Abstract
Biomass pretreatment methods are commonly used to isolate carbohydrates from biomass, but they often lead to modification, degradation, and/or low yields of lignin. Catalytic fractionation approaches provide a possible solution to these challenges by separating the polymeric sugar and lignin fractions in the presence of a catalyst that promotes cleavage of the lignin into aromatic monomers. Here, we demonstrate an oxidative fractionation method conducted in the presence of a heterogeneous non-precious-metal Co-N-C catalyst and O2 in acetone as the solvent. The process affords a 15 wt% yield of phenolic products bearing aldehydes (vanillin, syringaldehyde) and carboxylic acids (p-hydroxybenzoic acid, vanillic acid, syringic acid), complementing the alkylated phenols obtained from existing reductive catalytic fractionation methods. The oxygenated aromatics derived from this process have appealing features for use in polymer synthesis and/or biological funneling to value-added products, and the non-alkaline conditions associated with this process support preservation of the cellulose, which remains insoluble at reaction conditions and is recovered as a solid.
Graphical Abstract
Introduction
Lignocellulosic biomass is an important renewable feedstock for the production of transportation fuels and valuable chemicals that could reduce reliance on fossil-based resources.1,2 Historical efforts on biomass conversion, ranging from the pulp and paper industry to more recent efforts on bioethanol production, have emphasized utilization of carbohydrates. Although these polymeric sugars represent the major fraction (70-85 wt%) of nonedible biomass,3,4 there is growing recognition that valorization of lignin is crucial to the economic viability of biorefineries.5-8 Lignin is a structurally complex heterogeneous aromatic biopolymer that represents the largest renewable source of aromatic chemicals (Figure 1a). Conventional methods for the isolation of carbohydrates from lignocellulosic biomass, however, often result in chemical modification or degradation of the lignin (Figure 1b) or use of only a small portion of the lignin backbone (Table 1). Although the lignin extracted from these processes has found some direct commercial application,9,10 it is commonly burned for energy production and is not well suited for large-scale conversion into aromatic chemicals. The challenges in lignin isolation often arise from side-reactions involving the benzyl alcohols present in the lignin backbone.11,12 Facile generation of carbocation intermediates at these sites under acidic conditions, or the formation of quinone methide and/or epoxides under alkaline conditions, can lead to polymer cross-linking and the formation of recalcitrant C–C bonds that prevent conversion of lignin into aromatic monomers. Stabilization methods have been developed to preserve the lignin structure during extraction and provide the basis for improved yields of aromatic monomers.13-16
Figure 1.
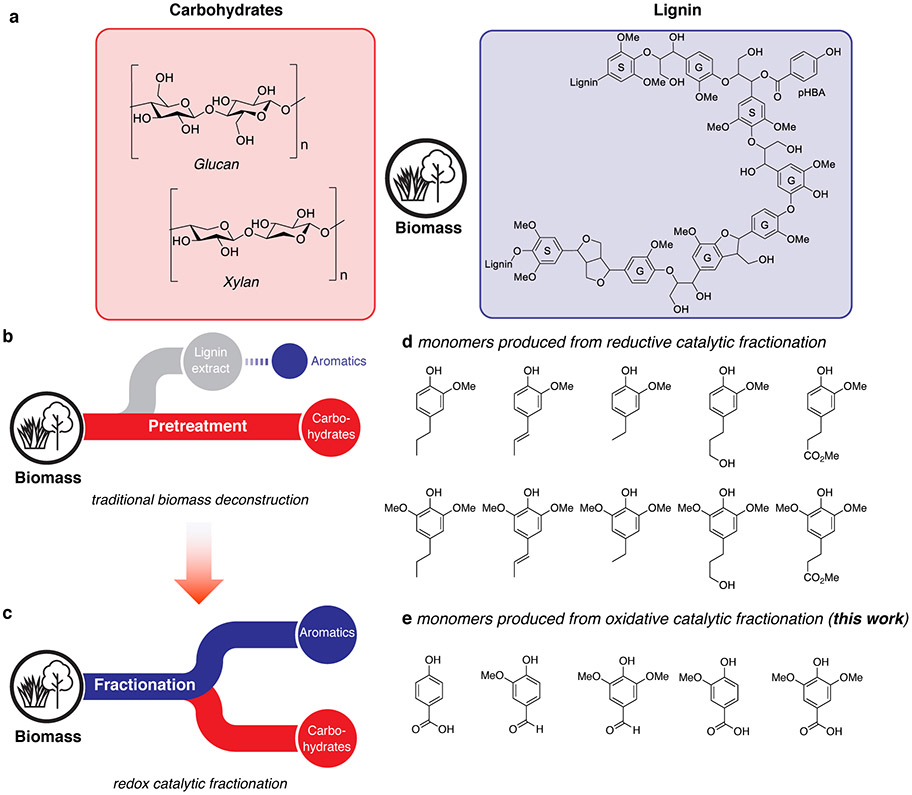
Overview of biomass deconstruction approaches. a) Model structures of carbohydrate and lignin components in lignocellulosic biomass b) Traditional cellulosic biomass isolation approaches focusing on obtaining high-quality carbohydrates and c) redox catalytic fractionation, focused on obtaining high-quality carbohydrates and lignin-derived aromatic monomers. d) Identity of representative monomers produced via reductive catalytic fractionation (RCF) and e) monomers produced via oxidative catalytic fractionation (OCF) in the present work. Biomass icon courtesy of Matthew Wisniewski (Wisconsin Energy Institute).
Table 1:
Oxygenated monomer yields from poplar treatment
| |||
|---|---|---|---|
| Lignin source material | Pretreatment lignin recovery (wt%) |
Oxygenated monomer yield (wt%) |
Monomer yield relative to total lignin (wt%) |
| Three-step sequence (pretreatment / oxidation / hydrolysis) | |||
| Mild acidolysis | 1579 | 41.648 | 6.2 |
| CuAHP | 3280 | 30.748 | 9.8 |
| GVL | 5581 | 23.448 | 12.9 |
| Propionaldehyde protection/acidolysis | 11914,a | 3214 | 3114 |
| One-step oxidative catalytic fractionation | |||
| Direct biomass | – | 15.0 | 15.0 |
The yield exceeds 100% because the aldehyde protection method adds mass to the lignin.
The issues noted above have contributed to growing interest in "catalytic fractionation" methods in which the biomass is processed under conditions that not only separate the lignin and carbohydrate fractions, but also promote catalytic conversion of the lignin into aromatic monomers (Figure 1c).17 Such processes provide a strategy to minimize lignin degradation pathways that occur during conventional pretreatment methods.11,18 Reductive catalytic fractionation (RCF) methods, which are conducted in the presence of a heterogeneous catalyst and source of H2 (hydrogen gas, formic acid, or alcohol solvent), have been extensively studied and are the subject of considerable ongoing development.19-30 In these methods, the lignin undergoes solvolytic extraction from the carbohydrates and in situ catalytic hydrogenolysis to afford aromatic monomers, commonly consisting of syringyl- and guaiacyl-derived phenols bearing (partially) deoxygenated hydrocarbon substituents (Figure 1d). Specific compositions vary with the catalyst identity (e.g., Pd/C, Ru/C, Ni/C), reaction conditions, and source of biomass, and the products are being explored as precursors to fuels and fuel additives,7 monomers for polymeric materials,31,32 and fine chemicals.33,34
The present study was initiated to explore prospects for oxidative catalytic fractionation of lignin. Oxygenated lignin-derived products, such as those in Figure 1e, would complement or offer advantages relative to the RCF products. The products in Figure 1e represent bifunctional phenols that could find use as monomers for bio-based polymers35-38 and represent appealing feedstocks for microbial conversion and biological funneling, due their increased water solubility relative to reduced products in Figure 1d and similarity (or identity) to known metabolic intermediates.39,40 The oxidation of lignin derived from conventional pretreatment methods (cf. Figure 1b) and lignin model compounds has been studied extensively.41-51 We speculated that fractionation of biomass in the presence of a suitable catalyst and O2 could provide the basis for an oxidative catalytic fraction method analogous to RCF, but capable of generating oxidized aromatic products while preserving the carbohydrate fraction. Important precedents for this concept have been demonstrated under alkaline aqueous conditions using copper salts, mixed-metal oxides or other catalyst or reagent compositions;52-54 however, the simultaneous production of good yields of oxidized aromatic monomers and high-quality cellulose has proven to be difficult. The present study employs non-basic conditions in organic solvent that allow for simultaneous production of oxidized aromatic chemicals from lignin and a high-quality cellulose stream.
Results and Discussion
Overview of strategy and reaction components
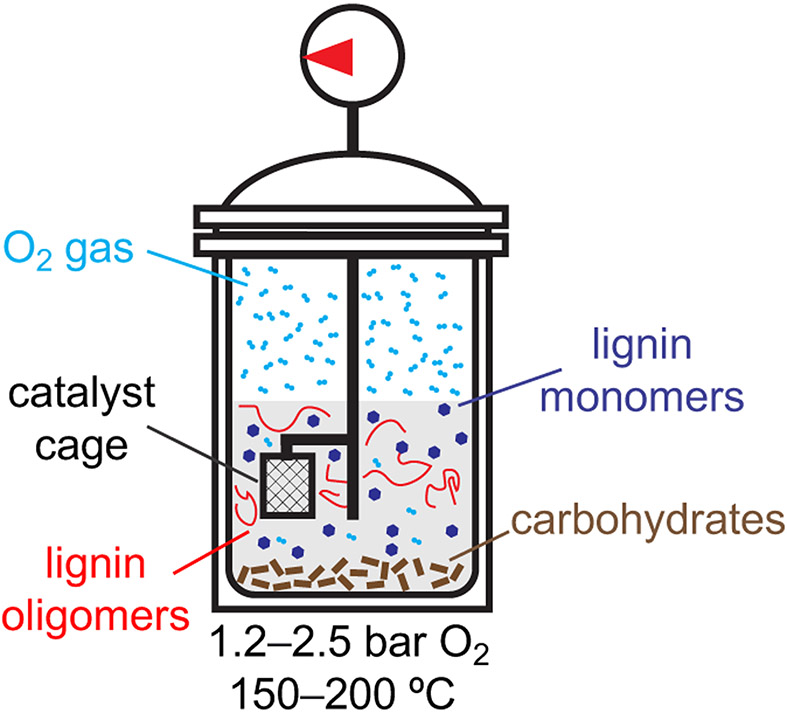
We envisioned that oxidation of biomass could take place in an organic solvent capable of promoting solvolytic separation of lignin from the carbohydrates23-29 under an atmosphere of oxygen gas. In an ideal scenario, the lignin will dissolve into solution and be susceptible to oxidation-initiated depolymerization and the carbohydrates will remain as a solid, thereby protecting them from oxidative degradation. Drawing on precedents from RCF studies,21,23,29 the heterogeneous catalyst for lignin oxidation/depolymerization can be integrated within a porous cage to avoid contamination of the solid carbohydrate fraction. The pores of the cage are designed to be large enough to allow soluble lignin to enter, but small enough to prevent passage of catalyst particles into the reaction vessel. A schematic diagram of the assembled reactor and various components of the reaction mixture is shown in Figure 2.
Figure 2.
Overview of OCF process development and schematic of OCF reactor.
Several different variables were evaluated during the course of this study. The majority of the work was conducted with poplar, as a representative hardwood biomass source, but the optimized conditions were also evaluated with pine (softwood), and miscanthus (grass). Solvents included both aprotic organic solvents (acetone, acetonitrile, and ethyl acetate) and water. Alcohols, such as methanol, are commonly used in RCF methods, but were avoided in this study due their susceptibility to oxidation. A range of different catalyst compositions was tested, starting with metal oxides, similar to those used previously for biomass oxidation under alkaline conditions,55,56 and supported platinum-group metals (PGMs), which have been used as catalysts for RCF (see above) and aerobic alcohol oxidation.57 The latter function could contribute to lignin depolymerization.45,51 In addition, we evaluated metal-containing nitrogen-doped carbon catalysts (M-N-C). These materials were first developed as electrocatalysts for the oxygen reduction reaction,58 but more recently have been used to catalyze aerobic oxidation of organic molecules,59-61 including lignin model compounds.62-65 These catalysts are typically prepared by adsorption of a metal salt and a source of nitrogen onto a carbon support, followed by pyrolysis under an inert atmosphere. For example, Co-PANI-C, which was identified as an effective catalyst in the studies described below, uses polyaniline (PANI) derived from in situ polymerization of aniline on the carbon support as the nitrogen source.
Biomass oxidation and analysis of lignin-derived products
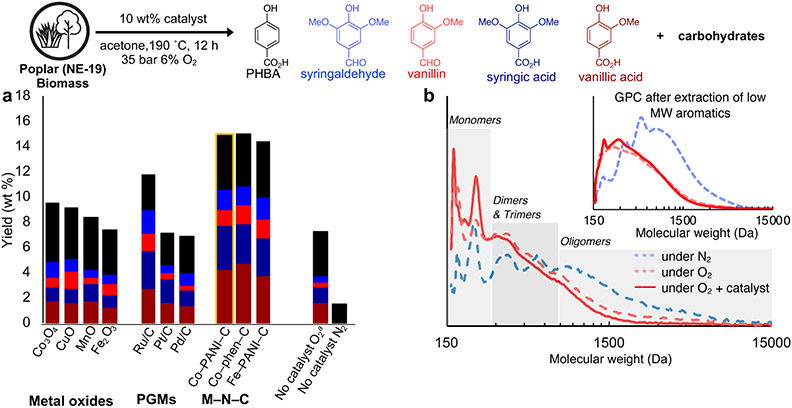
The oxidative catalytic fractionation of poplar wood chips (< 2 mm) was examined under a variety of conditions by testing various solvents, catalysts, and conditions to optimize the aromatic monomer yields.66 The reaction conditions employed O2 supplied as a gas mixture of 6% O2 in N2 to stay below the solvent flammability limits.67 Catalysts were used at 10 wt%, corresponding to metal loadings of 0.3-0.5 wt% with respect to biomass.66 Early studies suggested maximum lignin derived product yields are obtained following a 12 h reaction time, with lower yields observed at longer times. The preferred temperature and pressure (190 °C and 2 bar pO2) was identified via design-of-experiment studies. The reactions generated soluble and insoluble fractions. Analysis of the insoluble fraction showed that it mostly contains carbohydrate materials (discussed in the next section), while the soluble fraction primarily consists of lignin-derived materials, including the aromatic monomers. Analysis of the soluble lignin fraction by HPLC enabled identification and quantification of the low molecular weight products. Five aromatic compounds, including p-hydroxybenzoic acid (PHBA), vanillic acid, syringic acid, vanillin, and syringaldehyde, were identified as monomeric products of the reactions, together with a collection of higher molecular weight products (Figure 3). Among the different catalysts, Co-PANI-C and Co-phen-C (3 wt% Co) gave the best results (Figure 3a), affording a 15.0 wt% total yield of aromatic monomers relative to the mass of lignin in the starting biomass sample. Catalyst recycle studies indicate that the Co-PANI-C catalyst may be reused without substantial deactivation over five cycles: similar monomer yields were obtained in all five runs, with a reduction in monomer yield of only 1.4 wt% observed from the first to the last trial in this sequence (see Figure S6 in the Supporting Information). Preliminary assessment of acetone stability under the reaction conditions was conducted by distilling the solvent from the mixture after reaction completion and resulted in 92% recovery (see Section 2 in the Supporting Information for experimental details; larger scale applications would likely lead to improved solvent recovery). The overall solvent stability is consistent with the use of acetone in organosolv methods for biomass pretreatment at high temperature (140–180 °C).68,69 Good monomer yields were also observed in acetonitrile (13.6 wt% monomers; see Figure S5 in the Supporting Information), highlighting opportunities for overall system optimization in future work, guided by technoeconomic analysis.
Figure 3.
a) Catalyst screen for the oxidative catalytic fractionation of raw poplar biomass. Conditions: 0.1 g poplar, 10 wt% heterogeneous catalyst, 20 mL acetone, 190 °C, 35 bar 6% O2 in N2, 12 h. b) GPC analysis of lignin-derived oligomers and aromatics after lignin extraction under N2 and O2 without catalyst and under O2 with Co-PANI-C and GPC analysis of lignin-derived aromatic oligomers with the low molecular weight aromatics removed after lignin extraction under N2 and O2 without catalyst and under O2 with Co-PANI-C. Different molecular weight regions are defined as follows: monomers (<300 Da), dimers & trimers (300-750 Da), and oligomers (>750 Da) aA Teflon liner was used to isolate the reactants from the metal surface of the reactor. Biomass icon courtesy of Matthew Wisniewski (Wisconsin Energy Institute).
Ru/C is the most effective PGM catalyst (11.5 wt% yield), but several non-precious metal catalysts outperform the PGM catalysts, including all three of the M-N-C catalysts (14.3-15.0 wt% yield) (Figure 3a). Treatment of poplar with O2 under the optimized reaction conditions in the absence of catalyst also leads to the formation of monomers, but only in 7.2 wt% yield. Negligible lignin-derived monomers are obtained from the reaction under N2 in the absence of catalyst, with only 1–2 wt% yield of PHBA. This result indicates that acetone-promoted solvolysis of the lignin does not contribute to monomer formation.
Gel-permeation chromatography (GPC) was used to analyze the higher molecular weight products obtained from three different variations of the optimized reaction conditions: (a) the standard conditions under 2 bar O2 partial pressure with Co-PANI-C as the catalyst, (b) the standard conditions under 2 bar O2 partial pressure, but in the absence of a catalyst, and (c) the standard conditions, under anaerobic conditions (35 bar N2) in the absence of catalyst (Figure 3b). The material obtained from the anaerobic catalyst-free conditions (c) exhibited a molecular weight distribution of Mw and Mn values of 1578 and 307 Da, respectively. These values are consistent with the lack of significant monomer formation under these conditions, even while they are lower than the estimated molecular weight of native poplar lignin (Mw/Mn ~ 7.9/1.9 kDa).49 The aerobic conditions lead to lower molecular weight materials, with Mw and Mn values of 663 and 234 Da, respectively, in the absence of catalyst and 604 and 241 Da, respectively, in the presence of the Co-PANI-C catalyst. 2D HSQC NMR was used as an additional method to analyze the materials obtained from the soluble fraction obtained from the three different reaction conditions (see Figure S3 in the Supporting Information). The 2D NMR data indicate that the soluble lignin material from the anaerobic catalyst-free conditions retains only a small quantity of β-O-4 units, with signals indicating some condensation of the lignin. The NMR data indicated that the sample from aerobic catalyst-free conditions contains phenolic dimers, trimers, and oligomers with oxidized side chains, while the sample from aerobic conditions with catalyst has no native or oxidized β-O-4 signals, reflecting cleavage of these units. Collectively, the monomer yield, GPC, and NMR data obtained from the three different conditions provide the following insights: (i) some lignin cleavage occurs during solvolysis by acetone at the elevated temperatures, even under anaerobic conditions, but this process does not lead to significant formation of aromatic monomers; (ii) the presence of O2 alone contributes to further depolymerization, including the formation of low yields of monomers; and (iii) the presence of both catalyst and O2 leads to the highest levels of lignin depolymerization and monomer formation.
The results obtained with O2 alone (i.e., without a catalyst) are consistent with previous reports of "oxygen delignification" of Kraft pulp70 and oxygen-promoted treatment of lignin streams under non-catalyzed conditions.71,72 Such precedents and data from the present study suggest that O2 can promote direct autoxidation of lignin, leading to depolymerization and some monomer formation. Even better results are evident in the presence of a catalyst, however. The catalyst appears to have beneficial effects beyond promoting lignin oxidation. Specifically, control experiments show that the presence of the catalyst stabilizes the phenolic monomers under the reaction conditions, slowing their degradation and supporting higher product yields (see Figure S8 in the Supporting Information). We speculate that the catalyst controls reactive radical species derived from molecular oxygen that can lead to decomposition of the phenol-based products. Overall, the complementary behavior observed under O2 in the absence and presence of catalyst suggests that the catalyst supplements and/or modulates the autoxidation chemistry that contributes to lignin chain cleavage and side-chain oxidation.
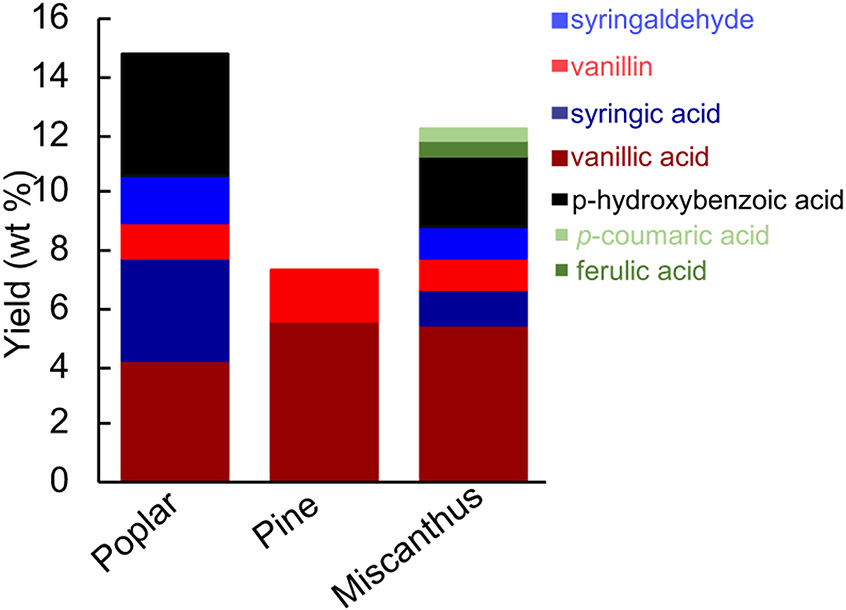
Two other biomass sources were tested under the optimized OCF conditions (Figure 4). Pine, a softwood with no syringyl subunits, affords a lower yield of 7.3 wt%, mainly consisting of vanillic acid. Miscanthus, a grass, generates the five monomers observed for poplar, in addition to ferulic and coumaric acids, with an overall yield of 11.1 wt%.
Figure 4.
OCF results using other biomass sources, including hardwood (poplar), softwood (pine), grass (miscanthus). Conditions: 0.1 g biomass, 10 wt% Co-PANI-C, 20 mL acetone, 190 °C, 35 bar 6% O2 in N2, 12 h.
Analysis of carbohydrate residue
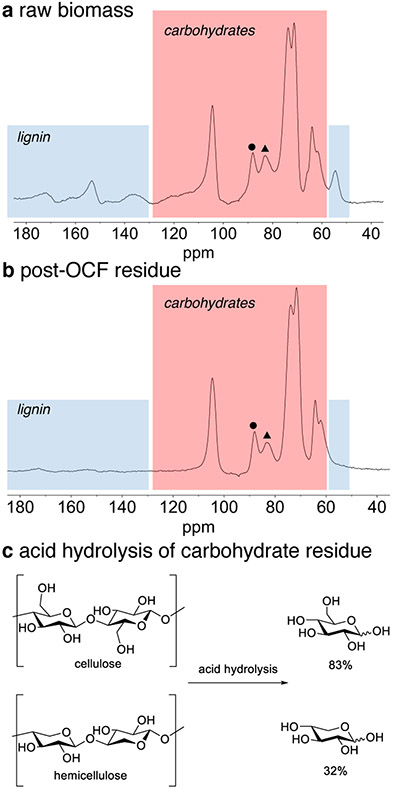
In this OCF process, the carbohydrate residue is readily isolated from post-reaction mixture by simple filtration. The solid material obtained in this manner constitutes 55% of the initial poplar mass, after removal of the solvent. Solid-state 1D 13C CPMAS NMR spectra of the original poplar biomass (Figure 5a) and post-OCF solids (Figure 5b) show that a large portion of the lignin is removed from the final solids, consistent with solvolysis of the lignin as described above. Meanwhile, the carbohydrate resonances are retained, with an increase in crystallinity relative to amorphous cellulose, evident from the change in relative peak heights of the amorphous C4 resonance at 83 ppm (Figure 5a/b, filled triangle) compared to the crystalline C4 resonance at 88 ppm (Figure 5a/b, filled circle).73 Powder X-ray diffraction patterns confirm an increase in the relative crystallinity index from 48.1 to 55.6 (see Figure S9 in the Supporting Information). Similar changes have been observed in carbohydrates observed from previously reported organosolv lignin extraction with acetone.74
Figure 5.
1D 13C CPMAS NMR spectra of raw biomass and the solid material obtained following OCF treatment (a, b) (filled triangle denotes amorphous C4 resonance; filled circle denotes crystalline C4 resonance), and results of acid hydrolysis of solid carbohydrate material obtained from OCF treatment (c).75
The quality of the carbohydrate residue was evaluated by acid hydrolysis, by using a modified version of the NREL biomass compositional analysis procedure.75 The solid material was rinsed with fresh acetone, dried, and subjected to an acid hydrolysis with H2SO4. This protocol afforded sugar yields of 83% glucose and 32% xylose, relative to the cellulose and hemicellulose present in the original raw biomass (Figure 5c). These results suggest that the OCF-derived carbohydrates should be suitable further conversion into glucose, ethanol, and other carbohydrate derived products.
Overall mass balance from oxidative catalytic fractionation
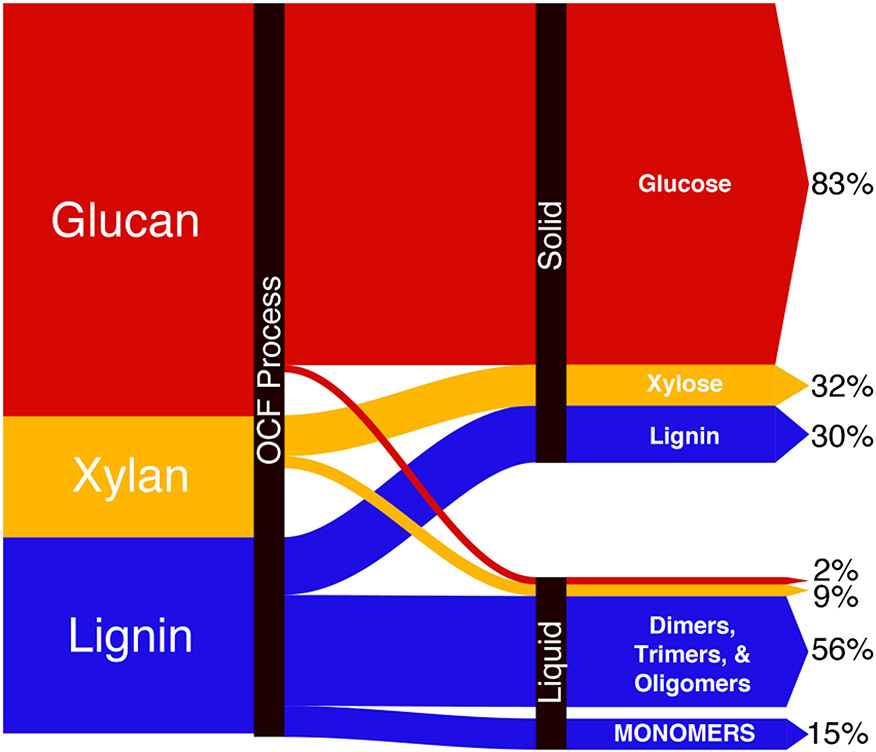
Analysis of the overall mass balance of the poplar OCF process is shown in the Sankey diagram in Figure 6. The initial poplar biomass consists of 21.4 wt% lignin, 13.9 wt% xylans, and 47.5 wt% glucans, in addition to 5.6% water and 11.6% unidentified mass.75 The lignin is fractionated into a dissolved portion, including 15.0 wt% monomers and 56 wt% higher molecular weight species (dimers, oligomers), while the solid fraction retains 30 wt% of the original lignin.76 Collectively, these fractions account for 101 ± 5 wt% of the original lignin. Precise mass balance is complicated by addition of mass arising from oxygenation of lignin-based aromatics and loss of mass from low molecular weight by-products arising from cleavage of the aliphatic linkers in lignin. Xylan-derived products partition between the soluble (9%) and insoluble (32%) fractions, while the vast majority of the glucan material (83%) is captured as the solid fraction recovered from the OCF process.
Figure 6.
Mass balance of recovered carbohydrates (glucans + xylans) and valorized lignin in poplar biomass. Product percentages reported are relative to the original biomass.
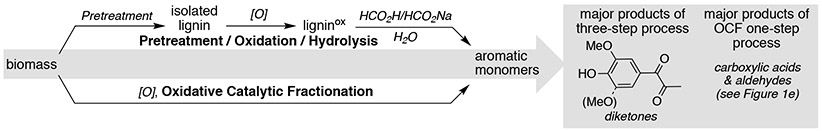
The yields of monoaromatic products obtained from this OCF process are not as high as those that have been accessed via RCF of hardwoods (yields are often 30-50%);14,28,53,77,78 however, meaningful future comparisons will need to account for the relative value of carboxylic acids and aldehydes from OCF relative to the alkylaromatics obtained from RCF (cf. Figure 1d and 1e) for the desired target applications, among other technoeconomic considerations.17 The present OCF results also may be compared to previously reported multi-step oxidative lignin depolymerization routes.14,44,48,51 The latter methods use lignin derived from pretreatment methods, which typically do not recover all of the lignin from biomass. Subsequent oxidative depolymerization methods result in further losses. The most effective routes thus far employ a three-step pre-treatment/oxidation/depolymerization sequence, with examples involving different pretreatment lignin sources summarized in Table 1. After accounting for the yields from each of three steps, the yields of oxidized aromatic compounds range from 6.2–31%. The best of these uses propionaldehyde to protect the lignin during an acidolysis pretreatment, followed by DDQ-mediated oxidation of the protected lignin, and HCO2H/HCO2Na-promoted hydrolysis of the oxidized lignin. The major products of this method are syringyl- and guaiacyl-derived diketones (see graphic above Table 1), rather than carboxylic acids and aldehydes.
The appeal of the one-step OCF process, similar to the RCF methods, is the ability to convert the biomass directly into lignin-based aromatics and a carbohydrate stream. Reducing the number of unit operations will inevitably reduce the overall process cost, offsetting differences in total product yield. It is reasonable to expect that future efforts will lead to new catalysts, process conditions, or privileged feedstocks to significantly improve the OCF product yields. The resulting aldehyde and carboxylic acid functional groups are highly appealing products, either as direct commercial products (vanillin) or as feedstocks for microbial and chemical upgrading.35-40 For example, the aromatic aldehydes and carboxylic acids have proven much more amenable to microbial conversion relative to the diketone products.39,40
Conclusion
In summary, the present study has demonstrated an important first step toward non-alkaline oxidative catalytic fractionation of raw biomass. The process directly generates valuable bifunctional oxygenated aromatic monomers from lignin in parallel with high-quality carbohydrate solids amenable to further processing. These OCF results introduce a valuable complement to reductive catalytic fractionation or other biomass fractionation approaches and set the stage for optimization efforts designed to improve the aromatic monomer yields and begin technoeconomic analysis to guide process improvements that could improve the viability of this approach.
Supplementary Material
Acknowledgements
The authors would like to thank Christopher Holland for helpful discussions and Junyong Zhu for samples of lodgepole pine and birch. Financial support for this project was provided by the Great Lakes Bioenergy Research Center, U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research under Award Numbers DE-SC0018409 and DE-FC02-07ER64494. The NMR facilities were supported by the NIH (S10 OD012245), by a generous gift from Paul J. and Margaret M. Bender, and by the University of Wisconsin-Madison UW2020 program. The pXRD facilities were supported by a generous gift from Paul J. and Margaret M. Bender.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c08635. Experimental details for catalytic OCF reactions, additional experimental and screening data, solvent recovery test, mass balance details, and spectroscopic data (PDF).
Competing interests
A provisional patent application has been filed based on this work.
References.
- 1).Tuck CO; Pérez E; Horváth IT; Sheldon RA; Poliakoff M Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. [DOI] [PubMed] [Google Scholar]
- 2).Langholtz MH; Stokes BJ; Eaton LM 2016 Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy, Volume 1: Economic Availability of Feedstocks; Technical report for U.S. Department of Energy, Oak Ridge National Laboratory: Oak Ridge, TN. July2016. [Google Scholar]
- 3).McKendry P Energy Production from Biomass (part 1): Overview of Biomass. Bioresource Technol. 2002, 83, 37–46. [DOI] [PubMed] [Google Scholar]
- 4).Mosier N; Wyman C; Dale B; Elander R; Lee YY; Holtzapple M; Ladisch M Features of Promising Technologies for Pretreatment of Lignocellulosic Biomass. Bioresource Technol. 2005, 96, 673–686. [DOI] [PubMed] [Google Scholar]
- 5).Ragauskas AJ ; Beckham GT; Biddy MJ; Chandra R; Chen F; Davis MF; Davison BH; Dixon RA; Gilna P; Keller M; Langan P; Naskar A,K; Saddler JN; Tschaplinski TJ; Tuskan GA; Wyman CE Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [DOI] [PubMed] [Google Scholar]
- 6).Li C; Zhao X; Wang A; Huber GW; Zhang T Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev 2015, 115, 11559–11624. [DOI] [PubMed] [Google Scholar]
- 7).Sun Z; Fridrich B; de Santi A; Elangovan S; Barta K Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev 2018, 118, 614–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Schutyser W; Renders T; Van den Bosch S; Koelewijn S-F; Beckham GT; Sels BF Chemicals from Lignin: an Interplay of Lignocellulose Fractionation, Depolymerisation, and Upgrading. Chem. Soc. Rev 2018, 47, 852–908. [DOI] [PubMed] [Google Scholar]
- 9).Lora J Industrial Commercial Lignins: Sources, Properties and Applications. in Monomers, Polymers and Composites from Renewable Resources; Belgacem MN; Gandini A, Eds.; Elsevier Science; 2008; vol 1 pp 225–241. [Google Scholar]
- 10).Strassberger Z; Tanase S; Rothenberg G The Pros and Cons of Lignin Valorisation in an Integrated Biorefinery. RSC Adv. 2014, 4, 25310–25318. [Google Scholar]
- 11).Rinaldi R; Jastrzebski R; Clough MT; Ralph J; Kennema M; Bruijnincx PCA; Weckhuysen BM Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed 2016, 55, 8164–8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Renders T; Van den Bosch S; Koelewijn S-F; Schutyser W; Sels BF Lignin-First Biomass Fractionation: the Advent of Active Stabilisation Strategies. Energy Environ. Sci 2017, 10, 1551–1557. [Google Scholar]
- 13).Shui L; Amiri MT; Questell-Santiago YM; Héroguel F; Li Y; Kim H; Meilan R; Chapple C; Ralph J; Luterbacher JS Formaldehyde Stabilization Facilitates Lignin Monomer Production During Biomass Depolymerization. Science 2016, 354, 329–333. [DOI] [PubMed] [Google Scholar]
- 14).Lan W; de Bueren JB; Luterbacher JS Highly Selective Oxidation and Depolymerization of α-γ-Diol-Protected Lignin. Angew. Chem. Int. Ed 2019, 58, 2649–2654. [DOI] [PubMed] [Google Scholar]
- 15).Questell-Santiago YM; Galkin MV; Barta K; Luterbacher JS Stabilization Strategies in Biomass Depolymerization Using Chemical Functionalization. Nature Rev. Chem 2020, 4, 311–330. [DOI] [PubMed] [Google Scholar]
- 16).Deuss PJ; Scott M; Tran F; Westwood NJ; de Vries JG; Barta K Aromatic Monomers by in Situ Protection of Reactive Intermediates in Acid Catalyzed Depolymerization of Lignin. J. Am. Chem. Soc 2015, 137, 7456–7467. [DOI] [PubMed] [Google Scholar]
- 17).Abu-Omar MM; Barta K; Beckham GT; Luterbacher JS; Ralph J; Rinaldi R; Román-Leshkov Y; Samec JSM; Sels BF; Wang F Guidelines for Performing Lignin-First Biorefining. Energy Environ. Sci 2021, 14, 262–292. [Google Scholar]
- 18).Renders T; Van den Bossche G; Vangeel T; Van Aelst K; Sels B Reductive Catalytic Fractionation: State of the Art of Lignin-First Biorefinery. Curr. Opin. Biotech 2019, 56, 193–201. [DOI] [PubMed] [Google Scholar]
- 19).Li C; Zheng M; Wang A; Zhang T One-Pot Catalytic Hydrocracking of Raw Woody Biomass into Chemicals Over Supported Carbide Catalysts: Simultaneous Conversion of Cellulose, Hemicellulose, and Lignin. Energy Environ. Sci 2012, 5, 6383–6390. [Google Scholar]
- 20).Song Q; Wang F; Cai J; Wang Y; Zhang J; Yu W; Xu J Lignin Depolymerization (LDP) in Alcohol over Nickel-Based Catalysts via a Fragmentation-Hydrogenolysis Process. Energy Environ. Sci 2013, 6, 994–1007. [Google Scholar]
- 21).Klein I; Marcum C; Kenttämaa H; Abu-Omar MM Mechanistic Investigation of the Zn/Pd/C Catalyzed Cleavage and Hydrodeoxygenation of Lignin. Green Chem. 2016, 18, 2399–2405. [Google Scholar]
- 22).Luo H; Klein IM; Jiang Y; Zhu H; Liu B; Kenttämaa HI; Abu-Omar MM Total Utilization of Miscanthus Biomass, Lignin and Carbohydrates, Using Earth Abundant Nickel Catalyst. ACS Sustainable Chem. Eng 2016, 4, 2316–2322. [Google Scholar]
- 23).Huang X; Morales Gonzalez OM; Zhu J; Korányi TI; Boot MD Hensen EJM Reductive Fractionation of Woody Biomass into Lignin Monomers and Cellulose by Tandem Metal Triflate and Pd/C Catalysis. Green Chem. 2017, 19, 175–187. [Google Scholar]
- 24).Van den Bosch S; Renders T; Kennis S; Koelewijn S-F; Van den Bossche G; Deneyer A; Depuydt D; Courtin CM; Thevelein JM; Schutyser W; Sels BF Integrating Lignin Valorization and Bio-Ethanol Production: on the Role of Ni-Al2O3 Catalyst Pellets during Lignin-First Fractionation. Green Chem. 2017, 19, 3313–3326. [Google Scholar]
- 25).Anderson EM; Stone ML; Katahira R; Reed M; Beckham GT; Román-Leshkov Y Flowthrough Reductive Catalytic Fractionation of Biomass. Joule 2017, 1, 613–622. [Google Scholar]
- 26).Kumaniaev I; Subbotina E; Sävmarker J; Larhed M; Galken MV; Samec JSM Lignin Depolymerization to Monophenolic Compounds in a Flow-Through System. Green Chem. 2017, 19, 5767–5771. [Google Scholar]
- 27).Anderson EM; Stone ML; Hülsey MJ; Beckham GT; Román-Leshkov Y Kinetic Studies of Lignin Solvolysis and Reduction by Reductive Catalytic Fractionation Decoupled in Flow-Through Reactors. ACS Sustainable Chem. Eng 2018, 6, 7951–7959. [Google Scholar]
- 28).Liao Y; Koelewijn S-F; Van den Bossche G; Van Aelst J; Van den Bosch S; Renders T; Navare K; Nicolaiï T; Van Aelst K; Maesen M; Matsushima H; Thevelein JM; Van Acker K; Lagrain B; Verboekend D; Sels BF A Sustainable Wood Biorefinery for Low Carbon Footprint Chemicals Production. Science 2020, 367, 1385–1390. [DOI] [PubMed] [Google Scholar]
- 29).Liu B; Abu-Omar MM Lignin Extraction and Valorization Using Heterogeneous Transition Metal Catalysts. In Advances in Inorganic Chemistry: Catalysis in Biomass Conversion; Ford PC; van Eldik R Eds. Academic Press, Cambridge, MA, 2021; Vol. 77, pp 137–174. [Google Scholar]
- 30).Arts W; Ruijten D; Van Aelst K; Trullemans L; Sels B The RCF Biorefinery: Building on a Chemical Platform from Lignin. In Advances in Inorganic Chemistry: Catalysis in Biomass Conversion; Ford PC; van Eldik R Eds. Academic Press, Cambridge, MA, 2021; Vol. 77, pp 241–297. [Google Scholar]
- 31).Koelewijn S-F; Cooreman C; Renders T; Saiz CA; Van den Bosch S; Schutyser W; De Leger W; Smet M; Van Puyvelde P; Witters H; Van der Bruggen B; Sels BF Promising Bulk Production of a Potentially Benign Bisphenol A Replacement from a Hardwood Lignin Platform. Green Chem. 2018, 20, 1050–1058. [Google Scholar]
- 32).Wang S; Shuai L; Saha B; Vlachos DG; Epps TH III. From Tree to Tape: Direct Synthesis of Pressure Sensitive Adhesives from Depolymerized Raw Lignocellulosic Biomass. ACS Cent. Sci 2018, 4, 701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Parsell T; Yohe S; Degenstein J; Jarrel T; Klein I; Gencer E; Hewetson B; Hurt M; Kim JI; Choudhari H; Saha B; Meilan R; Mosier N; Ribeiro F; Delgass WN; Chapple C; Kenttämaa HI; Agrawal R; Abu-Omar MM A Synergistic Biorefinery based on Catalytic Conversion of Lignin Prior to Cellulose Starting from Lignocellulosic Biomass. Green Chem. 2015, 17, 1492–1499. [Google Scholar]
- 34).Elangovan S; Afansenko A; Haupenthal J; Sun Z; Liu Y; Hirsch AKH; Barta K From Wood to Tetrahydro-2-benzazepines in Three Waste-Free Steps: Modular Synthesis of Biologically Active Lignin-Derived Scaffolds. ACS Cent. Sci 2019, 5, 1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Llevot A; Grau E; Carlotti S; Grelier S; Cramail H From Lignin-derived Aromatic Compounds to Novel Biobased Polymers. Macromol. Rapid Comm 2016, 37, 9–28. [DOI] [PubMed] [Google Scholar]
- 36).Xanthopoulou E; Terzopoulou Z; Zamboulis A; Papadopoulos L; Tsongas K; Tzetzis D; Papageorgiou GZ; Bikiaris DN; Poly(Propylene Vanillate): A Sustainable Lignin-Based Semicrystalline Engineering Polyester. ACS Sustainable. Chem. Eng 2021, 9, 1383–1397. [Google Scholar]
- 37).Bassett AW; Honnig AE; Breyeta CM; Dunn IC; La Scala JJ; Stanzione JF III. Vanillin-Based Resin for Additive Manufacturing. ACS Sustainable Chem. Eng 2020, 8, 5626–5635. [Google Scholar]
- 38).Fang Z; Nikafshar S; Hegg EL; Nejad M Biobased Divanillin as a Precursor for Formulating Biobased Epoxy Resin. ACS Sustainable Chem. Eng 2020, 8, 9095–9103. [Google Scholar]
- 39).Beckham GT; Johnson CW; Karp EM; Salvachúa D; Vardon DR Opportunities and Challenges in Biological Lignin Valorization. Curr. Opin. Biotech 2016, 42, 40–53. [DOI] [PubMed] [Google Scholar]
- 40).Perez JM; Kontur WS; Alherech M; Coplein J; Karlen SD; Stahl SS; Donohue TJ; Noguera DR Funneling Aromatic Products of Chemically Depolymerized Lignin into 2-Pyrone-4-6-Dicarboxylic Acid with Novosphingobium Aromaticivorans. Green Chem. 2019, 21, 1340–1350. [Google Scholar]
- 41).Ma R; Xu Y; Zhang X Catalytic Oxidation of Biorefinery Lignin to Value-added Chemicals to Support Sustainable Biofuel Production. ChemSusChem 2015, 8, 24–51. [DOI] [PubMed] [Google Scholar]
- 42).Behling R; Valange S; Chatel G Heterogeneous Catalytic Oxidation for Lignin Valorization into Valuable Chemicals: What Results? What Limitations? What Trends? Green Chem. 2016, 18, 1839–1854. [Google Scholar]
- 43).Vangeel T; Schutyser W; Renders T; Sels BF Perspective on Lignin Oxidation: Advances, Challenges, and Future Directions. Top. Curr. Chem 2018, 376, 30. [DOI] [PubMed] [Google Scholar]
- 44).Rahimi A; Azarpira A; Kim H; Ralph J; Stahl SS Chemoselective Metal-Free Aerobic Alcohol Oxidation in Lignin. J. Am. Chem. Soc 2013, 135, 6415–6418. [DOI] [PubMed] [Google Scholar]
- 45).Rahimi A; Ulbrich A; Coon JJ; Stahl SS Formic-Acid-Induced Depolymerization of Oxidized Lignin to Aromatics. Nature 2014, 515, 249–252. [DOI] [PubMed] [Google Scholar]
- 46).Lancefield CS; Ojo OS; Tran F; Westwood NJ Isolation of Functionalized Phenolic Monomers through Selective Oxidation and C-O Bond Cleavage of the β-O-4 Linkages in Lignin. Angew. Chem. Int. Ed 2015, 54, 258–262. [DOI] [PubMed] [Google Scholar]
- 47).Bosque T; Magallanes G; Rigoulet M; Kärkäs MD; Stephenson CRJ Redox Catalysis Facilitates Lignin Depolymerization. ACS Cent. Sci 2017, 3, 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Das A; Rahimi A; Ulbrich A; Alherech M; Motagamwala AH; Bhalla A; Sousa LC; Balan V; Dumesic JA; Hegg EL; Dale BE; Ralph J; Coon JJ; Stahl SS Lignin Conversion to Low-Molecular-Weight Aromatics via an Aerobic Oxidation-Hydrolysis Sequence: Comparison of Different Lignin Sources. ACS Sustainable Chem. Eng 2018, 6, 3367–3374. [Google Scholar]
- 49).Rafiee M; Alherech M; Karlen SD; Stahl SS Electrochemical Aminoxyl-Mediated Oxidation of Primary Alcohols in Lignin to Carboxylic Acids: Polymer Modifications and Depolymerization. J. Am. Chem. Soc 2019, 141, 15266–15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Song Y; Mobley JK; Motagamwala AH; Isaacs M; Dumesic JA; Ralph J; Lee AF; Wilson K; Crocker M Gold-Catalyzed Conversion of Lignin to Low Molecular Weight Aromatics. Chem. Sci 2018, 9, 8127–8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Cui Y; Goes SL; Stahl SS Sequential Oxidation – Depolymerization Strategies for Lignin Conversion to Low Molecular Weight Aromatic Chemicals. In Advances in Inorganic Chemistry: Catalysis in Biomass Conversion; Ford PC; van Eldik R Eds.; Academic Press: Cambridge, MA, 2021; Vol. 77, pp 99–136. [Google Scholar]
- 52).Tarabanko VE; Kaygorodov KL; Skiba EA; Tarabanko N; Chelbina YV; Baybakova OV; Kuznetsov BN; Djakovitch L Processing Pine Wood into Vanillin and Glucose by Sequential Catalytic Oxidation and Enzymatic Hydrolysis. J. Wood Chem. Tech 2017, 37, 43–51. [Google Scholar]
- 53).Schutyser W; Kruger JS; Robinson AM; Katahira R; Bradner DG; Cleveland NS; Mittal A; Peterson DJ; Meilan R; Román-Leshkov Y; Beckham GT Revisiting Alkaline Aerobic Lignin Oxidation. Green Chem. 2018, 20, 3828–3844. [Google Scholar]
- 54).While the present manuscript was under review, a modified oxidative catalytic fractionation method was reported in methanol/water solvent mixtures with polyoxometalate catalysts: Du X; Tricker AW; Yang W; Katahira R; Liu W; Kwok TT; Gogoi P; Deng Y Oxidative Catalytic Fractionation and Depolymerization of Lignin in a One-Pot Single-Catalyst System. ACS Sustainable Chem. Eng 2021, 9, 7719–7727. [Google Scholar]
- 55).Hdidou L; Khallouk K; Solhy A; Manoun B; Oukarroum A; Barakat A Synthesis of CoFeO Mixed Oxides via an Alginate Gelation Process as Efficient Heterogeneous Catalysts for Lignin Depolymerization in Water. Catal. Sci. Technol 2018, 8, 5445–5453. [Google Scholar]
- 56).Tarabanko VE; Tarabanko N Catalytic Oxidation of Lignins into the Aromatic Aldehydes: General Process Trends and Development Prospects. Int. J. Mol. Sci 2017, 18, 2421–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Mallat T; Baiker A Oxidation of Alcohols with Molecular Oxygen on Solid Catalysts. Chem. Rev 2004, 104, 3037–3058. [DOI] [PubMed] [Google Scholar]
- 58).Sun T; Tian B; Lu J; Su C Recent Advances in Fe (or Co)/N/C Electrocatalysts for the Oxygen Reduction Reaction in Polymer Electrolyte Membrane Fuel Cells. J. Mater. Chem. A 2017, 5, 18933–18950. [Google Scholar]
- 59).He L; Weniger F; Neumann H; Beller M Synthesis, Characterization, and Application of Metal Nanoparticles Supported on Nitrogen-Doped Carbon: Catalysis Beyond Electrochemistry. Angew. Chem. Int. Ed 2016, 55, 12582–12594. [DOI] [PubMed] [Google Scholar]
- 60).Jagadeesh RV; Junge H; Pohl M-M; Radnik J; Brükner A; Beller M Selective Oxidation of Alcohols to Esters Using Heterogeneous Co3O4-N@C Catalysts under Mild Conditions. J. Am. Chem. Soc 2013, 135, 10776–10782. [DOI] [PubMed] [Google Scholar]
- 61).Preger Y; Gerken JB; Biswas S; Anson CW; Johnson MR; Root TW; Stahl SS Quinone-Mediated Electrochemical O2 Reduction Accessing High Power Density with an Off-Electrode Co-N/C Catalyst. Joule 2018, 2, 2722–2731. [Google Scholar]
- 62).Luo H; Wang L; Li G; Shang S; Lv Y; Niu J; Gao S Nitrogen-Doped Carbon-Modified Cobalt-Nanoparticle-Catalyzed Oxidative Cleavage of Lignin β-O-4 Model Compounds under Mild Conditions. ACS Sustainable Chem. Eng 2018, 6, 14188–14196. [Google Scholar]
- 63).Liu S; Bai L; van Muyden AP; Huang Z; Cui X; Fei Z; Li X; Hu X, Dyson PJ Oxidative Cleavage of β-O-4 Bonds in Lignin Model Compounds with a Single-Atom Co Catalyst. Green Chem. 2019, 21, 1974–1981. [Google Scholar]
- 64).Sun K; Chen S; Zhang J; Lu G-P; Cai C Cobalt Nanoparticles Embedded in N-Doped Porous Carbon Derived from Bimetallic Zeolitic Imidazolate Frameworks for One-Pot Selective Oxidation Depolymerization of Lignin. ChemCatChem 2019, 11, 1264–1271. [Google Scholar]
- 65).Luo H; Wang L; Shang S; Li G; Lv Y; Gao S; Dai W Cobalt Nanoparticles-Catalyzed Widely Applicable Successive C-C Bond Cleavage in Alcohols to Access Esters. Angew. Chem. Int. Ed 2020, 59, 19268–19274. [DOI] [PubMed] [Google Scholar]
- 66).See section 2 of the Supporting Information for full experimental details.
- 67).Osterberg PM; Niemeier JK; Welch CJ; Hawkins JM; Martinelli JR; Johnson TE; Root TW; Stahl SS; Experimental Limiting Oxygen Concentrations for Nine Organic Solvents at Temperatures and Pressures Relevant to Aerobic Oxidations in the Pharmaceutical Industry. Org. Process Res. Dev 2015, 19, 1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).Jafari Y; Amiri H; Karimi K Acetone Pretreatment for Improvement of Acetone, Butanol, and Ethanol production from Sweet Sorghum Bagasse. Appl. Energy 2016, 168, 216–225. [Google Scholar]
- 69).Smit A; Huijgen W Effective Fractionation of Lignocellulose in Herbaceous Biomass and Hardwood using a Mild Acetone Organosolv Process. Green Chem. 2017, 19, 5505–5514. [Google Scholar]
- 70).Jafari V; Labafzadeh SR; King A; Kilpeläinen I; Sixta H; van Heiningen A Oxygen Delignification of Conventional and High Alkali Cooked Softwood Kraft Pulps, and Study of the Residual Lignin Structure. RCS Adv. 2014, 4, 17469–17477. [Google Scholar]
- 71).Lyu G; Yoo CG; Pan X Alkaline Oxidative Cracking for Effective Depolymerization of Biorefining Lignin to Mono-Aromatic Compounds and Organic Acids with Molecular Oxygen. Biomass Bioenergy 2018, 108, 7–14. [Google Scholar]
- 72).Prado R; Brandt A; Erdocia X; Hallet J; Welton T; Labidi J Lignin Oxidation and Depolymerization in Ionic Liquids. Green Chem. 2016, 18, 834–841. [Google Scholar]
- 73).Sathitsuksanoh N; Zhu Z; Wi S; Zhang Y-H, Cellulose P Solvent-Based Biomass Pretreatment Breaks Highly Ordered Hydrogen Bonds in Cellulose Fibers of Switchgrass. Biotechnol. Bioeng 2011, 108, 521–529. [DOI] [PubMed] [Google Scholar]
- 74).Sindhu R; Binod P; Janu KU; Sukumaran RK; Pandey A Organosolvent Pretreatment and Enzymatic Hydrolysis of Rice Straw for the Production of Bioethanol. World J. Microbiol Biotechnol 2012, 28, 473–483. [DOI] [PubMed] [Google Scholar]
- 75).Sluiter A; Hames R; Ruiz R; Scarlata C; Sluiter J; Templeton D; Crocker D; Determination of Structural Carbohydrates and Lignin in Biomass, Laboratory Analytical Procedure. Technical report by National Renewable Energy Laboratory, Office of Energy Efficiency and Renewable Energy, U.S. Department of Energy. Golden, CO, August2012. [Google Scholar]
- 76).Lu F; Wang C; Chen M; Yue F; Ralph J A Facile Method for Measuring Lignin Content in Lignocellulosic Biomass. Green Chem. 2021, 23, 5106–5112. [Google Scholar]
- 77).Luo H; Abu-Omar MM Lignin Extraction and Catalytic Upgrading from Genetically Modified Poplar. Green Chem. 2018, 20, 745–753. [Google Scholar]
- 78).Van den Bosch S; Schutyser W; Vanholme R; Driessen T; Koelewijn S-F; Renders T; De Meester B; Huijgen WJJ; Dehaen W; Courtin CM; Lagrain B; Boerjan W; Sels B; Reductive Lignocellulose Fractionation into Soluble Lignin-Derived Phenolic Monomers and Dimers and Processable Carbohydrate Pulps. Energy Environ. Sci 2015, 8, 1748–1763. [Google Scholar]
- 79).Zijlstra DS; Lahive CW; Analbers CA; Figueirêdo MB; Wang Z; Lancefield CS; Deuss PJ Mild Organosolv Lignin Extraction with Alcohols: The Importance of Benzylic Alkoxylation. ACS Sustainable Chem. Eng 2020, 8, 13, 5119–5131. [Google Scholar]
- 80).Bhalla A; Bansal N; Stoklosa RJ; Fountain M; Ralph J; Hodge DB; Hegg EL Effective Alkaline Metal-Catalyzed Oxidative Delignification of Hybrid Poplar. Biotechnol. Biofuels 2016, 9, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81).Luterbacher JS; Rand JM; Alonso DM; Han J; Youngquist JT; Maravelias CT; Pfleger BF; Dumesic JA Nonenzymatic Sugar Production from Biomass Using Biomass Derived γ-Valerolactone. Science 2014, 343, 277–280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.