Abstract
Long non-coding RNAs (lncRNAs) refer to a class of RNA molecules that are more than 200 nucleotides in length and usually lack protein-coding capacity. LncRNAs play important roles in regulating gene expression as well as many aspects of normal physiological processes. Dysregulations of lncRNA expressions and functions are considered to be critically involved in the development and progression of many diseases especially cancer. The lncRNA research in the field of cancer biology over the past decade reveals that a large number of lncRNAs are dysregulated in various types of cancer and that dysregulated lncRNAs may play important roles in cancer initiation, metastasis and therapeutic responses. Metal carcinogens and other common environmental carcinogens such as polycyclic aromatic hydrocarbons, fine particular matters, cigarette smoke, ultraviolet and ionizing radiation are important cancer etiology factors. However, the mechanisms of how metal carcinogens and other common environmental carcinogen exposures initiate cancer and promote cancer progression remain largely unknown. Accumulating evidence show that exposure to metal carcinogens and other common environmental carcinogens dysregulate lncRNA expression in various model systems, which may offer novel mechanistic insights for environmental carcinogenesis. This review will first provide a brief introduction about lncRNA biology and the mechanisms of lncRNA functions, followed by summarizing and discussing recent studies about lncRNA dysregulation by metal carcinogen and other common environment carcinogen exposures and the potential roles of dysregulated lncRNAs in environmental carcinogenesis. A perspective for future studies in this emerging and important field is also presented.
Keywords: Long non-coding RNAs, metals, arsenic, cadmium, chromium, nickel, environmental carcinogens, metal carcinogenesis, environmental carcinogenesis, epigenetics
1. Introduction
Long non-coding RNAs (lncRNAs) represent a class of RNA molecules transcribed from the genome with a length of ≥ 200 nucleotides, which usually have little or no protein-coding capacity and are involved in the regulation of protein-coding genes (PCGs) or other non-coding RNA (ncRNA) family members [1–3]. Recent advances in the large-scale genomic and transcriptomic sequencing technology and the completion of Encyclopedia of DNA Elements (ENCODE) Projects have made much headway in the identification of huge amounts of ncRNAs especially the lncRNAs [4–6]. Currently, the updated dataset in the lncRNA database LNCipedia 5 has released 56,946 lncRNA genes and 127,802 transcripts [7], The lncRNA database NONCODE has recently updated the number of human lncRNA genes to 96,308 and transcripts to 172,216, respectively [8], Generally, lncRNAs share many characteristics with messenger RNAs (mRNAs). They are usually transcribed by RNA polymerase II, processed with 5′ cap structure and 3′-end polyadenylation polyadenylation followed by RNA splicing and editing to generate isoform transcripts. The genomic loci encoding lncRNAs are putatively intronic, intergenic or overlapped with protein-coding genes in either sense or antisense orientation, which can affect the downstream target gene expression through cis- or trans-regulatory mechanisms [9, 10]. Moreover, more and more studies have revealed that lncRNAs can function as versatile gene expression regulators at the transcriptional, posttranscriptional, translational or posttranslational levels [11–13]. Accumulating evidence has also shown that lncRNAs are essential in various physiological processes, including cell growth and division, differentiation and development, homeostasis, stem cell pluripotency, X-chromosome inactivation (XCI) and genomic imprinting [14–16]. Importantly, aberrant expressions of lncRNAs has been observed extensively during the development of human diseases especially cancers and lncRNA dysregulations are increasingly recognized to play critical roles in cancer development and progression [17–21].
Cancer is the leading cause of human deaths around the world including the United States, with 1,806,590 new cases and 606,520 deaths that are projected to occur in 2020 [22], indicating that cancer remains the most important challenge for global health. Therefore, it is an urgent need to comprehensive understand the mechanism of how cancer develops and progresses. Among many factors, metal carcinogens and other environmental chemical carcinogens are considered as important cancer etiology factors playing critical roles in cancer development and progression [23–25]. While it is generally accepted that the genotoxic effects play important roles in the carcinogenicity of many chemical carcinogens; however, non-genotoxic effects are reported to play critical roles in the carcinogenicity of metal carcinogens such as arsenic, cadmium and nickel [23, 26, 27]. Over recent years, studies have shown that exposure to metal carcinogens or other environmental chemical carcinogens dysregulates lncRNA expressions. Given the important roles of lncRNA in regulating gene expression and many biological processes, it is envisioned that dysregulations of lncRNAs could be critically involved in metal carcinogens and other environmental chemical carcinogen exposure-induced carcinogenesis. The goals of this review are to discuss recent exciting findings showing lncRNA dysregulations resulting from exposures to metal carcinogens and other environmental chemical carcinogens and the potential contributions of lncRNA dysregulations to environmental carcinogenesis. The other carcinogens discussed in this review include cigarette smoke extracts (CSEs) and fine particular matters (PM2.5), polycyclic aromatic hydrocarbons (PAHs) and their metabolites, ultraviolet (UV) and ionizing radiation (IR). The CSEs, PM2.5, PAHs are common chemical carcinogens and usually co-exist with metal carcinogens. UV and IR are common physical carcinogens and they are included in this review to show that lncRNAs can be dysregulated not only by exposure to metal carcinogens and metal carcinogen-associated chemical carcinogens but also by exposure to physical carcinogens as well. Perspectives for further studies in the field are also presented.
2. Mechanisms of lncRNA functions
Increasing evidence shows that lncRNAs may exert their important functions by regulating gene expressions at transcriptional and posttranscriptional levels. The detailed mechanisms of lncRNA regulation of gene expression are the subjects of recent excellent reviews [1, 12, 28–30] and are beyond the scope of this review. Here we present brief summaries on a variety of mechanisms of gene expression regulation by lncRNAs.
Wang and Chang discussed the molecular mechanism of lncRNA functions and indicated that lncRNAs execute four archetypes of molecular functions [28]. Briefly, Wang and Chang described that lncRNAs act as (i) signals in response to various stimuli or combinatorial actions of transcription factors (TFs); (ii) guides to recruit histone-modifying enzymes or chromatin modifiers to the positions of target genes either in cis or in trans action; (iii) decoys or sponges to titrate either TFs or other DNA-binding proteins away from chromatins, or preclude access of protein regulators to DNA targets; (iv) scaffolds to recruit protein partners together in order to form functional ribonucleoprotein (RNP) complexes [28].
While lncRNAs and mRNAs share some common features, lncRNAs also have some unique features that distinguish lncRNAs from mRNAs. One of these features is lncRNA’s nuclear localization and their existences in other specific cellular compartments. It has been increasingly recognized that lncRNA’s biological functions are closely associated with their unique subcellular localization patterns [29], How lncRNA localizations are linked to their important functions has been discussed in details elsewhere [12, 29].
More recently, the mechanism of lncRNA functions have also been discussed in details based on their interactions with DNA, RNA or proteins [1, 30], The mechanisms of lncRNA functions are more likely to be determined by which specific molecular complexes or partnerships are formed together with interacting DNA, RNA and/or proteins. By interaction with DNA directly or indirectly, lncRNAs can change gene expression at transcriptional levels through modulating chromatin structures. The interactions of lncRNAs with mRNAs significantly affect mRNA splicing, stability and translation, regulating gene expression at posttranscriptional and translational levels. LncRNAs also interact with microRNAs (miRNAs) functioning as miRNA sponges to sequester miRNAs from binding their endogenous mRNA targets. LncRNA interacting with proteins has significant impacts on proteins’ stability and localization, thus changing cellular signaling events and ultimately affecting gene expression [1, 30], Strikingly, a recent study reported for the first time that a lncRNA directly interacts with a phospholipid second messenger phosphatidylinositol-3,4,5-trisphosphate [PtdIns(3,4,5)P3 or PIP3] to facilitate Akt activation promoting oncogenic signaling [31], The mechanisms of lncRNA functions discussed above are briefly summarized in Figure 1.
Figure 1.
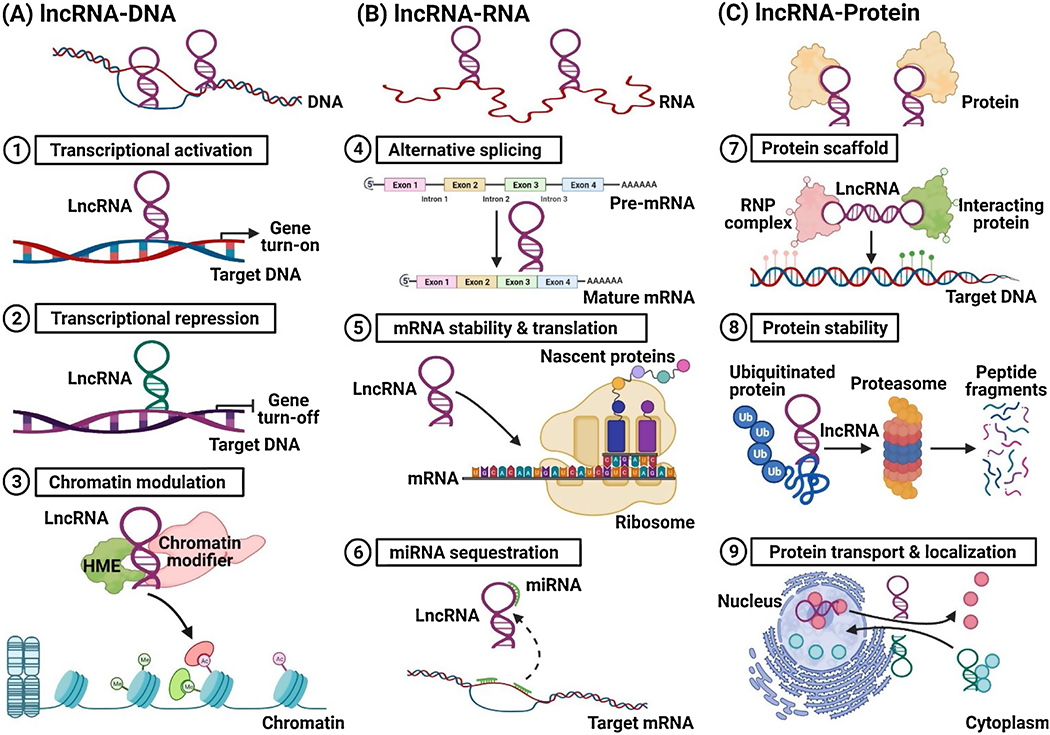
A briefly summary on the mechanisms of lncRNA functions. (A) LncRNAs interact with DNA or chromatin. LncRNAs can bind to DNAs directly or indirectly, regulating target genes at its transcription level. They also can recruit specific histone-modifying enzymes (HMEs) or chromatin modification complexes to different chromatin regions, activating or silencing gene expressions. (B) LncRNAs interact with mRNA or miRNAs. LncRNAs can modulate mRNA maturation by interacting with specific RNA-binding protein (RBP) components in spliceosomes. They also affect target mRNA stability and translation through modulating its post-transcriptional level. Moreover, they can function as sponges or decoys to sequester miRNA biofunctions. (C) LncRNAs interact with multiple proteins. LncRNAs can serve as scaffolds for assembly of ribonucleoprotein (RNP) complexes interacting with other protein partners. In addition, they can enhance protein stability or participate in the regulation of protein turnover. Furthermore, they can facilitate nuclear protein transport to various subcellar location, or vice versa.
3. LncRNA dysregulations in environmental carcinogenesis
3.1. Metal carcinogens-caused lncRNA dysregulations
Due to their wide spread use in industrial and consumer products, toxic metals are among the most common and potent environmental pollutants and carcinogens. In particular, arsenic, cadmium, hexavalent chromium [(Cr(VI))] and nickel are classified as group I carcinogens by the International agency for Research on Cancer [32], representing an important group cancer etiology factors. Chronic exposure to these metal carcinogens causes lung cancer and other types of cancer in humans and experimental animals. However, the mechanisms of metal carcinogenesis remain largely unknown. Recent studies showed that metal carcinogen exposure causes lncRNA dysregulations; which may play important roles in metal carcinogenesis.
3.1.1. Arsenic
MALAT1 (Metastasis-associated lung adenocarcinoma transcript 1), also known as NEAT2 (Nuclear-enriched transcript 2), locates on chromosome 11q13.1 and is one of the well-characterized lncRNA. High MALAT1 expression has been shown to be highly correlate with tumor stage and metastasis of non-small cell lung cancer (NSCLC) [33], Luo et al reported that MALAT1 expression levels are increased in the sera of people exposed to arsenite as well as in that of hepatocellular carcinomas (HCC) patients [34], The expression level of MALAT1 was highly correlated with the clinicpathological characteristics of HCC. It was determined that hypoxia-inducible factor 2α (HIF2α) and MALAT1 expression levels are up-regulated in HCC tissues and arsenite-transformed human hepatic epithelial (L-02) cells. Functionally, the up-regulated MALAT1 and HIF-2α promoted the invasive capability of HCC-LM3 cells and arsenite-transformed L-02 cells. Mechanistically, it was found that arsenite-induced MALAT1 disassociates the von Hippel-Lindau (VHL) protein from HIF-2α decreasing VHL-mediated HIF-2α ubiquitination and causing HIF-2α accumulation. Interestingly, it was further determined that HIF-2α transcriptionally regulates the expression of MALAT1, thus forming a positive feedback loop to up-regulate the expression of MALAT1 and HIF-2α in arsenite-exposed cells. Given the important roles of MALAT1 and HIF-2α in cancer, these findings provide evidence suggesting that lncRNA MALAT1 up-regulation may contribute to arsenic exposure-induced liver carcinogenesis.
In a similar study from this same group, it was found that arsenite exposure increases glycolysis in human hepatic epithelial (L-02) cells [35], In addition to the up-regulation of HIF-2α as discussed above, arsenite exposure also up-regulates the expression HIF-1α through the lncRNA MALAT1 in a similar mechanism: MALAT1 enhances the disassociation of the tumor suppressor VHL from HIF-1α and reduces VHL-mediated HIF-1α ubiquitination increasing HIF-1α accumulation. Moreover, it was further determined that arsenite exposure increases glycolysis by MALAT1-mediated stabilization of HIF-1α, but not HIF-2α [35], It is now well accepted that increased-glycolysis plays a critical role in cancer initiation and progression [36–38]. These findings provide additional evidence supporting an important role of MALAT1 up-regulation in arsenic carcinogenesis.
Interestingly, it was recently reported by the same group that MALAT1 levels are increased in exosomes derived from arsenite-treated L-02 cells [39], Moreover, the MALAT1 in exosomes from arsenite-treated L-02 cells could be transferred into the co-cultured-hepatic stellate cells (HSCs) causing activation of the HSCs, as evidenced by the fact that downregulation of MALAT1 reduced the MALAT1 levels in exosomes derived from arsenite-treated L-02 cells and inhibited the activation of LX-2 cells. Furthermore, it was found that the expression level of MALAT1 is also up-regulated during the progression of mouse liver fibrosis induced by arsenite exposure. These findings provide evidence showing that arsenite exposure not only up-regulates MALAT1 expression levels in cultured cells but also in mice, implying an important role of MALAT1 in arsenic carcinogenicity and other toxicity.
Some recent studies showed that arsenite exposure also up-regulates the expression of several lncRNAs such as HOTAIR, LincRNA-p21, and H19 in cell culture and mouse models [40, 41], However, the significance of these up-regulated lncRNAs in arsenic carcinogenesis are currently unknown.
3.1.2. Cadmium
Interestingly, it was reported that the expression levels of MALAT1 are also up-regulated in cadmium-transformed human bronchial epithelial 16HBE cells and in the lungs of cadmium-exposed rats [42]. Moreover, the blood levels of MALAT1 are positively correlated with blood/urinary cadmium concentrations in cadmium-exposed rats. In addition, this same group also reported that the expression levels of the lncRNA ENST00000414355 are also significantly increased in cadmium-transformed 16HBE cells and in the lung of cadmium-exposed rats in a dose-dependent manner [43]. Similarly, a significant positive correlation was observed between blood ENST00000414355 levels and the urinary/blood cadmium concentrations in cadmium-exposed rats. Furthermore, a recent study showed that the expression levels of another lncRNA ENST00000446135 are also significantly increased in cadmium-transformed 16HBE cells and in the lung of cadmium-exposed rats in a dose-dependent manner [44]. The blood levels of ENST00000446135 are also significantly positively correlated with the urinary/blood cadmium concentrations in cadmium-exposed rats. While these studies showed dysregulations of lncRNAs in cadmium-transformed cells and cadmium-exposed rat lung tissues, it remains to be determined whether these dysregulated lncRNAs play important roles in cadmium carcinogenesis. However, the findings from these studies suggest that the dysregulated lncRNAs may be used to monitor cadmium exposure.
3.1.3. Hexavalent chromium
Much less studies have been done to determine the effect of hexavalent chromium [Cr(VI)] exposure on lncRNA expression. Recently. Hu et al examined the effect of acute Cr(VI) exposure on lncRNA expression in cultured human bronchial epithelial 16HBE cells using the lncRNA microarray analysis [45]. It was found that a 24 h treatment with 10 μM of K2Cr2O7 caused 1868 lncRNAs to be significantly up-regulated and 2203 lncRNAs to be significantly down-regulated in 16HBE cells. Further bioinformatics analysis suggests that the differentially-expressed LncRNAs resulting from acute Cr(VI) treatment are associated with immune response, cell cycle, DNA damage and repair, etc. Whether chronic lower dose of Cr(VI) exposure dysregulates lncRNA expression similarly and whether dysregulated lncRNAs contribute significantly to Cr(VI) carcinogenesis are currently unknown.
3.1.4. Nickel
Similarly, fewer studies have been done to determine the effect of nickel exposure on lncRNA expression. However, a recent study by Zhou et al. showed that down-regulation of the lncRNA maternally expressed gene 3 (MEG3) contributes significantly to nickel-induced malignant transformation of human bronchial epithelial cells [46], MEG3 locates on chromosome 14q32 with reported tumor suppressive and tumor promoting effects [47, 48]. Importantly, MEG3 downregulation is a primary feature in multiple cancer tissues including lung cancers. Zhou et al first found that nickel exposure significantly down-regulates MEG3 expression levels in two types of human bronchial epithelial cells (BEAS-2B and BEP2D) [46], It was further determined that stably overexpressing MEG3 in BEAS-2B cells significantly reduces chronic nickel exposure-induced cell transformation. Mechanistically, nickel exposure down-regulating MEG3 expression was due to nickel-induced MEG3 promoter hypermethylation via increasing DNMT3b expression. Moreover, MEG3 down-regulation led to c-Jun-mediated PHLPP1 transcriptional inhibition and HIF-1α protein translation upregulation, which in turn enhances nickel-induced cell malignant transformation. Further mechanistic studies revealed that HIF-1α protein translation upregulation is attributed to the activation of the Akt/p70S6K/S6 axis resultant from nickel-caused PHLPP1 inhibition. This study provided the first the first evidence showing that a lncRNA dysregulation could play an important role in metal carcinogen-induced cell malignant transformation. Future further studies on the role of dysregulated lncRNAs in metal carcinogenesis are warranted.
The lncRNAs dysregulated by metal carcinogen exposures and their roles in lung cancer and other types of cancers and the involved potential mechanisms are summarized in Table 1.
Table 1:
LncRNAs dysregulated by metal carcinogen exposures.
| Carcinogens | LncRNAs | Cancers | Models | Upstream genes | Downstream genes | Phenotype | References |
|---|---|---|---|---|---|---|---|
| Arsenic | H19 (↑) | Lung cancer | Mice, human blood | – | let7a, c-Myc, TGF-α1 | Promote M2 polarization of macrophages and myofibroblast differentiation | [41] |
| HOTAIR (↑) | Lung cancer | Human blood and urine | – | – | – | [40] | |
| LincRNA-p21 (↑) | Lung cancer | Human blood and urine | – | – | – | [40] | |
| MALAT1 (↑) | HCC | Human l-02, HCC cell lines, mice, human blood and HCC | HIF-2α | HIF-2α | Promote invasiveness and metastasis in arsenite-transformed cells, induce inflammatory responses | [34] | |
| MALAT1 (↑) | HCC | Human l-02 cells | – | HIF-1α, HK-2, Eno-1, Glut-4 | Promote glycolysis in arsenite-transformed cells | [35] | |
| MALAT1 (↑) | HCC | Human l-02 and LX-2 cells, mice, human blood | – | miR-26b, COL1A2 | Promote HSC activation in liver fibrosis | [39] | |
| Cadmium | ENST00000414355 (↑) | Lung cancer | Human 16HBE cells, rats, human blood and urine | – | DNA-damage related genes (ATM, ATR and ATRIP), DNA-repair related genes (DDB1, DDB2, OGG1, ERCC1, MSH2, RAD50, XRCC1 and BARD1) | Induce the growth in DNA-damaged cells | [43] |
| ENST00000446135 (↑) | Lung cancer | Human 16HBE cells, rats, human blood and urine | – | DNA-damage related genes (ATM, ATR and ATRIP), DNA-repair related genes (DDB1, DDB2, OGG1, ERCC1, MSH2, RAD50, XRCC1 and BARD1) | Induce the growth in DNA-damaged cells | [44] | |
| MALAT1 (↑) | Lung cancer | Human 16HBE cells, rats, human blood and urine | – | FOXC2, STAT, BAX, EGFR, TGF-β1, BCL-2 | Promote cadmium-induced cell apoptosis, migration and invasion | [42] | |
| Chromium (VI) | RP11–388M20.9 (↑) | Lung cancer | Human 16HBE cells | – | Involved in Cr(VI)-induced DNA damage | [45] | |
| Nickel | MEG3 (↓) | Lung cancer | Human BEAS-2B and BEP2D cells, human lung SCC | DNMT3b | c-Jun, PHLPP1, Akt/p70S6K/S6, HIF-1α | Inhibit nickel-induced lung tumorigenesis | [46] |
(↑) Upregulation; (↓) Downregulation after exposure of environmental carcinogens; HCC, Hepatocellular carcinoma; HSC, Hepatic stellate cell; PM, Particulate matter, SCC, Squamous cell carcinoma.
3.2. Cigarette smoke extracts and fine particular matters-caused lncRNA dysregulations
Lung cancer has been one of the major leading cause of all cancer deaths worldwide including the United States, with 228,820 new cases and 135,720 deaths that are projected to occur in 2020 [22, 49], Importantly, cigarette smoke extracts (CSEs) have been shown as one of the most common carcinogens and the strongest risk factors of lung cancer [50–52], Fine particulate matters with an aerodynamic diameter of ≤ 2.5 μm (PM2.5) are another group of common and potent toxic air pollutants [53], PM2.5 particular matters are usually produced by fuel combustion or vehicle exhaust emissions, and the majority of PM2.5 constituents encompass a complex mixture of pollutants including organic and inorganic compounds, metals, sulfate, nitrate, ammonium and other ions [54, 55]. Due to their small sizes, PM2.5 particular matters can easily penetrate deeply into human respiratory tract, thus becoming another important risk factor of lung cancer [56]. However, the mechanisms of how CSEs and PM2.5 particular matters exposure causes lung cancer has not been well understood. Recent studies showed that exposure to CSEs or PM2.5 particular matters dysregulate lncRNA expressions, which may play important roles in their lung carcinogenic effects.
3.2.1. Cigarette smoke extracts (CSEs)
CCAT1 (Colon cancer-associated transcript 1), also called CARLO5 (Cancer-associated region long noncoding RNA 5) locates on chromosome 8q24.21 within the MYC enhancer region and has a regulatory role in cell cycle and tumor development in colorectal cancer (CRC) cells [57, 58], Studies have shown that CCAT1 is elevated in different types of cancer and CCAT1 overexpression has a positive correlation with tumor progression and metastasis in cancer patients [59], It was reported that high levels of CCAT1 were found in the sera of heavy smokers when compared to non-smokers [60], suggesting that CCAT1 may contribute to cigarette smoke-caused lung cancer. Indeed, Lu et al. found that CCAT1 levels are up-regulated in CSE-transformed human bronchial epithelial HBE cells and knockdown CCAT1 in CSE-transformed HBE cells reduced their transformed phenotypes [60], Together, the findings from these studies imply that the lncRNA CCAT1 may be involved in cigarette smoke-caused lung cancer.
HOTAIR (Hox transcript antisense intergenic RNA) is an intergenic lncRNA located on chromosome 12q13.13 and transcribed between HOXC11 and HOXC12 [61], HOTAIR overexpression has been shown to promote tumorigenesis and metastasis in several cancers by direct interacting and guiding polycomb repression complex 2 (PRC2) to downstream target genes associated with chromatin modulation [62], Specifically, HOTAIR can function as a scaffold for the assembly of distinct histone modification complexes. For example, the 5’ domain of HOTAIR can directly bind to histone methyltransferase, enhancer of zeste homolog 2 (EZH2), to mediate EZH2-induced Histone 3 lysine 27 trimethylation (H3K27me3), whereas its 3’ domain can interact with lysine-specific demethylase 1 (LSD1)/CoREST/REST repressor complex to mediate LSD1-dependent Histone 3 lysine 4 (H3K4) demethylation [63], indicating that HOTAIR have a pivotal role in regulating cancer epigenome. HOTAIR has been recognized as an epigenetic regulator of lung cancer and shows great potentials as a diagnostic and therapeutic marker of lung cancer [64]. Interestingly, it was reported that exposure to CSEs causes upregulation of lncRNA HOTAIR expression levels as well as signal transducer and activator of transcription 3 (STAT3) activation [65]. STAT3 activation increased HOTAIR expression levels by directly binding to its promoter region, enhancing CSE treatment-induced epithelial to mesenchymal transition (EMT) and cancer stem cell (CSC)-like property in HBE cells. Moreover, siRNA knockdown of HOTAIR expression partially reversed CSE treatment-induced phenotypes in HBE cells [65]. In a follow up study from the same group, it was further determined that HOTAIR cooperates with EZH2 to epigenetically down-regulate the expression of cyclin-dependent kinase inhibitor 1 (p21Cip1) by enriching H3K27me3 level on p21promoter region, accelerating cell cyle G1/S phase transition and cell cycle dysregulation in CSE-treated HBE cells [66]. Taken together, these findings provided evidence suggesting that the lncRNA HOTAIR may be critically involved in cigarette smoke-induced lung carcinogenesis.
LCPAT1 (LCPAT1 (Lung cancer progression-association transcript 1), also called RCC2-AS1 (RCC2 antisense RNA 1), locates on chromosome 1p36.13 and is highly expressed in lung tumors. Previous report revealed a positive correlation between the levels of LCPAT1 and RCC2, which was highly associated with tumor progression and poor patient survival, suggesting that LCPAT1 may acts as a tumor-promoting lncRNA in NSCLC [67]. Recently, Lin et al reported that cigarette smoking and PM2.5 pollution may act synergistically in increasing lung cancer risk [68]. Further experimental studies showed that CSEs and PM2.5 particles combination treatment increases the expression of LCPAT1 and enhances lung cancer cell migration, invasion, EMT and autophagy. SiRNA knockdown of LCPAT1 expression in lung cancer cells impaired the effect of CSEs and PM2.5 particles combination treatment on lung cancer cells. Mechanistically, LCPAT1 could physically interact with RCC2 and they coordinately regulated CSE- and PM2.5-induced autophagy and EMT [68], Most recently, it was also found that the LCPAT1-RCC2 signaling axis is involved in regulating CSE-induced DNA damage in human bronchial epithelial BEAS-2B cells, which was supported by decreased number of γ-H2AX foci after siRNA treatment targeting either LCPAT1 or RCC2 [69], Collectively, these findings suggest that LCPAT1 is involved in both CSE- and PM2.5-induced lung carcinogenesis.
LINC00152 (Long intergenic non-coding RNA 152), also called CYTOR (Cytoskeleton regulator RNA), is an oncogenic lncRNA located on chromosome 2p11.2. High linc00152 level was found in many cancer types including lung adenocarcinoma [70, 71], Previous studies have demonstrated that LINC00152 promotes tumor progression and metastasis by either activating pro-survival signaling pathways or recruiting EZH2 to IL-24 promoter, indicating that LINC00152 might have potential to serve as biomarkers or therapeutic target for lung cancer patients [72–74], Liu et al recently reported that the expression levels of lncRNA LINC00152 is significantly higher in CSE-transformed human bronchial epithelial 16HBE cells than that in the control 16HBE cells [75], Further analysis revealed that sera LINC00152 levels in cigarette smokers are also significantly higher than that in non-smokers and the sera levels of LINC00152 are positively correlated with pack-years of smoking. It was further determined that linc00152 is critically involved in regulation of cell adhesion, EMT and other malignant phenotypes in CSE-transformed 16HBE cells. SiRNA down-regulation of linc00152 expression caused G1/S cell cycle arrest and inhibited the proliferation of CSE-transformed 16HBE cells and human lung cancer H1299 cells. Mechanistic study revealed that linc00152 functions as an endogenous competitive RNA to target miR-193b and promote cyclin D1 expression and cell cycle G1/S transition [75].
SCAL1 (Smoke and cancer-associated lncRNA 1), also called LUCAT1 (Lung cancer-associated transcript 1), is an intergenic lncRNA located on chromosome 5q14.3 between arrestin domain-containing 3 (ARRDC3) and G protein-coupled receptor 98 (GPR98). Thai et al. reported that the expression of SCAL1 is induced by CSE treatment both in vitro and in vivo and SCAL1 levels are also up-regulated in many lung cancer cell lines [76]. Biochemical analysis determined that the expression of SCAL1 is regulated transcriptionally by nuclear factor erythroid 2-related factor (NRF2) as evidenced by findings from using siRNA knockdown of NRF2 and kelch-like ECH-associated protein 1 (KEAP1) experiments. Further functional analysis revealed that siRNA knockdown of SCAL1 in human bronchial epithelial cells shows a significant potentiation of cytotoxicity induced by CSE in vitro [76]. Although the expression levels of SCAL1 are up-regulated in multiple lung cancer cells, the role SCAL1 in CSE-induced cell malignant transformation and carcinogenesis remains to be determined.
3.2.2. Fine particular matters (PM2.5)
LINC00341 (Long intergenic non-coding RNA 341) is an intergenic lncRNA located on chromosome 14q32.13 and the prognostic value of LINC00341 has been evaluated in diverse cancer types [77]. It was reported that PM2.5 treatment causes cell cycle arrest at G2/M phase and inhibits 16HBE cell proliferation in a dose-dependent manner [78]. The lncRNA microarray analysis showed that PM2.5 treatment alters lncRNA expression profile in 16HBE cells and RT-PCR analysis confirmed that the lncRNA LINC00341 expression levels are significantly up-regulated in 16HBE cell after PM2.5 treatment [78]. Further mechanistic studies revealed that knockdown of lncRNA LINC00341 reverses PM2.5 treatment-induced p21 expression and cell cycle arrest of G2/M phase. Further studies are needed to determine the role of this lncRNA in PM2.5 exposure-induced cell transformation and lung carcinogenesis.
LOC146880, also called ARHGAP27P1 (Rho GTPase activating protein 27 pseudogene 1), locates on chromosome 17q24.1. It was found that lung tumor tissues express significantly higher levels of LOC146880 than the adjacent normal lung tissues [67]. In addition, LOC146880 higher expression was also positively correlated with malignant phenotypes of tumors and poor patient survival, suggesting that LOC146880 may have an oncogenic role in progression of NSCLC [67]. A follow up study from the same group showed that PM2.5 exposure significantly increases LOC146880 expression levels in human lung cancer A549 cells [79]. It was further determined that PM2.5 exposure generates reactive oxygen species (ROS), which contributes to PM2.5 exposure-induced LOC146880 expression. Functional analysis suggests that up-regulation of LOC146880 expression enhances lung cancer cell migration and invasion [79].
Luo et al. found that treatment with PM2.5 organic extracts increases the lncRNA MALAT1 expression level and causes EMT and transformation of human bronchial epithelial cells [80]. It was further determined that MALAT1 interacts with miR-204 reversing the inhibitory effect of miR-204 on its target gene ZEB1 and contributing to PM2.5 exposure-caused EMT and cell transformation [80]. Given the important role of MALAT1 in lung cancer, these findings suggest that MALAT1 up-regulation may play an important role in PM2.5 exposure-caused lung cancer.
The lncRNAs dysregulated by cigarette smoke extracts and fine particular matters exposures and their roles in lung cancer and the involved potential mechanisms are summarized in Table 2.
Table 2:
LncRNA dysregulated by cigarette smoke extracts and fine particular matters exposures.
| Carcinogens | LncRNAs | Cancers | Models | Upstream genes | Downstream genes | Phenotype | References |
|---|---|---|---|---|---|---|---|
| CSE | CCAT1 (↑) | Lung cancer | Human HBE cells, human blood | – | miR-218, BMI1 | Promote CSE-induced G1/S transition | [60] |
| HOTAIR (↑) | Lung cancer | Human HBE cells, mice | IL6, STAT3 | – | Promote CSE-induced EMT and CSC formation | [65] | |
| HOTAIR (↑) | Lung cancer | Human HBE cells | – | EZH2, p21 | Promote CSE-induced G1/S transition | [66] | |
| LCPAT1 (↑) | Lung cancer | Human lung cancer cell lines | – | RCC2 | Promote CSE and PM2.5-induced autophagy and EMT | [68] | |
| LCPAT1 (↑) | Lung cancer | Human BEAS-2B cells | – | RCC2 | Involved in CSE-induced DNA damage | [69] | |
| LINC00152 (T) (↑) | Lung cancer | Human 16HBE cells, lung cancer cell lines, mice, human blood and lung cancer tissues | – | miR-193b, CCND1 | Promote CSE-induced G1/S transition and cell proliferation | [75] | |
| SCAL1 (↑) | Lung cancer | Human HBE cells, lung cancer cell lines | NRF2 | – | Potentiate CSE-induced cytotoxicity after siRNA knockdown | [76] | |
| PM2.5 | LCPAT1 (↑) | Lung cancer | Human lung cancer cell lines | – | RCC2 | Promote CSE and PM2.5-induced autophagy and EMT | [68] |
| LINC00341 (↑) | Lung cancer | Human 16HBE cells | – | p21 | Promote PM2.5-induced G2/M arrest | [78] | |
| LOC146880 (↑) | Lung cancer | Human A549 cells, human blood and NSCLC | – | LC3B, Beclin1 | Promote PM2.5-induced autophagy, tumor cell migration and invasion | [79] | |
| MALAT1 (↑) | Lung cancer | Human 16HBE cells | NF-κB | miR-204, ZEB1 | Promote PM2.5-induced EMT and transformation | [80] |
(↑) Upregulation; (↓) Downregulation after exposure of environmental carcinogens; COPD, Chronic obstructive pulmonary disease; CSC, Cancer stem cell; CSE, Cigarette smoke extract; EMT, Epithelial-mesenchymal transition; NSCLC, Non-small cell lung cancer; PM, Particulate matter.
3.3. Polycyclic aromatic hydrocarbons (PAHs) and their metabolites-caused lncRNA dysregulations
Polycyclic aromatic hydrocarbons (PAHs) are carbon compounds with more than two annelated aromatic rings and have been demonstrated to possess high potentials to cause carcinogenicity or mutagenicity [81]. Certain members of PAHs are recognized as human carcinogens, including benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, dibenzo[a,h]anthracene, indeno[1,2,3-cd]pyrene and benzo[g,h,i]perylene. In particular, benzo[a]pyrene (B[a]P) is the most potent PAH carcinogen and considered to be highly toxic [82, 83]. Biotransformation of B[a]P is mediated by metabolic enzyme cytochrome P450A1 (CYP1A) and 1B1 (CYP1B1) and converted to anti-benzo[a]pyrene diol epoxide (anti-BPDE), which ultimately triggers DNA mutations and cytotoxicity contributing to its carcinogenicity [84, 85].
Recent studies showed that PAHs and their metabolite exposures cause lncRNA expression dysregulation, which may play important roles in PAH carcinogenesis. Gao et al. firstly reported that lncRNA dysregulations may be critically involved in BaP-induced cell transformation and tumorigenesis [86]. LncRNA-DQ786227 is an uncharacterized lncRNA with unknown function. Gao et al found that the expression levels of DQ786227 are significantly up-regulated in BaP-transformed human bronchial epithelial BEAS-2B cells. Knockdown of DQ786227 expression in BaP-transformed BEAS-2B cells inhibited cell proliferation and colony formation and increased apoptosis. Moreover, downregulation of DQ786227 induced cell apoptosis and suppressed tumor growth by BaP-transformed cells in mice [86].
Similarly, studies showed that BaP key metabolite BPDE treatment also dysregulates lncRNA expression. Yang et al. reported that expression levels of the lncRNA AF118081 are significantly up-regulated in anti-BPDE-transformed 16HBE cells as wells in human lung cancer cells and lung cancer tissues [87]. Functional analysis showed that knockdown AF118081 expression significantly reduces BPDE-transformed 16HBE cells growth and injection of transformed 16HBE cell-produced tumor growth as well [87]. In a follow up study, this same group also found that the expression level of another lncRNA LOC728228, also called LINC01433 (an intergenic lncRNA located on chromosome 20p13) are significantly up-regulated in BPDE-transformed 16HBE cells as well as human lung cancer cells [88]. Similarly, knockdown of LOC728228 expression caused BPDE-transformed cell cycle arrest at G0/G1 phase and reduced cell proliferation, colony formation, migration possibly through regulating cyclin D1 (CCND1) expression. Moreover, knockdown of LOC728228 also greatly decreased tumor growth in nude mice injected with BPDE-transformed cells [88]. Together, these studies provide evidence showing that dysregulated lncRNAs may contribute to BPDE-induced cell transformation and tumorigenesis.
The effect of PAH exposure on lncRNA expression in humans occupationally-exposed to PAH has also been examined. To investigate whether occupational PAHs exposure and DNA damage is correlated with the changes of lncRNA expression, Gao et al. examined PAHs external and internal exposure, DNA damage and lncRNAs (HOTAIR, MALAT1, TUG1 and GAS5) expression in peripheral blood lymphocytes (PBLCs) of 150 male coke oven workers and 60 non-PAHs exposure workers [89]. It was first found that the expression levels of HOTAIR, MALAT1, and TUG1 are elevated in PBLCs of coke oven workers and positively correlated with the levels of external PAHs exposure. Further analysis showed that only HOTAIR and MALAT1 levels are significantly associated with the level of internal PAHs exposure (urinary 1-hydroxypyrene). Moreover, the extent of DNA damage was found to be positively associated with MALAT1 and HOTAIR expression levels in PBLCs of all subjects [89]. These findings alone with cell culture studies discussed above suggest that exposure to PAH parent compounds and their metabolites causes multiple lncRNA expression dysregulations, which may be critically involved in PAH carcinogenesis.
The lncRNAs dysregulated by polycyclic aromatic hydrocarbons (PAHs) and their metabolites exposures and their roles in lung cancer and the involved potential mechanisms are summarized in Table 3.
Table 3:
LncRNAs dysregulated by polycyclic aromatic hydrocarbons and their metabolites exposures.
| Carcinogens | LncRNAs | Cancers | Models | Upstream genes | Downstream genes | Phenotype | References |
|---|---|---|---|---|---|---|---|
| Anti-BPDE | AF118081 (↑) | Lung cancer | Human 16HBE cells, lung cancer cell lines, mice, human lung cancer tissues | – | – | Promote cell proliferation, migration and invasion, promote tumorigenesis in vivo | [87] |
| Anti-BPDE | LOC728228 (↑) | Lung cancer | Human 16HBE cells, lung cancer cell lines, mice | – | CCND1 | Promote G1/S transition, cell proliferation and migration, promote tumorigenesis in vivo | [88] |
| B[a]P | LncRNA-DQ786227 (↑) | Lung cancer | Human BEAS-2B cells, lung cancer cell lines, mice | – | – | Promote cell proliferation and inhibit apoptosis, promote tumorigenesis in vivo | [86] |
| PAH | HOTAIR (↑) | Lung cancer | Human blood | – | – | Promote PAH-induced DNA damage | [89] |
| MALAT1 (↑) | Lung cancer | Human blood | – | – | Promote PAH-induced DNA damage | [89] |
(↑) Upregulation; (↓) Downregulation after exposure of environmental carcinogens; Anti-BPDE, Anti-benzo[a]pyrene-7,8-diol-910-epoxide; BaP, benzo[a]pyrene; PAH, Polycyclic aromatic hydrocarbon.
3.4. Ultraviolet (UV) and ionizing radiation (IR)-caused lncRNA dysregulations
Ultraviolet (UV) irradiation is a part of sun-produced natural energy and the types of UV relevant to human health are mainly UVA and UVB, which are a common type of environmental carcinogens causing skin cancer [90]. Ionizing radiation (IR) is any form of radiation with enough energy to alter an atom such as X-rays, gamma-rays or other radioactive particles. Exposure to a high dose of ionizing radiation causes tissue damages and cancer [91]. It is generally accepted that UV and ionizing radiation are genotoxic causing DNA damages and mutations, which play important roles in their carcinogenic effects. However, recent studies showed that non-genotoxic effects such as changing of lncRNA expressions may also contribute significantly to UV and ionizing radiation carcinogenesis.
3.4.1. Ultraviolet (UV) irradiation
Zeng et al. firstly explored whether UV exposure changes lncRNA expression in primary melanocytes and whether abnormally-expressed lncRNAs play a role in stress responses to UV exposure [92]. It was found that expression levels of 807 lncRNAs are changed more than two-fold in human primary melanocytes 24 h after 20 mJ/cm2 UVB irradiation. The up-regulation of some lncRNAs such as Lnc-GKN2-1:1 and lnc-CD1D-2:1 was likely due to the increased ROS production by UVB irradiation since a ROS scavenger (NAC) treatment reduced UVB-induced ROS generation and inhibited UVB-induced upregulation of lnc-GKN2-1:1 and lnc-CD1D-2:1. It was further determined that siRNA knockdown of Lnc-CD1D-2:1 significantly reduced the UVB-induced tyrosinase activation and p38 phosphorylation [92]. This study provided evidence showing that UVB irradiation is capable of changing lncRNA expression and the dysregulated lncRNAs are involved in UVB-induced stress response.
Kim et al further explored lncRNA expression profile changes in human primary epidermal keratinocytes after UVB (30 mJ/cm2) irradiation [93]. Similarly, the lncRNA microarray analysis revealed that UVB irradiation changed the expression levels of a good number of lncRNAs in human primary epidermal keratinocytes. Further bioinformatics analysis showed that lncRNAs up-regulated by UVB irradiation are mainly associated with the regulation of gene transcription; whereas lncRNAs down-regulated by UVB irradiation area mainly associated with tumorigenesis. Moreover, a panel of 41 lncRNAs that are differentially expressed in both UVB-irradiated keratinocytes and non-melanoma skin cancers (20 up- and 21 down-regulated) were identified [93]. The findings from this study not only provide additional evidence demonstrating that exposure to UVB irradiation dysregulates lncRNA expressions, but also suggest that dysregulated lncRNAs may play important role in UV irradiation-caused skin cancer.
3.4.2. Ionizing radiation (IR)
While a substantial number of studies have been done to show that IR treatment changes lncRNA expressions in a variety of cellular models especially in cancer cells [94], fewer studies have been done to determine the role of dysregulated lncRNAs in IR exposure-induced carcinogenesis. Occupational exposure to ionizing radiation is linked to increased risk of thyroid cancer [95]. Recently, Shi et al performed lncRNA microarray analysis and found that a total of 23 lncRNAs were aberrantly expressed in the occupational radiation exposure-related thyroid carcinoma cases [96]. Specifically, lncRNA n336302, n335249, n335243 and ENST00000509033 were highly expressed in thyroid tumor tissue samples when compared to adjacent nonneoplastic thyroid tissues, whereas lncRNA NR_024380 and ENST00000516478 were downregulated. Further gene ontology and pathway analyses showed that these differentially-expressed lncRNAs may affect many pathways, including those involved in cysteine and methionine metabolism, Huntington disease, propanoate metabolism, and carcinogenesis [96]. The findings of this study suggest that lncRNA dysregulations may be involved in thyroid cancer development due to occupational medical radiation exposure.
The lncRNAs dysregulated by ultraviolet (UV) and ionizing radiation (IR) exposures and their roles in cancer and the involved potential mechanisms are summarized in Table 4.
Table 4:
LncRNAs dysregulated by ultraviolet and ionizing radiation exposures.
| Carcinogens | LncRNAs | Cancers | Models | Upstream genes | Downstream genes | Phenotype | References |
|---|---|---|---|---|---|---|---|
| Medical occupational radiation | ENST00000516478, NR_024380 (↓) | Thyroid cancer | Human thyroid carcinoma | – | – | Decreased levels in patients with occupational exposure to radiation | [96] |
| LncRNA n335243, n335249, n336302, ENST00000509033 (↑) | Thyroid cancer | Human thyroid carcinoma | – | – | Increased levels in patients with occupational exposure to radiation | [96] | |
| UV irradiation | AC005971.3 (↓) | Non-melanoma skin cancer | Human primary epidermal keratinocytes | – | CENPV | Increased expression in UV-irradiated non-melanoma skin cancer | [93] |
| AC068302.3 (↑) | Non-melanoma skin cancer | Human primary epidermal keratinocytes | – | MFI2 | Increased expression in UV-irradiated non-melanoma skin cancer | [93] | |
| BC028022 (↓) | Non-melanoma skin cancer | Human primary epidermal keratinocytes | – | FAM111A | Increased expression in UV-irradiated non-melanoma skin cancer | [93] | |
| CYP17A10S (↓) | Non-melanoma skin cancer | Human primary epidermal keratinocytes | – | CYP17A1 | Increased expression in UV-irradiated non-melanoma skin cancer | [93] | |
| Lnc-CD1D-2:1 (↑) | Non-melanoma skin cancer | Human primary melanocytes | – | p38 | Involved in UVB-induced melanogenesis | [92] | |
| Lnc-GKN2–1:1 (↑) | Non-melanoma skin cancer | Human primary melanocytes | – | – | Involved in UVB-induced melanogenesis | [92] | |
| RP11–137J7.2 (↑) | Non-melanoma skin cancer | Human primary epidermal keratinocytes | – | NMBR | Increased expression in UV-irradiated non-melanoma skin cancer | [93] | |
| RP11–62121.1 (↑) | Non-melanoma skin cancer | Human primary epidermal keratinocytes | – | KIF26B | Increased expression in UV-irradiated non-melanoma skin cancer | [93] | |
| RP11–656D10.6 (↑) | Non-melanoma skin cancer | Human primary epidermal keratinocytes | – | DEM1 | Increased expression in UV-irradiated non-melanoma skin cancer | [93] |
(↑) Upregulation; (↓) Downregulation after exposure of environmental carcinogens; UV, Ultraviolet.
4. Conclusion remarks and future perspectives
LncRNAs have been increasingly recognized to have many important biological functions, implying that dysregulations of lncRNA expression levels or subcellular localizations could interfere with important normal physiological processes leading to the development and progression of many diseases. Indeed, many studies have shown that a large number of lncRNAs are abnormally expressed in cancer, although it remains to be poorly understood how dysregulated lncRNAs promote cancer initiation and progression. Metals and other environmental carcinogens are important cancer etiology factors. Accumulating evidence clearly shows that exposure to metals and other common environmental carcinogens dysregulates lncRNA expressions in various model systems as discussed in this review. While most studies reported the correlation between environmental carcinogen exposure and the abnormal lncRNA expressions, some studies did show that the dysregulated lncRNAs are linked to some malignant phenotypes induced by metals and other environmental carcinogen exposures. A brief summary on the proposed roles and mechanisms of lncRNA dysregulations in environmental carcinogenesis is presented in Figure 2.
Figure 2.

A schematic description on the proposed effects and mechanisms of environmental carcinogen exposure-caused lncRNA dysregulations and their roles in environmental carcinogenesis.
Although recent studies clearly demonstrate that metals and other environmental carcinogen exposures could dysregulate lncRNA expressions, our understanding on the role and mechanisms of dysregulated lncRNAs in environmental carcinogenesis is very limited. Further studies in following areas are needed: (i) Determine whether dysregulated lncRNAs causally contribute to metals and other environment carcinogen-induced cell malignant transformation and tumorigenesis. Most of recent studies only revealed a correlation between environmental carcinogen exposure and the abnormal expression of lncRNAs. The role of dysregulated lncRNAs in environmental carcinogenesis remains largely unknown. (ii) Determine the mechanisms of how environmental carcinogen exposure changes lncRNA expressions and how abnormally-expressed lncRNAs contribute to environmental carcinogenesis. The progression in lncRNA biology research indicates that many lncRNA expressions are regulated at the transcriptional and posttranscriptional levels in similar ways to that of mRNAs, providing critical clues for investigating the mechanisms of lncRNA dysregulation by environmental carcinogen exposure. The advances in understanding the mechanisms of lncRNA regulation of gene expression offers many opportunities to further study the mechanisms of how dysregulated lncRNAs promote or inhibit environmental carcinogenesis. A better understanding on the mechanism of how lncRNA dysregulations promote environmental carcinogenesis may identify new targets for more efficiently prevent and treat cancers resulting from environmental carcinogen exposure. (iii) Determine whether dysregulated lncRNAs may serve as novel biomarkers for monitoring environmental carcinogen exposure. Unlike mRNA and microRNAs, lncRNA expressions display tissue- and species-specificities, rendering lncRNAs as excellent candidates for disease markers. Future studies using proper model systems are expected to identify time- and tissue-dependent and differentially expressed lncRNAs, which may serve as valuable biomarkers for monitoring environmental carcinogen exposure.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (R01ES026151; R01ES028256; R01ES029496; R01ES029942).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
All authors declare no conflicts of interest.
REFERENCES
- [1].Statello L, Guo C-J, Chen L-L, Huarte M, Gene regulation by long non-coding RNAs and its biological functions, Nature Reviews Molecular Cell Biology (2020) 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tsagakis I, Douka K, Birds I, Aspden JL, Long non- coding RNAs in development and disease: conservation to mechanisms, The Journal of pathology 250(5) (2020) 480–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rinn JL, Chang HY, Long noncoding RNAs: molecular modalities to organismal functions, Annual review of biochemistry 89 (2020) 283–308. [DOI] [PubMed] [Google Scholar]
- [4].Mattick JS, The state of long non-coding RNA biology, Non-coding RNA 4(3) (2018) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Uszczynska-Ratajczak B, Lagarde J, Frankish A, Guigó R, Johnson R, Towards a complete map of the human long non-coding RNA transcriptome, Nature Reviews Genetics 19(9) (2018) 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Moore JE, Purcaro MJ, Pratt HE, Epstein CB, Shoresh N, Adrian J, Kawli T, Davis CA, Dobin A, Kaul R, Expanded encyclopaedias of DNA elements in the human and mouse genomes, Nature 583(7818) (2020) 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Volders P-J, Anckaert J, Verheggen K, Nuytens J, Martens L, Mestdagh P, Vandesompele J, LNCipedia 5: towards a reference set of human long non-coding RNAs, Nucleic acids research 47(D1) (2019) D135–D139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fang S, Zhang L, Guo J, Niu Y, Wu Y, Li H, Zhao L, Li X, Teng X, Sun X, NONCODEV5: a comprehensive annotation database for long non-coding RNAs, Nucleic acids research 46(D1) (2018) D308–D314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ulitsky I, Bartel DP, lincRNAs: genomics, evolution, and mechanisms, Cell 154(1) (2013) 26–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Guttman M, Rinn JL, Modular regulatory principles of large non-coding RNAs, Nature 482(7385)(2012) 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Quinn JJ, Chang HY, Unique features of long non-coding RNA biogenesis and function, Nature Reviews Genetics 17(1) (2016) 47. [DOI] [PubMed] [Google Scholar]
- [12].Yao R-W, Wang Y, Chen L-L, Cellular functions of long noncoding RNAs, Nature cell biology 21(5) (2019) 542–551. [DOI] [PubMed] [Google Scholar]
- [13].Geisler S, Coller J, RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts, Nature reviews Molecular cell biology 14(11) (2013) 699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Flynn RA, Chang HY, Long noncoding RNAs in cell-fate programming and reprogramming, Cell stem cell 14(6) (2014) 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fatica A, Bozzoni I, Long non-coding RNAs: new players in cell differentiation and development, Nature Reviews Genetics 15(1) (2014) 7–21. [DOI] [PubMed] [Google Scholar]
- [16].Lee JT, Bartolomei MS, X-inactivation, imprinting, and long noncoding RNAs in health and disease, Cell 152(6) (2013) 1308–1323. [DOI] [PubMed] [Google Scholar]
- [17].Cheetham S, Gruhl F, Mattick J, Dinger M, Long noncoding RNAs and the genetics of cancer, British journal of cancer 108(12) (2013) 2419–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Huarte M, The emerging role of lncRNAs in cancer, Nature medicine 21(11) (2015) 1253. [DOI] [PubMed] [Google Scholar]
- [19].Schmitt AM, Chang HY, Long noncoding RNAs in cancer pathways, Cancer cell 29(4) (2016) 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Evans JR, Feng FY, Chinnaiyan AM, The bright side of dark matter: lncRNAs in cancer, The Journal of clinical investigation 126(8) (2016) 2775–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bhan A, Soleimani M, Mandal SS, Long noncoding RNA and cancer: a new paradigm, Cancer research 77(15) (2017) 3965–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2020, CA: A Cancer Journal for Clinicians 70(1) (2020) 7–30. [DOI] [PubMed] [Google Scholar]
- [23].Wang Z, Yang C, Metal carcinogen exposure induces cancer stem cell-like property through epigenetic reprograming: A novel mechanism of metal carcinogenesis, Seminars in cancer biology, Elsevier, 2019, pp. 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lewandowska AM, Rudzki M, Rudzki S, Lewandowski T, Laskowska B, Environmental risk factors for cancer-review paper, Annals of agricultural and environmental medicine: AAEM 26(1) (2019) 1–7. [DOI] [PubMed] [Google Scholar]
- [25].Casey SC, Vaccari M, Al-Mulla F, Al-Temaimi R, Amedei A, Barcellos-Hoff MH, Brown DG, Chapellier M, Christopher J, Curran CS, The effect of environmental chemicals on the tumor microenvironment, Carcinogenesis 36(Suppl_1) (2015) S160–S183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhu Y, Costa M, Metals and molecular carcinogenesis, Carcinogenesis 41(9) (2020) 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Humphries B, Wang Z, Yang C, The role of microRNAs in metal carcinogen-induced cell malignant transformation and tumorigenesis, Food and Chemical Toxicology 98 (2016) 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang KC, Chang HY, Molecular mechanisms of long noncoding RNAs, Molecular cell 43(6) (2011) 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen L-L, Ling-Ling Chen: linking long noncoding RNA processing and function to RNA biology, Trends in biochemical sciences 41(9) (2016) 733–734. [DOI] [PubMed] [Google Scholar]
- [30].Kazimierczyk M, Kasprowicz MK, Kasprzyk ME, Wrzesinski J, Human long noncoding RNA interactome: detection, characterization and function, International journal of molecular sciences 21(3) (2020) 1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lin A, Hu Q, Li C, Xing Z, Ma G, Wang C, Li J, Ye Y, Yao J, Liang K, The LINK-A lncRNA interacts with PtdIns (3, 4, 5) P 3 to hyperactivate AKT and confer resistance to AKT inhibitors, Nature cell biology 19(3) (2017) 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].I. IARC, monographs vol. 100C Evaluation of carcinogenic risks to humans, International Agency for Research on Cancer, Lyon, France: (2012). [Google Scholar]
- [33].Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, MALAT-1, a novel noncoding RNA, and thymosin β 4 predict metastasis and survival in early-stage non-small cell lung cancer, Oncogene 22(39) (2003) 8031–8041. [DOI] [PubMed] [Google Scholar]
- [34].Luo F, Sun B, Li H, Xu Y, Liu Y, Liu X, Lu L, Li J, Wang Q, Wei S, A MALAT1/HIF-2α feedback loop contributes to arsenite carcinogenesis, Oncotarget 7(5) (2016) 5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Luo F, Liu X, Ling M, Lu L, Shi L, Lu X, Li J, Zhang A, Liu Q, The lncRNA MALAT1, acting through HIF-1α stabilization, enhances arsenite-induced glycolysis in human hepatic L-02 cells, Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1862(9) (2016) 1685–1695. [DOI] [PubMed] [Google Scholar]
- [36].Vander Heiden MG, DeBerardinis RJ, Understanding the intersections between metabolism and cancer biology, Cell 168(4) (2017) 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yu L, Chen X, Sun X, Wang L, Chen S, The glycolytic switch in tumors: how many players are involved?, Journal of Cancer 8(17) (2017) 3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mirzaei H, Hamblin MR, Regulation of glycolysis by non-coding RNAs in cancer: Switching on the Warburg effect, Molecular Therapy-Oncolytics (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dai X, Chen C, Xue J, Xiao T, Mostofa G, Wang D, Chen X, Xu H, Sun Q, Li J, Exosomal MALAT1 derived from hepatic cells is involved in the activation of hepatic stellate cells via miRNA-26b in fibrosis induced by arsenite, Toxicology Letters 316 (2019) 73–84. [DOI] [PubMed] [Google Scholar]
- [40].Tan J, Sun M, Luo Q, Sun H, Wang M, Jiang C, Li S, He Y, Arsenic exposure increased expression of HOTAIR and LincRNA-p21 in vivo and vitro, Environmental Science and Pollution Research 28(1) (2021) 587–596. [DOI] [PubMed] [Google Scholar]
- [41].Xiao T, Zou J, Xue J, Syed BM, Sun J, Dai X, Shi M, Li J, Wei S, Tang H, LncRNA H19-mediated M2 polarization of macrophages promotes myofibroblast differentiation in pulmonary fibrosis induced by arsenic exposure, Environmental Pollution 268 (2020) 115810. [DOI] [PubMed] [Google Scholar]
- [42].Huang Q, Lu Q, Chen B, Shen H, Liu Q, Zhou Z, Lei Y, LncRNA-MALAT1 as a novel biomarker of cadmium toxicity regulates cell proliferation and apoptosis, Toxicology research 6(3) (2017) 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhou Z, Liu H, Wang C, Lu Q, Huang Q, Zheng C, Lei Y, Long non-coding RNAs as novel expression signatures modulate DNA damage and repair in cadmium toxicology, Scientific reports 5 (2015) 15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhou Z, Huang Z, Chen B, Lu Q, Cao L, Chen W, LncRNA-ENST00000446135 is a novel biomarker of cadmium toxicity in 16HBE cells, rats, and Cd-exposed workers and regulates DNA damage and repair, Toxicology Research 9(6) (2020) 823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hu G, Feng H, Long C, Zhou D, Li P, Gao X, Chen Z, Wang T, Jia G, LncRNA expression profiling and its relationship with DNA damage in Cr (Vl)-treated 16HBE cells, Science of The Total Environment 655 (2019) 622–632. [DOI] [PubMed] [Google Scholar]
- [46].Zhou C, Huang C, Wang J, Huang H, Li J, Xie Q, Liu Y, Zhu J, Li Y, Zhang D, LncRNA MEG3 downregulation mediated by DNMT3b contributes to nickel malignant transformation of human bronchial epithelial cells via modulating PHLPP1 transcription and HIF-1α translation, Oncogene 36(27) (2017) 3878–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ghafouri-Fard S, Taheri M, Maternally expressed gene 3 (MEG3): A tumor suppressor long non coding RNA, Biomedicine & Pharmacotherapy 118 (2019) 109129. [DOI] [PubMed] [Google Scholar]
- [48].Al-Rugeebah A, Alanazi M, Panne NR, MEG3: an oncogenic long non-coding RNA in different cancers, Pathology & Oncology Research 25(3) (2019) 859–874. [DOI] [PubMed] [Google Scholar]
- [49].Barta JA, Powell CA, Wisnivesky JP, Global epidemiology of lung cancer, Annals of global health 85(1) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pope CA III, Burnett RT, Turner MC, Cohen A, Krewski D, Jerrett M, Gapstur SM, Thun MJ, Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure-response relationships, Environmental health perspectives 119(11) (2011) 1616–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].de Groot P, Munden RF, Lung cancer epidemiology, risk factors, and prevention, Radiologic Clinics 50(5) (2012) 863–876. [DOI] [PubMed] [Google Scholar]
- [52].Hecht SS, Lung carcinogenesis by tobacco smoke, International journal of cancer 131(12) (2012) 2724–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Morawska L, Airborne particles and health, Air Quality and Climate Change 44(2) (2010) 13–15. [Google Scholar]
- [54].Adams K, Greenbaum DS, Shaikh R, van Erp AM, Russell AG, Particulate matter components, sources, and health: Systematic approaches to testing effects, Journal of the Air & Waste Management Association 65(5) (2015) 544–558. [DOI] [PubMed] [Google Scholar]
- [55].Lippmann M, Chen LC, Gordon T, Ito K, Thurston GD, National Particle Component Toxicity (NPACT) Initiative: integrated epidemiologic and toxicologic studies of the health effects of particulate matter components, Research Report (Health Effects Institute) (177) (2013) 5–13. [PubMed] [Google Scholar]
- [56].Xing Y-F, Xu Y-H, Shi M-H, Lian Y-X, The impact of PM2. 5 on the human respiratory system, Journal of thoracic disease 8(1) (2016) E69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kim T, Cui R, Jeon Y-J, Lee J-H, Lee JH, Sim H, Park JK, Fadda P, Tili E, Nakanishi H, Long-range interaction and correlation between MYC enhancer and oncogenic long noncoding RNA CARLo-5, Proceedings of the National Academy of Sciences 111(11) (2014) 4173–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ling H, Spizzo R, Atlasi Y, Nicoloso M, Shimizu M, Redis RS, Nishida N, Gafà R, Song J, Guo Z, CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer, Genome research 23(9) (2013) 1446–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Liu Z, Chen Q, Hann SS, The functions and oncogenic roles of CCAT1 in human cancer, Biomedicine & Pharmacotherapy 115 (2019) 108943. [DOI] [PubMed] [Google Scholar]
- [60].Lu L, Xu H, Luo F, Liu X, Lu X, Yang Q, Xue J, Chen C, Shi L, Liu Q, Epigenetic silencing of miR-218 by the lncRNA CCAT1, acting via BMI1, promotes an altered cell cycle transition in the malignant transformation of HBE cells induced by cigarette smoke extract, Toxicology and applied pharmacology 304 (2016) 30–41. [DOI] [PubMed] [Google Scholar]
- [61].Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs, cell 129(7) (2007) 1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai M-C, Hung T, Argani P, Rinn JL, Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis, Nature 464(7291) (2010) 1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Tsai M-C, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY, Long noncoding RNA as modular scaffold of histone modification complexes, Science 329(5992) (2010) 689–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Loewen G, Jayawickramarajah J, Zhuo Y, Shan B, Functions of lncRNA HOTAIR in lung cancer, Journal of hematology & oncology 7(1) (2014) 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Liu Y, Luo F, Xu Y, Wang B, Zhao Y, Xu W, Shi L, Lu X, Liu Q, Epithelial-mesenchymal transition and cancer stem cells, mediated by a long non-coding RNA, HOTAIR, are involved in cell malignant transformation induced by cigarette smoke extract, Toxicology and applied pharmacology 282(1) (2015) 9–19. [DOI] [PubMed] [Google Scholar]
- [66].Liu Y, Wang B, Liu X, Lu L, Luo F, Lu X, Shi L, Xu W, Liu Q, Epigenetic silencing of p21 by long non-coding RNA HOTAIR is involved in the cell cycle disorder induced by cigarette smoke extract, Toxicology letters 240(1) (2016) 60–67. [DOI] [PubMed] [Google Scholar]
- [67].Feng N, Ching T, Wang Y, Liu B, Lin H, Shi O, Zhang X, Zheng M, Zheng X, Gao M, Analysis of microarray data on gene expression and methylation to identify long non-coding RNAs in non-small cell lung cancer, Scientific reports 6 (2016) 37233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lin H, Zhang X, Feng N, Wang R, Zhang W, Deng X, Wang Y, Yu X, Ye X, Li L, LncRNA LCPAT1 mediates smoking/particulate matter 2.5-induced cell autophagy and epithelial-mesenchymal transition in lung cancer cells via RCC2, Cellular Physiology and Biochemistry 47(3) (2018) 1244–1258. [DOI] [PubMed] [Google Scholar]
- [69].Gao S, Lin H, Yu W, Zhang F, Wang R, Yu H, Qian B, LncRNA LCPAT1 is involved in DNA damage induced by CSE, Biochemical and biophysical research communications 508(2) (2019)512–515. [DOI] [PubMed] [Google Scholar]
- [70].Li N, Feng XB, Tan Q, Luo P, Jing W, Zhu M, Liang C, Tu J, Ning Y, Identification of circulating long noncoding RNA Linc00152 as a novel biomarker for diagnosis and monitoring of non-small-cell lung cancer, Disease markers 2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yu Y, Yang J, Li Q, Xu B, Lian Y, Miao L, LINC 00152: A pivotal oncogenic long non-coding RNA in human cancers, Cell Proliferation 50(4) (2017) e12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zhang Y, Xiang C, Wang Y, Duan Y, Liu C, Jin Y, Zhang Y, lncRNA LINC00152 knockdown had effects to suppress biological activity of lung cancer via EGFR/PI3K/AKT pathway, Biomedicine & Pharmacotherapy 94 (2017) 644–651. [DOI] [PubMed] [Google Scholar]
- [73].Feng S, Zhang J, Su W, Bai S, Xiao L, Chen X, Lin J, Reddy RM, Chang AC, Beer DG, Overexpression of LINC00152 correlates with poor patient survival and knockdown impairs cell proliferation in lung cancer, Scientific reports 7(1) (2017) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Chen Q.-n., Chen X, Chen Z.-y., Nie F.-q., Wei C.-c., Ma H.-w., Wan L, Yan S, Ren S.-n., Wang Z.-x., Long intergenic non-coding RNA 00152 promotes lung adenocarcinoma proliferation via interacting with EZH2 and repressing IL24 expression, Molecular cancer 16(1) (2017) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Liu Z, Liu A, Nan A, Cheng Y, Yang T, Dai X, Chen L, Li X, Jia Y, Zhang N, The linc00152 controls cell cycle progression by regulating CCND1 in 16HBE cells malignantly transformed by cigarette smoke extract, Toxicological Sciences 167(2) (2019) 496–508. [DOI] [PubMed] [Google Scholar]
- [76].Thai P, Statt S, Chen CH, Liang E, Campbell C, Wu R, Characterization of a novel long noncoding RNA, SCAL1, induced by cigarette smoke and elevated in lung cancer cell lines, American journal of respiratory cell and molecular biology 49(2) (2013) 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Liao M, Li B, Zhang S, Liu Q, Liao W, Xie W, Zhang Y, Relationship between LINC00341 expression and cancer prognosis, Oncotarget 8(9) (2017) 15283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Xu Y, Wu J, Peng X, Yang T, Liu M, Chen L, Dai X, Wang Z, Yang C, Yan B, LncRNA LINC00341 mediates PM2.5-induced cell cycle arrest in human bronchial epithelial cells, Toxicology letters 276 (2017) 1–10. [DOI] [PubMed] [Google Scholar]
- [79].Deng X, Feng N, Zheng M, Ye X, Lin H, Yu X, Gan Z, Fang Z, Zhang H, Gao M, PM2.5 exposure-induced autophagy is mediated by lncRNA loc146880 which also promotes the migration and invasion of lung cancer cells, Biochimica et Biophysica Acta (BBA)-General Subjects 1861(2) (2017) 112–125. [DOI] [PubMed] [Google Scholar]
- [80].Luo F, Wei H, Guo H, Li Y, Feng Y, Bian Q, Wang Y, LncRNA MALAT1, an lncRNA acting via the miR-204/ZEB1 pathway, mediates the EMT induced by organic extract of PM2.5 in lung bronchial epithelial cells, American Journal of Physiology-Lung Cellular and Molecular Physiology 317(1) (2019) L87–L98. [DOI] [PubMed] [Google Scholar]
- [81].Yu H, Environmental carcinogenic polycyclic aromatic hydrocarbons: photochemistry and phototoxicity, Journal of Environmental Science and Health, Part C 20(2) (2002) 149–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Srogi K, Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: a review, Environmental Chemistry Letters 5(4) (2007) 169–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].I.W.G.o.t.E.o.C.R.t. Humans, Chemical agents and related occupations, IARC monographs on the evaluation of carcinogenic risks to humans 100(PT F) (2012) 9. [PMC free article] [PubMed] [Google Scholar]
- [84].Shimada T, Fujii- Kuriyama Y, Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and1B1, Cancer science 95(1) (2004) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Shiizaki K, Kawanishi M, Yagi T, Modulation of benzo [a] pyrene-DNA adduct formation by CYP1 inducer and inhibitor, Genes and Environment 39(1) (2017) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Gao L, Mai A, Li X, Lai Y, Zheng J, Yang Q, Wu J, Nan A, Ye S, Jiang Y, LncRNA-DQ786227-mediated cell malignant transformation induced by benzo (a) pyrene, Toxicology letters 223(2) (2013) 205–210. [DOI] [PubMed] [Google Scholar]
- [87].Yang Q, Zhang S, Liu H, Wu J, Xu E, Peng B, Jiang Y, Oncogenic role of long noncoding RNA AF118081 in anti-benzo [a] pyrene-trans-7, 8-dihydrodiol-9, 10-epoxide-transformed 16HBE cells, Toxicology letters 229(3) (2014) 430–439. [DOI] [PubMed] [Google Scholar]
- [88].Hu G, Yang T, Zheng J, Dai J, Nan A, Lai Y, Zhang Y, Yang C, Jiang Y, Functional role and mechanism of lncRNA LOC728228 in malignant 16HBE cells transformed by anti-benzopyrene-trans- 7, 8-dihydrodiol- 9, 10- epoxide, Molecular carcinogenesis 54(S1) (2015) E192–E204. [DOI] [PubMed] [Google Scholar]
- [89].Gao C, He Z, Li J, Li X, Bai Q, Zhang Z, Zhang X, Wang S, Xiao X, Wang F, Specific long non-coding RNAs response to occupational PAHs exposure in coke oven workers, Toxicology reports 3 (2016) 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kim I, He Y-Y, Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation, Genes & diseases 1(2) (2014) 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Barcellos-Hoff MH, Nguyen DH, Radiation carcinogenesis in context: how do irradiated tissues become tumors?, Health physics 97(5) (2009) 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Zeng Q, Wang Q, Chen X, Xia K, Tang J, Zhou X, Cheng Y, Chen Y, Huang L, Xiang H, Analysis of lncRNAs expression in UVB-induced stress responses of melanocytes, Journal of Dermatological Science 81(1) (2016) 53–60. [DOI] [PubMed] [Google Scholar]
- [93].Kim K-H, Kim H-J, Lee TR, Epidermal long non-coding RNAs are regulated by ultraviolet irradiation, Gene 637 (2017) 196–202. [DOI] [PubMed] [Google Scholar]
- [94].Podralska M, Ciesielska S, Kluiver J, van den Berg A, Dzikiewicz-Krawczyk A, Slezak-Prochazka I, Non-Coding RNAs in Cancer Radiosensitivity: MicroRNAs and lncRNAs as Regulators of Radiation-Induced Signaling Pathways, Cancers 12(6) (2020) 1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Aschebrook-Kilfoy B, Ward MH, Della Valle CT, Friesen MC, Occupation and thyroid cancer, Occupational and environmental medicine 71(5) (2014) 366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Shi F, Liu Y, Li M, Wen P, Qian QQ, Fan Y, Huang R, Analysis of lncRNA and mRNA Transcriptomes Expression in Thyroid Cancer Tissues Among Patients With Exposure of Medical Occupational Radiation, Dose-Response 17(3) (2019) 1559325819864223. [DOI] [PMC free article] [PubMed] [Google Scholar]


