Abstract
The human microbiome is constituted by an extensive network of organisms that lie at the host/environment interface and transduce signals that play vital roles in human health and disease across the lifespan. Frailty is a critical aging-related syndrome marked by diminished physiologic reserve and heightened vulnerability to stress, predictive of major adverse clinical outcomes including death. While recent studies suggest the microbiome may impact key pathways critical to frailty pathophysiology, direct evaluation of the microbiome-frailty relationship remains limited. In this article, we review the complex interplay of biological, behavioral and environmental factors that may influence shifts in gut microbiome composition and function in aging populations and the putative implications of such shifts for progression to frailty. We discuss HIV infection as a key prototype for elucidating the complex pathways via which the microbiome may precipitate frailty. Finally, we review considerations for future research efforts.
Introduction
There have been notable gains in life expectancy across the globe over the last century, with projections for future gains across many countries in the coming decade.1 With aging populations has come an increasing burden of aging-associated conditions and aging-related syndromes. Frailty is a critical aging related syndrome of major public health importance that precipitates substantive disparities in health outcomes across aging populations worldwide.2,3 Frailty is characterized by dysregulation across multiple core physiologic systems. Understanding upstream determinants of such dysregulation is critical to developing novel and effective frailty-targeted interventions.
With recent advances in molecular techniques, there has been notable expansion in the investigation of the role that the human microbiome may play in health and disease. While these studies suggest that the microbiome may significantly influence several core frailty pathophysiologic systems, direct studies of the role of the microbiome in frailty have been limited.
Here, we first will introduce key microbiome concepts and factors identified as having influence on microbiome constitution. We will discuss how microbiome biology and frailty pathophysiological pathways may intersect. We will examine HIV as a prototype for investigation of the microbiome-frailty relationship. Finally, we will discuss opportunities for microbiome modulation as a putative intervention for frailty and future research needs. For this review, our primary focus will be on factors related to the gut microbiome.
Microbiome in health and disease
Microbiome composition
The human host is inhabited by an extensive network of microbial organisms (microbiome) considered essential to maintaining vital functions of a healthy host. This network consists of trillions of organisms at the host/environment interface, including the skin, urogenital tract, respiratory tract and gastrointestinal system.4
The human microbiome consists of communities of bacteria, viruses, fungi, protozoa and archaea.5 Microbiome composition varies between different host compartments and even along the course of a single host compartment.6,7 The gastrointestinal compartment has been one of the most extensively studied and hosts the largest proportion of human microbes, containing 5 primary bacterial phyla - Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria and Verrucomicrobia.8 While phyla composition across healthy adults is similar, significant interindividual variation occurs at the genus and species levels.9,10 Viral microbiome components including bacteriophages, chromosomal elements and those that infect human cells, as well as fungal, protozoan and archaeal elements have been less well studied.11
Microbiome function
The human microbiome is critical for a range of key host physiologic functions, including host energy metabolism, drug metabolism, and body defense.6,12,13 The human microbiome exerts its effect through an integrated gene pool encoding critical metabolic pathways that mediate host-microbe interactions and signaling to human physiologic systems. 7,8,14 The microbiome signals through microbial components or microbially derived metabolites that engage with neural, endocrine, immune and metabolic systems.8
Analyzing microbiome composition and function
Culture independent 16S rRNA sequencing techniques are among the most commonly used methodologies for evaluation of the bacterial microbiome composition. Through imputed methodology of 16S rRNA sequencing output, some aspects of microbiome function may be inferred. However, there is not always full correlation between the imputed and actual microbiome metagenome. More direct evaluation of microbiome function can be achieved through high throughput DNA sequencing methods, evaluation of RNA transcripts via metatranscriptomics, protein content and activity via metaproteomics, and metabolite content and processes via metabolomics.15 Evaluation of the virome (viruses) and mycobiome (fungi) may require more targeted techniques, such as directed viral PCR and pan fungal internal transcribed spacer sequences.11
Through these modalities, one can identify the number of taxa present in the microbiome ecological community (richness); relative abundance of taxa (evenness); and diversity, a combined measure of richness and evenness. Diversity within 1 sample type or community (alpha diversity) and differentiation in microbiome constitution between sample types or ecologic niches (beta diversity) are key outputs.
Additional data reduction techniques exist to facilitate methodological evaluation of microbiome relationships in health and disease.16 The altered state of the microbiome associated with disease has been termed dysbiosis, though what constitutes “dysbiosis” is debated and may differ depending on the disease state and the microbiota described.17
Several animal models facilitate extension beyond associative studies in evaluation of the microbiome compartment. Among the most commonly applied are germ free mice. Germ free or gnotobiotic mice are mice that are sterile or free of all microbial life including bacteria, viruses, fungi, archaea and protozoa. They provide an effective platform for the direct functional investigation of defined microbial communities, and serve as a key translational model for causal evaluation of the role of the gut microbiome in health and disease.18
Factors that impact microbiome composition and function
Employing the aforementioned modalities, both intrinsic and extrinsic factors impacting microbiome composition and function have been identified. These factors may be heterogeneous across individuals and may evolve over the human lifespan. Here, we will describe factors that influence the microbiome with particular focus on the gastrointestinal microbiota, for which the most robust data on the microbiome relationship to aging-related conditions exist to date.
Early development of the microbiome
Early microbiome development is influenced by maternal factors, including mode of delivery, maternal antimicrobial exposure, mode of feeding (breast vs. formula), and gestational age at birth.8,19 Low alpha diversity and high interindividual variability exist in early infancy.19 With the shift from breast feeding, increased diversity and a shift to a more adult-like core microbiota occurs.8,19
Intrinsic/genetic factors
Host genetics significantly influence the microbiome, with substantial heritable elements observed.20,21 Microbiome constituents between family members are more similar than between unrelated individuals, and are more similar between monozygotic than dizygotic twins.20 Genes suggested to play a role in gut microbiome constitution include those involved in innate and adaptive immune pathways.22
Diet
Substantial shifts in gut microbiome composition occur with differential dietary intake.23–27 Characteristic gut microbial enterotypes have been defined in association with long term dietary patterns.28 Transient changes in the gut microbiome occur with transient changes in diet but such changes revert to baseline soon after diet reversion.29 In general, intake of high levels of saturated fat, high sugar, and low fiber diets have been associated with a less diverse and a more pro-inflammatory microbiome.30,31 Conversely, the microbiome and its metabolites may influence dietary intake through signaling to appetite-controlling hypothalamic centers.32
Geography
Distinct microbiome clustering has been observed by residence, including country of origin, urban vs. rural setting, and even floor residence location.24,33–36 From these studies, a role for residence environment and culture on microbiome composition has been inferred.
Disease states and medication treatment
Disease states and the associated medications used for their treatment also can influence microbiome constitution. Antimicrobial agents exert particular influence.37 Antibiotic-mediated shifts in the gut microbiome can be relatively rapid with decreases in microbiome diversity within 3–4 days. The consequent shifts can last weeks to months and possibly even longer.38,39 Other therapeutic agents can influence microbiome constitution by altering luminal pH or mucosal structures leading to selection of specific taxa.40,41 Some medications may exert a bacteriostatic or bactericidal effect despite their not falling under classification as antimicrobial therapeutics.42,43
Polypharmacy, the use of multiple drugs in a single person at the same time, also has been associated with decreased microbiome diversity and dysbiosis. Multimorbidity, the co-occurrence of multiple comorbid conditions, is a primary determinant of polypharmacy, and can significantly influence microbiome constitution as well.44–46
Environmental, lifestyle and behavioral factors and brain-gut axis
The microbiome demonstrates plasticity in response to a range of environmental and lifestyle or behavioral factors and may play a key role in transducing such signals for the host.22,47 Data suggest socioenvironmental factors and health behaviors may positively or negatively impact the host through microbiome-related pathways. Gut microbiome composition has been found to shift across different socioeconomic strata.48–50
Stress-inducing social factors also may impact the microbiome via the brain-gut axis. In this construct, activation of physiologic stress response systems, such as the hypothalamic-pituitary-adrenal (HPA) axis, may influence microbiome composition. Further, a feedback loop in which gut microbiota produce neuroactive metabolites that may modulate gut-brain signaling via neural and endocrine pathways has been postulated, with potential impact on mood and leading to other neuropsychiatric consequences.4,8,51,52
Other lifestyle factors, including alcohol and tobacco use may influence microbiome composition.53–56 Opiate use has the potential to alter gut motility, the latter associated with gut dysbiosis.57–59 Gut dysbiosis also may adversely influence behavioral responses to drug use by augmenting a negative feedback cycle to sustain active drug use and further promote deleterious microbiota changes.60
Reduced exercise and low physical activity have been associated with unfavorable changes in microbiome composition.46,61 Conversely, exercise or increased physical activity has been associated with increased microbiome diversity, as well as increased abundance of several organisms and microbial metabolites with putative beneficial health impact.62–66 These positive microbiota-related changes have been reported to dissipate with an individual’s transition back to a sedentary lifestyle.67
Microbiome in disease
Numerous studies have noted reduced gut microbiome diversity to be associated with disease or ill health.68–72 Alterations in microbiome composition and function have been reported in many conditions including obesity, insulin resistance, non-alcoholic fatty liver disease/non-alcoholic steatohepatitis, type 2 diabetes, atherosclerosis/thrombosis and stroke, allergic asthma, autism spectrum disorder, anxiety, depression, colorectal cancer, inflammatory bowel disease, systemic lupus and other autoimmune disorders.8,17,19,23,73,74
The potential causal role of the microbiome in disease pathogenesis across these states has been supported in multiple cases by germ free mice adoptive transfer models.9,75,76 Ultimately, microbiome-related pathways have been associated with many of the leading disease-related causes of death, many of these aging and inflammatory-related conditions.4
Frailty and Heterogeneity of Aging
Frailty is a critical aging-related syndrome, marked by a cumulative loss in physiologic reserve with diminished homeostasis, decreased resilience and heightened vulnerability to stress, precipitating major adverse clinical outcomes including increased hospitalization, institutionalization and death.2,77–79 While aging-related, frailty is not equivalent to chronologic age, reflecting the heterogeneity of the aging process between individuals.
Multiple instruments have been developed over the last several decades for frailty assessment.80 While there has been no definitive gold standard measure, the physical frailty phenotype (PFP) first operationalized by Fried and colleagues has been most commonly applied in the research literature to date. This phenotype is composed of 5 primary domains – weight loss, exhaustion, muscle weakness, low physical activity, and slow gait. Meeting 3 or more of these criteria classifies an individual as frail.2,81
Of other frailty instruments, the deficit accumulation index (DAI) construct also has been commonly applied.82–86 While differing somewhat in operationalization and conceptual basis, both the Fried PFP and the DAI are strongly predictive of significant adverse health outcomes in older adults.81–85,87 Given this association, frailty remains a key target for reducing the marked disparities in health outcomes that exist across populations.
Frailty biology
Understanding the biological pathways to frailty is critical to developing effective interventions to reduce frailty-related disparities in health outcomes. Studies of frailty biology have found associations with a range of aberrant, dynamic, multisystem physiologic stress responses among older adults, affecting neuroendocrine, metabolic, and musculoskeletal systems.77,88–91 Dysregulated immunity and inflammation have emerged as particularly prominent components of aberrant responses in frailty biology.91–97
Role of inflammation and immune dysregulation in frailty pathogenesis
Markers of inflammation increase with age. Heightened inflammation has been significantly associated with a range of prevalent and incident age-associated disease conditions. 98–104 Heightened inflammation also has been strongly associated with premature mortality in aging populations.98,102,103 Multiple studies in older adults have demonstrated a significant association of dysregulated inflammation with frailty.105,106 There have been multiple proposed drivers of heightened inflammation in aging populations. Dysregulated systemic immunity and disruption of immune homeostasis at the host/environment interface can play a key putative role in this regard.91,107
There has been significant recent interest in the putative linkage between the microbiome and frailty. Such interest stems in part from the linkage of dysregulated inflammation and immunity to frailty, along with substantive data linking the microbiome to inflammation, immune dysregulation and immune homeostasis, as well as other frailty pathophysiologic pathways. We will summarize several of these potential linkages below.
The microbiome and its metabolites may influence inflammation, immune dysregulation and immune homeostasis
Multiple studies suggest that the human microbiome plays a critical role in the human host’s attempt for a fine continuous balance between tolerance to self and innocuous non-pathogen, non-self and protection against pathogen non-self, through key roles in modulating immune development, immune homeostasis and immune dysregulation alike.108–110
Role of microbiome in immune system development
Several studies support the microbiome’s role in the early development, maturation and consequently the effective functioning of mucosal and systemic immune systems.18,108,111,112 Host-microbiota communication has been found critical to development of both innate immunity and cellular and humoral adaptive immune compartments.18,113,114 This developmental relationship is bidirectional, appreciating that the host immune system itself can significantly shape microbiome composition and function.108
Role of microbiome in host defense at the mucosal host/environment interface
Beyond its role in early immune system development, the microbiome has been considered to play a critical role in immunity throughout the lifespan. The role of the microbiome in immune function and immune homeostasis has been most well defined in the gut.6,8,115,116
Gut mucosa as a prototype of host mucosal defense
The gut is lined by a single layer of mucosal epithelial cells that form a tight physical barrier mediated by junctional proteins. Mucus, antimicrobial peptides and IgA production by the mucosal immune epithelial cell network constitute an additional barrier to pathogens. Innate immune responses mediated further by neutrophils and intestinal lymphoid cells play a role in containing commensal bacteria. Mucosal antigen presenting cells (APCs) mediate innate induction of adaptive immunity. These APCs could be pro-inflammatory and bactericidal or immunoregulatory (tolerogenic).19,117
Gut regulatory T cells, epithelial and stromal cells all produce TGF beta and IL-10 which induce tolerogenic APCs and immunosuppressive pathways, and in a positive feedback loop further induce regulatory T cell production. Activation of pattern recognition receptors such as Toll like receptors on dendritic cells leads to IL-23 production which in turn induces IL-17 production and differentiation of CD4 T cells into Th17 cells. Th17 cells themselves have a pro-inflammatory role and are critical for the adaptive immune defense against microbial pathogens at mucosal surfaces.19,30
Role of the microbiome in host mucosal defense
In healthy individuals, the gut microbiome plays multiple roles in maintaining a robust mucosal defense system, while ensuring immune homeostasis is sustained. Commensal microbes help maintain the mucus barrier by stimulating pattern recognition receptors on cells of the mucosal immune epithelial network. Commensal bacteria can promote tolerogenic APCs and help control mucus production and antimicrobial peptide expression. IgA secretion is also modulated by microbiota.30,118,119
Gut microbiota directly or via metabolites further play a role in epithelial cell proliferation and the expression of tight junction proteins, necessary for mucosal repair and maintenance of gut mucosal barrier integrity. Commensal bacteria also are necessary for the production of metabolites with key anti-inflammatory properties.30,120,121 The microbiota-derived metabolite butyrate also stimulates regulatory T cell production through induction of IL-10 and TGF beta.122,123 Butyrate also promotes mucin production and reduces production of pro-inflammatory cytokines.108,124 Commensal gut microbiota additionally may directly promote resistance to pathogen colonization in the gut via several modalities, including pH modification, production of antimicrobial products, competition for microbial adhesion sites, and nutrient competition.117
Role of dysbiosis in promoting aberrant mucosal and systemic inflammatory responses
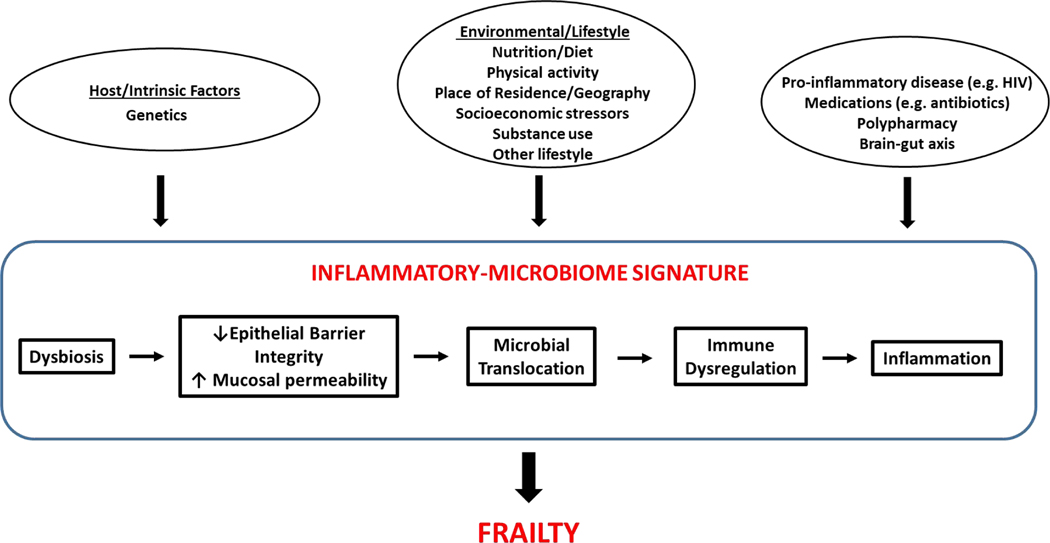
Several studies suggest an association of pro-inflammatory alterations in diversity, composition, and function of the microbiome with adverse inflammatory conditions.8,23,30,70,125–128 These include changes such as reductions in butyrate producing gut microbiota.23,129,130 Multiple studies suggest that a putative contributor to the pathogenesis of such conditions is an inflammatory-microbiome signature (Figure 1).19,23,110,113,114,131,132 Such a signature has been postulated to be characterized by adverse alterations in microbiome composition and function that may lead to impaired mucosal epithelial integrity, precipitating translocation of commensal and pathogenic microbes and their products across the mucosal barrier. Such translocation further has been postulated to promote both local mucosal and systemic immune activation, and ultimately sustained systemic inflammation that may trigger onset of inflammatory-related disease states.5,133–140
Figure 1. Inflammatory Microbiome Signature and Frailty.
Putative integrative pathway via which the human microbiome may mediate frailty onset and progression. This proposed pathway is based on existing data derived from animal models and human studies to date. In this model, host genetic factors, pro-inflammatory clinical parameters, and environmental, lifestyle and behavioral factors promote an inflammatory-microbiome signature characterized by pro-inflammatory alterations in the microbiome (particularly the gut) and functional metagenome that precipitate decreased epithelial barrier function with consequent increased mucosal permeability, translocation of microbial products, dysregulated innate and adaptive immunity, and a heightened systemic inflammatory state. This inflammatory-microbiome signature may intersect further with microbiome-mediated dysregulation of other key human physiologic systems, including neuroendocrine and energy metabolic pathways to precipitate frailty.
The putative causative role for the microbiome in local and systemic inflammatory pathways and disease is supported by animal models. Whereas the focus of these studies primarily has been on the bacterial microbiome, incipient data support a role for the virome in precipitating mucosal and systemic inflammation as well.11
Association of the microbiome with aging pathophysiology
Microbiome shift to a pro-inflammatory phenotype with increased age
Whereas rapid changes in microbiome composition occur in the first 3 years of life, the gut microbiome subsequently takes on a relatively stable composition over multiple decades in a healthy host.35,141,142 Several shifts in microbiome structure have been reported to occur with late age, including reductions in microbiome diversity and increased interindividual variation.9,46,110,129,143 Also observed among elderly adults is a reduction in commensal microbiota with putative beneficial functions to the host, such as maintenance of mucus production and mucosal barrier integrity.23,35,133,144–147
Concurrently, emergence of pathobionts has been observed.131,146 These pathobionts are symbiotic bacteria with the capacity to become pathogenic and precipitate chronic systemic inflammation. Overall, these findings all reflect a potential shift towards a pro-inflammatory microbiome phenotype with increased age. Among centenarians, models of longevity and healthy aging, enrichment of microbiota known to promote anti-inflammatory activity, metabolic homeostasis, and positive immune function have been observed.22,133,135,148
Changes in host physiology with aging can impact microbiome composition and function
While the microbiome may impact host aging phenotypes, several changes in host behavior and physiology with increased age simultaneously may influence microbiome composition and function through impacts on dietary patterns and gut nutrient delivery. These include reduced appetite, reduced salivary production, changes in dentition and masticatory function, changes in gut enzyme production, and increased orocecal and colonic transit times.40,149,150
Immunosenescence, cellular senescence and the microbiome
Cellular senescence and immunosenescence are central hallmarks of aging and also have been raised as cellular and physiological processes with potential influence on the host microbiome relationship. Cellular senescence is characterized by arrest of cellular proliferation that may occur in response to cellular damage or stress. This process plays a key role in body repair, including in targeting damaged cells for removal. Senescent cells have been found to accumulate with age across body compartments. Accumulation of senescent cells or decrease in their clearance with age has been associated with a senescence-associated secretory phenotype characterized in part by chronic pro-inflammatory cytokine production.91,151,152
Nascent data suggests a putative relationship of gut microbiota and cellular senescence. Associations of gut microbiota with cellular senescence have been particularly observed in the gut epithelial cellular compartment.153–155 A few studies additionally have found associations of gut microbiota metabolites with cellular senescence at distal organ sites.156 However, data on the relationship of the gut microbiome to cellular senescence remains limited and warrants further study.
Related yet distinct to the concept of cellular senescence is immunosenescence. The term immunosenescence has been used to refer collectively to the multiple changes observed across immune system compartments with increased age.157–159 Such changes have been characterized as pro-inflammatory and considered possibly deleterious to the host, with significant associations observed with aging-related syndromes, morbidity and mortality.157,159
These shifts involve both innate and adaptive immune compartments. Decreased phagocytic function, reduced antigen uptake, and impaired innate immune cell activation with decreased chemotaxis, diminished costimulatory molecule expression and dysregulated cytokine production resulting in impaired innate signaling to the adaptive immune system are among the multiple changes observed in the innate immune compartment with increased age.159–161 Depletion of naïve lymphocytes, a relative shift to a memory lymphocyte population, impaired B cell function with reduced levels of antibody production and avidity, and shifts in the representative distribution of T cell subpopulations are among the multiple changes in the adaptive immune compartment that have been observed with increased age.151,157,159–161 These aging-related immunological shifts may reduce the ability for the aging host to effectively respond to microbial pathogens or pathobionts and alter the host response to commensal organisms, consequently altering the composition and function of the microbiome compartment.
Whereas changes in the innate and adaptive immune compartments with age may precipitate changes in microbiome composition and function, it also has been postulated that the microbiome compartment may bidirectionally promote immunosenescencent shifts as well. The human virome has been postulated to play a particular role in the induction of immunosenescence.
The human virome consists of viruses that perpetually reside in host cells, endogenous viral elements integrated into cellular components, and viruses that infect other microbiota elements, particularly bacteriophages which inhabit bacteria.162,163 The herpesviridae family member cytomegalovirus (CMV) has been particularly prominently identified as having a significant association with the immunosenescent phenotype. CMV is highly ubiquitous in the general population, mostly latent in immunocompetent hosts and persistent in its presence in aging populations.162,164 Clonal expansion of memory T cells specific for CMV antigens has been observed with age and also associated with heightened pro-inflammatory cytokine production. Marked CMV clonal expansion in older adults has been postulated to restrict the T cell repertoire, with the potential to impair responses to other microbial antigens.159 While CMV has traditionally been identified as inducing a deleterious, pro-inflammatory effect via the immunosenescent pathway, more recent data has raised the question as to whether CMV might also find a putative beneficial role in the immunocompetent host with potential for enhancing immune responses in some scenarios such as vaccination.157,159,165 The role of CMV in immune aging requires further investigation.
The composition of the human virome also varies by host compartment. The gut virome constitutes the largest human virome compartment with an estimated 109 viral particles per gram of intestinal content.159,162,163 This compartment consists predominantly of bacteriophages that infect prokaryotic cells, primarily inhabiting the gut bacterial microbiome.158 Studies suggest that bacteriophages can modify the function of the bacterial microbiome. In particular, bacteriophages have been found to induce the production of inflammatory cytokines in vitro.158,166 Through this and other pathways, the bacteriophage component of the human microbiome may play a putative role in adverse aging-related conditions. However, very little data currently exist on aging-related changes associated with bacteriophages. The role of these organisms, other viral elements and other microbiota components on immunosenescence as a putative pathway to frailty and other aging-related syndromes merits further study.
Aging, impaired gut mucosal integrity and inflammatory-microbiome signature
With age, impairments in intestinal mucosal integrity also have been observed. This increased intestinal mucosal permeability has been observed in both mice and nonhuman primate models alike.134,138,167,168 Age-associated dysfunction of the gut mucosal barrier may lead to translocation of microbes and their products into systemic circulation and contribute to a systemic inflammatory signature.135,169,170
Several animal models support the role for the gut microbiome in precipitating an inflammatory-microbiome signature. In studies of Drosophila, microbiome alterations precede and predict the onset of intestinal barrier dysfunction in aged flies. Intestinal dysbiosis in these studies was characterized by expansion of Gammaproteobacteria and associated with systemic immune activation and heightened mortality.171 In other experimental models, age-associated changes in the gut microbiota promoted mucosal permeability, increased systemic bacterial products, and increased levels of central inflammatory cytokines such as IL-6 and TNFα.139 In murine models, transfer of microbiota from old mice to young germ free mice triggers inflammatory responses. These responses included increased intestinal inflammation, upregulation of inflammatory cytokine genes, and increased circulating systemic inflammatory markers.140 In humans, increased enterocyte apoptosis has been observed in biopsies of elderly adults.172 However, definitive evidence for increased gut permeability with translocation of pro-inflammatory microbial products in healthy older adults in the absence of overt inflammatory disease has been limited.45
Microbiome and chronologic vs. biologic age
Changes in diversity, microbiome composition and function with increased age have been reported across multiple studies, yet not universally observed. In several studies, reduced gut microbiome diversity and the putative pathological shifts in microbiome composition and function that have been observed correlate less closely with chronologic age than with aging-related shifts in health status and measures of “biological age”, namely markers considered to capture the underlying dysregulated multisystem physiology that account for the heterogeneity of aging-related outcomes, such as conceptualized in the frailty construct.34,40,110,173 Understanding the role of the microbiome in frailty pathophysiology ultimately may help determine its potential use as a key target to improve health outcomes for frail adults.
Association of the microbiome with frailty
Studies on the relationship of the human microbiome to the frailty construct
Despite the significant relationship of gut dysbiosis to inflammation and dysregulated immunity, and the aforementioned microbiome relationships with neuroendocrine dysfunction, and altered energy metabolism, all identified as core pathways in frailty pathophysiology, studies directly evaluating the relationship of the human microbiome to the frailty phenotype have been limited. The limited studies that exist vary in instrument used and population studied. We describe several of these early studies here.
Physical frailty phenotype and microbiome
Applying the Fried physical frailty phenotype in a cross-sectional analysis of 1551 community dwelling older adults, Verdi et al. found the frailty phenotype to be significantly associated with a reduction in microbiome diversity, a feature of the microbiome evidenced in multiple other adverse disease states.174
Also utilizing the Fried PFP, Buigues et al. performed a randomized double-blind clinical trial of daily administration of an inulin/fructooligosaccharide prebiotic versus placebo among 60 nursing home resident ambulatory adults. No significant change in frailty score was observed with this intervention, though significant improvements in 2 frailty domain criteria exhaustion and grip strength were observed.175
Ghosh TS et al. applied the Fried PFP in a very recent study of the microbiome relationship to frailty. This study was a single-blind randomized controlled trial of 612 adults across 5 European countries investigating a 1 year Mediterranean diet intervention in relationship to inflammation and frailty. Gut microbiota taxa enriched by adherence to the Mediterranean diet were significantly associated with reduced frailty and reductions in inflammatory markers including CRP and IL-17.176
Frailty indices and microbiome
Most other studies seeking to examine the relationship of the human microbiome to frailty have applied an index approach to frailty assessment. One of the earliest studies to explore the relationship of the microbiome with frailty, used the Groningen frailty indicator, a 15 item questionnaire based on mobility, physical fitness, comorbidity, weight loss, vision, hearing, cognition and psychosocial resources. Adverse scores on this instrument were associated with reductions in putative health promoting, anti-inflammatory organisms such as Faecalibacterium praunsnitzii and increased Enterobacteriaceae abundance, the latter considered to be pro-inflammatory.177
Jackson et al. applied a 39 item index comprised of measures of comorbidity, physical function, mental health, self-reported general health, disability, social functioning, polypharmacy and pain. This index was applied in a cross-sectional analysis of community dwelling cohort of 728 female twins. In this study, higher index score was significantly associated with a reduction in alpha diversity, even after adjustments for age, diet, alcohol intake, tobacco use and BMI. Higher index score was associated with significant reductions in microbiome butyrate producers, a characteristic with known anti-inflammatory effects.69
Maffei et al. applied a 34 item index of health history and functional variables to a cohort of 85 community dwelling adults. In cross-sectional analysis, this index significantly correlated with decreased gut microbiome richness, with no association of microbiota richness with chronologic age. These findings persisted after adjustment for chronologic age, sex, BMI and antibiotic usage.34
Using the 9 item Rockwood clinical frailty scale (RCFS) in a cross-sectional study of 76 patients hospitalized for acute illness, Ticinesi et al. found no difference in alpha diversity between top and bottom tertiles of the CFS score. Several compositional associations were observed with the CFS including differential abundance of Prevotella, Oscillospira, Porphyromonas, Peptococcus, and Fonticella.44
Also using the RCFS, Haran et al. performed a prospective observational study of 23 nursing home residents with stool collection at regular intervals over a 4 month period. Higher scores indicative of poorer health status were associated with reductions in butyrate producing bacteria and an increase in LPS and peptidoglycan synthesis pathways, agents known to have the capacity to induce systemic inflammation.178
In a posthoc analysis of a randomized controlled trial designed to examine the impact of an inulin/fructooligosaccharide prebiotic mixture versus placebo among 50 nursing home residents, prebiotic administration was associated with a small reduction in a 62 item frailty index.179
Microbiome and other measures of heterogeneous aging
Beyond application of the most commonly employed measures of frailty, one of the seminal studies of the relationship of the microbiome to syndromes known to capture differential health status among older adults was performed by Claesson et al. In this cross-sectional study of 178 elderly adults, the microbiota of community dwelling adults was compared to that of adults in long term residential care.
Long term stay was considered as a proxy for frail status in this study, with these subjects having poor scores on key geriatric markers, including comorbidity, functional impairment, disability, and cognitive impairment. Adults in long term residential care demonstrated a significantly different microbiome signature than community dwelling adults. The long term stay signature was characterized by decreased microbiome diversity, increases in Bacteroidetes phyla and decreases in Firmicutes phyla. This signature strongly correlated with markers of heightened inflammation (TNFα, IL-6, IL-8, CRP) and reductions in fecal microbial metabolites such as butyrate known to have anti-inflammatory and health promoting effects.23
Ogawa et al. similarly compared the salivary microbiome of nursing home residents to community dwelling controls. For this study, nursing home residence required certification of an inability to lie at home based on frail status, as per the opinion of a medical physician. The study focus was on the salivary microbiota, which was found to differ significantly between the community dwelling and nursing home groups. Study of the oral microbiome is emerging as an important entity, with some studies suggesting a linkage of this compartment with systemic physiology and adverse aging phenotypes.180,181 Additionally, while notable regional differences have been reported, there is emerging data in support of a putative linkage between the upper and lower GI tracts relative to microbiome composition.182,183 In this study, the salivary microbiome taxa that correlated with inferred frailty status included several taxa known to colonize the lower GI tract as well.184
In sum, frail status as defined across these different measures was associated with reduced microbiome diversity, reduction in putative beneficial health promoting anti-inflammatory microbes, reduction in health promoting microbiome metabolites (including those with a role in energy metabolism and a positive brain-gut axis), and microbiome compositional changes associated with heightened inflammation.
The primary limitation across these studies is the wide variability in the methodology applied in the attempt to capture frailty. In addition to limiting comparisons across studies, the application of distinct instruments raises the question as to whether the same underlying biological latent construct is being captured across studies. Additional limitations for several of these studies include use of fecal samples, which may not fully capture the full microbiome compartment; the limitations of 16S rRNA sequencing relative to microbiome functional assessment, absence of data on key putative confounders that may significantly additionally influence the microbiome such as antibiotics, diet and substance use, and the cross- sectional nature of most studies which limits causal inference.
Relationship of the microbiome to frailty components and sub-manifestations of frailty
The relationship of the microbiome to additional pathophysiologic mechanisms considered key components of frailty pathophysiology including changes in musculoskeletal physiology such as sarcopenia and endocrine physiology such as insulin resistance, as well as the microbiome relationship to key frailty phenotypic subdomains such as reduced muscle strength and gait speed has been preliminarily explored.
Sarcopenia
Sarcopenia, characterized by an aging-related decline in muscle mass and quality, has been considered significantly associated with frailty pathophysiology. Studies suggest the microbiome may influence multiple pathways with potential impact on muscle mass and quality. First, the gut microbiome can influence host appetite through endocrine pathways with potential impact on dietary protein intake necessary for muscle formation.46 Gut microbiota composition influences protein metabolism and bioavailability, with adverse impact on the bioavailability of amino acids specific to muscle protein synthesis.129,185 Synthesis of several vitamins, including B12, folate, and riboflavin necessary for skeletal muscle anabolism also is influenced by the microbiome.129,186
Conversely gut dysbiosis has been associated with impaired mitochondrial quality in myocytes with impaired antioxidant capacity. Accumulation of dysfunctional mitochondria can contribute to muscle atrophy. Further, induction of intramuscular fat infiltration can occur with gut dysbiosis, with potential adverse impact for muscle function and strength.129,187,188
Several key microbiome metabolites can play a key role in these pathways. Short chain fatty acids (SCFAs) play a role in skeletal muscle glucose uptake and protein deposition, modulating the balance between muscle anabolism and muscle breakdown.189,190 Microbiota-derived SCFAs may promote mitochondrial activity with positive impact on skeletal muscle cell function. Decrease in SCFA production with aging is considered to precipitate decreased mitochondrial fatty acid oxidation and increased intramuscular fatty acid deposition, resulting in reduced muscle strength and quality as a key putative precipitant to frailty.46
Several animal model studies support the putative causal influence of the gut microbiome on muscle mass and quality. Distinct gut microbiome signatures have been observed between rats with sarcopenia relative to those with normal muscle mass.191 Muscle atrophy in mice is associated with a pro-inflammatory gut microbiome with depletion of butyrate producing bacteria.192 Further, administration of the SCFA butyrate to aged mice has been shown to improve lean muscle mass.190
Grip strength
Little direct data exist in humans associating gut microbiota with skeletal muscle mass. However, low grip strength – a key functional readout of muscle strength and a primary frailty domain – has been associated with an increased abundance of pro-inflammatory microbiota, decreased abundance of anti-inflammatory microbiota, and reduction in fecal SCFA levels.175,193
Insulin resistance
Insulin resistance also has been identified as an important pathophysiologic pathway in frailty. Healthy gut microbiota can play a role in reducing insulin resistance.129 Microbiota-derived SCFAs promote insulin sensitivity, promoting glucose tolerance through increased energy expenditure.194 SCFAs promote release of systemic IGF-1.195 IGF-1 release can positively influence insulin sensitivity and inflammation, and is strongly associated with reduced frailty burden.129,196,197 Secondary bile acids are additional microbiota metabolites which can activate pathways that protect against insulin resistance.198,199 Conversely, gut dysbiosis can result in increased circulating lipopolysaccharide (LPS) which itself can induce insulin resistance and inflammation. Insulin resistance can result in accelerated loss of muscle mass and strength.122
Microbiome and gait speed
Gait speed has been an important predictor of adverse aging outcomes among older adults and is a key domain in the frailty phenotypic construct. In animal models, germ free mice demonstrate hyperactive locomotor behavior compared to animals with gut microbial colonization. Lactobacillus administration was shown to correct this hyperactive locomotor behavior.200
In the very few human studies examining the relationship of the gut microbiome to gait speed to date, some changes in gut microbiota composition have been observed. These included increased abundance of Enterobacteriaceae and decreased Lachnospiraceae with impaired gait in patients with Parkinson’s disease compared to controls; reduced Bacteroides with reduced gait speed in an exercise intervention study of sedentary women; and increased gait speed with probiotic supplementation in a probiotic randomized controlled trial targeting cirrhotic patients. Though, for the latter no changes in the fecal microbiome were observed.201–204 Overall, data on the relationship of the microbiome to frailty and its components in humans remains limited.
Frailty, HIV Infection and the Inflammatory Microbiome
HIV infection may serve as a key prototype for elucidating the complex intersection of biology, behavior and environment for the inflammatory microbiome-frailty relationship in disease states in aging populations.
Antiretroviral therapy (ART) has dramatically increased the lifespan of HIV-infected patients. Consequently, the proportion of people living with HIV worldwide who are over 50 years of age has significantly increased, and the number of older HIV-infected adults is anticipated to rise markedly over the next several decades.205–207 Despite these survival gains, disparities in morbidity and mortality remain for Persons Living with HIV (PLWH) even on effective ART. 208–210 Such disparities are due in part to the rising burden of aging-related conditions in the HIV-infected population, for which PLWH have been found to be disproportionately at risk. This heightened risk has been attributed to both aberrant HIV pathophysiologic pathways that persist even with ART-mediated virologic suppression, as well as behavioral and environmental factors that may disproportionately impact HIV-infected populations.2,211
Multiple recent studies have demonstrated a heightened frailty burden for PLWH relative to their HIV-uninfected counterparts.2 As for older HIV-uninfected adults, this heightened frailty burden in HIV has been shown to be predictive of major adverse clinical outcomes, including hospitalization, falls, fractures, disability, low quality of life and increased mortality.212–218 In a study by Piggott et al., being both frail and HIV-infected conferred an over 7-fold increased risk of death, independent of comorbidity or HIV disease stage.219 Thus, as for HIV-uninfected older adults, understanding pathways to frailty in HIV remains critical to reducing the marked disparities in morbidity and mortality persistent in this population.
Role of inflammation in aging-related phenotypes and frailty in HIV
As detailed above, heightened inflammation has been identified as central to frailty pathophysiology among HIV-uninfected older adults. Dysregulated inflammation is central to HIV pathophysiology, and a key driver of aging-related morbidity and premature death among PLWH as well. 220–225 Even amongst PLWH who are virally suppressed on effective ART, inflammation persists.220 What drives this persistent inflammation remains an area of active study. Putative mechanisms suggested include persistent low-level residual viremia, co-infections (such as CMV), persistent activation of pattern recognition receptors, telomere shortening, as well as inflammatory-promoting behavioral and environmental factors.105
The findings of several recent cross-sectional studies support a significant role for inflammation in frailty pathogenesis in HIV. 105,226 In more recent longitudinal evaluation using a nuclear factor kappa B (NFkB)-derived aggregate inflammatory index, reduced inflammation was associated with both reduced frailty progression and greater frailty recovery.227
Elucidating drivers of frailty-associated inflammation in HIV thus may be critical to reducing frailty-related disparities in this population. Significant interest has surrounded the possibility that an inflammatory microbiome signature may occur in HIV as well, constituted by microbiome changes that may lead to a dysregulated gut epithelial border, associated increases in microbial translocation and immune activation, chronic systemic inflammation and consequently frailty in HIV.
HIV as a putative precipitant of a pro-inflammatory microbiome signature
The intersection of key aspects of HIV biology, microbiome biology, and inflammatory pathways has raised the possibility that the microbiome may play an important role in driving inflammation and frailty in HIV.
HIV infection causes massive depletion of CD4 T cells in the gut, as well as dysregulation of the immune-epithelial network, which may not be fully restored even with effective ART. Depletion of gut mucosal CD4 T cells occurs very early in acute HIV infection, with particular adverse impact on the Th17 compartment. Gut CD4 T cell depletion is accompanied by impairment in gut mucosal barrier function secondary to inflammatory cytokine-mediated disruption of tight junctional proteins.10,228 CD4 T cell loss and particularly loss of IL-17 and IL-22 producing cells further impairs capacity for epithelial cell regeneration, together with mucus and antimicrobial peptide production that serve key mucosal defense functions as well.117
Enterocyte apoptosis and epithelial cell degeneration which occur with HIV infection further precipitate impairment in gut integrity.10 Markers associated with epithelial injury and increased gut permeability including I-FABP and Zonulin remain elevated and are predictive of increased mortality, even in ART-treated patients.222 Impaired gut integrity in HIV also has been significantly associated with translocation of microbial products from the gut lumen to peripheral blood.10,229,230 These microbial products retain capacity for immune activation and consequently systemic inflammation.10
There is interest in whether HIV-mediated shifts in gut immunity and gut barrier integrity may substantially shift host-microbe interactions and consequently alter microbiome composition and function in a manner that may promote an inflammatory-microbiome signature in HIV.5,17 It is possible that the loss of IL-17 production and antimicrobial peptides could significantly shift microbiome composition as well. In murine models, loss of IL-17 production has been associated with an inflammatory microbiome signature characterized by microbiota changes significantly associated with heightened inflammation.231,232
HIV may additionally adversely impact gut physiology in ways that may further alter microbiome composition and function. Such effects include reducing gastric acidity which may facilitate an increased presence of opportunistic pathogens and decreasing gastric emptying which may further impact colonization of the GI tract.117 HIV-mediated impairment of macrophage phagocytic activity also may alter microbiome composition and precipitate local and systemic inflammation.233,234
Epidemiologic observational shifts in microbiome composition and function in HIV
Many studies have compared the gut microbiota in HIV-infected and HIV-uninfected individuals.235–267 Despite the putative intersection of HIV biology and host microbiome biology as described above, a consistent HIV-associated microbiota signature has been slow to emerge, potentially reflective of the complexity of the host-microbiome relationship.
Several studies have reported a significant association of HIV infection with alterations in the gut microbiota. In acute SIV models, increased abundance of bacterial taxa with pathogenic potential has been observed with a corresponding decrease of putatively beneficial microbes such as Lactobacillus. These microbiome changes have been associated with impaired pattern recognition receptor expression and gut mucosal Th17 loss in these studies.268–270 In chronic SIV infection, gut microbiota changes have not been universally observed.17
Multiple human studies have been performed seeking to identify a distinct HIV-microbiome relationship. These studies have employed mostly 16S rRNA gene sequencing methodologies and results remain mixed. In human studies in which HIV-associated microbiome compositional changes have been observed, HIV-associated gut dysbiosis often manifests as reduced gut microbial diversity. 240,244,246,271 As detailed above, reduced gut microbiome diversity has been associated with aging-related conditions and frail status in older HIV-uninfected populations as well.
While many studies report significant differences in alpha diversity between PLWH and their HIV-uninfected counterparts, such findings have not been universal. 271–280 For example, in a recent study of HIV-infected women with longstanding HIV infection compared to HIV-uninfected women from the same geography, no difference in alpha diversity was observed.281 In a large individual level meta-analysis, Tuddenham et al. found decreases in alpha-diversity in HIV-infected patients as compared to HIV-uninfected patients. However, this reduction was observed only amongst women and men who have sex with women (MSW), but not in the men who have sex with men (MSM) population.282
Many (though not all) studies have reported differences in individual taxa between HIV-infected and HIV-uninfected individuals.271,272,274–280 Where changes in taxa have been observed, unique compositional and functional profiles in HIV distinct from other inflammatory conditions have been suggested. In one study of the gut microbiome from individuals with Clostridium difficile infection, lupus, HIV infection and healthy controls – 139 metabolic biomarkers were suggested to differentiate HIV infection from other conditions.283
Several studies suggest HIV-associated microbiota changes may skew towards pro-inflammatory organisms, though the specific changes observed across studies have differed.5,72,236,238,249,261,264,284 Early studies reported increases in Prevotella abundance with HIV infection. However, more recent studies suggest the increase in Prevotella in HIV possibly to be consequent to the confounding influence of MSM status as detailed further below.
Though significantly less studied, changes in the gut virome have also been reported with HIV infection. Contrary to the relationship observed with the bacterial microbiome, both HIV and SIV infection have been associated with an increased diversity and expansion of the enteric virome. SIV infection also has been associated with changes in the plasma virome.11,285 Increased abundance specifically of pathogenesis-associated viruses Adenoviridae and Anelloviridae have been observed with HIV infection.286
HIV-associated dysbiosis and mucosal and systemic inflammation
Several studies have found correlations of HIV-associated changes in both the bacterial microbiome and virome with immune activation and heightened systemic inflammation. Conversely, several microbiota observed in several studies to be in lower abundance with HIV infection positively correlate with markers of protective immunity.236,239,243,244,262,264,273,275,286
Some microbiota communities reported to be increased in abundance with HIV infection in several studies demonstrate an increased capacity to catabolize tryptophan to kynurenine. An increased kynurenine to tryptophan ratio has been shown to correlate with loss of mucosal Th17 cells, as well as heightened inflammation in HIV.257,264,287 Various microbiome composition changes across different taxa have been reported otherwise in association with microbial translocation, immune activation and systemic inflammation.236,239,243,244,246,248,273,288
More broadly, limitations of studies to date exploring the relationship of HIV infection to the microbiome include small sample sizes, a need for appropriate controls, and potential confounding effects of key putative microbiome modulating factors (including MSM status). Given the heterogeneity of findings to date, the exact role of HIV infection in modulating the microbiome remains to be determined.
Role of ART
ART itself has been considered a putative intervention to reduce inflammation through positive microbiome modulation. Yet, studies of ART in microbiome restoration have yielded discordant findings. A few studies suggest ART may promote gut dysbiosis, 246,289,290 while others show only partial recovery even with virologic suppression.240,242,252,261,264 Functional gene profile analyses of gut microbiota in ART treated subjects may still demonstrate persistent alterations in genes involved in bacterial translocation, systemic immune dysfunction and inflammatory pathways.248,291
In one recent study examining alpha diversity changes prospectively with ART treatment, changes in alpha diversity were observed with ART treatment at CD4 cell counts less than 300 but not with higher counts.292 The challenge of several studies of the relationship of ART to the microbiome include the heterogeneity of ART regimens used and as for studies exploring the role of HIV infection itself, the ability to differentiate ART specific effects from the influence of multiple potential microbiota-modulating confounders.
Putative drivers of inflammatory-microbiome signature in HIV beyond HIV-driven biological pathways
Many prior studies examining the role that HIV infection may play in aging-related phenotypes have sought to tease out the intersecting roles of HIV biology itself with comorbidity, medication effects, and behavior or lifestyle factors on adverse aging phenotypes. In order to elucidate the role that HIV biology, namely HIV-specific pathophysiologic pathways, may have on the gut microbiome in treated and untreated states, it is important to consider additional extrinsic factors that could strongly influence microbiome composition and function in the HIV-infected population.
Several factors known to influence the microbiome as detailed above, are known to disproportionately burden HIV-infected populations. These include maternal antibiotic use and mode of feeding; dietary factors consequent to malnutrition; bacterial infections requiring antibiotic use; multimorbidity and polypharmacy; tobacco use, alcohol use, opiate use, and other substance use; and mood disorders with potential impact for the bidirectional brain-gut axis. 207,293–300
MSM/Sexual behavior
Of particular relevance to the HIV-infected population, MSM status has been recently identified as being significantly associated with shifts in gut microbiome composition. Emerging data suggest that the gut microbiota of at least a subset of MSM is characterized by increased Prevotella and decreased Bacteroides.277,279,301 In one study, when the microbiota of MSM was transplanted into mouse models, elevated levels of gut-associated activated T cells were observed, independent of HIV status. 301 These findings, along with other data suggest that the microbiota of MSM is distinct and may have potentially pro-inflammatory immunologic effects. 302 However, what specific factor (e.g. receptive anal intercourse) associated with MSM status may drive such changes is still under investigation. 277,303–305
With the recognition of MSM status as a key putative confounder, new studies have attempted to control for this factor, either statistically or by balancing HIV-infected cases and HIV uninfected controls with respect to MSM status. Results remained mixed, and larger, carefully controlled studies accounting for MSM and other key putative microbiome-related factors that differentially burden HIV-infected populations will be needed to fully elucidate relationships between the microbiome, HIV, inflammation and frailty.
Microbiome modulation as putative target for frailty intervention
Increasing data suggest targeted modulation of an unfavorable, pathogenic gut microbiome via probiotics, prebiotics or fecal microbial transplantation (FMT) may restore health across a range of deleterious inflammatory conditions. 117,306–308 Probiotics are live microorganisms that confer a health benefit to the human host. Probiotics may promote health by positively impacting the immune system, increasing production of mucus, antimicrobial peptides and IgA secretion; limiting pathogen colonization through competitive adherence; controlling gut inflammation, and improving mucosal barrier functions and restoring gut integrity.110,309–311
Prebiotics are non-digestible substances considered to positively impact the host through selective growth of probiotic species.49,312 In both HIV-infected and uninfected populations, probiotics and prebiotics have been associated with reduced inflammation, immune activation, and microbial translocation, as well as recovery of gut mucosal integrity. 310,313–322
FMT robustly alters microbial communities and is emerging as a safe, effective, durable and innovative tool for microbiome modulation of inflammatory-related disease. In very limited HIV studies to date, FMT was found to be safe, well tolerated and to enhance immunity. 323–325 Importantly, the ability of probiotics and FMT to reduce inflammation and restore health has been strain, donor, disease and host dependent. 117,283,326–330 What probiotic strains, and prebiotic or FMT formulations will find optimal efficacy in preventing or reversing aberrant physiologic events in specific target populations or in specific pathogenic states, particularly frailty requires focused evaluation.
Future directions
While there have been significant separate advances in the elucidation of the biology of frailty and that of the human microbiome respectively, limited direct evaluation of the relationship of the human microbiome to the frailty construct exists in the literature to date. Detailed characterization of key microbiota targets, metabolites and other gene products that distinguish a frail microbiome from a non-frail microbiota could significantly inform interventions to rationally modulate the microbiome to combat frailty and promote healthy aging outcomes in HIV-infected and uninfected populations alike.
The few studies of the microbiome-frailty relationship to date have been primarily cross-sectional and correlative in nature. Longitudinal evaluation, use of translational animal models, and ultimately clinical intervention studies will be needed to determine causative pathways and application to combating frailty and promoting health. Beyond conventional 16S rRNA sequencing and metagenomics, future targeted metatranscriptomic, metaproteomic, and metabolomic studies will help elucidate additional novel targets for frailty prevention or recovery. Further, methodological considerations around instruments used for frailty assessment for future microbiome studies will be important for elucidation of common biology.
While most microbiome research has focused on bacterial communities, future studies are needed of the role of the virome, mycobiome, and other microbiome components, as well as the role of non-luminal and extra-intestinal microbiome compartments on frailty and other key aging phenotypes.
Disentangling the impact of HIV infection itself from lifestyle and behavioral factors, comorbidities, ART and other population-relevant microbiota-related factors on the microbiome will require sustained focused attention, appreciating both the promise and the complexity of linking the microbiome to complex and heterogeneous biological pathways such as inflammation across populations in which these multiple influences may be significantly integrated contributors to the microbiome signature.
Ultimately, evaluation of the role of the microbiome in frailty pathophysiology may require a systems approach in which the microbiome is appreciated as a highly complex ecosystem with multiple dynamic inputs, operating as an integrated biological network for host effect. Such an approach may provide optimized targeted opportunities for potential precision interventions to combat frailty and promote healthy aging across populations.
Acknowledgements
DAP is supported by National Institutes of Health grant R01AG060825 and by a Robert Wood Johnson Foundation Harold Amos Award. ST is supported by National Institutes of Health grant 1K23AI125715, a Willowcroft Foundation grant, and a 2017 developmental grant from the Johns Hopkins University Center for AIDS Research (JHU-CFAR), an NIH-funded program (P30AI094189). ST is the international co-PI on a small Indian government supported (SPARC) program grant for HIV gut microbiome research.
Footnotes
The authors have reviewed the journal’s policy on disclosure of potential conflicts of interest. The authors have read the journal’s authorship agreement. The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017;389(10076):1323–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piggott DA, Erlandson KM, Yarasheski KE. Frailty in HIV: Epidemiology, Biology, Measurement, Interventions, and Research Needs. Current HIV/AIDS reports. 2016. [DOI] [PMC free article] [PubMed]
- 3.Xue QL. The frailty syndrome: definition and natural history. Clinics in geriatric medicine. 2011;27(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finlay BB, Pettersson S, Melby MK, Bosch TCG. The Microbiome Mediates Environmental Effects on Aging. Bioessays. 2019;41(10):e1800257. [DOI] [PubMed] [Google Scholar]
- 5.Vujkovic-Cvijin I, Somsouk M. HIV and the Gut Microbiota: Composition, Consequences, and Avenues for Amelioration. Current HIV/AIDS reports. 2019;16(3):204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuddenham S, Sears CL. The intestinal microbiome and health. Curr Opin Infect Dis. 2015;28(5):464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroeder BO, Backhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nature medicine. 2016;22(10):1079–1089. [DOI] [PubMed] [Google Scholar]
- 9.Kim S, Jazwinski SM. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology. 2018;64(6):513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mudd JC, Brenchley JM. Gut Mucosal Barrier Dysfunction, Microbial Dysbiosis, and Their Role in HIV-1 Disease Progression. The Journal of infectious diseases. 2016;214 Suppl 2:S58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou S, Caler L, Colombini-Hatch S, Glynn S, Srinivas P. Research on the human virome: where are we and what is next. Microbiome. 2016;4(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136(1):65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7(7):688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez KB, Leone V, Chang EB. Microbial metabolites in health and disease: Navigating the unknown in search of function. J Biol Chem. 2017;292(21):8553–8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van de Wijgert JH, Jespers V. Incorporating microbiota data into epidemiologic models: examples from vaginal microbiota research. Ann Epidemiol. 2016;26(5):360–365. [DOI] [PubMed] [Google Scholar]
- 17.Williams B, Landay A, Presti RM. Microbiome alterations in HIV infection a review. Cell Microbiol. 2016;18(5):645–651. [DOI] [PubMed] [Google Scholar]
- 18.Bhattarai Y, Kashyap PC. Germ-Free Mice Model for Studying Host-Microbial Interactions. Methods Mol Biol. 2016;1438:123–135. [DOI] [PubMed] [Google Scholar]
- 19.Peloquin JM, Goel G, Villablanca EJ, Xavier RJ. Mechanisms of Pediatric Inflammatory Bowel Disease. Annual review of immunology. 2016;34:31–64. [DOI] [PubMed] [Google Scholar]
- 20.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, Spector TD, Clark AG, Ley RE. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim MY, You HJ, Yoon HS, Kwon B, Lee JY, Lee S, Song YM, Lee K, Sung J, Ko G. The effect of heritability and host genetics on the gut microbiota and metabolic syndrome. Gut. 2017;66(6):1031–1038. [DOI] [PubMed] [Google Scholar]
- 22.Dato S, Rose G, Crocco P, Monti D, Garagnani P, Franceschi C, Passarino G. The genetics of human longevity: an intricacy of genes, environment, culture and microbiome. Mechanisms of ageing and development. 2017;165(Pt B):147–155. [DOI] [PubMed] [Google Scholar]
- 23.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O’Sullivan O, Fitzgerald GF, Deane J, O’Connor M, Harnedy N, O’Connor K, O’Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O’Toole PW. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–184. [DOI] [PubMed] [Google Scholar]
- 24.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(33):14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faith JJ, McNulty NP, Rey FE, Gordon JI. Predicting a human gut microbiota’s response to diet in gnotobiotic mice. Science. 2011;333(6038):101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muegge BD, Kuczynski J, Knights D, Clemente JC, Gonzalez A, Fontana L, Henrissat B, Knight R, Gordon JI. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332(6032):970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Science translational medicine. 2009;1(6):6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drewes JL, Housseau F, Sears CL. Sporadic colorectal cancer: microbial contributors to disease prevention, development and therapy. British journal of cancer. 2016;115(3):273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell host & microbe. 2014;15(3):317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol Hepatol. 2017;2(10):747–756. [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Sung J, Lee J, Ko G. Comparison of the gut microbiotas of healthy adult twins living in South Korea and the United States. Applied and environmental microbiology. 2011;77(20):7433–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maffei VJ, Kim S, Blanchard Et, Luo M, Jazwinski SM, Taylor CM, Welsh DA. Biological Aging and the Human Gut Microbiota. J Gerontol A Biol Sci Med Sci. 2017;72(11):1474–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyakht AV, Kostryukova ES, Popenko AS, Belenikin MS, Pavlenko AV, Larin AK, Karpova IY, Selezneva OV, Semashko TA, Ospanova EA, Babenko VV, Maev IV, Cheremushkin SV, Kucheryavyy YA, Shcherbakov PL, Grinevich VB, Efimov OI, Sas EI, Abdulkhakov RA, Abdulkhakov SR, Lyalyukova EA, Livzan MA, Vlassov VV, Sagdeev RZ, Tsukanov VV, Osipenko MF, Kozlova IV, Tkachev AV, Sergienko VI, Alexeev DG, Govorun VM. Human gut microbiota community structures in urban and rural populations in Russia. Nat Commun. 2013;4:2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152(1–2):39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108 Suppl 1:4554–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jernberg C, Lofmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156(Pt 11):3216–3223. [DOI] [PubMed] [Google Scholar]
- 40.An R, Wilms E, Masclee AAM, Smidt H, Zoetendal EG, Jonkers D. Age-dependent changes in GI physiology and microbiota: time to reconsider? Gut. 2018;67(12):2213–2222. [DOI] [PubMed] [Google Scholar]
- 41.Freedberg DE, Toussaint NC, Chen SP, Ratner AJ, Whittier S, Wang TC, Wang HH, Abrams JA. Proton Pump Inhibitors Alter Specific Taxa in the Human Gastrointestinal Microbiome: A Crossover Trial. Gastroenterology. 2015;149(4):883–885 e889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H, Patil KR, Bork P, Typas A. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Pedersen HK, Arumugam M, Kristiansen K, Voigt AY, Vestergaard H, Hercog R, Costea PI, Kultima JR, Li J, Jorgensen T, Levenez F, Dore J, Meta HITc, Nielsen HB, Brunak S, Raes J, Hansen T, Wang J, Ehrlich SD, Bork P, Pedersen O. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ticinesi A, Milani C, Lauretani F, Nouvenne A, Mancabelli L, Lugli GA, Turroni F, Duranti S, Mangifesta M, Viappiani A, Ferrario C, Maggio M, Ventura M, Meschi T. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Scientific reports. 2017;7(1):11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zapata HJ, Quagliarello VJ. The microbiota and microbiome in aging: potential implications in health and age-related diseases. J Am Geriatr Soc. 2015;63(4):776–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ticinesi A, Nouvenne A, Cerundolo N, Catania P, Prati B, Tana C, Meschi T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients. 2019;11(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Candela M, Biagi E, Maccaferri S, Turroni S, Brigidi P. Intestinal microbiota is a plastic factor responding to environmental changes. Trends Microbiol. 2012;20(8):385–391. [DOI] [PubMed] [Google Scholar]
- 48.Bowyer RCE, Jackson MA, Le Roy CI, Ni Lochlainn M, Spector TD, Dowd JB, Steves CJ. Socioeconomic Status and the Gut Microbiome: A TwinsUK Cohort Study. Microorganisms. 2019;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jayanama K, Theou O. Effects of Probiotics and Prebiotics on Frailty and Ageing: A Narrative Review. Curr Clin Pharmacol. 2019. [DOI] [PubMed]
- 50.Miller GE, Engen PA, Gillevet PM, Shaikh M, Sikaroodi M, Forsyth CB, Mutlu E, Keshavarzian A. Lower Neighborhood Socioeconomic Status Associated with Reduced Diversity of the Colonic Microbiota in Healthy Adults. PloS one. 2016;11(2):e0148952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain, behavior, and immunity. 2011;25(3):397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szyszkowicz JK, Wong A, Anisman H, Merali Z, Audet MC. Implications of the gut microbiota in vulnerability to the social avoidance effects of chronic social defeat in male mice. Brain, behavior, and immunity. 2017;66:45–55. [DOI] [PubMed] [Google Scholar]
- 53.Allais L, Kerckhof FM, Verschuere S, Bracke KR, De Smet R, Laukens D, Van den Abbeele P, De Vos M, Boon N, Brusselle GG, Cuvelier CA, Van de Wiele T. Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut. Environmental microbiology. 2016;18(5):1352–1363. [DOI] [PubMed] [Google Scholar]
- 54.Biedermann L, Zeitz J, Mwinyi J, Sutter-Minder E, Rehman A, Ott SJ, Steurer-Stey C, Frei A, Frei P, Scharl M, Loessner MJ, Vavricka SR, Fried M, Schreiber S, Schuppler M, Rogler G. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PloS one. 2013;8(3):e59260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Capurso G, Lahner E. The interaction between smoking, alcohol and the gut microbiome. Best Pract Res Clin Gastroenterol. 2017;31(5):579–588. [DOI] [PubMed] [Google Scholar]
- 56.Savin Z, Kivity S, Yonath H, Yehuda S. Smoking and the intestinal microbiome. Arch Microbiol. 2018;200(5):677–684. [DOI] [PubMed] [Google Scholar]
- 57.Galligan JJ. HIV, opiates, and enteric neuron dysfunction. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2015;27(4):449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiles TJ, Jemielita M, Baker RP, Schlomann BH, Logan SL, Ganz J, Melancon E, Eisen JS, Guillemin K, Parthasarathy R. Host Gut Motility Promotes Competitive Exclusion within a Model Intestinal Microbiota. PLoS biology. 2016;14(7):e1002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meng J, Yu H, Ma J, Wang J, Banerjee S, Charboneau R, Barke RA, Roy S. Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PloS one. 2013;8(1):e54040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kiraly DD, Walker DM, Calipari ES, Labonte B, Issler O, Pena CJ, Ribeiro EA, Russo SJ, Nestler EJ. Alterations of the Host Microbiome Affect Behavioral Responses to Cocaine. Scientific reports. 2016;6:35455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mailing LJ, Allen JM, Buford TW, Fields CJ, Woods JA. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc Sport Sci Rev. 2019;47(2):75–85. [DOI] [PubMed] [Google Scholar]
- 62.Barton W, Penney NC, Cronin O, Garcia-Perez I, Molloy MG, Holmes E, Shanahan F, Cotter PD, O’Sullivan O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2018;67(4):625–633. [DOI] [PubMed] [Google Scholar]
- 63.Estaki M, Pither J, Baumeister P, Little JP, Gill SK, Ghosh S, Ahmadi-Vand Z, Marsden KR, Gibson DL. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fiuza-Luces C, Santos-Lozano A, Joyner M, Carrera-Bastos P, Picazo O, Zugaza JL, Izquierdo M, Ruilope LM, Lucia A. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol. 2018;15(12):731–743. [DOI] [PubMed] [Google Scholar]
- 65.Zhao X, Zhang Z, Hu B, Huang W, Yuan C, Zou L. Response of Gut Microbiota to Metabolite Changes Induced by Endurance Exercise. Frontiers in microbiology. 2018;9:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bressa C, Bailen-Andrino M, Perez-Santiago J, Gonzalez-Soltero R, Perez M, Montalvo-Lominchar MG, Mate-Munoz JL, Dominguez R, Moreno D, Larrosa M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PloS one. 2017;12(2):e0171352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, Holscher HD, Woods JA. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med Sci Sports Exerc. 2018;50(4):747–757. [DOI] [PubMed] [Google Scholar]
- 68.Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, Gougis S, Rizkalla S, Batto JM, Renault P, consortium ANRM, Dore J, Zucker JD, Clement K, Ehrlich SD. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500(7464):585–588. [DOI] [PubMed] [Google Scholar]
- 69.Jackson MA, Jeffery IB, Beaumont M, Bell JT, Clark AG, Ley RE, O’Toole PW, Spector TD, Steves CJ. Signatures of early frailty in the gut microbiota. Genome medicine. 2016;8(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jorgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clement K, Dore J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T, Meta HITc, Bork P, Wang J, Ehrlich SD, Pedersen O. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–546. [DOI] [PubMed] [Google Scholar]
- 71.Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, Roca J, Dore J. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55(2):205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Desai SN, Landay AL. HIV and aging: role of the microbiome. Current opinion in HIV and AIDS. 2018;13(1):22–27. [DOI] [PubMed] [Google Scholar]
- 73.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(34):13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. [DOI] [PubMed] [Google Scholar]
- 75.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016;167(6):1469–1480 e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buchner DM, Wagner EH. Preventing frail health. Clinics in geriatric medicine. 1992;8(1):1–17. [PubMed] [Google Scholar]
- 78.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–263. [DOI] [PubMed] [Google Scholar]
- 79.Walston JD, Bandeen-Roche K. Frailty: a tale of two concepts. BMC medicine. 2015;13:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buta BJ, Walston JD, Godino JG, Park M, Kalyani RR, Xue QL, Bandeen-Roche K, Varadhan R. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research G. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 82.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. TheScientificWorldJournal. 2001;1:323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mitnitski AB, Song X, Rockwood K. The estimation of relative fitness and frailty in community-dwelling older adults using self-report data. J Gerontol A Biol Sci Med Sci. 2004;59(6):M627–632. [DOI] [PubMed] [Google Scholar]
- 84.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62(7):738–743. [DOI] [PubMed] [Google Scholar]
- 85.Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54(6):975–979. [DOI] [PubMed] [Google Scholar]
- 86.Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2011;183(8):E487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262–266. [DOI] [PubMed] [Google Scholar]
- 88.Fried LP, Hadley EC, Walston JD, Newman AB, Guralnik JM, Studenski S, Harris TB, Ershler WB, Ferrucci L. From bedside to bench: research agenda for frailty. Science of aging knowledge environment : SAGE KE. 2005;2005(31):pe24. [DOI] [PubMed] [Google Scholar]
- 89.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54(6):991–1001. [DOI] [PubMed] [Google Scholar]
- 90.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fedarko NS. The biology of aging and frailty. Clinics in geriatric medicine. 2011;27(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, Fried LP. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med. 2007;167(7):635–641. [DOI] [PubMed] [Google Scholar]
- 93.Collerton J, Martin-Ruiz C, Davies K, Hilkens CM, Isaacs J, Kolenda C, Parker C, Dunn M, Catt M, Jagger C, von Zglinicki T, Kirkwood TB. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mechanisms of ageing and development. 2012;133(6):456–466. [DOI] [PubMed] [Google Scholar]
- 94.Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50(7):1268–1271. [DOI] [PubMed] [Google Scholar]
- 95.Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55(6):864–871. [DOI] [PubMed] [Google Scholar]
- 96.Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf). 2005;63(4):403–411. [DOI] [PubMed] [Google Scholar]
- 97.Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–2341. [DOI] [PubMed] [Google Scholar]
- 98.Newman AB, Sachs MC, Arnold AM, Fried LP, Kronmal R, Cushman M, Psaty BM, Harris TB, Robbins JA, Burke GL, Kuller LH, Lumley T. Total and cause-specific mortality in the cardiovascular health study. J Gerontol A Biol Sci Med Sci. 2009;64(12):1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH Jr., Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106(5):506–512. [DOI] [PubMed] [Google Scholar]
- 100.Jenny NS, Yanez ND, Psaty BM, Kuller LH, Hirsch CH, Tracy RP. Inflammation biomarkers and near-term death in older men. American journal of epidemiology. 2007;165(6):684–695. [DOI] [PubMed] [Google Scholar]
- 101.Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61(6):575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roubenoff R, Parise H, Payette HA, Abad LW, D’Agostino R, Jacques PF, Wilson PW, Dinarello CA, Harris TB. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115(6):429–435. [DOI] [PubMed] [Google Scholar]
- 103.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10(3):319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Walston JD, Matteini AM, Nievergelt C, Lange LA, Fallin DM, Barzilai N, Ziv E, Pawlikowska L, Kwok P, Cummings SR, Kooperberg C, LaCroix A, Tracy RP, Atzmon G, Lange EM, Reiner AP. Inflammation and stress-related candidate genes, plasma interleukin-6 levels, and longevity in older adults. Exp Gerontol. 2009;44(5):350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fukui SM, Piggott DA, Erlandson KM. Inflammation Strikes Again: Frailty and HIV. Current HIV/AIDS reports. 2018;15(1):20–29. [DOI] [PubMed] [Google Scholar]
- 106.Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, Sergi G, Isik AT, Manzato E, Maggi S, Maggio M, Prina AM, Cosco TD, Wu YT, Veronese N. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8. [DOI] [PubMed] [Google Scholar]
- 107.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cram JA, Hager KW, Kublin JG. Utilizing gnotobiotic models to inform the role of the microbiome in vaccine response heterogeneity. Current opinion in HIV and AIDS. 2018;13(1):1–8. [DOI] [PubMed] [Google Scholar]
- 109.Forbes JD, Van Domselaar G, Bernstein CN. The Gut Microbiota in Immune-Mediated Inflammatory Diseases. Frontiers in microbiology. 2016;7:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.O’Toole PW, Jeffery IB. Microbiome-health interactions in older people. Cellular and molecular life sciences : CMLS. 2018;75(1):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330(6012):1768–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lima MT, Andrade AC, Oliveira GP, Calixto RS, Oliveira DB, Souza EL, Trindade GS, Nicoli JR, Kroon EG, Martins FS, Abrahao JS. Microbiota is an essential element for mice to initiate a protective immunity against Vaccinia virus. FEMS Microbiol Ecol. 2016;92(2). [DOI] [PubMed] [Google Scholar]
- 113.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535(7610):75–84. [DOI] [PubMed] [Google Scholar]
- 114.Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535(7610):65–74. [DOI] [PubMed] [Google Scholar]
- 115.Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140(6):859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haag LM, Siegmund B . Intestinal Microbiota and the Innate Immune System - A Crosstalk in Crohn’s Disease Pathogenesis. Front Immunol. 2015;6:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.D’Angelo C, Reale M, Costantini E. Microbiota and Probiotics in Health and HIV Infection. Nutrients. 2017;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol. 2014;14(1):9–23. [DOI] [PubMed] [Google Scholar]
- 119.Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang JP, Brown EM, Frankel G, Levy M, Katz MN, Philbrick WM, Elinav E, Finlay BB, Flavell RA. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. 2014;156(5):1045–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saad MJ, Santos A, Prada PO. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology (Bethesda). 2016;31(4):283–293. [DOI] [PubMed] [Google Scholar]
- 123.Shapiro H, Thaiss CA, Levy M, Elinav E. The cross talk between microbiota and the immune system: metabolites take center stage. Curr Opin Immunol. 2014;30:54–62. [DOI] [PubMed] [Google Scholar]
- 124.Postler TS, Ghosh S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 2017;26(1):110–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C, Anrather J. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nature medicine. 2016;22(5):516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dejea CM, Wick EC, Hechenbleikner EM, White JR, Mark Welch JL, Rossetti BJ, Peterson SN, Snesrud EC, Borisy GG, Lazarev M, Stein E, Vadivelu J, Roslani AC, Malik AA, Wanyiri JW, Goh KL, Thevambiga I, Fu K, Wan F, Llosa N, Housseau F, Romans K, Wu X, McAllister FM, Wu S, Vogelstein B, Kinzler KW, Pardoll DM, Sears CL. Microbiota organization is a distinct feature of proximal colorectal cancers. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(51):18321–18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Casati M, Ferri E, Azzolino D, Cesari M, Arosio B. Gut microbiota and physical frailty through the mediation of sarcopenia. Exp Gerontol. 2019;124:110639. [DOI] [PubMed] [Google Scholar]
- 130.Ticinesi A, Lauretani F, Milani C, Nouvenne A, Tana C, Del Rio D, Maggio M, Ventura M, Meschi T. Aging Gut Microbiota at the Cross-Road between Nutrition, Physical Frailty, and Sarcopenia: Is There a Gut-Muscle Axis? Nutrients. 2017;9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkila J, Monti D, Satokari R, Franceschi C, Brigidi P, De Vos W. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PloS one. 2010;5(5):e10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guigoz Y, Dore J, Schiffrin EJ. The inflammatory status of old age can be nurtured from the intestinal environment. Current opinion in clinical nutrition and metabolic care. 2008;11(1):13–20. [DOI] [PubMed] [Google Scholar]
- 133.Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, Consolandi C, Quercia S, Scurti M, Monti D, Capri M, Brigidi P, Candela M. Gut Microbiota and Extreme Longevity. Curr Biol. 2016;26(11):1480–1485. [DOI] [PubMed] [Google Scholar]
- 134.Di Sabatino A, Lenti MV, Cammalleri L, Corazza GR, Pilotto A. Frailty and the gut. Dig Liver Dis. 2018;50(6):533–541. [DOI] [PubMed] [Google Scholar]
- 135.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Grosicki GJ, Fielding RA, Lustgarten MS. Gut Microbiota Contribute to Age-Related Changes in Skeletal Muscle Size, Composition, and Function: Biological Basis for a Gut-Muscle Axis. Calcif Tissue Int. 2018;102(4):433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Odenwald MA, Turner JR. Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol. 2013;11(9):1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mitchell EL, Davis AT, Brass K, Dendinger M, Barner R, Gharaibeh R, Fodor AA, Kavanagh K. Reduced Intestinal Motility, Mucosal Barrier Function, and Inflammation in Aged Monkeys. J Nutr Health Aging. 2017;21(4):354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP, Jury J, Foley KP, Schertzer JD, Larche MJ, Davidson DJ, Verdu EF, Surette MG, Bowdish DM. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell host & microbe. 2017;21(4):455–466 e454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fransen F, van Beek AA, Borghuis T, Aidy SE, Hugenholtz F, van der Gaast-de Jongh C, Savelkoul HFJ, De Jonge MI, Boekschoten MV, Smidt H, Faas MM, de Vos P. Aged Gut Microbiota Contributes to Systemical Inflammaging after Transfer to Germ-Free Mice. Front Immunol. 2017;8:1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, Gordon JI. The long-term stability of the human gut microbiota. Science. 2013;341(6141):1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hill CJ, Lynch DB, Murphy K, Ulaszewska M, Jeffery IB, O’Shea CA, Watkins C, Dempsey E, Mattivi F, Tuohy K, Ross RP, Ryan CA, PW OT, Stanton C. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, Stanton C, van Sinderen D, O’Connor M, Harnedy N, O’Connor K, Henry C, O’Mahony D, Fitzgerald AP, Shanahan F, Twomey C, Hill C, Ross RP, O’Toole PW. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proceedings of the National Academy of Sciences of the United States of America. 2011;108 Suppl 1:4586–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mariat D, Firmesse O, Levenez F, Guimaraes V, Sokol H, Dore J, Corthier G, Furet JP. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC microbiology. 2009;9:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Makivuokko H, Tiihonen K, Tynkkynen S, Paulin L, Rautonen N. The effect of age and non-steroidal anti-inflammatory drugs on human intestinal microbiota composition. Br J Nutr. 2010;103(2):227–234. [DOI] [PubMed] [Google Scholar]
- 146.Rampelli S, Candela M, Turroni S, Biagi E, Collino S, Franceschi C, O’Toole PW, Brigidi P. Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging (Albany NY). 2013;5(12):902–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zwielehner J, Liszt K, Handschur M, Lassl C, Lapin A, Haslberger AG. Combined PCR-DGGE fingerprinting and quantitative-PCR indicates shifts in fecal population sizes and diversity of Bacteroides, bifidobacteria and Clostridium cluster IV in institutionalized elderly. Exp Gerontol. 2009;44(6–7):440–446. [DOI] [PubMed] [Google Scholar]
- 148.Turroni F, Ventura M, Butto LF, Duranti S, O’Toole PW, Motherway MO, van Sinderen D. Molecular dialogue between the human gut microbiota and the host: a Lactobacillus and Bifidobacterium perspective. Cellular and molecular life sciences : CMLS. 2014;71(2):183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Giezenaar C, Chapman I, Luscombe-Marsh N, Feinle-Bisset C, Horowitz M, Soenen S. Ageing Is Associated with Decreases in Appetite and Energy Intake--A Meta-Analysis in Healthy Adults. Nutrients. 2016;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Roager HM, Hansen LB, Bahl MI, Frandsen HL, Carvalho V, Gobel RJ, Dalgaard MD, Plichta DR, Sparholt MH, Vestergaard H, Hansen T, Sicheritz-Ponten T, Nielsen HB, Pedersen O, Lauritzen L, Kristensen M, Gupta R, Licht TR. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat Microbiol. 2016;1(9):16093. [DOI] [PubMed] [Google Scholar]
- 151.Leng SX, Margolick JB. Aging, sex, inflammation, frailty, and CMV and HIV infections. Cell Immunol. 2020;348:104024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cougnoux A, Dalmasso G, Martinez R, Buc E, Delmas J, Gibold L, Sauvanet P, Darcha C, Dechelotte P, Bonnet M, Pezet D, Wodrich H, Darfeuille-Michaud A, Bonnet R. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut. 2014;63(12):1932–1942. [DOI] [PubMed] [Google Scholar]
- 154.Frey N, Venturelli S, Zender L, Bitzer M. Cellular senescence in gastrointestinal diseases: from pathogenesis to therapeutics. Nat Rev Gastroenterol Hepatol. 2018;15(2):81–95. [DOI] [PubMed] [Google Scholar]
- 155.Saito Y, Murata-Kamiya N, Hirayama T, Ohba Y, Hatakeyama M. Conversion of Helicobacter pylori CagA from senescence inducer to oncogenic driver through polarity-dependent regulation of p21. J Exp Med. 2010;207(10):2157–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. [DOI] [PubMed] [Google Scholar]
- 157.Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front Immunol. 2017;8:1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Viruses Hurme M. and immunosenescence - more players in the game. Immun Ageing. 2019;16:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nikolich-Zugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol. 2018;19(1):10–19. [DOI] [PubMed] [Google Scholar]
- 160.Amsterdam D, Ostrov BE. The Impact of the Microbiome on Immunosenescence. Immunol Invest. 2018;47(8):801–811. [DOI] [PubMed] [Google Scholar]
- 161.Tibbs TN, Lopez LR, Arthur JC. The influence of the microbiota on immune development, chronic inflammation, and cancer in the context of aging. Microb Cell. 2019;6(8):324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Barrera-Vazquez OS, Gomez-Verjan JC. The Unexplored World of Human Virome, Mycobiome, and Archaeome in Aging. J Gerontol A Biol Sci Med Sci. 2019. [DOI] [PubMed]
- 163.Zarate S, Taboada B, Yocupicio-Monroy M, Arias CF. Human Virome. Arch Med Res. 2017;48(8):701–716. [DOI] [PubMed] [Google Scholar]
- 164.Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53(5):747–754. [DOI] [PubMed] [Google Scholar]
- 165.Furman D, Jojic V, Sharma S, Shen-Orr SS, Angel CJ, Onengut-Gumuscu S, Kidd BA, Maecker HT, Concannon P, Dekker CL, Thomas PG, Davis MM. Cytomegalovirus infection enhances the immune response to influenza. Science translational medicine. 2015;7(281):281ra243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Van Belleghem JD, Clement F, Merabishvili M, Lavigne R, Vaneechoutte M. Pro- and anti-inflammatory responses of peripheral blood mononuclear cells induced by Staphylococcus aureus and Pseudomonas aeruginosa phages. Scientific reports. 2017;7(1):8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hollander D, Tarnawski H. Aging-associated increase in intestinal absorption of macromolecules. Gerontology. 1985;31(3):133–137. [DOI] [PubMed] [Google Scholar]
- 168.Katz D, Hollander D, Said HM, Dadufalza V. Aging-associated increase in intestinal permeability to polyethylene glycol 900. Dig Dis Sci. 1987;32(3):285–288. [DOI] [PubMed] [Google Scholar]
- 169.Buford TW, Carter CS, VanDerPol WJ, Chen D, Lefkowitz EJ, Eipers P, Morrow CD, Bamman MM. Composition and richness of the serum microbiome differ by age and link to systemic inflammation. Geroscience. 2018;40(3):257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sovran B, Hugenholtz F, Elderman M, Van Beek AA, Graversen K, Huijskes M, Boekschoten MV, Savelkoul HFJ, De Vos P, Dekker J, Wells JM. Age-associated Impairment of the Mucus Barrier Function is Associated with Profound Changes in Microbiota and Immunity. Scientific reports. 2019;9(1):1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Clark RI, Salazar A, Yamada R, Fitz-Gibbon S, Morselli M, Alcaraz J, Rana A, Rera M, Pellegrini M, Ja WW, Walker DW. Distinct Shifts in Microbiota Composition during Drosophila Aging Impair Intestinal Function and Drive Mortality. Cell Rep. 2015;12(10):1656–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ciccocioppo R, Di Sabatino A, Luinetti O, Rossi M, Cifone MG, Corazza GR. Small bowel enterocyte apoptosis and proliferation are increased in the elderly. Gerontology. 2002;48(4):204–208. [DOI] [PubMed] [Google Scholar]
- 173.O’Toole PW, Jeffery IB. Gut microbiota and aging. Science. 2015;350(6265):1214–1215. [DOI] [PubMed] [Google Scholar]
- 174.Verdi S, Jackson MA, Beaumont M, Bowyer RCE, Bell JT, Spector TD, Steves CJ. An Investigation Into Physical Frailty as a Link Between the Gut Microbiome and Cognitive Health. Front Aging Neurosci. 2018;10:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Buigues C, Fernandez-Garrido J, Pruimboom L, Hoogland AJ, Navarro-Martinez R, Martinez-Martinez M, Verdejo Y, Mascaros MC, Peris C, Cauli O. Effect of a Prebiotic Formulation on Frailty Syndrome: A Randomized, Double-Blind Clinical Trial. Int J Mol Sci. 2016;17(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ghosh TS, Rampelli S, Jeffery IB, Santoro A, Neto M, Capri M, Giampieri E, Jennings A, Candela M, Turroni S, Zoetendal EG, Hermes GDA, Elodie C, Meunier N, Brugere CM, Pujos-Guillot E, Berendsen AM, De Groot L, Feskins EJM, Kaluza J, Pietruszka B, Bielak MJ, Comte B, Maijo-Ferre M, Nicoletti C, De Vos WM, Fairweather-Tait S, Cassidy A, Brigidi P, Franceschi C, O’Toole PW. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. 2020. [DOI] [PMC free article] [PubMed]
- 177.van Tongeren SP, Slaets JP, Harmsen HJ, Welling GW. Fecal microbiota composition and frailty. Applied and environmental microbiology. 2005;71(10):6438–6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Haran JP, Bucci V, Dutta P, Ward D, McCormick B. The nursing home elder microbiome stability and associations with age, frailty, nutrition and physical location. J Med Microbiol. 2018;67(1):40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Theou O, Jayanama K, Fernandez-Garrido J, Buigues C, Pruimboom L, Hoogland AJ, Navarro-Martinez R, Rockwood K, Cauli O. Can a Prebiotic Formulation Reduce Frailty Levels in Older People? The Journal of frailty & aging. 2019;8(1):48–52. [DOI] [PubMed] [Google Scholar]
- 180.Graves DT, Correa JD, Silva TA. The Oral Microbiota Is Modified by Systemic Diseases. Journal of dental research. 2019;98(2):148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sampaio-Maia B, Caldas IM, Pereira ML, Perez-Mongiovi D, Araujo R. The Oral Microbiome in Health and Its Implication in Oral and Systemic Diseases. Adv Appl Microbiol. 2016;97:171–210. [DOI] [PubMed] [Google Scholar]
- 182.Iwauchi M, Horigome A, Ishikawa K, Mikuni A, Nakano M, Xiao JZ, Odamaki T, Hironaka S. Relationship between oral and gut microbiota in elderly people. Immun Inflamm Dis. 2019;7(3):229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Martinez-Guryn K, Leone V, Chang EB. Regional Diversity of the Gastrointestinal Microbiome. Cell host & microbe. 2019;26(3):314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ogawa T, Hirose Y, Honda-Ogawa M, Sugimoto M, Sasaki S, Kibi M, Kawabata S, Ikebe K, Maeda Y. Composition of salivary microbiota in elderly subjects. Scientific reports. 2018;8(1):414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lin R, Liu W, Piao M, Zhu H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids. 2017;49(12):2083–2090. [DOI] [PubMed] [Google Scholar]
- 186.LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. 2013;24(2):160–168. [DOI] [PubMed] [Google Scholar]
- 187.Leduc-Gaudet JP, Picard M, St-Jean Pelletier F, Sgarioto N, Auger MJ, Vallee J, Robitaille R, St-Pierre DH, Gouspillou G. Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget. 2015;6(20):17923–17937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Marzetti E, Calvani R, Lorenzi M, Tanganelli F, Picca A, Bossola M, Menghi A, Bernabei R, Landi F. Association between myocyte quality control signaling and sarcopenia in old hip-fractured patients: Results from the Sarcopenia in HIp FracTure (SHIFT) exploratory study. Exp Gerontol. 2016;80:1–5. [DOI] [PubMed] [Google Scholar]
- 189.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nature reviews Endocrinology. 2015;11(10):577–591. [DOI] [PubMed] [Google Scholar]
- 190.Walsh ME, Bhattacharya A, Sataranatarajan K, Qaisar R, Sloane L, Rahman MM, Kinter M, Van Remmen H. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell. 2015;14(6):957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Siddharth J, Chakrabarti A, Pannerec A, Karaz S, Morin-Rivron D, Masoodi M, Feige JN, Parkinson SJ. Aging and sarcopenia associate with specific interactions between gut microbes, serum biomarkers and host physiology in rats. Aging (Albany NY). 2017;9(7):1698–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wu CS, Wei Q, Wang H, Kim DM, Balderas M, Wu G, Lawler J, Safe S, Guo S, Devaraj S, Chen Z, Sun Y. Protective effects of ghrelin on fasting-induced muscle atrophy in aging mice. J Gerontol A Biol Sci Med Sci. 2018. [DOI] [PMC free article] [PubMed]
- 193.Bjorkhaug ST, Aanes H, Neupane SP, Bramness JG, Malvik S, Henriksen C, Skar V, Medhus AW, Valeur J. Characterization of gut microbiota composition and functions in patients with chronic alcohol overconsumption. Gut microbes. 2019;10(6):663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kimura I, Inoue D, Hirano K, Tsujimoto G. The SCFA Receptor GPR43 and Energy Metabolism. Front Endocrinol (Lausanne). 2014;5:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yan J, Charles JF. Gut Microbiota and IGF-1. Calcif Tissue Int. 2018;102(4):406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fried LP, Xue QL, Cappola AR, Ferrucci L, Chaves P, Varadhan R, Guralnik JM, Leng SX, Semba RD, Walston JD, Blaum CS, Bandeen-Roche K. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64(10):1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kalyani RR, Varadhan R, Weiss CO, Fried LP, Cappola AR. Frailty status and altered dynamics of circulating energy metabolism hormones after oral glucose in older women. J Nutr Health Aging. 2012;16(8):679–686. [DOI] [PubMed] [Google Scholar]
- 198.Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5: a valuable metabolic target. Dig Dis. 2011;29(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Prawitt J, Caron S, Staels B. Bile acid metabolism and the pathogenesis of type 2 diabetes. Curr Diab Rep. 2011;11(3):160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Schretter CE, Vielmetter J, Bartos I, Marka Z, Marka S, Argade S, Mazmanian SK. A gut microbial factor modulates locomotor behaviour in Drosophila. Nature. 2018;563(7731):402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Barichella M, Severgnini M, Cilia R, Cassani E, Bolliri C, Caronni S, Ferri V, Cancello R, Ceccarani C, Faierman S, Pinelli G, De Bellis G, Zecca L, Cereda E, Consolandi C, Pezzoli G. Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov Disord. 2019;34(3):396–405. [DOI] [PubMed] [Google Scholar]
- 202.Morita E, Yokoyama H, Imai D, Takeda R, Ota A, Kawai E, Hisada T, Emoto M, Suzuki Y, Okazaki K. Aerobic Exercise Training with Brisk Walking Increases Intestinal Bacteroides in Healthy Elderly Women. Nutrients. 2019;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Roman E, Nieto JC, Gely C, Vidal S, Pozuelo M, Poca M, Juarez C, Guarner C, Manichanh C, Soriano G. Effect of a Multistrain Probiotic on Cognitive Function and Risk of Falls in Patients With Cirrhosis: A Randomized Trial. Hepatol Commun. 2019;3(5):632–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30(3):350–358. [DOI] [PubMed] [Google Scholar]
- 205.Autenrieth CS, Beck EJ, Stelzle D, Mallouris C, Mahy M, Ghys P. Global and regional trends of people living with HIV aged 50 and over: Estimates and projections for 2000–2020. PloS one. 2018;13(11):e0207005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mills EJ, Barnighausen T, Negin J. HIV and aging--preparing for the challenges ahead. N Engl J Med. 2012;366(14):1270–1273. [DOI] [PubMed] [Google Scholar]
- 207.Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, Sighem A, de Wolf F, Hallett TB, cohort Ao. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. The Lancet Infectious diseases. 2015;15(7):810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Marcus JL, Chao CR, Leyden WA, Xu L, Quesenberry CP Jr, Klein DB, Towner WJ, Horberg MA, Silverberg MJ. Narrowing the Gap in Life Expectancy Between HIV-Infected and HIV-Uninfected Individuals With Access to Care. Journal of acquired immune deficiency syndromes. 2016;73(1):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gueler A, Moser A, Calmy A, Gunthard HF, Bernasconi E, Furrer H, Fux CA, Battegay M, Cavassini M, Vernazza P, Zwahlen M, Egger M, Swiss Hiv Cohort Study SNC. Life expectancy in HIV-positive persons in Switzerland: matched comparison with general population. Aids. 2017;31(3):427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, Burchell AN, Cohen M, Gebo KA, Gill MJ, Justice A, Kirk G, Klein MB, Korthuis PT, Martin J, Napravnik S, Rourke SB, Sterling TR, Silverberg MJ, Deeks S, Jacobson LP, Bosch RJ, Kitahata MM, Goedert JJ, Moore R, Gange SJ, North American ACCoR, Design of Ie DEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS one. 2013;8(12):e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Piggott DA, Muzaale AD, Varadhan R, Mehta SH, Westergaard RP, Brown TT, Patel KV, Walston JD, Leng SX, Kirk GD. Frailty and Cause-Specific Hospitalization Among Persons Aging With HIV Infection and Injection Drug Use. J Gerontol A Biol Sci Med Sci. 2016. [DOI] [PMC free article] [PubMed]
- 213.Sharma A, Shi Q, Hoover DR, Tien PC, Plankey MW, Cohen MH, Golub ET, Gustafson D, Yin MT. Frailty predicts fractures among women with and at-risk for HIV. Aids. 2019;33(3):455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tassiopoulos K, Abdo M, Wu K, Koletar SL, Palella FJ Jr., Kalayjian R, Taiwo B, Erlandson KM. Frailty is strongly associated with increased risk of recurrent falls among older HIV-infected adults. Aids. 2017;31(16):2287–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sharma A, Hoover DR, Shi Q, Gustafson DR, Plankey MW, Tien PC, Weber KM, Yin MT. Frailty as a predictor of falls in HIV-infected and uninfected women. Antivir Ther. 2019;24(1):51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Erlandson KM, Perez J, Abdo M, Robertson K, Ellis RJ, Koletar SL, Kalayjian R, Taiwo B, Palella FJ Jr., Tassiopoulos K. Frailty, Neurocognitive Impairment, or Both in Predicting Poor Health Outcomes Among Adults Living With Human Immunodeficiency Virus. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2019;68(1):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kelly SG, Wu K, Tassiopoulos K, Erlandson KM, Koletar SL, Palella FJ. Frailty Is an Independent Risk Factor for Mortality, Cardiovascular Disease, Bone Disease, and Diabetes Among Aging Adults With Human Immunodeficiency Virus. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2019;69(8):1370–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rubtsova AA, Sabbag S, Sundermann E, Nguyen AL, Ellis RJ, Moore DJ, Letendre S, Jeste DV, Marquine MJ. Frailty and Neurocognitive Impairment: Impacts on Quality of Life in HIV. J Assoc Nurses AIDS Care. 2019. [DOI] [PMC free article] [PubMed]
- 219.Piggott DA, Muzaale AD, Mehta SH, Brown TT, Patel KV, Leng SX, Kirk GD. Frailty, HIV Infection, and Mortality in an Aging Cohort of Injection Drug Users. PloS one. 2013;8(1):e54910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39(4):633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hsu DC, Ma YF, Hur S, Li D, Rupert A, Scherzer R, Kalapus SC, Deeks S, Sereti I, Hsue PY. Plasma IL-6 levels are independently associated with atherosclerosis and mortality in HIV-infected individuals on suppressive antiretroviral therapy. Aids. 2016;30(13):2065–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, Robinson J, Huang Y, Epling L, Martin JN, Deeks SG, Meinert CL, Van Natta ML, Jabs DA, Lederman MM. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. The Journal of infectious diseases. 2014;210(8):1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, Paton NI, Neaton JD, Group ISS. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS medicine. 2008;5(10):e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.So-Armah KA, Tate JP, Chang CC, Butt AA, Gerschenson M, Gibert CL, Leaf D, Rimland D, Rodriguez-Barradas MC, Budoff MJ, Samet JH, Kuller LH, Deeks SG, Crothers K, Tracy RP, Crane HM, Sajadi MM, Tindle HA, Justice AC, Freiberg MS, Team VP. Do Biomarkers of Inflammation, Monocyte Activation, and Altered Coagulation Explain Excess Mortality Between HIV Infected and Uninfected People? Journal of acquired immune deficiency syndromes. 2016;72(2):206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, Plants J, Seth A, Wilson CC, Deeks SG, Lederman MM, Landay AL. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. The Journal of infectious diseases. 2014;210(8):1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Piggott DA, Varadhan R, Mehta SH, Brown TT, Li H, Walston JD, Leng SX, Kirk GD. Frailty, Inflammation, and Mortality Among Persons Aging With HIV Infection and Injection Drug Use. J Gerontol A Biol Sci Med Sci. 2015. [DOI] [PMC free article] [PubMed]
- 227.Piggott DA, Bandeen-Roche K, Mehta SH, Brown TT, Yang H, Walston JD, Leng SX, Kirk GD. Frailty Progression and Recovery among Persons Aging with HIV and Substance Use. Conference on Retroviruses and Opportunistic Infections; 2017, 2017; Seattle, WA.
- 228.Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS pathogens. 2010;6(4):e1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, Paiardini M, Barclay GR, Smedley J, Pung R, Oliveira KM, Hirsch VM, Silvestri G, Douek DC, Miller CJ, Haase AT, Lifson J, Brenchley JM. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS pathogens. 2010;6(8):e1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Klatt NR, Harris LD, Vinton CL, Sung H, Briant JA, Tabb B, Morcock D, McGinty JW, Lifson JD, Lafont BA, Martin MA, Levine AD, Estes JD, Brenchley JM. Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal immunology. 2010;3(4):387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kumar P, Monin L, Castillo P, Elsegeiny W, Horne W, Eddens T, Vikram A, Good M, Schoenborn AA, Bibby K, Montelaro RC, Metzger DW, Gulati AS, Kolls JK. Intestinal Interleukin-17 Receptor Signaling Mediates Reciprocal Control of the Gut Microbiota and Autoimmune Inflammation. Immunity. 2016;44(3):659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, Stoel M, Zhou Y, Sodergren E, Weinstock GM, Bevins CL, Williams CB, Bos NA. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11(1):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN, Gordon JI, Onderdonk AB, Glimcher LH. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell host & microbe. 2010;8(3):292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131(1):33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Dillon SM, Lee EJ, Kotter CV, Austin GL, Gianella S, Siewe B, Smith DM, Landay AL, McManus MC, Robertson CE, Frank DN, McCarter MD, Wilson CC. Gut dendritic cell activation links an altered colonic microbiome to mucosal and systemic T-cell activation in untreated HIV-1 infection. Mucosal immunology. 2016;9(1):24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK, Gianella S, Siewe B, Smith DM, Landay AL, Robertson CE, Frank DN, Wilson CC. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal immunology. 2014;7(4):983–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Dillon SM, Lee EJ, Donovan AM, Guo K, Harper MS, Frank DN, McCarter MD, Santiago ML, Wilson CC. Enhancement of HIV-1 infection and intestinal CD4+ T cell depletion ex vivo by gut microbes altered during chronic HIV-1 infection. Retrovirology. 2016;13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Dillon SM, Kibbie J, Lee EJ, Guo K, Santiago ML, Austin GL, Gianella S, Landay AL, Donovan AM, Frank DN, Mc CM, Wilson CC. Low abundance of colonic butyrate-producing bacteria in HIV infection is associated with microbial translocation and immune activation. Aids. 2017;31(4):511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Dinh DM, Volpe GE, Duffalo C, Bhalchandra S, Tai AK, Kane AV, Wanke CA, Ward HD. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. The Journal of infectious diseases. 2015;211(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Dubourg G, Lagier JC, Hue S, Surenaud M, Bachar D, Robert C, Michelle C, Ravaux I, Mokhtari S, Million M, Stein A, Brouqui P, Levy Y, Raoult D. Gut microbiota associated with HIV infection is significantly enriched in bacteria tolerant to oxygen. BMJ open gastroenterology. 2016;3(1):e000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lozupone CA, Rhodes ME, Neff CP, Fontenot AP, Campbell TB, Palmer BE. HIV-induced alteration in gut microbiota: driving factors, consequences, and effects of antiretroviral therapy. Gut microbes. 2014;5(4):562–570. [DOI] [PubMed] [Google Scholar]
- 242.Lozupone CA, Li M, Campbell TB, Flores SC, Linderman D, Gebert MJ, Knight R, Fontenot AP, Palmer BE. Alterations in the gut microbiota associated with HIV-1 infection. Cell host & microbe. 2013;14(3):329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Monaco CL, Gootenberg DB, Zhao G, Handley SA, Ghebremichael MS, Lim ES, Lankowski A, Baldridge MT, Wilen CB, Flagg M, Norman JM, Keller BC, Luevano JM, Wang D, Boum Y, Martin JN, Hunt PW, Bangsberg DR, Siedner MJ, Kwon DS, Virgin HW. Altered Virome and Bacterial Microbiome in Human Immunodeficiency Virus-Associated Acquired Immunodeficiency Syndrome. Cell host & microbe. 2016;19(3):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Mutlu EA, Keshavarzian A, Losurdo J, Swanson G, Siewe B, Forsyth C, French A, Demarais P, Sun Y, Koenig L, Cox S, Engen P, Chakradeo P, Abbasi R, Gorenz A, Burns C, Landay A. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS pathogens. 2014;10(2):e1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Pinto-Cardoso S, Lozupone C, Briceno O, Alva-Hernandez S, Tellez N, Adriana A, Murakami-Ogasawara A, Reyes-Teran G. Fecal Bacterial Communities in treated HIV infected individuals on two antiretroviral regimens. Scientific reports. 2017;7:43741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Nowak P, Troseid M, Avershina E, Barqasho B, Neogi U, Holm K, Hov JR, Noyan K, Vesterbacka J, Svard J, Rudi K, Sonnerborg A. Gut microbiota diversity predicts immune status in HIV-1 infection. Aids. 2015;29(18):2409–2418. [DOI] [PubMed] [Google Scholar]
- 247.Serrano-Villar S, Vazquez-Castellanos JF, Vallejo A, Latorre A, Sainz T, Ferrando-Martinez S, Rojo D, Martinez-Botas J, Del Romero J, Madrid N, Leal M, Mosele JI, Motilva MJ, Barbas C, Ferrer M, Moya A, Moreno S, Gosalbes MJ, Estrada V. The effects of prebiotics on microbial dysbiosis, butyrate production and immunity in HIV-infected subjects. Mucosal immunology. 2017;10(5):1279–1293. [DOI] [PubMed] [Google Scholar]
- 248.Vazquez-Castellanos JF, Serrano-Villar S, Latorre A, Artacho A, Ferrus ML, Madrid N, Vallejo A, Sainz T, Martinez-Botas J, Ferrando-Martinez S, Vera M, Dronda F, Leal M, Del Romero J, Moreno S, Estrada V, Gosalbes MJ, Moya A. Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal immunology. 2015;8(4):760–772. [DOI] [PubMed] [Google Scholar]
- 249.Sun Y, Ma Y, Lin P, Tang YW, Yang L, Shen Y, Zhang R, Liu L, Cheng J, Shao J, Qi T, Tang Y, Cai R, Guan L, Luo B, Sun M, Li B, Pei Z, Lu H. Fecal bacterial microbiome diversity in chronic HIV-infected patients in China. Emerging microbes & infections. 2016;5:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Villanueva-Millan MJ, Perez-Matute P, Recio-Fernandez E, Lezana Rosales JM, Oteo JA. Differential effects of antiretrovirals on microbial translocation and gut microbiota composition of HIV-infected patients. J Int AIDS Soc. 2017;20(1):21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Volpe GE, Ward H, Mwamburi M, Dinh D, Bhalchandra S, Wanke C, Kane AV. Associations of cocaine use and HIV infection with the intestinal microbiota, microbial translocation, and inflammation. Journal of studies on alcohol and drugs. 2014;75(2):347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ling Z, Jin C, Xie T, Cheng Y, Li L, Wu N. Alterations in the Fecal Microbiota of Patients with HIV-1 Infection: An Observational Study in A Chinese Population. Scientific reports. 2016;6:30673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Deusch S, Serrano-Villar S, Rojo D, Martinez-Martinez M, Bargiela R, Vazquez-Castellanos JF, Sainz T, Barbas C, Moya A, Moreno S, Gosalbes MJ, Estrada V, Seifert J, Ferrer M. Effects of HIV, antiretroviral therapy and prebiotics on the active fraction of the gut microbiota. Aids. 2018;32(10):1229–1237. [DOI] [PubMed] [Google Scholar]
- 254.Missailidis C, Neogi U, Stenvinkel P, Troseid M, Nowak P, Bergman P. The microbial metabolite trimethylamine-N-oxide in association with inflammation and microbial dysregulation in three HIV cohorts at various disease stages. Aids. 2018;32(12):1589–1598. [DOI] [PubMed] [Google Scholar]
- 255.San-Juan-Vergara H, Zurek E, Ajami NJ, Mogollon C, Pena M, Portnoy I, Velez JI, Cadena-Cruz C, Diaz-Olmos Y, Hurtado-Gomez L, Sanchez-Sit S, Hernandez D, Urruchurtu I, Di-Ruggiero P, Guardo-Garcia E, Torres N, Vidal-Orjuela O, Viasus D, Petrosino JF, Cervantes-Acosta G. A Lachnospiraceae-dominated bacterial signature in the fecal microbiota of HIV-infected individuals from Colombia, South America. Scientific reports. 2018;8(1):4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhou Y, Ou Z, Tang X, Zhou Y, Xu H, Wang X, Li K, He J, Du Y, Wang H, Chen Y, Nie Y. Alterations in the gut microbiota of patients with acquired immune deficiency syndrome. Journal of cellular and molecular medicine. 2018;22(4):2263–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Hoel H, Hove-Skovsgaard M, Hov JR, Gaardbo JC, Holm K, Kummen M, Rudi K, Nwosu F, Valeur J, Gelpi M, Seljeflot I, Ueland PM, Gerstoft J, Ullum H, Aukrust P, Nielsen SD, Troseid M. Impact of HIV and Type 2 diabetes on Gut Microbiota Diversity, Tryptophan Catabolism and Endothelial Dysfunction. Scientific reports. 2018;8(1):6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Liu J, Johnson R, Dillon S, Kroehl M, Frank DN, Tuncil YE, Zhang X, Ir D, Robertson CE, Seifert S, Higgins J, Hamaker B, Wilson CC, Erlandson KM. Among older adults, age-related changes in the stool microbiome differ by HIV-1 serostatus. EBioMedicine. 2019;40:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Maurice JB, Garvey L, Tsochatzis EA, Wiltshire M, Cooke G, Guppy N, McDonald J, Marchesi J, Nelson M, Kelleher P, Goldin R, Thursz M, Lemoine M. Monocyte-macrophage activation is associated with nonalcoholic fatty liver disease and liver fibrosis in HIV monoinfection independently of the gut microbiome and bacterial translocation. Aids. 2019;33(5):805–814. [DOI] [PubMed] [Google Scholar]
- 260.Rocafort M, Noguera-Julian M, Rivera J, Pastor L, Guillen Y, Langhorst J, Parera M, Mandomando I, Carrillo J, Urrea V, Rodriguez C, Casadella M, Calle ML, Clotet B, Blanco J, Naniche D, Paredes R. Evolution of the gut microbiome following acute HIV-1 infection. Microbiome. 2019;7(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.McHardy IH, Li X, Tong M, Ruegger P, Jacobs J, Borneman J, Anton P, Braun J. HIV Infection is associated with compositional and functional shifts in the rectal mucosal microbiota. Microbiome. 2013;1(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Vazquez-Castellanos JF, Serrano-Villar S, Jimenez-Hernandez N, Soto Del Rio MD, Gayo S, Rojo D, Ferrer M, Barbas C, Moreno S, Estrada V, Rattei T, Latorre A, Moya A, Gosalbes MJ. Interplay between gut microbiota metabolism and inflammation in HIV infection. The ISME journal. 2018;12(8):1964–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Qing Y, Xie H, Su C, Wang Y, Yu Q, Pang Q, Cui F. Gut Microbiome, Short-Chain Fatty Acids, and Mucosa Injury in Young Adults with Human Immunodeficiency Virus Infection. Dig Dis Sci. 2019;64(7):1830–1843. [DOI] [PubMed] [Google Scholar]
- 264.Vujkovic-Cvijin I, Dunham RM, Iwai S, Maher MC, Albright RG, Broadhurst MJ, Hernandez RD, Lederman MM, Huang Y, Somsouk M, Deeks SG, Hunt PW, Lynch SV, McCune JM. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Science translational medicine. 2013;5(193):193ra191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Paquin-Proulx D, Ching C, Vujkovic-Cvijin I, Fadrosh D, Loh L, Huang Y, Somsouk M, Lynch SV, Hunt PW, Nixon DF, SenGupta D. Bacteroides are associated with GALT iNKT cell function and reduction of microbial translocation in HIV-1 infection. Mucosal immunology. 2017;10(1):69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tincati C, Merlini E, Braidotti P, Ancona G, Savi F, Tosi D, Borghi E, Callegari ML, Mangiavillano B, Barassi A, Bulfamante G, d’Arminio Monforte A, Romagnoli S, Chomont N, Marchetti G. Impaired gut junctional complexes feature late-treated individuals with suboptimal CD4+ T-cell recovery upon virologically suppressive combination antiretroviral therapy. Aids. 2016;30(7):991–1003. [DOI] [PubMed] [Google Scholar]
- 267.Sortino O, Hullsiek KH, Richards E, Rupert A, Schminke A, Tetekpor N, Quinones M, Prosser R, Schacker T, Sereti I, Baker JV. The Effects of Recombinant Human Lactoferrin on Immune Activation and the Intestinal Microbiome Among Persons Living with Human Immunodeficiency Virus and Receiving Antiretroviral Therapy. The Journal of infectious diseases. 2019;219(12):1963–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Glavan TW, Gaulke CA, Santos Rocha C, Sankaran-Walters S, Hirao LA, Raffatellu M, Jiang G, Baumler AJ, Goulart LR, Dandekar S. Gut immune dysfunction through impaired innate pattern recognition receptor expression and gut microbiota dysbiosis in chronic SIV infection. Mucosal immunology. 2016;9(3):677–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Moeller AH, Shilts M, Li Y, Rudicell RS, Lonsdorf EV, Pusey AE, Wilson ML, Hahn BH, Ochman H. SIV-induced instability of the chimpanzee gut microbiome. Cell host & microbe. 2013;14(3):340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Vujkovic-Cvijin I, Swainson LA, Chu SN, Ortiz AM, Santee CA, Petriello A, Dunham RM, Fadrosh DW, Lin DL, Faruqi AA, Huang Y, Apetrei C, Pandrea I, Hecht FM, Pilcher CD, Klatt NR, Brenchley JM, Lynch SV, McCune JM. Gut-Resident Lactobacillus Abundance Associates with IDO1 Inhibition and Th17 Dynamics in SIV-Infected Macaques. Cell Rep. 2015;13(8):1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Yu G, Fadrosh D, Ma B, Ravel J, Goedert JJ. Anal microbiota profiles in HIV-positive and HIV-negative MSM. Aids. 2014;28(5):753–760. [DOI] [PubMed] [Google Scholar]
- 272.Cook RR, Fulcher JA, Tobin NH, Li F, Lee D, Javanbakht M, Brookmeyer R, Shoptaw S, Bolan R, Aldrovandi GM, Gorbach PM. Effects of HIV viremia on the gastrointestinal microbiome of young MSM. Aids. 2019;33(5):793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lee SC, Chua LL, Yap SH, Khang TF, Leng CY, Raja Azwa RI, Lewin SR, Kamarulzaman A, Woo YL, Lim YAL, Loke P, Rajasuriar R. Enrichment of gut-derived Fusobacterium is associated with suboptimal immune recovery in HIV-infected individuals. Scientific reports. 2018;8(1):14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Machiavelli A, Duarte RTD, Pires MMS, Zarate-Blades CR, Pinto AR. The impact of in utero HIV exposure on gut microbiota, inflammation, and microbial translocation. Gut microbes. 2019;10(5):599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Rhoades N, Mendoza N, Jankeel A, Sureshchandra S, Alvarez AD, Doratt B, Heidari O, Hagan R, Brown B, Scheibel S, Marbley T, Taylor J, Messaoudi I. Altered Immunity and Microbial Dysbiosis in Aged Individuals With Long-Term Controlled HIV Infection. Front Immunol. 2019;10:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Vesterbacka J, Rivera J, Noyan K, Parera M, Neogi U, Calle M, Paredes R, Sonnerborg A, Noguera-Julian M, Nowak P. Richer gut microbiota with distinct metabolic profile in HIV infected Elite Controllers. Scientific reports. 2017;7(1):6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Armstrong AJS, Shaffer M, Nusbacher NM, Griesmer C, Fiorillo S, Schneider JM, Preston Neff C, Li SX, Fontenot AP, Campbell T, Palmer BE, Lozupone CA. An exploration of Prevotella-rich microbiomes in HIV and men who have sex with men. Microbiome. 2018;6(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Guillen Y, Noguera-Julian M, Rivera J, Casadella M, Zevin AS, Rocafort M, Parera M, Rodriguez C, Arumi M, Carrillo J, Mothe B, Estany C, Coll J, Bravo I, Herrero C, Saz J, Sirera G, Torrella A, Navarro J, Crespo M, Negredo E, Brander C, Blanco J, Calle ML, Klatt NR, Clotet B, Paredes R. Low nadir CD4+ T-cell counts predict gut dysbiosis in HIV-1 infection. Mucosal immunology. 2019;12(1):232–246. [DOI] [PubMed] [Google Scholar]
- 279.Noguera-Julian M, Rocafort M, Guillen Y, Rivera J, Casadella M, Nowak P, Hildebrand F, Zeller G, Parera M, Bellido R, Rodriguez C, Carrillo J, Mothe B, Coll J, Bravo I, Estany C, Herrero C, Saz J, Sirera G, Torrela A, Navarro J, Crespo M, Brander C, Negredo E, Blanco J, Guarner F, Calle ML, Bork P, Sonnerborg A, Clotet B, Paredes R. Gut Microbiota Linked to Sexual Preference and HIV Infection. EBioMedicine. 2016;5:135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Sortino OP N, Schuetz A, Ortiz AM, Chomchey N, Belkaid Y, Davis J, Mystakelis HA, Quinones M, Deleage C, Ingram B, Rerknimitr R, Pinyakorn S, Rupert A, Robb ML Anaworanich J, Brenchley J, Sereti I, on behalf of the RV254/SEARCH010 Study Group Impact of Acute HIV Infection and Early Antiretroviral Therapy on the Human Gut Microbiome. OFID. 2019;Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 281.Wang Z, Usyk M, Sollecito CC, Qiu Y, Williams-Nguyen J, Hua S, Gradissimo A, Wang T, Xue X, Kurland IJ, Ley K, Landay AL, Anastos K, Knight R, Kaplan RC, Burk RD, Qi Q. Altered Gut Microbiota and Host Metabolite Profiles in HIV-infected Women. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2019. [DOI] [PMC free article] [PubMed]
- 282.Tuddenham SA, Koay WLA, Zhao N, White JR, Ghanem KG, Sears CL, Consortium HIVMR-a. The Impact of Human Immunodeficiency Virus Infection on Gut Microbiota alpha-Diversity: An Individual-level Meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020;70(4):615–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Serrano-Villar S, Rojo D, Martinez-Martinez M, Deusch S, Vazquez-Castellanos JF, Sainz T, Vera M, Moreno S, Estrada V, Gosalbes MJ, Latorre A, Margolles A, Seifert J, Barbas C, Moya A, Ferrer M. HIV infection results in metabolic alterations in the gut microbiota different from those induced by other diseases. Scientific reports. 2016;6:26192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Gori A, Tincati C, Rizzardini G, Torti C, Quirino T, Haarman M, Ben Amor K, van Schaik J, Vriesema A, Knol J, Marchetti G, Welling G, Clerici M. Early impairment of gut function and gut flora supporting a role for alteration of gastrointestinal mucosa in human immunodeficiency virus pathogenesis. Journal of clinical microbiology. 2008;46(2):757–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Handley SA, Thackray LB, Zhao G, Presti R, Miller AD, Droit L, Abbink P, Maxfield LF, Kambal A, Duan E, Stanley K, Kramer J, Macri SC, Permar SR, Schmitz JE, Mansfield K, Brenchley JM, Veazey RS, Stappenbeck TS, Wang D, Barouch DH, Virgin HW. Pathogenic simian immunodeficiency virus infection is associated with expansion of the enteric virome. Cell. 2012;151(2):253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Gootenberg DB, Paer JM, Luevano JM, Kwon DS. HIV-associated changes in the enteric microbial community: potential role in loss of homeostasis and development of systemic inflammation. Curr Opin Infect Dis. 2017;30(1):31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, Barbour JD, Lowe MM, Jayawardene A, Aweeka F, Huang Y, Douek DC, Brenchley JM, Martin JN, Hecht FM, Deeks SG, McCune JM. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Science translational medicine. 2010;2(32):32ra36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Perez-Santiago J, Gianella S, Massanella M, Spina CA, Karris MY, Var SR, Patel D, Jordan PS, Young JA, Little SJ, Richman DD, Smith DM. Gut Lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. Aids. 2013;27(12):1921–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Klase Z, Ortiz A, Deleage C, Mudd JC, Quinones M, Schwartzman E, Klatt NR, Canary L, Estes JD, Brenchley JM. Dysbiotic bacteria translocate in progressive SIV infection. Mucosal immunology. 2015;8(5):1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Nowak RG, Bentzen SM, Ravel J, Crowell TA, Dauda W, Ma B, Liu H, Blattner WA, Baral SD, Charurat ME, Group TS. Rectal microbiota among HIV-uninfected, untreated HIV, and treated HIV-infected in Nigeria. Aids. 2017;31(6):857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Tincati C, Douek DC, Marchetti G. Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection. AIDS Res Ther. 2016;13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Ji Y, Zhang F, Zhang R, Shen Y, Liu L, Wang J, Yang J, Tang Q, Xun J, Qi T, Wang Z, Song W, Tang Y, Chen J, Lu H. Changes in intestinal microbiota in HIV-1-infected subjects following cART initiation: influence of CD4+ T cell count. Emerging microbes & infections. 2018;7(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Levin AM, Sitarik AR, Havstad SL, Fujimura KE, Wegienka G, Cassidy-Bushrow AE, Kim H, Zoratti EM, Lukacs NW, Boushey HA, Ownby DR, Lynch SV, Johnson CC. Joint effects of pregnancy, sociocultural, and environmental factors on early life gut microbiome structure and diversity. Scientific reports. 2016;6:31775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ford N, Shubber Z, Meintjes G, Grinsztejn B, Eholie S, Mills EJ, Davies MA, Vitoria M, Penazzato M, Nsanzimana S, Frigati L, O’Brien D, Ellman T, Ajose O, Calmy A, Doherty M. Causes of hospital admission among people living with HIV worldwide: a systematic review and meta-analysis. Lancet HIV. 2015;2(10):e438–444. [DOI] [PubMed] [Google Scholar]
- 295.Pacek LR, Cioe PA. Tobacco Use, Use Disorders, and Smoking Cessation Interventions in Persons Living With HIV. Current HIV/AIDS reports. 2015;12(4):413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, Stone J, Cunningham EB, Trickey A, Dumchev K, Lynskey M, Griffiths P, Mattick RP, Hickman M, Larney S. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. The Lancet Global health. 2017;5(12):e1192–e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Mathers BM, Degenhardt L, Ali H, Wiessing L, Hickman M, Mattick RP, Myers B, Ambekar A, Strathdee SA, Reference Group to the UNoHIV, Injecting Drug U. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet. 2010;375(9719):1014–1028. [DOI] [PubMed] [Google Scholar]
- 298.Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH. Alcohol Use and Human Immunodeficiency Virus (HIV) Infection: Current Knowledge, Implications, and Future Directions. Alcoholism, clinical and experimental research. 2016;40(10):2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Ivers LC, Cullen KA, Freedberg KA, Block S, Coates J, Webb P. HIV/AIDS, undernutrition, and food insecurity. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;49(7):1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Edelman EJ, Rentsch CT, Justice AC. Polypharmacy in HIV: recent insights and future directions. Current opinion in HIV and AIDS. 2020;15(2):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Li SX, Sen S, Schneider JM, Xiong KN, Nusbacher NM, Moreno-Huizar N, Shaffer M, Armstrong AJS, Severs E, Kuhn K, Neff CP, McCarter M, Campbell T, Lozupone CA, Palmer BE. Gut microbiota from high-risk men who have sex with men drive immune activation in gnotobiotic mice and in vitro HIV infection. PLoS pathogens. 2019;15(4):e1007611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Palmer CD, Tomassilli J, Sirignano M, Romero-Tejeda M, Arnold KB, Che D, Lauffenburger DA, Jost S, Allen T, Mayer KH, Altfeld M. Enhanced immune activation linked to endotoxemia in HIV-1 seronegative MSM. Aids. 2014;28(14):2162–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Fulcher JA, Hussain SK, Cook R, Li F, Tobin NH, Ragsdale A, Shoptaw S, Gorbach PM, Aldrovandi GM. Effects of Substance Use and Sex Practices on the Intestinal Microbiome During HIV-1 Infection. The Journal of infectious diseases. 2018;218(10):1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Haaland RE, Fountain J, Hu Y, Holder A, Dinh C, Hall L, Pescatore NA, Heeke S, Hart CE, Xu J, Hu YJ, Kelley CF. Repeated rectal application of a hyperosmolar lubricant is associated with microbiota shifts but does not affect PrEP drug concentrations: results from a randomized trial in men who have sex with men. J Int AIDS Soc. 2018;21(10):e25199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Kelley CF, Kraft CS, de Man TJ, Duphare C, Lee HW, Yang J, Easley KA, Tharp GK, Mulligan MJ, Sullivan PS, Bosinger SE, Amara RR. The rectal mucosa and condomless receptive anal intercourse in HIV-negative MSM: implications for HIV transmission and prevention. Mucosal immunology. 2017;10(4):996–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wang Y, Li X, Ge T, Xiao Y, Liao Y, Cui Y, Zhang Y, Ho W, Yu G, Zhang T. Probiotics for prevention and treatment of respiratory tract infections in children: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016;95(31):e4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Ganji-Arjenaki M, Rafieian-Kopaei M. Probiotics are a good choice in remission of inflammatory bowel diseases: A meta analysis and systematic review. Journal of cellular physiology. 2018;233(3):2091–2103. [DOI] [PubMed] [Google Scholar]
- 308.Guandalini S. Probiotics for prevention and treatment of diarrhea. J Clin Gastroenterol. 2011;45 Suppl:S149–153. [DOI] [PubMed] [Google Scholar]
- 309.Delcenserie V, Martel D, Lamoureux M, Amiot J, Boutin Y, Roy D. Immunomodulatory effects of probiotics in the intestinal tract. Curr Issues Mol Biol. 2008;10(1–2):37–54. [PubMed] [Google Scholar]
- 310.Ohland CL, Macnaughton WK. Probiotic bacteria and intestinal epithelial barrier function. American journal of physiology Gastrointestinal and liver physiology. 2010;298(6):G807–819. [DOI] [PubMed] [Google Scholar]
- 311.Sanders ME. Impact of probiotics on colonizing microbiota of the gut. J Clin Gastroenterol. 2011;45 Suppl:S115–119. [DOI] [PubMed] [Google Scholar]
- 312.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125(6):1401–1412. [DOI] [PubMed] [Google Scholar]
- 313.Bandera A, De Benedetto I, Bozzi G, Gori A. Altered gut microbiome composition in HIV infection: causes, effects and potential intervention. Current opinion in HIV and AIDS. 2018;13(1):73–80. [DOI] [PubMed] [Google Scholar]
- 314.d’Ettorre G, Ceccarelli G, Giustini N, Serafino S, Calantone N, De Girolamo G, Bianchi L, Bellelli V, Ascoli-Bartoli T, Marcellini S, Turriziani O, Brenchley JM, Vullo V. Probiotics Reduce Inflammation in Antiretroviral Treated, HIV-Infected Individuals: Results of the “Probio-HIV” Clinical Trial. PloS one. 2015;10(9):e0137200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.d’Ettorre G, Rossi G, Scagnolari C, Andreotti M, Giustini N, Serafino S, Schietroma I, Scheri GC, Fard SN, Trinchieri V, Mastromarino P, Selvaggi C, Scarpona S, Fanello G, Fiocca F, Ceccarelli G, Antonelli G, Brenchley JM, Vullo V. Probiotic supplementation promotes a reduction in T-cell activation, an increase in Th17 frequencies, and a recovery of intestinal epithelium integrity and mitochondrial morphology in ART-treated HIV-1-positive patients. Immun Inflamm Dis. 2017;5(3):244–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Gonzalez-Hernandez LA, Jave-Suarez LF, Fafutis-Morris M, Montes-Salcedo KE, Valle-Gutierrez LG, Campos-Loza AE, Enciso-Gomez LF, Andrade-Villanueva JF. Synbiotic therapy decreases microbial translocation and inflammation and improves immunological status in HIV-infected patients: a double-blind randomized controlled pilot trial. Nutr J. 2012;11:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Gori A, Rizzardini G, Van’t Land B, Amor KB, van Schaik J, Torti C, Quirino T, Tincati C, Bandera A, Knol J, Benlhassan-Chahour K, Trabattoni D, Bray D, Vriesema A, Welling G, Garssen J, Clerici M. Specific prebiotics modulate gut microbiota and immune activation in HAART-naive HIV-infected adults: results of the “COPA” pilot randomized trial. Mucosal immunology. 2011;4(5):554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Klatt NR, Canary LA, Sun X, Vinton CL, Funderburg NT, Morcock DR, Quinones M, Deming CB, Perkins M, Hazuda DJ, Miller MD, Lederman MM, Segre JA, Lifson JD, Haddad EK, Estes JD, Brenchley JM. Probiotic/prebiotic supplementation of antiretrovirals improves gastrointestinal immunity in SIV-infected macaques. The Journal of clinical investigation. 2013;123(2):903–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Ortiz AM, Klase ZA, DiNapoli SR, Vujkovic-Cvijin I, Carmack K, Perkins MR, Calantone N, Vinton CL, Riddick NE, Gallagher J, Klatt NR, McCune JM, Estes JD, Paiardini M, Brenchley JM. IL-21 and probiotic therapy improve Th17 frequencies, microbial translocation, and microbiome in ARV-treated, SIV-infected macaques. Mucosal immunology. 2016;9(2):458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Stiksrud B, Nowak P, Nwosu FC, Kvale D, Thalme A, Sonnerborg A, Ueland PM, Holm K, Birkeland SE, Dahm AE, Sandset PM, Rudi K, Hov JR, Dyrhol-Riise AM, Troseid M. Reduced Levels of D-dimer and Changes in Gut Microbiota Composition After Probiotic Intervention in HIV-Infected Individuals on Stable ART. Journal of acquired immune deficiency syndromes. 2015;70(4):329–337. [DOI] [PubMed] [Google Scholar]
- 321.Villar-Garcia J, Guerri-Fernandez R, Moya A, Gonzalez A, Hernandez JJ, Lerma E, Guelar A, Sorli L, Horcajada JP, Artacho A, G DA, Knobel H . Impact of probiotic Saccharomyces boulardii on the gut microbiome composition in HIV-treated patients: A double-blind, randomised, placebo-controlled trial. PloS one. 2017;12(4):e0173802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Villar-Garcia J, Hernandez JJ, Guerri-Fernandez R, Gonzalez A, Lerma E, Guelar A, Saenz D, Sorli L, Montero M, Horcajada JP, Knobel Freud H. Effect of probiotics (Saccharomyces boulardii) on microbial translocation and inflammation in HIV-treated patients: a double-blind, randomized, placebo-controlled trial. Journal of acquired immune deficiency syndromes. 2015;68(3):256–263. [DOI] [PubMed] [Google Scholar]
- 323.Di Bella S, Gouliouris T, Petrosillo N . Fecal microbiota transplantation (FMT) for Clostridium difficile infection: focus on immunocompromised patients. J Infect Chemother. 2015;21(4):230–237. [DOI] [PubMed] [Google Scholar]
- 324.Hensley-McBain T, Zevin AS, Manuzak J, Smith E, Gile J, Miller C, Agricola B, Katze M, Reeves RK, Kraft CS, Langevin S, Klatt NR. Effects of Fecal Microbial Transplantation on Microbiome and Immunity in Simian Immunodeficiency Virus-Infected Macaques. Journal of virology. 2016;90(10):4981–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Kelly CR, Ihunnah C, Fischer M, Khoruts A, Surawicz C, Afzali A, Aroniadis O, Barto A, Borody T, Giovanelli A, Gordon S, Gluck M, Hohmann EL, Kao D, Kao JY, McQuillen DP, Mellow M, Rank KM, Rao K, Ray A, Schwartz MA, Singh N, Stollman N, Suskind DL, Vindigni SM, Youngster I, Brandt L. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109(7):1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sanchez B, Delgado S, Blanco-Miguez A, Lourenco A, Gueimonde M, Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res. 2017;61(1). [DOI] [PubMed] [Google Scholar]
- 327.Gathe JC Jr., Diejomaoh EM, Mayberry CC, Clemmons JB. Fecal Transplantation for Clostridium Difficile-”All Stool May Not Be Created Equal”. J Int Assoc Provid AIDS Care. 2016;15(2):107–108. [DOI] [PubMed] [Google Scholar]
- 328.Vujkovic-Cvijin I, Rutishauser RL, Pao M, Hunt PW, Lynch SV, McCune JM, Somsouk M. Limited engraftment of donor microbiome via one-time fecal microbial transplantation in treated HIV-infected individuals. Gut microbes. 2017;8(5):440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Carter GM, Esmaeili A, Shah H, Indyk D, Johnson M, Andreae M, Sacks HS. Probiotics in Human Immunodeficiency Virus Infection: A Systematic Review and Evidence Synthesis of Benefits and Risks. Open Forum Infect Dis. 2016;3(4):ofw164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Habil N, Al-Murrani W, Beal J, Foey AD. Probiotic bacterial strains differentially modulate macrophage cytokine production in a strain-dependent and cell subset-specific manner. Benef Microbes. 2011;2(4):283–293. [DOI] [PubMed] [Google Scholar]