Abstract
Azoles are important motifs in medicinal chemistry, and elaboration of their structures via direct N–H/C–H coupling could have broad utility in drug discovery. The ambident reactivity of many azoles, however, presents significant selectivity challenges. Here, we report a copper-catalyzed method that achieves site-selective cross coupling pyrazoles and other N–H heterocycles with substrates bearing (hetero)benzylic C–H bonds. Excellent N-site selectivity is achieved, with the preferred site controlled by the identity of co-catalytic additives. This cross-coupling strategy features broad scope for both the N–H heterocycle and benzylic C–H coupling partners, enabling application of this method to complex molecule synthesis and medicinal chemistry.
Graphical Abstract
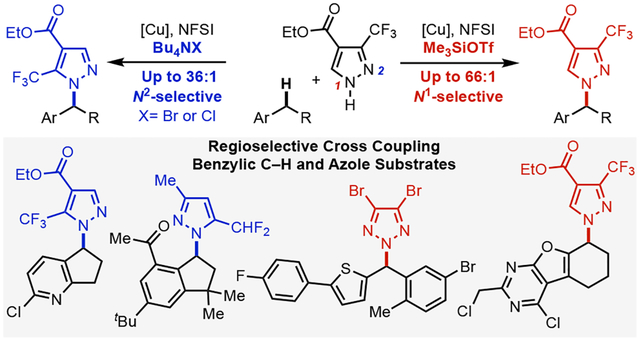
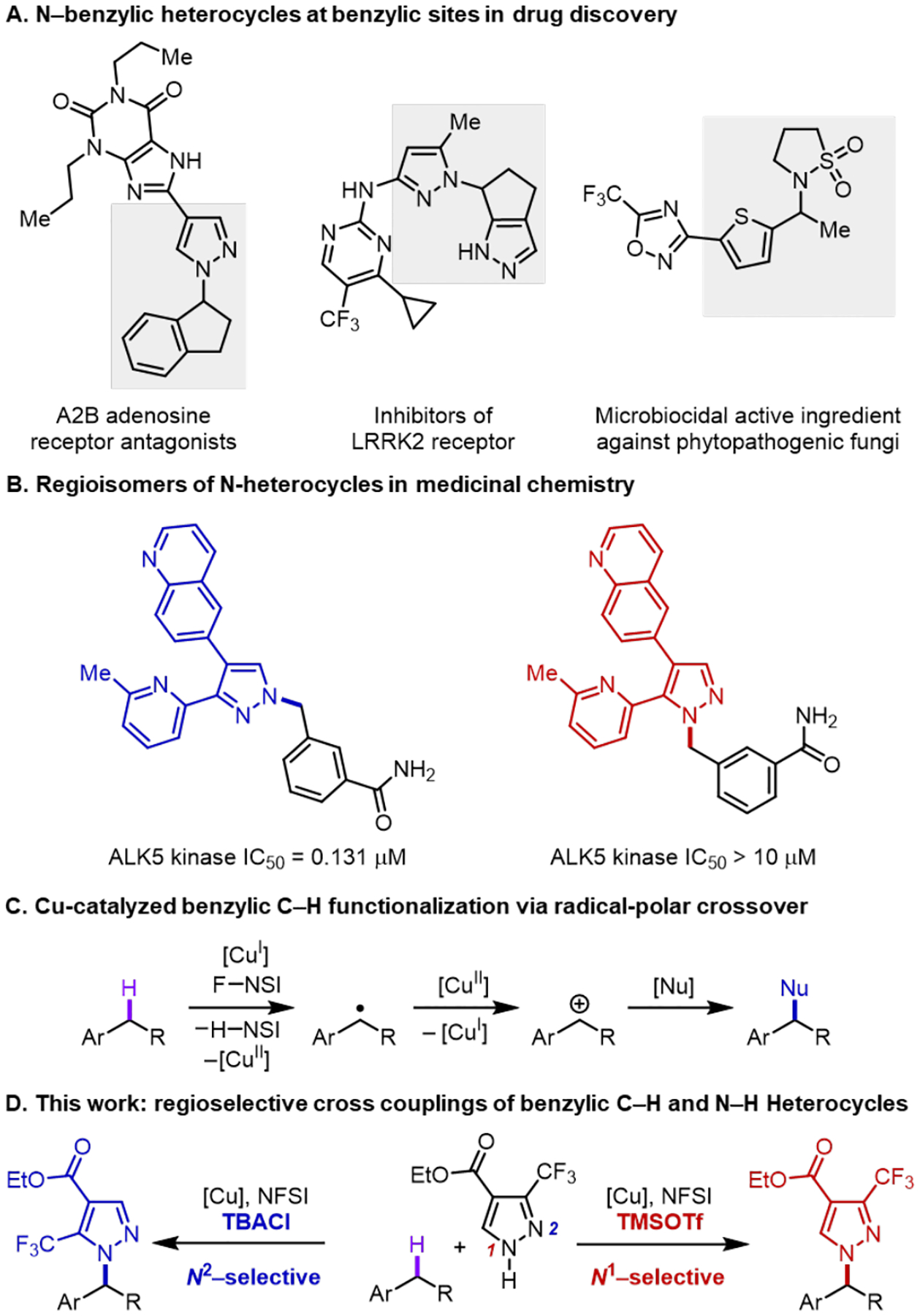
Pd-1 and Cu-catalyzed2 C(sp2)–N cross coupling reactions are some of the most widely used methods for pharmaceutical synthesis.3 Recent efforts have begun prioritizing complementary methods for C(sp3)–N coupling4 as a means to expand the topological diversity and physiochemical properties of the resulting molecules.3, 5 C–N coupling methods that directly functionalize C(sp3)–H bonds bypass the need for pre-functionalized alkyl electrophiles and represent important targets for medicinal chemistry.6 Significant progress has been made in C(sp3)–H amination reactions that install ammonia surrogates via nitrene transfer7 or azidation,8 while C–H/N–H cross-coupling reactions, for example, with secondary amines/amides or N–H heterocycles, are more limited9 and often require excess C–H substrate.10 Here, we report a copper-catalyzed method for selective cross-coupling of azoles with (hetero)benzylic C–H substrates as the limiting reagent, affording N-benzylic heterocycles featured in pharmaceutical and agrochemical compounds (Figure 1A).11–14 In addition to the challenge of C–H site selectivity, these reactions feature a second selectivity challenge arising from azoles that incorporate two (or more) nucleophilic nitrogen atoms.15 This issue has important implications for medicinal chemistry because regioisomeric N-substituted azoles can have very different properties and/or bioactivity (Figure 1B).16 The method described herein achieves excellent C–H site selectivity via hydrogen-atom transfer (HAT) from (hetero)benzylic C–H bonds (Figure 1C). Reaction with the azole coupling partners via CuII-mediated radical-polar crossover exhibits excellent N1/N2 site-selectivity (e.g., with pyrazoles), and variation of the reaction conditions lead to selective formation of either regioisomeric N-benzyl product with many coupling partners (Figure 1D).
Figure 1.
(A) Importance of benzylic N-azoles in drug discovery. (B) Impact of regioselectivity of heterocyclic compounds in medicinal chemistry. (C) Copper-catalyzed regioselective cross couplings of benzylic C–H bonds and N–H heterocycles enabled by various additives.
Investigation of oxidative cross coupling of benzylic C–H substrates and N–H azoles started with ethylbenzene (1a) and ethyl 3-(trifluoromethyl)-1H-pyrazole-4-carboxylate (2a), a pyrazole featured in previous drug discovery efforts.17 Potential reaction conditions were inspired by recent studies involving the use of copper catalysts in combination with N-fluorobenzenesulfonimide (NFSI) as the oxidant. Diverse nucleophilic coupling partners have been used in these reactions, including pseudohalides (cyanide,18 azide,8d isocyanate19), alcohols,20 carbamates,21 and carbon-based nucleophiles (Zn(CF3)2,22 ArB(OH)2,23 alkynes24)25,26 CuI-mediated activation of NFSI generates an N-centered radical that promotes selective HAT from benzylic C–H bonds, and subsequent coupling with heteroatom nucleophiles appears to favor a radical-polar crossover pathway involving formation of a benzylic cation intermediate.8d,19,20 Complementary studies have shown that mild reductants, such as dialkylphosphites, can promote these reactions by buffering the redox state of the Cu catalyst and ensuring that both CuI and CuII are present in the reaction.19,20,27
The above mechanistic considerations guided a survey of reaction conditions. Initial screening data showed that Cu/NFSI-catalyzed oxidative coupling of 1a and 2a favors the N2 isomeric product 3aa when the reaction is conducted in chlorinated solvents PhCl and dichloromethane (DCM) (Figure 2A, entries 1–2; see Supporting Information for full screening details). Use of more polar solvents [hexafluoroisopropanol (HFIP) and MeNO2] led to a switch in selectivity, favoring the N1 isomer 3aa’ (entries 3–4). Tetrabutylammonium (TBA) bromide helped to solubilize the Cu catalyst and improved the reactivity and selectivity. Conventional ancillary ligands, such as phenanthroline or bioxazolines, led to lower yields (see Table S1 and S2 for detailed information). A mixture of DCM:HFIP (7:3) enhanced the conversion of 1a and improved the product yield to 71% (3aa + 3aa’, entry 5). Increasing the loading of TBABr to 0.3 equiv further improved the reaction yield and increased the N2:N1 selectivity to 36:1 (entry 6). Optimal results results were obtained upon replacing TBABr with TBACl, affording 3aa in 75% yield (entry 7). Further screening experiments revealed that the N2/N1 regioselectivity could be switched to favor the N1 product 3aa’ with certain additives (Figure 2B, see Table S2 for additional data). The initial entries in Figure 2B show that additives with halide anions favor formation of the N2 regioisomer 3aa. In contrast, additives with triflate anion or Lewis acids, such as silyl triflates or BF3•OEt2, favor formation of the N1 regioisomer 3aa’. Optimal results for the formation of 3aa’ were achieved with trimethylsilyl triflate (TMSOTf) as the additive at 60 °C (67% yield; N2:N1 = 1:66).
Figure 2.
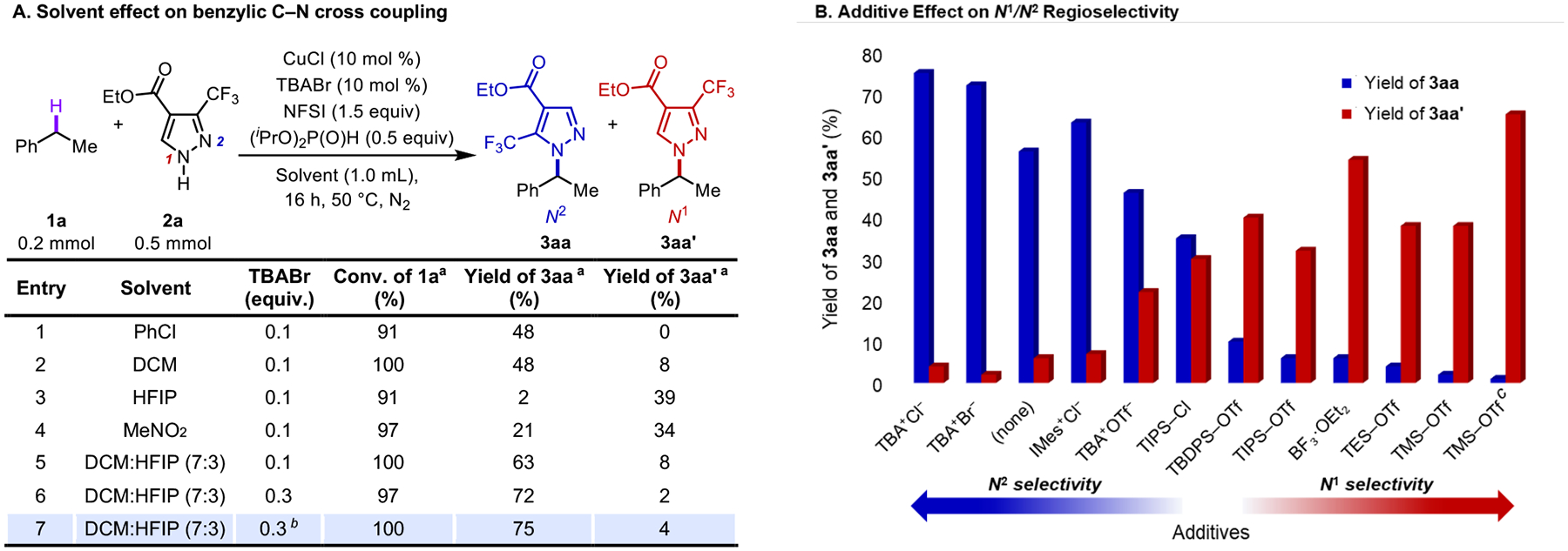
Evaluation of effects of various solvents and additives on the regioselectivity. (A) Effects of solvents on benzylic C–N cross coupling reaction. (B) Regioselectivity switch observed in cross coupling of 1a and 2a with different additives. Conditions identical to those shown in part A, using 10 mol% additive instead of TBABr, 10 mol % CuBr2 instead of CuCl, and DCM:HFIP (7:3) solvent. a Monitored by 1H NMR spectroscopy, yield determined using 0.2 mmol mesitylene as internal standard. TBA, tetrabutylammonium; DCM, dichloromethane; HFIP, hexafluoroisopropanol. b Reaction run with TBA+Cl− instead of TBABr. c Reaction run at 60 °C.
The pyrazole reagent 2a has an N–H bond at the N1 position, as revealed by X-ray crystallography and depicted in Figure 2A (see Section 7 in the Supporting Information for details). This structure is consistent with previous reports for other electron-deficient, 3-substituted pyrazoles.28 We postulated that the switch in pyrazole regioselectivity could arise from kinetic versus thermodynamic control over the C–N bond-forming step. Experimental data supported this hypothesis: addition of TMSOTf to 3aa induced isomerization of this compound to 3aa’, whereas no isomerization of 3aa’ was observed in the presence of TBABr (Figure 3A). These results suggest 3aa is the kinetic product, and they are rationalized by the non-basic reaction conditions, which result in alkylation of the pyrazole nitrogen atom lacking the proton. Reactivity with the pyrazole N2 lone pair (Figure 3B, top) contrasts previously reported reactivity with deprotonated pyrazolide reagents which react preferentially at the N1 site.28b,29 The isomerization data in Figure 3A suggests that strong Lewis acids, such as trimethylsilyl (TMS) cation, can promote isomerization via the benzylic cation and N2-TMS species, to the thermodynamically favored N1 product.28b,30 The data in Figure 2A, entries 1–4 suggest that solvents capable of stabilizing charged intermediates also favor the thermodynamic product.
Figure 3.
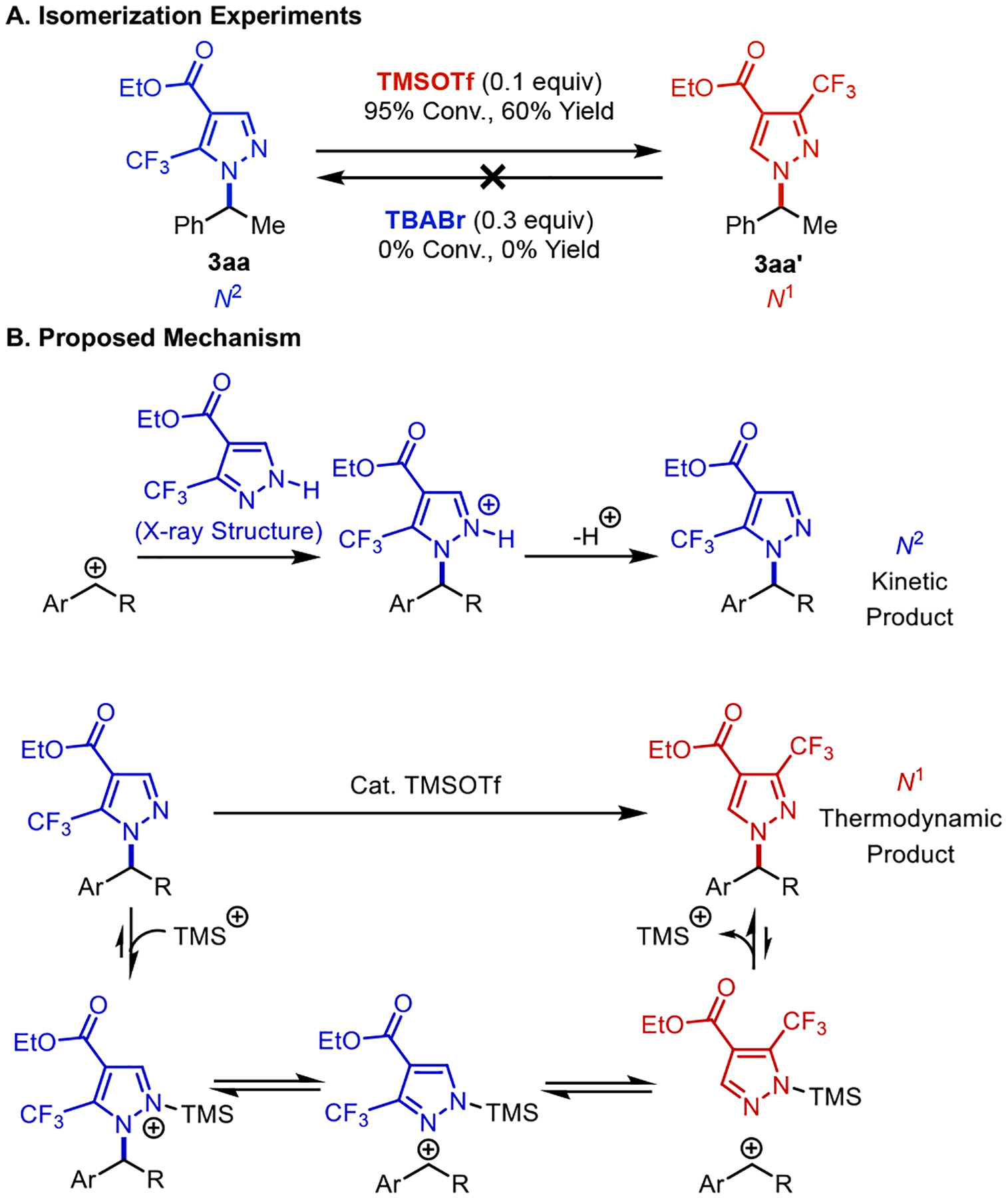
Mechanistic origin of pyrazole regioselectivity. (A) N1/N2-isomerization test, implicating the the N1. (B) Proposed mechanism rationalizing the influence of TMSOTf (and other Lewis acids) on the N2/N1 regioselectivity.
Access to both isomeric pyrazole coupling products is noteworthy because most coupling reactions with pyrazoles employ a base and access only the thermodynamic products.29,31 For example, reaction of (1-bromoethyl)benzene with pyrazole 2a affords exclusively the N1 isomer 3aa’ (see Section 4 in the Supporting Information for details). To explore the scope of this reactivity, we evaluated numerous other C–H/N–H cross-coupling reactions with (hetero)benzylic and azole coupling partners (Figures 4 and 5). Reactions with (hetero)benzylic C–H substrates were initially tested using the TBACl conditions, which tend to be more robust and promote formation of the unique N2-pyrazolyl product (Figure 4A). Ethylbenzene derivatives with different p-substituents are well tolerated (70–86%, 3aa-3da). While the N2 product is typically observed under these conditions, only the N1 product 3ba’ is observed with p-MeO-ethylbenzene (1b). This result is rationalized by the stability of the benzylic carbocation, which facilitates isomerization to the thermodynamic product (cf. Figure 3). The lack of C–N coupling at tertiary C–H sites in reactions with isobutyl- and isopentylbenzene (3ea, 3fa) highlights the exquisite site-selectivity of the HAT steps with the NFSI-derived imidyl radical.8d,20 The strongly electron-withdrawing nitro group in the indane substrate 1g reduces the product yield (33%, 3ga). Benzhydryl N-azoles, a class of compounds that exhibit aromatase inhibitory activity,32 exhibit good reactivity (86%, 3ha; 95%, 3ia) and form only the N1 regioisomers, again rationalized by the benzylic cation stability.
Figure 4.
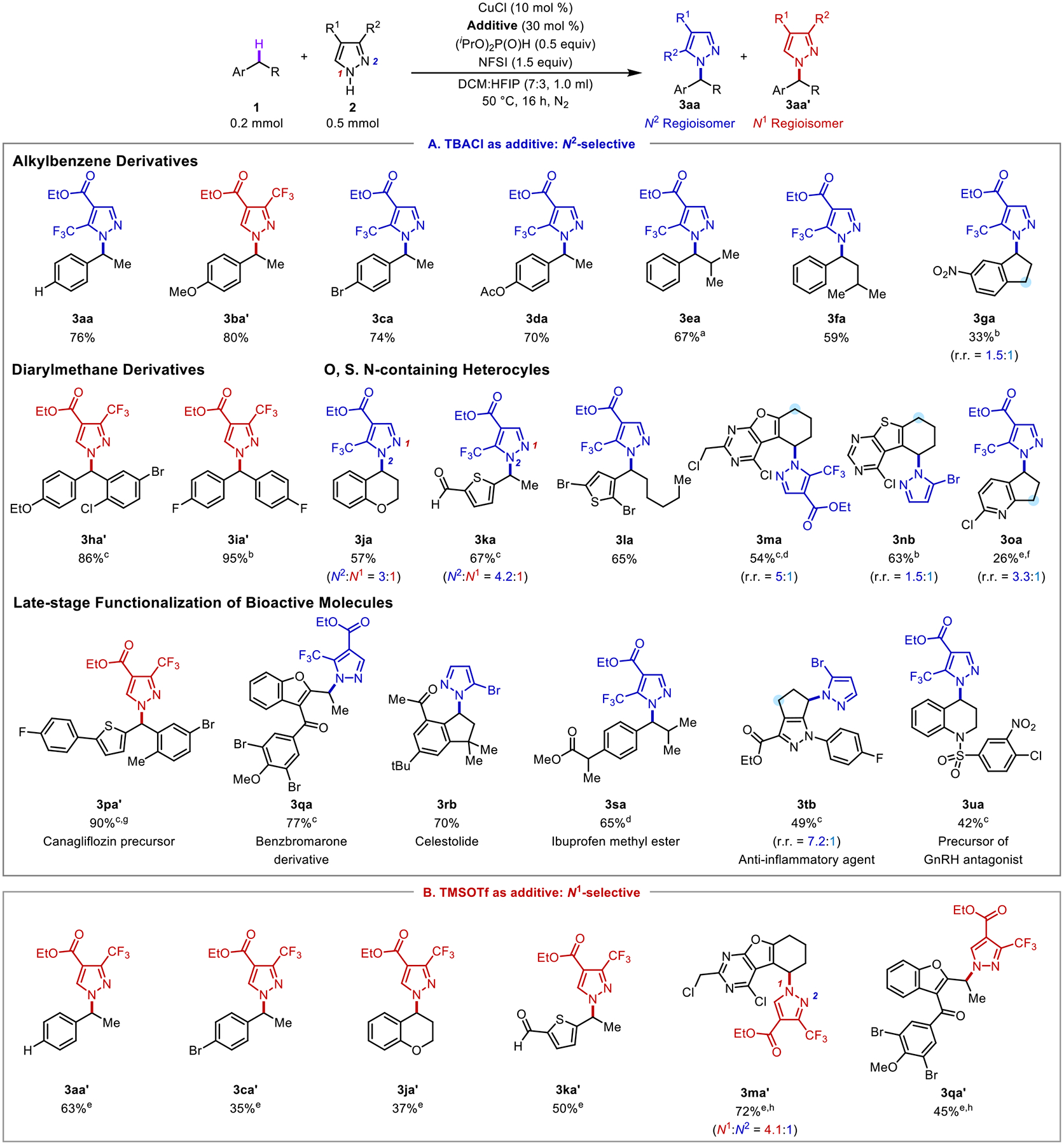
Assessment of various benzylic C–H substrates in cross coupling reactions with N–H heterocycles with (A) TBACl as the additive for N2 regioselectivity and (B) with TMSOTf as the additive for N1 regioselectivity. Regioisomers >5% were isolated and reported. aConducted in 0.5 mL DCM:HFIP (7:3). bConducted with 10 mol % CuBr2 and 30 mol % TBABr. c Conducted with 10 mol % TBACl. dConducted at 40 °C. eConducted at 60 °C. fConducted in DCM. gConducted at 30 °C. hConducted with 10 mol % BF3•OEt2.
Figure 5.
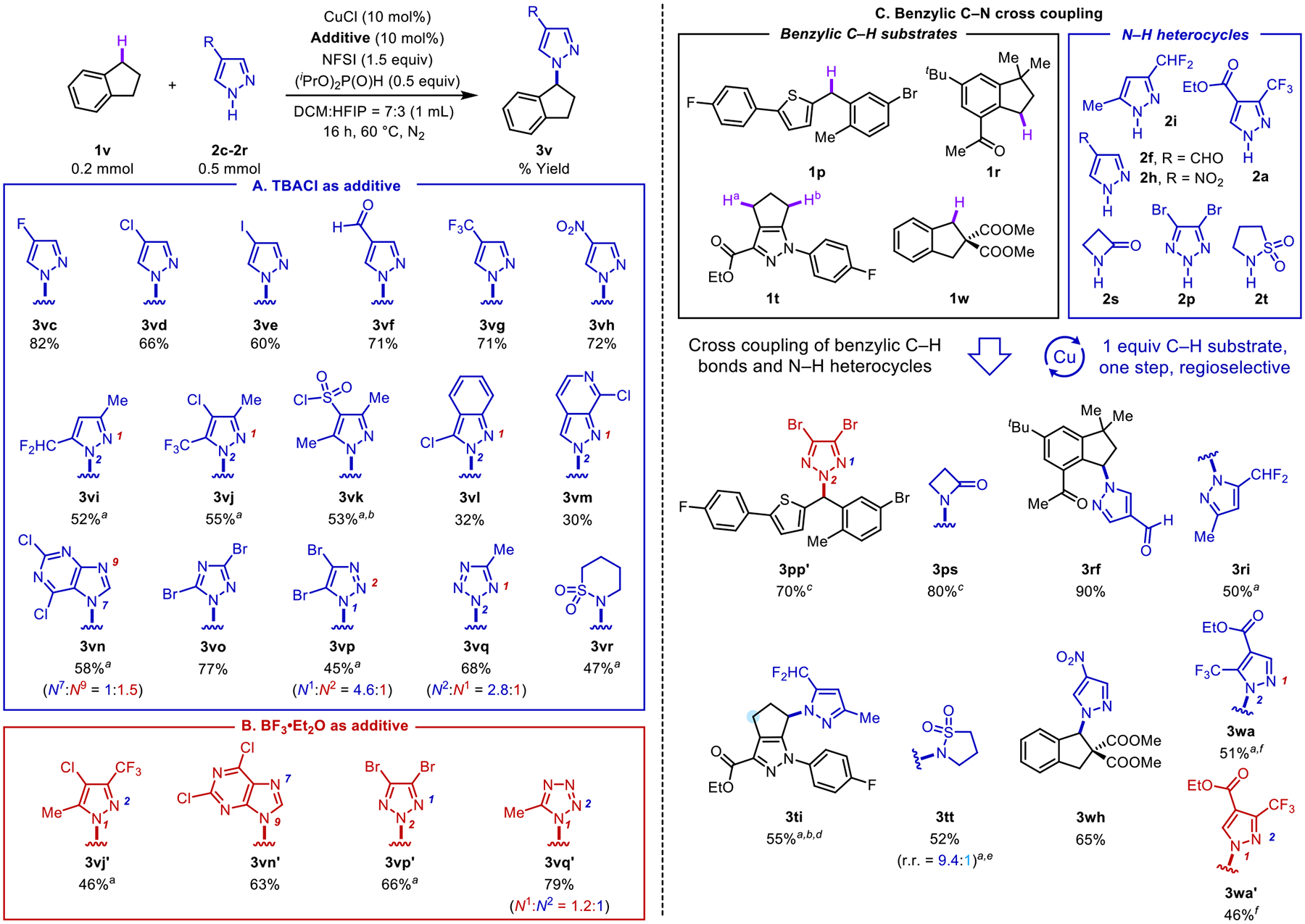
Assessment of various N–H heterocycles in cross coupling reactions. Substrate scope with diverse N–H heterocycles and indane under kinetically controlled TBACl conditions (A) and in the presence of BF3•OEt2 as a Lewis acid cocatalyst (B). Exploration of cross-coupling reactions of diverse N–H heterocycles and (hetero)benzylic C–H scaffolds (C) under the TBACl conditions of Figure 5A, unless noted otherwise. Regioisomers formed in >5% yield were isolated. aConducted in DCM. bConducted at 50 °C. cConducted at 30 °C. dTrace amount of the other benzylic regioisomer was observed. eConducted at 40 °C. fConducted with 10 mol % BF3•OEt2.
Benzylic pyrazoles of chromans,33 thiophene,14,34 and other substrates bearing oxygen-, sulfur- and nitrogen-containing heterocycles react successfully (1j–1u). These reactions demonstrate a tolerance for a formyl group (3ka), heteroarylbromides (3la), and pyrimidines (3ma, 3nb).35 The pyridine substrate 1o reacts in lower yield (26%, 3oa). Substrates with two possible (hetero)benzylic sites (1g, 1m, 1n, and 1o) generate a mixture of products, with moderate to good regioisomeric ratios (r.r.).
Pyrazoles 2a and 3-bromopyrazole (2b) undergo successful late-stage reactivity with bioactive molecules. Examples include an antidiabetic intermediate 1p, which affords only the N1 coupling product (90%, 3pa’); benzbromarone methyl ether (77%, 3qa), a derivative of a xanthine oxidase inhibitor;36 celestolide (70%, 3rb); ibuprofen methyl ester (65%, 3sa); an anti-inflammatory anti-allergy agent37 (49%, 3tb, r.r. = 7.2:1) and a precursor to a GnRH antagonist38 (42%, 3ua). These results were complemented with a focused assessment of the Lewis acid co-catalytic conditions (Figure 4B). These reactions lead to exclusive or high N1 pyrazole site-selectivity in the C–N coupling reactions.
Different pyrazoles and other azole coupling partners were then tested in reactions with indane, motivated by the relevance of N-indanyl azoles in medicinal chemistry (Figure 5A).39 Symmetrical 4-substituted pyrazoles, bearing fluoro, chloro, iodo, formyl, trifluoromethyl and nitro substituents, undergo coupling in good-to-excellent yields (60–82%, 3vc-3vh). Other di- and trisubstituted pyrazoles with di- and trifluoromethyl (2i, 2j)17a and sulfonyl chloride (2k) substituents react effectively (52–55% yields, 3vi-3vk). Successful reactivity, but lower yields are observed with indazole coupling partners 2l and 2m (32%, 3vl; 30%, 3vm).40,41 The dichloropurine derivative 2n generates both N9 and N7 regioisomeric products (58% yield, N7:N9 = 1:1.5, 3vn). Triazoles, including 1,2,4- and 1,2,3-isomers 2o and 2p and a tetrazole 2q undergo successful reactivity (77%, 3vo; 45%, 3vp; 68%, 3vq). The reaction with 2p favors formation of the less common N1 regioisomer (N1:N2 = 4.6:1),42 consistent with the mechanistic rationale above.
Inclusion of BF3•OEt2 in these reactions enabled modulation of the azole regioselectivity (results with BF3•OEt2 were slightly better than with TMSOTf). In each of the four cases shown in Figure 5B, the favored regioisomer is different from that observed in the TBACl conditions. For example, the reaction with 2j completely switches from N2 to N1 selectivity (46%, 3vj’). In other cases, use of BF3•OEt2 ensures only a single isomer is formed (63%, 3vn’; 66%, 3vp’).
In a final assessment of the method, we explored C–N cross coupling reaction with different (hetero)benzylic and N-heterocyclic partners (Figure 5C). These reactions proceed in moderate-to-excellent yields. The reaction conditions show promise for heterocyclic coupling partners beyond azoles, including the beta-lactam 2s (80%, 3ps) and sultams 2r and 2t (47%, 3vr, Figure 5A; 52%, 3tt, Figure 5C). Reactions of the azoles 2a, 2i, and 2p react with the regioselectivity expected from the observation elaborated above. For example, 1p affords the thermodynamically favored coupling product upon reaction with 2p, reflecting the stability of benzylic cation. On the other hand, the reaction of 1w and 2a afforded the kinetically favored the N2 regioisomer even with BF3•OEt2 as a cocatalyst (51%, 3wa). The combined effect of BF3•OEt2 cocatalyst and HFIP as a cosolvent, however, supported a switch in the observed regioselectivity (46%, 3wa’). The reaction of 1t and 2i affords only one of the four possible C–N coupling products (55%, 3ti), demonstrating both C–H and N-nucleophile selectivity.
In summary, the results outlined herein introduce a unique synthetic method for direct C(sp3)–H/N–H coupling of (hetero)benzylic substrates and azoles. Multiple features contribute to the potential impact of these methods. Perhaps the most notable feature is the ability to control the azole N-site selectivity, often enabling access to either regioisomeric product. Other highlights include the high-to-exclusive (hetero)benzylic C–H site selectivity, use of the C–H substrate as the limiting reagent, excellent scope for both coupling partners, and broad functional group compatibility. Overall, these C(sp3)–N coupling methods provide efficient access to complex, pharmaceutically relevant structures, and they should find widespread utility for library synthesis and exploration of chemical space in medicinal chemistry and related disciplines.43
Supplementary Material
ACKNOWLEDGMENT
The authors thank Ilia A. Guzei and Amelia M. Wheaton for assistance with the X-ray crystallographic characterization of 2a and [PhN(CH3)3]2[CuBr4]. This work was supported by funding from the NIH (R35 GM134929). Spectroscopic instrumentation was supported by a gift from Paul. J. Bender, the NSF (CHE-1048642), and the NIH (S10 OD020022).
Footnotes
Supporting Information.
Experimental details with supplemental notes, characterization data, and NMR spectra (PDF).
CCDC 2095281 and 2095282 contains the supplementary crystallographic data for this paper. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.
The authors declare no competing financial interest.
REFERENCES
- (1).Ruiz-Castillo P; Buchwald SL Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev 2016, 116, 12564–12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).(a) West MJ; Fyfe JWB; Vantourout JC; Watson AJB Mechanistic Development and Recent Applications of the Chan–Lam Amination. Chem. Rev 2019, 119, 12491–12523. [DOI] [PubMed] [Google Scholar]; (b) Qiao JX; Lam PYS Copper-Promoted Carbon–Heteroatom Bond Cross-Coupling with Boronic Acids and Derivatives. Synthesis 2011, 6, 829–856. [Google Scholar]
- (3).Brown DG; Boström J Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone? J. Med. Chem 2016, 59, 4443–4458. [DOI] [PubMed] [Google Scholar]
- (4).Trowbridge A; Walton SM; Gaunt MJ New Strategies for the Transition-Metal Catalyzed Synthesis of Aliphatic Amines. Chem. Rev 2020, 120, 2613–2692. [DOI] [PubMed] [Google Scholar]
- (5).(a) Lovering F; Bikker J; Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem 2009, 52, 6752–6756. [DOI] [PubMed] [Google Scholar]; (b) Morley AD; Pugliese A; Birchall K; Bower J; Brennan P; Brown N; Chapman T; Drysdale M; Gilbert IH; Hoelder S; Jordan A; Ley SV; Merritt A; Miller D; Swarbrick ME; Wyatt PG Fragment-Based Hit Identification: Thinking in 3D. Drug Discov. Today 2013, 18, 1221–1227. [DOI] [PubMed] [Google Scholar]
- (6).Cernak T; Dykstra KD; Tyagarajan S; Vachal P; Krska SW The Medicinal Chemist’s Toolbox for Late Stage Functionalization of Drug-Like Molecules. Chem. Soc. Rev 2016, 45, 546–576. [DOI] [PubMed] [Google Scholar]
- (7).(a) Clark JR; Feng K; Sookezian A; White MC Manganese-Catalysed Benzylic C(sp3)–H Amination for Late-Stage Functionalization. Nat. Chem 2018, 10, 583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chiappini ND; Mack JBC; Du Bois J Intermolecular C(sp3)–H Amination of Complex Molecules. Angew. Chem. Int. Ed 2018, 57, 4956–4959. [DOI] [PubMed] [Google Scholar]
- (8).(a) Sharma A; Hartwig JF Metal-Catalysed Azidation of Tertiary C–H Bonds Suitable for Late-Stage Functionalization. Nature 2015, 517, 600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Huang X; Bergsten TM; Groves JT Manganese-Catalyzed Late-Stage Aliphatic C–H Azidation. J. Am. Chem. Soc 2015, 137, 5300–5303. [DOI] [PubMed] [Google Scholar]; (c) Margrey KA; Czaplyski WL; Nicewicz DA; Alexanian EJ A General Strategy for Aliphatic C–H Functionalization Enabled by Organic Photoredox Catalysis. J. Am. Chem. Soc 2018, 140, 4213–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Suh S-E; Chen S-J; Mandal M; Guzei IA; Cramer CJ; Stahl SS Site-Selective Copper-Catalyzed Azidation of Benzylic C–H Bonds. J. Am. Chem. Soc 2020, 142, 11388–11393. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Niu L; Jiang C; Liang Y; Liu D; Bu F; Shi R; Chen H; Chowdhury AD; Lei A Manganese-Catalyzed Oxidative Azidation of C(sp3)–H Bonds under Electrophotocatalytic Conditions. J. Am. Chem. Soc 2020, 142, 17693–17702. [DOI] [PubMed] [Google Scholar]
- (9).(a) Pandey G; Laha R; Singh D Benzylic C(sp3)–H Functionalization for C–N and C–O Bond Formation via Visible Light Photoredox Catalysis. J. Org. Chem 2016, 81, 7161–7171. [DOI] [PubMed] [Google Scholar]; (b) Yang Y-Z; Song R-J; Li J-H Intermolecular Anodic Oxidative Cross-Dehydrogenative C(sp3)–N Bond-Coupling Reactions of Xanthenes with Azoles. Org. Lett 2019, 21, 3228–3231. [DOI] [PubMed] [Google Scholar]; (c) Hou Z-W; Liu D-J; Xiong P; Lai X-L; Song J; Xu H-C Site-Selective Electrochemical Benzylic C–H Amination. Angew. Chem. Int. Ed 2020, 60, 2943–2947. [DOI] [PubMed] [Google Scholar]; (d) Hou Z-W; Li L; Wang L Organocatalytic Electrochemical Amination of Benzylic C–H Bonds. Org. Chem. Front 2021, DOI: 10.1039/D1QO00746G. [DOI] [Google Scholar]
- (10).(a) Song C; Dong X; Yi H; Chiang C-W; Lei A DDQ-Catalyzed Direct C(sp3) – H Amination of Alkylheteroarenes: Synthesis of Biheteroarenes under Aerobic and Metal-Free Conditions. ACS Catal 2018, 8, 2195–2199. [Google Scholar]; (b) Ruan Z; Huang Z; Xu Z; Zeng S; Feng P; Sun P-H Late-Stage Azolation of Benzylic C‒H Bonds Anabled by Electrooxidation. Sci. China. Chem 2021, 64, 800–807. [Google Scholar]
- (11).Eight of top 50 best-selling drugs contain N-benzylic heterocycles:; Baumann M; Baxendale IR; Ley SV; Nikbin N Beilstein J. Org. Chem 2011, 7, 442–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Taylor RD; MacCoss M; Lawson ADG Rings in Drugs. J. Med. Chem 2014, 57, 5845–5859. [DOI] [PubMed] [Google Scholar]
- (13).Estrada AA; Feng JA; Lyssikatos JP; Sweeney ZK Compounds, Compositions, and Methods. Worldwide patent, WO2017087905 A1, May26, 2017.
- (14).Hoffman TJ; Stierli D; Pitterna T; Rajan R Microbiocidal Oxadiazole Derivatives. Worldwide patent2018158365 A1, September07, 2018.
- (15).Blakemore DC; Castro L; Churcher I; Rees DC; Thomas AW; Wilson DM; Wood A Organic Synthesis Provides Opportunities to Transform Drug Discovery. Nat. Chem 2018, 10, 383–394. [DOI] [PubMed] [Google Scholar]
- (16).Adams ND; Adams JL; Burgess JL; Chaudhari AM; Copeland RA; Donatelli CA; Drewry DH; Fisher KE; Hamajima T; Hardwicke MA; Huffman WF; Koretke-Brown KK; Lai ZV; McDonald OB; Nakamura H; Newlander KA; Oleykowski CA; Parrish CA; Patrick DR; Plant R; Sarpong MA; Sasaki K; Schmidt SJ; Silva DJ; Sutton D; Tang J; Thompson CS; Tummino PJ; Wang JC; Xiang H; Yang J; Dhanak D Discovery of GSK1070916, a Potent and Selective Inhibitor of Aurora B/C Kinase. J. Med. Chem 2010, 53, 3973–4001. [DOI] [PubMed] [Google Scholar]
- (17).(a) Mykhailiuk PK Fluorinated Pyrazoles: From Synthesis to Applications. Chem. Rev 2021, 121, 1670–1715. [DOI] [PubMed] [Google Scholar]; (b) Davie RL; Edwards HJ; Evans DM; Hodgson ST; Miller I; Novak AR; Smith AJ; Stocks MJ Heterocyclic Derivates. Worldwide Patent2014188211 A1, November27, 2014.
- (18).Zhang W; Wang F; McCann SD; Wang D; Chen P; Stahl SS; Liu G Enantioselective Cyanation of Benzylic C–H Bonds via Copper-Catalyzed Radical Relay. Science 2016, 353, 1014–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Suh S-E; Nkulu LE; Lin S; Krska S; Stahl SS Benzylic C–H Isocyanation/Amine Coupling Sequence Enabling High-Throughput Synthesis of Pharmaceutically Relevant Ureas. Chem. Sci 2021, DOI: 10.1039/D1SC02049H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Hu H; Chen S-J; Mandal M; Pratik SM; Buss JA; Krska SW; Cramer CJ; Stahl SS Copper-Catalysed Benzylic C–H Coupling with Alcohols via Radical Relay Enabled by Redox Buffering. Nat. Catal 2020, 3, 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Liu S; Achou R; Boulanger C; Pawar G; Kumar N; Lusseau J; Robert F; Landais Y Copper-Catalyzed Oxidative Benzylic C(sp3)–H Amination: Direct Synthesis of Benzylic Carbamates. Chem. Commun 2020, 56, 13013–13016. [DOI] [PubMed] [Google Scholar]
- (22).Xiao H; Liu Z; Shen H; Zhang B; Zhu L; Li C Copper-Catalyzed Late-Stage Benzylic C(Sp3)–H Trifluoromethylation. Chem 2019, 5, 940–949. [DOI] [PubMed] [Google Scholar]
- (23).(a) Zhang W; Chen P; Liu G Copper-Catalyzed Arylation of Benzylic C–H bonds with Alkylarenes as the Limiting Reagents. J. Am. Chem. Soc 2017, 139, 7709–7712. [DOI] [PubMed] [Google Scholar]; (b) Zhang W; Wu L; Chen P; Liu G Enantioselective Arylation of Benzylic C–H Bonds by Copper-Catalyzed Radical Relay. Angew. Chem. Int. Ed 2019, 58, 6425–6429. [DOI] [PubMed] [Google Scholar]
- (24).Fu L; Zhang Z; Chen P; Lin Z; Liu G Enantioselective Copper-Catalyzed Alkynylation of Benzylic C–H Bonds via Radical Relay. J. Am. Chem. Soc 2020, 142, 12493–12500. [DOI] [PubMed] [Google Scholar]
- (25).Wang F; Chen P; Liu G Copper-Catalyzed Radical Relay for Asymmetric Radical Transformation. Acc. Chem. Res 2018, 51, 2036–2046. [DOI] [PubMed] [Google Scholar]
- (26).For a survey of other examples of radical C–H functionalization reactions, see:; Zhang C; Li Z-L; Gu Q-S; Liu X-Y Catalytic Enantioselective C(sp3)‒H Functionalization Involving Radical Intermediates. Nat. Commun 2021, 12, 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).(a) Vasilopoulos A; Golden DL; Buss JA; Stahl SS Copper-Catalyzed C–H Fluorination/Functionalization Sequence Enabling Benzylic C–H Cross Coupling with Diverse Nucleophiles. Org. Lett 2020, 22, 5753–5757. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Buss JA; Vasilopoulos A; Golden DL; Stahl SS Copper-Catalyzed Functionalization of Benzylic C–H Bonds with N -Fluorobenzenesulfonimide: Switch from C–N to C–F Bond Formation Promoted by a Redox Buffer and Brønsted Base. Org. Lett 2020, 22, 5749–5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).(a) Stanovnik B; Svete J Product Class 1: Pyrazoles. Science of Synthesis; Neier R; Bellus D, Eds.; Thieme: Stuttgart, 2002; Vol. 12, pp 15–225. [Google Scholar]; (b) Ivanova AE; Burgart YV; Saloutin VI; Slepukhin PA; Borisevich SS; Khursan SL Ambident Polyfluoroalkyl-Substituted Pyrazoles in the Methylation Reactions. J. Fluor. Chem 2017, 195, 47–56. [Google Scholar]; (c) Jaćimović Željko. K.; Novaković SB; Bogdanović GA; Kosović M; Libowitzky E; Giester G Crystal Structure of Ethyl 3-(Trifluoromethyl)-1H-Pyrazole-4-Carboxylate, C7H7F3N2O2. Z. Kristallogr.-New Cryst. Struct 2020, 235, 1189–1190. [Google Scholar]
- (29).Huang A; Wo K; Lee SYC; Kneitschel N; Chang J; Zhu K; Mello T; Bancroft L; Norman NJ; Zheng S-L Regioselective Synthesis, NMR, and Crystallographic Analysis of N1-Substituted Pyrazoles. J. Org. Chem 2017, 82, 8864–8872. [DOI] [PubMed] [Google Scholar]
- (30).For selected examples where cations have been used to influence N-regioselectivity, see:; (a) Goikhman R; Jacques TL; Sames D C-H Bonds as Ubiquitous Functionality: A General Approach to Complex Arylated Pyrazoles via Sequential Regioselective C-Arylation and N-Alkylation Enabled by SEM-Group Transposition. J. Am. Chem. Soc 2009, 131, 3042–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang X; Wang Q; Xue Y; Sun K; Wu L; Zhang B An Organoselenium-Catalyzed N1- and N2-selective Aza-Wacker Reaction of Alkenes with Benzotriazoles. Chem. Commun 2020, 56, 4436–4439. [DOI] [PubMed] [Google Scholar]
- (31).Hilpert LJ; Sieger SV; Haydl AM; Breit B Palladium- and Rhodium-Catalyzed Dynamic Kinetic Resolution of Racemic Internal Allenes Towards Chiral Pyrazoles. Angew. Chem. Int. Ed 2019, 58, 3378–3381. [DOI] [PubMed] [Google Scholar]
- (32).Jones CD; Winter MA; Hirsch KS; Stamm N; Taylor HM; Holden HE; Davenport JD; Krumkalns EV; Suhr RG Estrogen Synthetase Inhibitors. 2. Comparison of the in Vitro Aromatase Inhibitory Activity for a Variety of Nitrogen Heterocycles Substituted with Diarylmethane or Diarylmethanol Groups. J. Med. Chem 1990, 33, 416–429. [DOI] [PubMed] [Google Scholar]
- (33).Wang L; Doherty G; Wang X; Tao Z-F; Brunko M; Kunzer AR; Wendt MD; Song X; Frey R; Hansen TM; Sullivan GM; Judd A; Souers A Apoptosis-Inducing Agents for the Treatment of Cancer and Immune and Autoimmune Diseases. Worldwide patent2013055895 A1, April18, 2013.
- (34).Zhi L; Grote M; Reddy RK; Li W; Craigo W Glucagon Receptor Antagonists. Worldwide patent2018035172 A1, February22, 2018.
- (35).(a) Gangjee A Tricyclic Compounds Having Antimitotic and/or Antitumor Activity and Method of Use Thereof. Worldwide patent2010006032A1, January14, 2010.; (b) Aponte JC; Vaisberg AJ; Castillo D; Gonzalez G; Estevez Y; Arevalo J; Quiliano M; Zimic M; Verástegui M; Málaga E; Gilman RH; Bustamante JM; Tarleton RL; Wang Y; Franzblau SG; Pauli GF; Sauvain M; Hammond GB Trypanoside, Anti-Tuberculosis, Leishmanicidal, and Cytotoxic Activities of Tetrahydrobenzothienopyrimidines. Bioorg. & Med. Chem 2010, 18, 2880–2886. [DOI] [PubMed] [Google Scholar]
- (36).Schepers G Benzbromarone Therapy in Hyperuricaemia; Comparison with Allopurinol and Probenecid. J. Int. Med. Res 1981, 9, 511–515. [DOI] [PubMed] [Google Scholar]
- (37).Smith HW Cyclopentapyrazole and tetrahydroindazole compounds. Worldwide patent8607357, December18, 1986.
- (38).Fushimi N; Yonekubo S; Ohno K; Miyagi T Nitrogen-Containing Fused Ring Derivatives, Pharmaceutical Compositions Containing Them and Their Pharmaceutical Use. Japan patent5308342B2, October9, 2013.
- (39).Shu S; Cai X; Li J; Feng Y; Dai A; Wang J; Yang D; Wang M-W; Liu H Design, Synthesis, Structure–Activity Relationships, and Docking Studies of Pyrazole-Containing Derivatives as a Novel Series of Potent Glucagon Receptor Antagonists. Bioorg. & Med. Chem 2016, 24, 2852–2863. [DOI] [PubMed] [Google Scholar]
- (40).Indazoles tend to exist as 1H-tautomers:; Minkin VI; Garnovskii AD; Elguero J; Katritzky AR; Denisko OV The Tautomerism of Heterocycles: Five-Membered Rings with Two or More Heteroatoms. In Advances in Heterocyclic Chemistry; Elsevier, 2000; Vol. 76, pp 157–323. [Google Scholar]
- (41).Selected methods that achieved indazole N2-alkylations:; (a) Cheung M; Boloor A; Stafford JA Efficient and Regioselective Synthesis of 2-Alkyl-2H-indazoles. J. Org. Chem 2003, 68, 4093–4095. [DOI] [PubMed] [Google Scholar]; (b) Luo G; Chen L; Dubowchik G Regioselective Protection at N-2 and Derivatization at C-3 of Indazoles. J. Org. Chem 2006, 71, 5392–5395. [DOI] [PubMed] [Google Scholar]; (c) Slade DJ; Pelz NF; Bodnar W; Lampe JW; Watson PS Indazoles: Regioselective Protection and Subsequent Amine Coupling Reactions. J. Org. Chem 2009, 74, 6331–6334. [DOI] [PubMed] [Google Scholar]
- (42).Wang X; Zhang L; Krishnamurthy D; Senanayake CH; Wipf P General Solution to the Synthesis of N-2-Substituted 1,2,3-Triazoles Org. Lett 2010, 12, 4632–4635. [DOI] [PubMed] [Google Scholar]
- (43).(a) Gordon EM; Barrett RW; Dower WJ; Fodor SPA; Gallop MA Applications of Combinatorial Technologies to Drug Discovery. 2. Combinatorial Organic Synthesis, Library Screening Strategies, and Future Directions. J. Med. Chem 1994, 37, 1385–1401. [DOI] [PubMed] [Google Scholar]; (b) Thompson LA; Ellman JA Synthesis and Applications of Small Molecule Libraries. Chem. Rev 1996, 96, 555–600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


