Abstract
Background
Bronchodilators are a central component for treating exacerbations of chronic obstructive pulmonary disease (COPD) all over the world. Clinicians often use nebulisers as a mode of delivery, especially in the acute setting, and many patients seem to benefit from them. However, evidence supporting this choice from systematic analysis is sparse, and available data are frequently biased by the inclusion of asthma patients. Therefore, there is little or no formal guidance regarding the mode of delivery, which has led to a wide variation in practice between and within countries and even among doctors in the same hospital. We assessed the available randomised controlled trials (RCTs) to help guide practice in a more uniform way.
Objectives
To compare the effects of nebulisers versus pressurised metered dose inhalers (pMDI) plus spacer or dry powder inhalers (DPI) in bronchodilator therapy for exacerbations of COPD.
Search methods
We searched the Cochrane Airways Group Trial Register and reference lists of articles up to 1 July 2016.
Selection criteria
RCTs of both parallel and cross‐over designs. We included RCTs during COPD exacerbations, whether measured during hospitalisation or in an outpatient setting. We excluded RCTs involving mechanically ventilated patients due to the different condition of both patients and airways in this setting.
Data collection and analysis
Two review authors independently assessed studies for inclusion, extracted data and assessed the risk of bias. We report results with 95% confidence intervals (CIs).
Main results
This review includes eight studies with a total of 250 participants comparing nebuliser versus pMDI plus spacer treatment. We identified no studies comparing DPI with nebulisers. We found two studies assessing the primary outcome of 'change in forced expiratory volume in one second (FEV1) one hour after dosing'. We could not pool these studies, but both showed a non‐significant difference in favour of the nebuliser group, with similar frequencies of serious adverse events. For the secondary outcome, 'change in FEV1 closest to one hour after dosing': we found a significant difference of 83 ml (95% CI 10 to 156, P = 0.03) in favour of nebuliser treatment. For the secondary outcome of adverse events, we found a non‐significant odds ratio of 1.65 (95% CI 0.42 to 6.48) in favour of the pMDI plus spacer group.
Authors' conclusions
There is a lack of evidence in favour of one mode of delivery over another for bronchodilators during exacerbations of COPD. We found no difference between nebulisers versus pMDI plus spacer regarding the primary outcomes of FEV1 at one hour and safety. For the secondary outcome 'change in FEV1 closest to one hour after dosing' during an exacerbation of COPD, we found a greater improvement in FEV1 when treating with nebulisers than with pMDI plus spacers.
A limited amount of data are available (eight studies involving 250 participants). These studies were difficult to pool, of low quality and did not provide enough evidence to favour one mode of delivery over another. No data of sufficient quality have been published comparing nebulisers versus DPIs in this setting. More studies are required to assess the optimal mode of delivery during exacerbations of COPD.
Plain language summary
Bronchodilators delivered by nebuliser versus inhalers for lung attacks of chronic obstructive pulmonary disease
Review question
When someone is suffering from a lung attack due to chronic obstructive pulmonary disease (COPD), are inhalers with a spacer as good as nebulisers?
Background
Someone experiencing a lung attack suffers from shortness of breath because the airways are narrowed. Bronchodilators are a type of drug that helps to open these airways, but the best way to deliver them to the body is unknown. We searched for the best delivery device during lung attacks, focusing on whether there is a difference between wet nebulisers, which allow people to breathe in medicine as a mist using a mask or mouthpiece, compared with inhalers.
What evidence did we find?
We found eight studies including 250 participants in a search of the available studies up to 1 July 2016. All of the studies took place in a hospital.
What do the studies tell us?
The primary outcomes of the review showed no difference between the inhaler with a spacer and the nebuliser. However, in our secondary outcomes, we found some evidence that nebuliser treatment improves lung function more than inhalers with a spacer, but the quality and quantity of the data is limited. We found no difference between the therapies in terms of side effects or for reducing breathlessness. There are no studies available testing dry powder inhalation against a nebuliser.
Conclusion
Due to the low quality and quantity of the data, it is not clear whether nebulisers or inhalers with spacers are better for lung attacks. We found no difference between an inhaler with a spacer and the nebuliser in lung function after one hour or in unwanted side effects during lung attacks of COPD. The secondary outcome for lung function did favour nebulisers over inhalers with a spacer.
Summary of findings
for the main comparison.
| Bronchodilators delivered by nebuliser versus pMDI with spacer for exacerbations of COPD | ||||||
|
Patient or population: participants with an exacerbation of COPD; people with asthma excluded from our analysis Settings: treatment was allowed at home or in the clinic or hospital. Intervention: nebuliser Comparison: pMDI with spacer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| pMDI with spacer | Nebuliser | |||||
| Change in FEV1 1 h after dosing in ml | The mean change in FEV1 1 h after dosing in the pMDI group was 103 ml | The mean change in FEV1 1 h after dosing in the nebuliser group was 36 ml more (from 38 ml fewer to 110 ml more) | — | 40 (1) | ⊕⊝⊝⊝ Very lowa | — |
| Serious adverse events | 88 per 1000 | 88 per 1000 (17 to 348) | OR 1.00 ( 0.18 to 5.53) | 70 (2) | ⊕⊕⊝⊝ Lowb | — |
| Change in FEV1 closest to 1 h after dosing in ml | The mean change in FEV1 closest to 1 h after dosing in the pMDI group is93 ml | The mean change in FEV1 closest to 1 h after dosing in the nebuliser groups was 83 ml more (10 to 156 ml more) | — | 126 (4) | ⊕⊕⊝⊝ Lowb | — |
| Change in dyspnoea score during the first 24 h after dosing | The mean change in dyspnoea score during the first 24 h after dosing−1.28 points on the Borg scale (lower score indicates reduced dyspnoea) | The mean change in dyspnoea score during the first 24 h after dosing was 0.12 points worse (0.56 better to 0.79 worse) on the Borg scale in the nebuliser groups | — | 74 (2) | ⊕⊕⊝⊝ Lowb | A lower Borg score indicates reduced dyspnoea |
| Adverse events/side effects | 56 per 1000 | 89 per 1000 (24 to 278) |
OR 1.65 ( 0.42 to 6.48) |
110 (3) | ⊕⊕⊝⊝ Lowb | — |
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; ml: millilitres; FEV1 : forced expiratory volume in 1 second; pMDI: pressurised metered dose inhaler. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded for sample size (only one small study included in the analysis) (−2) and indirectness (e.g. older trials, so devices used may not be relevant to clinical practice today, and heterogeneity in dose between the groups) (−1) bDowngraded for sample size of the included trials (−1) and indirectness (e.g.older trials, so devices used may not be relevant to clinical practice today and heterogeneity in dose between the groups) (−1)
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is one of the most important respiratory diseases and the third leading cause of death worldwide (WHO 2014). It is generally caused by exposure to smoke or pollution. It is characterised by lung function decline and is associated with a decreased quality of life. Patients with COPD may have episodes with worsening of respiratory symptoms that require additional treatment (Burge 2003). These COPD exacerbations are the main driver of quality of life and survival in COPD. Exacerbations consist of a heterogenous spectrum of pathobiological changes compared to stable COPD, including inflammation, infection and hyperinflation (Lopez‐Campos 2015; Van Geffen 2015a; Van Geffen 2016). Exacerbations account for between 34% and 70% of all costs incurred in COPD (Oostenbrink 2004).
Description of the intervention
Bonchodilation is important in the medical treatment of COPD, both in stable state and during exacerbations (GOLD 2015). The choice of drug, dose and device all contribute to the success of inhaled medication in their own way, but remarkable differences exist in the prescribing habits of individual clinicians in all of these areas.
The inhaled bronchodilators used in COPD are short‐acting beta2‐agonists (SABA), long‐acting beta2‐agonists (LABA), and short‐and long‐acting anticholinergics. These are administered through various devices (GOLD 2015).
Many clinicians choose to treat patients with nebulisers, especially in the acute setting, and many patients claim to benefit from them (Zheng 2014). However, evidence supporting this choice from systematic analysis is lacking, and the available data are frequently biased by the inclusion of asthma patients (Greene 1988; Jasper 1987; Mandelberg 1997; Turner 1997).
This Cochrane review will assess the evidence available on nebulised bronchodilator treatment versus delivery by pressurised metered dose inhalers (pMDI) with spacer or by dry powder inhalers (DPI) for acute exacerbations of COPD. We published our planned strategy and methods earlier as a protocol (Van Geffen 2015b).
How the intervention might work
Prior research has clearly established the benefit of bronchodilation in treating patients with COPD. Several systematic reviews have shown this for bronchodilators in a stable state of COPD (Appleton 2006; Kew 2014). During exacerbations, experts also recommend the use of bronchodilation (GOLD 2015). Hence, bronchodilators are common in treatment of COPD exacerbations all over the world. However, less is known about the best mode of delivery for these treatments, especially during exacerbations. Important features known to affect the deposition include particle size, choice of the device, respiration pattern and inhalation technique. During exacerbations of COPD, nebulisers, as well as pMDIs and DPIs, have been shown to be useful in delivering medication into the lungs (Demoly 2014; Mazhar 2008). However, there are differences between device types, which may lead to differences in efficacy. For instance, the use of nebulisers is more time‐consuming compared with pMDI/DPI, and patients require a better technique to inhale their bronchodilators by DPI and especially pMDI without spacer. Due to the nature of exacerbations, the best choice of a delivery method for bronchodilators may differ from stable state.
Why it is important to do this review
Although there is consensus on the use of bronchodilators, there has been little attention to the mode of delivery. As a consequence, wide variations in practice exist between and within countries and even among doctors in the same hospital. We assessed the available RCTs to help guide practice in a more uniform way.
Objectives
To compare the effects of nebulisers versus pressurised metered dose inhalers (pMDI) plus spacer or dry powder inhalers (DPI) in bronchodilator therapy for exacerbations of COPD.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of both parallel and cross‐over designs.
Types of participants
We included studies in participants with an exacerbation of COPD receiving treatment at home, in the clinic or in hospital. We excluded RCTs involving mechanically ventilated patients due to the different condition of both patients and airways in this setting. We also excluded people with asthma from our analysis.
Types of interventions
We included trials comparing a bronchodilator medication by nebuliser with the same bronchodilator medication by either pMDI (with or without spacer) or DPI. We allowed co‐interventions including inhaled steroids.
Types of outcome measures
Primary outcomes
Change in forced expiratory volume in one second (FEV1), one hour after dosing
Serious adverse events
Secondary outcomes
Change in peak FEV1
Change in FEV1 closest to one hour after dosing
Change in FEV1 at other time points during the first 24 hours after dosing
Change in dyspnoea score during the first 24 hours after dosing
Change in quality of life on the first day of dosing
Admission rates
Time in hospital emergency department
Length of hospital stay
Change in oxygen saturation
Hospital readmission in 30 days
Adverse events/side effects
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Information Specialist for the Cochrane Airways Group. The CAGR contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see Appendix 1 for further details). We searched all records in the CAGR using the search strategy in Appendix 2 up to 1 July 2016.
We also searched ClinicalTrials.gov (clinicaltrials.gov) and the World Health Organization (WHO) trials portal (who.int/ictrp/en/). We searched both databases from their inception 1 July 2016, and we imposed no restriction on language of publication.
Searching other resources
We checked reference lists of all primary trials and review articles for additional references. We searched for errata and retractions from included trials published in full‐text on PubMed (www.ncbi.nlm.nih.gov/pubmed) to 1 July 2016.
Data collection and analysis
Selection of studies
Two review authors (WG and HK) independently screened titles and abstracts for inclusion of all the potential trials identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. Based on the consensus reached, we retrieved the full texts for assessment. Two review authors independently screened the full‐text records and identified trials for inclusion. We reported the reasons for excluding the ineligible trials in a 'Characteristics of excluded studies' table. We resolved any disagreements through discussion or, if required, we consulted a third review author. We identified and excluded duplicates and collated multiple reports of the same trial so that each trial rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1).
1.
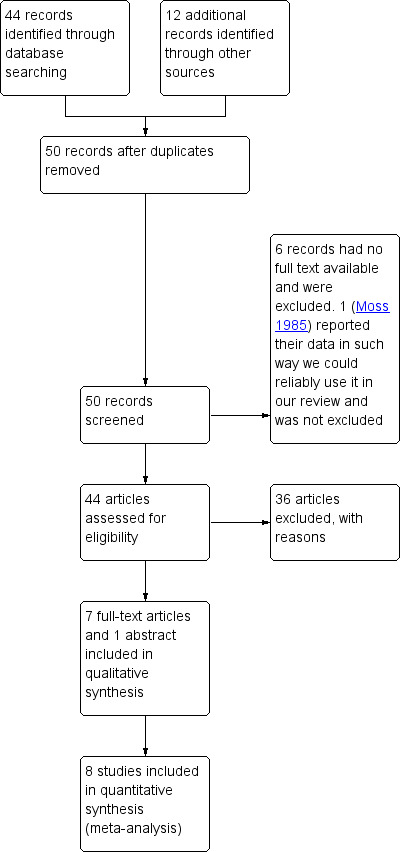
Study flow diagram
Data extraction and management
We used a data collection form, which we piloted on one included study, to record trial characteristics and outcome data. Two review authors extracted the following trial characteristics from included trials.
Methods: trial design, total duration of trial, details of any 'run‐in' period, number of trial centres and location, trial setting, withdrawals and date of trial.
Participants: N, mean age, age range, sex, severity of condition, diagnostic criteria, baseline lung function, smoking history, and inclusion and exclusion criteria.
Interventions: intervention, comparison, concomitant medications and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial and notable conflicts of interest of trial authors.
Two review authors extracted outcome data from the included trials. We noted in the 'Characteristics of included studies' table if outcome data was not reported in a usable way. We resolved disagreements by consensus or by involving a third review author. One review author, WG, transferred data into Review Manager (RevMan 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the trial reports. A second review author checked the papers' trial characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors assessed risk of bias for each trial using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving a third review author. We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as either 'high', 'low', or 'unclear' and provided a quote from the trial report or a justification for our judgment in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different trials for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐ reported pain scale). Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' summary (Figure 2).
2.
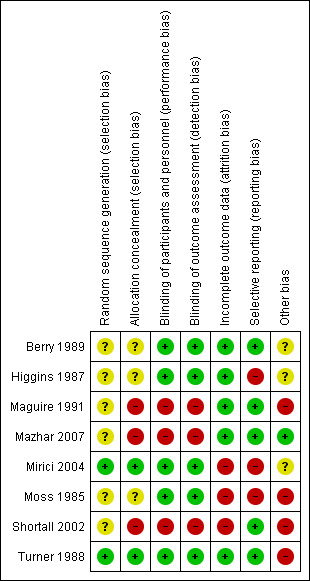
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
When considering treatment effects, we took into account the risk of bias for the trials that contributed to that outcome.
Assessment of bias in conducting the systematic review
The review was conducted according to the published protocol (Van Geffen 2015b), and we report any deviations from it in the 'Differences between protocol and review' section.
Measures of treatment effect
We analysed dichotomous data as odds ratios (OR) and continuous data as mean difference (MD) or standardised mean difference (SMD). We entered data presented as a scale with a consistent direction of effect. To analyse the cross‐over trials included in Analyses 1.1, 2.2 and 2.3, we used the generic inverse variance (GIV) method.
We undertook meta‐analyses only where it was meaningful to do so, that is, if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense.
We narratively described skewed data reported as medians and interquartile ranges.
For these studies, we expected to have to standardise the results of the studies to a uniform scale before combining them. The SMD expresses the size of the intervention effect in each study relative to the variability observed in that study. However, we could not use the SMD due to the cross‐over design of some of the included studies. In the studies where this was the case, we decided to present the data as a mean difference only.
Unit of analysis issues
If we had identified both cluster RCTs and individual RCTs, we planned to synthesise the acquired data. We planned to combine the results if we only detected a little heterogeneity between the trial designs, and we considered bias based on the choice of randomisation unit to be unlikely. Otherwise, we would have adjusted the sample sizes or standard errors using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
When we thought missing data could introduce serious bias, we explored the impact of including such studies in the overall assessment of results by performing a sensitivity analysis.
The studies we examined for the primary outcomes mostly had relatively short‐term outcomes. We found some missing data for the primary outcomes. In the case of Turner 1988, we managed to obtain original data, and we were able to calculate some of the missing data. We did not impute or extrapolate existing data.
Assessment of heterogeneity
We used the I2 statistic to assess heterogeneity among the studies in each analysis. Where we identified substantial heterogeneity, we have reported it and explored possible causes.
Assessment of reporting biases
Had we been able to pool more than 10 studies, we would have created and examined funnel plots to explore possible small trial and publication biases. However, we did not reach a pool of 10 studies.
Data synthesis
We used a random‐effects model and performed a sensitivity analysis with a fixed‐effect model. We used the standard deviations to standardise the mean differences to a single scale and compute trial weights.
Subgroup analysis and investigation of heterogeneity
We planned to analyse data according to bronchodilators used,mechanism (anticholinergic or beta‐adrenergic), and short‐acting versus long‐acting beta2‐agonists, analysing subgroups separately for SABA, LABA, SAMA, LAMA, and SABA/LAMA combinations. We also planned to analyse the data from single dose trials in the primary outcomes, and to analyse a subgroup of multiple treatment (doses) trials for the primary and secondary outcomes. However, due to the small number of studies included in our review, subgroup analyses (e.g. for dose or device) were underpowered. Therefore, we decided to assess all data pooled.
Sensitivity analysis
We assessed the risk of introducing bias due to missing data through a sensitivity analysis of our primary outcomes by comparing Berry 1989 and Mazhar 2007 with the other studies assessed as being at low risk of bias.
'Summary of findings' table
We created a 'Summary of findings' table using both the primary and secondary outcomes (Table 1). We used the five GRADE considerations (trial limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence as it relates to the trials contributing data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of Higgins 2011, using GRADEpro software (GRADEpro GDT). We detailed all decisions to downgrade or upgrade the quality of trials in the 'Summary of findings' table footnotes and made comments to aid readers' understanding of the review where necessary.
Results
Description of studies
Results of the search
We found 1082 records from the Cochrane Airways Group Specialised Register. After scanning titles and abstracts, we selected 44 for full‐text review. In addition, we identified 277 records from ClinicalTrials.gov (clinicaltrials.gov) and 80 from the WHO trials portal (www.who.int/ictrp/en/). Of these, we selected only one additional ongoing study (NCT02291016), with no available data. We found 12 additional references through other sources. We analysed those 56 articles in detail, as reported in Figure 1.
Included studies
See the 'Characteristics of included studies' for full details. We identified eight studies with an appropriate design to evaluate our predefined outcomes. A total of 250 participants with COPD were randomised to doses of aerosol with an inhaler plus spacer or a nebuliser treatment. Six out of the eight included studies reported excluding participants experiencing the most severe exacerbations, using criteria such as pH < 7.30 kPa, inability to perform spirometry or stand unsupported, respiratory failure or requiring mechanical ventilation. We identified no studies reporting on dry powder inhaler versus a nebuliser. We included studies with single or multiple dose and cross‐over designs. The studies took place in hospital settings in the United States (Berry 1989; Maguire 1991; Moss 1985; Shortall 2002; Turner 1988), the United Kingdom (Higgins 1987; Mazhar 2007), and Turkey (Mirici 2004). The studies used different beta2‐agonists, anticholinergics, pMDIs, spacers and nebulisers. We noticed a difference in dosage ratio between the pMDI/spacer and nebuliser in the studies. This ratio varies from 1:1 in Higgins 1987 to 1:11.5 in Maguire 1991.
Excluded studies
See the 'Characteristics of excluded studies' table for full details. Most commonly, we excluded studies in the ventilation setting, studies without an appropriate comparator to answer our hypothesis and studies mixing results for asthma and COPD.
Risk of bias in included studies
See Figure 2 for the 'Risk of bias' summary. For each study, we describe the 'Risk of bias' assessment in the 'Characteristics of included studies' table. The methodological quality of the studies included varied. Most of the studies did not describe the method of sequence generation, allocation concealment, or blinding of outcome assessment. None of the included studies reported the use of an intention‐to‐treat analysis or a power analysis. One study did not adequately describe the use of a spacer in their manuscript (Moss 1985). However, we decided to include Moss 1985 in our analysis based on the following arguments: we estimated that they did use a spacer in their study; according to our protocol, we had agreed to include studies that did not use a spacer; based on the reported trial design, we assessed this study to be of sufficient quality to be included in this analysis; and the study has been included in another meta‐analysis (Turner 1997).
Allocation
Only Mirici 2004 and Turner 1988 reported the use of a computer‐generated list of random numbers; the other six included studies may have been influenced by selection bias. Mirici 2004 adequately described their allocation blinding, and based on the overall quality of Turner 1988, we deemed the risk for selection bias due to allocation concealment methods to be low.
Blinding
Three studies were not blinded (Maguire 1991; Mazhar 2007; Shortall 2002), so the risk of performance and detection bias in these studies is high. The other studies were all double‐blinded.
Incomplete outcome data
The risk of attrition bias was high in three of the studies using peak expiratory flow (PEF) measurements in the analysis, because FEV1 measurements after hospitalisation were not available for all participants (Mirici 2004; Moss 1985; Shortall 2002). Moss 1985 was never published as a full paper. Shortall 2002 reported that 4 participants of the oral/pMDI group and 12 in the intravenous/nebuliser group did not complete the trial. It remains unclear why these participants dropped out and what caused the imbalance between the groups in the number of drop‐outs.
Selective reporting
We observed a risk of selective reporting bias in three studies where authors described a change in FEV1 in the methods but did not report it (Higgins 1987; Mirici 2004; Moss 1985). Mirici 2004 did not report FEV1 and forced vital capacity (FVC) measurements after hospitalisation. The abstract of Moss 1985 was not published as a full paper, leading to a high risk of reporting bias.
Other potential sources of bias
An important issue to consider is a difference in dose ratio between the pMDI/spacer and the nebuliser in the studies. This ratio varies from 1:1 in Higgins 1987 to 1:11.5 in Maguire 1991.
Effects of interventions
See: Table 1
Primary outcomes
Change in FEV1 one hour after dosing
We analysed the change in FEV1 one hour after dosing in Berry 1989 and Mazhar 2007. Due to different measurement units and the cross‐over design of the studies, we could not pool them. A separate analysis of both studies showed a non‐significant difference in favour of the nebuliser group. Mazhar 2007 found a mean absolute increase in FEV1 of 4.3% ± 4.8 in the nebuliser group, compared with 2.6% ± 3.3 in the pMDI group. Berry 1989 found a mean relative increase in FEV1 of 16.7% ± 17 in the nebuliser group compared with 13.4% ± 20.5 for the pMDI group. Change in FEV1 one hour after dosing did not show a significant difference between the pDMI and nebuliser group (MD 36 ml, 95% CI −38 to 110, N = 40, Analysis 1.1). Most other included studies reported two separate values for FEV1 instead of a change in FEV1 at this time point, making meta‐analysis of their data impossible.
1.1. Analysis.
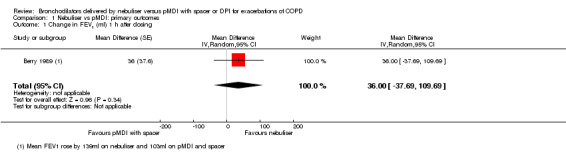
Comparison 1 Nebuliser vs pMDI: primary outcomes, Outcome 1 Change in FEV1 (ml) 1 h after dosing.
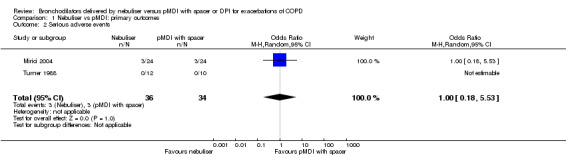
Serious adverse events
There were no significant differences in the occurrence of serious adverse events between the two delivery methods in the two trials that reported on this outcome (Mirici 2004; Turner 1988). Turner 1988 reported none. Mirici 2004 reported that two participants developed a pneumothorax and one participant required mechanical ventilation in the nebuliser group, and three participants developed a pneumothorax in the pMDI group (Figure 3).
3.

Forest plot of comparison: Primary endpoint: Nebuliser vs pMDI/DPI, outcome: Serious adverse events.
Secondary outcomes
Change in peak FEV1
There were no data available regarding change in peak FEV1.
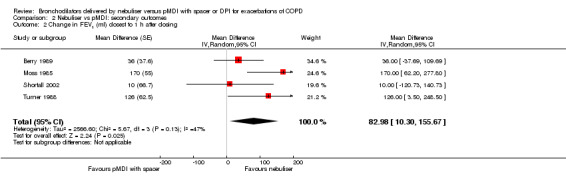
Change in FEV1 closest to one hour after dosing
We pooled reported data change in millilitres. According to our protocol, we could include cross‐over designs (Van Geffen 2015b). This resulted in the fact that studies reporting a different scale of data could not be included in the meta‐analysis. The forest plot shows a significant difference of 83 ml (95% CI 10 to 156, P = 0.03) in favour of the nebuliser treatment (Figure 4). If multiple time points were available, we included the time points closest to one hour of dosing. Moss 1985 measured FEV1 at 20 minutes after the dose, while we included the measurements from Turner 1988 at a 30 minute time point. The measurements from Berry 1989 were performed at one hour. Shortall 2002 did not report data about the timing of measurements; however, based on their trial design, we assumed they were performed at a sufficient time point to include them in this analysis. Due to a different unit of reporting, we could not include data from Maguire 1991 and Mazhar 2007 in this meta‐analysis. However, their results also show a non‐significant difference in favour of the nebuliser group. We calculated the standard error for the GIV analysis from the formula in Section 16.4.6.1 of Higgins 2011.
4.
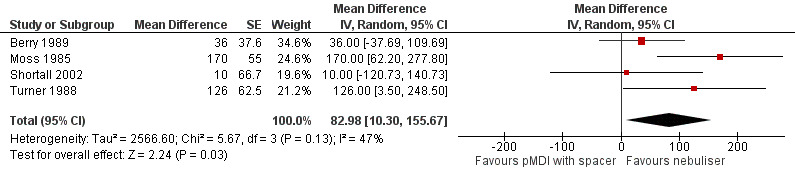
Forest plot of comparison: Secondary endpoint: Nebuliser vs pMDI/DPI, outcome: Change in FEV1 (mL) closest to one hour after dosing.
Change in FEV1 at other time points during the first 24 hours after dosing
We were not able to find data about additional time points other than those reported in the analyses above. Therefore, we did not deem a separate analysis to be meaningful for this outcome.
Change in dyspnoea score during the first 24 hours after dosing
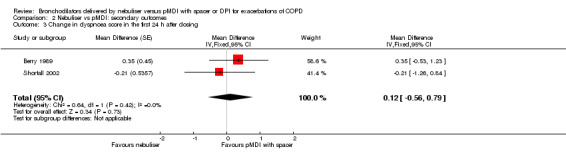
Based on data from two studies measuring dyspnoea with Borg's scale, we found no significant change in dyspnoea score (Berry 1989; Shortall 2002). One additional study also used this scale, reporting no significant difference between the groups (Turner 1988). However, we were not able to obtain the raw data for this outcome to recalculate their numbers to our previously defined outcome. Based on the included data, we found a non‐significant difference of 0.12 points (95% CI −0.56 to 0.79; P = 0.73) in favour of the pMDI group (Figure 5).
5.
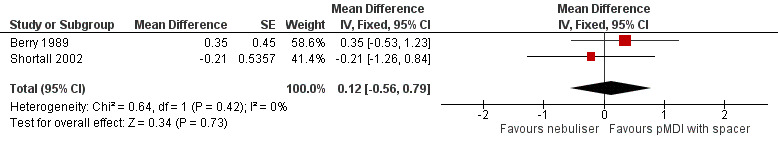
Forest plot of comparison: Secondary endpoint: Nebuliser vs pMDI/DPI, outcome: Change in dyspnoea score in the first 24 hours after dosing.
Change in quality of life on the first day of dosing
There were no data available about change in quality of life on the first day of dosing.
Admission rates
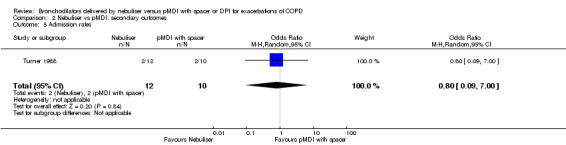
We found no significant difference in admission rate. Turner 1988 took place at the emergency department, reporting two admissions in both the pMDI and nebuliser group. We nevertheless found a non‐significant difference in favour of the nebuliser group (OR: 0.80, 95% CI 0.09 to 7.00) because the nebuliser group contained slightly more participants.
Time in hospital emergency department
Although Turner 1988 was performed at the emergency department, it did not report on time in the emergency department. Thus we could not extract data about this outcome.
Length of hospital stay
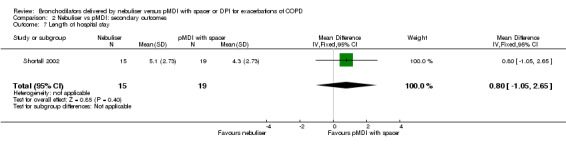
We found no significant difference in hospital stay in the one study reporting this outcome: Shortall 2002 reported a non‐significant difference in favour of the pMDI group of 0.80 days (95% CI −1.05 to 2.65, P = 0.40).
Change in oxygen saturation
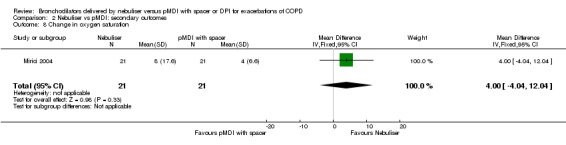
Mirici 2004 reported a change in oxygen saturation at several time points after inclusion. There were no significant changes at 30 minutes after the first dose or at the other reported time points (6 h, 24 h, 48 h or 10 d).
Hospital readmission in 30 days
There were no data available about hospital readmission rates in 30 days.
Adverse events/side effects
We found no significant differences between the groups concerning adverse events in the three studies reporting on this outcome (Higgins 1987; Mirici 2004; Turner 1988). Turner 1988 reported two adverse events in the nebuliser group; however, they did not explain the nature of these events. One participant in Higgins 1987 developed a marked fall in saturation from 88% to 73% 15 minutes after taking the nebuliser treatment. As stated earlier in the primary outcome section, Mirici 2004 reported two participants developing a pneumothorax and one participant requiring mechanical ventilation in the nebuliser group, and three participants developing a pneumothorax in the pMDI group (Figure 6).
6.
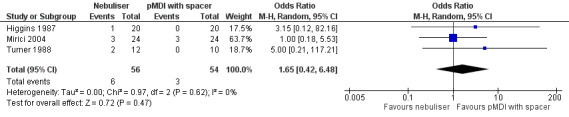
Forest plot of comparison: 2 Secondary endpoint: Nebuliser vs pMDI/DPI, outcome: 2.10 Adverse events/side effects.
Discussion
Summary of main results
There is a lack of evidence favouring one mode of bronchodilator delivery over another during exacerbations of COPD. We found no difference between nebulisers and pMDI plus spacer regarding the primary outcomes FEV1 at one hour and safety. The secondary outcome 'change in FEV1 closest to one hour after dosing' showed a greater improvement in FEV1 when treating with nebulisers than with pMDI plus spacers. A limited amount of data are available (eight studies involving 250 participants). These studies were difficult to pool. There were no available study data to enable us to include data about DPIs in our analysis.
Bronchusobstruction
The search for better parameters for acute, severe COPD exacerbations is ongoing (Van Geffen 2015a), but for now, FEV1 continues to be an important parameter in clinical trials for COPD exacerbations. This review assessed change in FEV1 at several time points. We found no significant differences between the pMDI and nebuliser group for a change in FEV1 at one hour after dosing, but we could not pool the available data. The secondary outcome, 'change in FEV1 closest to one hour after dosing', showed a greater improvement in FEV1 in the nebuliser group than in the pMDI plus spacers group. Overall, there is a lack of evidence favouring one mode of delivery over another for bronchodilators during exacerbations of COPD with regard to bronchus obstruction.
Adverse events
Three studies reported on adverse events (Higgins 1987; Mirici 2004; Turner 1988). This is the first time the data have been pooled and assessed systematically. Adverse and especially serious adverse events might influence the device choice for physicians when treating patients with COPD exacerbations. However, with current available data in this systematic review, we found no significant differences between pMDI and nebuliser treatment. Overall, there is a lack of evidence favouring one mode of delivery for bronchodilators over another during exacerbations of COPD with regard to adverse events.
Dyspnoea and quality of life
Patient‐reported outcomes are becoming more important in current practice. Patient‐reported outcomes include scoring of dyspnoea and quality of life. The analysis of dyspnoea showed no significant differences between pMDI and nebuliser treatment. We did not identify any data about quality of life. Overall, there is a lack of evidence favouring one mode of delivery for bronchodilators over another during exacerbations of COPD with regard to dyspnoea and quality of life.
Clinically important outcomes
This systematic review assessed additional clinically important outcomes, used both by physicians and policymakers on a daily basis. We were surprised by the lack of data about admission rates, time in the hospital emergency department, length of hospital stay, and hospital readmission within 30 days. These are perhaps parameters that have only recently become more important, and additionally necessitate longer trials. Overall. there is a lack of evidence favouring one mode of delivery for bronchodilators over another during exacerbations of COPD with regard to these outcomes.
Overall completeness and applicability of evidence
The overall completeness of the evidence is low. Due to differences in outcome reporting we could not calculate the change in parameters from all studies. The evidence gathered related only to the comparison of nebulisers versus pMDIs. We found no studies investigating DPIs versus nebulisers using the same substance, nor studies with nebulised long‐acting bronchodilators. Data about important clinical parameters, hospital readmission in 30 days, change in peak FEV1' and change in quality of life were not available. Participants in the included studies were all treated in a hospital setting rather than at home. Turner 1988 reported on an emergency department setting, from which most participants were not admitted. We recognise that the setting in which a patient receives treatment may have an impact on the choice of treatment mode, beyond concerns solely about the efficacy of the method. The paucity of data in this review has not allowed us to comment on the effect of the trial setting on the outcomes. We noticed a lack of standardised definitions in both COPD and exacerbations, which might influence the generalisability of the findings, although this lack of standardised definitions is also present in regular clinical practice. Thus, it is not entirely clear whether our results apply to all patients who present to a hospital with an exacerbation of COPD.
Additional studies could prove useful in providing further evidence towards the difference we signalled in bronchodilator effects in favour of the nebuliser treatment. However, readers should keep in mind that the mean clinically important difference for the FEV1 is generally reported to be 100 to 140 ml (Cazzola 2008; Jones 2014).
Many practitioners commonly prescribe nebulisers for the acute exacerbation of COPD. Based on the results of our review, there is no evidence to either support or refute this practice. This might influence the applicability of the evidence; however, given the lack of evidence provided in this review, it is even more important to adequately assess the individual patient, the available modes of nebulisers and the available pMDIs and spacers. There are several important differences between different types of modern nebulisers, for instance regarding inhaled dose, delivered dose and the use of the compressor (De Boer 2003; Le Brun 1999). In the absence of good quality evidence, such an assessment might provide guidance to select the optimal treatment for each patient.
Quality of the evidence
We used the GRADE assessment to qualify the amount of evidence of the outcomes, reporting this in the Table 1. Overall the quality of the evidence was low and sometimes even lacking. The studies that were included in this review are relatively small, and we downgraded the quality of the outcomes to reflect this. Especially for the primary outcome measuring FEV1 at one hour, we could only include one older trial (Berry 1989). We therefore downgraded the evidence for this outcome. Heterogeneity varied across individual outcomes, ranging from I2 = 0% to I2 for = 47% for change in FEV1 (ml) closest to one hour after dosing.
The evidence was relatively old, with studies performed from at least 9 years and up to 31 years prior to this systematic review. This might influence the results, since modern nebulisers, pMDIs and DPIs may work in a different way than the ones used 30 years ago.
It is important to note the lack of standardised dose of bronchodilators between the different designs. Although actual lung deposition is generally held to be lower by nebuliser than by pMDI when using the same dose in both devices, good data are sparse. We noticed a significant variation in dose between the studies. Additionally, the type of nebuliser, compressor and pMDI used in trials will influence the actual lung deposition (De Boer 2003; Le Brun 1999; Mazhar 2007). This might influence results, although it is unclear to what extent. We downgraded the quality of the evidence due to the combination of relatively old studies and dose variation.
Potential biases in the review process
A potential bias in our review process is publication bias. We found several studies reported only as abstracts. Although we tried, we could not retrieve a full data set from the study authors for several reasons. The data reported in the abstracts were not sufficient to allow recalculation for our outcomes, except in the case of the study by Moss 1985.
Agreements and disagreements with other studies or reviews
Although the data for the primary outcome did not show significant differences, this systemic review suggests for the first time that treatment with nebulisers during an exacerbation of COPD may improve FEV1 more than pMDI with a spacer. However, it is very difficult to interpret this result correctly due to the previously discussed bias. We therefore concur with the earlier findings from Turner 1997 and Dolovich 2005. They did not find significant differences and concluded that there is not enough evidence to favour a mode of delivery for bronchodilators during exacerbations of COPD. Both reviews used asthma patients in their analysis, and both focused on FEV1 or peak flow. A systematic review in mechanically ventilated patients with a need for aerosol bronchodilator therapy found no difference in bronchodilator effects, although they were only able to pool two studies with 28 participants in total for this outcome (Holland 2013).
Authors' conclusions
Implications for practice.
Due to inconclusive findings for our primary outcomes and all but one of our secondary outcomes, risk of bias, and relatively low numbers of studies and participants (eight studies involving 250 participants), the existing published data do not provide enough evidence to firmly favour one mode of delivery for bronchodilators over another during exacerbations of COPD. One secondary outcome suggests that treatment with nebulisers during an exacerbation of COPD slightly outperforms pMDI plus spacer with regard to improving FEV1; however, this finding should be interpreted with care. Limited data about nebulisers versus pMDIs plus spacer are available. No data of sufficient quality have been published comparing nebulisers in this setting versus DPIs. We did not identify any studies of nebulised long‐acting drugs. Most studies tested on one day only, in a cross‐over design.
Implications for research.
More studies are required to assess the optimal mode of delivery during exacerbations of COPD. In particular, data about DPIs versus nebulisers are lacking. There seems to be a larger effect on FEV1 with the nebuliser. However, larger studies could shed more light on this and should take into account the considerable difference in the total administered dose between nebulisation and pMDI, and indeed the differences between different nebuliser designs and inhalers devices. The outcomes of these studies have traditionally focused at bronchodilating effects. Future studies should also assess different parameters such as adverse events, dyspnoea and quality of life. Patients, both in the acute setting and even in a stable state of COPD, seem to be more satisfied with nebulised administration than can be understood from the bronchodilatory data. Further research may be required to investigate the acceptability of different drug delivery modes in patients who may be accustomed to receiving nebulised treatment during an exacerbation. In times of strain on the medical system and its costs, length of stay and time to readmission would be valuable additional parameters for trials to consider. Investigators should report data about patients with COPD, asthma or an overlap syndrome separately. Future research evaluating nebuliser treatment compared with pMDI or DPI during COPD exacerbations should report findings as a change in means with standard error or standard deviation, or studies should provide sufficient data in the study report to enable calculation of these values. This will enable a meta‐analysis of the study findings. We would also advise researchers to perform a power analysis when planning any new trials.The value of long‐acting bronchodilators in the treatment of exacerbations, as well as their optimal modes of delivery, is totally unknown but would be valuable to study, especially since they have been shown to reduce hyperinflation and improve dyspnoea in stable state and are the standard of care after discharge (Van Geffen 2015a).
Acknowledgements
We would like to thank the Cochrane Airways Group editorial staff and especially Chris Cates, Emma Welsh and Jessica Thomas, who helped us tremendously during several stages of this review and who also helped us by obtaining additional data about the trial of Turner 1988 and by sharing the knowledge they obtained during the writing of their review (Cates 2013).
We would like to thank William OC Cookson for helping us to extract data from Higgins 1987.
We would like to thank Cheng Xu in helping us translating Qian 2008.
We would like to thank Judith Vonk for statistical support with the data extraction from Shortall 2002 and assessment of Higgins 1987 and Mazhar 2007.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
Rebecca Normansell was the Editor for this review and commented critically on the review.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the CAGR
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (the Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to identify relevant trials from the CAGR
#1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All
#2 MeSH DESCRIPTOR Bronchitis, Chronic
#3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)
#4 COPD:MISC1
#5 (COPD OR COAD OR COBD):TI,AB,KW
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 nebuli*:TI,AB,KW
#8 PMDI:TI,AB,KW
#9 DPI:TI,AB,KW
#10 metered* NEAR3 inhaler*:TI,AB,KW
#11 MDI:TI,AB,KW
#12 inhaler*:TI,AB,KW
#13 spacer*:TI,AB,KW
#14 inhal* NEAR3 device*:TI,AB,KW
#15 MeSH DESCRIPTOR Nebulizers and Vaporizers Explode All
#16 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15
#17 #6 AND #16
[Note: in search line #4, MISC1 denotes the field in the record in which the reference has been coded for condition, in this case, COPD]
Data and analyses
Comparison 1. Nebuliser vs pMDI: primary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 (ml) 1 h after dosing | 1 | Mean Difference (Random, 95% CI) | 36.0 [‐37.69, 109.69] | |
| 2 Serious adverse events | 2 | 70 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.18, 5.53] |
1.2. Analysis.
Comparison 1 Nebuliser vs pMDI: primary outcomes, Outcome 2 Serious adverse events.
Comparison 2. Nebuliser vs pMDI: secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in peak FEV1 [%] | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Change in FEV1 (ml) closest to 1 h after dosing | 4 | Mean Difference (Random, 95% CI) | 82.98 [10.30, 155.67] | |
| 3 Change in dyspnoea score in the first 24 h after dosing | 2 | Mean Difference (Fixed, 95% CI) | 0.12 [‐0.56, 0.79] | |
| 4 Change in quality of life on the first day of dosing | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission rates | 1 | 22 | Odds Ratio (M‐H, Random, 95% CI) | 0.8 [0.09, 7.00] |
| 6 Time in hospital emergency department | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Length of hospital stay | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐1.05, 2.65] |
| 8 Change in oxygen saturation | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐4.04, 12.04] |
| 9 Hospital readmission in 30 d | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Adverse events/side effects | 3 | 110 | Odds Ratio (M‐H, Random, 95% CI) | 1.65 [0.42, 6.48] |
2.2. Analysis.
Comparison 2 Nebuliser vs pMDI: secondary outcomes, Outcome 2 Change in FEV1 (ml) closest to 1 h after dosing.
2.3. Analysis.
Comparison 2 Nebuliser vs pMDI: secondary outcomes, Outcome 3 Change in dyspnoea score in the first 24 h after dosing.
2.5. Analysis.
Comparison 2 Nebuliser vs pMDI: secondary outcomes, Outcome 5 Admission rates.
2.7. Analysis.
Comparison 2 Nebuliser vs pMDI: secondary outcomes, Outcome 7 Length of hospital stay.
2.8. Analysis.
Comparison 2 Nebuliser vs pMDI: secondary outcomes, Outcome 8 Change in oxygen saturation.
2.10. Analysis.
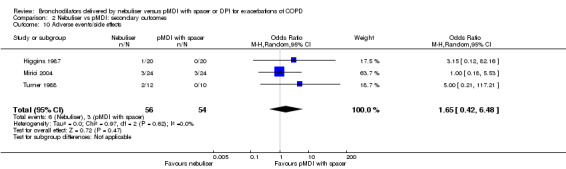
Comparison 2 Nebuliser vs pMDI: secondary outcomes, Outcome 10 Adverse events/side effects.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Berry 1989.
| Methods | Cross‐over RCT Power analysis: not presented |
|
| Participants | Location: Long Beach, CA, USA 20 participants aged 60‐91, mean 67.9 (SD 7.1) Baseline characteristics: comparable Setting: admitted through emergency department COPD definition: long history of chronic airflow obstruction and smoking Exclusion criteria: pH < 7.30 kPa |
|
| Interventions | 2 treatment blocks on a single day separated by 4 h with salbutamol, either by pMDI and spacer or by nebuliser, and placebo in the other device Beta2 ‐agonist: salbutamol (albuterol) pMDI: brand not reported Spacer: InspirEase Nebuliser: Airlife misty nebuliser Dosage ratio spacer/nebuliser: 1:7 Co‐interventions: aminophylline IV, corticosteroids IV Medication adherence rates: not reported |
|
| Outcomes | FEV1, FVC, Borg scale Time points: at baseline and 1 hour after treatment with each device |
|
| Notes | Funded by a grant from the Schering Group and VA Research Service | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "We undertook a study to .... using a more typical inpatient schedule of drug administration with a randomised double blind cross‐over protocol." |
| Allocation concealment (selection bias) | Unclear risk | Randomisation protocol not described Comment: unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "double blind cross‐over protocol." Comment: Probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotation: "double blind cross‐over protocol" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: We detected no missing data |
| Selective reporting (reporting bias) | Low risk | For all groups all data was reported |
| Other bias | Unclear risk | COPD was defined only as a long history of chronic airflow obstruction and smoking. No definition of COPD exacerbation was provided Only males were included, potentially limiting the extrapolation of results to males only. Possible sequence effect in cross‐over trial not reported on. Comment: this might lead to bias |
Higgins 1987.
| Methods | Cross‐over RCT Power analysis: not presented |
|
| Participants | Location: Oxford, England 20 participants, mean age 71.1 years (SEM: 1.5) Baseline characteristics: comparable Setting: admitted in hospital COPD definition: not reported Exclusion criteria: not reported |
|
| Interventions | Measurements on 1 day, 3 sessions, 4 h apart. Sessions were 4 mg terbutaline either by nebuliser or pMDI with Nebuhaler and placebo in the other device and sessions with placebo in both devices in random sequence. Beta2 ‐agonist: terbutaline pMDI: brand not reported Spacer: Nebuhaler Nebuliser: brand not reported Dosage ratio spacer/nebuliser: 1:1 Co‐interventions: not reported Medication adherence rates: not reported |
|
| Outcomes | Changes in FEV1, % changes in tcCO2, % change in SaO2 Time points: FEV1 was measured at baseline and 20 minutes after treatment, tcCOand SaO2 were measured continously during 30 minutes after treatment |
|
| Notes | Financial support by Astra Pharmaceuticals. Author Cookson received a WA and MG Saw Medical Research Fellowship | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotation: "the order of the sessions was randomised" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation protocol not described Comment: unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotation: "double Blind" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotation: "double Blind" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: we detected no missing data |
| Selective reporting (reporting bias) | High risk | Change in FEV1 was described in the Methods but not reported |
| Other bias | Unclear risk | COPD was not defined; no definition of COPD exacerbation was provided. Possible sequence effect in cross‐over trial not reported upon. Comment: this might lead to bias |
Maguire 1991.
| Methods | Cross‐over RCT Power analysis: was not presented |
|
| Participants | Location: Valhalla, NY, USA 7 participants with COPD; mean smoking 82.8 pack‐years and onset of symptoms after age 40. 10 asthma participants were also included, but were reported separately Baseline characteristics: not reported separately for the COPD group; comparable between trial arms Setting: hospital COPD definition: 1990 ATS definition. Two of the COPD group had bronchitis and did not meet the ATS criteria of that time. Exacerbation was defined as acute onset of increasing respiratory symptoms Exclusion criteria: not able to stand unsupported next to the bed or to perform spirometry |
|
| Interventions | Metaproterenol in pMDI‐Spacer (2 x 0.65 mg), or handheld nebuliser (15 mg). Each participant received both devices. Treatment was separated by 2.96 ± 0.27 h (mean ± SEM) Beta2 ‐agonist: metaproterenol pMDI: brand not reported Spacer: InspirEase, Key Pharmaceuticals, Miami, FL (USA) Nebuliser: Travenol, Travenol corporation, Edison, NJ (USA) Dosage ratio spacer/nebuliser: 1:11.5 Co‐interventions: unclear Medication adherence rates: not reported |
|
| Outcomes | Change in FEV1, FVC and FEF 25‐75, FEV1, FVC and FEF 25‐75 Time points: each participant tested 4 times (30 min before and after each treatment) |
|
| Notes | Trial was supported by a grant from the National Institute of Health (NIHR) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotation: "randomly selected" Randomisation protocol not described |
| Allocation concealment (selection bias) | High risk | Randomisation protocol not described Comment: given probable lack of blinding, this also probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: we detected no missing data |
| Selective reporting (reporting bias) | Low risk | Comment: we detected no missing outcomes |
| Other bias | High risk | Compared to the other studies, a very high dosage medication by nebuliser was administered (ratio spacer/nebuliser 1:11.5). Possible sequence effect in cross‐over trial not reported upon |
Mazhar 2007.
| Methods | Cross‐over RCT Power analysis: not presented |
|
| Participants | Location: Huddersfield, England 11 participants,19 asthma participants were also included and reported separately Baseline characteristics: reported to be comparable but not specified Setting: ward COPD definition: not reported Exclusion criteria: respiratory failure |
|
| Interventions | On the 2nd and 4th day of admission, regular terbutaline dose was replaced by a salbutamol study dose. Five 100 µg salbutamol doses were inhaled from a metered dose inhaler plus spacer (pMDI + SP) or 5 mg was nebulised (NEB) Beta2 ‐agonist: salbutamol pMDI: Ventolin Evohaler; GlaxoSmithKline, Brentford, UK Spacer: Volumatic (GlaxoSmithKline) large volume spacer Nebuliser: Sidestream chamber (Respironics, Tangmere, UK) Dosage ratio spacer/nebuliser: 1:10 Co‐interventions: terbutaline, other were not reported Medication adherence rates: not reported |
|
| Outcomes | Urinary salbutamol excretion, change in FEV1 Time points: 30 min and 24 h (salbutamol excretion) and baseline and after 1 hour for FEV1 |
|
| Notes | Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotation: "The inhalation method to be used on the study days was randomized." Randomisation protocol not described |
| Allocation concealment (selection bias) | High risk | Randomisation protocol not described Comment: Given probable lack of blinding, this also probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding or the use of placebo was not described in the manuscript. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding or the use of placebo was not described in the manuscript. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: none detected |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | No data provided regarding possible sequence effect in cross‐over trial |
Mirici 2004.
| Methods | Randomised, double dummy, parallel group trial. Power analysis: not reported |
|
| Participants | Location: Erzum, Turkey 48 participants were randomised Baseline characteristics: comparable except for pH. Participants in the nebuliser group had a significantly lower pH than the pMDI group. Setting: hospital COPD definition: 1995 ATS guidelines, known FEV1/ FVC < 70% and maximum FEV1 < 80% Definition of the exacerbation: one of the following: increased dyspnoea, increased production and purulence, leading to a change of treatment Exclusion criteria: presence of other conditions such as cystic fibrosis, asthma, severe bronchiectasia, pneumonia, severe hypertension, and severe exacerbation requiring invasive or noninvasive mechanical ventilation. Patients who were not using the pMDI/spacer with the appropriate technique were also not included the study. |
|
| Interventions | Participants were randomised to pMDI/spacer group receiving 100 µg salbutamol and 20 µg ipratropium (4 times 4 puffs) and placebo nebuliser or the nebuliser group receiving 2.5 mg salbutamol and 500 µg ipratropium 4 times a day and placebo pMDI/spacer Beta2 ‐agonist: salbutamol Anticholinergic: ipratropium pMDI: not reported Spacer: yes, type not reported Nebuliser: not reported Dosage ratio spacer/nebuliser: 1:6.25 Co‐interventions: oral steroids, theophylline, antibiotics and supplementary oxygen Medication adherence rates: not reported |
|
| Outcomes | Cost effectiveness, FEV1 (not reported), PEF, PaO2, PaCO2, SaO2, pH Time points: baseline, 30 min, 24 h, 48 h and 10 d |
|
| Notes | Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation protocol described a computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Low risk | Quotation: "randomization was performed using unmarked, ordered, sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: fully blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quotation: "PEFR measurements were used in the analysis, because FEV1 measurements after hospitalisation were not available for all patients." |
| Selective reporting (reporting bias) | High risk | Quotation: "PEFR measurements were used in the analysis, because FEV1 measurements after hospitalization were not available for all patients." FVC was not reported although baseline measurements were performed |
| Other bias | Unclear risk | The groups were relatively small (21 vs 22 participants) Comment: small and power calculation was not provided, leaving room for a type II error |
Moss 1985.
| Methods | Double blind randomised cross‐over design Power analysis: not reported |
|
| Participants | Location: St John's Mercy Medical Center, St Louis, MO, USA 15 participants were randomised in a cross‐over design Baseline characteristics: stated to be comparable Setting: hospital COPD definition: not provided Exclusion criteria: not provided |
|
| Interventions | Participants were treated with either 15 mg metaproterenol by compressor driven nebuliser or 1.95 mg by pMDI. 4 h later they were treated with the other device. Beta2 ‐agonist: metaproterenol Anticholinergic: none pMDI: not reported Spacer: not reported Nebuliser: not reported Dosage ratio MDI/nebuliser: 1:7.69 Co‐interventions: not reported Medication adherence rates: not reported |
|
| Outcomes | Change in FEV1 and subjective improvement by a numerical scale Time points: 20 min after treatment with each device |
|
| Notes | This study was only published as an abstract. Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotation: "randomized" Randomisation protocol not described |
| Allocation concealment (selection bias) | Unclear risk | Randomisation protocol not described Comment: unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotation "double Blind" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotation "double Blind" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data is not provided, the abstract was never published as a full paper |
| Selective reporting (reporting bias) | High risk | Comment: the trial was not reported as a full‐text paper. Also the data about subjective improvement were not reported other than it was not significantly different. |
| Other bias | High risk | No definition of COPD, exacerbation or other details were provided |
Shortall 2002.
| Methods | RCT Power analysis: not reported |
|
| Participants | Location: Eastern Maine Medical Center and St Joseph Hospital, Bangor, ME, USA 50 participants were randomised; however, only 34 were analysed. Dropouts were imbalanced (4 in pMDI group and 12 in the nebuliser group) Baseline characteristics: comparable Setting: hospital COPD definition: FEV1 < 60% predicted, FEV1/FVC < 60% after bronchodilators. Postbronchodilator < 15 % increase in FEV1. Exacerbations were defined as an increase of dyspnoea, cough or mucus production plus one or more of the following: inadequate response to outpatient treatment, marked decrease in exercise capacity, inability to eat or sleep due to dyspnoea, worsening hypoxaemia and a new or worsening hypercapnia Exclusion criteria: acute pneumonia, status asthmaticus, bronchiectasis, cystic fibrosis, upper airway obstruction, left ventricular dysfunction, positive blood culture, (non)invasive ventilation, inability to cooperate with pMDI or mask inhalation. |
|
| Interventions | Participants were randomised to either an oral/pMDI regimen or an IV/nebuliser regime. The oral/pMDI regimen was: methylprednisolone; 40 mg per os every 6 h until wheeze‐free, then 40 mg per os per day. Albuterol; 1 puff per 30 s the first 2 min, then 1 puff every min up to 20 puffs per 4 h and 1 puff every h as needed until alleviation of dyspnoea. Ipratropium; 1 puff per 30 s the first 2 min, 1 puff every min, up to 8 puffs per 4 h, and 1 puff every h as needed. Cefuroxim 500 mg per os twice a day. The IV/nebuliser regime was methylprednisolone; 40 mg intravenously each 6 h until wheeze‐free, then 40 mg per os per day. Nebulised 2.5 mg albuterol and 0.5 mg ipratropium every 4 h while awake and every h as needed. Cefuroxim 1.5 intravenously every 8 h Beta2 ‐agonist: albuterol Anticholinergic: ipratropium pMDI: not reported Spacer: AeroChamber, Monaghan Medical Plattsburgh, NY, USA Nebuliser: Airlife mistyneb nebulizer (Allegiance Healthcare, McGraw, IL, USA) Dosage ratio spacer/nebuliser: not reported Co‐interventions: theophylline and supplementary oxygen Medication adherence rates: not reported |
|
| Outcomes | FEV1 (L), change in FEV1 (L), mean length of stay, treatment failure, change in Borg scale Time points: not reported |
|
| Notes | Investigators did not report SDs or confidence intervals, and we could not reach them to obtain these. With help from our statistician, we calculated the SDs (assuming they were equal in both groups) The raw data provided us with a mean change in FEV1 in litres of 0.12 (oral/pMDI group, N = 19) and 0.13 in the (IV/NEB group, N = 15). Based on the formula t = (y1 − y2)/ (SD * √(1/N1+1/N2)) t = 0.15 based on the P value provided by the article, y = change. Thiswould make y1 = 0.12, y2 = 0.13, N1 = 19 and N2 = 15; we calculated the SD to be 0.193. We used a similar calculation to calculate the SD for the Borg score. Here t was 0.41 based on the provided P value. SD was calculated to be 1.55. For length of stay t = 0.85 SD was calculated to be 2.73. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotation: "randomized" Randomisation protocol not described |
| Allocation concealment (selection bias) | High risk | Randomisation protocol not described Comment: Given lack of blinding, probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quotation: 4 participants of the oral/pMDI group and 12 of the IV/nebuliser group did not complete the trial. It is unclear why the patients in each group dropped out. |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | High risk | The treatment groups differed not only by nebulised vs pMDI device for inhalation, but also by IV vs oral administration of systemic steroids and antibiotics, rendering all comparisons difficult to interpret. Comment: the timing of the performed lung function measurements was not reported 58% of the potential study participants were not studied for several reasons |
Turner 1988.
| Methods | RCT Power analysis: not reported |
|
| Participants | Location: San Francisco, CA, USA 22 participants: mean age 55 years (SD 4), pMDI‐spacer group; 57 years (SD 3), nebuliser group. 53 asthma patients were also included and reported separately Baseline characteristics: comparable Setting: emergency room COPD definition: ≥10 pack‐years of smoking and onset of symptoms ≥ age 30 Exclusion criteria: younger than 18, older than 75, acute myocardial infarction, heart failure, intubation, inability to perform an FEV1, allergy towards metaproterenol or pregnant |
|
| Interventions | Randomly assigned to 3 x 0.65 mg metaproterenol via pMDI + spacer + placebo, or nebuliser metaproterenol 15 mg + placebo. Each treatment was given 3 times at 30 min intervals. Beta2 ‐agonist: metaproterenol pMDI: brand not reported Spacer: InspirEase, Key Pharmaceuticals, USA Nebuliser: Acorn II, Marquest Medical, USA Dosage ratio spacer/nebuliser: 1:7.7 Co‐interventions: oxygen, IV steroids; theophylline was withheld Medication adherence rates: not reported |
|
| Outcomes | FEV1, Borg scale, pulse, respiratory rate, blood pressure Time points: baseline, after 30 and 90 minutes |
|
| Notes | Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation protocol was described in unpublished data quote: "therapy was then determined . . . by a random list of numbers" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Randomisation protocol was described in unpublished data quote: "therapy was then determined . . . by a random list of numbers". Comment: based on the quality of the trial and procedures for a trial performed in this decade, we estimate the risk to be low |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotation: "double blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotation: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: none detected |
| Selective reporting (reporting bias) | Low risk | Change in FEV1 was not reported; we obtained access to the full data and calculated this ourselves |
| Other bias | High risk | COPD was defined only as ≥10 pack years of smoking and onset of symptoms ≥ age 30, and therefore less than fully clearly separated from asthma. Comment: this might lead to bias, especially since asthmatics tend to have larger bronchodilator responses |
ATS: American Thoracic Society; COPD: chronic obstructive pulmonary disease; FEF: forced expiratory flow; FEV1 : forced expiratory volume in 1 second; FVC: forced vital capacity; IV: Intravenous;PaCO2 : partial pressure of carbon dioxide in arterial blood; PaO2 : partial pressure of oxygen in arterial blood; PEF(R): peak expiratory flow (rate); pH: potential of hydrogen; pMDI: pressurised metered‐dose inhaler; SaO2 : symbol for the percentage of oxygen saturation of arterial blood; SEM: standard error of the mean; SD: standard deviation;tcCO2: transcutaneous carbon dioxide.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdelrahim 2011 | Non‐invasive ventilation setting and no pMDI or DPI was used as comparator |
| Bai 1993 | Mechanical ventilation setting |
| Broeders 2004 | No nebuliser was used as comparator |
| Brunetti 2015 | No pMDI or DPI was used as comparator |
| Carpenter 2008 | Trial was performed in stable state only |
| Cazzola 2002a | No nebuliser was used as comparator |
| Cazzola 2002b | No nebuliser was used as comparator |
| Chu 1989 | Trial was performed in stable state only |
| Dhand 2002 | No abstract or full‐text available. Prof Dhand informed us that this trial was performed in stable state |
| Duarte 2000 | Mechanical ventilation setting |
| Finnerty 1999 | No abstract or full‐text available; authors could not be reached |
| Foresi 2002 | No full‐text available; authors could not be reached. Based on FEV1/FVC ratio, not all participants had COPD and reported stable state only |
| Formgren 1994 | No nebuliser was used as comparator |
| Greene 1988 | Combination of asthma and COPD exacerbations. No results for COPD were reported |
| Guerin 1999 | Mechanical ventilation setting |
| Haynes 2012 | No pMDI or DPI was used as comparator |
| Ikeda 1994 | Trial was performed in stable state only |
| Jasper 1987 | Combination of asthma and COPD exacerbations. Insufficient results for COPD were reported |
| Kaminski 1997 | Trial was performed in stable state only |
| Lai 1990 | Trial was performed in stable state only |
| Lees 1980 | Trial was performed in stable state only |
| Li 2011 | No full‐text available; authors could not be reached |
| Mandelberg 1997 | Combination of asthma and COPD exacerbations. No separate results for COPD were reported |
| Marlin 1986 | Trial was performed in stable state only |
| Marta 1997 | Stable state, combination of asthma and COPD |
| Martos 1990 | No full‐text available; authors could not be reached |
| Mestitz 1988 | Trial was performed in stable state only. Combination of asthma and COPD |
| Mouloudi 1998 | Non‐invasive ventilation setting and no nebuliser was used as comparator |
| Nair 2005 | No pMDI or DPI was used as comparator |
| Numata 2002 | The trial lacked specific data for the nebuliser group. We contacted Prof Schwartzman; however the raw data has been lost. |
| Pappalettera 2005 | No full‐text available; authors could not be reached |
| Petrova 2001 | No full‐text available; authors could not be reached |
| Qian 2008 | No pMDI or DPI was used as comparator |
| Quinn 2014 | Trial was performed in stable state only, no nebuliser was used as comparator |
| Rebuck 1987 | No pMDI or DPI was used as comparator |
| Schleufe 2004 | No nebuliser was used as comparator |
| Segreti 2013 | Different types of bronchodilators used |
| Summer 1989 | Combination of asthma and COPD exacerbations. No results for COPD were reported |
| Willaert 2002 | Different types of bronchodilators used |
| Wisthal 1997 | Trial was performed in stable state only |
| Wu 2012 | Mechanical ventilation setting |
| Zanen 1997 | Trial was performed in stable state only |
COPD: chronic obstructive pulmonary disease; DPI: dry powder inhaler; FEV1 : forced expiratory volume in 1 second; FVC: forced vital capacity; pMDI: pressurised metered‐dose inhaler
Characteristics of ongoing studies [ordered by study ID]
NCT02291016.
| Trial name or title | COPD aerosol study comparing the efficacy of nebulizers versus dry powder inhalers |
| Methods | Registered, currently running and unpublished randomised interventional trial. |
| Participants | Location: University of Tennessee,TN, USA Target: 30 participants Setting: unknown COPD definition: FEV1/FVC ratio ≤ 70% |
| Interventions | Comparison of dosage administered via a nebuliser versus dosage administered via a dry powder inhaler. 12 µg formoterol with the dry powder inhaler and 20 µg (solution form) of formoterol with the nebuliser Beta2 ‐agonist: formoterol DPI: brand not reported Nebuliser: brand not reported Dosage ratio DPI/nebuliser: 1:1.667 |
| Outcomes | The difference between the values of area under the response curve for FEV1 from baseline through 4 h (AUC FEV1 0 to 4 h), |
| Starting date | 2015 |
| Contact information | Contact: Lauren Davis; lsdavis@mc.utmck.edu; principal investigator: Rajiv Dhand, MD |
| Notes | NCT02291016 |
AUC: area under the curve; FEV1 : forced expiratory volume in 1 second; FVC: forced vital capacity
Differences between protocol and review
We published our planned strategy and methods earlier as a protocol (Van Geffen 2015b).
We updated the Background section to include the most recent literature.
We excluded RCTs involving asthma patients and mechanically ventilated patients due to the differences in patients, inhalation and airways in this setting. We did not describe this in our protocol because we did not expect these trials to turn up during our search. There are separate Cochrane reviews for these groups (Cates 2013; Holland 2013).
As reported in the protocol, safety is an important outcome of the review; to clarify this, we added safety assessment as an objective to the review.
Initially, we aimed only to include full‐text papers; however, based on both Cochrane policy and optimal data gathering, we decided to include abstracts in the review.
The included studies defined COPD in several different ways although we anticipated all studies to follow the American Thoracic Society (ATS) or Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria. We reported the definition used by each study in the 'Characteristics of included studies' table, and we reported it specifically in our risk of bias tables if we considered that the definition introduced bias.
We initially formulated one of our secondary outcomes, 'Change in FEV1 closest to one hour after dosing', as within six hours after dosing. However, one study did not report the time of their measurements (Shortall 2002), so we cannot claim with certainty that it happened within six hours after dosing. Nevertheless, one can assume based on their manuscript that the measurements actually were performed within six hours. For this reason we chose not to exclude this report for the meta‐analysis.
Due to the cross‐over design of the included trials, we used the GIV method to analyse outcomes 1.1, 2.2 and 2.3.
We had planned several subgroup analyses, but due to the small number of included studies, we were not able to provide a meaningful pooled analysis by mechanism (anticholinergic or beta‐adrenergic), by short‐acting versus long‐acting agent, or to analyse subgroups separately for SABA, LABA, SAMA, LAMA, and SABA/LAMA combinations. We could not perform a stratification for treatment setting (hospital or outpatient) based on the included studies.
Contributions of authors
WG performed the search, screened titles and abstracts, screened the full‐text records and identified trials for inclusion, performed data extraction, transferred data into RevMan and analysis, assessed risk of bias and wrote the first draft of the manuscript.
WD reviewed the manuscript and functioned as third review author during discussions.
DJS reviewed the manuscript and functioned as third review author during discussions.
HK screened titles and abstracts, screened the full‐text records and identified trials for inclusion, extracted outcome data, double checked transferred data in RevMan, checked papers' trial characteristics, assessed risk of bias and reviewed the manuscript.
Sources of support
Internal sources
The authors declare that no such funding was received for this systematic review, Other.
External sources
The authors declare that no such funding was received for this systematic review, Other.
Declarations of interest
Dr van Geffen reports a grant for an investigator initiated trial to the institution from Novartis, outside the submitted work.
Dr Douma reports no conflicts of interest.
Dr Slebos reports grants, personal fees, non‐financial support and others from PneumRx/BTG (CA, USA); grants, personal fees, non‐financial and other support from Boston Scientific (Europe/USA); grants and non‐financial support from Aeris Therapeutics (USA); grants, personal fees, non‐financial support and other from Holaira, Inc (MN, USA); personal fees from Olympus Europe (Germany); and grants, personal fees, non‐financial support and other from PulmonX (CA, USA); all outside the submitted work.
Dr Kerstjens reports fees paid to his institution from Boehringer Ingelheim and Pfizer based on advisory board participation, lectures, and patient recruitment in trials; from GlaxoSmithKline for advisory board participation and patient recruitment in trials; from Novartis for advisory board participation, lectures, and a grant for an investigator‐initiated trial; and from Almirall and Chiesi for advisory board participation, all outside the submitted work. All fees were paid to the institution.
New
References
References to studies included in this review
Berry 1989 {published data only}
- Berry RB, Shinto RA, Wong FH, Despars JA, Light RW. Nebulizer vs spacer for bronchodilator delivery in patients hospitalized for acute exacerbations of COPD. Chest 1989;96(6):1241‐6. [DOI] [PubMed] [Google Scholar]
Higgins 1987 {published data only}
- Higgins RM, Cookson WO, Chadwick GA. Changes in blood gas levels after nebuhaler and nebulizer administration of terbutaline in severe chronic airway obstruction. Bulletin Européen de Physiopathologie Respiratoire 1987;23(3):261‐4. [PUBMED: 3117150] [PubMed] [Google Scholar]
Maguire 1991 {published data only}
- Maguire GP, Newman T, DeLorenzo LJ, Brown RB, Stone D. Comparison of a hand‐held nebulizer with a metered dose inhaler‐spacer combination in acute obstructive pulmonary disease. Chest 1991;100(5):1300‐5. [DOI] [PubMed] [Google Scholar]
Mazhar 2007 {published data only}
- Mazhar SH, Ismail NE, Newton DA, Chrystyn H. Relative lung deposition of salbutamol following inhalation from a spacer and a Sidestream jet nebulizer following an acute exacerbation. British Journal of Clinical Pharmacology 2008;65(3):334‐7. [PUBMED: 17922883] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mirici 2004 {published data only}
- Mirici A, Meral M, Akgun M, Kaynar H, Kiris S. Comparison of cost‐effectiveness of bronchodilator drugs via inhaler or nebulizer route in exacerbations of chronic obstructive pulmonary disease. Turkish Respiratory Journal 2004;5(3):169‐74. [Google Scholar]
Moss 1985 {published data only}
- Moss K, McDonald A, Ferrara L, Myles D, Brischetto M. Metered dose inhaler vs compressor driven nebuliser in the delivery of metaproterenol. Diseases of Chest 1985;88:53S. [Google Scholar]
Shortall 2002 {published data only}
- Shortall SP, Blum J, Oldenburg FA, Rodgerson L, Branscombe JM, Harrow EM. Treatment of patients hospitalized for exacerbations of chronic obstructive pulmonary disease: comparison of an oral/metered‐dose inhaler regimen and an intravenous/nebulizer regimen. Respiratory Care 2002;47(2):154‐8. [PubMed] [Google Scholar]
Turner 1988 {published and unpublished data}
- Turner JR, Corkery KJ, Eckman D, Gelb AM, Lipavsky A, Sheppard D. Equivalence of continuous flow nebulizer and metered‐dose inhaler with reservoir bag for treatment of acute airflow obstruction. Chest 1988;93(3):476‐81. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdelrahim 2011 {published data only}
- Abdelrahim ME, Plant PK, Chrystyn H. The relative lung and systemic bioavailability of terbutaline following nebulisation in non‐invasively ventilated patients. International Journal of Pharmaceutics 2011;420(2):313‐8. [DOI] [PubMed] [Google Scholar]
Bai 1993 {published data only}
- Bai CX, Niu SF, Rong L, Xu FP, Cai YY. Comparison of salbutamol rebreathing aerosol therapy vs salbutamol nebulizing therapy in COPD patients requiring mechanical ventilation. European Respiratory Journal 1993;6(Suppl):298S. [Google Scholar]
Broeders 2004 {published data only}
- Broeders MEAC, Molema J, Hop WCJ, Vermue NA, Folgering HTHM. The course of inhalation profiles during an exacerbation of obstructive lung disease. Respiratory Medicine 2004;98(12):1173‐9. [DOI] [PubMed] [Google Scholar]
Brunetti 2015 {published data only}
- Brunetti L, Poiani G, Dhanaliwala F, Poppiti K, Kang H, Suh DC. Clinical outcomes and treatment cost comparison of levalbuterol versus albuterol in hospitalized adults with chronic obstructive pulmonary disease or asthma. American Journal of Health‐System Pharmacy 2015;72(12):1026‐35. [DOI] [PubMed] [Google Scholar]
Carpenter 2008 {published data only}
- Carpenter M, Kim KT, Denis‐Mize K, Rinehart M. A pharmacokinetic study in COPD patients comparing fomoterol fumarate delivered by nebulization and DPI [Abstract]. American Thoracic Society International Conference; 2008 May 16‐21; Toronto. 2008:Poster F14.
Cazzola 2002a {published data only}
- Cazzola M, Grella E, Matera MG, Mazzarella G, Marsico SA. Onset of action following formoterol Turbuhaler and salbutamol pMDI in reversible chronic airway obstruction. Pulmonary Pharmacology & Therapeutics 2002;15(2):97‐102. [DOI] [PubMed] [Google Scholar]
Cazzola 2002b {published data only}
- Cazzola M, Matera MG, D'Amato M, Noschese P, Califano C, Perna F, et al. Long‐acting beta2‐agonists in the treatment of acute exacerbations of COPD. Clinical Drug Investigation 2002;22(6):369‐76. [Google Scholar]
Chu 1989 {published data only}
- Chu WM, Lee YC, Dai LKNNS, Tam HC, Ng YY. Comparison of nebuhaler and conventional metered‐dose inhaler in elderly patients. Current Therapeutic Research, Clinical and Experimental 1989;46(4):735‐9. [Google Scholar]
Dhand 2002 {published data only}
- Dhand R, Giri V, Noth I, Lissin D, Fishman R, Taylor K, et al. Bronchodilator response to ipratropium delivered via Aerodose(r) inhaler or pari LC plus nebulizer in COPD [abstract]. American Journal of Respiratory and Critical Care Medicine 2002;165(8):A593. [Google Scholar]
Duarte 2000 {published data only}
- Duarte AG, K Momii K, Bidani A. Bronchodilator therapy with metered‐dose inhaler and spacer versus nebulizer in mechanically ventilated patients: comparison of magnitude and duration of response [Comment]. Respiratory Care 2000;45(7):817‐23. [PubMed] [Google Scholar]
Finnerty 1999 {published data only}
- Finnerty JP, Bullough I, Keeping IM. Trial of route of inhaled albuterol: comparison of nebuliser versus dry powder delivery in patients with COPD [Abstract]. European Respiratory Society; 1999 Oct 9‐13; Madrid. 1999:P3383.
Foresi 2002 {published data only}
- Foresi A, Malorgio R, Cavigioli G, Aiello M, Castagnaro A, Pelucchi A, et al. Changes in FEV and inspiratory capacity (IC) induced by increasing doses of salbutamol delivered by MDI or nebulizer in unselected patients with airflow obstruction. American Journal of Respiratory and Critical Care Medicine 2002;165:A581. [Google Scholar]
Formgren 1994 {published data only}
- Formgren H, Sjökvist A, Stahl E, Wiren JE. Terbutaline in COPD comparison between Turbuhaler and chlorofluorocarbon (CFC) inhaler. Lung 1994;172(5):271‐80. [DOI] [PubMed] [Google Scholar]
Greene 1988 {published data only}
- Greene AB Jr, Jackson CL. Terbutaline metered‐dose inhalation vs metaproterenol by hand‐held nebulization: a comparison in black inner‐city COPD patients. Journal of the National Medical Association 1988;80(4):393‐6. [PUBMED: 3385785] [PMC free article] [PubMed] [Google Scholar]
Guerin 1999 {published data only}
- Guerin C, Chevre A, Dessirier P, Poncet T, Becquemin MH, Dequin PF, et al. Inhaled fenoterol‐ipratropium bromide in mechanically ventilated patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1999;159(4):1036‐42. [DOI] [PubMed] [Google Scholar]
Haynes 2012 {published data only}
- Haynes JM. Randomized controlled trial of a breath‐activated nebulizer in patients with exacerbation of COPD. Respiratory Care 2012;57(9):1385‐90. [DOI] [PubMed] [Google Scholar]
Ikeda 1994 {published data only}
- Ikeda A, Nishimura K, Koyama H, Izumi T. Comparison of the bronchodilator effect of salbutamol delivered from a dry powder inhaler, a metered‐dose inhaler and a wet nebulizer in patients with COPD. European respiratory journal. Supplement 1994;7:435s. [DOI] [PubMed] [Google Scholar]
Jasper 1987 {published data only}
- Jasper AC, Mohsenifar Z, Kahan S, Goldberg HS, Koerner SK. Cost‐benefit comparison of aerosol bronchodilator delivery methods in hospitalized patients. Chest 1987;91(4):614‐8. [DOI] [PubMed] [Google Scholar]
Kaminski 1997 {published data only}
- Kaminski D, Sliwinski P, Zielinski J. Nebuliser vs MDI ‐ comparison of efficacy in severe COPD patients. European Respiratory Journal 1997;10(Suppl):107S. [Google Scholar]
Lai 1990 {published data only}
- Lai CKW, Legge JS, Friend JAR. Air driven nebulised high dose salbutamol (S) in severe chronic obstructive airways disease (COPD): is it safe?. European Respiratory Journal 1990;3(Suppl):118S. [Google Scholar]
Lees 1980 {published data only}
- Lees AW, Allan GW, Smith J. Nebulised ipratropium bromide and salbutamol in chronic bronchitis. British Journal of Clinical Practice 1980;34(11):340‐2. [PubMed] [Google Scholar]
Li 2011 {published data only}
- Li W, Wen J, Lu W, Yin S‐J. Clinical analysis of nebulized budesonide suspension combined with terbutaline sulphate to treat acute exacerbations in chronic obstructive pulmonary disease of elderly people [Abstract]. Respirology 2011;16:1098‐103. [Google Scholar]
Mandelberg 1997 {published data only}
- Mandelberg A, Chen E, Noviski N, Priel IE. Nebulized wet aerosol treatment in emergency department‐‐is it essential? Comparison with large spacer device for metered‐dose inhaler. Chest 1997;112(6):1501‐5. [DOI] [PubMed] [Google Scholar]
Marlin 1986 {published data only}
- Marlin GE. Studies of ipratropium bromide and fenoterol administered by metered‐dose inhaler and aerosolized solution. Respiration; International Review of Thoracic Diseases 1986;50:290‐3. [DOI] [PubMed] [Google Scholar]
Marta 1997 {published data only}
- Marta D, Dutu ST, Bistriceanu G, Stoicescu P, Mihaltan FL, Marica C, et al. Comparison of the bronchodilating effect of salbutamol delivered via a multidose powder inhaler versus a conventional metered dose inhaler [Abstract]. European Respiratory Journal 1997;10(Suppl):238S. [Google Scholar]
Martos 1990 {published data only}
- Martos JA, Montserrat JM, Castells A, Salo J, Llena J, Mellis G, et al. Spacer‐devices versus nebulizers for the delivery of salbutamol in COPD and asthmatic patients during exacerbations. European Respiratory Journal 1990;3(Suppl):94S. [Google Scholar]
Mestitz 1988 {published data only}
- Mestitz H, Copland JM, McDonald. Nebulised versus multidose inhaler bronchodilator therapy in patients with chronic airflow obstruction [Abstract]. Australian and New Zealand Journal of Medicine 1988;18(3):538. [Google Scholar]
Mouloudi 1998 {published data only}
- Mouloudi E, Katsanoulas K, Anastasaki M, Askitopoulou E, Georgopoulos D. Bronchodilator delivery by metered‐dose inhaler in mechanically ventilated COPD patients: influence of end‐inspiratory pause. European Respiratory Journal 1998;12(1):165‐9. [DOI] [PubMed] [Google Scholar]
Nair 2005 {published data only}
- Nair S, Thomas E, Pearson SB, Henry MT. A randomized controlled trial to assess the optimal dose and effect of nebulized albuterol in acute exacerbations of COPD. Chest 2005;128(1):48‐54. [DOI] [PubMed] [Google Scholar]
Numata 2002 {published data only}
- Numata Y, Bourbeau J, Ernst P, Duquette G, Schwartzman K. Teaching time for metered‐dose inhalers in the emergency setting. Chest 2002;122(2):498‐504. [DOI] [PubMed] [Google Scholar]
Pappalettera 2005 {published data only}
- Pappalettera M, Canetta C, Aliberti S, Folli C, Milani G, Brambilla A. Aerosol vs. nebulisation: comparison of two methods of salbutamol administration in AECB hypercapnic respiratory failure requiring NIMV [Abstract]. European Respiratory Journal 2005;26:Abstract No. 3108. [Google Scholar]
Petrova 2001 {published data only}
- Petrova, I Ryzhova, M. The effect of nebulized fenoterol‐ipratropium bromide compared with combined fenoterol‐ipratropium bromide and ambroxol in patients with acute attacks of chronic obstructive pulmonary disease. Pneumologie (Stuttgart, Germany) 2001;55:S50. [Google Scholar]
Qian 2008 {published data only}
- Qian XY. Effectiveness observation on bricanyl and ipratropium bromide with oxygen nebulizer inhalation for acute exacerbation of chronic obstructive pulmonary disease (COPD). Xian Dai Zhong Xi Yi Jie He Za Zhi [Modern Journal of Integrated Traditional Chinese and Western Medicine] 2008;17(1):33‐4. Chinese. [Google Scholar]
Quinn 2014 {published data only}
- Quinn D, Seale JP, Reisner C, Fischer T, Golden M, Fernandez C, et al. A randomized study of formoterol fumarate in a porous particle metered‐dose inhaler in patients with moderate‐to‐severe COPD. Respiratory Medicine 2014;108(9):1327‐35. [PUBMED: 25060541] [DOI] [PubMed] [Google Scholar]
Rebuck 1987 {published data only}
- Rebuck AS, Chapman KR, Abboud R, Pare PD, Kreisman H, Wolkove N, et al. Nebulized anticholinergic and sympathomimetic treatment of asthma and chronic obstructive airways disease in the emergency room. American Journal of Medicine 1987;82(1):59‐64. [DOI] [PubMed] [Google Scholar]
Schleufe 2004 {published data only}
- Schleufe P, Domurath H, Piepenbrock S. Beta(2)‐agonist delivery via a resuscitator bag (Ambu MediBag): a comparison with a metered‐dose inhaler using the Volumatic‐Spacer. Resuscitation 2004;61(3):327‐31. [DOI] [PubMed] [Google Scholar]
Segreti 2013 {published data only}
- Segreti A, Fiori E, Calzetta L, Sabatini M, Segreti V, Rogliani P, et al. The effect of indacaterol during an acute exacerbation of COPD. Pulmonary Pharmacology & Therapeutics 2013;26(6):630‐4. [DOI] [PubMed] [Google Scholar]
Summer 1989 {published data only}
- Summer W, Elston R, Tharpe L, Nelson S, Haponik EF. Aerosol bronchodilator delivery methods. Relative impact on pulmonary function and cost of respiratory care. Archives of Internal Medicine 1989;149(3):618‐23. [PUBMED: 2919938] [DOI] [PubMed] [Google Scholar]
Willaert 2002 {published data only}
- Willaert W, Daenen M, Bomans P, Verleden G, Decramer M. What is the optimal treatment strategy for chronic obstructive pulmonary disease exacerbations?. European Respiratory Journal 2002;19(5):928‐35. [DOI] [PubMed] [Google Scholar]
Wisthal 1997 {published data only}
- Wisthal B, Schonrock B, Hermann R, Peukert M. Clinical investigation comparing the bronchodilator effects of a salbutamol multiple‐dose powder inhaler versus a salbutamol metered‐dose inhaler [Abstract]. European Respiratory Journal 1997;10(Suppl):238S. [Google Scholar]
Wu 2012 {published data only}
- Wu HP, Liu YC, Lin SC, Chien MY, Liao FC, Chang SC, et al. Comparison of respiratory parameters and plasma cytokine levels between treatment with Salmeterol/fluticasone and ipratropium/terbutaline/budesonide in mechanically ventilated COPD patients. Chang Gung Medical Journal 2012;35(5):373‐81. [DOI] [PubMed] [Google Scholar]
Zanen 1997 {published data only}
- Zanen P. The efficacy of a low dosed, monodisperse parasympathicolytic aerosol compared to a standard aerosol from a metered dose inhaler [Abstract]. European Respiratory Journal 1997;10(Suppl):128S. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02291016 {published data only}
- NCT02291016. COPD aerosol study comparing the efficacy of nebulizers versus dry powder inhalers. https://clinicaltrials.gov/ct2/show/NCT02291016 accessed 22 July 2016.
Additional references
Appleton 2006
- Appleton S, Jones T, Poole P, Pilotto L, Adams R, Lasserson TJ, et al. Ipratropium bromide versus short acting beta‐2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD001387.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burge 2003
- Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. European Respiratory Journal 2003;41(Suppl):46s‐53S. [DOI] [PubMed] [Google Scholar]
Cates 2013
- Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta‐agonist treatment of acute asthma. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD000052.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cazzola 2008
- Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. European Respiratory Journal 2008;31(2):416‐69. [PUBMED: 18238951] [DOI] [PubMed] [Google Scholar]
De Boer 2003
- Boer AH, Hagedoorn P, Frijlink HW. The choice of a compressor for the aerosolisation of tobramycin (TOBI) with the PARI LC PLUS reusable nebuliser. International Journal of Pharmaceutics 2003;268(1‐2):59‐69. [PUBMED: 14643977] [DOI] [PubMed] [Google Scholar]
Demoly 2014
- Demoly P, Hagedoorn P, Boer AH, Frijlink HW. The clinical relevance of dry powder inhaler performance for drug delivery. Respiratory Medicine 2014;108(8):1195‐203. [DOI] [PubMed] [Google Scholar]
Dolovich 2005
- Dolovich MB, Ahrens RC, Hess DR, Anderson P, Dhand R, Rau JL, et al. Device selection and outcomes of aerosol therapy: evidence‐based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest 2005;127(1):335‐71. [PUBMED: 15654001] [DOI] [PubMed] [Google Scholar]
GOLD 2015
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2015. From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2015. http://www.goldcopd.org/ (accessed 27 July 2015).
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 22 July 2016. Hamilton, Ontario: GRADE Working Group, McMaster University, 2014.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holland 2013
- Holland A, Smith F, Penny K, McCrossan G, Veitch L, Nicholson C. Metered dose inhalers versus nebulizers for aerosol bronchodilator delivery for adult patients receiving mechanical ventilation in critical care units. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD008863.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2014
- Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. American Journal of Respiratory and Critical Care Medicine 2014;189(3):250‐5. [PUBMED: 24383418] [DOI] [PubMed] [Google Scholar]
Kew 2014
- Kew KM, Dias S, Cates CJ. Long‐acting inhaled therapy (beta‐agonists, anticholinergics and steroids) for COPD: a network meta‐analysis. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD010844.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Le Brun 1999
- Brun PP, Boer AH, Gjaltema D, Hagedoorn P, Heijerman HG, Frijlink HW. Inhalation of tobramycin in cystic fibrosis. Part 1: the choice of a nebulizer. International Journal of Pharmaceutics 1999;189(2):205‐14. [PUBMED: 10536249] [DOI] [PubMed] [Google Scholar]
Lopez‐Campos 2015
- Lopez‐Campos JL, Agusti A. Heterogeneity of chronic obstructive pulmonary disease exacerbations: a two‐axes classification proposal. The Lancet Respiratory Medicine 2015;3(9):729‐34. [DOI] [PubMed] [Google Scholar]
Mazhar 2008
- Mazhar SH, Ismail NE, Newton DA, Chrystyn H. Relative lung deposition of salbutamol following inhalation from a spacer and a Sidestream jet nebulizer following an acute exacerbation. British Journal of Clinical Pharmacology 2008;65(3):334‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oostenbrink 2004
- Oostenbrink JB, Rutten‐van Molken MP. Resource use and risk factors in high‐cost exacerbations of COPD. Respiratory Medicine 2004;98(9):883‐91. [PUBMED: 15338802] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Turner 1997
- Turner MO, Patel A, Ginsburg S, FitzGerald JM. Bronchodilator delivery in acute airflow obstruction. A meta‐analysis. Archives of Internal Medicine 1997;157(15):1736‐44. [PubMed] [Google Scholar]
Van Geffen 2015a
- Geffen WH, Slebos DJ, Kerstjens HA. Hyperinflation in COPD exacerbations. The Lancet Respiratory Medicine 2015;3(12):e43‐4. [DOI: 10.1016/S2213-2600(15)00459-2] [DOI] [PubMed] [Google Scholar]
Van Geffen 2016
- Geffen WH, Bruins M, Kerstjens HA. Diagnosing viral and bacterial respiratory infections in acute COPD exacerbations by an electronic nose: a pilot study. Journal of Breath Research 2016;10(3):036001. [PUBMED: 27310311] [DOI] [PubMed] [Google Scholar]
WHO 2014
- World Health Organization. The top 10 causes of death. Fact sheet N°310. http://www.who.int/mediacentre/factsheets/fs310/en/ accessed 22 July 2016.
Zheng 2014
- Zheng Z, Jinping Z, Zhongping W, Yanqing X, Yi G, Liping Z, et al. Clinical practice of nebulized therapy in China: a national questionnaire survey. Journal of Aerosol Medicine and Pulmonary Drug Delivery 2014;27(5):386‐91. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Van Geffen 2015b
- Geffen WH, Douma WR, Slebos DJ, Kerstjens HA. Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD011826] [DOI] [PMC free article] [PubMed] [Google Scholar]


