicted by the 6MWT. Patient recruitment and screening are currently ongoing.
Our preliminary results from one study patient show that MLR-DBS is feasible and safe. The efficacy of MLR-DBS to enhance training and promote functional recovery in human SCI patients can now be tested in an appropriate number of individuals.
Supplementary Material
Acknowledgments
We thank our patient for her courage and enthusiasm to participate in this study, and Medtronic, Minneapolis, Minnesota, USA, who provide the implants required. We also thank all collaborators involved in the study who have agreed to provide treatment and assessments as in-kind contribution of the Departments of Neurosurgery, Neurology, Neuroradiology, Anaesthesiology and Psychiatry of the University Hospital Zurich, the Spinal Cord Injury Center of the Balgrist University Hospital, the Institute for Regenerative Medicine of the University of Zurich, and the Swiss Federal Institute of Technology Zurich. The study has been presented at the 'European Society for Stereotactic and Functional Neurosurgery (ESSFN) Meeting' 2018 in Edinburgh, Scotland, and the 'EANS Trauma & Critical Care Update meeting' 2018 in Lund, Sweden.
Footnotes
LHS and A-SH contributed equally.
Contributors: LHS and A-SH contributed equally to the manuscript and are joint first authors. MES, LR and ACurt are joint senior authors. LHS, A-SH, MB, CB, LI, LR, MES and ACurt designed the study, created and refined the study protocol, and supervise the study. LHS, MFO, A-SH and LR perform surgeries. MB, LF, RW, ACathomen, CM and ACurt designed assessments of motor function and perform testing and analysis. MS, MH, CB and LI designed and conduct electrophysiological measurements. TMK conceptualised and performs assessments of lower urinary tract function. IK and AP assist with study coordination and conduct questionnaire-based assessments. All authors are involved in the development and implementation of the study as well as in data collection and analysis. A-SH and LHS designed the figures and drafted the manuscript. All authors critically revised the manuscript and approved its final version.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Implanted hardware (electrodes, impulse generators, extension wires and patient programming devices) including replacements for a period of 10 years after implantation in case of, for example, battery depletion is provided by Medtronic, Minneapolis, Minnesota, USA, for five patients free of charge. Beyond that, we do not receive any financial support by Medtronic. The study is financed by the Department of Neurosurgery, University Hospital Zurich, the Spinal Cord Injury Center, Balgrist University Hospital, and the Department of Neurology, University Hospital Zurich.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Not applicable.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Ditunno PL, Patrick M, Stineman M, et al. Who wants to walk? preferences for recovery after SCI: a longitudinal and cross-sectional study. Spinal Cord 2008;46:500–6. 10.1038/sj.sc.3102172 [DOI] [PubMed] [Google Scholar]
- 2.Côté M-P, Murray M, Lemay MA. Rehabilitation strategies after spinal cord injury: inquiry into the mechanisms of success and failure. J Neurotrauma 2017;34:1841–57. 10.1089/neu.2016.4577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hofer A-S, Schwab ME. Enhancing rehabilitation and functional recovery after brain and spinal cord trauma with electrical neuromodulation. Curr Opin Neurol 2019;32:828–35. 10.1097/WCO.0000000000000750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Spinal Cord Injury Statistical Center (NSCISC) . Spinal cord injury (sci)-facts and figures at a glance, 2019. Available: https://www.nscisc.uab.edu/ [Accessed 17 Dec 2019].
- 5.Dietz V, Schwab ME. From the rodent spinal cord injury model to human application: promises and challenges. J Neurotrauma 2017;34:1826–30. 10.1089/neu.2016.4513 [DOI] [PubMed] [Google Scholar]
- 6.Dietz V. Body weight supported gait training: from laboratory to clinical setting. Brain Res Bull 2008;76:459–63. 10.1016/j.brainresbull.2008.02.034 [DOI] [PubMed] [Google Scholar]
- 7.Taccola G, Sayenko D, Gad P, et al. And yet it moves: recovery of volitional control after spinal cord injury. Prog Neurobiol 2018;160:64–81. 10.1016/j.pneurobio.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hubli M, Dietz V. The physiological basis of neurorehabilitation--locomotor training after spinal cord injury. J Neuroeng Rehabil 2013;10:5. 10.1186/1743-0003-10-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musienko P, Heutschi J, Friedli L, et al. Multi-system neurorehabilitative strategies to restore motor functions following severe spinal cord injury. Exp Neurol 2012;235:100–9. 10.1016/j.expneurol.2011.08.025 [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Ríos M, Guertin PA, Rivera-Oliver M. Neuromodulation of spinal locomotor networks in rodents. Curr Pharm Des 2017;23:1741–52. 10.2174/1381612823666170124111729 [DOI] [PubMed] [Google Scholar]
- 11.Gill ML, Grahn PJ, Calvert JS, et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med 2018;24:1677–82. 10.1038/s41591-018-0175-7 [DOI] [PubMed] [Google Scholar]
- 12.Marques MR, Nicola FC, Sanches EF, et al. Locomotor training promotes time-dependent functional recovery after experimental spinal cord contusion. Neuroscience 2018;392:258–69. 10.1016/j.neuroscience.2018.08.033 [DOI] [PubMed] [Google Scholar]
- 13.Rejc E, Angeli CA. Spinal cord epidural stimulation for lower limb motor function recovery in individuals with motor complete spinal cord injury. Phys Med Rehabil Clin N Am 2019;30:337–54. 10.1016/j.pmr.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 14.Inanici F, Samejima S, Gad P, et al. Transcutaneous electrical spinal stimulation promotes long-term recovery of upper extremity function in chronic tetraplegia. IEEE Trans Neural Syst Rehabil Eng 2018;26:1272–8. 10.1109/TNSRE.2018.2834339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer C, Hofstoetter US, Hubli M, et al. Immediate effects of transcutaneous spinal cord stimulation on motor function in chronic, sensorimotor incomplete spinal cord injury. J Clin Med 2020;9:3541. 10.3390/jcm9113541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachmann LC, Matis A, Lindau NT, et al. Deep brain stimulation of the midbrain locomotor region improves paretic hindlimb function after spinal cord injury in rats. Sci Transl Med 2013;5:208ra146-208ra146. 10.1126/scitranslmed.3005972 [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Rill E, Skinner RD. The mesencephalic locomotor region. II. projections to reticulospinal neurons. Brain Res 1987;411:13–20. 10.1016/0006-8993(87)90676-7 [DOI] [PubMed] [Google Scholar]
- 18.Steeves JD, Jordan LM. Autoradiographic demonstration of the projections from the mesencephalic locomotor region. Brain Res 1984;307:263–76. 10.1016/0006-8993(84)90480-3 [DOI] [PubMed] [Google Scholar]
- 19.Edwards SB, de Olmos JS. Autoradiographic studies of the projections of the midbrain reticular formation: ascending projections of nucleus cuneiformis. J Comp Neurol 1976;165:417–31. 10.1002/cne.901650403 [DOI] [PubMed] [Google Scholar]
- 20.Shik ML, Severin FV, Orlovskiĭ GN. [Control of walking and running by means of electric stimulation of the midbrain]. Biofizika 1966;11:659–66. [PubMed] [Google Scholar]
- 21.Skinner RD, Garcia-Rill E. The mesencephalic locomotor region (MLR) in the rat. Brain Res 1984;323:385–9. 10.1016/0006-8993(84)90319-6 [DOI] [PubMed] [Google Scholar]
- 22.Ryczko D, Dubuc R. The multifunctional mesencephalic locomotor region. Curr Pharm Des 2013;19:4448–70. 10.2174/1381612811319240011 [DOI] [PubMed] [Google Scholar]
- 23.Caggiano V, Leiras R, Goñi-Erro H, et al. Midbrain circuits that set locomotor speed and gait selection. Nature 2018;553:455–60. 10.1038/nature25448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stefani A, Lozano AM, Peppe A, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe parkinson's disease. Brain 2007;130:1596–607. 10.1093/brain/awl346 [DOI] [PubMed] [Google Scholar]
- 25.Pereira EA, Muthusamy KA, De Pennington N, et al. Deep brain stimulation of the pedunculopontine nucleus in parkinson’s disease. preliminary experience at Oxford. Br J Neurosurg 2008;22:S41–4. 10.1080/02688690802448335 [DOI] [PubMed] [Google Scholar]
- 26.Mazzone P, Lozano A, Stanzione P, et al. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in parkinson's disease. Neuroreport 2005;16:1877–81. 10.1097/01.wnr.0000187629.38010.12 [DOI] [PubMed] [Google Scholar]
- 27.Moro E, Hamani C, Poon Y-Y, et al. Unilateral pedunculopontine stimulation improves falls in Parkinson's disease. Brain 2010;133:215–24. 10.1093/brain/awp261 [DOI] [PubMed] [Google Scholar]
- 28.Josset N, Roussel M, Lemieux M, et al. Distinct contributions of mesencephalic locomotor region nuclei to locomotor control in the freely behaving mouse. Curr Biol 2018;28:884–901. 10.1016/j.cub.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 29.Capelli P, Pivetta C, Soledad Esposito M, Esposito MS, et al. Locomotor speed control circuits in the caudal brainstem. Nature 2017;551:373–7. 10.1038/nature24064 [DOI] [PubMed] [Google Scholar]
- 30.Fluri F, Malzahn U, Homola GA, et al. Stimulation of the mesencephalic locomotor region for gait recovery after stroke. Ann Neurol 2017;82:828–40. 10.1002/ana.25086 [DOI] [PubMed] [Google Scholar]
- 31.Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med 2006;355:896–908. 10.1056/NEJMoa060281 [DOI] [PubMed] [Google Scholar]
- 32.Kleiner-Fisman G, Herzog J, Fisman DN, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord 2006;21 Suppl 14:S290–304. 10.1002/mds.20962 [DOI] [PubMed] [Google Scholar]
- 33.Hartmann CJ, Fliegen S, Groiss SJ, et al. An update on best practice of deep brain stimulation in Parkinson's disease. Ther Adv Neurol Disord 2019;12:1756286419838096. 10.1177/1756286419838096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu F, Ma W, Huang Y, et al. Deep brain stimulation of pallidal versus subthalamic for patients with Parkinson's disease: a meta-analysis of controlled clinical trials. Neuropsychiatr Dis Treat 2016;12:1435–44. 10.2147/NDT.S105513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin S, Wu Y, Li H, et al. Deep brain stimulation of the globus pallidus internus versus the subthalamic nucleus in isolated dystonia. J Neurosurg 2019;132:1–12. 10.3171/2018.12.JNS181927 [DOI] [PubMed] [Google Scholar]
- 36.Cury RG, Fraix V, Castrioto A, et al. Thalamic deep brain stimulation for tremor in parkinson disease, essential tremor, and dystonia. Neurology 2017;89:1416–23. 10.1212/WNL.0000000000004295 [DOI] [PubMed] [Google Scholar]
- 37.Shi Y, Liu R, He L. Recent development of implantable and flexible nerve electrodes. Smart Mater. Med 2020;1. 10.1016/j.smaim.2020.08.002 [DOI] [Google Scholar]
- 38.Zhao S, Li G, Tong C, et al. Full activation pattern mapping by simultaneous deep brain stimulation and fMRI with graphene fiber electrodes. Nat Commun 2020;11:1788. 10.1038/s41467-020-15570-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shan Y, Feng H, Li Z. Electrical stimulation for nervous system injury: research progress and prospects. Wuli Huaxue Xuebao/ Acta Phys - Chim Sin 2020;36. 10.3866/PKU.WHXB202005038 [DOI] [Google Scholar]
- 40.Thevathasan W, Debu B, Aziz T, et al. Pedunculopontine nucleus deep brain stimulation in Parkinson's disease: a clinical review. Mov Disord 2018;33:10-20. 10.1002/mds.27098 [DOI] [PubMed] [Google Scholar]
- 41.Nudo RJ, Masterton RB. Descending pathways to the spinal cord: a comparative study of 22 mammals. J Comp Neurol 1988;277:53–79. 10.1002/cne.902770105 [DOI] [PubMed] [Google Scholar]
- 42.Nathan PW, Smith M, Deacon P. Vestibulospinal, reticulospinal and descending propriospinal nerve fibres in man. Brain 1996;119:1809–33. 10.1093/brain/119.6.1809 [DOI] [PubMed] [Google Scholar]
- 43.Ballermann M, Fouad K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur J Neurosci 2006;23:1988–96. 10.1111/j.1460-9568.2006.04726.x [DOI] [PubMed] [Google Scholar]
- 44.Kakulas BA. A review of the neuropathology of human spinal cord injury with emphasis on special features. J Spinal Cord Med 1999;22:119–24. 10.1080/10790268.1999.11719557 [DOI] [PubMed] [Google Scholar]
- 45.Baker SN, Perez MA. Reticulospinal contributions to gross hand function after human spinal cord injury. J Neurosci 2017;37:9778–84. 10.1523/JNEUROSCI.3368-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zörner B, Bachmann LC, Filli L, et al. Chasing central nervous system plasticity: the brainstem's contribution to locomotor recovery in rats with spinal cord injury. Brain 2014;137:1716–32. 10.1093/brain/awu078 [DOI] [PubMed] [Google Scholar]
- 47.Noga BR, Kriellaars DJ, Jordan LM. The effect of selective brainstem or spinal cord lesions on treadmill locomotion evoked by stimulation of the mesencephalic or pontomedullary locomotor regions. J Neurosci 1991;11:1691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Enright PL. The six-minute walk test. Respir Care 2003;48:783–5. [PubMed] [Google Scholar]
- 49.Chan A-W, Tetzlaff JM, Altman DG, et al. Spirit 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Opris I, Dai X, Johnson DMG, et al. Activation of brainstem neurons during mesencephalic locomotor region-evoked locomotion in the cat. Front Syst Neurosci 2019;13:69. 10.3389/fnsys.2019.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang SJ, Santamaria AJ, Sanchez FJ, et al. Deep brain stimulation of midbrain locomotor circuits in the freely moving pig. Brain Stimul 2021;14:467–76. 10.1016/j.brs.2021.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Hedel HJA, Dietz V, Curt A. Assessment of walking speed and distance in subjects with an incomplete spinal cord injury. Neurorehabil Neural Repair 2007;21:295–301. 10.1177/1545968306297861 [DOI] [PubMed] [Google Scholar]
- 53.Vallery H, Lutz P, von Zitzewitz J, et al. Multidirectional transparent support for overground gait training. IEEE Int Conf Rehabil Robot 2013;2013:6650512. 10.1109/ICORR.2013.6650512 [DOI] [PubMed] [Google Scholar]
- 54.Easthope C, Traini L, Awai L. Multidirectional transparent body weight support engages specific kinematic response patterns in controls and spinal cord injury patients. International Neurorehabilitations Symposium, 2017. [Google Scholar]
- 55.Killeen T, Easthope CS, Demkó L, et al. Minimum toe clearance: probing the neural control of locomotion. Sci Rep 2017;7:1922. 10.1038/s41598-017-02189-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Filli L, Sutter T, Easthope CS, et al. Profiling walking dysfunction in multiple sclerosis: characterisation, classification and progression over time. Sci Rep 2018;8:4984. 10.1038/s41598-018-22676-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Betz R, Biering-Sørensen F, Burns SP. The 2019 revision of the international standards for neurological classification of spinal cord injury (ISNCSCI)-what's new? Spinal Cord 2019;57:815–7. 10.1038/s41393-019-0350-9 [DOI] [PubMed] [Google Scholar]
- 58.Haas BM, Bergström E, Jamous A, et al. The inter rater reliability of the original and of the modified Ashworth scale for the assessment of spasticity in patients with spinal cord injury. Spinal Cord 1996;34:560–4. 10.1038/sc.1996.100 [DOI] [PubMed] [Google Scholar]
- 59.Bluvshtein V, Front L, Itzkovich M, et al. A new grading for easy and concise description of functional status after spinal cord lesions. Spinal Cord 2012;50:42–50. 10.1038/sc.2011.84 [DOI] [PubMed] [Google Scholar]
- 60.Ditunno JF, Ditunno PL, Scivoletto G, et al. The walking index for spinal cord injury (WISCI/WISCI II): nature, metric properties, use and misuse. Spinal Cord 2013;51:346–55. 10.1038/sc.2013.9 [DOI] [PubMed] [Google Scholar]
- 61.Groen J, Pannek J, Castro Diaz D, et al. Summary of European association of urology (EAU) guidelines on Neuro-Urology. Eur Urol 2016;69:324–33. 10.1016/j.eururo.2015.07.071 [DOI] [PubMed] [Google Scholar]
- 62.Panicker JN, Fowler CJ, Kessler TM. Lower urinary tract dysfunction in the neurological patient: clinical assessment and management. Lancet Neurol 2015;14:720–32. 10.1016/S1474-4422(15)00070-8 [DOI] [PubMed] [Google Scholar]
- 63.Jimenez-Cidre MA, Lopez-Fando L, Esteban-Fuertes M, et al. The 3-day bladder diary is a feasible, reliable and valid tool to evaluate the lower urinary tract symptoms in women. Neurourol Urodyn 2015;34:128–32. 10.1002/nau.22530 [DOI] [PubMed] [Google Scholar]
- 64.Costa P, Perrouin-Verbe B, Colvez A, et al. Quality of life in spinal cord injury patients with urinary difficulties. development and validation of qualiveen. Eur Urol 2001;39:107–13. 10.1159/000052421 [DOI] [PubMed] [Google Scholar]
- 65.Rosen R, Brown C, Heiman J, et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000;26:191–208. 10.1080/009262300278597 [DOI] [PubMed] [Google Scholar]
- 66.Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther 2005;31:1–20. 10.1080/00926230590475206 [DOI] [PubMed] [Google Scholar]
- 67.Rosen RC, Riley A, Wagner G, et al. The International index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822–30. 10.1016/s0090-4295(97)00238-0 [DOI] [PubMed] [Google Scholar]
- 68.Johns MW. A new method for measuring daytime sleepiness: the epworth Sleepiness scale. Sleep 1991;14:540–5. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 69.Flachenecker P, Kümpfel T, Kallmann B, et al. Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Mult Scler 2002;8:523–6. 10.1191/1352458502ms839oa [DOI] [PubMed] [Google Scholar]
- 70.Warner FM, Cragg JJ, Jutzeler CR, et al. Progression of neuropathic pain after acute spinal cord injury: a meta-analysis and framework for clinical trials. J Neurotrauma 2019;36:1461–8. 10.1089/neu.2018.5960 [DOI] [PubMed] [Google Scholar]
- 71.Cragg JJ, Haefeli J, Jutzeler CR, et al. Effects of pain and pain management on motor recovery of spinal cord-injured patients: a longitudinal study. Neurorehabil Neural Repair 2016;30:1461–8. 10.1177/1545968315624777 [DOI] [PubMed] [Google Scholar]
- 72.Franz S, Schulz B, Wang H, et al. Management of pain in individuals with spinal cord injury: guideline of the German-speaking medical society for spinal cord injury. Ger Med Sci 2019;17:Doc05. 10.3205/000271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Franz S, Schuld C, Wilder-Smith EP, et al. Spinal cord injury pain instrument and paindetect questionnaire: convergent construct validity in individuals with spinal cord injury. Eur J Pain 2017;21:1642–56. 10.1002/ejp.1069 [DOI] [PubMed] [Google Scholar]
- 74.Bryce TN, Richards JS, Bombardier CH, et al. Screening for neuropathic pain after spinal cord injury with the spinal cord injury pain instrument (SCIPI): a preliminary validation study. Spinal Cord 2014;52:407–12. 10.1038/sc.2014.21 [DOI] [PubMed] [Google Scholar]
- 75.Kalsi-Ryan S, Beaton D, Curt A, et al. The graded redefined assessment of strength sensibility and prehension: reliability and validity. J Neurotrauma 2012;29:905–14. 10.1089/neu.2010.1504 [DOI] [PubMed] [Google Scholar]
- 76.Kalsi-Ryan S, Curt A, Verrier MC, et al. Development of the graded redefined assessment of strength, sensibility and prehension (GRASSP): reviewing measurement specific to the upper limb in tetraplegia. J Neurosurg Spine 2012;17:65–76. 10.3171/2012.6.AOSPINE1258 [DOI] [PubMed] [Google Scholar]
- 77.Steeves JD, Lammertse D, Curt A, et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord 2007;45:206–21. 10.1038/sj.sc.3102008 [DOI] [PubMed] [Google Scholar]
- 78.Ware JE. Sf-36 health survey update. Spine 2000;25:3130–9. 10.1097/00007632-200012150-00008 [DOI] [PubMed] [Google Scholar]
- 79.Curt A, Dietz V. Electrophysiological recordings in patients with spinal cord injury: significance for predicting outcome. Spinal Cord 1999;37:157–65. 10.1038/sj.sc.3100809 [DOI] [PubMed] [Google Scholar]
- 80.Hubli M, Kramer JLK, Jutzeler CR, et al. Application of electrophysiological measures in spinal cord injury clinical trials: a narrative review. Spinal Cord 2019;57:909–23. 10.1038/s41393-019-0331-z [DOI] [PubMed] [Google Scholar]
- 81.Cruccu G, Aminoff MJ, Curio G, et al. Recommendations for the clinical use of somatosensory-evoked potentials. Clin Neurophysiol 2008;119:1705–19. 10.1016/j.clinph.2008.03.016 [DOI] [PubMed] [Google Scholar]
- 82.Harkema SJ, Schmidt-Read M, Lorenz DJ, et al. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch Phys Med Rehabil 2012;93:1508–17. 10.1016/j.apmr.2011.01.024 [DOI] [PubMed] [Google Scholar]
- 83.Wirz M, Zemon DH, Rupp R, et al. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Arch Phys Med Rehabil 2005;86:672–80. 10.1016/j.apmr.2004.08.004 [DOI] [PubMed] [Google Scholar]
- 84.Morita H, Hass CJ, Moro E, et al. Pedunculopontine nucleus stimulation: where are we now and what needs to be done to move the field forward? Front Neurol 2014;5:243. 10.3389/fneur.2014.00243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Golestanirad L, Elahi B, Graham SJ, et al. Efficacy and safety of pedunculopontine nuclei (PPN) deep brain stimulation in the treatment of gait disorders: a meta-analysis of clinical studies. Can J Neurol Sci 2016;43:120–6. 10.1017/cjn.2015.318 [DOI] [PubMed] [Google Scholar]
- 86.Noga BR, Sanchez FJ, Villamil LM, et al. LFP oscillations in the mesencephalic locomotor region during voluntary locomotion. Front Neural Circuits : 2017;11:34. 10.3389/fncir.2017.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Herrity AN, Aslan SC, Ugiliweneza B, et al. Improvements in bladder function following activity-based recovery training with epidural stimulation after chronic spinal cord injury. Front Syst Neurosci 2020;14:614691. 10.3389/fnsys.2020.614691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hubscher CH, Herrity AN, Williams CS, et al. Improvements in bladder, bowel and sexual outcomes following task-specific locomotor training in human spinal cord injury. PLoS One 2018;13:e0190998. 10.1371/journal.pone.0190998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Filli L, Engmann AK, Zörner B, et al. Bridging the gap: a reticulo-propriospinal detour bypassing an incomplete spinal cord injury. J Neurosci 2014;34:13399–410. 10.1523/JNEUROSCI.0701-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Afshar F, Watkins ES, Yap J. Stereotaxic atlas of the human brainstem and cerebellar nuclei: a variability study. Raven Press, 1978. [Google Scholar]
- 91.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6 edn, 2009. [DOI] [PubMed] [Google Scholar]
- 92.Mai JK, Paxinos G, Voss T. Atlas of the human brain. 3 edn. Acad Press, 2008: 181–91. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.