Abstract
Nicotine is the major alkaloid present in cigarettes that induces various biochemical and behavioral changes. Nanonaringenin (NNG) and vitamin E are antioxidants that are reported to mitigate serious impairments caused by some toxins and oxidants. Thus, we aimed to investigate the efficacy of NNG, vitamin E, and their combinations to ameliorate behavioral, biochemical, and histological alterations induced by nicotine in rats. Adult male albino rats were randomly grouped into six equal groups (10 rats/group): control, N (nicotine 1 mg/kg b.w./day S/C from 15th to 45th day, 5 days a week), NNG (25 mg/kg b.w./day orally for 45 days), N + NNG, N + E (nicotine + vitamin E 200 mg/kg b.w./day orally), and N + NNG + E (nicotine + NNG + vitamin E at the aforementioned doses). Behavioral tests were conducted on day 15 and 30 postnicotine injection, while memory tests, brain neurotransmitters, antioxidants, and histopathological examination were examined at day 30 only. As a result, nicotine impaired rats' activity (hypoactivity and hyperactivity) and memory, induced anxiolytic and anxiogenic effects on rats, and altered neurotransmitters (acetylcholinesterase, serotonin, and dopamine), and redox markers (MDA, H2O2, GSH, and catalase) levels in brain homogenates. Thickening and congestion of the meninges and degeneration of the cerebral neurons and glia cells were observed. Cosupplementation with NNG, vitamin E, and their combination with nicotine was beneficial in the alleviation of activity impairments and improved short memory and cognition defects and exploratory behaviors. Our results indicate the antioxidant potential of NNG and vitamin E by modulating redox markers and neurotransmitters in the brain. Thus, data suggest that the prophylactic use of NNG, vitamin E, and/or their combination for (45 days) may have a successful amelioration of the disrupted behavior and cognition and biochemical and histopathological alterations induced by nicotine.
1. Introduction
Tobacco smoking caused death to 100 million persons worldwide in the 20th century and is expected to kill about one billion people in the 21st century if the same manners remain [1]. The major alkaloid that existed in cigarettes is nicotine (N) about 1–2 mg/ml and was detected in the smoker's blood [2]. Nicotine activates the nicotinic acetylcholine receptors (nAChRs) present in the brain [3]. The nAChRs activation enhances the liberation of various neurotransmitters such as acetylcholine, glutamate, dopamine (DA), noradrenaline (NDA), and gamma amino butyric acid (GABA) in the brain [4, 5]. These neurotransmitters play an important role in modulating a great number of behaviors, including locomotion, anxiety, exploration, learning, and memory [3]. In addition, nicotine is implicated in the production of free radicals and reactive oxygen species (ROS) and generates oxidative stress [6, 7] which leads to mitochondrial dysfunction that causes neural death [8]. Therefore, antioxidants are highly needed by brain for the high oxygen demands and long-life duration of the neurons [9]. Recently, natural antioxidants have attracted more attention to face free radical damage in different tissues in rats induced by various oxidants [10–14]. Flavonoids are a group of natural products that have valuable biological activities, including antioxidant, anti-inflammation, and antitumor properties [15–17]. Among flavonoids, naringenin (NG) known as 4′,5,7-trihydroxy flavanone, a citrus flavanone, has rapid circulation bioavailability [18]. Naringenin has antioxidant [19], anti-inflammatory [20], and antidepressant effects [21]. In addition, vitamin E is an antioxidant that could compete for oxidative stress status, such as neurodegenerative diseases [22]. Hence, NG and vitamin E could successfully alleviate learning and cognition deficits induced by lipopolysaccharide and aesthesia, neurotoxicity, chronic stress in Alzheimer's disease model and diabetic rats [23–28]. Moreover, NG mitigated anxiety-like effects induced by iron and hypoxic stress in rats [29, 30].
The larger surface area of nanoparticles per mass unit and their smaller size can lead to faster drug delivery and higher bioactivity compared to bulky particles [31–33]. Thus, nanosize form naringenin (NNG) may have more ameliorative action against the serious effects of oxidants than NG. Moreover, little is known about the prophylactic use of nanosize form naringenin (NNG) and vitamin E combination to mitigate behavioral and memory disruption caused by oxidants such as nicotine.
Therefore, we aimed to evaluate the prophylactic role of NNG, vitamin E, and their combination in amelioration of impaired activity, anxiety, and learning induced by nicotine as well as biochemical and histological alterations posed by subcutaneous nicotine administration in rats.
2. Materials and Methods
2.1. Chemicals
Nicotine hydrogen tartrate salt (C10H14N2) (MW = 462.41 anhydrous 95% nicotine, with CAS number 65-31-6) and naringenin (C15H12O5) (4′,5,7-Trihydroxyflavanone MW = 272.26, purity $95% with CAS Number: 67604-48-2) were purchased from Glentham Life Sciences Ltd., England Unit 5 Ingoldmells Court Edinburgh Way, Corsham Wiltshire SN13 9XN, the United Kingdom. Vitamin E (dl-Alpha Tocopheryl Acetate) (C29H50O2) with item number 1770 was purchased from Puritan's Pride, INC Ronkonkoma, NY 11779, USA. The commercial diagnostic kits used for assaying of the reduced glutathione (GSH), malondialdehyde (MDA), catalase, hydrogen peroxide (H2O2), and acetylcholinesterase (AchE) were obtained from Biodiagnostic Company for Research Kits, Egypt. Enzyme-linked immunosorbent assay (ELISA) kits for rat dopamine (Catalog Number: MBS725908) and rat 5-hydroxytryptamine (5-HT) (Catalog Number: MBS725497) were provided by R&D System, USA. All other chemicals were of analytical grade.
2.2. Nanonaringenin Preparation
A high-energy ball milling technique was used to prepare nanonaringenin according to the method of Gusev and Kurlov [34]. NNG was prepared at Nanotechnology Lab, Faculty of Postgraduate Studies for Advanced Sciences, Beni-Suef University.
2.3. NNG Characterization
TEM electron microscope (Model: JEM-2100, JEOL Ltd., Tokyo, Japan) was used for the characterization of NNG at the National Research Center, Dokki, Giza, Egypt. A droplet of a freshly prepared suspension was poured onto copper grids and left to dry in the air, then observed by high-resolution TEM. The TEM indicated that NNG droplets were nearly spherical crystal shaped with homogeneous nanometric size spreading (Figure 1).
Figure 1.
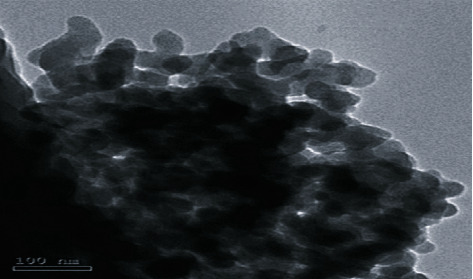
TEM images of NNG showing the shape and size of NNG, which appear as spherical particles with an average size of 100 nm.
2.4. Experimental Design
The present study was carried out on 60 adult male albino rats ranging between 120 and 150 g body weight. They were obtained from Helwan Farm of Laboratory Animals, Cairo, Egypt. Rats were acclimatized for 2 weeks, then they were housed in groups in metal cages under good ventilation and illumination conditions at room temperature (24°C ± 2°C), humidity (68%) under 12 hours light-dark cycle during the period of the experiment. Rats had free access to water and diet ad libitum. All experimental measures were performed in a strict guideline according to the recommendations for the care and use of laboratory animals and approved by the Institutional Animal Care and Use Committee at Beni-Suef University.
The rats were randomized into six equal groups (n = 10/group).
2.4.1. Control Group
The rats were injected subcutaneously with 0.2 ml/kg b.w. of 0.9% physiological saline only (vehicle).
2.4.2. Nicotine Group (N)
The rats were injected subcutaneously with freshly prepared nicotine dissolved in physiological saline (1 mg/kg b.w./day) for 30 days (15th–45th day) 5 days a week [35]. This dose delivered a plasma nicotine level in rats equal to the quantity absorbed from the smoking of 20 cigarettes by human persons [36]. The LD50 of nicotine is 50 mg/kg for rats [37].
2.4.3. Nanonaringenin Group (NNG)
The rats received NNG (25 mg/kg b.w./day) orally with a gastric tube for 45 days [38, 39].
2.4.4. Nicotine + Nanonaringenin Group (N + NNG)
The rats were given NNG (25 mg/kg b.w./day) for 45 days orally with a gastric tube, interrupted by nicotine injected subcutaneously (1 mg/kg b.w.) at the 15th–30th day. Nicotine was given an hour before NNG administration.
2.4.5. Nicotine + Vitamin E Group (N + E)
The rats were given vitamin E dissolved in corn oil orally with a gastric tube for 45 days (200 mg/kg b.w./day) [40], interrupted by nicotine injected subcutaneously (1 mg/kg b.w.) at the 15th–30th day. Nicotine was given an hour before vitamin E administration.
2.4.6. Nicotine + Nanonaringenin + Vitamin E Group (N + NNG + E)
The rats were given NNG (25 mg/kg b.w./day) and vitamin E (200 mg/kg b.w./day) orally with a gastric tube for 45 days interrupted by nicotine injected subcutaneously (1 mg/kg b.w.) at the 15th–30th day.
2.5. Behavioral Measurements
The following behavioral tests were performed to evaluate the effect of the nicotine, NNG and/or vitamin E with nicotine injection on rats' activity and memory. Five rats per group were used. The tests were performed two hours after the nicotine injection.
2.5.1. Open Field Test
This test was conducted on days 15 and 30 after injection of nicotine. This test measured locomotion, exploration, and anxiety, according to Gould et al. [41]. The apparatus was constructed according to Brown et al. [42]. The rat was placed into one of the four corners of the open field and permitted to discover the apparatus for 5 minutes. Behavior was analyzed according to Walsh and Cummins [43], Choleris et al. [44], and Kalueff and Tuohimaa [45].
For each rat, locomotion anxiety like behaviors (number of peripheral squares crossed with all four paws and rearing; frequency with which the rat stands against wall of the maze), freezing (immobility) time, exploration (number of center square entries with all four paws and time rats spent in them) were measured.
2.5.2. Learning and Memory Tests
(1) Y-Maze Test. This test was conducted on day 30 of the experiment. The test is used to measure spatial short-term working memory by recording the spontaneous alternative behavior in the maze arms [46]. According to the procedure described by Wall et al. [47], Rasoolijazi et al. [48], and Baluchnejadmojarad et al. [49], arms were marked as A, B, and C; then, each rat was placed at the beginning of (A) arm and left for eight minutes and then cleaning maze with 70% alcohol after each rat. Overlapping triplet sets (i.e., ABC CBA ABC) were used to calculate the sequence of arm entries. Spontaneous alternation behavior percentage (SAP) was measured in the Y-maze to evaluate working memory.
| (1) |
(2) Novel Object Recognition (NOR). The aim of this test is to evaluate the short-term memory and was performed at the end of the experiment. Novel object recognition test is used to evaluate learning and memory deficits in rats and mice depending on the natural behavior of a rat in interacting with novel objects more than familiar objects, which are known as recognition memory [50] according to the protocol designated by Bevins and Besheer [50], Leger et al. [51], and Lim et al. [52]. NOR was evaluated by calculation of discrimination index (DI).
| (2) |
2.6. Sampling and Tissue Preparations
Twenty-four hours following the last dose, the animals were killed by cervical dislocation. Brain tissues were collected and washed with physiological saline (NaCl 0.9%), then divided into three parts. The first portion was preserved in a neutral buffered formalin solution for histopathological examination. The second portion was homogenized in 10 volume phosphate buffer saline (pH: 7) using a homogenizer (Ortoalresa, Spain), then centrifuged at 20,000 ×g for 15 minutes at 4°C. The supernatant was kept at −80°C for further biochemical assays of GSH, MDA, Catalase, H2O2 levels, and AchE activities. The third portion of brain tissue was kept at −80°C for ELISA assays of dopamine and serotonin.
2.7. Biochemical Assays
The brain homogenate was used for the measurements of MDA, GSH, H2O2 levels, and catalase activity according to the methods described by Satoh [53], Beutler and Kelly [54], and Aebi [55], respectively. AchE activity was estimated in brain tissue homogenate according to the method of Kovarik et al. [56]. Levels of dopamine and serotonin in rat brain tissues were estimated by competitive enzyme- immunoassay technique (ELISA) [57] using rat ELISA kits for dopamine and rat 5-hydroxytryptamine (5-HT) or serotonin following the manufacturer's instructions.
2.8. Histopathological Examination
Brain tissues were dissected carefully and washed with physiological saline (NaCl 0.9%), then immersed in neutral buffered formalin solution 10%. Tissue specimens of the brain were dehydrated in ethyl alcohol, cleared in xylol, impregnated in soft paraffin, and embedded in hard paraffin. Sections of 4–6 μm were cut and mounted on clear and dry glass slides. The obtained slides were stained with Hematoxylin and Eosin (H & E) and Bielschowsky's silver stain for histopathological examination using LEICA (DFC290 HD system digital camera, Heerbrugg, Switzerland) connected to the light microscope using 10, 20, 40 objective lenses [58].
2.9. Statistical Analysis
The results were statistically analyzed by using Graph Pad In stat software (version 3, ISS-Rome, Italy). One-way analysis of variance (ANOVA) test followed by Tukey's post hoc test for experimental group comparison was used. The p values below 0.05 were accepted for significance.
3. Results
3.1. Behavioral Measurements
Table 1 shows that nicotine induced a marked decrease and increase in locomotor behaviors of the treated rats. On day 15 after injection of nicotine, the crossed peripheral squares number was decreased significantly (p < 0.05) in N and N + NNG groups compared to the control one. At day 30 of nicotine injection, a significant (p < 0.05) decrease was observed in N and N + E in comparison to the control group. On the contrary, on day 15, rearing showed a significant (p < 0.05) increase in the N group while a significant (p < 0.05) decrease was observed in N + NNG, N + E and N + NNG + E compared to the control and nicotine groups. At day 30 of nicotine injection, rearing was significantly (p < 0.05) reduced in N, N + NNG, and N + E compared to control. However, rats in N + NNG + E group performed a locomotor activity near to that of control group. In additions, nicotine significantly (p < 0.05) decreased the freezing time and immobility at day 15 of nicotine injection. However, NNG, N + NNG, N + E and N + NNG + E significantly (p < 0.05) prolonged the freezing time of rats at day 15 of nicotine injection. Moreover, N + NNG significantly (p < 0.05) increased the frequency and duration of rat's entrance into central squares at days 15 and 30 while N + NNG + E increased them significantly (p < 0.05) at day 15 only. Thus, the administration of nicotine-treated rats with NNG either alone in N + NNG group or in combination with vitamin E in N + NNG + E group could improve rats, exploratory behaviors.
Table 1.
Effect of treatments on locomotor and exploratory behavior of rats (in the open field test).
| Locomotor and anxiety like behaviors | Exploratory behaviors | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of peripheral square crosses | Rearing (frequency) | Freezing duration (sec) | No. of central square crosses | Central square time (sec) | ||||||
| 15 days | 30 days | 15 days | 30 days | 15 days | 30 days | 15 days | 30 days | 15 days | 30 days | |
| Control | 67.4 ± 2.79 ac | 38.2 ± 9.65a | 12.0 ± 1.58a | 8.4 ± 2.07a | 61.8 ± 6.49a | 119 ± 44.22a | 2.4 ± 1.34a | 1.8 ± 0.45a | 3.0 ± 2.1a | 1.6 ± 0.89a |
| N | 27.2 ± 5.63b | 16.8 ± 3.96b | 16.8 ± 3.3b | 2.4 ± 1.14b | 28.6 ± 3.21b | 103.2 ± 16.16a | 1.4 ± 0.55a | 1.4 ± 0.55a | 2.4 ± 1.1a | 2.4 ± 0.86a |
| NNG | 74.4 ± 4.7a | 36.4 ± 2.7a | 9.8 ± 0.84 ad | 8.52 ± 1.41a | 139.6 ± 8.26c | 114.6 ± 12.68a | 2.8 ± 1.92a | 1.2 ± 0.84a | 4.0 ± 1.2 ab | 1.0 ± 1.0a |
| N + NNG | 39.2 ± 9.5 bd | 30.8 ± 4.76a | 6.6 ± 0.89 cd | 3.2 ± 2.17b | 111 ± 23.01c | 122 ± 28.64a | 6.6 ± 2.88b | 5.2 ± 1.8b | 7.6 ± 1.52b | 7.2 ± 1.9b |
| N + E | 46.4 ± 19.8 cb | 16.0 ± 4.69b | 5.4 ± 1.14c | 2.0 ± 1.58b | 119 ± 18.84c | 138 ± 28.64a | 2.4 ± 2.70a | 1.4 ± 0.55a | 3.0 ± 1.58a | 1.6 ± 0.89a |
| N + NNG + E | 50.4 ± 15.6 cd | 37.2 ± 7.56a | 6.8 ± 1.3 cd | 10.0 ± 1.23a | 236 ± 23.02d | 130 ± 30.82a | 4.8 ± 0.45 ab | 1.6 ± 1.52a | 7.4 ± 3.36b | 1.8 ± 0.84a |
Table 2 illustrates that the working memory (SAP) was significantly (p < 0.05) decreased in N, N + NNG, and N + E groups, while it was moderately improved in the combination (N + NNG + E) group. DI was significantly (p < 0.05) decreased in the N group. The prophylactic administration of vitamin E and/or combination treatment with NNG and vitamin E showed an ameliorative effect on cognition by the restoration of the values to near normalcy.
Table 2.
Effect of treatments on learning and cognition of rats.
| Spontaneous alternation behavior percent (SAP) | Novel object recognition discrimination index (DI) | |
|---|---|---|
| Control | 33.8 ± 2.17a | 0.406 ± 0.10ab |
| N | 28.2 ± 3.03bc | 0.288 ± 0.08b |
| NNG | 32.0 ± 3.39ab | 0.464 ± 0.07a |
| N + NNG | 28.2 ± 1.79bc | 0.396 ± 0.07ab |
| N + E | 26.6 ± 3.21c | 0.536 ± 0.07a |
| N + NNG + E | 31.2 ± 2.39abc | 0.456 ± 0.06a |
3.2. Biochemical Changes
Figure 2 shows the activity of the AchE enzyme and the concentrations of dopamine and serotonin in brain tissue homogenates. AchE activity and serotonin content significantly increased (p < 0.05) in brain tissue of nicotine-treated rats compared with those in the control group. However, the concentration of dopamine significantly reduced (p < 0.05) in the nicotine group in comparison to the control group. Figure 2 also shows that the GSH concentration and catalase activity significantly decreased while the concentrations of MDA and H2O2 significantly increased (p < 0.05) in brain tissue homogenates of nicotine-treated rats compared with those in the control group. On the other hand, the prophylactic treatment with NNG and/or vitamin E parallel with nicotine administration showed an ameliorative effect by the restoration of these biomarkers' values to near normalcy. The supplementation of the nicotine-treated rats with either NNG or vitamin E induced the same degree of protection. In addition, NNG supplementation to normal rats did not alter these values in comparison to those in the control group, indicating no adverse effect of NNG on the brain.
Figure 2.
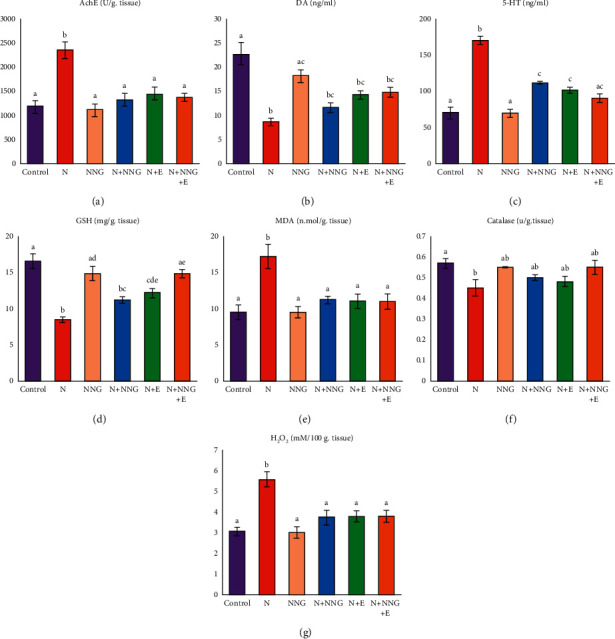
Effect of different treatments on (a) acetylcholinesterase activity, (b) dopamine (DA), (c) serotonin (5-HT), (d) GSH, (e) MDA, (f) catalase, and (g) H2O2 concentrations in the brain tissue of rats in different groups. Results are expressed as means ± SE (n = 10). Values with different letters in a column are significantly different at level p < 0.05. N: nicotine, NNG: nanonaringenin, E: vitamin E, DA: dopamine, 5-HT: serotonin, GSH: reduced glutathione, MDA: malondialdehyde, H2O2: hydrogen peroxide.
3.3. Histological Changes of the Brain Tissue
The influence of different treatments on the nicotine-induced histopathological alterations in brain tissue is presented in Figures 3–5. Nicotine administration caused a thickening and congestion of the meninges and degeneration of the cerebral neurons and glia cells. The nerve fibers appeared short and less branched. NNG and/or vitamin E exhibited an improvement in brain structure.
Figure 3.
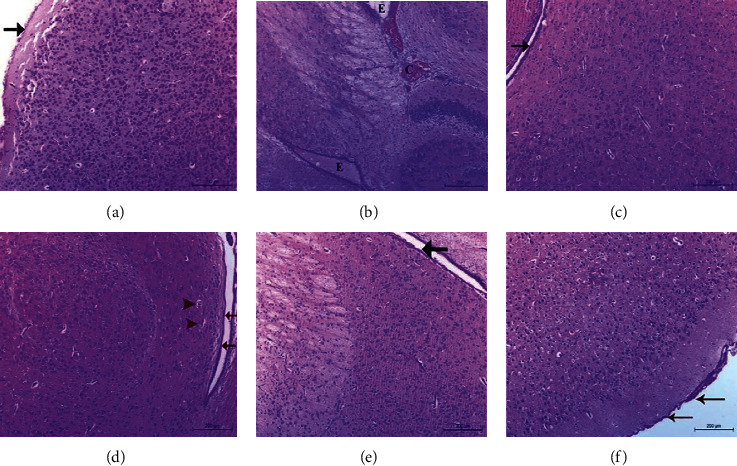
A photomicrograph of brain tissue in adult male albino rats showing the groups. (a) Control group, the brain tissue was covered by well-demarcated normal fine fibrous meninges (arrow). The brain tissue appeared normal in constituent with normal nerve cells and glial tissue. The meningeal and cerebral blood vessels appeared normal. (b) N group, the brain tissue covered with meninges suffered from thickening, congestion of meningeal blood vessels (C), and submeningeal edema (E). Both nerve cells and glial tissue showed degenerative changes. (c) NNG group, the brain tissue, and the meningeal layers (arrow) appeared normal. (d) N + E group, the brain tissue, and meninges (arrow) appeared normal. The cortical blood vessels suffered from congestion (arrow head). (e) N + NNG group, the brain tissue covered with normal meninges (arrow). The nerve cells and glial tissue appeared normal. (f) N + NNG + E group, the meningeal layers (arrow), and the brain tissue appeared normal. H&E stain X 100.
Figure 4.
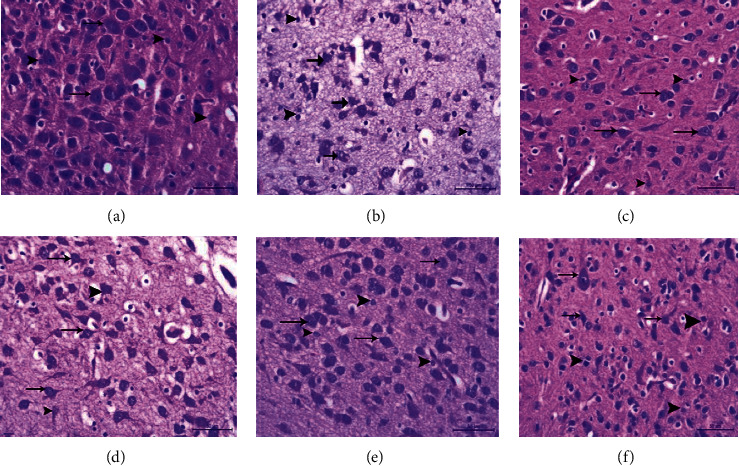
A higher magnification of brain tissue in adult male albino rats showing the groups. (a) Control group, showing normal nerve cells with a large spherical vesicular nucleus and basophilic cytoplasm (arrow). The neuroglia cells (arrow head) appeared normal and adjacent to the neurons. (b) N group, the majority of nerve cells appeared degenerated (arrow) the neuroglia cells (arrow head) appeared suffering from degeneration and necrosis. (c) NNG group, both nerve cells (arrow) and neuroglia (arrow head) appeared normal. (d) N + E group, nerve cells appeared normal (arrow) while other neurons suffered from degeneration (arrow head). (e) N + NNG group, the majority of neurons seemed to be normal (arrow) and the neuroglia cells appeared normal around neurons. (f) N + NNG + E group, the neurons (arrow) and the neuroglia cells appeared normal. H&E stain X 400.
Figure 5.
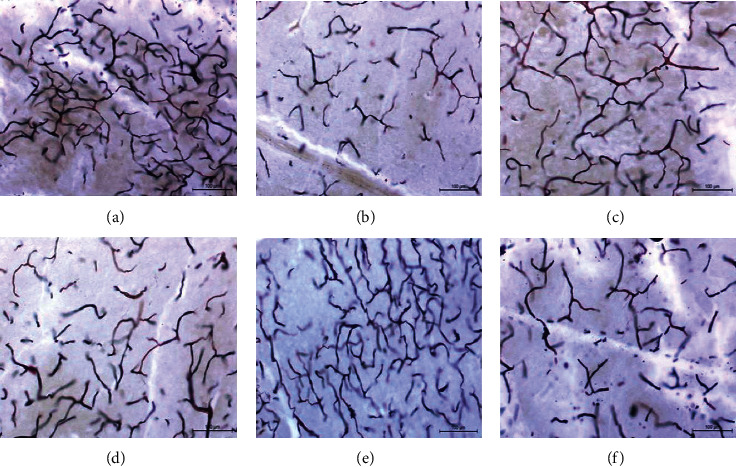
A histological picture of brain tissue in adult male albino rats showing the groups. (a) Control group, showing long and highly branched nerve fibers. (b) N group, the nerve fibers appeared short and less branched. (c) NNG group, the nerve fibers appeared long and highly branched. (d) N + E group, showing few nerve fibers appeared long and branched while the other nerve fibers appeared short and less branched. (e) N + NNG group, showing the majority of nerve fibers appeared long and highly branched. (f) N + NNG + E group, showing the nerve fibers appeared long and highly branched. Silver stain X 200.
4. Discussion
Nicotine stimulates the release of different neurotransmitters such as acetylcholine, dopamine, norepinephrine, and serotonin (5-HT) by nAChRs activation [3]. Nicotine effect on rat's behavior media by stimulation and desensitization of nAChRs is varied following acute and chronic nicotine administration [59]. The obtained data in our study indicated that nicotine might induce both rat's hypoactivity and hyperactivity. Nicotine was reported to reduce or increase activity (locomotion and rearing) in rats [60]. Repeated intermittent injections of nicotine may cause an increase in locomotor activity [61]. However, activity sensitization was not observed after continuous nicotine exposure [62]. According to Domino [63], the first dose of nicotine (0.32 mg/kg) could induce marked hypoactivity. Hence, the effect of nicotine on locomotion depends on sex [64], dose, and duration of exposure.
Monoamines are an important group of neuromodulators that are released onto spinal cord circuits and are critical for the expression of locomotion [65]. Dopamine plays a central role in the stimulation and modulation of the motor system in vertebrates and invertebrates [65, 66]. Animals with low DA levels exhibited hypoactivity and had impaired learning capability [67]. Thus, the recorded alteration in rats' activity is owing to the reported brain monoamines changes in our study.
The obtained results showed that the NNG and vitamin E did not ameliorate the prominent reduction in rat's activity caused by nicotine at 15 days of the experiment, while their combination successfully relieved the nicotine effect on locomotion at day 30. This indicated that the ameliorative effect of vitamin E [68, 69] and NNG became more potent at 30 days. Therefore, vitamin E and NNG combination is suggested in long-term nicotine administration.
Interestingly, NNG, vitamin E, and their combination prolonged immobility time at 15 days of the experiment. Tobacco was recorded to decrease vitamins C and E [68, 69]. In addition, adverse effects of some antioxidant supplementation on animal and human exercise performance were reported [70–72]. Hence, we hypothesize that the reported hypoactivity in N + NNG and N + E groups may be due to the high doses of their supplementation that were not decreased by the nicotine effect during the first 15 days of its administration.
The time rats spent freezing in the open field maze is an indicator of animal anxiety [44, 45]. In addition, the locomotion and rearing frequency of rats in a novel area are used to investigate their anxiety [44]. Less anxious rats in the novel area spent short freezing time and exhibited frequent motility such as rearing.
The observed behaviors declared that nicotine was anxiolytic on day 15 (decreased freezing time) and anxiogenic (decreased rearing) on day 30 of the experiment. These results are supported by early reports [3, 5, 73, 74] provided that nicotine had different effects on anxiety in both humans and animals depending on the species, strain, doses, route of administration, and experimental model used [5, 74, 75]. Acute injection of nicotine (0.01 mg/kg) was anxiogenic 5 min after injection and anxiolytic after 30 min [76]. Acute nicotine (0.35 mg/kg) injection had anxiogenic action [77] while chronic (15 days, 0.35 mg/kg) nicotine administration induced anxiolytic effect on the rat [77, 78]. In our experiment, the anxiogenic effect of nicotine on day 30 may be owing to the recorded decrease in brain DA [79].
Our data revealed that NNG could ameliorate the anxiogenic effect of nicotine. Similarly, NNG alleviated anxiety impairment induced by deltamethrin in rats [80]. This may be attributed to the capability of NNG to decrease AchE activities in addition to its neuroprotective and antistress effect [81].
The obtained results showed that the administration of NNG alone or in combination with vitamin E could successfully mitigate the nicotine effect on activity. Meanwhile, NNG improved the exploratory behavior of rats. Likewise, NNG treated rats exhibited a long exploration time [80]. Exploration is an indication of anxiety. Hence, NNG and vitamin E successfully decreased anxiety in treated rats. This may be due to the efficacy of NNG to decrease the anxiety that causes an increase in exploratory behavior and improves memory [82].
Our findings indicated that the tested dose of nicotine impaired the working memory and cognition of rats. In the early studies, nicotine caused impairments in learning and memory [83]. In additions, heavy smoking led to reduced cognitive function evaluated in mid-life [84]. On the other hand, nicotine enhanced cognitive function in experimental animals [85] or had no effect [86] on memory. Moreover, nicotine was found to improve learning at low doses, while higher doses impaired spatial memory in experimental animals [87]. Hence, the effect of nicotine on learning and memory seemed to depend critically upon some factors such as the test conducted, the administered dose of the nicotine, and the route of administration. In fact, the hippocampus had a significant role in learning and memory, especially spatial memory [88]. Also, intermittent injection of nicotine enhances nicotinic receptors and stimulates the mesolimbic and nigrostriatal dopaminergic pathways and the noradrenergic projections to the hippocampus, whereas continuous nicotine administration inhibits these responses [89]. Thus, we hypothesize that the deficits in spatial memory detected in the current study may be due to neuronal nicotinic receptors desensitization or the reported degenerative changes in cortical nerve cells and glial tissue. Furthermore, acetylcholine is one of the brain neurotransmitters included in cognitive and attention processes [90]. Thus, reported memory impairments may be attributed to the detected decrease in acetylcholine levels induced by the recorded increased brain AchE activity in our study.
Our data suggested that vitamin E had an ameliorative effect on the impaired cognition induced by nicotine. Meanwhile, a combination of vitamin E and NNG improved the cognition capability of rats. NNG could improve learning in experimental animals [25, 91]. Vitamin E and C administration in a small dose reduced memory deficit in APP/PSEN1 mice and improved performance of wild-type mice in the water maze while a higher dose of vitamin E with C had little decreasing oxidative stress effect than vitamin C only or the little dose of vitamins E and C, however, a combination of a great dose also weakened water maze performance in mice of both genotypes [92]. NNG has successfully ameliorated learning deficits mediated by inhibition of brain AchE activity [26] because it has an antiapoptotic effect [93]. Vitamin E alone was reported to attenuate the effect of hypoxia on the learning of rats [94, 95] and improved learning memory deficit [96]. On the contrary, no improvement or impairments in cognition were found following the administration of vitamin E [97–99]. Vitamin E ameliorated learning deficits induced by nicotine might be due to its antioxidant effect in the central nervous system [100].
The reported changes in brain monoamines (decrease DA and increase 5-HT) levels are consistent with Perez et al. [101], who found that prolonged nicotine treatment reduces the liberation of DA. Shearman et al. [102] reported that nicotine might induce a decrease or increase in DA and 5-HT levels. 5-HT was increased in response to high nicotine (0.5 mg/kg) doses while it decreased at low concentration (0.03 mg/kg). In addition, the reported increase in brain AchE is consistent with Saad et al. [103], who found that nicotine (1 mg/kg) increased brain AchE levels in the rat.
We suggest six explanations for decreased brain DA in the present study. The first is that prolonged exposure to nicotine (LTN) leads to increase nAChR desensitization that may alter the response of DA terminals to distinct firing patterns [101]. The second is the decreased endogenous acetylcholine levels induced by LTN treatment, which might decrease electrically evoked DA release [101, 104]. The third is LTN injection may induced alterations in the mesocorticolimbic pathway that may modify the dopaminergic terminals (in nucleus accumbens) response to an electrical stimulus. Indeed, nicotine treatment regulates mesolimbic α4β2 nAChRs on GABAergic neurons (in the ventral tegmental area; VTA), which in turn leads to an increase in their firing and decreases dopaminergic activity [105]. The altered GABAergic and glutamatergic activity in VTA may reduce dopaminergic activity in the nucleus [106]. Fourth, LTN exposure may increase the action of dopamine D2 receptor inhibition of dopaminergic function directly or/by reducing the activity of cholinergic interneurons [107]. Fifth, nicotine increases levels of circulating corticosteroids in both animals and humans due to stimulation of the hypothalamic-pituitary adrenal axis [108]. Consequently, chronic stress attenuates DA release [109]. Sixth, nicotine encourages the synthesis and release of catecholamines (noradrenaline/adrenaline) from the adrenal medulla. Nicotine controls the expression of tyrosine hydroxylase from the adrenal medulla (which is the rate-limiting enzyme in catecholamine biosynthesis), dopamine hydroxylase (which changes dopamine to noradrenaline), and neuropeptide Y (which is coliberated with the catecholamines) [110]. In addition, exposure to nicotine prenatally raises blood noradrenaline levels at baseline [111].
The prophylactic treatment with NNG and/or vitamin E or a combination of both for 45 days showed an ameliorative effect as the values returned nearly to control. The supplementation of nicotine-treated rats with either NNG or vitamin E induced the same degree of protection. Similarly, Rahigude et al. [112] and Khajevand-Khazaei et al. [26] stated that naringenin inhibits brain AchE in the rat. Liaquat et al. [113] reported that administration of naringenin improved cholinergic neurotransmission, which was detected by inhibited AchE activity, and increased acetylcholine levels in the whole brain of flavonoid fed rats. This decreased AchE activity led to increasing acetylcholine levels in synapses which resulted in improving cholinergic neurotransmission and eventually improved cognitive functions [113]. Therefore, it is believed that drugs that can increase acetylcholine levels and promote cholinergic functions may have the capacity for the therapeutic management of cognitive dysfunction such as dementia and Alzheimer's disease. In addition, naringenin prompted neuroprotective activity against neurotoxicity caused by 6-hydroxydopamine [114] via activation of Nrf2 signaling.
Nicotine promotes oxidative stress, implicates in several brain dysfunction, and prompts neurodegeneration. Experimental animal exposure to nicotine provokes oxidative stress and histopathological alterations due to diminishing the structural integrity and function of the brain [115, 116]. Peroxidizable fatty acids, which are present abundantly in neuronal membranes, are highly vulnerable to lipid peroxidation [117], which finally leads to the liberation of oxidative markers such as MDA and 4-hydroxynonenal [9]. ROS overproduction successfully caused lipid peroxidation in brain tissues of nicotine-treated rats, which was detected in the current study by a significant increase in MDA and H2O2 concentrations that, in turn, utilize GSH and catalase enzyme resulting in a significant decrease in GSH content and catalase activity in the brain tissue of nicotine-treated group compared to the control group (Figure 2). This data suggests that nicotine may exert its toxic action via redox imbalance. Similarly, Budzynska et al. [118] found that nicotine promoted lipid peroxidation in the brain tissues, which may have a major role in the development of brain dysfunction. The observed nicotine-induced significant depletion of brain GSH levels in the current study may indicate the decreasing of GSH synthesis and the more consumption of GSH for counteracting the nicotine-induced oxidative stress. Catalase catalyzes the breakdown of H2O2 into water and oxygen. The reduced tissue catalase activity detected in the current investigation may be owing to the increased ROS generation and the accumulation of superoxide radicals and H2O2 and, consequently, the increased utilization of these antioxidants to counter lipid peroxidation [118, 119]. In matching with our data, previous studies had reported similar results of nicotine toxicity in different rat tissues [120, 121]. Besides, Helen et al. [122] observed an increase in hydroperoxide levels in the lung, liver, and kidney of nicotine-administered rats. Cognitive impairment occurs due to reduced activity of the antioxidant defense system [113, 123].
Pre- and cotreatments of nicotine-treated rats with either NNG and/or vitamin E significantly raised the levels of antioxidants like GSH and catalase enzyme but significantly reduced MDA and H2O2 concentrations. This action was augmented by the histopathological picture of the brain tissue, especially neurons and neuroglia cells (Figures 3–5). Interestingly, the degree of improvement in MDA and GSH levels was markedly higher in the combination group reflecting the synergistic antioxidant effects of both treatments by augmenting antioxidant defense mechanisms than the single use. In this group, neurons appeared normal without any degree of impairment and the nerve fibers became highly branched.
Vitamin E, the major lipophilic antioxidant, plays an important role in the protection against oxidative stress and protects cell membranes from oxidative damage [124]. It is demonstrated that vitamin E can also protect against lipid peroxidation caused by nicotine in animals [125, 126] and humans [127, 128], therefore preventing lipid peroxidation and raising the antioxidant status in nicotine-treated rats [129, 130]. Vitamin E prevented nicotine-induced lipid peroxidation and GSH depletion in brain tissue, as it might have easily diffused to rat brain as a lipid-soluble antioxidant [131].
Several studies reported that flavonoids can cross Blood-brain barrier (BBB), where they act as potent antioxidants and protect nerve cells against oxidative damage [132, 133]. Naringenin is a well-known flavonoid abundant in citrus fruits [134]. The lipophilic nature of naringenin favors a good BBB-permeability [135, 136]. Naringenin uptake into the cerebral cortex and the striatum [137, 138] proposes that naringenin should allow protection of the neurons throughout the CNS. A previous study had postulated that naringenin could enhance learning and memory retention by improving antioxidant enzyme activities in rats [113]. Other reports documented that oral supplementation of naringenin significantly enhanced antioxidant enzyme activities [139, 140]. In our study, TEM analysis revealed that the prepared NNG possesses a size up to 100 nm with a spherical shape indicative of better uptake of nanoparticles by cells. Naringenin antioxidant properties have been earlier described [112]. Naringenin antioxidant properties are related to its potential scavenging effect on both O2−, OH−, and lipid peroxide owing to the presence of hydroxyl groups in its structure, which possesses electron-donating properties, thereby protecting membranes from the free radical attack [141].
As an anticholinesterase, NNG enhances memory inefficiency in diabetes [112] and cognitive deficit induced by intracerebroventricular-streptozotocin [139]. Other studies recommended that pretreatment with naringenin promotes locomotion and raised glutathione with reduced MDA content in the brain tissue of Parkinson's disease (PD) rat model [142]. All these findings indicated that NNG can be considered as a potential neuroprotective agent and improve learning and memory via its antioxidant activity.
In the current study, the histopathological examinations support the biochemical analysis where the nicotine caused marked pathological changes in the cerebral cortex indicated by structural alterations, congestion of meningeal and cerebral blood vessels, prevascular edema, and degeneration of the cortical nerve cells and glial tissue. Cotreatment with oral NNG and/or vitamin E attenuates these changes in cerebral structure. Structural observations have confirmed the cerebroprotection nature of NNG and/or vitamin E on nicotine-induced oxidative lesions.
5. Conclusion
Our findings suggested that nicotine may lead to oxidative stress and impairment in behavioral, biochemical, and histological variables. However, supplementation of nanonaringenin (NNG), vitamin E, and their combination for 45 days might exert successful amelioration of theses impairments as well as brain AchE, monoamines and redox markers alterations. Thus, NNG and vitamin E should be included in the diet to afford a protective effect against nicotine-induced cytotoxicity. Further studies are required to evaluate the efficacy of different doses of NNG and vitamin E to mitigate impairments induced by nicotine at different routes of administration and durations.
Acknowledgments
The authors thank all staff members of the Biochemistry, Histology and Animal and Poultry Management Departments, Beni-Suef University, Egypt, for their help and advice.
Data Availability
All data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
All experimental measures were performed according to the recommendations for the care and use of laboratory animals and in accordance with the local Animal Care and Use Committee at Beni-Suef University.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Mohammed A. Kandeil, Eman T. Mohammed, and Ghada M. Safwat designed the research and shared the methodology of the experiment, the analysis of data, and writing processes. Rania A. Radi performed the experiment and analysis of data. Fatma Khalil performed the behavioral study and analysis of data. Abdel-Razik H. performed the pathological study and analysis of data. All authors shared in the writing processes and proofreading of the manuscript.
References
- 1.Eriksen M., Mackay J., Schluger N. The Tobacco Atlas . 5th. Atlanta, GA, USA: American Cancer Society; 2015. [Google Scholar]
- 2.Hammond D., Collishaw N. E., Callard C. Secret science: tobacco industry research on smoking behaviour and cigarette toxicity. The Lancet . 2006;367(9512):781–787. doi: 10.1016/s0140-6736(06)68077-x. [DOI] [PubMed] [Google Scholar]
- 3.Zarrindast M.-R., Khakpai F. The modulatory role of nicotine on cognitive and non-cognitive functions. Brain Research . 2019;1710:92–101. doi: 10.1016/j.brainres.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Berrendero F., Robledo P., Trigo J. M., Martín-García E., Maldonado R. Neurobiological mechanisms involved in nicotine dependence and reward: participation of the endogenous opioid system. Neuroscience & Biobehavioral Reviews . 2010;35(2):220–231. doi: 10.1016/j.neubiorev.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piri M., Nasehi M., Shahab Z., Zarrindast M. R. The effects of nicotine on nitric oxide induced anxiogenic-like behaviors in the dorsal hippocampus. Neuroscience Letters . 2012;528(2):93–98. doi: 10.1016/j.neulet.2012.08.074. [DOI] [PubMed] [Google Scholar]
- 6.Mosbah R., Yousef M. I., Mantovani A. Nicotine-induced reproductive toxicity, oxidative damage, histological changes and haematotoxicity in male rats: the protective effects of green tea extract. Experimental & Toxicologic Pathology . 2015;67(3):253–259. doi: 10.1016/j.etp.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Ben Saad A., Rjeibi I., Alimi H., Ncib S., Bouhamda T., Zouari N. Protective, effects of mentha spicata against nicotine induced toxicity in liver and erythrocytes. Applied Physiology Nutrition and Metabolism . 2017;11:1–7. doi: 10.1139/apnm-2017-0144. [DOI] [PubMed] [Google Scholar]
- 8.Yan M. H., Wang X., Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radical Biology and Medicine . 2013;62:90–101. doi: 10.1016/j.freeradbiomed.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandhi S., Abramov A. Y. Mechanism of oxidative stress in neurodegeneration. Oxidative Medicine and Cellular Longevity . 2012;2012:11. doi: 10.1155/2012/428010.428010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kandeil M. A., Mohammed E. T., Hashem K. S., Aleya L., Abdel-Daim M. M. Moringa seed extract alleviates titanium oxide nanoparticles (TiO2-NPs)-induced cerebral oxidative damage, and increases cerebral mitochondrial viability. Environmental Science & Pollution Research . 2019;27(16):19169–19184. doi: 10.1007/s11356-019-05514-2. [DOI] [PubMed] [Google Scholar]
- 11.Mohammed E. T., Safwat G. M. Grape seed Proanthocyanidin extract mitigates titanium dioxide nanoparticle (TiO2-NPs)–induced hepatotoxicity through TLR-4/NF-κB signaling pathway. Biological Trace Element Research . 2020;196(2):579–589. doi: 10.1007/s12011-019-01955-5. [DOI] [PubMed] [Google Scholar]
- 12.Mohammed E. T., Hashem K. S., Ahmed A. E., Aly M. T., Aleya L., Abdel-Daim M. M. Ginger extract ameliorates bisphenol a (BPA)-induced disruption in thyroid hormones synthesis and metabolism: involvement of Nrf-2/HO-1 pathway. The Science of the Total Environment . 2020;703 doi: 10.1016/j.scitotenv.2019.134664. [DOI] [PubMed] [Google Scholar]
- 13.Mohammed E. T., Radi A. M., Aleya L., Abdel-Daim M. M. Cynara scolymus leaves extract alleviates nandrolone decanoate-induced alterations in testicular function and sperm quality in albino rats. Environmental Science and Pollution Research . 2020;27(5):5009–5017. doi: 10.1007/s11356-019-07302-4. [DOI] [PubMed] [Google Scholar]
- 14.Radi A. M., Mohammed E. T., Abushouk A. I., Aleya L., Abdel-Daim M. M. The effects of abamectin on oxidative stress and gene expression in rat liver and brain tissues: modulation by sesame oil and ascorbic acid. The Science of the Total Environment . 2020;701 doi: 10.1016/j.scitotenv.2019.134882.134882 [DOI] [PubMed] [Google Scholar]
- 15.Chen Y.-C., Yang L.-L., Lee T. J.-F., Oroxylin A. Oroxylin A inhibition of lipopolysaccharide-induced iNOS and COX-2 gene expression via suppression of nuclear factor-κB activation. Biochemical Pharmacology . 2000;59(11):1445–1457. doi: 10.1016/s0006-2952(00)00255-0. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y.-C., Shen S.-C., Lee W.-R., Hou W.-C., Yang L.-L., Lee T. J. F. Inhibition of nitric oxide synthase inhibitors and lipopolysaccharide induced inducible NOS and cyclooxygenase-2 gene expressions by rutin, quercetin, and quercetin pentaacetate in RAW 264.7 macrophages. Journal of Cellular Biochemistry . 2001;82(4):537–548. doi: 10.1002/jcb.1184. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y.-C., Shen S.-C., Lee W.-R., et al. Wogonin and fisetin induction of apoptosis through activation of caspase 3 cascade and alternative expression of p21 protein in hepatocellular carcinoma cells SK-HEP-1. Archives of Toxicology . 2002;76(5-6):351–359. doi: 10.1007/s00204-002-0346-6. [DOI] [PubMed] [Google Scholar]
- 18.Arafah A., Rehman M. U., Mir T. M., et al. Multi-therapeutic potential of naringenin (4′,5,7-trihydroxyflavonone): experimental evidence and mechanisms. Plants . 2020;9(12):p. 1784. doi: 10.3390/plants9121784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Da Pozzo E., Costa B., Cavallini C., et al. The citrus flavanone naringenin protects myocardial cells against age-associated damage. Oxidative Medicine and Cellular Longevity . 2017;2017:12. doi: 10.1155/2017/9536148.9536148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyane N. A., Tlaila T. B., Malefane T. G., Ndwandwe D. E., Owira P. M. O. Metformin-like antidiabetic, cardio-protective and non-glycemic effects of naringenin: molecular and pharmacological insights. European Journal of Pharmacology . 2017;803:103–111. doi: 10.1016/j.ejphar.2017.03.042. [DOI] [PubMed] [Google Scholar]
- 21.Budzynska B., Boguszewska-Czubara A., Kruk-Slomka M., et al. Effects of imperatorin on nicotine-induced anxiety- and memory-related responses and oxidative stress in mice. Physiology & Behavior . 2013;122(122):46–55. doi: 10.1016/j.physbeh.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Ricciarelli R., Argellati F., Pronzato M. A., Domenicotti C. Vitamin E and neurodegenerative diseases. Molecular Aspects of Medicine . 2007;28(5-6):591–606. doi: 10.1016/j.mam.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Trofimiuk E., Braszko J. J. Long-term administration of cod liver oil ameliorates cognitive impairment induced by chronic stress in rats. Lipids . 2011;46(5):417–423. doi: 10.1007/s11745-011-3551-3. [DOI] [PubMed] [Google Scholar]
- 24.Sabogal-Guáqueta A. M., Muñoz-Manco J. I., Ramírez-Pineda J. R., Lamprea-Rodriguez M., Osorio E., Cardona-Gómez G. P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology . 2015;93:134–145. doi: 10.1016/j.neuropharm.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hua F.-Z., Ying J., Zhang J., et al. Naringenin pre-treatment inhibits neuroapoptosis and ameliorates cognitive impairment in rats exposed to isoflurane anesthesia by regulating the PI3/Akt/PTEN signalling pathway and suppressing NF-κB-mediated inflammation. International Journal of Molecular Medicine . 2016;38(4):1271–1280. doi: 10.3892/ijmm.2016.2715. [DOI] [PubMed] [Google Scholar]
- 26.Khajevand-Khazaei M.-R., Ziaee P., Motevalizadeh S.-A., et al. Naringenin ameliorates learning and memory impairment following systemic lipopolysaccharide challenge in the rat. European Journal of Pharmacology . 2018;826(826):114–122. doi: 10.1016/j.ejphar.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 27.de Souza C. P., Gambeta E., Stern C. A. J., Zanoveli J. M. Posttraumatic stress disorder-type behaviors in streptozotocin-induced diabetic rats can be prevented by prolonged treatment with vitamin E. Behavioural Brain Research . 2019;359(359):749–754. doi: 10.1016/j.bbr.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Krishna Chandran A. M., Christina H., Das S., Mumbrekar K. D., Satish Rao B. S. Neuroprotective role of naringenin against methylmercury induced cognitive impairment and mitochondrial damage in a mouse model. Environmental Toxicology and Pharmacology . 2019;71 doi: 10.1016/j.etap.2019.103224.103224 [DOI] [PubMed] [Google Scholar]
- 29.Chtourou Y., Slima A. B., Gdoura R., Fetoui H. Naringenin mitigates iron-induced anxiety-like behavioral impairment, mitochondrial dysfunctions, ectonucleotidases and acetylcholinesterase alteration activities in rat Hippocampus. Neurochemical Research . 2015;40(8):1563–1575. doi: 10.1007/s11064-015-1627-9. [DOI] [PubMed] [Google Scholar]
- 30.Olugbemide A. S., Ben-Azu B., Bakre A. G., Ajayi A. M., Femi-Akinlosotu O., Umukoro S. Naringenin improves depressive- and anxiety-like behaviors in mice exposed to repeated hypoxic stress through modulation of oxido-inflammatory mediators and NF-kB/BDNF expressions. Brain Research Bulletin . 2021;169:214–227. doi: 10.1016/j.brainresbull.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Nair H. B., Sung B., Yadav V. R., Kannappan R., Chaturvedi M. M., Aggarwal B. B. Delivery of antiinflammatory nutraceuticals by nanoparticles for the prevention and treatment of cancer. Biochemical Pharmacology . 2010;80(12):1833–1843. doi: 10.1016/j.bcp.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhadoriya S. S., Mangal A., Madoriya N., Dixit P. Bioavailability and bioactivity enhancement of herbal drugs by “nanotechnology”: a review. International Journal of Current Pharmaceutical Research . 2011;8:1–7. [Google Scholar]
- 33.Kushwaha S. K., Rastogl A., Rai A. K., Singh S. Novel drug delivery system for anticancer drug: a review. International Journal of PharmTech Research . 2012;4(2):542–553. [Google Scholar]
- 34.Gusev A. I., Kurlov A. S. Production of nanocrystalline powders by high-energy ball milling: model and experiment. Nanotechnology . 2008;19(26) doi: 10.1088/0957-4484/19/26/265302.265302 [DOI] [PubMed] [Google Scholar]
- 35.Maritz G. S., Dennis H. Maternal nicotine exposure during gestation and lactation interferes with alveolar development in the neonatal lung. Reproduction, Fertility and Development . 1998;10(3):255–262. doi: 10.1071/r98036. [DOI] [PubMed] [Google Scholar]
- 36.Kershbaum A., Bellet S., Khorsandian R. Elevation of serum cholesterol after administration of nicotine. American Heart Journal . 1965;69(2):206–210. doi: 10.1016/0002-8703(65)90038-4. [DOI] [PubMed] [Google Scholar]
- 37.Okamoto M., Kita T., Okuda H., Tanaka T., Nakashima T. Effects of aging on acute toxicity of nicotine in rats. Pharmacology & Toxicology . 1994;75(1):1–6. doi: 10.1111/j.1600-0773.1994.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y., An W., Gao A. Protective effects of naringenin in cardiorenal syndrome. Journal of Surgical Research . 2016;203(2):416–423. doi: 10.1016/j.jss.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Chen C., Jie X., Ou Y., et al. Nanoliposome improves inhibitory effects of naringenin on nonalcoholic fatty liver disease in mice. Nanomedicine . 2017;12(15):1791–1800. doi: 10.2217/nnm-2017-0119. [DOI] [PubMed] [Google Scholar]
- 40.An L., Zhang T. Vitamins C and E reverse melamine-induced deficits in spatial cognition and hippocampal synaptic plasticity in rats. Neurotoxicology . 2014;44:132–139. doi: 10.1016/j.neuro.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Gould T. D., Dao D. T., Kovacsics C. E. The open field test. Mood and Anxiety Related Phenotypes in Mice . 2009;42:1–20. doi: 10.1007/978-1-60761-303-9_1. [DOI] [Google Scholar]
- 42.Brown R. E., Corey S. C., Moore A. K. Differences in measures of exploration and fear in MHC-congenic C57BL/6J and B6-H-2K mice. Behavior Genetics . 1999;29(4):263–271. doi: 10.1023/a:1021694307672. [DOI] [Google Scholar]
- 43.Walsh R. N., Cummins R. A. The open-field test: a critical review. Psychological Bulletin . 1976;83(3):482–504. doi: 10.1037/0033-2909.83.3.482. [DOI] [PubMed] [Google Scholar]
- 44.Choleris E., Thomas A. W., Kavaliers M., Prato F. S. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neuroscience & Biobehavioral Reviews . 2001;25(3):235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- 45.Kalueff A. V., Tuohimaa P. Experimental modeling of anxiety and depression. Acta Neurobiologiae Experimentalis . 2004;64(4):439–448. doi: 10.55782/ane-2004-1526. [DOI] [PubMed] [Google Scholar]
- 46.Jalali M. R., Roghani M. The effect of nigella sativa on learning and memory in male diabetic rats. Basic and Clinical Neuroscience . 2009;1(1):32–34. [Google Scholar]
- 47.Wall P., Blanchard R. J., Markham C., Yang M., Blanchard D. C. Infralimbic D1 receptor agonist effects on spontaneous novelty exploration and anxiety-like defensive responding in CD-1 mice. Behavioural Brain Research . 2004;152(1):67–79. doi: 10.1016/j.bbr.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 48.Rasoolijazi H., Joghataie M. T., Roghani M., Nobakht M. The beneficial effect of (-)-Epigallocatechin-3-Gallate in an experimental model of alzheimer’s disease in rat: a behavioral analysis. IBJ . 2007;114:237–243. [PubMed] [Google Scholar]
- 49.Baluchnejadmojarad T., Roghani M., Kamran M., Karimi N. The effect of alpha-lipoic acid on learning and memory deficit in a rat model of temporal lobe epilepsy. Basic and Clinical Neuroscience . 2012;3(3):58–66. [Google Scholar]
- 50.Bevins R. A., Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study “recognition memory”. Nature Protocols . 2006;1(3):11306–11311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- 51.Leger M., Quiedeville A., Bouet V., et al. Object recognition test in mice. Nature Protocols . 2013;8(12):2531–2537. doi: 10.1038/nprot.2013.155. [DOI] [PubMed] [Google Scholar]
- 52.Lim S., Moon M., Oh H., Kim H. G., Kim S. Y., Oh M. S. Ginger improves cognitive function via NGF-induced ERK/CREB activation in the hippocampus of the mouse. The Journal of Nutritional Biochemistry . 2014;25(10):1058–1065. doi: 10.1016/j.jnutbio.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clinica Chimica Acta; International Journal of Clinical Chemistry . 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 54.Beutler E., Kelly B. M. D. O. Improved method for the determination of blood glutathione. The Journal of Laboratory and Clinical Medicine . 1963;61:882–888. [PubMed] [Google Scholar]
- 55.Aebi H. [13] Catalase in vitro. Methods in Enzymology . 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 56.Kovarik Z., Radić Z., Berman H. A., Simeon-Rudolf V., Reiner E., Taylor P. Acetyl cholinesterase active centre and gorge conformations analyzed by combinatorial mutations and enantiomeric phosphonates. Biochemical Journal . 2003;1(73):33–40. doi: 10.1042/bj20021862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tietz N. W. Clinical Guide to Laboratory Tests (ELISA) 3rd. Philadelphia, PA, USA: W.B. Saunders, Co.; 1995. pp. 22–23. [Google Scholar]
- 58.Bancroft J. D., Gamble M. Theory and Practice of Histological Techniques . 5th. London, UK: Churchill Livingstone; 2008. [Google Scholar]
- 59.Picciotto M. R., Brunzell D. H., Caldarone B. J. Effect of nicotine and nicotinic receptors on anxiety and depression. NeuroReport . 2002;13(9):1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 60.Ksir C. Acute and chronic nicotine effects on measures of activity in rats: a multivariate analysis. Psychopharmacology . 1994;115(1-2):105–109. doi: 10.1007/bf02244758. [DOI] [PubMed] [Google Scholar]
- 61.Vezina P., McGehee D. S., Green W. N. Exposure to nicotine and sensitization of nicotine-induced behaviors. Progress in Neuro-Psychopharmacology and Biological Psychiatry . 2007;31(8):1625–1638. doi: 10.1016/j.pnpbp.2007.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malin D. H. Nicotine dependence. Pharmacology Biochemistry and Behavior . 2001;70(4):551–559. doi: 10.1016/s0091-3057(01)00699-2. [DOI] [PubMed] [Google Scholar]
- 63.Domino E. F. Nicotine induced behavioral locomotor sensitization. Progress in Neuro-Psychopharmacology and Biological Psychiatry . 2001;25(1):59–71. doi: 10.1016/s0278-5846(00)00148-2. [DOI] [PubMed] [Google Scholar]
- 64.Caldarone B. J., King S. L., Picciotto M. R. Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neuroscience Letters . 2008;439(2):187–191. doi: 10.1016/j.neulet.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharples S. A., Koblinger K., Humphreys J. M., Whelan P. J. Dopamine: a parallel pathway for the modulation of spinal locomotor networks. Frontiers in Neural Circuits . 2014;8(55):p. 55. doi: 10.3389/fncir.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lambert A. M., Bonkowsky J. L., Masino M. A. The conserved dopaminergic diencephalospinal tract mediates vertebrate locomotor development in zebrafish larvae. Journal of Neuroscience . 2012;32(39):13488–13500. doi: 10.1523/jneurosci.1638-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beninger R. J. The role of dopamine in locomotor activity and learning. Brain Research Reviews . 1983;6(2):173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- 68.Preston A. M. Cigarette smoking-nutritional implications. Progress in Food & Nutrition Science . 1991;15(4):183–217. [PubMed] [Google Scholar]
- 69.Banerjee K. K., Marimuthu P., Sarkar A., Chaudhuri R. N. Influence of cigarette smoking on Vitamin C, glutathione and lipid peroxidation status. Indian Journal of Public Health . 1998;42(1):20–23. [PubMed] [Google Scholar]
- 70.Gomez-Cabrera M.-C., Domenech E., Romagnoli M., et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. American Journal of Clinical Nutrition . 2008;87(1):142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 71.Ristow M., Zarse K., Oberbach A., et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proceedings of the National Academy of Sciences . 2009;106(21):8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nikolaidis M. G., Kerksick C. M., Lamprecht M., McAnulty S. R. Does vitamin C and E supplementation impair the favorable adaptations of regular exercise? Oxidative Medicine and Cellular Longevity . 2012;2012:11. doi: 10.1155/2012/707941.707941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zarrindast M.-R., Naghdi-Sedeh N., Nasehi M., Sahraei H., Bahrami F., Asadi F. The effects of dopaminergic drugs in the ventral hippocampus of rats in the nicotine-induced anxiogenic-like response. Neuroscience Letters . 2010;475(3):156–160. doi: 10.1016/j.neulet.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 74.Nasehi M., Mafi F., Oryan S., Nasri S., Zarrindast M. R. The effects of dopaminergic drugs in the dorsal hippocampus of mice in the nicotine-induced anxiogenic-like response. Pharmacology Biochemistry and Behavior . 2011;98(3):468–473. doi: 10.1016/j.pbb.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 75.Zarrindast M. R., Nasehi M., Piri M., Heidari N. Effects of cholinergic system of dorsal hippocampus of rats on MK-801 induced anxiolytic-like behavior. Neuroscience Letters . 2011;505(2):65–70. doi: 10.1016/j.neulet.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 76.Irvine E. E., Cheeta S., File S. E. Time-course of changes in the social interaction test of anxiety following acute and chronic administration of nicotine. Behavioural Pharmacology . 1999;10(6):691–697. doi: 10.1097/00008877-199911000-00016. [DOI] [PubMed] [Google Scholar]
- 77.Olausson P., Åkesson P., Engel J. A., Söderpalm B. Effects of 5-HT1A and 5-HT2 receptor agonists on the behavioral and neurochemical consequences of repeated nicotine treatment. European Journal of Pharmacology . 2001;420(1):45–54. doi: 10.1016/s0014-2999(01)00939-6. [DOI] [PubMed] [Google Scholar]
- 78.Olausson P., Engel J. A., Söderpalm B. Behavioral sensitization to nicotine is associated with behavioral disinhibition; counteraction by citalopram. Psychopharmacology . 1999;142(2):111–119. doi: 10.1007/s002130050869. [DOI] [PubMed] [Google Scholar]
- 79.Espejo E. F. Selective dopamine depletion within the medial prefrontal cortex induces anxiogenic-like effects in rats placed on the elevated plus maze. Brain Research . 1997;762(1-2):281–284. doi: 10.1016/s0006-8993(97)00593-3. [DOI] [PubMed] [Google Scholar]
- 80.Mani V. M., Sadiq A. M. M. Naringin modulates the impairment of memory, anxiety, locomotor, and emotionality behaviors in rats exposed to deltamethrin; a possible mechanism association with oxidative stress, acetylcholinesterase and ATPase. Biomedicine & Preventive Nutrition . 2014;4(4):527–533. doi: 10.1016/j.bionut.2014.08.006. [DOI] [Google Scholar]
- 81.Rendeiro C., Guerreiro J. D. T., Williams C. M., Spencer J. P. E. Flavonoids as modulators of memory and learning: molecular interactions resulting in behavioural effects. Proceedings of the Nutrition Society . 2012;71(2):246–262. doi: 10.1017/s0029665112000146. [DOI] [PubMed] [Google Scholar]
- 82.Kim H.-J., Song J. Y., Park H. J., Park H.-K., Yun D. H., Chung J.-H. Naringin protects against rotenone-induced apoptosis in human neuroblastoma SH-SY5Y cells. Korean Journal of Physiology and Pharmacology . 2009;13(4):281–285. doi: 10.4196/kjpp.2009.13.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dunnett S. B., Martel F. L. Proactive interference effects on short-term memory in rats: I. Basic parameters and drug effects. Behavioral Neuroscience . 1990;104(5):655–665. doi: 10.1037/0735-7044.104.5.655. [DOI] [PubMed] [Google Scholar]
- 84.Richards M., Jarvis M. J., Thompson N., Wadsworth M. E. J. Cigarette smoking and cognitive decline in midlife: evidence from a prospective birth cohort study. American Journal of Public Health . 2003;93(6):994–998. doi: 10.2105/ajph.93.6.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Levin E., Rezvani A. Nicotinic treatment for cognitive dysfunction. Current Drug Targets-CNS & Neurological Disorders . 2002;1(4):423–431. doi: 10.2174/1568007023339102. [DOI] [PubMed] [Google Scholar]
- 86.Attaway C. M., Compton D. M., Turner M. D. The effects of nicotine on learning and memory. Physiology & Behavior . 1999;67(3):421–431. doi: 10.1016/s0031-9384(99)00081-5. [DOI] [PubMed] [Google Scholar]
- 87.Scerri C., Stewart C. A., Breen K. C., Balfour D. J. The effects of chronic nicotine on spatial learning and bromodeoxyuridine incorporation into the dentate gyrus of the rat. Psychopharmacology . 2006;184(3-4):540–546. doi: 10.1007/s00213-005-0086-4. [DOI] [PubMed] [Google Scholar]
- 88.Jacobs L. F., Schenk F. Unpacking the cognitive map: the parallel map theory of hippocampal function. Psychological Review . 2003;110(2):285–315. doi: 10.1037/0033-295x.110.2.285. [DOI] [PubMed] [Google Scholar]
- 89.Benwell M. E. M., Balfour D. J. K. Regional variation in the effects of nicotine on catecholamine overflow in rat brain. European Journal of Pharmacology . 1997;325(1):13–20. doi: 10.1016/s0014-2999(97)00101-5. [DOI] [PubMed] [Google Scholar]
- 90.Khakpai F., Nasehi M., Haeri-Rohani A., Eidi A., Zarrindast M. R. Scopolamine induced memory impairment; possible involvement of NMDA receptor mechanisms of dorsal hippocampus and/or septum. Behavioural Brain Research . 2012;231(1):1–10. doi: 10.1016/j.bbr.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 91.Yang W., Ma J., Liu Z., Lu Y., Hu B., Yu H. Effect of naringenin on brain insulin signaling and cognitive functions in ICV-STZ induced dementia model of rats. Neurological Sciences . 2014;35(5):741–751. doi: 10.1007/s10072-013-1594-3. [DOI] [PubMed] [Google Scholar]
- 92.Harrison F. E., Allard J., Bixler R., et al. Antioxidants and cognitive training interact to affect oxidative stress and memory in APP/PSEN1 mice. Nutritional Neuroscience . 2009;12(5):203–218. doi: 10.1179/147683009X423364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang J.-Y., Jin P., He Q., et al. Naringenin ameliorates hypoxia/reoxygenation-induced endoplasmic reticulum stress-mediated apoptosis in H9c2 myocardial cells: involvement in ATF6, IRE1α and PERK signaling activation. Molecular and Cellular Biochemistry . 2017;424(1-2):111–122. doi: 10.1007/s11010-016-2848-1. [DOI] [PubMed] [Google Scholar]
- 94.Fukui K., Omoi N.-O., Hayasaka T., et al. Cognitive impairment of rats caused by oxidative stress and aging, and its prevention by vitamin E. Annals of the New York Academy of Sciences . 2002;959(1):275–284. doi: 10.1111/j.1749-6632.2002.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 95.Fukui K., Takatsu H., Shinkai T., Suzuki S., Abe K., Urano S. Appearance of amyloid β-like substances and delayed-type apoptosis in rat hippocampus CA1 region through aging and oxidative stress. Journal of Alzheimer’s Disease . 2005;8(3):299–309. doi: 10.3233/jad-2005-8309. [DOI] [PubMed] [Google Scholar]
- 96.Mehrabadi S., Sadr S. S. Administration of Vitamin D3 and E supplements reduces neuronal loss and oxidative stress in a model of rats with Alzheimer’s disease. Neurological Research . 2020;42(10):862–868. doi: 10.1080/01616412.2020.1787624. [DOI] [PubMed] [Google Scholar]
- 97.Socci D. J., Crandall B. M., Arendash G. W. Chronic antioxidant treatment improves the cognitive performance of aged rats. Brain Research . 1995;693(1-2):88–94. doi: 10.1016/0006-8993(95)00707-w. [DOI] [PubMed] [Google Scholar]
- 98.Sumien N., Heinrich K. R., Sohal R. S., Forster M. J. Short-term vitamin E intake fails to improve cognitive or psychomotor performance of aged mice. Free Radical Biology and Medicine . 2004;36(11):1424–1433. doi: 10.1016/j.freeradbiomed.2004.02.081. [DOI] [PubMed] [Google Scholar]
- 99.McDonald S. R., Sohal R. S., Forster M. J. Concurrent administration of coenzyme Q10 and α-tocopherol improves learning in aged mice. Free Radical Biology and Medicine . 2005;38(6):729–736. doi: 10.1016/j.freeradbiomed.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 100.Min Y. N., Niu Z. Y., Sun T. T., et al. Vitamin E and vitamin C supplementation improves antioxidant status and immune function in oxidative-stressed breeder roosters by up-regulating expression of GSH-Px gene. Poultry Science . 2018;97(4):1238–1244. doi: 10.3382/ps/pex417. [DOI] [PubMed] [Google Scholar]
- 101.Perez X. A., Ly J., McIntosh J. M., Quik M. Long-term nicotine exposure depresses dopamine release in nonhuman primate nucleus accumbens. Journal of Pharmacology and Experimental Therapeutics . 2012;342(2):335–344. doi: 10.1124/jpet.112.194084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shearman E., Fallon S., Sershen H., Lajtha A. Nicotine-induced monoamine neurotransmitter changes in the brain of young rats. Brain Research Bulletin . 2008;76(6):626–639. doi: 10.1016/j.brainresbull.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 103.Saad A. B., Rjeibi I., Brahmi N., Elaloui E., Zouari N. Nicotine-induced oxidative stress, testis injury, AChE inhibition and brain damage alleviated by Mentha spicata. Inflammopharmacology . 2020;28(4):939–948. doi: 10.1007/s10787-019-00650-0. [DOI] [PubMed] [Google Scholar]
- 104.Exley R., Clements M. A., Hartung H., McIntosh J. M., Cragg S. J. α6-Containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology . 2008;33(9):2158–2166. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- 105.Nashmi R., Xiao C., Deshpande P., et al. Chronic nicotine cell specifically upregulates functional 4∗ nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. Journal of Neuroscience . 2007;27(31):8202–8218. doi: 10.1523/jneurosci.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tzschentke T. M., Schmidt W. J. Functional relationship among medial prefrontal cortex, nucleus accumbens, and ventral tegmental area in locomotion and reward. Critical Reviews in Neurobiology . 2000;14(2) doi: 10.1615/critrevneurobiol.v14.i2.20. [DOI] [PubMed] [Google Scholar]
- 107.Novak G., Seeman P., Foll B. L. Exposure to nicotine produces an increase in dopamine D2HighReceptors: a possible mechanism for dopamine hypersensitivity. International Journal of Neuroscience . 2010;120(11):691–697. doi: 10.3109/00207454.2010.513462. [DOI] [PubMed] [Google Scholar]
- 108.Caggiula A. R., Epstein L. H., Antelman S. M., et al. Conditioned tolerance to the anorectic and corticosterone-elevating effects of nicotine. Pharmacology Biochemistry and Behavior . 1991;40(1):53–59. doi: 10.1016/0091-3057(91)90319-w. [DOI] [PubMed] [Google Scholar]
- 109.Kumar A., Rinwa P., Kaur G., Machawal L. Stress: neurobiology, consequences and management. Journal of Pharmacy and Bioallied Sciences . 2013;5(2):91–97. doi: 10.4103/0975-7406.111818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hiremagalur B., Sabban E. L. Nicotine elicits changes in expression of adrenal catecholamine biosynthetic enzymes, neuropeptide Y and immediate early genes by injection but not continuous administration. Molecular Brain Research . 1995;32(1):109–115. doi: 10.1016/0169-328x(95)00068-4. [DOI] [PubMed] [Google Scholar]
- 111.Yu F., Li Y., Yang J., Qian J., Li X., Liu C. Prenatal nicotine exposure results in the inhibition of baroreflex sensitivity induced by intravenous injection angiotensin II in the adult male offspring rats. Cardiovascular Toxicology . 2017;17(2):200–207. doi: 10.1007/s12012-016-9375-x. [DOI] [PubMed] [Google Scholar]
- 112.Rahigude A., Bhutada P., Kaulaskar S., Aswar M., Otari K. Participation of antioxidant and cholinergic system in protective effect of naringenin against type-2 diabetes-induced memory dysfunction in rats. Neuroscience . 2012;226:62–72. doi: 10.1016/j.neuroscience.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 113.Liaquat L., Ahmad S., Sadir S., et al. Development of AD like symptoms following co-administration of AlCl3 and D-gal in rats: a neurochemical, biochemical and behavioral study. Pakistan Journal of Pharmaceutical Sciences . 2017;30:647–653. [PubMed] [Google Scholar]
- 114.Lou H., Jing X., Wei X., Shi H., Ren D., Zhang X. Naringenin protects against 6-OHDA-induced neurotoxicity via activation of the Nrf2/ARE signaling pathway. Neuropharmacology . 2014;79:380–388. doi: 10.1016/j.neuropharm.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 115.Alkam T., Nabeshima T. Molecular mechanisms for nicotine intoxication. Neurochemistry International . 2019;125:117–126. doi: 10.1016/j.neuint.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 116.Tjoncke J.-A., Goncalves R., Castaing N., Molimard M., Tovagliaro F., Titier K. Death related to nicotine replacement therapy: a case report. Forensic Science International . 2020;309 doi: 10.1016/j.forsciint.2020.110223.110223 [DOI] [PubMed] [Google Scholar]
- 117.Halliwell B. Reactive oxygen species and the central nervous system. Journal of Neurochemistry . 1992;59(5):1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 118.Budzynska B., Boguszewska-Czubara A., Kruk-Slomka M., Kurzepa J., Biala G. Mephedrone and nicotine: oxidative stress and behavioral interactions in animal models. Neurochemical Research . 2015;40(5):1083–1093. doi: 10.1007/s11064-015-1566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Motaghinejad M., Motevalian M., Fatima S., Faraji F., Mozaffari S. The neuroprotective effect of curcumin against nicotine-induced neurotoxicity is mediated by CREB-BDNF signaling pathway. Neurochemical Research . 2017;42(10):2921–2932. doi: 10.1007/s11064-017-2323-8. [DOI] [PubMed] [Google Scholar]
- 120.Dhouib H., Jallouli M., Draief M., Bouraoui S., El-Fazâa S. Oxidative damage and histopathological changes in lung of rat chronically exposed to nicotine alone or associated to ethanol. Pathologie Biologie . 2015;63(6):258–267. doi: 10.1016/j.patbio.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 121.Elsonbaty S. M., Ismail A. F. M. Nicotine encourages oxidative stress and impairment of rats’ brain mitigated by spirulina platensis lipopolysaccharides and low-dose ionizing radiation. Archives of Biochemistry and Biophysics . 2020;689(689):p. 108382. doi: 10.1016/j.abb.2020.108382. [DOI] [PubMed] [Google Scholar]
- 122.Helen A., Krishnakumar K., Vijayammal P. L., Augusti K. T. A comparative study of antioxidants S-allyl cysteine sulfoxide and vitamin E on the damages induced by nicotine in rats. Pharmacology . 2003;67(3):113–117. doi: 10.1159/000067796. [DOI] [PubMed] [Google Scholar]
- 123.Haider S., Liaquat L., Shahzad S., et al. A high dose of short term exogenous D-galactose administration in young male rats produces symptoms simulating the natural aging process. Life Sciences . 2015;124:110–119. doi: 10.1016/j.lfs.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 124.Ray G., Husain S. A. Oxidants, antioxidants and carcinogenesis. Indian Journal of Experimental Biology . 2002;42:1213–1232. [PubMed] [Google Scholar]
- 125.Helen A., Krishnakumar K., Vijayammal P. L., Augusti K. T. Antioxidant effect of onion oil (Allium cepa. Linn) on the damages induced by nicotine in rats as compared to alpha-tocopherol. Toxicology Letters . 2000;116(1-2):61–68. doi: 10.1016/s0378-4274(00)00208-3. [DOI] [PubMed] [Google Scholar]
- 126.Chattopadhyay K., Chattopadhyay B. D. Effect of nicotine on lipid profile, per oxidation and antioxidant enzymes in female rats with restricted dietary protein. Indian Journal of Medical Research . 2008;127:571–576. [PubMed] [Google Scholar]
- 127.Brown K. M., Morrice P. C., Arthur J. R., Duthie G. G. Effects of vitamin E supplementation on erythrocyte antioxidant defence mechanisms of smoking and non-smoking men. Clinical Science . 1996;91(1):107–111. doi: 10.1042/cs0910107. [DOI] [PubMed] [Google Scholar]
- 128.Valk E. E., Hornstra G. Relationship between vitamin E requirement and polyunsaturated fatty acid intake in man: a review. International Journal for Vitamin and Nutrition Research . 2000;70(2):31–42. doi: 10.1024/0300-9831.70.2.31. [DOI] [PubMed] [Google Scholar]
- 129.Das S., Gautam N., Dey S. K., Maiti T., Roy S. Oxidative stress in the brain of nicotine-induced toxicity: protective role of andrographis paniculata nees and vitamin E. Applied Physiology Nutrition and Metabolism . 2009;34(2):124–135. doi: 10.1139/H08-147. [DOI] [PubMed] [Google Scholar]
- 130.Gumustekin K., Taysi S., Alp H. H., et al. Vitamin E and Hippophea rhamnoides L. extract reduce nicotine-induced oxidative stress in rat heart. Cell Biochemistry and Function . 2010;28(4):329–333. doi: 10.1002/cbf.1663. [DOI] [PubMed] [Google Scholar]
- 131.Brigelius-Flohe R., Traber M. G. Vitamin E: function and metabolism. The FASEB Journal . 1999;13:1145–1155. [PubMed] [Google Scholar]
- 132.Ishisaka A., Ichikawa S., Sakakibara H., et al. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Radical Biology and Medicine . 2011;51(7):1329–1336. doi: 10.1016/j.freeradbiomed.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 133.Raza S. S., Khan M. M., Ahmad A., et al. Neuroprotective effect of naringenin is mediated through suppression of NF-κB signaling pathway in experimental stroke. Neuroscience . 2013;230:157–171. doi: 10.1016/j.neuroscience.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 134.Ahmed L. A., Obaid A. A. Z., Zaki H. F., Agha A. M. Naringenin adds to the protective effect of L-arginine in monocrotaline-induced pulmonary hypertension in rats: favorable modulation of oxidative stress, inflammation and nitric oxide. European Journal of Pharmaceutical Sciences . 2014;62:161–170. doi: 10.1016/j.ejps.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 135.Tsai T.-H. Determination of naringin in rat blood, brain, liver, and bile using microdialysis and its interaction with cyclosporin A, a P-glycoprotein modulator. Journal of Agricultural and Food Chemistry . 2002;50(23):6669–6674. doi: 10.1021/jf020603p. [DOI] [PubMed] [Google Scholar]
- 136.Youdim K. A., Dobbie M. S., Kuhnle G., Proteggente A. R., Abbott N. J., Rice-Evans C. Interaction between flavonoids and the blood-brain barrier: in vitro studies. Journal of Neurochemistry . 2003;85(1):180–192. doi: 10.1046/j.1471-4159.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- 137.Peng H. W., Cheng F. C., Huang Y. T., Chen C. F., Tsai T. H. Determination of naringenin and its glucuronide conjugate in rat plasma and brain tissue by high-performance liquid chromatography. Journal of Chromatography B: Biomedical Sciences and Applications . 1998;714(2):369–374. doi: 10.1016/s0378-4347(98)00204-7. [DOI] [PubMed] [Google Scholar]
- 138.Youdim K. A., Qaiser M. Z., Begley D. J., Rice-Evans C. A., Abbott N. J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radical Biology and Medicine . 2004;36(5):592–604. doi: 10.1016/j.freeradbiomed.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 139.Khan M. B., Khan M. M., Khan A., et al. Naringenin ameliorates Alzheimer’s disease (AD)-type neurodegeneration with cognitive impairment (AD-TNDCI) caused by the intracerebroventricular-streptozotocin in rat model. Neurochemistry International . 2012;61(7):1081–1093. doi: 10.1016/j.neuint.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 140.Sriraksa N., Wattanathorn J., Muchimapura S., Tiamkao S., Brown K., Chaisiwamongkol K. Cognitive-enhancing effect of quercetin in a rat model of Parkinson’s disease induced by 6-hydroxydopamine. Evidence-Based Complementary and Alternative Medicine . 2012;2012:9. doi: 10.1155/2012/823206.823206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Amic D., Davidovic-Amic D., Beslo D., Trinajstic N. Structure radical scavenging activity relationships of flavonoids. Croatica Chemica Acta . 2003;76(1):55–61. [Google Scholar]
- 142.El Madani M. A., Elsalam R. M. A., Attia A. S., El-shenawy S. M., Arbid M. S. Neuropharmacological effects of naringenin, harmine and adenosine on parkinsonism induced in rats. Scholars Research Library . 2016;8(5):45–57. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used to support the findings of this study are available from the corresponding author upon request.


