Abstract
Background
Smoking cessation reduces lung cancer mortality. However, little is known about whether diagnosis of lung cancer impacts changes in smoking behaviors. Furthermore, the effects of smoking cessation on the risk of second primary lung cancer (SPLC) have not been established yet. This study aims to examine smoking behavior changes after initial primary lung cancer (IPLC) diagnosis and estimate the effect of smoking cessation on SPLC risk following IPLC diagnosis.
Methods
The study cohort consisted of 986 participants in the Multiethnic Cohort Study who were free of lung cancer and active smokers at baseline (1993-1996), provided 10-year follow-up smoking data (2003-2008), and were diagnosed with IPLC in 1993-2017. The primary outcome was a change in smoking status from “current” at baseline to “former” at 10-year follow-up (ie, smoking cessation), analyzed using logistic regression. The second outcome was SPLC incidence after smoking cessation, estimated using cause-specific Cox regression. All statistical tests were 2-sided.
Results
Among 986 current smokers at baseline, 51.1% reported smoking cessation at 10-year follow-up. The smoking cessation rate was statistically significantly higher (80.6%) for those diagnosed with IPLC between baseline and 10-year follow-up vs those without IPLC diagnosis (45.4%) during the 10-year period (adjusted odds ratio = 5.12, 95% confidence interval [CI] = 3.38 to 7.98; P < .001). Incidence of SPLC was statistically significantly lower among the 504 participants who reported smoking cessation at follow-up compared with those without smoking cessation (adjusted hazard ratio = 0.31, 95% CI = 0.14 to 0.67; P = .003).
Conclusion
Lung cancer diagnosis has a statistically significant impact on smoking cessation. Quitting smoking after IPLC diagnosis may reduce the risk of developing a subsequent malignancy in the lungs.
Lung cancer is the second most common cancer and the leading cause of cancer death in the United States (1). Past research has found smoking to be the strongest risk factor for lung cancer, increasing the disease incidence up to 20 times (2,3). Smoking cessation has been shown to reduce the risk of lung cancer incidence and mortality (4-6). A large prospective cohort study reported a 50%-80% reduction of lung cancer risk among individuals who had either recently quit smoking or were stable former smokers (7), and another historical cohort study reported a substantially reduced lung cancer-specific mortality after smoking cessation, with greater survival benefits associated with an earlier age at cessation (8).
Several studies have examined the impact of smoking cessation on lung cancer incidence and mortality (4-9), however, little is known about whether lung cancer diagnosis is associated with changes in smoking behavior. For example, it is unknown whether lung cancer diagnosis potentially leads to smoking cessation among active smokers. Prior studies on smoking cessation among lung cancer patients were primarily based on smoking data measured cross-sectionally, with few studies fully incorporating individual-level longitudinal changes in smoking data to assess smoking cessation and its impact on health outcomes (10,11).
With advances in early detection and therapeutic techniques for lung cancer, the number of lung cancer survivors is rapidly increasing (12). It has been shown that the risk of developing second primary lung cancer (SPLC) among lung cancer survivors is 5-6 times higher than the risk of a person in the general population developing initial primary lung cancer (IPLC) (13,14). However, despite the well-established role of smoking on IPLC risk, the impact of smoking on the subsequent risk of SPLC has remained controversial until recently; prior literature reported inconsistent results (15,16) in part due to heterogeneous smoking exposure measures examined across different studies, low statistical power from small sample sizes in single institution–based studies, and a lack of appropriate statistical methods. Recently, we reported statistically significant associations between SPLC risk and smoking pack-years and smoking intensity (as measured by cigarettes per day) among IPLC cases (17). A preliminary analysis was conducted by our group to evaluate the association between smoking cessation and SPLC risk, however, it was based on a small subset of data (n < 200), and a more comprehensive analysis using larger data is warranted to robustly capture the effect of smoking cessation on SPLC risk.
In this study, we aimed to examine smoking behavioral changes after IPLC diagnosis using a large, prospective, population-based multi-ethnic cohort with a long follow-up time. We aimed to evaluate the factors associated with smoking cessation among active smokers, which include time of IPLC diagnosis, race and ethnicity, family history of lung cancer, and body mass index (BMI). Additionally, we examined the impact of smoking cessation on SPLC risk by estimating the cumulative incidence of SPLC based on smoking cessation status.
Methods
Participants, Study Design, and Outcomes
The Multiethnic Cohort (MEC) is a prospective, population-based cohort that follows 215 251 participants aged 45 to 75 years at enrollment (1993-1996) from 5 main racial and ethnic groups (18). Baseline epidemiologic data including sociodemographic and lifestyle factors and medical histories were collected through a self-reported questionnaire at enrollment. Smoking-related variables were assessed longitudinally at baseline (1993-1996) and at 10-year follow-up (2003-2008).
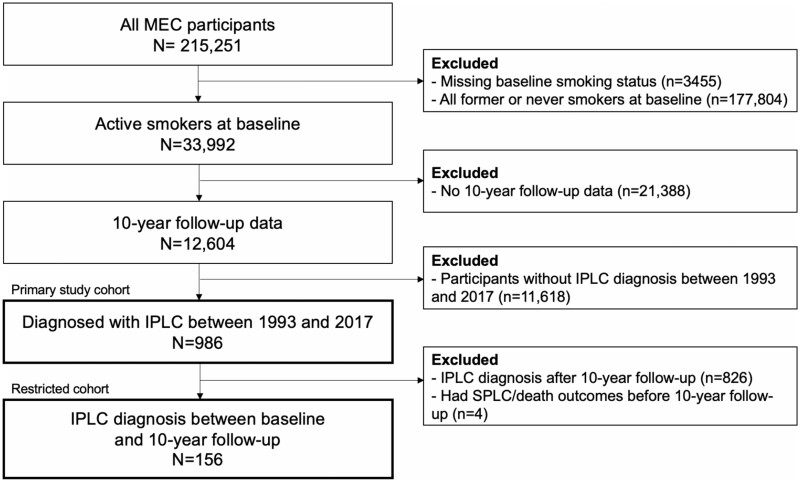
We defined our study cohort (n = 986) as the MEC participants who were free of lung cancer and active smokers at baseline, had provided updated smoking information at 10-year follow-up, and were diagnosed with IPLC sometime between 1993 and 2017 (see Figure 1).
Figure 1.
Cohort selection diagram. IPLC = initial primary lung cancer; MEC = Multiethnic Cohort; SPLC = second primary lung cancer.
The primary outcome of this study was smoking cessation, as defined by a change in smoking status from “current” at baseline to “former” at the 10-year follow-up survey. To evaluate the impact of IPLC diagnosis on this outcome, we assessed the association between smoking cessation and IPLC diagnoses that had specifically occurred between baseline and 10-year follow-up. The secondary outcome was the incidence of SPLC, as followed from the time of IPLC diagnosis (mean 10.9 [SD = 1.33] years). SPLC was defined using the well-established Martini and Melamed criteria (19). Information on IPLC and SPLC diagnosis was obtained via Surveillance, Epidemiology, and End Results (SEER) registries and collected through 2017.
Statistical Analysis
For the primary outcome, we used multivariable logistic regression to evaluate the association between smoking cessation and IPLC diagnosis that occurred between baseline enrollment and 10-year follow-up. The covariates for the multivariable logistic regression model were selected using a set of univariable logistic models (P < .05) that evaluated the association between smoking cessation and an individual demographic or clinical variable.
For the secondary outcome, we evaluated the association between smoking cessation and SPLC risk by applying cause-specific Cox proportional hazards regression (n = 982), with death from all causes as the competing risk (20). Out of the primary cohort (n = 986), 4 participants were diagnosed with SPLC before 10-year follow-up and were excluded to accurately measure the effect of smoking cessation on SPLC risk. This analysis aimed to evaluate whether smoking cessation is associated with a reduced risk of SPLC, regardless of when smoking cessation occurred (ie, before or after IPLC diagnosis). The cause-specific Cox regression was adjusted for age at initial diagnosis and stage of IPLC based on prior literature (13).
In addition, we conducted a restricted analysis that evaluated the association between SPLC risk and smoking cessation that occurred after IPLC diagnosis by limiting the cohort to those who had IPLC diagnosed before 10-year follow-up (n = 156). By doing so, we aimed to examine whether smoking cessation that specifically occurred after IPLC diagnosis was associated with a decreased risk of SPLC. The cause-specific Cox model was refitted based on this restricted cohort and was adjusted for age at initial diagnosis and IPLC stage derived from the SEER summary stage.
The cumulative risk of SPLC was estimated using the Aalen-Johansen estimator to account for the competing risk of death among participants at risk at a specific time point. All statistical analyses were conducted using R version 3.6.0. Cause-specific Cox regression was done using the riskRegression package (TA Gerds, University of Copenhagen), and cumulative incidence plots were created using the prodlim package (TA Gerds, University of Copenhagen). The 2-sided Wald test was used to assess for statistical significance, with P values less than .05 being considered statistically significant.
Missing Data
The prevalence of missing data for all variables included in our study was low (0%-2%), with the exception of IPLC stage (4.9%) (Supplementary Table 1, available online). The IPLC stage variable was derived from the SEER summary stage, categorized by early for localized and regional and advanced for distant stage. We examined the missing data mechanism for IPLC stage and determined that it was not missing completely at random based on descriptive analysis stratified by missing status of IPLC stage (Supplementary Table 2, available online) (21). Given that IPLC stage was not self-reported but was linked through the SEER registry, selective nonresponse (missing not at random) is also unlikely. Therefore, we performed multiple imputation by chain equations (21,22) in our primary analysis to impute missing data 10 times using all the patient characteristics. We applied Rubin rules to obtain the pooled estimates of regression analyses from multiple imputed datasets (23). Additionally, we conducted a complete-case analysis for the secondary outcome using individuals with complete data for all variables included in our study (n = 934).
Sensitivity Analysis
Sensitivity analyses were performed to assess the robustness of the association between IPLC diagnosis and smoking cessation. We evaluated the association in different subgroups defined by smoking pack-years (<30 pack-years vs ≥30 pack-years) and age at baseline (younger than 60, 60-69, older than 69 years) and under a different set of covariates selected using a more stringent threshold defined by Bonferroni criteria (P < .005) from the univariable logistic analysis.
Given that the exact timing of smoking cessation was not available for those who reported smoking cessation at follow-up in the MEC, we assumed that patients who were diagnosed with IPLC between baseline and 10-year follow-up and had quit smoking during this time frame did so following their IPLC diagnosis; thus, some misclassification may have occurred with regards to the chronological sequence of smoking cessation and IPLC diagnosis. Therefore, we conducted a sensitivity analysis by limiting the patients who were diagnosed with IPLC between baseline and 10-year follow-up in the primary cohort to only those who were diagnosed within 5 years from baseline, which may help increase the likelihood of smoking cessation occurring after IPLC diagnosis.
Results
Participant Characteristics
The primary cohort was comprised of 23.9% Whites, 23.4% African Americans, 23.5% Japanese Americans, 13.9% Latinos, and 11.9% Native Hawaiians (Table 1). The 986 participants in the primary cohort had a mean (SD) of 31.5 (16.2) pack-years and a mean of 17.9 (7.8) cigarettes per day at baseline. Of 986 individuals, 160 (16.4%) were diagnosed with IPLC between baseline and 10-year follow-up, and the remaining 826 (83.6%) individuals developed IPLC after 10-year follow-up through 2017.
Table 1.
Population characteristics stratified by smoking cessation status at 10-year follow-up in the MECa
| Variable | Overall | No smoking cessation (ie, current-current)b | Smoking cessation (ie, current-former)b | P c |
|---|---|---|---|---|
| Total, No. (%) | 986 | 482 (48.9) | 504 (51.1) | |
| Mean age at baseline (SD), y | 59.55 (7.23) | 58.76 (7.23) | 60.29 (7.17) | .001 |
| Mean BMI (SD), kg/m2 | 25.66 (5.00) | 26.25 (5.62) | 25.09 (4.26) | <.001 |
| Male sex, No. (%) | 527 (53.4) | 259 (49.1) | 268 (50.9) | .91 |
| Education, No. (%) | .87 | |||
| High school or less | 460 (46.7) | 221 (48.0) | 239 (52.0) | |
| Some college or graduate | 423 (43.0) | 209 (49.4) | 214 (50.6) | |
| Postgraduate | 101 (10.3) | 51 (50.5) | 50 (49.5) | |
| Family history of lung cancer, No. (%) | 81 (8.2) | 30 (37.0) | 51 (63.0) | .04 |
| Prior history of cancer, No. (%) | 226 (22.9) | 108 (47.8) | 118 (52.2) | .76 |
| Pack-years, mean (SD) | 31.50 (16.21) | 34.15 (15.83) | 28.98 (16.18) | <.001 |
| Cigarettes per day, mean (SD) | 17.86 (7.84) | 19.18 (7.74) | 16.61 (7.74) | <.001 |
| Race, No. (%) | .14 | |||
| White | 236 (23.9) | 107 (45.3) | 129 (54.7) | |
| African American | 231 (23.4) | 118 (51.1) | 113 (48.9) | |
| Japanese American | 232 (23.5) | 108 (46.6) | 124 (53.4) | |
| Latino | 137 (13.9) | 69 (50.4) | 68 (49.6) | |
| Native Hawaiian | 117 (11.9) | 68 (58.1) | 49 (41.9) | |
| Other | 33 (3.3) | 12 (36.4) | 21 (63.6) | |
| IPLC diagnosis, No. (%) | <.001 | |||
| No | 826 (83.6) | 445 (54.6) | 370 (45.4) | |
| Yesd | 160 (16.4) | 31 (19.4) | 129 (80.6) |
Based on n = 986 patients who were 1) active smokers at baseline, 2) had 10-year follow-up information, and 3) were diagnosed with IPLC between 1993 and 2017. BMI = body mass index; IPLC = initial primary lung cancer; MEC = Multiethnic Cohort.
P value was calculated across smoking cessation stratum using the χ2 test for categorical data and the t test for continuous data.
IPLC diagnosis between baseline (1993-1996) and 10-year follow-up (2003-2008).
Smoking-related variables were measured at baseline and updated with 10-year follow-up data prior to lung cancer diagnosis. Current-current denotes patients who were current smokers at baseline and remained as smokers at 10-year follow-up; current-former denotes patients who were current smokers at baseline and quit smoking sometime between baseline and 10-year follow-up.
Smoking Cessation and Initial Primary Lung Cancer Diagnosis
Among the 986 participants who were active smokers at baseline, 504 (51.1%) participants reported having quit smoking at 10-year follow-up, and 482 (48.9%) participants remained active smokers. Notably, those who were diagnosed with IPLC between baseline and 10-year follow-up had a higher smoking cessation rate (80.6%) at follow-up vs those who were not diagnosed with IPLC between baseline and 10-year follow-up (45.4%) (Table 1). In addition, compared with the participants who remained active smokers, those who reported smoking cessation at follow-up tended to be older at baseline (60.29 vs 58.76 years), had a lower mean smoking pack-years (28.98 vs 34.15 pack-years) and smoking intensity (16.61 vs 19.18 cigarettes per day) measured at baseline, had a lower mean BMI (25.09 vs 26.25 kg/m2), and a higher proportion of family history of lung cancer (63.0% vs 37.0%) (Table 1).
Table 2 presents the results of the multivariable logistic analysis to evaluate the association between smoking cessation and IPLC diagnosis during the 10-year follow-up period, adjusting for factors that were selected by univariable logistic regression analysis (P < .05; Supplementary Table 3, available online). The results show that those who were diagnosed with IPLC between baseline and 10-year follow-up questionnaire were more likely to have quit smoking (adjusted odds ratio [aOR] = 5.12, 95% confidence interval [CI] = 3.38 to 7.98; P < .001) at the follow-up questionnaire.
Table 2.
Multivariate logistic regression analysis for factors associated with smoking cessation at 10-year follow-up in the MECa
| Variable | aOR (95% CI) | P b |
|---|---|---|
| IPLC diagnosis (%) | ||
| No | 1.00 (Referent) | |
| Yesc | 5.12 (3.38 to 7.98) | <.001 |
| BMI (kg/m2) | 0.97 (0.95 to 1.00) | .07 |
| Age at baseline, y | 1.03 (1.01 to 1.05) | .003 |
| Pack-years | 0.97 (0.96 to 0.98) | <.001 |
| Family history of lung cancer, % | ||
| No | 1.00 (Referent) | |
| Yes | 1.77 (1.08 to 2.95) | .03 |
| Race (%) | ||
| White | 1.00 (Referent) | |
| African American | 0.60 (0.39 to 0.92) | .02 |
| Japanese American | 0.89 (0.60 to 1.33) | .58 |
| Latino | 0.57 (0.35 to 0.92) | .02 |
| Native Hawaiian | 0.51 (0.31 to 0.84) | .009 |
| Other | 1.18 (0.52 to 2.75) | .70 |
Based on n = 986 patients who were 1) current smokers at baseline, 2) had 10-year follow-up information, and 3) were diagnosed with IPLC between 1993 and 2017. aOR = adjusted odds ratio; CI = confidence interval; BMI = body mass index; IPLC = initial primary lung cancer; MEC = Multiethnic Cohort.
P value was calculated using the 2-sided Wald test.
IPLC diagnosis between baseline (1993-1996) and 10-year follow-up (2003-2008).
Given that the exact timing of smoking cessation was not available for those who reported smoking cessation at follow-up in the MEC, we assumed that smoking cessation occurred after IPLC diagnosis for patients who were diagnosed with IPLC between baseline and 10-year follow-up (median diagnosis time 8.34 years after baseline) (Supplementary Figure 2, A, available online). To better account for this chronological sequence of events, we conducted a sensitivity analysis where we limited the patients in the primary cohort who were diagnosed with IPLC between baseline and 10-year follow-up to only those who were diagnosed within 5 years from baseline; analysis under this limiting criterion yielded a higher odds ratio (aOR = 7.14, 95% CI = 3.80 to 14.68; P < .001) compared with the odds ratio found for the primary analysis, consistent with the expectation that lung cancer diagnosis increases the likelihood of smoking cessation occurring.
Sensitivity analyses by evaluating a more stringent set of covariates (Supplementary Table 4, available online), limiting the duration of time between baseline assessment and IPLC diagnosis (Supplementary Table 5, available online), and evaluating across different subgroups (Supplementary Figure 1, available online) showed consistent findings on the association between smoking cessation and IPLC diagnosis.
Smoking Cessation and SPLC Risk
In the primary study cohort of 986 participants who were diagnosed with IPLC between 1993 and 2017, 33 participants (3.4%) developed SPLC after IPLC, and 644 participants died before developing SPLC (65.3%). The median of the time elapsed between IPLC and SPLC was 4.34 years (interquartile range = 1.25 and 8.75, respectively) (Supplementary Figure 2, B, available online).
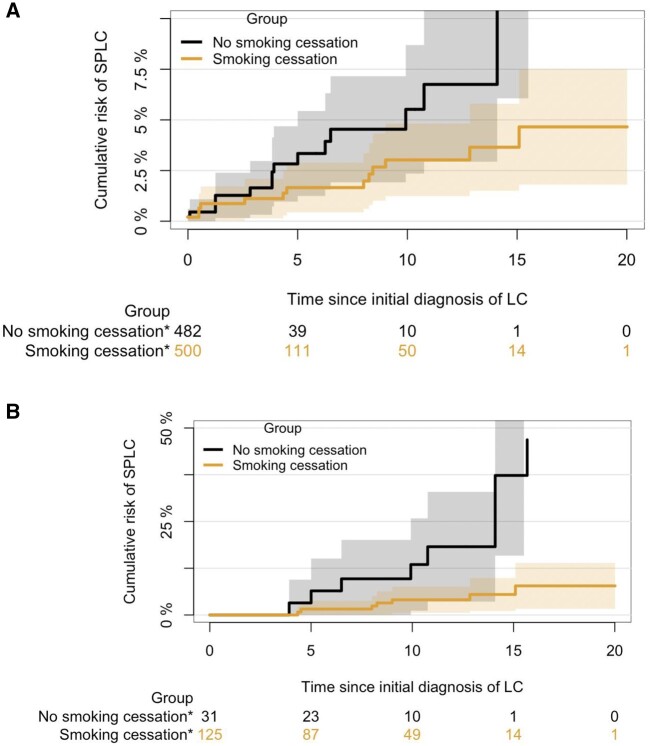
In the cohort of 982 participants used to evaluate the association between SPLC risk and smoking cessation, the 10-year and 15-year cumulative risk of SPLC since IPLC diagnosis was 3.8% (95% CI = 2.3% to 5.4%) and 5.6% (95% CI = 3.3% to 7.9%), respectively. Cause-specific hazards analysis showed that any smoking cessation that occurred after baseline—regardless of whether it was before or after IPLC diagnosis—was statistically significantly associated with a decrease in SPLC risk (hazard ratio [HR] = 0.31, 95% CI = 0.14 to 0.67; P = .003) (Table 3). Advanced IPLC stage and older age were associated with a lower risk of SPLC because of the competing risk of death. Complete-case analysis excluding those with missing IPLC stage information (n = 934) showed consistent results (Supplementary Table 6, available online). The cumulative incidence of SPLC based on smoking cessation status is shown in Figure 2, A.
Table 3.
Cause-specific hazard ratios of factors associated with risk of second primary lung cancer in the primary cohorta,b
| Variable | aHR (95% CI) | P c |
|---|---|---|
| Smoking cessation b,d | ||
| No, current-current | 1.00 (Referent) | |
| Yes, current-former) | 0.31 (0.14 to 0.67) | .003 |
| Age at initial diagnosis, y | 0.97 (0.93 to 1.02) | .27 |
| Stage at initial diagnosis | ||
| Early, I-III | 1.00 (Referent) | |
| Advanced, IV | 0.42 (0.14 to 1.23) | .11 |
Based on n = 982 patients who were 1) current smokers at baseline, 2) had 10-year follow-up information, and 3) had a SPLC outcome that occurred after their 10-year follow-up. aHR = adjusted hazard ratio; CI = confidence interval.
Based on 10 completed datasets (n = 982 x 10) by multiple imputation in the Multiethnic Cohort. Hazards of the associated factors were fitted in each of imputed datasets and pooled using Rubin rules.
P value was calculated using the 2-sided Wald test.
Surveyed at baseline and 10-year follow-up.
Figure 2.
Cumulative risk of second primary lung cancer (SPLC) by smoking cessation status. A) Cumulative risk of SPLC by smoking cessation status based on the primary cohort for SPLC analysis (n = 982) B) Cumulative risk of SPLC by smoking cessation status based on the restricted cohort (n = 156) of individuals who had initial primary lung cancer diagnosed before 10-year follow-up. Asterisk denotes number of people who are at risk at each time point. LC = lung cancer
To evaluate the impact of smoking cessation that occurred after IPLC diagnosis on SPLC risk, we limited the cohort to only those whose IPLC diagnosis occurred before the 10-year follow-up (n = 156). Analysis under this reduced cohort showed a stronger association between reduced SPLC risk and smoking cessation after IPLC diagnosis (HR = 0.14, 95% CI = 0.05 to 0.40; P < .001) compared with the primary cohort (Table 4 and Figure 2, B).
Table 4.
Cause-specific hazard ratios of factors associated with risk of second primary lung cancer in restricted cohorta,b
| Variable | aHR (95% CI) | P c |
|---|---|---|
| Smoking cessationb,d | ||
| No, current-current | 1.00 (Referent) | |
| Yes, current-former | 0.14 (0.049 to 0.40) | <.001 |
| Age at initial diagnosis, y | 1.00 (0.93 to 1.08) | .97 |
| Stage at initial diagnosis | ||
| Early, I-III | 1.00 (Referent) | |
| Advanced, IV | 0.27 (0.03 to 2.10) | .21 |
Based on n = 156 patients who were 1) diagnosed with initial lung cancer between baseline and 10-year follow up and 2) had a SPLC outcome that occurred after 10-year follow-up. aHR = adjusted hazard ratio; CI = confidence interval.
Based on 10 completed datasets (n = 156 x 10) by multiple imputation in the MEC. Hazards of the associated factors were fitted in each of imputed datasets and pooled using Rubin rules.
P value was calculated using the 2-sided Wald test.
Surveyed at baseline and 10-year follow-up.
Discussion
In this study, we evaluated smoking behavioral changes after lung cancer diagnosis and the impact of smoking cessation on SPLC risk using a large, population-based prospective cohort. We showed that the smoking cessation rate was substantially higher among those diagnosed with IPLC between baseline and 10-year follow-up compared with those without IPLC diagnosis during this period. Our analysis also showed that those who reported smoking cessation at follow-up questionnaire had a statistically significantly lower risk of SPLC following IPLC diagnosis compared with those who continued to smoke.
The impact of smoking cessation has been examined previously among general populations (4–8) and among patients after diagnosis of lung cancer (10) and other diseases (24–26). However, these studies primarily related smoking cessation to mortality or disease incidence as the study outcome; studies specific for cancer patients, especially those linking the effect of cancer diagnosis with smoking cessation, are very limited. One recent study reported on the statistically significant association between thoracic surgery and higher smoking cessation (27), showing the plausibility that a major life event, such as lung cancer diagnosis or thoracic surgery, might have a considerable impact on smoking behavioral changes. Another study examined the effect of a pulmonary finding on subsequent smoking behavioral changes and found that the more severe the pulmonary abnormality, the greater the likelihood of smoking cessation (28). However, these studies did not specifically focus on confirmed lung cancer diagnoses but instead examined all pulmonary screening results or a wide range of patients with thoracic comorbidities.
Our analysis confirms previous findings that reported the statistically significant association between smoking cessation and other smoking behaviors and sociodemographic factors, including race and ethnicity (9,29). In our study, smoking cessation at 10-year follow-up was statistically significantly lower for African Americans, Latinos, and Native Hawaiians compared with Whites; these observations indicate the need to tailor smoking cessation support based on race and ethnicity. Of note, participants in the MEC with a higher number of smoking pack-years were less likely to quit smoking. These results concur with previous general population studies (30,31), which found that heavy smokers were less likely to change their smoking habits compared with light or intermittent smokers, who were more sensitive to external cues and more likely to change their smoking patterns because of lower nicotine dependence (32,33).
The Centers for Disease Control and Prevention’s National Comprehensive Cancer Control Program supports increasing the knowledge and availability of evidence-based smoking cessation services (eg, counseling, pharmacotherapy) among cancer survivors (34). Clinical evidence has found that participation in low-dose computed tomography (LDCT) screening programs can substantially increase the likelihood of smoking abstinence (35) and that LDCT screening and smoking cessation together lead to maximum lung cancer mortality reduction (36). Taken together with this evidence, our findings emphasize the potential for integrating smoking cessation with screening (37), especially among lung cancer survivors who have a high risk of developing SPLC. Smoking cessation coupled with screening may help lower lung cancer survivors’ risk of developing SPLC, leading to greater reduction of lung cancer mortality overall; however, further investigation is needed to confirm the efficacy of LDCT screening on mortality reduction among lung cancer survivors.
To our knowledge, this study presents the first effort to examine the impact of lung cancer diagnosis on smoking cessation and to investigate the effect of smoking cessation on SPLC risk using a population-based prospective cohort. The large and racially and ethnically diverse population evaluated in this study coupled with the long follow-up time provided comprehensive and structured smoking data for our analysis. Additionally, our study examined the effect of smoking cessation by considering temporal smoking behavior changes and by conducting longitudinal analysis rather than cross-sectional analysis (ie, comparing current smokers to former or never smokers at baseline only). Thorough sensitivity analyses were performed to evaluate the robustness of the findings, which largely showed consistent results.
This study has several limitations. Given that all exposure data were self-reported, results could be biased by recall bias. Additionally, the lung cancer cases in MEC have relatively lower current smoking prevalence (41%) compared with lung cancer patients from previous studies (42%-83%) (10). However, as this analysis focused exclusively on current smokers at baseline, differential biases based on smoking status were minimized. To assess longitudinal smoking behavior changes, the present study included only active smokers at baseline who provided smoking status information at 10-year follow-up, leading to a reduced sample size and potential selection bias. Furthermore, the exact timing of smoking cessation was also not available for those who reported smoking cessation at 10-year follow-up in MEC, which may have occurred any time between baseline and follow-up. However, a sensitivity analysis to better account for the chronological sequence of events (ie, smoking cessation following IPLC diagnosis) yielded results consistent with the expectation that lung cancer diagnosis increases the likelihood of smoking cessation occurring. In addition, our cohort consisted only of lung cancer cases, a population subset that may be different from other active smokers in the MEC who did not go on to develop lung cancer (ie, our cohort may be inclusive of heavier smokers). If true, we hypothesize that if the control group derived from our cohort had consisted of patients who were light smokers, the odds ratio for smoking cessation would be lower, as patients in this new control condition would be more likely to quit smoking compared with those in our current control group (32,33).
To conclude, through analyzing a large and racially diverse population-based cohort, we find that lung cancer diagnosis has a statistically significant impact on smoking cessation and that among lung cancer survivors, smoking cessation after the diagnosis of IPLC reduces SPLC risk. The present finding suggests the potential for integrating smoking cessation into surveillance and screening programs for SPLC among lung cancer survivors. A well-integrated smoking cessation program combined with LDCT screening may have a synergistic effect on SPLC prevention among lung cancer survivors and, thus, may help reduce the burden of lung cancer mortality overall.
Funding
This study is supported by grant from the National Institutes of Health (1R37CA226081). The MEC is supported by National Institutes of Health grant U01 CA164973.
Notes
Role of the funders: The funders had no role in study design, data collection, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author contributions: Conceptualization, SSH, SL, EC, JVA; Data Curation, SSH, SL, EC, LRW, LLM; Formal Analysis, SSH, SL, EC; Writing—original draft, SL, EC; Writing—review & editing, all authors; Validation, all authors; Supervision, SSH.
Disclosures: Dr Tammemagi is a consultant for Johnson & Johnson/Janssen, Medial EarlySign, Nucleix, bioAffinity Technologies, and AstraZeneca. Dr Wakelee reports grants from Gilead; personal fees from Janssen, Daiichi Sankyo, INC, Helsinn, Mirati, UpToDate; personal fees and non-financial support from AztraZeneca; personal fees and research funding to the institution from Xcovery; non-financial support from Takeda, CellWorks, Clinical Care Options Oncology, LLC, Fishawack Facilitate LTD, Medscape, Onclive/Intellisphere LLC, Phillips Gilmore Oncology, Physicians Education Resource LLC/MJH (Targeted Oncology), Potomac Center for Medical Education (Rockpointe), Prime Oncology LLC, Primo, Research to Practice, WebMD Health, Novartis, RGCON—Rajiv Gandi Conference, JLCS—Japanese Lung Cancer Society, KSMO—Korean Society of Medical Oncology, Stanford University, ITMIG; non-financial support and research funding to the institution from Genentech/Roche, Merck; research funding to the institution from ACEA Biosciences, Arrys Therapeutics, AztraZeneca/MedImmune, BMS, Celgene, Clovis Oncology, Exelixis, Lilly, Pfizer, Pharmacyclics, all outside the submitted work. All remaining authors report no other disclosures.
Data Availability
The data underlying this analysis were provided by the Multiethnic Cohort Study (MEC) under data use agreement. Researchers interested in the MEC data may submit an inquiry online: https://www.uhcancercenter.org/for-researchers/mec-data-sharing .
Supplementary Material
References
- 1.Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Gandini S, Botteri E, Iodice S, et al. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122(1):155–164. [DOI] [PubMed] [Google Scholar]
- 3.Rojewski AM, Tanner NT, Dai L, et al. Tobacco dependence predicts higher lung cancer and mortality rates and lower rates of smoking cessation in the national lung screening trial. Chest. 2018;154(1):110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341–350. [DOI] [PubMed] [Google Scholar]
- 5.Pirie K, Peto R, Reeves GK, Green J, Beral V; for the Million Women Study Collaborators. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381(9861):133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zha L, Sobue T, Kitamura T, et al. Changes in smoking status and mortality from all causes and lung cancer: a longitudinal analysis of a population-based study in Japan. J Epidemiol. 2019;29(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godtfredsen NS, Prescott E, Osler M.. Effect of smoking reduction on lung cancer risk. Jama. 2005;294(12):1505–1510. [DOI] [PubMed] [Google Scholar]
- 8.Doll R, Peto R, Boreham J, Sutherland I.. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328(7455):1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stram DO, Park SL, Haiman CA, et al. Racial/ethnic differences in lung cancer incidence in the multiethnic cohort study: an update. J Natl Cancer Inst. 2019;111(8):811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parsons A, Daley A, Begh R, Aveyard P.. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR.. Smoking at diagnosis and survival in cancer patients. Int J Cancer. 2013;132(2):401–410. [DOI] [PubMed] [Google Scholar]
- 12.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. [DOI] [PubMed] [Google Scholar]
- 13.Han SS, Rivera GA, Tammemägi MC, et al. Risk stratification for second primary lung cancer. J Clin Oncol. 2017;35(25):2893–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakur MK, Ruterbusch JJ, Schwartz AG, et al. Risk of second lung cancer in patients with previously treated lung cancer: analysis of Surveillance, Epidemiology, and End Results (SEER) data. J Thorac Oncol. 2018;13(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyle JM, Tandberg DJ, Chino JP, et al. Smoking history predicts for increased risk of second primary lung cancer: a comprehensive analysis. Cancer. 2015;121(4):598–604. [DOI] [PubMed] [Google Scholar]
- 16.Ripley RT, McMillan RR, Sima CS, et al. Second primary lung cancers: smokers versus nonsmokers after resection of stage I lung adenocarcinoma. Ann Thorac Surg. 2014;98(3):968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aredo JV, Luo SJ, Gardner RM, et al. Tobacco smoking and risk of second primary lung cancer. J Thorac Oncol. 2021;16(6):968–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151(4):346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martini N, Melamed MR.. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70(4):606–612. [PubMed] [Google Scholar]
- 20.Prentice RL, Kalbfleisch JD, Peterson AV Jr, et al. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34(4):541–554. [PubMed] [Google Scholar]
- 21.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schafer JL.Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3–15. [DOI] [PubMed] [Google Scholar]
- 23.Miles A.Obtaining predictions from models fit to multiply imputed data. Sociol Methods Res. 2016;45(1):175–185. [Google Scholar]
- 24.Duncan MS, Freiberg MS, Greevy RA Jr, et al. Association of smoking cessation with subsequent risk of cardiovascular disease. JAMA. 2019;322(7):642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Y, Zong G, Liu G, et al. Smoking cessation, weight change, type 2 diabetes, and mortality. N Engl J Med. 2018;379(7):623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramanujam R, Hedström AK, Manouchehrinia A, et al. Effect of smoking cessation on multiple sclerosis prognosis. JAMA Neurol. 2015;72(10):1117–1123. [DOI] [PubMed] [Google Scholar]
- 27.Mustoe MM, Clark JM, Huynh TT, et al. Engagement and effectiveness of a smoking cessation Quitline Intervention in a Thoracic Surgery Clinic. JAMA Surg. 2020;155(9):816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tammemägi MC, Berg CD, Riley TL, Cunningham CR, Taylor KL.. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst. 2014;106(6):dju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trinidad DR, Pérez-Stable EJ, White MM, Emery SL, Messer K.. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am J Public Health. 2011;101(4):699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J.. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84(7):791–799. [DOI] [PubMed] [Google Scholar]
- 31.Hymowitz N, Cummings KM, Hyland A, et al. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tob Control. 1997;6(suppl 2):S57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swayampakala K, Thrasher J, Carpenter MJ, et al. Level of cigarette consumption and quit behavior in a population of low-intensity smokers—longitudinal results from the International Tobacco Control (ITC) survey in Mexico. Addict Behav. 2013;38(4):1958–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tindle HA, Shiffman S.. Smoking cessation behavior among intermittent smokers versus daily smokers. Am J Public Health. 2011;101(7):e1–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallaway MS, Huang B, Chen Q, et al. Smoking and smoking cessation among persons with tobacco- and non-tobacco-associated cancers. J Community Health. 2019;44(3):552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Aalst CM, van den Bergh KA, Willemsen MC, de Koning HJ, van Klaveren RJ.. Lung cancer screening and smoking abstinence: 2 year follow-up data from the Dutch-Belgian randomised controlled lung cancer screening trial. Thorax. 2010;65(7):600–605. [DOI] [PubMed] [Google Scholar]
- 36.Tanner NT, Kanodra NM, Gebregziabher M, et al. The association between smoking abstinence and mortality in the national lung screening trial. Am J Respir Crit Care Med. 2016;193(5):534–541. [DOI] [PubMed] [Google Scholar]
- 37.Fucito LM, Czabafy S, Hendricks PS, et al. ; for the Association for the Treatment of Tobacco Use and Dependence/Society for Research on Nicotine and Tobacco Synergy Committee. Pairing smoking-cessation services with lung cancer screening: a clinical guideline from the Association for the Treatment of Tobacco Use and Dependence and the Society for Research on Nicotine and Tobacco. Cancer. 2016;122(8):1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this analysis were provided by the Multiethnic Cohort Study (MEC) under data use agreement. Researchers interested in the MEC data may submit an inquiry online: https://www.uhcancercenter.org/for-researchers/mec-data-sharing .