Abstract
Objectives
To determine s.c. tocilizumab (s.c.-TCZ) dosing regimens for systemic JIA (sJIA) and polyarticular JIA (pJIA).
Methods
In two 52-week phase 1 b trials, s.c.-TCZ (162 mg/dose) was administered to sJIA patients every week or every 2 weeks (every 10 days before interim analysis) and to pJIA patients every 2 weeks or every 3 weeks with body weight ≥30 kg or <30 kg, respectively. Primary end points were pharmacokinetics, pharmacodynamics and safety; efficacy was exploratory. Comparisons were made to data from phase 3 trials with i.v. tocilizumab (i.v.-TCZ) in sJIA and pJIA.
Results
Study participants were 51 sJIA patients and 52 pJIA patients aged 1–17 years who received s.c.-TCZ. Steady-state minimum TCZ concentration (Ctrough) >5th percentile of that achieved with i.v.-TCZ was achieved by 49 (96%) sJIA and 52 (100%) pJIA patients. In both populations, pharmacodynamic markers of disease were similar between body weight groups. Improvements in Juvenile Arthritis DAS-71 were comparable between s.c.-TCZ and i.v.-TCZ. By week 52, 53% of sJIA patients and 31% of pJIA patients achieved clinical remission on treatment. Safety was consistent with that of i.v.-TCZ except for injection site reactions, reported by 41.2% and 28.8% of sJIA and pJIA patients, respectively. Infections were reported in 78.4% and 69.2% of patients, respectively. Two sJIA patients died; both deaths were considered to be related to TCZ.
Conclusion
s.c.-TCZ provides exposure and risk/benefit profiles similar to those of i.v.-TCZ. S.c. administration provides an alternative administration route that is more convenient for patients and caregivers and that has potential for in-home use.
Trial registration
ClinicalTrials.gov, http://clinicaltrials.gov, NCT01904292 and NCT01904279
Keywords: autoinflammatory conditions, biologic therapies, cytokines and inflammatory mediators, inflammation, juvenile idiopathic arthritis
Rheumatology key messages
Subcutaneous tocilizumab dosing in JIA was determined by bridging to data for intravenous tocilizumab.
Subcutaneously administered tocilizumab has a risk/benefit profile similar to that of intravenous tocilizumab dosing regimens.
Subcutaneous tocilizumab provides an alternative administration route that is more convenient for patients and caregivers.
Introduction
JIA is classified into mutually exclusive categories [1–3]. Children with polyarticular JIA (pJIA) have a more refractory disease course than those who have fewer affected joints, and they are at risk for severe disability [4, 5]. Systemic JIA (sJIA) is characterized by autoinflammatory and autoimmune disease features [6, 7].
IL-6 plays a central role in the pathogenesis of JIA, and levels correlate positively with the severity of joint involvement, systemic disease features, and markers of inflammation such as CRP [8–11]. Tocilizumab (TCZ) is a humanized monoclonal anti–IL-6 receptor (IL-6R)-alpha antibody that inhibits IL-6 function [12, 13]. Phase 3 randomized controlled trials demonstrated that i.v.-TCZ was an effective treatment for sJIA [14], pJIA [15] and RA [16, 17]. In RA, s.c.-TCZ has efficacy and safety comparable with those of i.v.-TCZ [18, 19]. S.c. injections offer a convenient alternative to i.v. administration by allowing administration by the patient or caregiver, eliminating the inconvenience of short hospital admission for infusions and reducing the stress of i.v. administration, which is particularly relevant for younger children [20, 21].
We report results of two clinical trials in children with sJIA and pJIA conducted to identify optimal dosing regimens of s.c.-TCZ.
Patients and methods
Study designs
The sJIA trial (ClinicalTrials.gov, NCT01904292) and the pJIA trial (ClinicalTrials.gov, NCT01904279) were 52-week, open-label, multicentre, pharmacokinetic (PK), pharmacodynamic (PDy) and safety phase 1 b studies designed to identify s.c.-TCZ dosing regimens that would achieve TCZ exposure [steady-state minimum TCZ concentration (Ctrough)] comparable with that of i.v.-TCZ dosing regimens [14, 15]. Based on PK analyses from i.v.-TCZ trials in sJIA and pJIA (Supplementary Data S1, available at Rheumatology online), a dose of 162 mg s.c.-TCZ once weekly [QW; 162 mg every 10 days (Q10D) for patients weighing <30 kg] for sJIA and 162 mg s.c.-TCZ every 2 weeks [Q2W; 162 mg every 3 weeks (Q3W) for patients weighing <30 kg] for pJIA was expected to provide exposures comparable with those of i.v.-TCZ. CONSORT [22] reporting was followed as applicable. A sample size of 48 patients in each study was considered adequate to provide ≥80% power to have the 95% CI fall within 60% and 140% of the population mean estimates for the PK parameters in paediatric age groups [23].
Patients
Children aged 1–17 years (12–17 years in Russia) with sJIA or pJIA (RF-positive or RF-negative polyarticular and extended oligoarticular JIA) according to the ILAR criteria were eligible [24]. sJIA patients were enrolled from 15 August 2013 through 28 June 2016 at 26 centres, and pJIA patients were enrolled from 24 July 2013 through 27 May 2015 at 24 centres in 11 countries in the Paediatric Rheumatology INternational Trials Organisation (PRINTO) and the Paediatric Rheumatology Collaborative Study Group (PRCSG) networks [25, 26].
Patients in the sJIA trial had inadequate responses to NSAIDs and glucocorticoids. Patients in the pJIA trial had inadequate responses or intolerance to MTX. Eligible patients were TCZ-naive or had achieved well-controlled sJIA or pJIA with i.v.-TCZ (TCZ-prior). Patients for whom i.v.-TCZ was discontinued for lack of efficacy or safety reasons were excluded. Full eligibility criteria are in Supplementary Data S2, available at Rheumatology online. To adequately characterize the absorption of s.c.-TCZ, ≥50% of the total population was TCZ-naive at baseline. TCZ-prior patients received their first dose of s.c.-TCZ at their next scheduled date of i.v.-TCZ. Concomitant treatment with oral glucocorticoids (doses at the investigator’s discretion), NSAIDs and non-biologic DMARDs, including MTX, was allowed. Both studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice, and informed consent was obtained from all patients and/or parents. The protocols were approved by institutional review boards or independent ethics committees at each site.
Assessments
Primary PK and PDy objectives were to evaluate s.c.-TCZ in patients with sJIA or pJIA to identify fixed-dosing regimens of TCZ 162 mg that provide TCZ exposures similar to those achieved with i.v.-TCZ. Blood samples for PK/PDy analysis were obtained per protocol (Supplementary Data S3, available at Rheumatology online). Safety assessments included adverse events (AEs), serious AEs (SAEs) and laboratory parameters. AEs were classified using the Medical Dictionary for Regulatory Activities 20.0 (sJIA) or 19.0 (pJIA). Efficacy was an exploratory objective. Anti-TCZ antibodies were assayed at prespecified intervals with a tiered testing strategy [27].
Exploratory efficacy measures included disease activity measured by Juvenile Arthritis DAS including 71 joints (JADAS-71) (cut-off scores for pJIA: high disease activity, >10.5; moderate disease activity, 3.9–10.5; low disease activity, ≤3.8; inactive disease, ≤1) [28–31], proportions of patients with inactive disease (JIA ACR inactive disease criteria) [32] and clinical remission on treatment (inactive disease for ≥6 months continuously), functional ability using the Childhood HAQ–Disability Index (CHAQ-DI; range, 0–3) [33] and growth measured using height velocity (cm/year).
Statistical analysis
Descriptive statistical analyses were planned. Population PK models previously developed for i.v.-TCZ in sJIA and pJIA were used to delineate the weight-based s.c.-TCZ dose regimens. Subsequently, individual model–computed PK parameters were estimated after data were incorporated from the s.c.-TCZ trials [Ctrough, area under the concentration-time curve (AUC), and maximum concentration (Cmax) at steady-state]. Dense sampling after the first s.c.-TCZ dose allowed for characterization of absorption parameters.
At the week 14 interim analysis, a dosing change from Q10D to Q2W was introduced for sJIA patients weighing <30 kg, because the Q10D regimen resulted in exposures higher than those observed with i.v.-TCZ for some patients.
All enrolled patients who received ≥1 dose of s.c.-TCZ were included in the PK/PDy, safety and intention-to-treat analysis populations.
Results
Patients
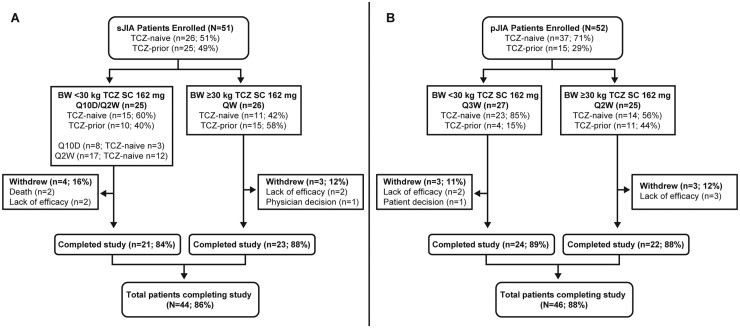
Among 51 sJIA patients, 44 (86%) completed 52 weeks (Fig. 1A), 4 withdrew for lack of efficacy, 1 withdrew based on the physician’s decision (because of persistently low neutrophil counts) and 2 died. Among 52 pJIA patients (Fig. 1B), 46 (89%) completed 52 weeks, 5 withdrew for lack of efficacy and 1 withdrew based on the patient’s decision.
Fig. 1.
Patient disposition in the trial of (A) patients with sJIA and (B) patients with pJIA
Withdrawal by patient: TCZ-naive, n = 1. Withdrawal because of lack of efficacy: TCZ-naive, n = 4; TCZ-prior, n = 1. BW: body weight; Q10D: every 10 days; QW: every week; Q2W: every 2 weeks; TCZ: tocilizumab; pJIA: polyarticular JIA; sJIA: systemic JIA.
Demographic and disease characteristics at baseline were balanced between body weight groups in both studies (Table 1). Four patients were younger than 2 years of age at baseline—three in the sJIA study (aged 17, 19 and 22 months) and one in the pJIA study (aged 23 months). Demographics of TCZ-naive patients with sJIA and pJIA were similar to those in the TCZ-prior groups, except for higher JADAS-71 and CHAQ-DI scores at baseline (Table 1).
Table 1.
Baseline demographics and disease characteristics of patients with sJIA and patients with pJIA
| Characteristic | sJIA |
pJIA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCZ-naive n = 26 |
TCZ-prior n = 25 |
All TCZ n = 51 |
TCZ-naive n = 37 |
TCZ-prior n = 15 |
All TCZ n = 52 |
|||||||
| <30 kg Q10D/Q2W n = 15 | ≥30 kg QW n = 11 | <30 kg Q10D/Q2W n = 10 | ≥30 kg QW n = 15 | <30 kg Q10D/Q2W n = 25 | ≥30 kg QW n = 26 | <30 kg Q3W n = 23 | ≥30 kg Q2W n = 14 | <30 kg Q3W n = 4 | ≥30 kg Q2W n = 11 | <30 kg Q3W n = 27 | ≥30 kg Q2W n = 25 | |
| Age, years, mean (S.D.) | 5.2 (3.0) | 13.1 (3.2) | 4.9 (3.6) | 13.5 (3.2) | 5.1 (3.2) | 13.3 (3.2) | 5.3 (2.1) | 14.9 (2.2) | 6.8 (1.0) | 12.7 (2.9) | 5.5 (2.1) | 13.9 (2.7) |
| Females, n (%) | 7 (46.7) | 6 (54.5) | 6 (60.0) | 10 (66.7) | 13 (52.0) | 16 (61.5) | 14 (60.9) | 10 (71.4) | 4 (100.0) | 8 (72.7) | 18 (66.7) | 18 (72.0) |
| Weight, kg, mean (S.D.) | 19.1 (5.4) | 52.2 (14.3) | 18.1 (6.4) | 51.3 (12.8) | 18.7 (5.7) | 51.7 (13.2) | 19.2 (4.9) | 60.4 (15.1) | 22.2 (1.3) | 51.9 (15.3) | 19.7 (4.7) | 56.7 (15.5) |
| No. of active joints, mean (S.D.) | 11.6 (11.6) | 6.6 (8.1) | 1.3 (1.8) | 1.2 (2.2) | 7.5 (10.3) | 3.5 (6.0) | 9.4 (7.5) | 11.4 (8.5) | 1.3 (1.9) | 7.0 (11.2) | 8.2 (7.5) | 9.5 (9.8) |
| CHAQ-DI score, mean (S.D.) | 1.28 (0.93) | 0.78 (1.00) | 0.28 (0.42) | 0.45 (0.77) | 0.88 (0.90) | 0.59 (0.88) | 0.95 (0.66) | 0.88 (0.70) | 0.50 (0.51) | 0.59 (0.73) | 0.88 (0.65) | 0.76 (0.72) |
| JADAS-71 score, mean (S.D.) | 23.6 (15.6) | 15.9 (12.4) | 3.2 (3.3) | 3.5 (5.2) | 15.5 (15.8) | 8.7 (10.8) | 19.7 (9.8) | 21.0 (9.9) | 5.9 (7.1) | 11.4 (14.6) | 17.6 (10.6) | 16.8 (12.9) |
| Concurrent MTX use, n (%) | 8 (53.3) | 5 (45.5) | 5 (50.0) | 9 (60.0) | 13 (52.0) | 14 (53.8) | 18 (78.3) | 7 (50.0) | 3 (75.0) | 8 (72.7) | 21 (77.8) | 15 (60.0) |
| Concurrent glucocorticoid use, n (%)a | 14 (93.3) | 8 (72.7) | 6 (60.0) | 4 (26.7) | 20 (80.0) | 12 (46.2) | 11 (47.8) | 6 (42.9) | 0 | 6 (54.5) | 11 (40.7) | 12 (48.0) |
| Previous biologic use, n (%) | 4 (26.7) | 8 (72.7) | 10 (100.0) | 15 (100.0) | 14 (56.0) | 23 (88.5) | 6 (26.1) | 10 (71.4) | 4 (100) | 11 (100) | 10 (37.0) | 21 (84.0) |
CHAQ-DI: Childhood HAQ–Disability Index; JADAS-71: Juvenile Arthritis DAS including 71 joints; pJIA: polyarticular JIA; Q10D: every 10 days; QW: every week; Q2W: every 2 weeks; Q3W: every 3 weeks; sJIA systemic JIA; TCZ: tocilizumab.
Included all prior and concomitant use.
Pharmacokinetics
Ctrough values reached stable levels around week 12 or 14 in TCZ-naive sJIA and pJIA patients newly initiating s.c.-TCZ. Ctrough levels were maintained in TCZ-prior patients on switching to s.c.-TCZ.
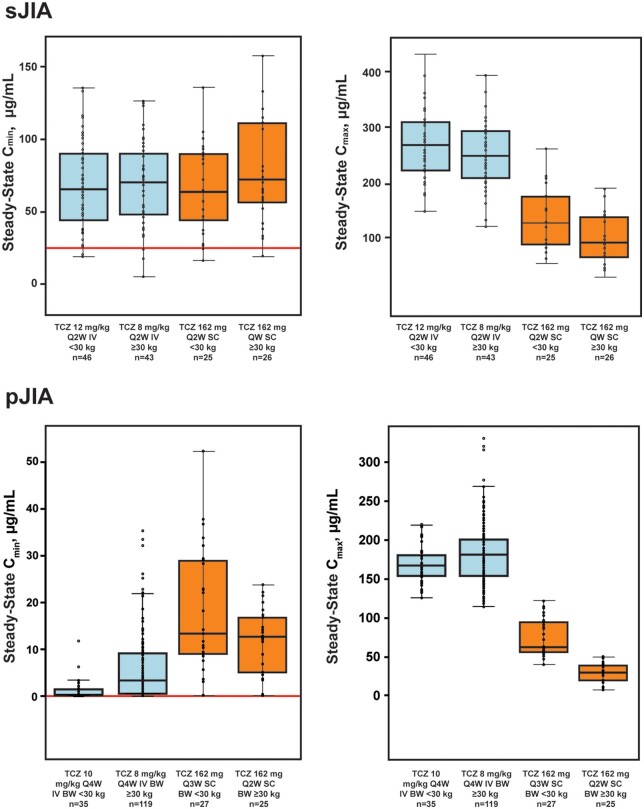
Median steady-state Ctrough levels were similar across body weight groups in sJIA patients after dose adjustment to Q2W in the <30-kg group (<30 kg, 64.2 µg/ml; ≥30 kg, 72.4 µg/ml) and in pJIA patients (<30 kg, 13.4 µg/ml; ≥30 kg, 12.7 µg/ml) (Fig. 2, Supplementary Table S1, available at Rheumatology online). Among TCZ-naive patients with sJIA, median steady-state Ctrough (range) was higher in the <30-kg group treated with s.c.-TCZ Q10D [116 (91.8–256.0) µg/ml] than with s.c.-TCZ Q2W [41.4 (12.8–114.0) µg/ml], which led to dose reduction to Q2W in this group after the interim analysis. For pJIA patients, and to a lesser extent sJIA patients, median steady-state Cmax and overall exposure (AUC) were slightly higher in patients weighing <30 kg than in those weighing ≥30 kg (Fig. 2, Supplementary Fig. S1, Supplementary Table S1, available at Rheumatology online). Comparison of exposure after s.c. vs i.v. administration indicated that, consistent with initial model predictions, a high percentage (96%; 49/51) of sJIA patients and all (100%) pJIA patients treated with s.c.-TCZ had a steady-state Ctrough at or above the 5th percentile of that achieved in the i.v.-TCZ trial across the body weight range (Fig. 2). Similarly, 80.4% (41/51) of sJIA patients and 78.8% (41/52) of pJIA patients had steady-state Ctrough values between the 5th and 95th percentiles of values achieved with i.v.-TCZ. As expected, the Cmax attained with s.c.-TCZ was lower than that attained with i.v.-TCZ (Fig. 2).
Fig. 2.
Model-computed median steady-state Cmin and Cmax from s.c. dosing vs i.v. dosing
Median values are designated by black lines in the centres of the boxes. Boxes indicate the IQR. Whiskers represent 1.5 × IQR. Horizontal red line denotes the model-computed 5th percentile from the i.v.-TCZ trials. The number of i.v.-TCZ sJIA patients includes all patients randomly assigned to TCZ in part 1 of the i.v.-TCZ trial and any patient who escaped from placebo to TCZ in part 1 for whom a PK sample was available. BW: body weight; Cmax: maximum concentration; Cmin: minimum concentration; IQR: interquartile range; PK: pharmacokinetic; QW: every week; Q2W: every 2 weeks; Q3W: every 3 weeks; TCZ: tocilizumab; sJIA: systemic JIA.
Ctrough values in the three sJIA patients aged <2 years were at the higher end of the exposure spectrum (above the 53rd percentile of exposure in the <30-kg group) following treatment with s.c.-TCZ, but were within the range of model-predicted exposures in sJIA patients aged ≥2 years (19.5–158 µg/ml).
Pharmacodynamics
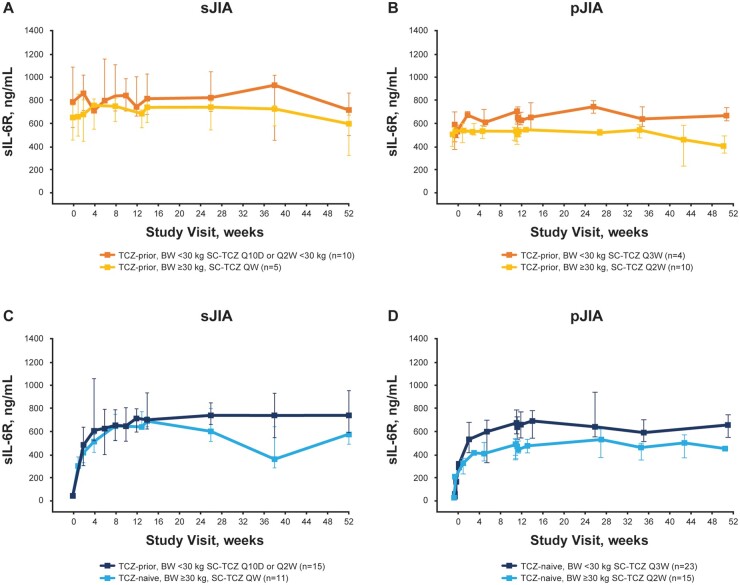
Changes in PDy parameters over time were comparable for TCZ-naive patients initiating treatment with s.c.-TCZ and i.v.-TCZ and remained stable for patients switching from i.v.-TCZ to s.c.-TCZ. Median soluble IL-6R (sIL-6R) serum concentrations increased rapidly in TCZ-naive patients after the first TCZ dose through week 12 and then stabilized between 500 and 800 ng/ml. Among TCZ-prior patients, median sIL-6R concentrations remained stable over time compared with baseline levels. Consistent with TCZ exposures, sIL-6R levels were slightly higher in patients weighing <30 kg than ≥30 kg over the dosing period in both disease populations (Fig. 3). Median IL-6 concentrations stabilized by week 12 (Supplementary Fig. S2, available at Rheumatology online). Median CRP levels and ESR decreased rapidly among TCZ-naive patients with s.c.-TCZ and remained within the normal range reported in the i.v.-TCZ studies [14, 15] among TCZ-prior patients (Supplementary Fig. S3, available at Rheumatology online).
Fig. 3.
sIL-6R concentration-time profiles with s.c.-TCZ treatment of TCZ-prior (A, B) and TCZ-naive (C, D) patients
Data are shown as median (IQR) values. Error bars = IQR. BW: body weight; IQR: interquartile range; Q10D: every 10 days; QW: every week; Q2W: every 2 weeks; Q3W: every 3 weeks; sIL-6R: serum IL-6 receptor; TCZ: tocilizumab.
Exploratory efficacy analysis
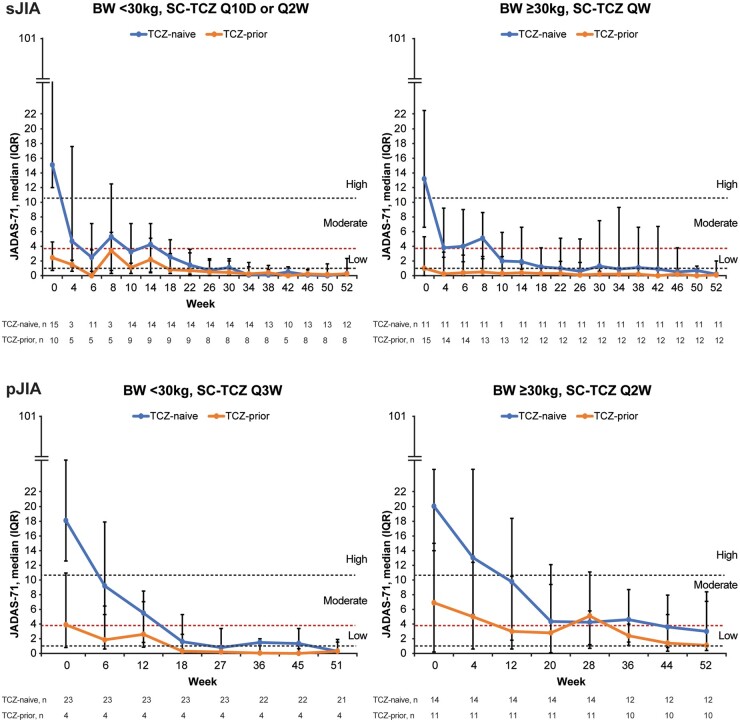
JADAS-71 improved in TCZ-naive sJIA and pJIA patients treated with s.c.-TCZ, similar to improvements observed with i.v.-TCZ, indicating that comparable efficacy was achieved with the s.c. and i.v. formulations (Supplementary Fig. S4, available at Rheumatology online). In addition, JADAS-71 was maintained in TCZ-prior patients (Fig. 4; Supplementary Fig. S4, available at Rheumatology online), indicating that patients could switch from i.v. to s.c. and maintain the same level of efficacy. Among all patients who had efficacy data at week 52, 3 of 43 (7.0%) children with sJIA and 8 of 47 (17.0%) children with pJIA were able to reach a status of moderate disease activity (JADAS-71 3.9–10.5), and 40 of 43 (93.0%) children with sJIA and 35 of 47 (74.5%) children with pJIA were able to reach a status of a low disease activity (≤3.8). By week 52, 68.6% (35/51) of sJIA patients and 63.5% (33/52) of pJIA patients had inactive disease; 52.9% (27/51) of sJIA and 30.8% (16/52) of pJIA patients achieved clinical remission on treatment.
Fig. 4.
JADAS-71 over time for sJIA and pJIA patients treated with s.c.-TCZ
Data are shown as median (IQR) valuesData points at weeks 4 and 8 for the <30kg group (left-hand sJIA panel) include only those for patients receiving Q10D dosing. Horizontal dashed lines represent inactive disease (JADAS-71 < 1.0), low disease activity (≤3.8), moderate disease activity (3.9–10.5) and high disease activity (>10.5). BW: body weight; IQR: interquartile range; JADAS-71: Juvenile Arthritis DAS including 71 joints; pJIA: polyarticular JIA; Q10D: every 10 days; QW: every week; Q2W: every 2 weeks; Q3W: every 3 weeks; s.c.-TCZ: subcutaneous tocilizumab; sJIA: systemic JIA; TCZ: tocilizumab.
The proportion of patients with sJIA receiving glucocorticoid therapy decreased from 27 of 51 (52.9%) at baseline to 7 of 51 (13.7%) at week 52 and from a mean dose of 2.7 mg/kg/day to 0.6 mg/kg/day, respectively, for patients <30 kg and from 0.3 mg/kg/day to 0.1 mg/kg/day, respectively, for patients ≥30 kg. The proportion of pJIA patients receiving glucocorticoid therapy decreased from 17 of 52 (32.7%) at baseline to 5 of 52 (9.6%) at week 52 and from a mean dose of 0.2 mg/kg/day to 0 mg/kg/day, respectively, for patients <30 kg and from 0.2 mg/kg/day to 0.1 mg/kg/day, respectively, for patients ≥30 kg (Supplementary Table S2, available at Rheumatology online). Exploratory analysis of growth showed that the distribution of height velocities was consistent with World Health Organization normative values (Supplementary Fig. S5, available at Rheumatology online) and in line with observations for pJIA and sJIA with i.v.-TCZ [34, 35].
Safety
A total of 46.7 patient-years (PY) of follow-up for sJIA patients was available for safety assessment: 23.0 PY in the <30-kg group and 23.8 PY in the ≥30-kg group. Total follow-up in the pJIA study was 50.4 PY overall: 26.6 PY for the <30-kg group and 23.8 PY for the ≥30-kg group.
Most AEs in both studies were mild or moderate in intensity and were considered unrelated to TCZ treatment. A higher AE rate was observed in patients in the ≥30-kg body weight group than the <30-kg group in both studies: 1378.7/100 PY (95% CI, 1233.5–1536.3) vs 1015.3/100 PY (889.1–1154.3) in sJIA patients and 944.2/100 PY (824.8–1076.0) vs 680.5/100 PY (584.9–787.1) in pJIA patients. Similar AE rates were observed in TCZ-naive patients and TCZ-prior patients with sJIA (1196.2/100 PY and 1205.0/100 PY), whereas in pJIA patients, the AE rate was higher in TCZ-naive patients than in TCZ-prior patients (876.1/100 PY vs 631.0/100 PY). The most common AEs were from the infections and infestations system organ class (Table 2), reported by a higher proportion of patients in the <30-kg group than in the ≥30-kg group (88% vs 69.2%, respectively, for sJIA; 74.1% vs 64%, respectively, for pJIA). Overall, nine SAEs occurred in seven sJIA patients (five serious infections, two sJIA flares, vertigo and pulmonary hemorrhage), and four SAEs occurred in three pJIA patients (croup, varicella, worsening of anorexia and arthralgia) (Supplementary Data S4, available at Rheumatology online). AEs and SAEs among the three sJIA patients who were aged <2 years at the time of enrolment were consistent with those observed in the older sJIA patients. No serious or clinically significant hypersensitivity reactions and no cases of anaphylaxis or macrophage activation syndrome, gastrointestinal perforations, serious hepatic AEs, malignancies, serious myocardial infarctions, opportunistic infections or serious strokes were reported during this study.
Table 2.
Safety profile of s.c.-TCZ in patients with sJIA and patients with pJIA
| Safety outcomes | sJIA |
pJIA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <30 kg Q10D/Q2W n = 25 |
≥30 kg QW n = 26 |
All TCZ n = 51 |
<30 kg Q3W n = 27 |
≥30 kg Q2W n = 25 |
All TCZ n = 52 |
|||||||
| TCZ- naive n = 15 | TCZ- prior n = 10 | TCZ- naive n = 11 | TCZ- prior n = 15 | TCZ- naive n = 26 | TCZ- prior n = 25 | TCZ- naive n = 23 | TCZ- prior n = 4 | TCZ- naive n = 14 | TCZ- prior n = 11 | TCZ naive n = 37 | TCZ- prior n = 15 | |
| PY of follow-up | 13.9 | 9.1 | 11.2 | 12.6 | 25.1 | 21.7 | 22.6 | 4.0 | 13.3 | 10.5 | 35.8 | 14.6 |
| Patients with ≥1 AE, n (%) | 15 (100) | 10 (100) | 11 (100) | 14 (93.3) | 26 (100) | 24 (96) | 21 (91.3) | 4 (100) | 13 (92.9) | 10 (90.9) | 34 (91.9) | 14 (93.3) |
| Total AEs, n | 131 | 102 | 169 | 159 | 300 | 261 | 167 | 14 | 147 | 78 | 314 | 92 |
| Patients with ≥1 SAE, n (%) | 3 (20) | 2 (20) | 1 (9.1) | 1 (6.7) | 4 (15.4) | 3 (12) | 1 (4.3) | 0 | 1 (7.1) | 1 (9.1) | 2 (5.4) | 1 (6.7) |
| AEs by system organ class, n (%)a | ||||||||||||
| Infections and infestationsb | 12 (80)e | 10 (100) | 9 (81.8) | 9 (60) | 21 (80.8) | 19 (76) | 18 (78.3) | 2 (50) | 9 (64.3) | 7 (63.6) | 27 (73) | 9 (60) |
| Musculoskeletal and CTDs | 3 (20) | 4 (40) | 3 (27.3) | 4 (26.7) | 6 (23.1) | 8 (32) | 11 (47.8) | 0 | 7 (50.0) | 5 (45.5) | 18 (48.6) | 5 (33.3) |
| Gastrointestinal disordersc | 5 (33.3) | 6 (60) | 3 (27.3) | 9 (60) | 8 (30.8) | 15 (60) | 8 (34.8) | 2 (50.0) | 7 (50.0) | 4 (36.4) | 15 (40.5) | 6 (40.0) |
| General disorders and administrative site conditions | 5 (33.3) | 4 (40) | 6 (54.5) | 12 (80) | 11 (42.3) | 16 (64) | 4 (17.4) | 2 (50.0) | 9 (64.3) | 5 (45.5) | 13 (35.1) | 7 (46.7) |
| Respiratory, thoracic and mediastinal disorders | 9 (60) | 4 (40) | 6 (54.5) | 6 (40) | 15 (57.7) | 10 (40) | 10 (43.5) | 1 (25.0) | 1 (7.1) | 6 (54.5) | 11 (29.7) | 7 (46.7) |
| Skin and s.c. tissue disorders | 5 (33.3) | 3 (30) | 6 (54.5) | 1 (6.7) | 11 (42.3) | 4 (16) | 5 (21.7) | 0 | 5 (35.7) | 2 (18.2) | 10 (27.0) | 2 (13.3) |
| Nervous system disorders | 0 | 0 | 2 (18.2) | 5 (33.3) | 2 (7.7) | 5 (20.0) | 2 (8.7) | 0 | 5 (35.7) | 3 (27.3) | 7 (18.9) | 3 (20.0) |
| Psychiatric disordersd | 0 | 0 | 1 (9.1) | 0 | 1 (3.8) | 0 | 3 (13.0) | 1 (25.0) | 3 (21.4) | 1 (9.1) | 6 (16.2) | 2 (13.3) |
| Blood and lymphatic system disorders | 5 (33.3) | 3 (30) | 4 (36.4) | 6 (40) | 9 (34.6) | 9 (36) | 3 (13.0) | 0 | 1 (7.1) | 0 | 4 (10.8) | 0 |
| Injury, poisoning and procedural complications | 3 (20) | 4 (40) | 5 (45.5) | 3 (20) | 8 (30.8) | 7 (28) | 3 (13.0) | 1 (25.0) | 2 (14.3) | 1 (9.1) | 5 (13.5) | 2 (13.3) |
| Investigations | 2 (13.3) | 3 (30) | 2 (18.2) | 1 (6.7) | 4 (15.4) | 4 (16) | 4 (17.4) | 0 | 2 (14.3) | 0 | 6 (16.2) | 0 |
Multiple occurrences of the same event in a patient were counted once.
AE: adverse event; BW: body weight; pJIA: polyarticular JIA; PY: patient years; Q10D: every 10 days; QW: every week; Q2W: every 2 weeks; Q3W: every 3 weeks; SAE: serious adverse event; sJIA: systemic JIA; SOC: system organ class; TCZ: tocilizumab.
SOC included if all-grade AEs within that SOC occurred in ≥15% of sJIA patients or pJIA patients overall.
The most common infections (≥10% of all patients) were nasopharyngitis (34.6%), gastroenteritis (11.5%) and upper respiratory tract infections (9.6%) in pJIA patients and viral upper respiratory tract infection (25.5%), upper respiratory tract infection (21.6%) and rhinitis (11.8%) in sJIA patients.
No patients experienced gastrointestinal perforations.
The most common psychiatric disorder in pJIA patients was insomnia (four TCZ-naive patients). Others included depression (one TCZ-naive patient) and fear of injections (one patient who previously received TCZ).
One patient was receiving QW dosing at the time of infection (serious sepsis infection that was fatal) after a body weight increase to ≥30 kg.
Two patients with sJIA died, both in the <30-kg group. Both deaths were considered related to TCZ. A TCZ-naive patient had oral candidiasis and pneumonia and died of pulmonary hemorrhage on day 15 after receiving a single TCZ dose on day 1, and another patient died of suspected sepsis on day 262. Both patients were receiving concomitant steroids and other medications (Supplementary Data S4, available at Rheumatology online). No deaths occurred during the pJIA study.
The AE profile for s.c.-TCZ was comparable with that observed with the i.v.-TCZ regimens in sJIA and pJIA (Supplementary Table S3, available at Rheumatology online) except for injection site reactions (ISRs), which are applicable only for patients receiving s.c.-TCZ. Overall, 41.2% of sJIA patients treated with s.c.-TCZ reported ≥1 ISR (20% of patients weighing <30 kg, 61.5% of patients weighing ≥30 kg), as did 28.8% of pJIA patients (14.8% of patients weighing <30 kg, 44.0% of patients weighing ≥30 kg). All ISRs were considered non-serious, and none required withdrawal from treatment. There were no serious or clinically significant hypersensitivity reactions (defined as hypersensitivity reactions leading to withdrawal) or confirmed anaphylaxis (Supplementary Data S4, available at Rheumatology online). Laboratory abnormalities were consistent with those expected in children treated with TCZ—most commonly, decrease in neutrophil count (sJIA, 54.9%; pJIA, 42.3%; all grade ≤3), elevated alanine aminotransferase levels (sJIA, 33.3%; pJIA 38.5%; all grade ≤3 except one grade 4 for sJIA) and elevated aspartate aminotransferase levels (sJIA, 23.5%; pJIA 25.0%; all grade ≤3) (Supplementary Data S5, available at Rheumatology online).
No patients with sJIA developed detectable anti-TCZ antibodies. Among pJIA patients, three who were TCZ-naive (one weighing <30 kg, two weighing ≥30 kg) developed anti-TCZ antibodies after baseline, but not of the immunoglobulin E isotype. One of these patients was withdrawn because of lack of efficacy (treating physician decision). One patient who developed anti-TCZ antibodies after baseline had injection site hematomas on days 2 and 16. The other two patients did not experience ISRs.
Discussion
Bridging of PK and PDy to efficacy data is recognized by the US Food and Drug Administration (FDA) [36] and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use [37] to support new dose regimens, new dosage forms and new formulations and routes of administration based on known exposure–response relationships from existing clinical trial data as particularly relevant for children. In the s.c.-TCZ trials, 162 mg QW dosing for sJIA patients weighing ≥30 kg (162 mg Q2W for patients <30 kg) and Q2W dosing for pJIA patients weighing ≥30 kg (162 mg Q3W for patients <30 kg) provided TCZ exposures similar to those of approved i.v.-TCZ regimens and resulted in comparable PDy, safety and exploratory efficacy. Based on these data, the FDA and the European Medicines Agency approved these s.c. dosing regimens for the treatment of sJIA and pJIA [38, 39].
A relationship between TCZ predose concentration (Ctrough or Cmin, as a measure of exposure) and efficacy has been established for patients with sJIA (data on file) and patients with pJIA [40]. Evaluation and comparison of TCZ exposure after s.c. administration formed the basis of bridging to the pivotal i.v.-TCZ trials in sJIA or pJIA. Consistent with comparable exposures, PDy responses measured by changes in sIL-6R, IL-6, CRP and ESR were comparable with those observed for i.v.-TCZ regimens. The increase observed in serum sIL-6R is consistent with the formation of sIL-6R/TCZ immune complexes [41]. Some measures of TCZ exposure, and correspondingly sIL-6R levels, were slightly higher in the <30-kg group but had no effect on safety or efficacy. The s.c.-TCZ trials confirmed that starting s.c.-TCZ in sJIA or pJIA patients previously treated with i.v.-TCZ can be achieved safely and effectively with the first s.c.-TCZ dose delivered when the next i.v.-TCZ dose is due.
The safety profile of s.c.-TCZ was consistent with that observed with the i.v.-TCZ regimen for sJIA and pJIA patients [14, 15]. The most common AEs were infections, consistent with the safety profile for TCZ. No new types of AE were observed except for ISRs (consistent with s.c. administration). ISR rates of 291.0/100 PY in sJIA patients and 93.2/100 PY in pJIA patients with s.c.-TCZ were higher than those observed in s.c.-TCZ trials in adult RA patients (11.5–26.1/100 PY) [18, 42] (data on file). Fewer patients in the <30-kg groups (sJIA 20%, pJIA 14.8%) than in the ≥30-kg groups (sJIA 61.5%, pJIA 44.0%) reported ISRs, possibly because of a more limited ability of younger patients to articulate certain ISR symptoms. Consistent with previous observations, there was a low incidence of anti-drug antibodies among pJIA patients and none among sJIA patients. Anti-drug antibodies in pJIA patients did not negatively affect the clinical effectiveness of TCZ. Two patients died in the s.c.-TCZ sJIA trial because of complications following serious infections compared with one death from suspected pneumothorax observed over a similar treatment duration in the i.v.-TCZ sJIA trial [14]; none died in the i.v.-TCZ pJIA trial [15]. Serious and sometimes fatal infections have been reported in patients receiving immunosuppressive therapies, including TCZ, highlighting the vigilance required for timely detection and treatment.
Given the similar PK/PDy results for s.c.-TCZ vs i.v.-TCZ, comparable efficacy can be expected. Patients newly initiating TCZ therapy had efficacy responses with s.c.-TCZ comparable with those observed in i.v.-TCZ sJIA and pJIA studies, and disease control was maintained for patients who switched from i.v.-TCZ to s.c.-TCZ at study entry and began s.c. injections when the next i.v.-TCZ dose was due. Safety assessment is ongoing in patients with sJIA or pJIA treated with s.c.-TCZ in a long-term extension study that includes ≤5 years of s.c.-TCZ treatment (ClinicalTrials.gov, NCT02165345).
The design of the s.c.-TCZ studies in patients with sJIA and pJIA allowed for robust confirmation of optimal s.c.-TCZ dosing regimens of the s.c. formulation. Eligibility criteria were similar to those of the i.v.-TCZ studies, enabling comparison for confirmation of the dosing strategy and of PK, PDy, safety and efficacy. This meant fewer patients had to undergo the clinical trial procedure, which accelerated the approval and subsequent availability of the s.c.-TCZ formulation compared with a traditional phase 3 trial approach. Limiting the number of TCZ-prior patients to <50% of the total population allowed more precise estimation of PK absorption parameters and determination of the onset of PDy effects after s.c. administration during collection of clinical data for patients switching from i.v.-TCZ to s.c.-TCZ.
Both studies had limitations. The number of patients across the body weight spectrum (at individual ages) might have been too small to enable detection of potentially important immunogenicity and safety differences between s.c.-TCZ and i.v.-TCZ. Other limitations include sparse data for children <2 years (n = 3, sJIA; n = 1, pJIA), the open-label nature of s.c.-TCZ administration and the lack of statistical comparisons (all comparisons were between-study and descriptive).
In conclusion, appropriate s.c.-TCZ dosing regimens were successfully identified from studies in patients with sJIA or pJIA that bridged to data from studies of i.v.-TCZ. The overall benefit/risk profile for s.c.-TCZ was favourable and comparable with that for i.v.-TCZ in patients with sJIA and in patients with pJIA. These findings highlight the ability of PK/PDy bridging to provide a path towards regulatory approval of agents for the treatment of paediatric patients.
Supplementary Material
Acknowledgements
The authors thank all additional PRINTO and PRCSG investigators for their participation in the study (see Supplementary Data S6, available at Rheumatology online, for the full list of investigators). Jianmei Wang of Genentech provided input into the conception and design of the study and analysis and interpretation of the data. Ruchi Upmanyu, Mohamed Kamal and Alysha Kadva of Roche Products Ltd provided assistance with study design and analysis and interpretation of the data. The study was designed jointly by the academic authors (N.R., H.I.B., A.M., D.L., andF.D.B.) and Roche, with data collected by PRINTO and PRCSG investigators. The first and subsequent versions of the manuscript were written by N.R., H.I.B. and F.D.B., and it was edited by A.M. and D.L. and revised critically by all remaining coauthors. All authors attest to the completeness and veracity of data and data analyses. Consistency in reporting the study data to healthcare authorities and institutional review boards was ensured by Roche. All authors had full access to the study data, and all authors reviewed and revised the manuscript for important intellectual content and approved the final version to be published. All authors were involved in the decision to submit the manuscript for publication and had the right to accept or reject comments or suggestions. Professional writing and editorial assistance was provided by Sara Duggan, PhD, of ApotheCom, on behalf of F. Hoffmann-La Roche Ltd. Acquisition of data: All PRINTO and PRCSG participating centres. Analysis and interpretation of data: M.B., K.B., H.I.B., F.D.B., W.D., J.C.H., N.L.M., N.R., C.W. and S.W.
Funding: This work was supported by the sponsor, Roche. Funding for manuscript preparation was provided by F. Hoffmann-La Roche Ltd. The sponsor was involved in the study design, analysis and interpretation of data, critical review of the manuscript and decision to submit the manuscript for publication.
Disclosure statement: N.R. – consultancy/honoraria/speaker fees: Ablynx, AbbVie, AstraZeneca-Medimmune, Biogen, Boehringer, Bristol-Myers Squibb, Eli-Lilly, EMD Serono, GlaxoSmithKline, Hoffmann-La Roche, Janssen, Merck, Novartis, Pfizer, R-Pharma, Sanofi, Servier, Sinergie, Sobi, Takeda; travel support: Hoffmann-La Roche; grants to institution: Bristol-Myers Squibb, Eli-Lilly, GlaxoSmithKline, F. Hoffmann-La Roche, Janssen, Novartis, Pfizer, Sobi. H.I.B. – consultancy: Bristol-Myers Squibb, Pfizer, Lilly, R-Pharm, Roche, Novartis, Janssen, EMD Serono, AstraZeneca; consulting fees/honorarium/travel support/review activities/speaker burePfizer; money paid to institution: Pfizer (presentation of abstracts at various national and international meetings), Novartis (development of educational presentations), Roche (development of educational presentations). A.V.R. – consultancy: AbbVie, Eli Lilly, Novartis, UCB; lectures/speaker bureaus: AbbVie, Roche, Sobi, UCB. G.H. – fees to institution: travel support from Roche and grant support for the BiKeR registry. R.C. – consultancy/lectures/speaker bureaus: Bristol-Myers Squibb, Centocor, GlaxoSmithKline, Lilly, Novartis, Pfizer, Roche, Sanofi Aventis. J.A. – consultancy/lectures/speaker bureaus: AbbVie, Pfizer, Roche, Gebro, Novartis, Sobi; grant: AbbVie, Gebro, Novartis, Pfizer, Roche, Sobi; travel support: AbbVie, Novartis, Pfizer, Roche, Sobi; grants to institution: AbbVie, Bristol-Myers Squibb, GlaxoSmithKline, Lilly, Novartis, Novimmune, Pfizer, Roche, Sanofi, Sobi. I.C.P. – expert testimony: Novartis, GlaxoSmithKline, AbbVie, Roche. K.M. – lectures/speaker bureaus: Sanofi, Roche; development of educational presentations: AbbVie; board membership: GlaxoSmithKline, Chugai, Sanofi. H.S. – travel support: Hoffmann-La Roche; grant to institution: Hoffmann-La Roche. M.H. – development of educational presentations: Novartis, Roche; payment to institution: Roche. K.N. – consultancy: Novartis; salary support: Abbott. J.C.H. – employee: Roche; stock/stock options: Roche. S.W. – former employee: Roche Products Ltd. C.W. – employee: Roche Products Ltd; stock/stock options: Roche Products Ltd. K.B. – former employee: Roche/Genentech. W.D. – employee: Roche Products Ltd. M.B. – employee: Roche; patent: subcutaneously administered anti IL-6 receptor antibody. N.L.M. – former employee: Roche. A.M. – consultancy/honoraria: Eli-Lilly, EMD Serono, Janssen, Novartis, Pfizer, AbbVie; consultancy/lectures/speaker bureaus: Eli-Lilly, EMD Serono, Janssen, Novartis, Pfizer, AbbVie; travel support: Hoffmann-LaRoche; grants to institution: Bristol-Myers Squibb, Eli-Lilly, GlaxoSmithKline, F. Hoffmann-La Roche, Janssen, Novartis, Pfizer, Sobi. D.L. – consultancy: Takeda; lectures/speaker bureaus: Genentech. Data Safety and Monitoring Board membership: Forest Research, National Institutes of Health; consultancy/paid to institution: Boehringer Ingelheim, Celgene, GlaxoSmithKline, Hoffmann-La Roche. Novartis, Pfizer, UBC; grants to institution: AbbVie, Bristol-Myers Squibb, Janssen, Novartis, Pfizer, Roche NIH, NIH/NICHD. F.D.B. – grants to institution: Roche, Novimmune, Sobi, Sanofi, Novartis, Pfizer.
Data availability statement
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
References
- 1.Ravelli A, Martini A.. Juvenile idiopathic arthritis. Lancet 2007;369:767–78. [DOI] [PubMed] [Google Scholar]
- 2.Prakken B, Albani S, Martini A.. Juvenile idiopathic arthritis. Lancet 2011;377:2138–49. [DOI] [PubMed] [Google Scholar]
- 3.Martini A, Ravelli A, Avcin T, for the Paediatric Rheumatology International Trials Organization (PRINTO) et al. Toward new classification criteria for juvenile idiopathic arthritis: first steps, Paediatric Rheumatology International Trials Organization International Consensus. J Rheumatol 2019;46:190–7. [DOI] [PubMed] [Google Scholar]
- 4.Guzman J, Oen K, Tucker LB. et al. The outcomes of juvenile idiopathic arthritis in children managed with contemporary treatments: results from the ReACCh-Out cohort. Ann Rheum Dis 2015;74:1854–60. [DOI] [PubMed] [Google Scholar]
- 5.Oen K, Guzman J, Dufault B, the Research in Arthritis in Canadian Children emphasizing Outcomes (ReACCh-Out) investigators et al. Health-related quality of life in an inception cohort of children with juvenile idiopathic arthritis: a longitudinal analysis. Arthritis Care Res 2018;70:134–44. [DOI] [PubMed] [Google Scholar]
- 6.Grevich S, Shenoi S.. Update on the management of systemic juvenile idiopathic arthritis and role of IL-1 and IL-6 inhibition. Adolesc Health Med Ther 2017;8:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canny S, Mellins E.. New frontiers in the treatment of systemic juvenile idiopathic arthritis. F1000Res 2017;6:971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Benedetti F, Massa M, Robbioni P. et al. Correlation of serum interleukin-6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Rheum 1991;34:1158–63. [DOI] [PubMed] [Google Scholar]
- 9.De Benedetti F, Robbioni P, Massa M. et al. Serum interleukin-6 levels and joint involvement in polyarticular and pauciarticular juvenile chronic arthritis. Clin Exp Rheumatol 1992;10:493–8. [PubMed] [Google Scholar]
- 10.Mangge H, Kenzian H, Gallistl S. et al. Serum cytokines in juvenile rheumatoid arthritis: correlation with conventional inflammation parameters and clinical subtypes. Arthritis Rheum 1995;38:211–20. [DOI] [PubMed] [Google Scholar]
- 11.De Benedetti F, Pignatti P, Gerloni V. et al. Differences in synovial fluid cytokine levels between juvenile and adult rheumatoid arthritis. J Rheumatol 1997;24:1403–9. [PubMed] [Google Scholar]
- 12.Mihara M, Kasutani K, Okazaki M. et al. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int Immunopharmacol 2005;5:1731–40. [DOI] [PubMed] [Google Scholar]
- 13.Rose-John S.IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci 2012;8:1237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Benedetti F, Brunner HI, Ruperto N. et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med 2012;367:2385–95. [DOI] [PubMed] [Google Scholar]
- 15.Brunner HI, Ruperto N, Zuber Z, for the Paediatric Rheumatology International Trials Organisation (PRINTO) and the Paediatric Rheumatology Collaborative Study Group (PRCSG) et al. Efficacy and safety of tocilizumab in patients with polyarticular-course juvenile idiopathic arthritis: results from a phase 3, randomised, double-blind withdrawal trial. Ann Rheum Dis 2015;74:1110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smolen JS, Beaulieu A, Rubbert-Roth A. et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008;371:987–97. [DOI] [PubMed] [Google Scholar]
- 17.Genovese MC, McKay JD, Nasonov EL. et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 2008;58:2968–80. [DOI] [PubMed] [Google Scholar]
- 18.Burmester GR, Rubbert-Roth A, Cantagrel A. et al. Efficacy and safety of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional DMARDs in patients with RA at week 97 (SUMMACTA). Ann Rheum Dis 2016;75:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Besada E.Potential patient benefit of a subcutaneous formulation of tocilizumab for the treatment of rheumatoid arthritis: a critical review. Patient Prefer Adherence 2014;8:1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barton JL.Patient preferences and satisfaction in the treatment of rheumatoid arthritis with biologic therapy. Patient Prefer Adherence 2009;3:335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chilton F, Collett RA.. Treatment choices, preferences and decision-making by patients with rheumatoid arthritis. Musculoskeletal Care 2008;6:1–14. [DOI] [PubMed] [Google Scholar]
- 22.Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Jadhav PR, Lala M, Gobburu JV.. Clarification on precision criteria to derive sample size when designing paediatric pharmacokinetic studies. J Clin Pharmacol 2012;52:1601–6. [DOI] [PubMed] [Google Scholar]
- 24.Petty RE, Southwood TR, Manners P, International League of Associations for Rheumatology et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. [PubMed] [Google Scholar]
- 25.Ruperto N, Martini A.. Networking in paediatrics: the example of the Paediatric Rheumatology International Trials Organisation (PRINTO). Arch Dis Child 2011;96:596–601. [DOI] [PubMed] [Google Scholar]
- 26.Brunner HI, Rider LG, Kingsbury DJ, for the PRCSG Advisory Council et al. Paediatric Rheumatology Collaborative Study Group—over four decades of pivotal clinical drug research in paediatric rheumatology. Pediatr Rheumatol Online J 2018;16:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stubenrauch K, Wessels U, Birnboeck H. et al. Subset analysis of patients experiencing clinical events of a potentially immunogenic nature in the pivotal clinical trials of tocilizumab for rheumatoid arthritis: evaluation of an antidrug antibody ELISA using clinical adverse event-driven immunogenicity testing. Clin Ther 2010;32:1597–609. [DOI] [PubMed] [Google Scholar]
- 28.Consolaro A, Ruperto N, Bazso A, Paediatric Rheumatology International Trials Organisation et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum 2009;61:658–66. [DOI] [PubMed] [Google Scholar]
- 29.Consolaro A, Bracciolini G, Ruperto N, for the Paediatric Rheumatology International Trials Organization et al. Remission, minimal disease activity, and acceptable symptom state in juvenile idiopathic arthritis: defining criteria based on the juvenile arthritis disease activity score. Arthritis Rheum 2012;64:2366–74. [DOI] [PubMed] [Google Scholar]
- 30.Consolaro A, Ruperto N, Bracciolini G, for the Paediatric Rheumatology International Trials Organization (PRINTO) et al. Defining criteria for high disease activity in juvenile idiopathic arthritis based on the juvenile arthritis disease activity score. Ann Rheum Dis 2014;73:1380–3. [DOI] [PubMed] [Google Scholar]
- 31.Consolaro A, Negro G, Chiara Gallo M. et al. Defining criteria for disease activity states in nonsystemic juvenile idiopathic arthritis based on a three-variable juvenile arthritis disease activity score. Arthritis Care Res 2014;66:1703–9. [DOI] [PubMed] [Google Scholar]
- 32.Wallace CA, Giannini EH, Huang B, Itert L, Ruperto N, Childhood Arthritis Rheumatology Research Alliance (CARRA) American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res 2011;63:929–36. [DOI] [PubMed] [Google Scholar]
- 33.Ruperto N, Ravelli A, Pistorio A. et al. Cross-cultural adaptation and psychometric evaluation of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ) in 32 countries: review of the general methodology. Clin Exp Rheumatol 2001;19:S1–9. [PubMed] [Google Scholar]
- 34.De Benedetti F, Brunner H, Ruperto N, for the Paediatric Rheumatology International Trials Organisation and the Paediatric Rheumatology Collaborative Study Group et al. Catch-up growth during tocilizumab therapy for systemic juvenile idiopathic arthritis: results from a phase III trial. Arthritis Rheumatol 2015;67:840–8. [DOI] [PubMed] [Google Scholar]
- 35.Bharucha KN, Brunner HI, Calvo Penades I, for the Paediatric Rheumatology International Trials Organisation and the Paediatric Rheumatology Collaborative Study Group et al. Growth during tocilizumab therapy for polyarticular-course juvenile idiopathic arthritis: 2-year data from a phase III clinical trial. J Rheumatol 2018;45:1173–9. [DOI] [PubMed] [Google Scholar]
- 36.US Food and Drug Administration. Guidance for Industry: Exposure–Response Relationships—Study Design, Data Analysis, and Regulatory Applications. Rockville, MD, April 2003. https://www.fda.gov/media/71277/download. (30 June 2020, date last accessed).
- 37.European Medicines Agency. ICH Topic E 5 (R1): Ethnic Factors in the Acceptability of Foreign Clinical Data. London, 1998. https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-5-r1-ethnic-factors-acceptability-foreign-clinical-data-step-5_en.pdf. (30 June 2020, date last accessed).
- 38.Genentech, Inc. ACTEMRA® (tocilizumab) injection, for intravenous or subcutaneous use. South San Francisco, CA: Genentech, Inc, December 2018. [Google Scholar]
- 39.Roche Products Ltd. RoActemra 20mg/ml concentrate for solution for infusion. Hertfordshire, UK: Roche Products Ltd, November 2018. [Google Scholar]
- 40.Zhang X, Chen YC, Terao K.. Clinical pharmacology of tocilizumab for the treatment of polyarticular-course juvenile idiopathic arthritis. Expert Rev Clin Pharmacol 2017;10:471–82. [DOI] [PubMed] [Google Scholar]
- 41.Nishimoto N, Terao K, Mima T. et al. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 2008;112:3959–64. [DOI] [PubMed] [Google Scholar]
- 42.Kivitz A, Wallace T, Olech E. et al. Long-term safety and efficacy of subcutaneously administered tocilizumab for adult rheumatoid arthritis: a multicenter phase 3b long-term extension study. Rheumatol Ther 2016;3:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).