Abstract
Polyethylene glycol (PEG) precipitation is one of the conventional methods for virus concentration. This technique has been used to detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in wastewater. The procedures and seeded surrogate viruses were different among implementers; thus, the reported whole process recovery efficiencies considerably varied among studies. The present study compared five PEG precipitation procedures, with different operational parameters, for the RT-qPCR-based whole process recovery efficiency of murine hepatitis virus (MHV), bacteriophage phi6, and pepper mild mottle virus (PMMoV), and molecular process recovery efficiency of murine norovirus using 34 raw wastewater samples collected in Japan. The five procedures yielded significantly different whole process recovery efficiency of MHV (0.070%–2.6%) and phi6 (0.071%–0.51%). The observed concentration of indigenous PMMoV ranged from 8.9 to 9.7 log (8.2 × 108 to 5.6 × 109) copies/L. Interestingly, PEG precipitation with 2-h incubation outperformed that with overnight incubation partially due to the difference in molecular process recovery efficiency. The recovery load of MHV exhibited a positive correlation (r = 0.70) with that of PMMoV, suggesting that PMMoV is the potential indicator of the recovery efficiency of SARS-CoV-2. In addition, we reviewed 13 published studies and found considerable variability between different studies in the whole process recovery efficiency of enveloped viruses by PEG precipitation. This was due to the differences in operational parameters and surrogate viruses as well as the differences in wastewater quality and bias in the measurement of the seeded load of surrogate viruses, resulting from the use of different analytes and RNA extraction methods. Overall, the operational parameters (e.g., incubation time and pretreatment) should be optimized for PEG precipitation. Co-quantification of PMMoV may allow for the normalization of SARS-CoV-2 RNA concentration by correcting for the differences in whole process recovery efficiency and fecal load among samples.
Keywords: SARS-CoV-2, Surrogates, Polyethylene glycol precipitation, Virus concentration, Wastewater-based epidemiology
Graphical abstract
1. Introduction
Development of sensitive methods for detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in wastewater is a key imperative owing to the increasing attention on wastewater-based epidemiology (WBE) (Kitajima et al., 2020; Zhu et al., 2021). Highly efficient methods for primary concentration and the downstream molecular processes, including nucleic acid extraction, reverse transcription (RT), and quantitative polymerase chain reaction (qPCR), are required. Many researchers have recently proposed novel analytical methods (Ahmed et al., 2020a; Graham et al., 2020; Whitney et al., 2021) and compared the recovery efficiency of the various conventional methods (Chik et al., 2021; LaTurner et al., 2021; Pecson et al., 2021).
Polyethylene glycol (PEG) precipitation is one of the methods for virus concentration from environmental water samples (Haramoto et al., 2018; Lewis and Metcalf, 1988). The polymer, PEG, preferentially traps solvent and sterically excludes proteins (e.g., virion) from the solvent phase. This allows for concentration of the proteins and their precipitation once their concentrations exceed the saturated solubility (Atha and Ingham, 1981; Lewis and Metcalf, 1988). One of the advantages of PEG precipitation is that it can be performed with basic laboratory equipment (Ahmed et al., 2020b) with a relatively low running cost compared to other methods (e.g., ultrafiltration). Several studies have reported its applicability for the detection of SARS-CoV-2 RNA in wastewater (Hata et al., 2021; Kumar et al., 2020; Torii et al., 2021; Wu et al., 2020). However, the procedures of PEG precipitation highly depended on implementers. For example, various analytes (e.g., supernatant or filtrate of raw wastewater and non-pretreated raw wastewater) were added with a different concentration of salt and PEG. Moreover, the incubation time for the precipitation varied from 0 h to overnight incubation (Ahmed et al., 2020a; Alexander et al., 2020; Barril et al., 2021; Chavarria-Miró et al., 2021; D'Aoust et al., 2021; Gerrity et al., 2021; Graham et al., 2020; LaTurner et al., 2021; Pecson et al., 2021; Pérez-Cataluña et al., 2021; Philo et al., 2021; Sapula et al., 2021; Torii et al., 2021). A recent study reported that the whole process recovery efficiencies of human coronavirus OC43 by PEG precipitation were different depending on the procedures, ranging from 0.03% to 78%, even using the same wastewater samples (Pecson et al., 2021). Nevertheless, the impact of operational conditions on the recovery efficiency was unclear because different procedures use different downstream molecular processes, which may contribute to the variability of whole process recovery (Torii et al., 2021). To the best of our knowledge, no studies have compared the recovery efficiency for surrogate enveloped viruses among various PEG precipitation procedures using the same wastewater, surrogate viruses, and downstream molecular processes.
Due to the stringent containment policy requirement (i.e., biosafety level [BSL] 3) for the handling of SARS-CoV-2 in laboratory, various surrogate viruses were alternatively adopted to evaluate the recovery efficiency of specific methods. Murine hepatitis virus (MHV) is an enveloped and positive-sense single-stranded RNA virus with a diameter of 80–120 nm. MHV belongs to the genus Betacoronavirus that also includes SARS-CoV-2. MHV has been widely used as a surrogate for SARS-CoV-2 for comparing various virus concentration methods (Ahmed et al., 2020a; Graham et al., 2020) because of its phylogenetic similarity to SARS-CoV-2 and lower BSL requirement (i.e., BSL 2). Bacteriophage phi6 belongs to the genus Cystovirus and infects Pseudomonas syringae (King et al., 2012). The advantage of the use of phi6 as a surrogate virus is the minimum BSL requirement (i.e., BSL 1) and ease of propagation, allowing for broad use. However, few studies have confirmed the comparability to other surrogate viruses in terms of recovery efficiency (Torii et al., 2021). Pepper mild mottle virus (PMMoV) is a non-enveloped single-stranded RNA virus, with a rod-shaped structure (312-nm length). PMMoV belongs to the genus Tobamovirus (Kitajima et al., 2018). PMMoV is abundant (106–1010 copies/L) (Ahmed et al., 2020c; Symonds et al., 2018) in raw wastewater and thus is widely used as an indicator of virus reduction during (waste)water treatment (Asami et al., 2016; Canh et al., 2021a; Schmitz et al., 2016; Tandukar et al., 2020). This may allow for the use of PMMoV as potential control for assessing the recovery efficiency of a certain concentration method (Haramoto et al., 2020). However, the effect of the morphological differences between PMMoV and SARS-CoV-2 on the whole process recovery efficiency has not been well characterized.
In the present study, we compared five different PEG precipitation procedures for the RT-qPCR-based whole process recovery efficiency of MHV, phi6, and PMMoV and molecular process recovery efficiency of murine norovirus (MNV). Moreover, we assessed the association between the recovery efficiency of surrogate viruses to propose how PMMoV can be used for interpreting SARS-CoV-2 concentration in wastewater.
2. Materials and methods
2.1. Virus propagation
MHV A59 strain (VR-764, American Type Culture Collection, Manassas, VA, USA) was propagated on DBT cells. DBT cells were grown in Eagle's minimum essential medium (MEM) (Nissui Pharmaceutical, Tokyo, Japan) supplemented with 5% fetal bovine serum (FBS) in a 75 cm2 flask. Semi-confluent DBT cells were inoculated with MHV and incubated in MEM with 1% FBS at 37 °C (5% CO2) for 3 days. Then, the flask was frozen and thawed once to recover MHV from the cells. The suspensions were centrifuged at 3500g for 15 min. The supernatant was filtered through a 0.2-μm cellulose acetate membrane (DISMIC-25CS, Advantec, Tokyo, Japan).
Bacteriophage phi6 (NBRC 105899, National Institute of Technology and Evaluation (NITE), Tokyo, Japan) was propagated using P. syringae (NBRC14084, NITE) as the host bacterium. To prepare the phi6 stock, P. syringae was propagated in Luria-Bertani broth at 28 °C for 6 h, subsequently inoculated with phi6, and incubated overnight. The suspensions were centrifuged at 3500g for 15 min. The supernatant was filtered through a 0.2-μm cellulose acetate membrane (Advantec).
MNV S7-PP3 strain was propagated on RAW 264.7 cells, as described elsewhere (Kitajima et al., 2008). All propagated virus stocks were stored at 4 °C before the experiment.
2.2. Preparation of raw wastewater spiked with MHV and phi6
A total of 34 raw wastewater samples (Sample Nos.1–34) were used in the experiment. Samples of raw wastewater (300 mL) were collected weekly from July 1 to October 19, 2020 (17 consecutive weeks) at wastewater treatment plants (WWTPs) A and B, both of which are in Kanto area in Japan, and stored at −20 °C. Information pertaining to WWTPs A and B is provided in the supplemental information (SI) (see Table S1). The basic water quality parameters of raw wastewater at WWTP A were as follows: pH, 8.23 ± 0.20; UV254, 0.49 ± 0.07 cm−1; and conductivity, 83.8 ± 22.7 mS/m. Those of WWTP B were as follows: pH, 8.11 ± 0.09; UV254, 0.50 ± 0.04 cm−1; and conductivity, 52.0 ± 5.8 mS/m (mean ± standard deviation).
Each thawed raw wastewater sample was divided into five 41-mL aliquots. Each aliquot was spiked with 41 μL of propagated MHV and phi6 as whole process controls (WPCs) to obtain the final concentrations of 3.9 × 105 and 1.7 × 105 copies/mL, respectively. As a control, 41-mL MilliQ water (Millipore, Tokyo, Japan) spiked with the same amount of MHV, and phi6 was prepared in duplicate to determine the initial concentrations (see Fig. S1 for the results). The spiked raw wastewater and MilliQ water were immediately placed in −20 °C freezer and stored overnight. Each set of samples was transported at <−70 °C to five laboratories and stored in a freezer at −20 °C for up to one month to perform each PEG precipitation procedure, RNA extraction, and the RT process.
2.3. PEG precipitation
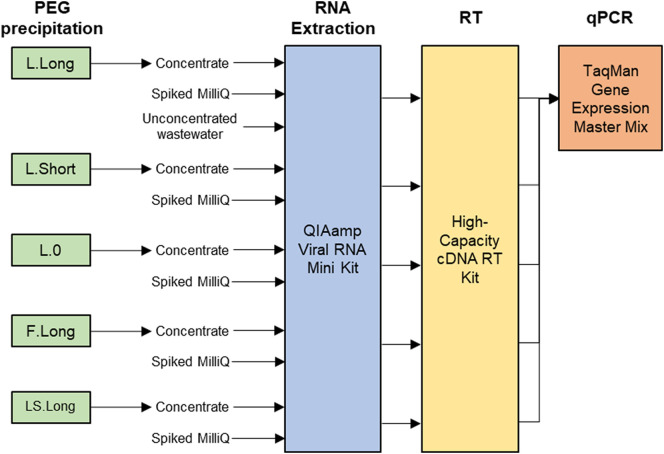
The whole experimental scheme is illustrated in Fig. 1 . A total of five PEG precipitation procedures, with different operational conditions, including analyte (liquid [L], filtrate [F], or liquid + solid [LS]) and incubation time (0 h [0], 2 h [Short], or overnight [Long]), were performed. Each procedure (L.Long, L.0, L.Short, F.Long, and LS.Long) was named based on its analyte and incubation time.
Fig. 1.
Flow diagram of sample processing for the comparison of five PEG precipitation procedures. PEG precipitation, RNA extraction, and RT were performed in each laboratory. At the laboratory of the University of Tokyo, the spiked wastewater was also directly subjected to RNA extraction, RT, and qPCR for the measurement of the indigenous PMMoV concentration in the unconcentrated samples. All the runs of qPCR were performed at the laboratory of the University of Tokyo.
Before each PEG precipitation, a 41-mL aliquot was thawed at room temperature, 40 mL of which was subjected to PEG precipitation. In the laboratory of the University of Tokyo, the remaining 1 mL was used for the measurement of indigenous PMMoV concentration in the unconcentrated samples.
2.3.1. L.Long
The raw wastewater sample was concentrated as described elsewhere (Hata et al., 2021; Torii et al., 2021). A 40-mL aliquot of raw wastewater was centrifuged at 3500g for 5 min to remove the suspended solids. The supernatant was supplemented with 4.0 g of PEG8000 and 2.4 g of NaCl to obtain the final concentrations of 10% (w/v) and 1.0 M, respectively. The mixture was incubated overnight in a shaker at 4 °C. Then, the mixture was centrifuged at 10,000g for 30 min. After carefully discarding the supernatant, the precipitate was resuspended with 10 mM phosphate buffer (0.5 mL). The final volume of the concentrate was 0.68 ± 0.05 mL.
2.3.2. L.Short
This procedure was identical to L.Long except for the incubation time. In this protocol, the incubation period was reduced to 2 h. The final volume of the concentrate was 0.62 ± 0.05 mL.
2.3.3. L.0
The raw wastewater sample was concentrated using the protocol recommended by the IDEXX Laboratories (Westbrook ME, USA) with slight modifications (https://www.idexx.com/files/sample-concentration-protocol-for-wastewater-surveillance.pdf). Briefly, a 40-mL aliquot of raw wastewater was centrifuged at 4700g for 30 min at 4 °C to remove the suspended solids. The supernatant was supplemented with 4.0 g of PEG8000 and 0.94 g of NaCl to obtain the final concentrations of 10% (w/v) and 0.4 M, respectively, followed by vortex mixing for 10 min. The mixture was directly subjected to centrifugation at 12,000g for 100 min at 4 °C without incubation. The supernatant was discarded, leaving approximately 5 mL of the mixture in the tube. The mixture was subsequently centrifuged at 12,000g for 5 min at 4 °C. After carefully discarding the supernatant, the precipitate was resuspended with 600 μL of PCR-grade water, followed by vortex mixing and spin down. The final volume of the concentrate was 0.65 ± 0.17 mL.
2.3.4. F.Long
A 40-mL aliquot of raw wastewater was filtered through a hydrophilic polytetrafluoroethylene membrane (Millipore) with a pore size of 0.2 μm. The filtrate was supplemented with 4.0 g of PEG6000 and 0.94 g of NaCl to obtain the final concentrations of 10% (w/v) and 0.4 M, respectively. The mixture was incubated overnight in a shaker at 4 °C. Then, the mixture was centrifuged at 12,000g for 60 min. After carefully discarding the supernatant, the precipitate was resuspended with 0.5 mL of TRIzol reagent (Thermo Fisher Scientific, MA, USA). The final volume of the concentrate was 0.66 ± 0.03 mL. Note that the TRIzol reagent was used as a suspension medium of PEG precipitates. The use of TRIzol reagent aimed to immediately lyse the protein in the precipitates, resulting in virus inactivation, which minimizes the microbial health risks for laboratory personnel. The suspensions were not treated by chloroform but were directly subjected to RNA extraction using QIAamp Viral RNA Mini Kit (see 2.4).
2.3.5. LS.Long
A 40-mL aliquot of raw wastewater was incubated at 60 °C for 90 min. The pretreated raw wastewater was supplemented with 3.2 g of PEG6000 and 0.94 g of NaCl to obtain the final concentrations of 8% (w/v) and 0.4 M, respectively. The mixture was incubated at 4 °C overnight in a shaker. Then, the mixture was centrifuged at 10,000g for 30 min. After carefully discarding the supernatant, the precipitate was resuspended with 0.5 mL of TRIzol reagent. The final volume of the concentrate was 1.14 ± 0.33 mL. The suspensions were not treated by chloroform but were directly subjected to RNA extraction using QIAamp Viral RNA Mini Kit (see 2.4).
2.4. RNA extraction and RT
RNA was extracted from the concentrates, the spiked MilliQ water, and unconcentrated wastewater samples at the laboratory of the University of Tokyo while RNA was extracted only from the concentrates and the spiked MilliQ water in the other four laboratories (Fig. 1).
Prior to RNA extraction, a 140-μL aliquot of each sample was seeded with 5-μL MNV as a molecular process control (MPC). Comparison of the MNV concentrations between the concentrates or unconcentrated wastewater samples and the spiked MilliQ water allows the evaluation of the total recovery efficiency of the molecular process (i.e., extraction-(RT-)qPCR efficiency) (Haramoto et al., 2018). The samples were processed by QIAamp Viral RNA Mini Kit (QIAGEN, Tokyo, Japan) to obtain an RNA extract with a final volume of 60 μL. The RNA extract was subjected to RT on the same day in each laboratory.
Before the RT reaction, 8-μL subsample of RNA extract was incubated at 95 °C for 5 min, followed by 4 °C for 1 min to denature the double-stranded RNA. Then, 8-μL heat-incubated RNA and 35-μL non-incubated RNA were subjected to RT to synthesize cDNA. High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) was used according to the manufacturer's instructions. The obtained cDNA was stored at −20 °C at each laboratory and transported with ice packs to the laboratory of the University of Tokyo. The transported cDNA was stored at −20 °C until qPCR.
2.5. qPCR
All qPCR assays were run with StepOnePlus™ Real-Time PCR System (Thermo Fisher Scientific) at the laboratory of the University of Tokyo. Quantification of MHV, phi6, PMMoV, and MNV was performed with TaqMan-based qPCR assays using previously reported primers and TaqMan (MGB) probe (Besselsen et al., 2002; Gendron et al., 2010; Haramoto et al., 2013; Kitajima et al., 2008; Zhang et al., 2006). SARS-CoV-2 RNA was detected with two TaqMan-based qPCR assays, CDC-N1 and CDC-N2, using the reported primers and probe (Centers for Disease Control and Prevention, 2020). The primers and probes used in the present study are listed in Table S2. The thermal cycle conditions are listed in Table S3. Briefly, 5 μL of synthesized cDNA was mixed with 15 μL of reaction mixture containing forward primer, reverse primer, and TaqMan (MGB) probe and 10 μL of TaqMan™ Gene Expression Master Mix (Thermo Fisher Scientific). All reactions were tested with duplicated qPCR reactions. Negative control was included for every qPCR run.
A standard curve was generated every run from ten-fold serial dilutions of gBlocks for the assays of MHV, phi6, PMMoV, and MNV (Integrated DNA Technologies, Coralville, IA, USA) or plasmid DNA for the assays of CDC-N1 and CDC-N2 (Cat: 10006625, Integrated DNA Technologies) containing the target sequence (5 × 105 to 5 × 100 or 5 × 104 to 5 × 100 copies/reaction). The number of viral genome copies per qPCR reaction was determined by each standard curve. For CDC-N1 and CDC-N2 assays, the samples that showed Ct values lower than 40 for at least one out of the duplicated reactions were considered positive for SARS-CoV-2.
The slope, intercept, R2, and amplification efficiency of the standard curves of each assay are presented in Table S4.
2.6. Data analysis and interpretation
The whole process recovery efficiency (W) (Eq. (1)) and molecular process recovery efficiency (M) (Eq. (2)) are given below. Note that the W depends on the efficiency of the concentration process and the downstream molecular processes (i.e., RNA extraction, RT, and qPCR), while M depends only on the efficiency of the molecular process.
| (1) |
| (2) |
where C conc_WPC represents the concentration of WPC (i.e., MHV and phi6) in concentrated samples (copies/reaction), C ini_WPC represents the concentration of WPC in the spiked MilliQ water (copies/reaction), x represents the concentration factor during PEG concentration, C conc_MPC represents the concentration of MPC (i.e., MNV) in concentrated samples (copies/reaction), and C ini_MPC represents the concentration of MPC in the spiked MilliQ water (copies/reaction).
The observed concentration of indigenous PMMoV, C obs_PMMoV (copies/L) (Haramoto et al., 2020) was calculated using Eq. (3).
| (3) |
where C conc_PMMoV represents the concentration of PMMoV in concentrated samples (copies/reaction).
The recovery load of viruses (N [copies]) was calculated using Eq. (4):
| (4) |
where V concentrate represents the concentrate volume.
2.7. Statistical analysis
All statistical analyses were performed using R 3.6.0 (R Core Team, 2019). A post hoc pairwise Wilcoxon rank-sum test was performed for the multiple comparisons of the log W or log concentrations of PMMoV among different PEG precipitation procedures using “pairwise.wilcox.test” function in {stats} package. Comparisons with a P-value <0.05 were considered significant. Note that the inequality sign (<) was placed only when a statistically significant difference was observed. Otherwise, an approximate symbol (≈) was placed. Spearman's rank correlation coefficient was determined to assess the relationship between the recovery load of each virus using “rcorr” function in {Hmisc} package.
3. Results and discussion
3.1. Comparison of whole process recovery efficiency among different PEG precipitation procedures
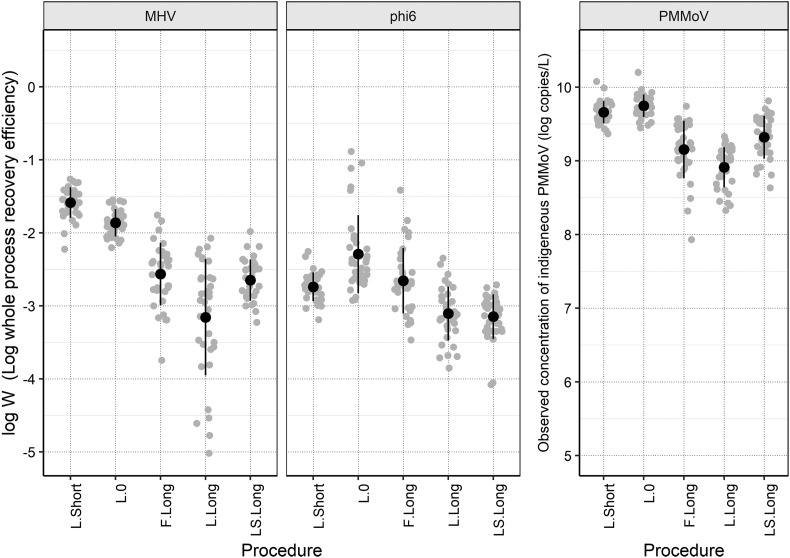
Fig. 2 shows the whole process recovery efficiency of MHV and phi6 and the observed concentrations of indigenous PMMoV with each PEG precipitation procedure. All data required for the calculation are also provided in the supplemental spreadsheet.
Fig. 2.
Log whole process recovery efficiency (log W) of MHV and phi6 and the observed concentrations of indigenous PMMoV with each PEG precipitation procedure (n = 34). Black circles represent the arithmetic mean of log W or the observed concentration of indigenous PMMoV, the error bars represent standard deviations, and the gray circles represent the individual data-points.
The whole process recovery efficiency of MHV differed with the following order: L.Long < LS.Long ≈ F.Long < L.0 < L.Short, ranging from −3.16 ± 0.80 log (geometric mean of 0.070%) to −1.59 ± 0.19 log (2.6%). Similarly, the observed concentration of indigenous PMMoV differed with the following order L.Long < F.Long ≈ LS.Long, < L.Short < L.0, ranging from 8.91 ± 0.27 log (8.2 × 108) to 9.74 ± 0.15 log (5.6 × 109) copies/L. Given that the indigenous PMMoV concentration in unconcentrated wastewater samples was determined to be 9.50 ± 0.30 log (3.2 × 109) copies/L, the estimated whole process recovery efficiency of PMMoV ranged from −0.88 ± 0.17 log (7.6%) to −0.05 ± 0.27 log (89%). Note that the whole process recovery efficiency of PMMoV may have been overestimated compared with MHV and phi6 because the initial concentration was directly determined from the unconcentrated wastewater, which may contain substances that may inhibit the molecular process. In fact, the MNV recovery of unconcentrated wastewater was −0.32 ± 0.20 log (48%) with the lowest value of −0.75 log (18%), suggesting slight underestimation of the concentrations of PMMoV in the unconcentrated samples. The whole process recovery efficiency of phi6 differed with the following order: LS.Long ≈ L.Long < L.Short ≈ F.Long < L.0, ranging from −3.15 ± 0.31 (0.071%) to −2.29 ± 0.54 log (0.51%).
It is of particular interest to assess whether shortening the incubation period for PEG precipitation reduces the recovery efficiency. This is because PEG precipitation is known for its lower throughput, owing to the long incubation time and requirement of centrifugation. The comparison of L.Long and L.Short, both of which adopted the same procedures except for incubation time (see Section 2.3), suggested that overnight incubation did not improve the recovery efficiency of MHV. Rather, we observed a negative impact on the whole process recovery. This may be partially attributable to the low molecular process recovery efficiency of L.Long. Fig. 3 shows the molecular process recovery efficiency of MNV with each PEG precipitation procedure. The MNV recovery differed with the following order: L.Long < LS.Long < L.Short ≈ L.0 < F.Long, ranging from −0.92 ± 0.17 log to 0.29 ± 0.27 log. L.Long showed significantly lower molecular process recovery efficiency than L.Short. One of the potential mechanisms is the inhibition during RT-qPCR. In a previous study, <10% efficiency of RT-qPCR was observed in 1.2%–15% of wastewater influent samples by PEG precipitation with overnight incubation (da Silva et al., 2007). Moreover, a relatively higher whole process recovery efficiency of MHV was observed with L.0 compared to the three procedures applying longer incubation time (i.e., L.Long, F.Long, and LS.Long). This also supports the finding that overnight incubation did not improve the recovery efficiency of MHV. Although further studies are required to understand this phenomenon, the incubation time can be shortened for precipitation, allowing higher recovery and throughput.
Fig. 3.
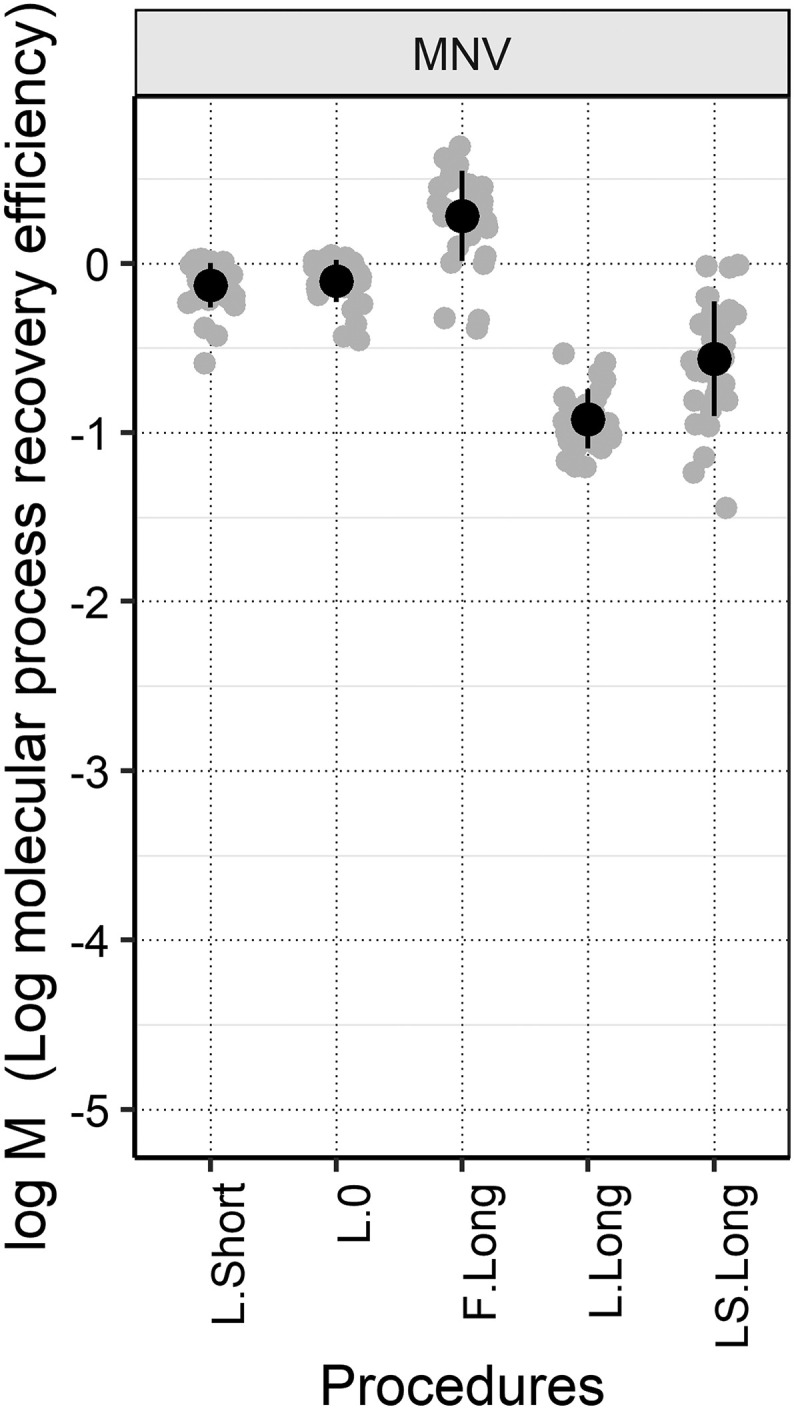
Log molecular process recovery efficiency (log M) of MNV with each PEG precipitation procedure (n = 34). Black circles represent the arithmetic means of log M, the error bars represent the standard deviations of log M, the gray circles represent the individual data-points.
3.2. Comparison of SARS-CoV-2 RNA detection among different PEG precipitation procedures
Table 1 shows the number of the samples that exhibited positive signals of CDC-N1 and CDC-N2 assays for SARS-CoV-2 with each PEG precipitation procedure. Two procedures, L.Short and LS.Long, yielded positive results of SARS-CoV-2 in one each of the 34 samples, respectively, while the others yielded negative results in all samples. Interestingly, the recoveries of the WPCs and PMMoV with LS.Long were comparable or lower than those with the procedures that did not yield positive results (i.e., L.0 and F.Long). A possible explanation is that SARS-CoV-2 RNA was detected from the solid fraction in LS.Long. This implies that the WPCs or PMMoV did not fully simulate the liquid-solid partitioning of indigenous SARS-CoV-2 in wastewater. In fact, some studies reported higher partitioning of SARS-CoV-2 to a solid phase in wastewater (Graham et al., 2020; Kitamura et al., 2021).
Table 1.
Number of samples from which SARS-CoV-2 RNA was detected by CDC-N1 and CDC-N2 assays.
| Procedure | CDC-N1 | CDC-N2 | Total number of positive samples |
|---|---|---|---|
| L.Long | 0 | 0 | 0/34 |
| L.Short | 1a | 0 | 1/34 |
| L.0 | 0 | 0 | 0/34 |
| F.Long | 0 | 0 | 0/34 |
| LS.Long | 1b | 0 | 1/34 |
Positive signal was observed at the sample collected on July 21 at WWTP A (Ct 39.0).
Positive signal was observed at the sample collected on July 21 at WWTP A (Ct 39.1).
It should be noted that the current sampling campaign yielded a small number of positive results of SARS-CoV-2, partially due to the limited numbers of infected people in the catchment area during the sampling period (see SI for the reported cases per million people in Kanto area). Moreover, our whole experimental scheme included several times of freeze-thawing, which may degrade the level of RNA in the sample (Fernandez-Cassi et al., 2021; Markt et al., 2021; Weidhaas et al., 2021). The obtained positive numbers are insufficient to statistically support our hypothesis and to judge whether the solid phase should be included in an analyte. It is also not clear whether the SARS-CoV-2 exists in feces or wastewater in the same form as the propagated stocks of surrogate viruses. Recent studies that adopted virion integrity-based RT-qPCR assays reported several states of SARS-CoV-2 RNA in wastewater, including protected and non-protected forms (Canh et al., 2021b; Wurtzer et al., 2021). Thus, further studies are required to investigate whether the WPCs and indigenous PMMoV can simulate the SARS-CoV-2 present in the solid fraction of raw wastewater and whether solid fraction should be included in the analysis for sensitive detection of SARS-CoV-2 RNA in wastewater.
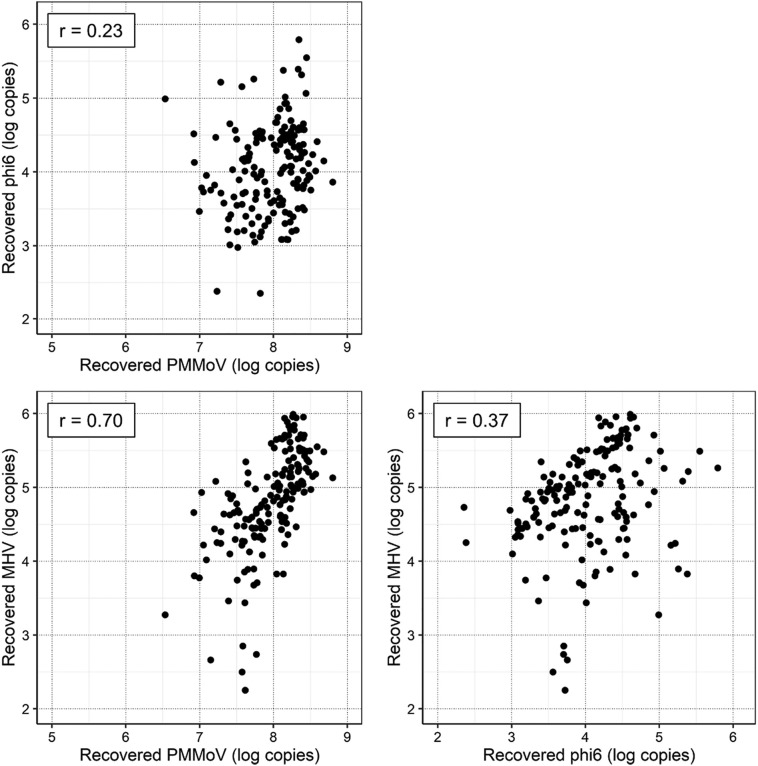
3.3. Correlation of recovery loads of MHV, phi6, and PMMoV
Fig. 4 shows the correlation between the recovery load of each virus. For comparison, the recovery load of viruses was adopted instead of log W to equalize the effect of systematic error. The differences in logW of MHV and phi6 among samples are only affected by those in C conc_WPC, while those of PMMoV are affected by variabilities derived from not only C conc_PMMoV but also C ini_PMMoV among samples.
Fig. 4.
Correlation between the recovery loads of each virus. Spearman's rank correlation coefficient is shown in the upper left of each panel.
A weak positive correlation was observed between the recovery loads of phi6 and PMMoV (Spearman correlation r = 0.23, P < 0.01) and those of phi6 and MHV (r = 0.37, P < 0.01), while a stronger positive correlation was observed between those of MHV and PMMoV (r = 0.70, P < 0.01). This indicates the potential of PMMoV as an indicator for the recovery efficiency of MHV. Although fecal load may differ among samples, there was no substantial variability among the samples with respect to the indigenous PMMoV concentration in this study (9.50 ± 0.30 log copies/L). Therefore, in the present study, the observed concentration of indigenous PMMoV was mainly affected by the recovery efficiency, rather than the variation in fecal load across the wastewater samples.
The advantages of monitoring the observed PMMoV concentrations are its simplicity and practicality. As distinct from MHV and phi6, PMMoV is endogenously present. Also, PMMoV is a single-stranded RNA virus and thus quantifiable along with SARS-CoV-2 without requiring an additional step (e.g., heat denaturation of double-stranded RNA of phi6). However, the whole process recovery efficiency of PMMoV seems to be higher than that of MHV (see Section 3.1). Therefore, the observed PMMoV concentration cannot be directly used for the back-calculation of the SARS-CoV-2 concentration but can be used as a normalization factor. Specifically, the SARS-CoV-2 concentration divided by observed indigenous PMMoV concentration can correct for the differences in whole process efficiency among samples and may help WBE implementers better capture the time-series trend of SARS-CoV-2. Several other studies have also proposed the advantages of co-investigation of fecal viral markers, including PMMoV (Graham et al., 2020; Wolfe et al., 2021) and crAssphage (Wilder et al., 2021).
The use of phi6 as a WPC needs to be further discussed despite their morphological similarities. A recent study showed a significantly lower whole process recovery of phi6 (i.e., 3.9-log lower recovery than HCoV OC43 (Pecson et al., 2021)). Another study showed 1–2-log higher whole process recovery by ultrafiltration compared with MHV (Fernandez-Cassi et al., 2021). The quantification method for double-stranded RNA virus (e.g., phi6) typically includes heat denaturation step, and the efficiency of denaturation depends on the temperature, time, and the ionic strength of the medium (Gendron et al., 2010; Steger et al., 1980). Although 95 °C is one of the most frequently used temperatures for denaturation, higher temperatures have been shown to provide a higher observed concentration of phi6 (Gendron et al., 2010). This implies that the heat denaturation step at 95 °C might not work well especially in case of higher ionic strength of the RNA extract due to the carryover of PEG concentrate. The potential factors responsible for variable recovery efficiency of phi6 compared to other surrogate viruses remain unclear and should be investigated in further studies.
Overall, the positive correlation between the recovery amount of MHV and PMMoV suggested that co-quantification of PMMoV along with SARS-CoV-2 can be used for the validation of the whole process recovery and normalization of obtained SARS-CoV-2 quantification data.
3.4. Implications for PEG precipitation as a primary concentration method for SARS-CoV-2
Table 2 shows the operational parameters of PEG precipitation along with the whole process recovery efficiency of spiked SARS-CoV-2 or enveloped surrogate viruses in previous studies. As a general trend, wastewater samples are centrifuged or filtered to remove large particles. Subsequently, the supernatant is mixed with PEG6000 or PEG8000 and NaCl and then incubated overnight. Some studies have performed this with pH adjustment, shorter incubation time, and/or without particle separation step (Ahmed et al., 2020a; Alexander et al., 2020; Barril et al., 2021; Pecson et al., 2021; Pérez-Cataluña et al., 2021b; Philo et al., 2021; Sapula et al., 2021). The reported whole process recovery considerably varied from 0.001% to 78%, depending on the surrogate viruses, PEG precipitation procedures, and the downstream molecular process (Table 2). The results of this study (MHV, 0.070%–2.6%; phi6, 0.071%–0.51%) were at a lower range compared to previously reported values.
Table 2.
Operational parameters of PEG precipitation along with the whole process recovery efficiency of spiked SARS-CoV-2 or enveloped surrogate viruses in previous studies and the present study.
| Virus | Conc. factor in PEG procedure | Particle separation (centrifugation speed [g], time [min]) | Elution from solid | pH adjustment | Heat treatment | PEG type | PEG conc. (%) | NaCl conc. (M) | Incubation time (h) | RNA extraction | Recovery (%)f | Citation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SARS-CoV-2 | 50 | y (3250,20) | n | 7.5 | n | 6000 | 10 | 0.1g | 1.5 | NA | 57 | (Alexander et al., 2020) |
| SARS-CoV-2 | 200 | y (3500,30) | y (pH 9.5) | 7.1 | n | 8000 | 20 | 0.3 | Overnight | Nucleospin RNA Virus Kit | 52.8 | (Pérez-Cataluña et al., 2021) |
| SARS-CoV-2 | 200 | y (3500,30) | y (pH 9.5) | 7.1 | n | 8000 | 20 | 0.3 | Overnight | Maxwell RSC Pure Food GMO and authentication kit | 44 | (Pérez-Cataluña et al., 2021) |
| SARS-CoV-2 | 200 | n | n | 6.5-7.2 | n | 6000 | 10 | 0.3 | 2 | Direct-zol RNA Miniprep Kit | 7.4-9.4 | (Barril et al., 2021) |
| SARS-CoV-2 | 1000 | y (5000,30) | n | n | n | 6000 | 15 | 0.3 | Overnight | A mix of TRIzol/chloroform and NucleoSpin RNA Virus Kit | 27.5-56.7 | (Sapula et al., 2021) |
| SARS-CoV-2 | 1000 | n | n | n | n | 6000 | 15 | 0.3 | Overnight | A mix of TRIzol/chloroform and NucleoSpin RNA Virus Kit | 8.69-41.1 | (Sapula et al., 2021) |
| Engineered alphavirus | 1000 | y (5000,30) | n | n | n | 6000 | 15 | 0.3 | Overnight | A mix of TRIzol/chloroform and NucleoSpin RNA Virus Kit | 24.4-40.4 | (Sapula et al., 2021) |
| PEDVa | 200 | y (3500,30) | y (pH 9.5) | 7.1 | n | 8000 | 20 | 0.3 | Overnight | Nucleospin RNA Virus Kit | 43.5 | (Pérez-Cataluña et al., 2021) |
| PEDVa | 200 | y (3500,30) | y (pH 9.5) | 7.1 | n | 8000 | 20 | 0.3 | Overnight | Maxwell RSC Pure Food GMO and authentication kit | 27.5 | (Pérez-Cataluña et al., 2021) |
| phi6 | 67 | y (3500,5) | n | n | n | 8000 | 10 | 1 | Overnight | QIAamp Viral RNA Mini Kit | 1.4-3.0 | (Torii et al., 2021) |
| phi6 | 67 | y (3500,5) | n | n | n | 8000 | 10 | 1 | Overnight | TRIzol | 29.8-49.8 | (Torii et al., 2021) |
| phi6 | 27 | y (5000,NA and filtration by 0.2 μm membrane) | n | n | n | 8000 | 10 | 0.4 | Overnight | TRIzol | Approx. 0.05 | (Pecson et al., 2021) |
| phi6 | 27 | y (5000,NA and filtration by 0.2 μm membrane) | n | n | y | 8000 | 10 | 0.4 | Overnight | TRIzol | Approx. 0.001 | (Pecson et al., 2021) |
| phi6 | 59 | y (3500,5) | n | n | n | 8000 | 10 | 1 | Overnight | QIAamp Viral RNA Mini Kit | 0.078 | This study (L.Long) |
| phi6 | 65 | y (3500,5) | n | n | n | 8000 | 10 | 1 | 2 | QIAamp Viral RNA Mini Kit | 0.18 | This study (L.Short) |
| phi6 | 62 | y (4700,30) | n | n | n | 8000 | 10 | 0.4 | 0 | QIAamp Viral RNA Mini Kit | 0.51 | This study (L.0) |
| phi6 | 61 | y (filtration by 0.2 μm membrane) | n | n | n | 6000 | 10 | 0.4 | Overnight | QIAamp Viral RNA Mini Kit | 0.22 | This study (F.Long) |
| phi6 | 35 | n | n | n | y | 6000 | 8 | 0.4 | Overnight | QIAamp Viral RNA Mini Kit | 0.071 | This study (LS.Long) |
| MHV | 63 | y (10,000,20) | y (pH 9.0) | Neutralized | n | 8000 | 10 | 0.3 | 2 | RNeasy PowerMicrobiome Kit | 44 | (Ahmed et al., 2020a) |
| MHV | 220 | y (24,000,15) | n | n | n | 8000 | 8 | 0.2 | Overnight | AllPrep PowerViral DNA/RNA Kit | 2-16 | (Graham et al., 2020) |
| MHV | 59 | y (3500,5) | n | n | n | 8000 | 10 | 1 | Overnight | QIAamp Viral RNA Mini Kit | 0.070 | This study (L.Long) |
| MHV | 65 | y (3500,5) | n | n | n | 8000 | 10 | 1 | 2 | QIAamp Viral RNA Mini Kit | 2.6 | This study (L.Short) |
| MHV | 62 | y (4700,30) | n | n | n | 8000 | 10 | 0.4 | 0 | QIAamp Viral RNA Mini Kit | 1.4 | This study (L.0) |
| MHV | 61 | y (filtration by 0.2 μm membrane) | n | n | n | 6000 | 10 | 0.4 | Overnight | QIAamp Viral RNA Mini Kit | 0.27 | This study (F.Long) |
| MHV | 35 | n | n | n | y | 6000 | 8 | 0.4 | Overnight | QIAamp Viral RNA Mini Kit | 0.23 | This study (LS.Long) |
| HCoV OC43b | 50 | n | n | n | n | 8000 | 14 | 0.2 | Overnight | QIAamp Viral RNA Mini Kit | 3.2 | (Philo et al., 2021) |
| HCoV OC43b | 18 | n | n | n | n | 8000 | 8 | 0.2 | Overnight | QIAamp Viral RNA kit | 4.5 | (Pecson et al., 2021) |
| HCoV OC43b | 27 | y (5000,NA and filtration by 0.2 μm membrane) | n | n | y | 8000 | 10 | 0.4 | Overnight | TRIzol | 65 | (Pecson et al., 2021) |
| HCoV OC43b | 27 | y (5000,NA and filtration by 0.2 μm membrane) | n | n | n | 8000 | 10 | 0.4 | Overnight | TRIzol | 78 | (Pecson et al., 2021) |
| HCoV OC43b | 263 | y (4700, 30) | n | n | y | 8000 | 10 | 0.4 | 0 | Water DNA/RNA Magnetic Bead kit | 1.4 | (Pecson et al., 2021) |
| HCoV OC43b | 263 | y (4700, 30) | n | n | n | 8000 | 10 | 0.4 | 0 | Water DNA/RNA Magnetic Bead kit | 2.2 | (Pecson et al., 2021) |
| HCoV OC43b | 56 | y (4700,45) | n | n | n | 8000 | 8 | 0.2 | Overnight | QIAamp Viral RNA Mini Kit | 36 | (Pecson et al., 2021) |
| HCoV OC43b | NA | y (filtration by 0.22 μm membrane) | n | n | n | 8000 | 12.5 | 0.3 | 2 | QIAamp Viral RNA Mini Kit | 0.51 | (Pecson et al., 2021) |
| HCoV OC43b | 24 | y (5000,10 and filtration by 0.22 μm membrane) | n | n | y | 8000 | 10 | 0.4 | Overnight | QIAamp Viral RNA Mini Kit | 0.85 | (Pecson et al., 2021) |
| HCoV OC43b | 1786 | y (4000,30 and filtration by 0.45 μm membrane) | n | 9.6 | y | 8000 | 10 | 0.5 | Overnight | QIAamp Viral RNA Mini Kit | 0.03 | (Pecson et al., 2021) |
| HCoV OC43b | NA | y (3200,30) | n | n | n | 8000 | 9 | 1 | Overnight | PureLink™ Viral RNA/DNA Mini Kit | 5.7 | (Pecson et al., 2021) |
| BCoVc | 200 | y (7140, 15 and filtration by 0.22 μm membrane) | n | n | n | 8000 | 8 | 0.5 | Overnight | Chemagic™ Prime Viral DNA/RNA 300 Kit H96 | 0.08 | (LaTurner et al., 2021) |
| BCoVc | 500 | y (3500, 15-30) | n | n | n | 8000 | 9 | 1 | Overnight | PureLink™ Viral RNA/DNA Mini Kit | 11 | (Gerrity et al., 2021) |
| TGEVd | 267 | NA | n | n | n | 6000 | 20 | NA | NA | NucliSENS miniMAG | 2.5 | (Chavarria-Miró et al., 2021) |
| VSVe | NA | n | n | n | n | 8000 | 8 | 0.3 | Overnight | RNeasy PowerMicrobiome Kit | 9.3 | (D'Aoust et al., 2021) |
n indicates that the corresponding procedure was not performed, while y indicates that the corresponding procedure was performed. NA indicate that the information was not available in the original literature.
PEDV represents porcine epidemic diarrhea virus (Alphacoronavirus).
HCoV OC43 represents human coronavirus OC43 strain (Betacoronavirus).
TGEV represents transmissible gastroenteritis coronavirus (Alphacoronavirus).
BCoV represents bovine coronavirus (Betacoronavirus).
VSV represents vesicular stomatitis virus (Vesiculovirus).
The mean or range of whole process recovery efficiency in each study was reported. Approx. was placed if the whole process recovery was not reported numerically but reported in the figure.
Not NaCl but MgSO4 was used as salt in the study.
One of the main explanations is the difference in the operational parameters. The present study showed that a difference in the procedure (e.g., incubation time) leads to significantly (up to 1.57 log) different recovery efficiency (Fig. 2). Other studies suggested that omitting the particle separation step may lead to stronger inhibition during RT-qPCR, resulting in lower whole process recovery efficiency (Sapula et al., 2021).
Additionally, the difference in wastewater quality may also lead to inconsistency in the whole process recovery. For example, the whole process recovery efficiency of phi6 by L.Long was 0.078% in this study, while that in a previous study (Torii et al., 2021) ranged 1.4%–3.0%. This is due to the lower molecular process recovery efficiency of MNV (−0.92 log) (Fig. 3) in the present study compared with that in our previous study (−0.02 to 0.07 log) (Torii et al., 2021).
Another potential explanation is an element of bias in the determination of the load of seeded viruses in each study (Kantor et al., 2021), partially due to the selection of RNA extraction kit or method of determining the recovery efficiency. A previous study showed differences in the recovery of some enteric viruses with different RNA extraction kits (Ahmed et al., 2021; Iker et al., 2013). This was also confirmed by our investigation (see Fig. S1); the loads of seeded viruses differed depending on the RNA extraction kit. A review suggested that direct quantification from the viral stock leads to biased results due to the inhibition effect or presence of free RNA (Rusiñol et al., 2020). Note that, in the present study, the positive control for the recovery test was prepared by spiking the viral stock into MilliQ water to achieve the same concentration in the wastewater sample.
Overall, the whole process recovery efficiency of PEG precipitation varied not only due to different operational parameters (such as incubation time and pretreatment) but also due to the differences in wastewater quality and bias resulting from the determination of the seeded load of viruses. Therefore, it is recommended to make the step-by-step procedure consistent to minimize quantification bias.
4. Conclusions
-
•
A total of five PEG precipitation procedures yielded significantly different whole process recovery efficiency of MHV (0.070%–2.6%) and phi6 (0.071%–0.51%). The observed indigenous PMMoV concentration also ranged from 8.2 × 108 to 5.6 × 109 copies/L.
-
•
PEG precipitation with a shorter incubation time (~ 2 h) yielded better recovery of MHV, phi6 and PMMoV and molecular process recovery of MNV, compared with longer incubation time (overnight).
-
•
The recovery load of PMMoV exhibited a positive correlation (r = 0.70) with that of MHV. This indicates that the observed indigenous PMMoV concentration can correct for the differences in whole process recovery efficiency among samples and aid in capturing the time-series trend of SARS-CoV-2 concentration in wastewater.
-
•
The reported whole process recovery by PEG precipitation varied in previous studies (0.001%–78%) and the present study (0.070%–2.6%), owing to the differences in surrogate viruses, operational parameters, water quality, and determination methods of the seeded load of surrogate viruses.
CRediT authorship contribution statement
Shotaro Torii, Wakana Oishi, Yifan Zhu, Ocean Thakali, Bikash Malla, Zaizhi Yu, Bo Zhao, and Chisato Arakawa performed the experiments. Shotaro Torii, Masaaki Kitajima, Akihiko Hata, Masaru Ihara, Shigeru Kyuwa, Daisuke Sano, Eiji Haramoto, and Hiroyuki Katayama designed the study. Shotaro Torii drafted the manuscript. All authors interpreted the results and edited and proofread the manuscript.
Declaration of competing interest
The authors declare no conflicts of interest associated with this manuscript.
Acknowledgment
This work was supported by JSPS KAKENHI Grant Numbers JP20J10268 and JP20H00259, JST J-RAPID Grant Number JPMJJR2001, the JST-Mirai Program Grant Numbers JPMJMI18DB, and JPMJMI18DA, GAP Fund Program of Kyoto University, and the “Startup Research Program for Post-Corona Society” of Academic Strategy Office, School of Engineering, the University of Tokyo. We really appreciate kind cooperation of Japan Society on Water Environment COVID-19 taskforce and thank Enago for the English language review. Graphical abstract was created with Biorender.com.
Editor: Damia Barcelo
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.150722.
Appendix A. Supplementary data
Supplemental information
Supplemental spreadsheet
References
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R., Mueller J.F., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bivins A., Bertsch P.M., Bibby K., Choi P.M., Farkas K., Gyawali P., Hamilton K.A., Haramoto E., Kitajima M., Simpson S.L., Tandukar S., Thomas K.V., Mueller J.F. Surveillance of SARS-CoV-2 RNA in wastewater: methods optimization and quality control are crucial for generating reliable public health information. Curr. Opin. Environ. Sci. Health. 2020;17:82–93. doi: 10.1016/j.coesh.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Kitajima M., Tandukar S., Haramoto E. Recycled water safety: current status of traditional and emerging viral indicators. Curr. Opin. Environ. Sci. Health. 2020;16:62–72. doi: 10.1016/j.coesh.2020.02.009. [DOI] [Google Scholar]
- Ahmed W., Simpson S., Bertsch P., Bibby K., Bivins A., Blackall L., Bofill-Mas S., Bosch A., Brandao J., Choi P., Ciesielski M., Donner E., D’Souza N., Farnleitner A., Gerrity D., Gonzalez R., Griffith J., Gyawali P., Haas C., Hamilton K., Hapuarachchi C., Harwood V., Haque R., Jackson G., Khan S., Khan W., Kitajima M., Korajkic A., Rosa G.La, Layton B., Lipp E., McLellan S., McMinn B., Medema G., Metcalfe S., Meijer W., Mueller J., Murphy H., Naughton C., Noble R., Payyappat S., Petterson S., Pitkanen T., Rajal V., Reyneke B., Roman F., Rose J., Rusinol M., Sadowsky M., Sala-Comorera L., Setoh Y.X., Sherchan S., Sirikanchana K., Smith W., Steele J., Sabburg R., Symonds E., Thai P., Thomas K., Tynan J., Toze S., Thompson J., Whiteley A., Wong J., Sano D., Wuertz S., Xagoraraki I., Zhang Q., Zimmer-Faust A., Shanks O. 2021. Minimizing Errors in RT-PCR Detection and Quantification of SARS-CoV-2 RNA for Wastewater Surveillance. preprints. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M., Rootes C.L., van Vuren P.J., Stewart C.R. Concentration of infectious SARS-CoV-2 by polyethylene glycol precipitation. J. Virol. Methods. 2020;286 doi: 10.1016/j.jviromet.2020.113977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asami T., Katayama H., Torrey J.R., Visvanathan C., Furumai H. Evaluation of virus removal efficiency of coagulation-sedimentation and rapid sand filtration processes in a drinking water treatment plant in Bangkok, Thailand. Water Res. 2016;101:84–94. doi: 10.1016/j.watres.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Atha D.H., Ingham K.C. Mechanism of precipitation of proteins by polyethylene glycols. analysis in terms of excluded volume. J. Biol. Chem. 1981;256:12108–12117. doi: 10.1016/S0021-9258(18)43240-1. [DOI] [PubMed] [Google Scholar]
- Barril P.A., Pianciola L.A., Mazzeo M., Ousset M.J., Jaureguiberry M.V., Alessandrello M., Sánchez G., Oteiza J.M. Evaluation of viral concentration methods for SARS-CoV-2 recovery from wastewaters. Sci. Total Environ. 2021;756 doi: 10.1016/j.scitotenv.2020.144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besselsen D., Wagner A., Loganbill J. Detection of rodent coronaviruses by use of fluorogenic reverse transcriptase-polymerase chain reaction analysis. Comp. Med. 2002;52:111–116. [PubMed] [Google Scholar]
- Canh V.D., Torii S., Furumai H., Katayama H. Application of capsid integrity (RT-)qPCR to assessing occurrence of intact viruses in surface water and tap water in Japan. Water Res. 2021;189 doi: 10.1016/j.watres.2020.116674. [DOI] [PubMed] [Google Scholar]
- Canh V.D., Torii S., Yasui M., Kyuwa S., Katayama H. Capsid integrity RT-qPCR for the selective detection of intact SARS-CoV-2 in wastewater. Sci. Total Environ. 2021;148342 doi: 10.1016/j.scitotenv.2021.148342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2020. CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel. [Google Scholar]
- Chavarria-Miró G., Anfruns-Estrada E., Martínez-Velázquez A., Vázquez-Portero M., Guix S., Paraira M., Galofré B., Sánchez G., Pintó R.M., Bosch A. Time-evolution of SARS-CoV-2 in wastewater during the first pandemic wave of COVID-19 in the metropolitan area of Barcelona. Appl. Environ. Microbiol. 2021;AEM.02750-20 doi: 10.1128/AEM.02750-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chik A.H.S., Glier M.B., Servos M., Mangat C.S., Pang X.-L., Qiu Y., D’Aoust P.M., Burnet J.-B., Delatolla R., Dorner S., Geng Q., Giesy J.P., McKay R.M., Mulvey M.R., Prystajecky N., Srikanthan N., Xie Y., Conant B., Hrudey S.E. Comparison of approaches to quantify SARS-CoV-2 in wastewater using RT-qPCR: results and implications from a collaborative inter-laboratory study in Canada. J. Environ. Sci. 2021;107:218–229. doi: 10.1016/j.jes.2021.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aoust P.M., Mercier E., Montpetit D., Jia J.-J., Alexandrov I., Neault N., Baig A.T., Mayne J., Zhang X., Alain T., Langlois M.-A., Servos M.R., MacKenzie M., Figeys D., MacKenzie A.E., Graber T.E., Delatolla R. Quantitative analysis of SARS-CoV-2 RNA from wastewater solids in communities with low COVID-19 incidence and prevalence. Water Res. 2021;188 doi: 10.1016/j.watres.2020.116560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva A.K., Jean-Claude L.S., Sylvain P., Monique P., Menachem E., S L.G.F. Evaluation of removal of noroviruses during wastewater treatment, using real-time reverse transcription-PCR: different behaviors of Genogroups I and II. Appl. Environ. Microbiol. 2007;73:7891–7897. doi: 10.1128/AEM.01428-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Cassi X., Scheidegger A., Bänziger C., Cariti F., Corzon A.T., Ganesanandamoorthy P., Lemaitre J.C., Ort C., Julian T.R., Kohn T. Wastewater monitoring outperforms case numbers as a tool to track COVID-19 incidence dynamics when test positivity rates are high. Water Res. 2021;117252 doi: 10.1016/j.watres.2021.117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron L., Verreault D., Veillette M., Moineau S., Duchaine C. Evaluation of filters for the sampling and quantification of RNA phage aerosols. Aerosol Sci. Technol. 2010;44:893–901. doi: 10.1080/02786826.2010.501351. [DOI] [Google Scholar]
- Gerrity D., Papp K., Stoker M., Sims A., Frehner W. Early-pandemic wastewater surveillance of SARS-CoV-2 in southern Nevada: methodology, occurrence, and incidence/prevalence considerations. Water Res. X. 2021;10 doi: 10.1016/j.wroa.2020.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham K.E., Loeb S.K., Wolfe M.K., Catoe D., Sinnott-Armstrong N., Kim S., Yamahara K.M., Sassoubre L.M., Mendoza Grijalva L.M., Roldan-Hernandez L., Li L., Langenfeld K., Wigginton K.R., Boehm A.B. SARS-CoV-2 RNA in wastewater settled solids is associated with COVID-19 cases in a large urban sewershed. Environ. Sci. Technol. 2020 doi: 10.1021/acs.est.0c06191. [DOI] [PubMed] [Google Scholar]
- Haramoto E., Kitajima M., Kishida N., Konno Y., Katayama H., Asami M., Akiba M. Occurrence of pepper mild mottle virus in drinking water sources in Japan. Appl. Environ. Microbiol. 2013;79:7413–7418. doi: 10.1128/AEM.02354-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Kitajima M., Hata A., Torrey J.R., Masago Y., Sano D., Katayama H. A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 2018;135:168–186. doi: 10.1016/j.watres.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Hara-Yamamura H., Meuchi Y., Imai S., Honda R. Detection of SARS-CoV-2 in wastewater in Japan during a COVID-19 outbreak. Sci. Total Environ. 2021;758 doi: 10.1016/j.scitotenv.2020.143578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iker B.C., Bright K.R., Pepper I.L., Gerba C.P., Kitajima M. Evaluation of commercial kits for the extraction and purification of viral nucleic acids from environmental and fecal samples. J. Virol. Methods. 2013;191:24–30. doi: 10.1016/j.jviromet.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Kantor R.S., Nelson K.L., Greenwald H.D., Kennedy L.C. Challenges in measuring the recovery of SARS-CoV-2 from wastewater. Environ. Sci. Technol. 2021;55:3514–3519. doi: 10.1021/acs.est.0c08210. [DOI] [PubMed] [Google Scholar]
- King A.M.Q., Adams M.J., Carsten E.B., Lefkowitz E.J. Elsevier Inc; 2012. Virus Taxonomy: Classification and Nomenclature of Viruses. Ninth Report of the International Committee on Taxonomy of Viruses. [DOI] [Google Scholar]
- Kitajima M., Tohya Y., Matsubara K., Haramoto E., Utagawa E., Katayama H., Ohgaki S. Use of murine norovirus as a novel surrogate to evaluate resistance of human norovirus to free chlorine disinfection in drinking water supply system. Environ. Eng. Res. 2008;45:361–370. doi: 10.11532/proes1992.45.361. [DOI] [Google Scholar]
- Kitajima M., Sassi H.P., Torrey J.R. Pepper mild mottle virus as a water quality indicator. NPJ Clean Water. 2018;1:19. doi: 10.1038/s41545-018-0019-5. [DOI] [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;139076 doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Sadamasu K., Muramatsu M., Yoshida H. Efficient detection of SARS-CoV-2 RNA in the solid fraction of wastewater. Sci. Total Environ. 2021;763 doi: 10.1016/j.scitotenv.2020.144587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746 doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaTurner Z.W., Zong D.M., Kalvapalle P., Gamas K.R., Terwilliger A., Crosby T., Ali P., Avadhanula V., Santos H.H., Weesner K., Hopkins L., Piedra P.A., Maresso A.W., Stadler L.B. Evaluating recovery, cost, and throughput of different concentration methods for SARS-CoV-2 wastewater-based epidemiology. Water Res. 2021;197 doi: 10.1016/j.watres.2021.117043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G.D., Metcalf T.G. Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis a virus and human rotavirus, from oyster, water, and sediment samples. Appl. Environ. Microbiol. 1988;54:1983–1988. doi: 10.1128/aem.54.8.1983-1988.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markt R., Mayr M., Peer E., Wagner A.O., Lackner N., Insam H. medRxiv; 2021. Detection and Stability of SARS-CoV-2 Fragments in Wastewater: Impact of Storage Temperature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecson B.M., Darby E., Haas C.N., Amha Y.M., Bartolo M., Danielson R., Dearborn Y., Di Giovanni G., Ferguson C., Fevig S., Gaddis E., Gray D., Lukasik G., Mull B., Olivas L., Olivieri A., Qu Y., Consortium S.-C.-2.I. Reproducibility and sensitivity of 36 methods to quantify the SARS-CoV-2 genetic signal in raw wastewater: findings from an interlaboratory methods evaluation in the U.S. Environ. Sci. Water Res. Technol. 2021;7:504–520. doi: 10.1039/D0EW00946F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Cataluña A., Cuevas-Ferrando E., Randazzo W., Falcó I., Allende A., Sánchez G. Comparing analytical methods to detect SARS-CoV-2 in wastewater. Sci. Total Environ. 2021;758 doi: 10.1016/j.scitotenv.2020.143870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philo S.E., Keim E.K., Swanstrom R., Ong A.Q.W., Burnor E.A., Kossik A.L., Harrison J.C., Demeke B.A., Zhou N.A., Beck N.K., Shirai J.H., Meschke J.S. A comparison of SARS-CoV-2 wastewater concentration methods for environmental surveillance. Sci. Total Environ. 2021;760 doi: 10.1016/j.scitotenv.2020.144215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . 2019. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Rusiñol M., Martínez-Puchol S., Forés E., Itarte M., Girones R., Bofill-Mas S. Concentration methods for the quantification of coronavirus and other potentially pandemic enveloped virus from wastewater. Curr. Opin. Environ. Sci. Health. 2020 doi: 10.1016/j.coesh.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapula S.A., Whittall J.J., Pandopulos A.J., Gerber C., Venter H. An optimized and robust PEG precipitation method for detection of SARS-CoV-2 in wastewater. Sci. Total Environ. 2021;785 doi: 10.1016/j.scitotenv.2021.147270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz B.W., Kitajima M., Campillo M.E., Gerba C.P., Pepper I.L. Virus reduction during advanced bardenpho and conventional wastewater treatment processes. Environ. Sci. Technol. 2016;50:9524–9532. doi: 10.1021/acs.est.6b01384. [DOI] [PubMed] [Google Scholar]
- Steger G., Müller H., Riesner D. Helix-coil transitions in double-stranded viral RNA. Fine resolution melting and ionic strength dependence. Biochim. Biophys. Acta, Nucleic Acids Protein Synth. 1980;606:274–284. doi: 10.1016/0005-2787(80)90037-4. [DOI] [PubMed] [Google Scholar]
- Symonds E.M., Nguyen K.H., Harwood V.J., Breitbart M. Pepper mild mottle virus: a plant pathogen with a greater purpose in (waste)water treatment development and public health management. Water Res. 2018;144:1–12. doi: 10.1016/j.watres.2018.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandukar S., Sherchan S.P., Haramoto E. Applicability of crAssphage, pepper mild mottle virus, and tobacco mosaic virus as indicators of reduction of enteric viruses during wastewater treatment. Sci. Rep. 2020;10:3616. doi: 10.1038/s41598-020-60547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii S., Furumai H., Katayama H. Applicability of polyethylene glycol precipitation followed by acid guanidinium thiocyanate-phenol-chloroform extraction for the detection of SARS-CoV-2 RNA from municipal wastewater. Sci. Total Environ. 2021;756 doi: 10.1016/j.scitotenv.2020.143067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidhaas J., Aanderud Z.T., Roper D.K., VanDerslice J., Gaddis E.B., Ostermiller J., Hoffman K., Jamal R., Heck P., Zhang Y., Torgersen K., Laan J.Vander, LaCross N. Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. Sci. Total Environ. 2021;775 doi: 10.1016/j.scitotenv.2021.145790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney O.N., Kennedy L.C., Fan V.B., Hinkle A., Kantor R., Greenwald H., Crits-Christoph A., Al-Shayeb B., Chaplin M., Maurer A.C., Tjian R., Nelson K.L. Sewage, salt, silica, and SARS-CoV-2 (4S): an economical kit-free method for direct capture of SARS-CoV-2 RNA from wastewater. Environ. Sci. Technol. 2021;55:4880–4888. doi: 10.1021/acs.est.0c08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder M.L., Middleton F., Larsen D.A., Du Q., Fenty A., Zeng T., Insaf T., Kilaru P., Collins M., Kmush B., Green H.C. Co-quantification of crAssphage increases confidence in wastewater-based epidemiology for SARS-CoV-2 in low prevalence areas. Water Res. X. 2021;11 doi: 10.1016/j.wroa.2021.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe M.K., Archana A., Catoe D., Coffman M.M., Dorevich S., Graham K.E., Kim S., Grijalva L.M., Roldan-Hernandez L., Silverman A.I., Sinnott-Armstrong N., Vugia D.J., Yu A.T., Zambrana W., Wigginton K.R., Boehm A.B. Scaling of SARS-CoV-2 RNA in settled solids from multiple wastewater treatment plants to compare incidence rates of laboratory-confirmed COVID-19 in their sewersheds. Environ. Sci. Technol. Lett. 2021 doi: 10.1021/acs.estlett.1c00184. [DOI] [PubMed] [Google Scholar]
- Wu F., Zhang J., Xiao A., Gu X., Lee W.L., Armas F., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N., Duvallet C., Poyet M., Moniz K., Washburne A.D., Erickson T.B., Chai P.R., Thompson J., Alm E.J. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems. 2020;5 doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Waldman P., Ferrier-Rembert A., Frenois-Veyrat G., Mouchel J.M., Boni M., Maday Y., Marechal V., Moulin L. Several forms of SARS-CoV-2 RNA can be detected in wastewaters: implication for wastewater-based epidemiology and risk assessment. Water Res. 2021;117183 doi: 10.1016/j.watres.2021.117183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Breitbart M., Lee W.H., Run J.-Q., Wei C.L., Soh S.W.L., Hibberd M.L., Liu E.T., Rohwer F., Ruan Y. RNA viral Community in Human Feces: prevalence of plant pathogenic viruses. PLoS Biol. 2006;4 doi: 10.1371/journal.pbio.0040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Oishi W., Maruo C., Saito M., Chen R., Kitajima M., Sano D. Early warning of COVID-19 via wastewater-based epidemiology: potential and bottlenecks. Sci. Total Environ. 2021;767 doi: 10.1016/j.scitotenv.2021.145124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental information
Supplemental spreadsheet