Abstract
The clinical importance of interstitial lung abnormality (ILA) is increasingly recognized. In July 2020, the Fleischner Society published a position paper about ILA. The purposes of this article are to summarize the definition, existing evidence, clinical management, and unresolved issues for ILA from a radiologic standpoint and to provide a practical guide for radiologists. ILA is a common incidental finding at CT and is often progressive and associated with worsened clinical outcomes. The hazard ratios for mortality range from 1.3 to 2.7 in large cohorts. Risk factors for ILA include age, smoking status, other inhalational exposures, and genetic factors (eg, gene encoding mucin 5B variant). Radiologists should systematically record the presence, morphologic characteristics, distribution, and subcategories of ILA (ie, nonsubpleural, subpleural nonfibrotic, and subpleural fibrotic), as these are informative for predicting progression and mortality. Clinically significant interstitial lung disease should not be considered ILA. Individuals with ILA are triaged into higher- and lower-risk groups depending on their risk factors for progression, and systematic follow-up, including CT, should be considered for the higher-risk group. Artificial intelligence-based automated analysis for ILA may be helpful, but further validation and improvement are needed. Radiologists have a central role in clinical management and research on ILA.
© RSNA, 2021
Summary
The importance of interstitial lung abnormality incidentally found at CT is increasingly recognized, and radiologists have a central role in management and research on interstitial lung abnormalities.
Essentials
■ Interstitial lung abnormalities (ILAs) are common incidental findings at CT, which progress over 5 years in more than 50% of individuals, and are associated with worsened clinical outcomes, including respiratory symptoms, exercise capacity, lung function, and mortality.
■ Radiologists should record the presence, morphologic characteristics, distribution, and subcategories (ie, nonsubpleural, subpleural nonfibrotic, and subpleural fibrotic) of ILA.
■ In the clinical management of ILA, clinically significant interstitial lung disease should be excluded, and individuals with risk factors for ILA progression require active monitoring.
■ Issues remain to be solved, such as validation of definition, the long-term outcomes of the management strategy, histopathologic correlation, treatment of progressive ILA, and lung injury risk during cancer therapies.
■ Quantitative and automatic analysis for ILA using artificial intelligence and machine learning will increase in importance, but further validations and improvements are necessary.
Introduction
The recognition of the importance of interstitial lung abnormality (ILA) at CT emerged from the systematic evaluation of large cohorts, showing a prevalence of 4%–9% in cigarette smokers and a prevalence of 2%–7% in never-smokers (Table 1) (1–9). Individuals with ILA have an increase in symptoms such as chronic cough and shortness of breath, decreased total lung capacity, reduced exercise capacity, and increased all-cause mortality. ILA may progress and may represent subclinical or early pulmonary fibrosis (Table 2) (10–16).
Table 1:
Major Results of ILA Studies in Large Cohorts
Table 2:
Associations between Imaging Features and ILA Progression and between Imaging Features and Mortality in AGES-Reykjavik Study

A recent Fleischner position paper provided an updated definition of ILA, with descriptive terms, a summary of risk factors and clinical outcomes, and a proposed schema for management (2). Our purpose is to provide a practical guide for radiologists regarding ILA that includes the definition of and terms for ILA, outcomes, possible strategies for clinical management, and future directions.
Definition and Terms
ILA is defined as incidental CT findings of nondependent abnormalities affecting more than 5% of any lung zone (ie, upper, middle, and lower lung zones are demarcated by the levels of the inferior aortic arch and right inferior pulmonary vein) at complete or partial chest CT (eg, abdominal or cardiac CT, including lower lung zones) where interstitial disease was not previously suspected (2). The findings include ground-glass or reticular abnormalities, lung distortion, traction bronchiectasis or bronchiolectasis, honeycombing, and nonemphysematous cysts (Figs 1–2) (17) (Appendix E1 [online]). All aforementioned abnormalities can also be defined according to their predominant distribution (Fig 3)—subpleural or central predominant in the axial plane and upper- or lower-lung predominant in the craniocaudal plane. The threshold extent of 5% is acknowledged to be arbitrary and subjective and is provided simply to exclude patients with minimal abnormality.
Figure 1:

Flowchart of definition and subcategories of interstitial lung abnormality (ILA). * = Fibrosis is characterized by the presence of architectural distortion with traction bronchiectasis or honeycombing (or both). (Adapted, with permission, from reference 2.)
Figure 2:
Thin-section CT scans show typical findings of interstitial lung abnormalities. (A) Ground-glass abnormality (arrows) is seen in peripheral lung zone. (B) Reticular abnormality with ground-glass opacity (arrows) is seen in subpleural area. (C) Lung distortion is suggested by volume loss and displacement of bronchi and vessels toward medial side (arrow). Distortion accompanies ground-glass abnormality and traction bronchiectasis. (D) Traction bronchiectasis (arrow) is conspicuous in left lower lobe. Ground-glass and reticular abnormalities are also seen in subpleural area. (E) Honeycombing is demonstrated as appearance of clustered cystic air spaces (arrow). (F) Nonemphysematous cysts can be seen as lucencies with well-defined walls (arrows). Surrounding ground-glass and reticular abnormalities are also seen.
Figure 3:
Distribution of interstitial lung abnormalities (ILAs). (A) ILA with subpleural predominance. Axial CT scan shows reticulation and ground-glass abnormality (arrowheads) in bilateral subpleural area. (B) ILA with central predominance. Axial CT scan shows patchy ground-glass abnormalities (arrowheads) in central area of left lower lobe. Nodular ground-glass abnormalities (arrow) are also seen in central area of right lower lobe. (C) ILA with upper-lobe predominance. Coronal CT scan shows subpleural reticulation and ground-glass abnormality (arrows) in both upper lobes. (D) ILA with lower-lobe, or basal, predominance. Coronal CT scan shows subpleural ground-glass abnormality (arrows) and nonemphysematous cyst in bilateral basal area.
ILA is subcategorized according to the distribution and the presence of fibrosis, that is, nonsubpleural, subpleural nonfibrotic, and subpleural fibrotic (Figs 1–4). Among patients with ILA, subpleural ILA is associated with a greater likelihood of progression (odds ratio [OR] = 6.6 [95% CI: 2.3, 19], P = .0004) (Table 2) (18). Fibrosis is characterized by the presence of architectural distortion with traction bronchiectasis or bronchiolectasis and/or honeycombing, and it is correlated with increased mortality when compared with individuals without ILA (hazard ratio [HR] = 1.5 [95% CI: 1.3, 1.6], P < .0001) (7,18).
Figure 4:
Typical interstitial lung abnormalities (ILAs). (A) Nonsubpleural and nonfibrotic ILA. CT scan shows multifocal ground-glass abnormalities (arrows) in central area of both lungs. (B) Subpleural nonfibrotic ILA. CT scan shows predominantly subpleural ground-glass and linear abnormalities (arrowheads) without evidence of fibrosis. (C) Subpleural fibrotic ILA. Besides subpleural ground-glass and linear abnormalities (arrowheads), CT scan shows traction bronchiectasis (arrow) and architectural distortion in left lower lobe, which suggests lung fibrosis.
ILA does not imply the absence of respiratory symptoms or functional impairment. When an individual with ILA has symptoms, clinical signs, or functional impairment, ILA may represent mild interstitial lung disease (ILD). Abnormalities identified during screening for ILD in high-risk groups (eg, those with connective tissue disease or familial ILD) are considered as subclinical or preclinical ILD and not as ILA because they are not incidental (19,20).
Entities Not Considered ILA
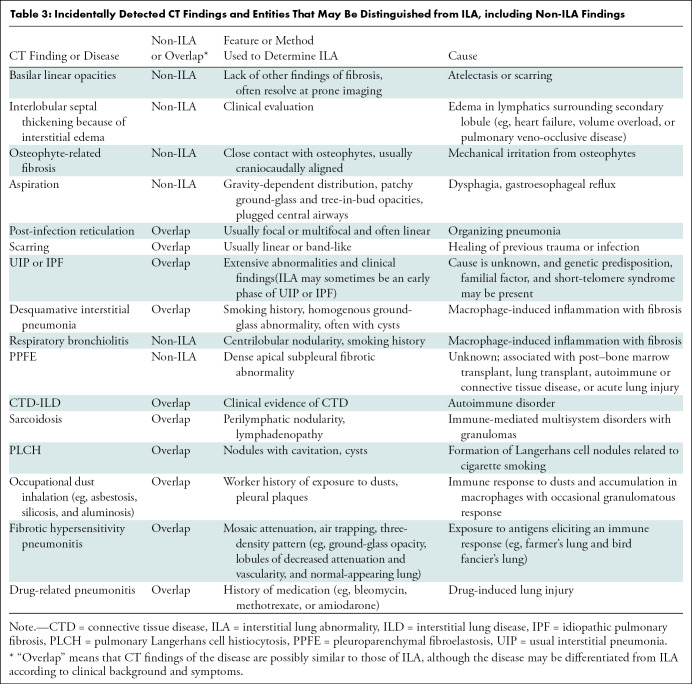
Potential chest CT pitfalls and overlaps in ILA are summarized in Table 3. Although centrilobular nodularity (Fig 5) was included in initial descriptions of ILA (3,4,6–10), it is not no longer included in the definition of ILA proposed in the position paper. The reason is that centrilobular nodularity is a typical manifestation of smoking-related respiratory bronchiolitis (21,22), and several studies have shown that it is usually nonprogressive (7,18,23). Pleuroparenchymal fibroelastosis is an unusual pulmonary disease with unique characteristics and suggested by multiple subpleural areas of consolidation with traction bronchiectasis or bronchiolectasis located predominantly in the bilateral upper lobes (24,25). Apical cap or pleuroparenchymal fibroelastosis–like lesions are sometimes identified at CT incidentally (26) but are not included in the definition of ILA. Pleuroparenchymal fibroelastosis is a distinct radiologic entity, which has not been systematically studied in asymptomatic patients.
Table 3:
Incidentally Detected CT Findings and Entities That May Be Distinguished from ILA, including Non-ILA Findings
Figure 5:
Pitfalls in diagnosis of interstitial lung abnormalities. (A) Centrilobular nodularity. CT scan shows numerous poorly defined ground-glass centrilobular nodules (arrows). (B) Apical cap or pleuroparenchymal fibroelastosis–like lesion. CT scan shows subpleural consolidative nodules with traction bronchiectasis (arrow) in right apical lung. Subpleural linear and nodular opacities (arrowheads) are also seen in left lung. (C) Osteophyte-related focal fibrosis. CT scan shows focal reticulation and ground-glass abnormality adjacent to osteophyte (circle). (D) Aspiration. CT scan shows ill-defined ground-glass abnormalities (arrowheads) in left lower lobe. Bronchial wall thickening (arrows) is also seen, which suggests that ground-glass abnormalities are related to aspiration.
Dependent abnormalities are equivocal unless they are persistent in the prone position (Fig 6). If the abnormality disappears in the prone position, it should be lung atelectasis. Mild focal or unilateral abnormality is also regarded as equivocal for ILA. Other non-ILA findings include focal paraspinal fibrosis in close contact with spine osteophytes, interstitial edema (eg, as in heart failure), and findings of aspiration such as patchy ground-glass and tree-in-bud opacities (Table 3 and Fig 5).
Figure 6:
Dependent abnormality. L = left. (A) Supine CT scan shows faint ground-glass abnormality (arrowhead) in dependent portion of right (R) lower lobe. (B) Abnormality disappeared (arrowhead) in same area on prone CT scan, indicating abnormality on A was atelectasis and not interstitial lung abnormality.
Associations and Outcomes of ILA
ILA may represent an early or subclinical form of pulmonary fibrosis (1). Many clinical studies have investigated various aspects of ILA such as risk factors (4,6,7,9,12,27,28), imaging progression (7,8,23), association with clinical outcomes (4,6,8–10,15,27,29–33), pathologic correlation (34,35), and quantification (19,31,36–38). These investigations include large cohort studies such as the Age, Gene/Environment Susceptibility (AGES)–Reykjavik Study, Genetic Epidemiology of Chronic Obstructive Pulmonary Disease (COPD) Study, Danish Lung Cancer Screening Trial, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints study, Framingham Heart Study, Multi-Ethnic Study of Atherosclerosis, Multicentric Italian Lung Detection trial, and National Lung Screening Trial. The major results of these large cohort studies are summarized in Table 1.
Risk Factors
Risk factors for the presence of ILA include increasing age, tobacco smoke exposure, other inhalational exposures (eg, vapors, gases, dusts, fumes, and traffic-related air pollution), and genetic factors (eg, gene encoding mucin 5B variant) (4,6,7,9,13,27). A common promoter polymorphism (rs35705950) in the gene encoding mucin 5B is associated with both idiopathic pulmonary fibrosis (IPF) and familial interstitial pneumonia (5,39). Similarly, the gene encoding mucin 5B promoter polymorphism has been shown to be associated with the presence of ILA (OR = 2.8 [95% CI: 2.0, 3.9], P < .001) (6) and ILA progression (OR = 2.8 [95% CI: 1.7, 4.4], P = .0001) (8). Increasing age is a clear risk factor for ILA (40,41). In the Genetic Epidemiology of COPD Study, the prevalence of ILA increased from 4% in individuals aged less than 60 years to 6% in those aged 70 years or older. In the Framingham Heart Study, the prevalence of ILA in those aged 70 or older was a remarkable 47%. In both populations, the presence of ILA was associated with increased levels of growth differentiation factor 15, a biomarker of aging (41). Male sex has been identified as a risk factor in some studies (9,16) but not in all studies.
The relationship between ILA and aging is important to clarify. Reticular abnormalities are common in older individuals and have sometimes been regarded as part of the normal spectrum of senescent lung (40,42). The presence of ILA is associated with some other biomarkers of aging (41). However, the observations that progression of ILA is more likely in older individuals (18), and that the increased risk of mortality persists independent of age, suggest that ILA remain clinically relevant in older patients.
Imaging Progression
The rate of imaging progression of ILA has ranged from 20% over 2 years in the National Lung Screening Trial (7) to 73% over 5 years in the AGES-Reykjavik study (Table 1, Fig 7) (18). When comparing the imaging features of patients with ILA who progressed with those who did not progress, several specific imaging features, including subpleural reticular changes, lower-lobe predominant changes, and traction bronchiectasis, were associated with ILA progression (subpleural reticular changes: OR = 6.6 [95% CI: 2.3, 19], P = .0004; lower-lobe predominant changes: OR = 6.7 [95% CI: 1.8, 25], P = .004; traction bronchiectasis: OR = 6.6 [95% CI: 2.3, 19], P = .0004) (Table 2) (18). Some patients with nonfibrotic ILA showed progression and developed signs of fibrosis at follow-up (Fig 7C, 7E).
Figure 7:
Progression of interstitial lung abnormalities (ILAs) in three individuals (A and B, C and D, and E and F). A, C, and, E are CT scans obtained 5 years before B, D, and F, respectively, in each individual. (A) Scan shows no evidence of ILA. (B) Scan shows newly developed ground-glass abnormalities (arrows) in subpleural area of bilateral lungs. (C) Scan shows slight ground-glass abnormality and nonemphysematous cysts (arrows) but no clear evidence of fibrosis. (D) Scan shows increased severity and extent of abnormalities (straight arrows) with new traction bronchiectasis indicating lung fibrosis (curved arrow). (E) Scan shows mild unilateral ground-glass abnormalities (arrows) suggesting ILAs. (F) Individual developed severe architectural distortion with traction bronchiectasis in both lower lobes (circles).
Clinical Outcomes and Mortality
The presence of ILA is associated with worsened clinical outcomes. Individuals with ILA have more respiratory symptoms than those without ILA at the initial CT examination (chronic cough in patients with ILA: 21 of 177 patients [12%] vs chronic cough in patients without ILA: 87 of 1370 patients [6%], P = .006; shortness of breath in patients with ILA: 31 of 177 patients [18%] vs shortness of breath in patients without ILA: 117 of 1370 patients [9%], P < .001) (6,15). ILA is associated with reduced exercise capacity (6-minute walk distance: –19 m [95% CI: –33, –5], P = .008) (10). The association between the presence of ILA and increased mortality is one of the most consistent findings, both in general population studies (ie, the Framingham Heart Study and the AGES-Reykjavik study) and among populations of smokers assessed for COPD (the Genetic Epidemiology of COPD Study and Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints study) or lung cancer screening (Danish Lung Cancer Screening Trial) (Table 1) (4,6,8,9,27,31). The HRs of increased mortality range from 1.3 to 2.7. In the AGES-Reykjavik study cohort, participants with ILA and fibrosis had increased mortality compared with participants without ILA (HR = 1.5 [95% CI: 1.3, 1.6], P < .0001) (Table 2) (18). In the same cohort, participants with ILA had a higher OR of death from a respiratory cause compared with those without ILA (OR = 2.4 [95% CI: 1.7, 3.4], P < .001) (9). Other studies showed that ILA was associated with an increased hazard of lung cancer mortality in the AGES-Reykjavik study (HR = 2.89 [95% CI: 1.80, 4.66], P < .0001) (43), the National Lung Screening Trial (HR = 1.51 [95% CI: 1.13, 2.03]) (44), and the Danish Lung Cancer Screening Trial (HR = 3.2 [95% CI: 1.7, 6.2], P < .001) (27). Imaging progression of ILA was also associated with the increase in mortality in the Framingham Heart Study (HR = 3.9 [95% CI: 1.3, 10.9], P = .01) (8) and AGES-Reykjavik study (HR = 1.4 [95% CI: 1.3, 1.5], P < .0001) (18). The presence of ILA was a significant factor for increased risk of death in patients with advanced stage 4 lung cancer treated with systemic therapy (HR = 2.09, P = .028) (29,33). In patients undergoing transcatheter aortic valve replacement, ILA was an independent predictor of worse mortality (HR = 3.29 [95% CI: 1.34, 8.08], P = .009) (30). In a cohort of patients with sepsis or systemic inflammatory response syndrome, patients with pre-existing ILA were more likely to develop acute respiratory distress syndrome (OR = 4.2 [95% CI: 2.1, 8.2], P < .0001) and to die within 28 days after intensive care unit admission (OR = 2.3 [95% CI: 1.2, 4.2], P = .01) (32).
Lung Function
The correlation between ILA and lung function decline has been also explored. The presence of ILA is associated with decreased total lung capacity (–0.444 L [95% CI: –0.596, –0.292], P < .001) (4,6). Individuals with ILA showed impaired gas exchange compared with those without ILA (mean diffusion capacity of carbon monoxide percent predicted in patients with ILA: 86% ± 14 [standard deviation] vs that in patients without ILA: 98% ± 15, P < .001) (6,15). The association between ILA and spirometry results is controversial. Significant differences in forced expiratory volume in 1 second, or FEV1, and the ratio of FEV1 to forced vital capacity between individuals with and without ILA have been reported in some cohorts but not in all cohorts (9). Some of these differences may be affected by older age and smoking history, which are common in both ILA and decline in spirometry values.
Incidence of Lung Cancer and Lung Injury Risk during Cancer Therapies
An association between ILA and increased hazard or incidence of lung cancer diagnosis is reported in the AGES-Reykjavik study (HR = 2.77 [95% CI: 1.76, 4.36], P < .0001) (43) and the National Lung Screening Trial (incidence rate ratio: 1.33 [95% CI: 1.07, 1.65]) (44).
ILA is an important risk factor for lung injury during cancer therapies. In patients with early-stage cancers treated with surgical resection, ILA was associated with a risk of postoperative pulmonary complications such as pneumonia, acute respiratory distress syndrome, respiratory failure, bronchopleural fistula or empyema, prolonged air leakage, and pneumothorax (OR = 1.91 [95% CI: 1.02, 1.13], P = .004) (45). Pre-existing ILA is associated with an increased risk of grade 3 or higher radiation pneumonitis in patients with small-cell lung cancer treated with 50–60 Gy of thoracic radiation therapy (cumulative incidence: patients with ILA, 36% [95% CI: 6.3, 60.4] vs patients without ILA, 9% [95% CI: 2.4, 15.1]; P = .005) (46) and with an increased risk of extensive radiation pneumonitis in patients with early-stage lung cancer treated with stereotactic body radiation therapy (patients with ILA: three of 16 patients [19%] vs patients without ILA: zero of 84 patients [0%], P = .0035) (47). Immune checkpoint inhibitors, such as nivolumab and pembrolizumab, are recently identified therapies for patients with advanced malignancies (48,49). Approximately 5% of patients treated with immune checkpoint inhibitors show drug-related pneumonitis, although this toxicity is manageable if recognized early and treated appropriately (50,51). It is reported that the incidence of immunotherapy-associated pneumonitis was significantly higher in patients with pre-existing ILA than in those without ILA (patients with pre-existing ILA: six of 14 patients [43%] vs patients without ILA: seven of 69 patients [10%], P = .007) (52).
Pathologic Correlation
Few studies have reported the pathologic correlation of ILA (34,35). Miller et al (34) evaluated paired chest CT scans and histopathologic samples obtained during lung nodule resections in 424 patients. Among 26 patients who had ILA at CT, histologic findings showed fibrosis in 19 individuals (73%) and usual interstitial pneumonia (UIP) in two (8%) individuals. It should be noted that 207 of 398 patients (52%) without ILA or indeterminate ILA status also showed histologic fibrosis, which suggests that CT cannot necessarily depict all cases of fibrosis because of its limited spatial resolution. Hung et al (35) also assessed 406 lung specimens from 397 patients and reported that ILA were present on CT images before surgery in three of 30 patients (10%) with histologic smoking-related interstitial fibrosis, in four of four patients (100%) with histologic UIP, and in one of three patients (33%) with histologic nonspecific interstitial pneumonia (35). The relationship between ILA and smoking-related interstitial fibrosis remains unclear because of the paucity of histologic correlation studies.
Quantification of ILA
Several approaches have sought to quantify ILA, including densitometry of high-attenuation areas, local histogram analysis, and deep learning–based textural evaluation (Fig 8). Densitometry assesses the proportion of high-attenuation areas between –600 and –250 HU, which are considered to reflect ground-glass and reticular abnormalities and exclude more dense areas, such as consolidation, nodules or masses, and blood vessels (12,13). Greater regional high-attenuation area was associated with clinical outcomes and higher odds of visually assessed ILA (OR range, 1.42–2.20, P ≤ .03) (36). However, the densitometry techniques appear to be neither sensitive nor specific for ILA sufficiently because high-attenuation areas are affected by various factors such as inadequate inspiration, obesity, pulmonary atelectasis, and scanner variation. A postprocessing software tool using local histogram analysis had a sensitivity of 87.8% and a specificity of 57.5% for the detection of ILA with a C statistic of 0.82 (37). This tool showed that the quantified interstitial features were associated with reduced lung function, worse quality of life, and increased mortality, and more of the lung was affected by interstitial features for each copy of the minor allele of the gene encoding mucin 5B promoter polymorphism (1; minor allele as heterozygote: 2; minor allele as homozygote) (31). Using a different local histogram-based system, 217 patients undergoing lung resection for stage I lung cancer were investigated (38). The percentage of fibrosis extent according to the tool correlated well with the fibrosis extent according to the radiologists’ analysis (Pearson correlation coefficient = 0.88; P < .001) and was an independent predictor of poorer disease-free survival (HR = 1.3 [95% CI: 1.1, 1.6], P = .001). A study in family members of patients with familial interstitial pneumonia showed that deep learning–based textural evaluation had a sensitivity of 84% and a specificity of 86% for the detection of early interstitial change (19). Suggested CT protocols for quantitative imaging analysis have been published by the Quantitative Imaging Biomarkers Alliance of the Radiological Society of North America (53).
Figure 8:
Use of data-driven texture analysis to quantify progression of interstitial lung abnormality (ILA) in Genetic Epidemiology of Chronic Obstructive Pulmonary Disease Study patient. (A) Baseline CT scan shows minimal ILA. (B) CT scan with data-driven texture analysis overlay precisely identifies fibrotic abnormality, quantified as 1.4% of lung volume. (C) CT scan obtained 5 years later shows clear disease progression. (D) Follow-up CT scan with data-driven texture analysis overlay shows extent of ILA progression, quantified as 5.6% of lung volume. (Figure 8 courtesy of Stephen M. Humphries, PhD, Quantitative Imaging Laboratory, National Jewish Health.)
Implications for Clinical Management
The existing evidence is insufficient to determine a definitive management plan for ILA identified in screening. The management plan introduced in this section (Fig 9, Table 4) was proposed by the Fleischner Society, based on the available published literature and consensus clinical opinion (2).
Figure 9:
Proposed triage rubric for interstitial lung abnormalities (ILAs) found at chest CT. Action items for radiologist are in blue, action items for treating physician or pulmonologist are in green, and action items for a pulmonologist, ideally with interstitial lung disease (ILD) experience, are in orange. * = Nontrivial abnormalities present in three or more lung zones (above bottom of aortic arch, between aortic arch and top of inferior pulmonary vein, and below inferior pulmonary vein). (Adapted, with permission, from reference 2.)
Table 4:
Risk Factors for ILA Progression

All individuals with ILA should be told of the future implications that the presence of ILA portends. The preventable causes of ILA should be explored (eg, cigarette smoking, other inhalational exposures, drug toxicity, connective tissue disorders, or recurrent aspiration). Patients with current clinically significant ILD should be identified according to the presence of respiratory symptoms, physical performance, diminished pulmonary function or gas exchange, and extensive findings at CT. Abnormalities involving three or more of the six lung zones (ie, upper, middle, and lower zones in right and left lung) are generally considered to be extensive. Patients suspected to have significant ILD should be referred for further pulmonary evaluation, ideally with multidisciplinary discussion. Management of a diagnosed ILD should follow standard ILD guidelines.
After excluding ILD, the individuals are separated into higher- and lower-risk groups depending on clinical and radiologic risk factors of ILA progression (Table 4). Individuals with one or more risk factors are at a higher risk, whereas those without such risk factors are at a lower risk. Both lower- and higher-risk groups are recommended to reduce risk factors such as cigarette smoking and other inhalational exposures. The lower-risk group is advised to return for evaluation if they develop any respiratory symptoms, whereas systematic follow-up should be considered for the higher-risk group. An initial clinical follow-up is performed at 3–12 months to check for any symptomatic or physiologic progression, and additional follow-up beyond 1 year is beneficial. Follow-up CT is performed with an interval of 12–24 months. If the patient develops symptoms or impaired pulmonary function, earlier CT is indicated. Because the optimal interval for clinical and radiologic follow-up is unknown due to the limited evidence, the aforementioned strategy is a suggestion by the experts in the Fleischner Society based on their clinical experience.
ILA progression can be defined as the development of respiratory symptoms and clinical signs (eg, exercise limitation and characteristic crackles at auscultation), development of abnormal pulmonary function, or increase in the extent of CT abnormalities, particularly with the fibrotic features. However, the optimal treatment of the patient with ILA progression is completely unknown. A prospective clinical trial is needed to determine the optimal management plan.
ILA should also be regarded as an important comorbidity in patients with lung cancers or other lung diseases. Clinicians must pay attention to the presence of ILA when the patients undergo lung surgery, chemotherapy, and radiation therapy because they are likely to be at an increased risk of lung injury (54–58). Considering the life-threatening nature of malignancies and the benefits of the therapy, clinicians should discuss the possible increased risk of pneumonitis in patients who have ILA. Medications that may cause ILD (eg, Bleomycin, Methotrexate, and Amiodarone) should be avoided in patients with ILA, if possible. Because the presence of ILA is associated with worse prognosis in patients with lung cancers (29,33,38), careful treatment planning and subsequent monitoring are required. Positive pressure ventilation might induce acute respiratory distress in patients with ILA. It may be appropriate for patients with ILA to adopt preventive strategies for ventilation-induced lung injury such as limitation of tidal volume or inspiratory pressure (59).
Abnormalities Identified during Screening for ILD in High-Risk Groups
CT findings in patients with known connective tissue disease, familial ILD, and exposure are beyond the scope of the definition of ILA provided by the original Fleischner position paper but are discussed in this section because the findings are often similar to ILA.
Connective Tissue Disease
When patients with connective tissue diseases are screened with thin-section CT, the prevalence of interstitial abnormality is high, particularly in scleroderma, where interstitial abnormalities may be found in about 60% of patients (60). Such cases are not included in the definition and the strategy of clinical management for ILA proposed by the Fleischner Society; the treatment of these patients is usually individualized according to the underlying connective tissue disease. However, it is possible that the management scheme proposed in Figure 9 may be applied in individuals with connective tissue disease and ILA-like CT findings. The overlaps between ILA and interstitial pneumonia with autoimmune features will also need to be addressed (61–64).
Familial ILD
When first-degree relatives of patients with familial pulmonary fibrosis are screened with CT, the prevalence of CT abnormality is 16%–22%. The prevalence of abnormality is increased in men, in older individuals, and in those who have smoked. In general, these patients are followed up with a sequential pulmonary function test and CT. In evaluating these patients, it is helpful to use the standardized radiologic descriptive terminology presented here.
Exposure-related Interstitial Abnormality
Thin-section CT has been used to screen for interstitial abnormality in workers exposed to a variety of environmental dusts, most frequently asbestos (65–68). In these populations, the prevalence of interstitial abnormality varies depending on the age of the individual, the smoking history, and the type, duration, and intensity of exposure. If interstitial abnormality is identified, it should be characterized according to the standardized descriptive terms for ILA.
Guidance for Radiologists
ILA was regarded as insignificant abnormalities in the past, but now radiologists should add these findings to their report. Radiologists are the first actors in the management of ILA, and they have the responsibility in the detection and radiologic factors of ILA. When ILA is suspected at CT, radiologists should record the morphologic characteristics, distribution, and extent of abnormality, and they should categorize the ILA according to the definitions provided here and in Figure 1 and Table 5 (Appendix E1 [online]). Individuals with subpleural fibrotic ILA should be further categorized according to the recently published diagnostic categories for UIP—typical UIP, probable UIP, indeterminate, and suggestive of an alternative diagnosis (69,70). The uncertain relationship between ILA and subsequent development of IPF is particularly true for ILA that is mild in extent (eg, subpleural reticulation without overt signs of lung fibrosis). However, typical UIP or a probable UIP pattern may be found in patients with underappreciated symptoms, and it has a much higher diagnostic likelihood for IPF. CT imaging of ILA and UIP may be understood as a spectrum (71).
Table 5:
Scenario and Radiologic Reporting Examples
The management of ILA is determined in the consideration of clinical information (Fig 9). For individuals with ILA, radiologists should recommend that clinicians evaluate for risk factors for ILA progression and exclude significant ILDs. If extensive ILA is identified at CT, it is appropriate to recommend a further pulmonary evaluation. Extensive ILA is defined as “Nontrivial abnormalities present in three or more lung zones (ie, above the bottom of the aortic arch, between the aortic arch and top of the inferior pulmonary vein, and below the inferior pulmonary vein)” as shown in Figure 9. Radiologists should recommend active monitoring if the individual has one or more risk factors for ILA progression.
ILA is ideally evaluated using a thin-section chest CT protocol reconstructed with thin sections (≤1.5 mm) and a high-spatial-frequency algorithm. Thin-section CT should be performed if the initial CT examination was performed without thin sections, using an ultra-low-dose protocol or using nonoptimal reconstruction methods such as soft-tissue setting and some iterative reconstructions that affect the image texture (72,73), that is, if the scan did not include all of the lungs (eg, abdominal CT) or if the identified abnormalities are equivocal for ILA. Prone CT scanning is often helpful in distinguishing dependent lung atelectasis from true ILA (74,75). Expiratory CT scanning is not required for the diagnosis of ILA, but it can help identify lobular air trapping suggestive of hypersensitivity pneumonitis (74,76,77). Subsequent CT to evaluate for progression should use scanning protocols similar to the initial CT scanning. In the future, the clinical and imaging management strategy will be updated when scientific evidence is accumulated.
Future Directions
Regarding ILA, we know the risk factors, the percentage of patients who progress according to imaging, and the association with clinical outcomes, including symptoms, exercise capacity, lung function, and mortality (4,6–10,12,15,29–33). These results have been shown in many studies using cohorts with a large sample size. However, many issues remain to be solved in future studies.
A major unresolved question is the relationship between ILA and subsequent development of IPF. The prevalence of ILA in older individuals (4%–9%) is substantially higher than the prevalence of IPF in a similar population (0.5%) (78). This suggests that although ILA is an important risk factor for IPF and other respiratory outcomes, the majority of individuals with ILA do not subsequently develop IPF. Several risk factors for progressive ILA have been discussed previously, but much more work needs to be done to understand how and why subclinical ILA can sometimes develop into clinical IPF.
The definition of ILA in the Fleischner position paper is preliminary and requires more robust evaluation to determine its reproducibility and application to clinical practice. The threshold of 5% of any lung zone is arbitrary and may need validation. Optimal thresholds for defining initial extent and progression at visual and quantitative evaluation should be determined according to subsequent physiologic progression, development of clinically significant disease, and mortality. The management plan proposed in the position paper is also preliminary, and its effect on intermediate- and long-term outcomes must be evaluated in the future.
Further histopathologic evaluation is required to validate the correlation between ILA and histologic fibrosis and also to assess the importance of incidental histologic fibrosis identified in lung resection specimens. The histologic characteristics, natural history, and biologic cause of nonfibrotic ILA remain unclear.
No established treatment is available for progressive ILA. Because antifibrotic treatment reduces the rate of progression in patients with fibrosing ILD, including IPF (79,80), early treatment could potentially reduce the rate of progression in a high-risk population with ILA. However, given the expense and adverse-effect profile of current antifibrotic drugs, a clear need exists for further investigations to identify groups at higher risk of ILA progression and to confirm the natural course from ILA to clinically significant pulmonary fibrosis before a clinical trial can be considered.
Several studies have suggested that ILA is associated with increased mortality in patients with cancer and is a risk factor for lung injury during cancer therapies (29,33,38), but further validations are needed. The cause of the increased mortality is not clear. A better understanding of the association between ILA and the clinical course of the patients with cancers may contribute to risk stratification during cancer therapies and the development of preventive strategies for complications. In addition, ILA should be considered as a specific subcategory under the significant other findings modifier in the Lung CT Screening Reporting and Data System scoring system (81). This subcategory will help both treatment of the patients and understanding of the natural course of ILA in the lung cancer screening population.
The relationship between ILA and acute interstitial pneumonia is of importance. Unsuspected subpleural fibrosis has been identified histologically in seven of 14 patients (50%) who die with acute interstitial pneumonia (82). Thus, ILA may be a predictor of acute interstitial pneumonia. It is reported that the extent of traction bronchiectasis or bronchiolectasis in ILA was associated with mortality in the AGES-Reykjavik study (mortality in patients with traction bronchiolectasis: HR = 2.2 [95% CI: 1.8, 2.6], P < .0001; mortality in patients with mild or moderate traction bronchiectasis: HR = 2.6 [95% CI: 2.2, 3.3], P < .0001; mortality in patients with severe traction bronchiectasis or honeycombing: HR = 6.8 [95% CI: 4.2, 11.1], P < .0001) (83). Risk stratification using this factor may be beneficial in clinical management. Further investigations validating these results and seeking risk factors for mortality or progression are required. CT scans obtained in some patients with prolonged dyspnea following COVID-19 pneumonia may demonstrate ILA-like appearances. However, the role of ILA as a risk factor for COVID-19 infection and the prognostic significance of ILA identified at CT after COVID-19 infection remain to be investigated.
The clinical management plan of ILA proposed in the position paper is a new practice, and it is mandatory to evaluate and improve this new practice. Radiologists should systematically record the presence, morphologic characteristics, distribution, and subcategory of ILA not only for the clinical work but for the validation of the importance and evaluation of the management plan in clinical settings.
Quantitative Imaging
Artificial intelligence and machine learning approaches have recently become a hot topic in radiology (84–87) and are increasingly used to quantify ILA, as discussed previously (19,37,38). They will also increase in importance for quantification and automatic classification of ILA. These approaches provide a rapid and objective measurement of abnormality extent and change over time compared with visual assessment. We have a greater opportunity to encounter incidental CT findings, including ILA, because of the growing number of CT examinations for lung cancer screening and other diagnostic purposes (88,89). Moreover, the clinical management plan proposed in the position paper recommends follow-up CT for individuals at high risk of ILA progression, resulting in a further increase of CT examinations related to ILA. Artificial intelligence– and machine learning–based approaches will help address the increasing demand for identifying progression of ILA, and they will potentially increase observer agreement for assessing disease extent and progression. In addition, artificial intelligence– and machine learning–based approaches will be also useful in research work and clinical trials as a reproducible biomarker for diagnosis or a monitoring tool for ILA.
Artificial intelligence– and machine learning–based quantitative and automatic analysis of ILA has been investigated in several studies (19,31,36–38), but many challenges exist. Quantification is sensitive to image variation caused by factors, including inspired lung volumes, image noise from CT exposure dose, reconstruction methods, and the difference in scanner make and model (90,91). The performance of machine learning algorithms depends on the quality of the training data sets, and overfitting may become a problem (84,92,93). In addition, the characteristics of ILA are likely to interfere with stable analysis by computers; ILA is often subtle and varied, which makes it difficult to differentiate ILA from the normal lung. The subpleural distribution of ILA results in challenges in segmenting the abnormality from the chest wall. Considering these challenges, the stability of the approaches must be tested and improved in further studies using multiple cohorts. New advances in artificial intelligence and deep learning techniques might overcome some of these challenges (94–100). Annotated data sets are necessary to provide a reference benchmark to establish the robustness of each approach.
The natural history of UIP and IPF, especially its early form, is still not fully understood, and it is also unclear what proportion of patients with ILA progress to significant pulmonary fibrosis. The recognition of ILA offers opportunities for identifying pathogenetic abnormalities in early pulmonary fibrosis. Continuous investigation of biomarkers in individuals with ILA might enable us to identify biologic abnormalities that result in the subsequent development of UIP and IPF. Radiologists’ insight into ILA will have an important role in this research activity.
Conclusion
Analogous to the finding of coronary artery calcifications as a harbinger of early heart disease, interstitial lung abnormality (ILA) is often an early finding of pulmonary fibrosis. ILA may progress to the commonly recognized CT features of idiopathic pulmonary fibrosis (IPF) and is correlated with worsened clinical outcomes. Radiologists should record the presence, morphologic characteristics, distribution, and subcategories (ie, nonsubpleural, subpleural nonfibrotic, and subpleural fibrotic) of ILA. Clinically significant interstitial lung disease should not be considered ILA. In the treatment of ILA, individuals should be triaged into higher- and lower-risk groups depending on their risk factors for ILA progression, and systematic follow-up, including CT, should be considered for the higher-risk group. Many issues remain to be solved in future studies, including refinement of the definition and the long-term outcomes of the proposed treatment plan. Artificial intelligence– and machine learning–based quantitative and automatic analysis for ILA will increase in importance, but further validations and improvements are needed. Continuous investigation of ILA might provide important clues for understanding the development of pulmonary fibrosis, usual interstitial pneumonia, and IPF. Radiologists have a central role in clinical management and research of ILA.
H.H. supported by the National Institutes of Health (grant nos. NIH R01CA203636, 5U01CA209414-03, NIH/NHLBI 2R01HL111024-06, NIH R01HL135142, and NIH/NHLBI 1R01HL130974).
Disclosures of Conflicts of Interest: A.H. disclosed no relevant relationships. M.L.S. is member of the Radiology editorial board: disclosed no relevant relationships. Activities not related to the present article: disclosed no relevant relationships. Other relationships: is a shareholder in Healthmyne, Stemina Biomarker Discovery, and X-Vac. D.A.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Parexel Imaging, Boehringer Ingelheim, Veracyte, Daiichi Sankyo, and Astra Zeneca. Other relationships: has U.S. patent issued. H.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution receives payment for research grants from Canon Medical Systems and Konica-Minolta; is a consultant for Mitsubishi Chemical; receives payment for advisory board membership from Canon Medical Systems. Other relationships: disclosed no relevant relationships.
Abbreviations:
- AGES
- Age
- COPD
- chronic obstructive pulmonary disease
- HR
- hazard ratio
- ILA
- interstitial lung abnormality
- ILD
- interstitial lung disease
- IPF
- idiopathic pulmonary fibrosis
- OR
- odds ratio
- UIP
- usual interstitial pneumonia
References
- 1. Hatabu H , Hunninghake GM , Lynch DA . Interstitial lung abnormality: Recognition and perspectives . Radiology 2019. ; 291 ( 1 ): 1 – 3 . [DOI] [PubMed] [Google Scholar]
- 2. Hatabu H , Hunninghake GM , Richeldi L , et al . Interstitial lung abnormalities detected incidentally on CT: a Position Paper from the Fleischner Society . Lancet Respir Med 2020. ; 8 ( 7 ): 726 – 737 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Washko GR , Lynch DA , Matsuoka S , et al . Identification of early interstitial lung disease in smokers from the COPDGene Study . Acad Radiol 2010. ; 17 ( 1 ): 48 – 53 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Washko GR , Hunninghake GM , Fernandez IE , et al . Lung volumes and emphysema in smokers with interstitial lung abnormalities . N Engl J Med 2011. ; 364 ( 10 ): 897 – 906 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seibold MA , Wise AL , Speer MC , et al . A common MUC5B promoter polymorphism and pulmonary fibrosis . N Engl J Med 2011. ; 364 ( 16 ): 1503 – 1512 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hunninghake GM , Hatabu H , Okajima Y , et al . MUC5B promoter polymorphism and interstitial lung abnormalities . N Engl J Med 2013. ; 368 ( 23 ): 2192 – 2200 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin GY, Lynch D, Chawla A, et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology 2013;268(2):563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Araki T , Putman RK , Hatabu H , et al . Development and progression of interstitial lung abnormalities in the Framingham Heart Study . Am J Respir Crit Care Med 2016. ; 194 ( 12 ): 1514 – 1522 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Putman RK , Hatabu H , Araki T , et al . Association Between Interstitial Lung Abnormalities and All-Cause Mortality . JAMA 2016. ; 315 ( 7 ): 672 – 681 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doyle TJ , Washko GR , Fernandez IE , et al . Interstitial lung abnormalities and reduced exercise capacity . Am J Respir Crit Care Med 2012. ; 185 ( 7 ): 756 – 762 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lederer DJ , Enright PL , Kawut SM , et al . Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study . Am J Respir Crit Care Med 2009. ; 180 ( 5 ): 407 – 414 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Podolanczuk AJ , Oelsner EC , Barr RG , et al . High attenuation areas on chest computed tomography in community-dwelling adults: the MESA study . Eur Respir J 2016. ; 48 ( 5 ): 1442 – 1452 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sack CS , Doney BC , Podolanczuk AJ , et al . Occupational Exposures and Subclinical Interstitial Lung Disease. The MESA (Multi-Ethnic Study of Atherosclerosis) Air and Lung Studies . Am J Respir Crit Care Med 2017. ; 196 ( 8 ): 1031 – 1039 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Podolanczuk AJ , Oelsner EC , Barr RG , et al . High-attenuation areas on chest computed tomography and clinical respiratory outcomes in community-dwelling adults . Am J Respir Crit Care Med 2017. ; 196 ( 11 ): 1434 – 1442 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsushima K , Sone S , Yoshikawa S , Yokoyama T , Suzuki T , Kubo K . The radiological patterns of interstitial change at an early phase: over a 4-year follow-up . Respir Med 2010. ; 104 ( 11 ): 1712 – 1721 . [DOI] [PubMed] [Google Scholar]
- 16. Pompe E , de Jong PA , Lynch DA , et al . Computed tomographic findings in subjects who died from respiratory disease in the National Lung Screening Trial . Eur Respir J 2017. ; 49 ( 4 ): 1601814 . [DOI] [PubMed] [Google Scholar]
- 17.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246(3):697–722. [DOI] [PubMed] [Google Scholar]
- 18. Putman RK , Gudmundsson G , Axelsson GT , et al . Imaging patterns are associated with interstitial lung abnormality progression and mortality . Am J Respir Crit Care Med 2019. ; 200 ( 2 ): 175 – 183 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mathai SK , Humphries S , Kropski JA , et al . MUC5B variant is associated with visually and quantitatively detected preclinical pulmonary fibrosis . Thorax 2019. ; 74 ( 12 ): 1131 – 1139 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salisbury ML , Hewlett JC , Ding G , et al . Development and Progression of Radiologic Abnormalities in Individuals at Risk for Familial Interstitial Lung Disease . Am J Respir Crit Care Med 2020. ; 201 ( 10 ): 1230 – 1239 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Remy-Jardin M , Remy J , Boulenguez C , Sobaszek A , Edme JL , Furon D . Morphologic effects of cigarette smoking on airways and pulmonary parenchyma in healthy adult volunteers: CT evaluation and correlation with pulmonary function tests . Radiology 1993. ; 186 ( 1 ): 107 – 115 . [DOI] [PubMed] [Google Scholar]
- 22. Remy-Jardin M , Remy J , Gosselin B , Becette V , Edme JL . Lung parenchymal changes secondary to cigarette smoking: pathologic-CT correlations . Radiology 1993. ; 186 ( 3 ): 643 – 651 . [DOI] [PubMed] [Google Scholar]
- 23. Putman RK , Gudmundsson G , Araki T , et al . The MUC5B promoter polymorphism is associated with specific interstitial lung abnormality subtypes . Eur Respir J 2017. ; 50 ( 3 ): 1700537 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chua F , Desai SR , Nicholson AG , et al . Pleuroparenchymal Fibroelastosis. A Review of Clinical, Radiological, and Pathological Characteristics . Ann Am Thorac Soc 2019. ; 16 ( 11 ): 1351 – 1359 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Watanabe K , Ishii H , Kiyomi F , et al . Criteria for the diagnosis of idiopathic pleuroparenchymal fibroelastosis: A proposal . Respir Investig 2019. ; 57 ( 4 ): 312 – 320 . [DOI] [PubMed] [Google Scholar]
- 26. Sumikawa H , Johkoh T , Iwasawa T , Nakanishi K , Tomiyama N . Pleuroparenchymal fibroelastosis-like lesions on chest computed tomography in routine clinical practice . Jpn J Radiol 2019. ; 37 ( 3 ): 230 – 236 . [DOI] [PubMed] [Google Scholar]
- 27. Hoyer N , Wille MMW , Thomsen LH , et al . Interstitial lung abnormalities are associated with increased mortality in smokers . Respir Med 2018. ; 136 ( 77 ): 82 . [DOI] [PubMed] [Google Scholar]
- 28. Sverzellati N , Guerci L , Randi G , et al . Interstitial lung diseases in a lung cancer screening trial . Eur Respir J 2011. ; 38 ( 2 ): 392 – 400 . [DOI] [PubMed] [Google Scholar]
- 29. Araki T , Dahlberg SE , Hida T , et al . Interstitial lung abnormality in stage IV non-small cell lung cancer: A validation study for the association with poor clinical outcome . Eur J Radiol Open 2019. ; 6 ( 128 ): 131 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kadoch M , Kitich A , Alqalyoobi S , et al . Interstitial lung abnormality is prevalent and associated with worse outcome in patients undergoing transcatheter aortic valve replacement . Respir Med 2018. ; 137 ( 55 ): 60 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ash SY , Harmouche R , Putman RK , et al . Clinical and Genetic Associations of Objectively Identified Interstitial Changes in Smokers . Chest 2017. ; 152 ( 4 ): 780 – 791 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Putman RK , Hunninghake GM , Dieffenbach PB , et al . Interstitial lung abnormalities are associated with acute respiratory distress syndrome . Am J Respir Crit Care Med 2017. ; 195 ( 1 ): 138 – 141 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nishino M , Cardarella S , Dahlberg SE , et al . Interstitial lung abnormalities in treatment-naïve advanced non-small-cell lung cancer patients are associated with shorter survival . Eur J Radiol 2015. ; 84 ( 5 ): 998 – 1004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miller ER , Putman RK , Vivero M , et al . Histopathology of interstitial lung abnormalities in the context of lung nodule resections . Am J Respir Crit Care Med 2018. ; 197 ( 7 ): 955 – 958 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hung YP , Hunninghake GM , Miller ER , et al . Incidental nonneoplastic parenchymal findings in patients undergoing lung resection for mass lesions . Hum Pathol 2019. ; 86 ( 93 ): 101 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Choi B , Kawut SM , Raghu G , et al . Regional distribution of high-attenuation areas on chest computed tomography in the Multi-Ethnic Study of Atherosclerosis . ERJ Open Res 2020. ; 6 ( 1 ): 00115 – 02019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ash SY , Harmouche R , Ross JC , et al . The Objective Identification and Quantification of Interstitial Lung Abnormalities in Smokers . Acad Radiol 2017. ; 24 ( 8 ): 941 – 946 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Iwasawa T , Okudela K , Takemura T , et al . Computer-aided Quantification of Pulmonary Fibrosis in Patients with Lung Cancer: Relationship to Disease-free Survival . Radiology 2019. ; 292 ( 2 ): 489 – 498 . [DOI] [PubMed] [Google Scholar]
- 39. Yang IV , Fingerlin TE , Evans CM , Schwarz MI , Schwartz DA . MUC5B and Idiopathic Pulmonary Fibrosis . Ann Am Thorac Soc 2015. ; 12 ( Suppl 2 ): S193 – S199 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Copley SJ , Wells AU , Hawtin KE , et al . Lung morphology in the elderly: comparative CT study of subjects over 75 years old versus those under 55 years old . Radiology 2009. ; 251 ( 2 ): 566 – 573 . [DOI] [PubMed] [Google Scholar]
- 41. Sanders JL , Putman RK , Dupuis J , et al . The Association of Aging Biomarkers, Interstitial Lung Abnormalities, and Mortality . Am J Respir Crit Care Med 2020 . 10.1164/rccm.202007-2993OC. Published online October 20,2020 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Winter DH , Manzini M , Salge JM , et al . Aging of the lungs in asymptomatic lifelong nonsmokers: findings on HRCT . Lung 2015. ; 193 ( 2 ): 283 – 290 . [DOI] [PubMed] [Google Scholar]
- 43. Axelsson GT , Putman RK , Aspelund T , et al . The associations of interstitial lung abnormalities with cancer diagnoses and mortality . Eur Respir J 2020. ; 56 ( 6 ): 1902154 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Whittaker Brown SA , Padilla M , Mhango G , et al . Interstitial Lung Abnormalities and Lung Cancer Risk in the National Lung Screening Trial . Chest 2019. ; 156 ( 6 ): 1195 – 1203 . [DOI] [PubMed] [Google Scholar]
- 45. Im Y , Park HY , Shin S , et al . Prevalence of and risk factors for pulmonary complications after curative resection in otherwise healthy elderly patients with early stage lung cancer . Respir Res 2019. ; 20 ( 1 ): 136 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li F , Zhou Z , Wu A , et al . Preexisting radiological interstitial lung abnormalities are a risk factor for severe radiation pneumonitis in patients with small-cell lung cancer after thoracic radiation therapy . Radiat Oncol 2018. ; 13 ( 1 ): 82 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamaguchi S , Ohguri T , Ide S , et al . Stereotactic body radiotherapy for lung tumors in patients with subclinical interstitial lung disease: the potential risk of extensive radiation pneumonitis . Lung Cancer 2013. ; 82 ( 2 ): 260 – 265 . [DOI] [PubMed] [Google Scholar]
- 48. Tang J , Yu JX , Hubbard-Lucey VM , Neftelinov ST , Hodge JP , Lin Y . Trial watch: The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors . Nat Rev Drug Discov 2018. ; 17 ( 12 ): 854 – 855 . [DOI] [PubMed] [Google Scholar]
- 49. Ribas A , Wolchok JD . Cancer immunotherapy using checkpoint blockade . Science 2018. ; 359 ( 6382 ): 1350 – 1355 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brahmer JR , Lacchetti C , Schneider BJ , et al . Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline . J Clin Oncol 2018. ; 36 ( 17 ): 1714 – 1768 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Puzanov I , Diab A , Abdallah K , et al . Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group . J Immunother Cancer 2017. ; 5 ( 1 ): 95 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nakanishi Y , Masuda T , Yamaguchi K , et al . Pre-existing interstitial lung abnormalities are risk factors for immune checkpoint inhibitor-induced interstitial lung disease in non-small cell lung cancer . Respir Investig 2019. ; 57 ( 5 ): 451 – 459 . [DOI] [PubMed] [Google Scholar]
- 53. QIBA Profile: Computed Tomography: Lung Densitometry. Radiological Society of North America Web site . https://qibawiki.rsna.org/images/a/a8/QIBA_CT_Lung_Density_Profile_090420-clean.pdf. Accessed February 6,2021 .
- 54. Licker M , Fauconnet P , Villiger Y , Tschopp JM . Acute lung injury and outcomes after thoracic surgery . Curr Opin Anaesthesiol 2009. ; 22 ( 1 ): 61 – 67 . [DOI] [PubMed] [Google Scholar]
- 55. Özkan M , Dweik RA , Ahmad M . Drug-induced lung disease . Cleve Clin J Med 2001. ; 68 ( 9 ): 782 – 785 . 789 – 795 . [DOI] [PubMed] [Google Scholar]
- 56. Limper AH . Chemotherapy-induced lung disease . Clin Chest Med 2004. ; 25 ( 1 ): 53 – 64 . [DOI] [PubMed] [Google Scholar]
- 57. Giuranno L , Ient J , De Ruysscher D , Vooijs MA . Radiation-Induced Lung Injury (RILI) . Front Oncol 2019. ; 9 877 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Käsmann L , Dietrich A , Staab-Weijnitz CA , et al . Radiation-induced lung toxicity - cellular and molecular mechanisms of pathogenesis, management, and literature review . Radiat Oncol 2020. ; 15 ( 1 ): 214 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Beitler JR , Malhotra A , Thompson BT . Ventilator-induced Lung Injury . Clin Chest Med 2016. ; 37 ( 4 ): 633 – 646 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Suliman YA , Dobrota R , Huscher D , et al . Brief Report: Pulmonary Function Tests: High Rate of False-Negative Results in the Early Detection and Screening of Scleroderma-Related Interstitial Lung Disease . Arthritis Rheumatol 2015. ; 67 ( 12 ): 3256 – 3261 . [DOI] [PubMed] [Google Scholar]
- 61. Fischer A , Antoniou KM , Brown KK , et al . An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features . Eur Respir J 2015. ; 46 ( 4 ): 976 – 987 . [DOI] [PubMed] [Google Scholar]
- 62. Fernandes L , Nasser M , Ahmad K , Cottin V . Interstitial Pneumonia With Autoimmune Features (IPAF) . Front Med (Lausanne) 2019. ; 6 209 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Graney BA , Fischer A . Interstitial pneumonia with autoimmune features . Ann Am Thorac Soc 2019. ; 16 ( 5 ): 525 – 533 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yoo H , Hino T , Han J , et al . Connective tissue disease-related interstitial lung disease (CTD-ILD) and interstitial lung abnormality (ILA): Evolving concept of CT findings, pathology and management . Eur J Radiol Open 2020. ; 8 100311 . [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65. Akira M , Yamamoto S , Yokoyama K , et al . Asbestosis: high-resolution CT-pathologic correlation . Radiology 1990. ; 176 ( 2 ): 389 – 394 . [DOI] [PubMed] [Google Scholar]
- 66. Gevenois PA , De Vuyst P , Dedeire S , Cosaert J , Vande Weyer R , Struyven J . Conventional and high-resolution CT in asymptomatic asbestos-exposed workers . Acta Radiol 1994. ; 35 ( 3 ): 226 – 229 . [PubMed] [Google Scholar]
- 67. Oksa P , Suoranta H , Koskinen H , Zitting A , Nordman H . High-resolution computed tomography in the early detection of asbestosis . Int Arch Occup Environ Health 1994. ; 65 ( 5 ): 299 – 304 . [DOI] [PubMed] [Google Scholar]
- 68. Staples CA , Gamsu G , Ray CS , Webb WR . High resolution computed tomography and lung function in asbestos-exposed workers with normal chest radiographs . Am Rev Respir Dis 1989. ; 139 ( 6 ): 1502 – 1508 . [DOI] [PubMed] [Google Scholar]
- 69. Lynch DA , Sverzellati N , Travis WD , et al . Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper . Lancet Respir Med 2018. ; 6 ( 2 ): 138 – 153 . [DOI] [PubMed] [Google Scholar]
- 70. Raghu G , Remy-Jardin M , Myers JL , et al . Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline . Am J Respir Crit Care Med 2018. ; 198 ( 5 ): e44 – e68 . [DOI] [PubMed] [Google Scholar]
- 71. Hino T , Lee KS , Han J , Hata A , Ishigami K , Hatabu H . Spectrum of Pulmonary Fibrosis from Interstitial Lung Abnormality to Usual Interstitial Pneumonia: Importance of Identification and Quantification of Traction Bronchiectasis in Patient Management . Korean J Radiol 2020. ; 21 e196 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yanagawa M , Gyobu T , Leung AN , et al . Ultra-low-dose CT of the lung: effect of iterative reconstruction techniques on image quality . Acad Radiol 2014. ; 21 ( 6 ): 695 – 703 . [DOI] [PubMed] [Google Scholar]
- 73. Hata A , Yanagawa M , Honda O , Miyata T , Tomiyama N . Ultra-low-dose chest computed tomography for interstitial lung disease using model-based iterative reconstruction with or without the lung setting . Medicine (Baltimore) 2019. ; 98 ( 22 ): e15936 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kazerooni EA . High-resolution CT of the lungs . AJR Am J Roentgenol 2001. ; 177 ( 3 ): 501 – 519 . [DOI] [PubMed] [Google Scholar]
- 75. Kashiwabara K , Kohshi S . Additional computed tomography scans in the prone position to distinguish early interstitial lung disease from dependent density on helical computed tomography screening patient characteristics . Respirology 2006. ; 11 ( 4 ): 482 – 487 . [DOI] [PubMed] [Google Scholar]
- 76. Arakawa H , Niimi H , Kurihara Y , Nakajima Y , Webb WR . Expiratory high-resolution CT: diagnostic value in diffuse lung diseases . AJR Am J Roentgenol 2000. ; 175 ( 6 ): 1537 – 1543 . [DOI] [PubMed] [Google Scholar]
- 77. Silva CIS , Churg A , Müller NL . Hypersensitivity pneumonitis: spectrum of high-resolution CT and pathologic findings . AJR Am J Roentgenol 2007. ; 188 ( 2 ): 334 – 344 . [DOI] [PubMed] [Google Scholar]
- 78. Raghu G , Chen SY , Yeh WS , et al . Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11 . Lancet Respir Med 2014. ; 2 ( 7 ): 566 – 572 . [DOI] [PubMed] [Google Scholar]
- 79. Richeldi L , du Bois RM , Raghu G , et al . Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis . N Engl J Med 2014. ; 370 ( 22 ): 2071 – 2082 . [DOI] [PubMed] [Google Scholar]
- 80. King TE Jr , Bradford WZ , Castro-Bernardini S , et al . A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis . N Engl J Med 2014. ; 370 ( 22 ): 2083 – 2092 . [DOI] [PubMed] [Google Scholar]
- 81. American College of Radiology . Lung-RADS version 1.1 . Reston, Va: : American College of Radiology, 2019. . [Google Scholar]
- 82. Araya J , Kawabata Y , Jinho P , Uchiyama T , Ogata H , Sugita Y . Clinically occult subpleural fibrosis and acute interstitial pneumonia a precursor to idiopathic pulmonary fibrosis? . Respirology 2008. ; 13 ( 3 ): 408 – 412 . [DOI] [PubMed] [Google Scholar]
- 83. Hida T , Nishino M , Hino T , et al . Traction Bronchiectasis/Bronchiolectasis is Associated with Interstitial Lung Abnormality Mortality . Eur J Radiol 2020. ; 129 : 109073 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chassagnon G , Vakalopolou M , Paragios N , Revel MP . Deep learning: definition and perspectives for thoracic imaging . Eur Radiol 2020. ; 30 ( 4 ): 2021 – 2030 . [DOI] [PubMed] [Google Scholar]
- 85. Lakhani P , Sundaram B . Deep learning at chest radiography: Automated classification of pulmonary tuberculosis by using convolutional neural networks . Radiology 2017. ; 284 ( 2 ): 574 – 582 . [DOI] [PubMed] [Google Scholar]
- 86. Wang S , Shi J , Ye Z , et al . Predicting EGFR mutation status in lung adenocarcinoma on computed tomography image using deep learning . Eur Respir J 2019. ; 53 ( 3 ): 1800986 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Walsh SLF , Calandriello L , Silva M , Sverzellati N . Deep learning for classifying fibrotic lung disease on high-resolution computed tomography: a case-cohort study . Lancet Respir Med 2018. ; 6 ( 11 ): 837 – 845 . [DOI] [PubMed] [Google Scholar]
- 88. Organization for Economic Co-operation and Development. Health at a Glance 2019 . http://www.oecd.org/health/health-systems/health-at-a-glance-19991312.htm. Published 2019. Accessed October 26, 2020. .
- 89. Power SP , Moloney F , Twomey M , James K , O’Connor OJ , Maher MM . Computed tomography and patient risk: Facts, perceptions and uncertainties . World J Radiol 2016. ; 8 ( 12 ): 902 – 915 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Coxson HO . Sources of variation in quantitative computed tomography of the lung . J Thorac Imaging 2013. ; 28 ( 5 ): 272 – 279 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Boes JL , Bule M , Hoff BA , et al . The Impact of Sources of Variability on Parametric Response Mapping of Lung CT Scans . Tomography 2015. ; 1 ( 1 ): 69 – 77 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Choy G , Khalilzadeh O , Michalski M , et al . Current applications and future impact of machine learning in radiology . Radiology 2018. ; 288 ( 2 ): 318 – 328 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yamashita R , Nishio M , Do RKG , Togashi K . Convolutional neural networks: an overview and application in radiology . Insights Imaging 2018. ; 9 ( 4 ): 611 – 629 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jun S , Kim N , Seo JB , Lee YK , Lynch DA . An Ensemble Method for Classifying Regional Disease Patterns of Diffuse Interstitial Lung Disease Using HRCT Images from Different Vendors . J Digit Imaging 2017. ; 30 ( 6 ): 761 – 771 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kim GB , Jung KH , Lee Y , et al . Comparison of Shallow and Deep Learning Methods on Classifying the Regional Pattern of Diffuse Lung Disease . J Digit Imaging 2018. ; 31 ( 4 ): 415 – 424 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Park SO , Seo JB , Kim N , et al . Feasibility of automated quantification of regional disease patterns depicted on high-resolution computed tomography in patients with various diffuse lung diseases . Korean J Radiol 2009. ; 10 ( 5 ): 455 – 463 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lee SM , Seo JB , Oh SY , et al . Prediction of survival by texture-based automated quantitative assessment of regional disease patterns on CT in idiopathic pulmonary fibrosis . Eur Radiol 2018. ; 28 ( 3 ): 1293 – 1300 . [DOI] [PubMed] [Google Scholar]
- 98. Park HJ , Lee SM , Song JW , et al . Texture-based automated quantitative assessment of regional patterns on initial CT in patients with idiopathic pulmonary fibrosis: Relationship to decline in forced vital capacity . AJR Am J Roentgenol 2016. ; 207 ( 5 ): 976 – 983 . [DOI] [PubMed] [Google Scholar]
- 99. Yoon RG , Seo JB , Kim N , et al . Quantitative assessment of change in regional disease patterns on serial HRCT of fibrotic interstitial pneumonia with texture-based automated quantification system . Eur Radiol 2013. ; 23 ( 3 ): 692 – 701 . [DOI] [PubMed] [Google Scholar]
- 100. Wu X , Kim GH , Salisbury ML , et al . Computed Tomographic Biomarkers in Idiopathic Pulmonary Fibrosis. The Future of Quantitative Analysis . Am J Respir Crit Care Med 2019. ; 199 ( 1 ): 12 – 21 . [DOI] [PubMed] [Google Scholar]