Abstract
A growing body of literature has reported the effects of dual tasks on gait performance in people with Parkinson’s disease (PD). The purpose of this meta-analysis was to synthesize the existing literature and quantify the overall influence of dual tasks on gait performance in PD. A thorough literature search was conducted, and 19 studies met the stringent inclusion criteria. Two moderator variable analyses examined the dual-task effect by: (a) mean single-task gait speed for each study (≥ 1.1 m/s or < 1.1 m/s), and (b) the type of dual task (arithmetic, language, memory, and motor). Three main findings were revealed by a random effects model analysis. First, a strong negative effect of dual tasks on walking performance (SMD = −0.68) confirmed that gait performance is adversely affected by dual tasks in people with PD. Second, the significant negative effect of dual tasks is present regardless of the mean level of single-task gait speed in a study. Third, dual-task walking speed deteriorates regardless of the type of dual task. Together, these results confirm that dual tasks severely affect walking performances in people with PD.
Keywords: Parkinson’s, Dual-task, cognition, interference, walk
1. Introduction
Parkinson’s disease is a progressive neurodegenerative disorder that affects over 10 million individuals globally [1,2]. People with Parkinson’s disease (PD) suffer movement deficits such as tremor, rigidity, and bradykinesia that impair the ability to complete common activities of daily living and increase reliance on assistive care [3]. Parkinsonian gait is characterized by diminished propulsive force resulting in small and slow steps, stooped posture, low foot clearance, and impaired inter-limb coordination [4,5]. Further, as many as 19–38% PD patients develop cognitive impairments that are significant predictors of disability [6,7]. Several cognitive functions deteriorate with disease progression in PD including executive function, working memory, processing speed, attentional, and language abilities [8]. Because walking depends on similar higher-level neurological systems and cognitive processes [9], gait impairment in PD may be exacerbated when walking is paired with a concurrent goal-directed task, referred to as a dual task [10].
Dual tasks have been studied extensively in healthy older adult populations [11–14]. The effects on performance of dual-task behaviors, measured as the change in performance from single to dual task, is indicative of ‘cognitive-motor’ interference [13–15]. Similarly, a growing number of studies have investigated the influence of dual-task performances on walking in PD [16,17]. Unfortunately, the breadth of methodological variations and outcomes reported in the existing literature makes drawing conclusions challenging.
Specifically, experimental designs vary widely across studies and seemingly lead to differential consequences on dual-task performance [18]. For example, the effect of dual-task performance on gait is influenced by the instructions given to the participants (e.g. prioritizing one aspect of a dual task or focusing on both) [19–21]. Further, study samples vary widely with respect to disease severity which directly influences both walking and cognitive performance [22,23]. Thus, differences in single-task gait speed may contribute to the mixed results in studies of idiopathic PD patients. A clear understanding of dual-tasking in PD is further complicated because cognitive-motor interference is evaluated using a wide range of concurrent cognitive tasks across studies (i.e. carrying a tray; generating words; counting backwards by three; or reacting to auditory or visual stimuli). Some studies suggest that more complex tasks that challenge working memory or executive function impair walking in PD more than simple cognitive tasks, [18,24] while others report little difference caused by the type of concurrent task [25]. Consequently, previous studies in the PD population do not clarify if distinct types of concurrent cognitive tasks affect dual-task walking to the same extent. A systematic review and meta-analysis are necessary to evaluate the consequences of dual tasks on PD gait.
2. Methods
2.1. Literature search and study selection
Consistent with PRISMA’s suggestions [26], a thorough search in several electronic databases for published and unpublished data in the field was conducted from May 2017 to January 2018. Literature searches were conducted in eight computerized databases: (1) Cochrane’s Database of Systematic Reviews, (2) EBSCOhost, (3) Embase, (4) ISI’s Web of Knowledge, (5) PubMed, (6) ProQuest, (7) ProQuest Dissertations and Theses Global, and (8) ResearchGate. The key words for the computerized search included: (a) Parkinson, (b) dual-task (c) walk, (d) gait, (e) cognition, and (f) concurrent. Two authors (TR and FT) conducted independent searches to confirm identical results and determined inclusion eligibility. Study eligibility criteria required human subjects with idiopathic Parkinson’s disease, full-length articles written in English, and direct empirical evidence. After combining the results from each database and removing duplicates, animal studies, foreign language articles, reviews, and conference abstracts, 230 studies remained. The next step involved assessing the articles for the predetermined inclusion criteria. Each study was required to evaluate single and dual-task walking over ground at a self-selected pace, excluding 124 studies that reported other types of dual tasks. See Figure 1 for the PRISMA flowchart. Studies were excluded if the information required for the meta-analysis were not reported.
The remaining 106 studies of dual-task walking in people with PD were evaluated for quantitative reports of single and dual-task gait speed, and 25 studies were removed because they did not report gait speed. For instance, studies that reported outcomes related to the cognitive task [27]; visual scanning [28]; or brain imaging [29] were not included.
The remaining 81 studies were coded by two independent reviewers and qualitative evaluations of validity were conducted. In harmony with traditional meta-analysis techniques [30], 62 studies that did not provide within-subject statistical comparisons of single and dual-task gait speed were not included in the analysis.
Nineteen studies qualified for inclusion in the analysis using data of single and dual-task gait speed from each study, representing a total of 510 participants (Figure 1, Table 1)
Figure 1.
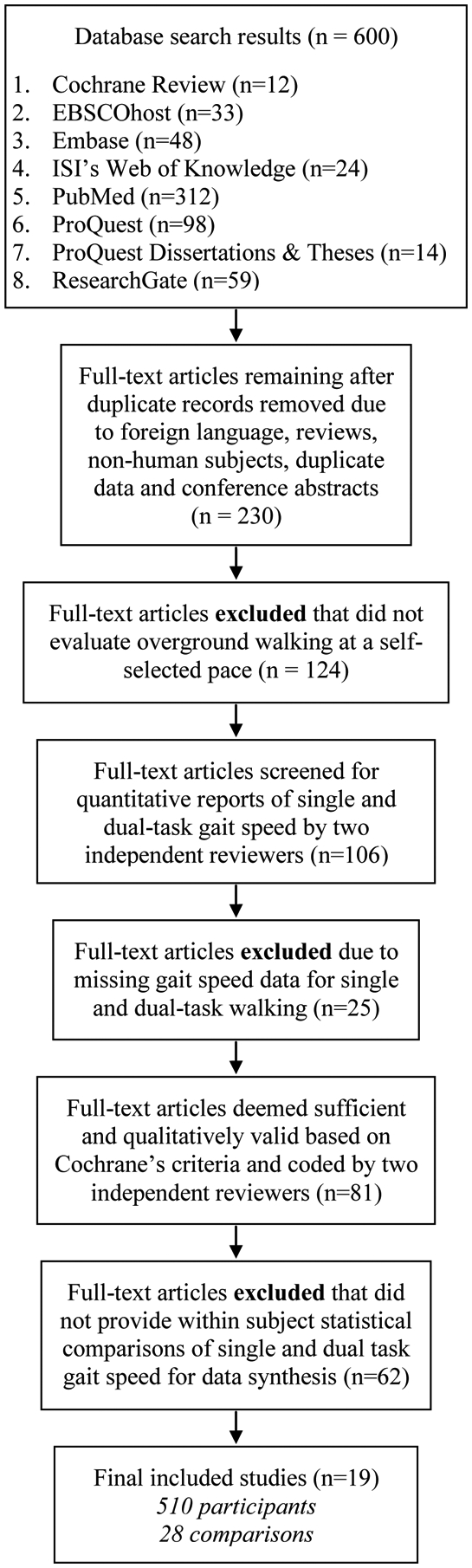
Search procedure and results according to PRISMA guidelines [26].
Table 1.
Included study demographics
| Study | UPDRS-III Mean (SD) |
Functional Impairment | Cognitive Task Type | Age | Sample Gender M/W |
Total N | Global Cognition |
|---|---|---|---|---|---|---|---|
| [31] Alcock et al., (2016) | -- | Pre-impaired | Memory | 68.5 (9.1) | 28/12 | 40 | ┼28.5 (1.2) |
| [32] Beck et al., (2015) | 24.7 (7.5) | Pre-impaired | Memory | 70.5 (8.4) | 18/2 | 20 | §26.5 (2.1) |
| [33] Bond et al., (2000) | -- | Impaired | Motor | 65.08 (10.3) | 11/1 | 12 | -- |
| [34] Canning et al., (2005) | 20.1 (4.8) | Pre-impaired | Motor | 65.4 (10.4) | 9/3 | 12 | ┼28.8 (1.4) |
| [35] Galletly et al., (2005) | 14.4 (6.1) | Pre-impaired | Arithmetic, Language, Motor | 65.0 (9.5) | 9/7 | 16 | ┼28.0 (3.0) |
| [36] Harrison et al., (2017) | 30.5 (11.8) | Pre-impaired | Language | 69.5 (7.6) | 13/10 | 23 | ┼29 (3.0) |
| [37] Lohnes et al., (2011) | 21.5 (6.7) | Pre-impaired | Language | 70.3 (6.8) | 4/7 | 11 | -- |
| [38] Lord et al., (2010) | 39.2 (15.4) | Impaired | Memory, Motor | 71.3 (7.4) | 19/10 | 29 | ┼26.9 (2.8) |
| [25] O’Shea et al., (2002) | -- | Pre-impaired | Arithmetic, Motor | 68.3 (6.6) | 12/3 | 15 | -- |
| [39] Plotnik et al., (2009) | -- | Impaired | Arithmetic | 71.9 (6.8) | 15/6 | 21 | -- |
| [10] Plotnik et al., (2011) | 19.8 (7.3) | Pre-impaired | Arithmetic | 65.9 (7.2) | 21/9 | 30 | ┼28.5 (1.3) |
| [40] Rochester et al., (2004) | -- | Impaired | Language, Motor | 64.6 (8.0) | 12/8 | 20 | ┼27.2 (2.0) |
| [41] Rochester et al., (2014) | 25.4 (10.4) | Pre-impaired | Memory | 67.0 (10.4) | 81/40 | 121 | ┼28.6 (1.3) |
| §25.1 (3.5) | |||||||
| [42] Salazar et al., (2017) | 20.6 (10.1) | Pre-impaired | Memory | 66.3 (5.6) | 11/8 | 19 | -- |
| [43] Stegemoller et al., (2014) | 24.0 (7.0) | Impaired | Arithmetic | 66.2 (8.5) | 28/7 | 35 | ┼29.2 (1.0) |
| [44] Wild et al., (2013) | 16.2 (7.9) | Impaired | Arithmetic, Language, Memory | 69.3 (2.3) | 8/10 | 18 | ┼26.4 (0.5) |
| [24] Yogev-Seligmann et al., (2005) | 17.5 (8.3) | Impaired | Arithmetic, Language, Memory | 70.9 (7.9) | 20/10 | 30 | 28.1 (1.6) |
| [45] Yogev-Seligmann et al., (2012) | 20.7 (8.9) | Pre-impaired | Language | 70.3 (6.6) | 12/8 | 20 | §26.4 (2.8) |
| [46] Yogev-Seligmann et al., (2013) | 23.2 (9.1) | Pre-impaired | Language | 68.7 (4.7) | 16/2 | 18 | §25.6 (1.7) |
Note: Unreported data marked as: --. Sample Gender: M=men, W=Women. Global cognition measures out of 30 points noted as § for the Montreal Cognitive Assessment (MoCA) and ┼ for the Mini-Mental State Exam (MMSE). Unified Parkinson’s Disease Rating Score, Part III (Motor) (UPDRS III).
Two authors (TR and FT) independently extracted the appropriate data from the studies [31–38,25,39,10,40–44,24,45,46] and coded the data according to two predetermined categories: (a) participant characteristics and (b) type of dual task. Participant characteristics included: (a) single-task gait speed mean and standard deviation, (b) Unified Parkinson’s Disease Rating Scale motor section score (UPDRS-III score), (c) age, (d) gender, (e) total N, and (f) cognitive assessment score (Mini-Mental State Exam (MMSE) or Montreal Cognitive Assessment (MoCA) score) (see Table 1). Type of concurrent tasks included: (a) arithmetic, (b) language, (c) memory, or (d) motor (Table 2).
Table 2.
Type of dual tasks, corresponding cognitive domain, and dual-task tests defined for each study
|
Arithmetic Executive function, working memory, attention |
| N-back: Count back from an assigned number by designated increment (n) (i.e., count by three from the number 197) [35,25,39,10,43,44,24] |
| Language |
| Executive function, language production and comprehension |
|
Letter Fluency: Recite words that begin from an assigned letter in the alphabet (i.e., name words beginning with ‘F’) [35,36,37,45,46] Extemporaneous Speech: Responding to repeated questions using autobiographical memory [40] Text Recall: Listen to passage during walking and answer questions about passage after walking [44,24] |
| Memory |
| Working memory, attention |
|
Digit Span Forward: Repeating increasingly long lists of numbers in the same order [31,41] Digit Recall: Listen to numbers while walking and count the number of times an assigned digit was recited after finishing walking [32] Count Tones: Count number of auditory tones while walking and report after walk [38] Phoneme Monitor: Listen to text and count the occurrence of two pre-assigned words [44,24] Oral Trails Test: Verbal responses pairing letters and numbers in order, ‘3C-4D-5E’ [42] |
| Motor |
| Manual motor coordination |
|
Tray Carry: Carrying a tray while walking [33,38,40] Tray +: Carry a tray with cups [34] Button: Buttoning shirt while walking [35] Coin: Transferring coin between pants pockets [25] |
2.2. Dual-task walking outcomes and moderator variable analysis
Consistent with traditional meta-analytic techniques, studies that compared gait speed in single and dual-task over ground walking conditions were included in the analysis. The meta-analysis determined a standardized effect size from 28 observations of the differences in gait speed between single-task walking and dual-task walking.
For a comprehensive understanding of the effect of a dual task on gait performance in PD, the authors conducted two pertinent moderator variable analyses. First, given that disease severity affects functional ability in people with PD, the authors examined the effect of baseline physical function on dual-task gait performance. Because mean gait speed is an indicator of physical function, cognitive impairment, and fall risk [10,3,47], single-task gait speed was used to define baseline mobility within in each study. Paul et al. [48] reported that people with PD who walk below 1.1 m/s were more likely to fall. Thus, we examined the effects of baseline mobility capability on dual-task detriments using mean single-task gait speed as a threshold for categorizing study samples into two groups: those studies reporting a mean single-task gait speed ≥ 1.1 m/s or studies with a mean single-task gait speed < 1.1 m/s. Of the analyzed studies, 12 studies reported a mean single-task gait speed ≥ 1.1 m/s and seven studies reported a mean single-task gait speed < 1.1 m/s (Table 1).
Second, because the existing literature includes a wide range of concurrent tasks that may influence gait differently in people with PD [18,49], we categorized each study according to the type of cognitive task (defined in Table 2). Four primary concurrent task types were identified and included in this moderator variable analysis: (a) arithmetic (seven comparisons), (b) language (eight comparisons), (c) memory (seven comparisons), and (d) motor (six comparisons) (Table 1, Table 2).
2.3. Data synthesis, measuring heterogeneity, and evaluating publication bias
Meta-analytic findings were calculated using the Comprehensive Meta-Analysis software (version 3.0, 2015; Englewood, NJ, USA) [50]. Gait speed was selected as the primary comparison outcome for single and dual-task walking (i.e., within-subjects analyses) from 19 studies. The data included for the analysis were 1) sample N, 2), mean gait speeds, 3) their respective standard deviations, and 4) p-values for within group comparisons of gait speed from single to dual-task walking. However, six studies reported multiple outcomes, and to be sure that including an extra 11 comparisons did not artificially inflate our effect size we conducted sensitivity analyses. Six permutations of sensitivity analyses were conducted by randomly selecting one of the individual dual-task effects from each study and determining the resultant random effects model SMD, 95% confidence interval, and p-value. The meta-analysis used the inverse variance weighting procedure to take into account sample size as well as variability between studies [51]. Two moderator variable analyses of mean single-task gait speed and cognitive dual-task domains provided additional SMDs.
Determining heterogeneity is critical in meta-analyses to accurately combine a broad and diverse group of studies [30,52]. To quantify heterogeneity three variability tests were used. First, Cochran’s Q indicates that heterogeneity between studies if the p-value is less than 0.05 [30]. Second, Higgins and Green’s I2 quantifies heterogeneity in the dual-task effect as a percentage of total variance across studies, and a percentage greater than 50% is considered indicative of moderate heterogeneity [53]. Lastly, T2 estimates the variability of the dual-task effect between studies in a random effects model. A T2 value of 1.0 specifies greater heterogeneity across studies [54,55].
Another critical meta-analytic technique is examining publication bias in the literature. The authors used three meta-analytic techniques to quantify publication bias and symmetry. First, funnel-plots provided a measure of symmetry and an estimated proportion of unpublished data by detailing the SMD versus standard errors for each comparison [56]. Second, the trim and fill technique was applied to the funnel plots to reveal a corrected funnel plot comparing the original standardized effect size with the corrected standardized effect size [56]. Third, the fail-safe N analysis was conducted to quantify the number of non-significant missing studies needed to reduce the overall significance of the effect to p ≥ 0.05 [30].
3. Results
3.1. The effect of dual tasks on walking speed in PD
The random effects meta-analysis compared 28 outcome measures from 19 studies of single-task and dual-task gait speed in people with PD and revealed a moderate to large standardized mean difference effect (SMD = −0.68, SE = 0.05; 95% CI = −0.78 to −0.58; p < 0.0001). The sensitivity analyses evaluated the influence of multiple individual effect sizes from six studies on the overall meta-analysis. The analyses revealed a range of SMD between −0.67 and −0.72, 95% CI with a lower limit of −0.84 and upper limit of −0.56, and p-values < 0.001 in each permutation. The results from the sensitivity analyses confirmed a minimal difference and permitted inclusion of all dual-task effects from six studies. The meta-analysis effect size confirms that adding dual tasks to walking degrades gait speed in people with PD. The Forest plot below shows the individual and overall SMD for each comparison and relative weight for each study (Figure 2).
Figure 2.
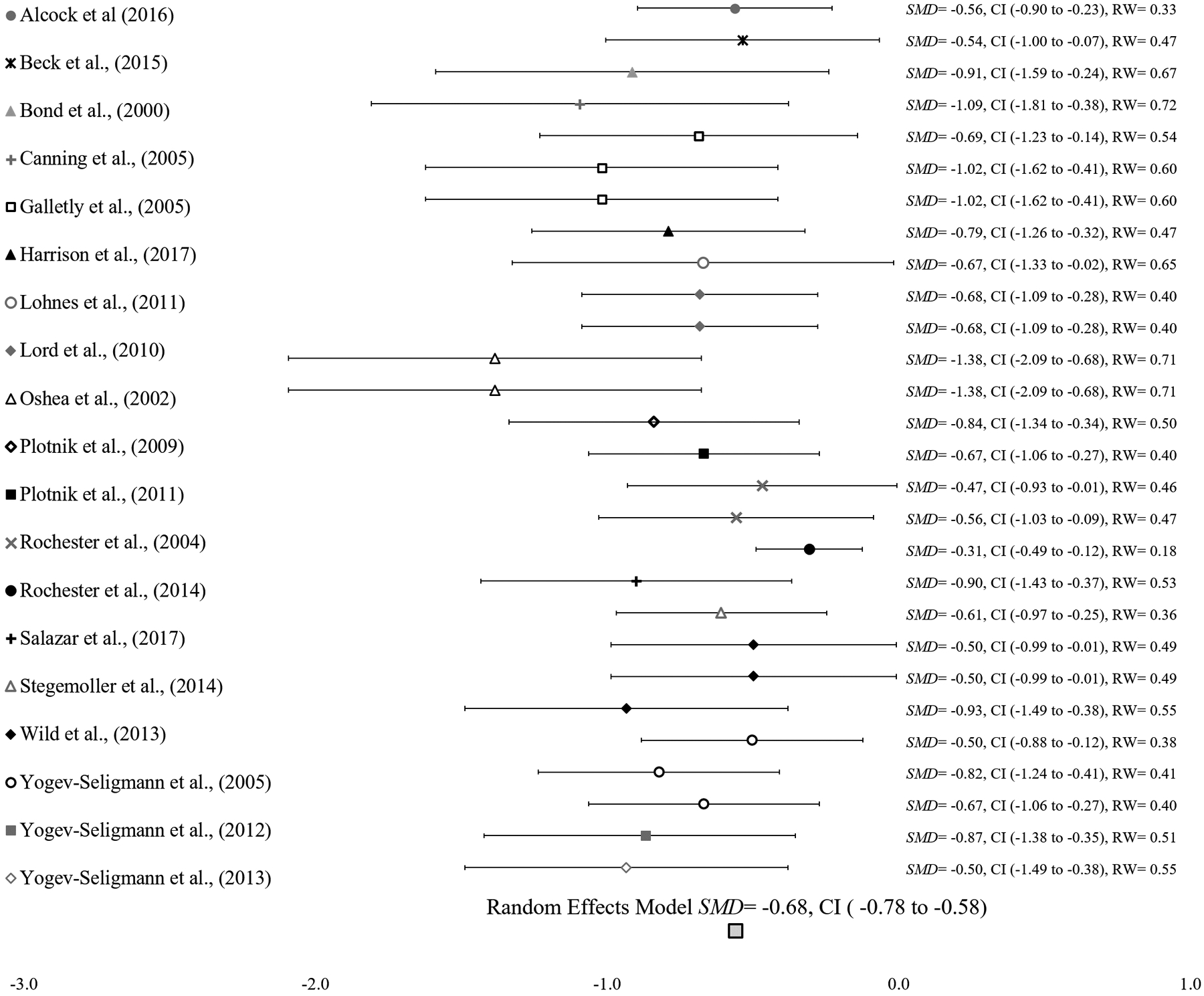
Forest plot of random effects model of 19 studies. Overall SMD is represented by a diamond on the bottom of the graph. SMD= Standardized Mean Difference, CI= 95% Confidence Interval, RW = Relative Weight.
3.2. Heterogeneity and publication bias
In the overall analysis, evaluations of heterogeneity across studies were not significant: (a) Cochrane’s Q (27) = 34.29, p= 0.16, (b) I2 = 21.27%, and (c) T2 = 0.01. Cumulatively, these measures verified the data were not heterogeneous. After applying the trim and fill technique, the analysis detected a minimal difference between an original overall effect size (i.e., white diamond: SMD = −0.68) and a corrected overall effect size (i.e., black diamond: SMD = −0.53, 95% CI = −1.31 to 0.27) as shown in Figure 3. Ten imputed values (black circles) were added to the right side of the funnel plot to adjust for symmetry (Figure 3). In addition, a classic fail-safe N analysis indicated that 1,831 null effect findings are necessary for reducing our significant overall effect size. These findings indicate minor publication bias in this meta-analysis.
Figure 3.
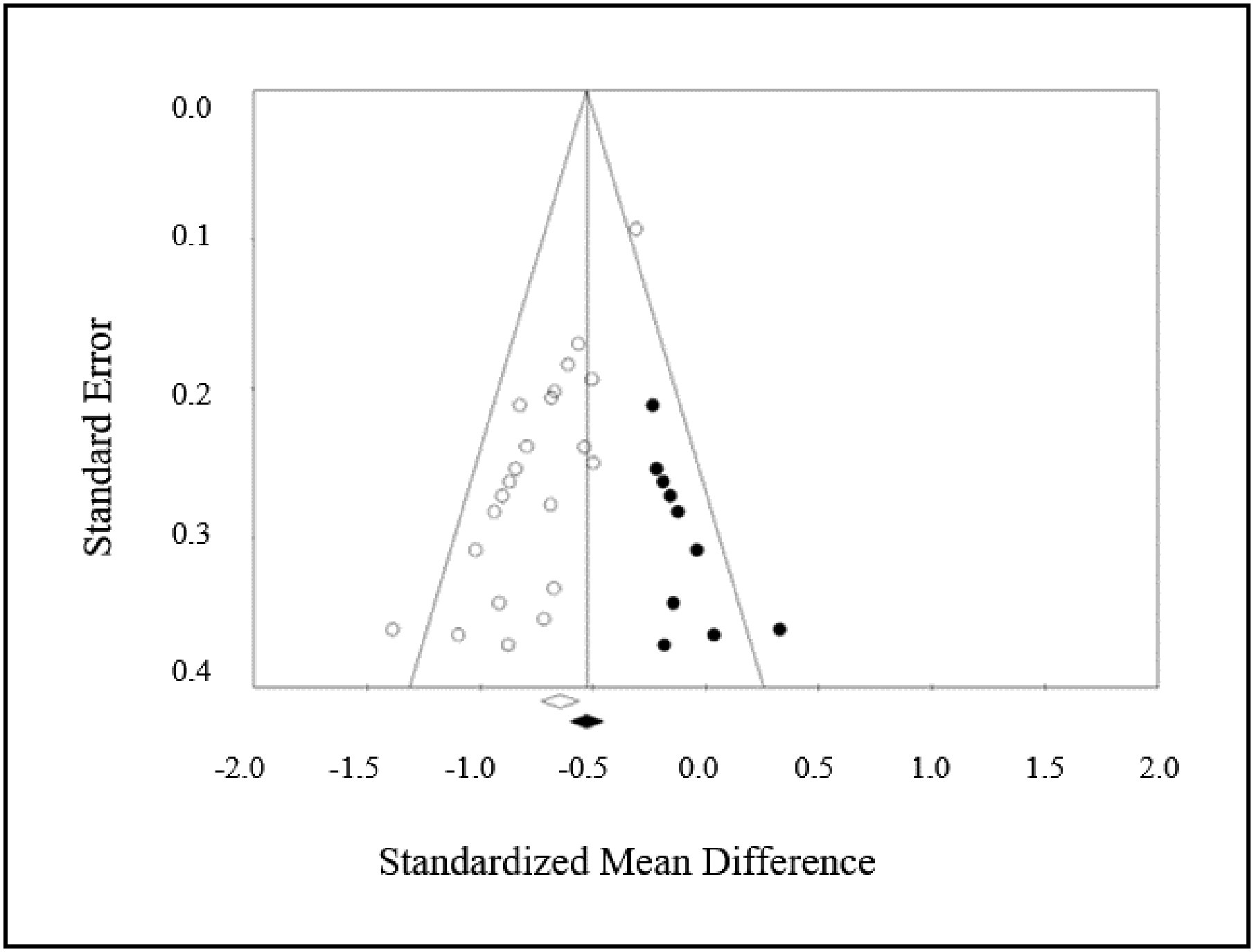
Trim and fill funnel plot of the comparison for random effects model. The x-axis indicates the SMD and the y-axis shows the standard error for each comparison. The white diamond on the x-axis represents the standardized effects of the 28 comparisons (SMD = −0.68) while the black diamonds represent the adjusted standardized effect (SMD =−0.53) after imputing ten values.
3.3. Moderator variable analyses
3.2.1. Baseline mobility level: mean single-task gait speed ≥1.1 m/s, mean single-task gait speed < 1.1 m/s
As reported earlier, the minimal change in the results of the sensitivity analyses reinforced our confidence in reporting the moderator variable analyses. A moderator variable analysis of the influence of a dual task on gait in studies grouped by baseline walking speed demonstrated significant differences between single and dual-task gait performance in studies including samples with a mean single-task gait speed ≥ 1.1 m/s (SMD = −0.78; SE = 0.09; 95% CI = −0.96 to −0.60; p < 0.0001) and a mean single-task gait speed < 1.1 m/s (SMD = −0.65; SE = 0.06; 95% CI = −0.77 to −0.53; p < 0.0001) individuals with PD. These findings indicate that persons with PD who demonstrate baseline gait deficiencies experience walking deficits with a dual task as well as more functional PD patients.
3.2.2. Cognitive dual task domains: arithmetic, language, memory, and motor tasks
Another objective was to determine whether types of dual tasks differentially influenced gait speed. A moderator variable analysis on cognitive task type was conducted with concurrent tasks categorized into four categories (Table 2). Each type of task adversely affected gait performances, shown by significant moderate to strong negative effects on walking: Arithmetic (SMD = −0.78; SE = 0.09; 95% CI = −0.95 to −0.60; p < 0.0001); Language (SMD = −0.76; SE = 0.09; 95% CI = −0.94 to −0.58; p < 0.0001); Memory (SMD = −0.49; SE = 0.07; 95% CI = −0.63 to −0.34; p < 0.0001); and Motor (SMD = −0.78; SE = 0.12; 95% CI = −1.02 to −0.54; p < 0.0001). These findings verify that all four types of dual tasks significantly impaired gait speed.
4. Discussion
4.1. Dual tasks severely disrupt walking in people with PD
For people with PD, difficulty walking while performing another task is frequently reported in the literature. However, variability in methodological approaches and selected outcome measures have confounded our understanding of the effect of dual tasks on PD gait. Herein, the authors synthesized existing literature with meta-analysis to determine the influence of dual tasks on gait speed in people with PD. The advantages to meta-analytic techniques enabled us to draw clear conclusions from a diverse body of literature in a vulnerable population. Importantly, our meta-analysis indicated that for people with PD, the performance of dual tasks while walking bears significant and meaningful consequences.
The profound effect of a dual task on walking in PD revealed in the meta-analysis was supported by a lack of heterogeneity and minimal publication bias in the existing literature. Moderator variable analyses revealed that dual tasks significantly affect gait speed regardless of baseline mean gait speed or the type of dual task (arithmetic, language, memory, and motor). Moreover, our results align with studies that show impaired performance in people with PD when a dual task is added to walking, but did not meet the criteria for inclusion in this analysis [18,22,49,57–60].
By overcoming the limitations of assorted dual-task paradigms, our results provide important insight about cognitive-motor performances in PD. When considering the effect of dual tasks on walking in healthy older adults, similar analyses by Smith et al., [14] determined that older adults who walked faster than 1.0 m/s at baseline were significantly negatively affected by a dual task, slowing gait from an average of 1.21 m/s to 1.02 m/s [14]. Because Smith et al., [14] used different methods, direct comparisons of effect sizes are not possible with our findings. However, similarly qualifying our findings revealed a clinically meaningful decline from single-task (1.07 m/s) to dual-task gait speed (0.92 m/s) in people with PD [61]. Thus, our analysis builds on previous findings that healthy older adults demonstrate significant negative effects on gait speed with dual tasks.
The magnitude of the effect sizes for the gait related negative consequences of dual-task performances reported herein are comparable to the magnitude of the effectiveness for improving gait from common PD therapeutic approaches. For example, deep brain stimulation therapy provides a positive SMD for gait speed of 0.60 [62]. Similarly, auditory feedback provides similar magnitudes of effects on gait speed (SMD: 0.54) [63]. Additionally, exercise interventions demonstrate a similar positive effect on gait performances [64]. Our results establish that the negative effect of a dual task is of a similar magnitude to the positive effect of effective walking interventions, affirming that the decrements to dual-task walking in PD represent severe and disruptive impairments to gait.
4.2. The negative effect of dual tasks persists in moderator variable analyses
4.2.1. The Effect of Baseline Walking Speed on Dual Task Walking Performance in PD
Regardless of mean baseline walking speed, people with PD must overcome significant decrements when performing dual tasks. Although a slower initial walking speed could limit the negative effect of a dual task on gait speed, our standardized effects revealed that in studies with single-task gait speed lower than 1.1 m/s a large effect of a dual task on walking speed is present. However, these studies showed a relatively smaller effect size than studies whose mean single task gait speed was higher than 1.1 m/s at baseline. The current findings suggest that disease progression influences dual-task walking such that patients whose single-task gait speed was lower than 1.1 m/s exhibit slightly smaller declines to gait speed with a dual task. Previous evidence suggests that patients with PD are unable to prioritize gait with a dual task, ultimately increasing their risk of falls [20,65]. Consistent with previous studies, [66,67] our results suggest that PD patients with adequate baseline walking speeds may be able to prioritize walking as a protective mechanism to prevent falls. These findings are closely aligned with some reports in the literature [65,68], but not others [69,70], which may have been hindered by small samples that range in disease severity.
4.2.3. The Effect of Type of Dual Task on PD Walking Speed
Given previous results suggesting that different types of dual tasks may affect gait performance differently [18] or not at all [25], we conducted moderator variable analyses to quantify changes from single to dual-task gait speed within four types of dual tasks. The meta-analytic findings showed that tasks that heavily involve executive function skills significantly hindered gait speed demonstrated by large standardized effect sizes in arithmetic, language, and motor tasks, but a relatively smaller standardized effect in memory tasks. The analyzed studies confirmed the clinical consequences of each type of dual task for people with PD such that gait speed declines between −0.11 m/s to −0.18 m/s, similar to a previous meta-analysis of older adult dual-task walking performances [13]. These decrements are beyond a clinically meaningful difference [61], further supporting the significant impact that concurrent cognitive task performance has on gait speed in persons with PD. Concurrent tasks that challenge executive processes are involved in performing many common daily tasks. For instance, walking while talking requires executive function and language processes to walk while retrieving words and forming sentences [71]. The degraded executive processes that impair the performance of such common dual tasks likely contribute to decreased community mobility and independence in PD patients [3]. Our meta-analysis demonstrated that despite variation between study paradigms there was sufficient evidence to conclude that dual-task effects on walking speed in the presence of each of the tasks examined.
5. Conclusions
Overall, the findings provide new evidence on the effects of dual-task studies and PD walking speed. In each study, persons with PD walked slower during dual tasks regardless of mean single task gait speed or the type of concurrent task.
Acknowledgements
The authors would like to acknowledge Grace Kellaher for her assistance in aggregating data.
Footnotes
Conflict of Interest
The authors report no conflict of interest.
References
- [1].Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K, Stages in the development of Parkinson’s disease-related pathology, Cell and Tissue Research. 318 (2004) 121–34. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- [2].National I Parkinson Foundation Quality Improvement Initiative, Understanding Parkinson’s Disease: Causes and Statistics, (2016). [Google Scholar]
- [3].Schrag A, Jahanshahi M, Quinn NP, What contributes to quality of life in patients with Parkinson’s disease, Journal of Neurology, Neurosurgery & Psychiatry. 69 (2000) 308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Morris ME, Huxham F, McGinley J, Dodd K, Iansek R, The biomechanics and motor control of gait in Parkinson’s disease, Clinical Biomechanics. 16 (2001) 459–470. [DOI] [PubMed] [Google Scholar]
- [5].Hass CJ, Malczak P, Nocera JR, Stegemoller EL, Shukla A, Malaty I, Jacobsen CE, Okun MS, McFarland N, Quantitative normative gait data in a large cohort of ambulatory persons with Parkinson’s disease, PloS One. 7 (2012) e-42337. doi: 10.1371/journal.pone.0042337.t001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aarsland D, Larsen JP, Lim NG, Tanderberg E, Mental symptoms in Parkinson’s disease are important contributors to caregiver distress, International Journal of Geriatric Psychiatry. 14 (1999) 866–874. [PubMed] [Google Scholar]
- [7].Palavra NC, Naismith SL, Lewis SJ, Mild cognitive impairment in Parkinson’s disease: a review of current concepts, Neurol Res Int. 2013 (2013). doi: 10.1155/2013/576091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cooper CA, Mikos AE, Wood MF, Kirsch-Darrow L, Jacobson CE, Okun MS, Rodriguez RL, Bowers D, Fernandez HH, Does laterality of motor impairment tell us something about cognition in Parkinson disease?, Parkinsonism & Related Disorders. 15 (2009) 315–7. doi: 10.1016/j.parkreldis.2008.07.009. [DOI] [PubMed] [Google Scholar]
- [9].Takakusaki K, Functional neuroanatomy for posture and gait control, Journal of Movement Disorders. 10 (2017) 1–17. doi: 10.14802/jmd.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Plotnik M, Dagan Y, Gurevich T, Giladi N, Hausdorff JM, Effects of cognitive function on gait and dual tasking abilities in patients with Parkinson’s disease suffering from motor response fluctuations, Experimental Brain Research. 208 (2011) 169–79. doi: 10.1007/s00221-010-2469-y. [DOI] [PubMed] [Google Scholar]
- [11].Lundin-Olsson L, Nyberg L, Gustafson Y, “Stops walking when talking” as a predictor of falls in elderly people, The Lancet. 349 (1997) 617. doi: 10.1016/s0140-6736(97)24009-2. [DOI] [PubMed] [Google Scholar]
- [12].Ayers EI, Tow AC, Holtzer R, Verghese J, Walking while talking and falls in aging, Gerontology. 60 (2014) 108–13. doi: 10.1159/000355119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, Cockburn J, Cognitive motor interference while walking: a systematic review and meta-analysis, Neuroscience and Biobehavioral Reviews. 35 (2011) 715–28. doi: 10.1016/j.neubiorev.2010.08.008. [DOI] [PubMed] [Google Scholar]
- [14].Smith E, Cusack T, Blake C, The effect of a dual task on gait speed in community dwelling older adults: a systematic review and meta-analysis, Gait & Posture. 44 (2016) 250–8. doi: 10.1016/j.gaitpost.2015.12.017. [DOI] [PubMed] [Google Scholar]
- [15].Woollacott MH, Shumway-Cook A, Attention and the control of posture: a review of an emerging area of research, Gait & Posture. 16 (2002) 1–14. [DOI] [PubMed] [Google Scholar]
- [16].Kelly VE, Eusterbrock AJ, Shumway-Cook A, A review of dual-task walking deficits in people with Parkinson’s disease: motor and cognitive contributions, mechanisms, and clinical implications, Parkinson’s Disease. 2012 (2012) 918719. doi: 10.1155/2012/918719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Strouwen C, Test-retest reliability of dual-task outcome measures in people with Parkinson disease, Phys Ther. 96 (2015) 1276–1286. [DOI] [PubMed] [Google Scholar]
- [18].Brauer SG, Morris ME, Can people with Parkinson’s disease improve dual tasking when walking?, Gait & Posture. 31 (2010) 229–233. doi: 10.1016/j.gaitpost.2009.10.011. [DOI] [PubMed] [Google Scholar]
- [19].Fernandes Â, Sousa ASP, Rocha N, Tavares JMRS, Parkinson’s disease and cognitive-motor dual-task: is motor prioritization possible in the early stages of the disease?, Journal of Motor Behavior. 48 (2016) 377–383. doi: 10.1080/00222895.2015.1105194. [DOI] [PubMed] [Google Scholar]
- [20].Bloem BR, Grimbergen YA, van Dijk JG, Munneke M, The “posture second” strategy: a review of wrong priorities in Parkinson’s disease, Journal of the Neurological Sciences. 248 (2006) 196–204. doi: 10.1016/j.jns.2006.05.010. [DOI] [PubMed] [Google Scholar]
- [21].Fok P, Farrell M, McMeeken J, The effect of dividing attention between walking and auxiliary tasks in people with Parkinson’s disease, Human Movement Science. 31 (2012) 236–46. doi: 10.1016/j.humov.2011.05.002. [DOI] [PubMed] [Google Scholar]
- [22].Canning CG, Ada L, Johnson JJ, McWhirter S, Walking capacity in mild to moderate Parkinson’s disease, Archives of Physical Medicine and Rehabilitation. 87 (2006) 371–5. doi: 10.1016/j.apmr.2005.11.021. [DOI] [PubMed] [Google Scholar]
- [23].Mamikonyan E, Moberg PJ, Siderowf A, Duda JE, Have TT, Hurtig HI, Stern MB, Weintraub D, Mild cognitive impairment is common in Parkinson’s disease patients with normal Mini-Mental State Examination (MMSE) scores, Parkinsonism & Related Disorders. 15 (2009) 226–31. doi: 10.1016/j.parkreldis.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM, Dual tasking, gait rhythmicity, and Parkinson’s disease: which aspects of gait are attention demanding?, The European Journal of Neuroscience. 22 (2005) 1248–56. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- [25].O’Shea S, Morris ME, Iansek R, Dual task interference during gait in people with Parkinson disease: effects of motor versus cognitive secondary tasks, Physical Therapy. 82 (2002) 888–898. [PubMed] [Google Scholar]
- [26].Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement, PLoS Med. 6 (2009) e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Brown RG, Marsden CD, Dual task performance and processing resources in normal subjects and patients with Parkinson’s disease, Brain : A Journal of Neurology. 114 (1991) 215–231. [PubMed] [Google Scholar]
- [28].Galna B, Lord S, Daud D, Archibald N, Burn D, Rochester L, Visual sampling during walking in people with Parkinson’s disease and the influence of environment and dual-task, Brain Research. 1473 (2012) 35–43. doi: 10.1016/j.brainres.2012.07.017. [DOI] [PubMed] [Google Scholar]
- [29].Wu T, Hallett M, Neural correlates of dual task performance in patients with Parkinson’s disease, Journal of Neurology, Neurosurgery, and Psychiatry. 79 (2008) 760–6. doi: 10.1136/jnnp.2007.126599. [DOI] [PubMed] [Google Scholar]
- [30].Borenstein M, Hedges L, Rothstein H, Introduction to Meta-Analysis, 2007.
- [31].Alcock L, Galna B, Lord S, Rochester L, Characterisation of foot clearance during gait in people with early Parkinsons disease: deficits associated with a dual task, Journal of Biomechanics. 49 (2016) 2763–2769. doi: 10.1016/j.jbiomech.2016.06.007. [DOI] [PubMed] [Google Scholar]
- [32].Beck EN, Martens KAE, Almeida QJ, Freezing of gait in Parkinson’s disease: an overload problem?, PLoS One; San Francisco. 10 (2015) e0144986. doi: 10.1371/journal.pone.0144986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bond J, Morris ME, Goal-directed secondary motor tasks: their effects on gait in subjects with Parkinson disease, Archives of Physical Medicine and Rehabilitation. 81 (2000) 110–116. [DOI] [PubMed] [Google Scholar]
- [34].Canning CG, The effect of directing attention during walking under dual-task conditions in Parkinson’s disease, Parkinsonism & Related Disorders. 11 (2005) 95–9. doi: 10.1016/j.parkreldis.2004.09.006. [DOI] [PubMed] [Google Scholar]
- [35].Galletly R, Brauer SG, Does the type of concurrent task affect preferred and cued gait in people with Parkinson’s disease?, Australian Journal of Physiotherapy. 51 (2005) 175–180. doi: 10.1016/S0004-9514(05)70024-6. [DOI] [PubMed] [Google Scholar]
- [36].Harrison EC, McNeely ME, Earhart GM, The feasibility of singing to improve gait in Parkinson disease, Gait & Posture. 53 (2017) 224–229. doi: 10.1016/j.gaitpost.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lohnes CA, Earhart GM, The impact of attentional, auditory, and combined cues on walking during single and cognitive dual tasks in Parkinson disease, Gait & Posture. 33 (2011) 478–83. doi: 10.1016/j.gaitpost.2010.12.029. [DOI] [PubMed] [Google Scholar]
- [38].Lord S, Rochester L, Hetherington V, Allcock LM, Burn D, Executive dysfunction and attention contribute to gait interference in ‘off’ state Parkinson’s Disease, Gait & Posture. 31 (2010) 169–174. doi: 10.1016/j.gaitpost.2009.09.019. [DOI] [PubMed] [Google Scholar]
- [39].Plotnik M, Giladi N, Hausdorff JM, Bilateral coordination of gait and Parkinson’s disease: the effects of dual tasking, Journal of Neurology, Neurosurgery, and Psychiatry. 80 (2009) 347–50. doi: 10.1136/jnnp.2008.157362. [DOI] [PubMed] [Google Scholar]
- [40].Rochester L, Hetherington V, Jones D, Nieuwboer A, Willems A-M, Kwakkel G, Van Wegen E, Attending to the task: interference effects of functional tasks on walking in Parkinson’s disease and the roles of cognition, depression, fatigue, and balance, Archives of Physical Medicine and Rehabilitation. 85 (2004) 1578–1585. doi: 10.1016/j.apmr.2004.01.025. [DOI] [PubMed] [Google Scholar]
- [41].Rochester L, Galna B, Lord S, Burn D, The nature of dual-task interference during gait in incident Parkinson’s disease, Neuroscience. 265 (2014) 83–94. doi: 10.1016/j.neuroscience.2014.01.041. [DOI] [PubMed] [Google Scholar]
- [42].Salazar RD, Ren X, Ellis TD, Toraif N, Barthelemy OJ, Neargarder S, Cronin-Golomb A, Dual tasking in Parkinson’s disease: cognitive consequences while walking., Neuropsychology. 31 (2017) 613–623. doi: 10.1037/neu0000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Stegemoller E, Wilson J, Hazamy AA, Shelley M, Okun MS, Altmann LJ, Hass CJ, Associations between cognitive and gait performance during single and dual-task walking in people with Parkinson’s disease, Phys Ther. 94 (2014) 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wild LB, de Lima DB, Balardin JB, Rizzi L, Giacobbo BL, Oliveira HB, de Lima A, Peyre-Tartaruga LA, Rieder CR, Bromberg E, Characterization of cognitive and motor performance during dual-tasking in healthy older adults and patients with Parkinson’s disease, Journal of Neurology. 260 (2013) 580–9. doi: 10.1007/s00415-012-6683-3. [DOI] [PubMed] [Google Scholar]
- [45].Yogev-Seligmann G, Rotem-Galili Y, Dickstein R, Giladi N, Hausdorff JM, Effects of explicit prioritization on dual task walking in patients with Parkinson’s disease, Gait & Posture. 35 (2012) 641–6. doi: 10.1016/j.gaitpost.2011.12.016. [DOI] [PubMed] [Google Scholar]
- [46].Yogev-Seligmann G, Giladi N, Gruendlinger L, Hausdorff JM, The contribution of postural control and bilateral coordination to the impact of dual tasking on gait, Experimental Brain Research. 226 (2013) 81–93. doi: 10.1007/s00221-013-3412-9. [DOI] [PubMed] [Google Scholar]
- [47].Studenski SA, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach JS, Chandler J, Cawthon P, Barrett Connor E, Nevitt M, Visser M, Kritchevsky SB, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik JM, Gait speed and survival in older adults, JAMA. 305 (2011) 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Paul SS, Canning CG, Sherrington C, Lord SR, Close JC, Fung VS, Three simple clinical tests to accurately predict falls in people with Parkinson’s disease, Movement Disorders: Official Journal of the Movement Disorder Society. 28 (2013) 655–62. doi: 10.1002/mds.25404. [DOI] [PubMed] [Google Scholar]
- [49].Strouwen C, Molenaar EA, Keus SH, Munks L, Heremans E, Vandenberghe W, Bloem BR, Nieuwboer A, Are factors related to dual-task performance in people with Parkinson’s disease dependent on the type of dual task?, Parkinsonism & Related Disorders. 23 (2016) 23–30. doi: 10.1016/j.parkreldis.2015.11.020. [DOI] [PubMed] [Google Scholar]
- [50].Borenstein M, Hedges LV, Higgins JPT, Comprehensive Meta Analysis Version 3.0, (2004). https://www.meta-analysis.com/downloads/Meta-Analysis%20Manual%20V3.pdf (accessed August 21, 2018).
- [51].Borenstein M, Hedges LV, Higgins JPT, Rothstein HR, Introduction to Meta-Analysis, John Wiley & Sons, 2011. [Google Scholar]
- [52].Higgins JPT, Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified, Int J Epidemiol. 37 (2008) 1158–1160. doi: 10.1093/ije/dyn204. [DOI] [PubMed] [Google Scholar]
- [53].Higgins JP, Green S, eds., Cochrane Handbook for Systematic Reviews of Interventions, John Wiley & Sons, Ltd, Chichester, UK, 2008. doi: 10.1002/9780470712184. [DOI] [Google Scholar]
- [54].Spiegelhalter DJ, Abrams KR, Myles JP, Bayesian approaches to clinical trials and health-care evaluation, John Wiley & Sons, Ltd, West Sussex, England, 2004. [Google Scholar]
- [55].Ren S, Oakley JE, Stevens JW, Incorporating Genuine Prior Information about Between-Study Heterogeneity in Random Effects Pairwise and Network Meta-analyses, Med Decis Making. 38 (2018) 531–542. doi: 10.1177/0272989X18759488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Duval S, Tweedie R, Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias and meta-analysis, Biometrics. 56 (2000) 455–463. [DOI] [PubMed] [Google Scholar]
- [57].Baker K, Rochester L, Nieuwboer A, The effect of cues on gait variability--reducing the attentional cost of walking in people with Parkinson’s disease, Parkinsonism & Related Disorders. 14 (2008) 314–20. doi: 10.1016/j.parkreldis.2007.09.008. [DOI] [PubMed] [Google Scholar]
- [58].Chenji G, Wright ML, Chou KL, Seidler RD, Patil PG, Parkinsonian gait improves with bilateral subthalamic nucleus deep brain stimulation during cognitive multi-tasking, Parkinsonism & Related Disorders. 38 (2017) 72–79. doi: 10.1016/j.parkreldis.2017.02.028. [DOI] [PubMed] [Google Scholar]
- [59].Mirelman A, Bernad-Elazari H, Thaler A, Giladi-Yacobi E, Gurevich T, Gana-Weisz M, Saunders-Pullman R, Raymond D, Doan N, Bressman SB, Marder KS, Alcalay RN, Rao AK, Berg D, Brockmann K, Aasly J, Waro BJ, Tolosa E, Vilas D, Pont-Sunyer C, Orr-Urtreger A, Hausdorff JM, Giladi N, Arm swing as a potential new prodromal marker of Parkinson’s disease, Movement Disorders: Official Journal of the Movement Disorder Society. 31 (2016) 1527–1534. doi: 10.1002/mds.26720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].LaPointe LL, Stierwalt JAG, Maitland CG, Talking while walking: cognitive loading and injurious falls in Parkinson’s disease, International Journal of Speech-Language Pathology. 12 (2010) 455–459. doi: 10.3109/17549507.2010.486446. [DOI] [PubMed] [Google Scholar]
- [61].Hass CJ, Bishop M, Moscovich M, Stegemöller EL, Skinner J, Malaty IA, Wagle Shukla A, McFarland N, Okun MS, Defining the clinically meaningful difference in gait speed in persons With Parkinson disease, Journal of Neurologic Physical Therapy. 38 (2014) 233–238. doi: 10.1097/NPT.0000000000000055. [DOI] [PubMed] [Google Scholar]
- [62].Roper JA, Kang N, Ben J, Cauraugh JH, Okun MS, Hass CJ, Deep brain stimulation improves gait velocity in Parkinson’s disease: a systematic review and meta-analysis, Journal of Neurology. 263 (2016) 1195–203. doi: 10.1007/s00415-016-8129-9. [DOI] [PubMed] [Google Scholar]
- [63].Spaulding SJ, Barber B, Colby M, Cormack B, Mick T, Jenkins ME, Cueing and gait improvement among people with Parkinson’s disease: a meta-analysis, Archives of Physical Medicine and Rehabilitation. 94 (2013) 562–70. doi: 10.1016/j.apmr.2012.10.026. [DOI] [PubMed] [Google Scholar]
- [64].Shen X, Wong-Yu IS, Mak MK, Effects of exercise on falls, balance, and gait ability in Parkinson’s disease: a meta-analysis, Neurorehabilitation and Neural Repair. 30 (2016) 512–27. doi: 10.1177/1545968315613447. [DOI] [PubMed] [Google Scholar]
- [65].Bloem BR, Grimbergen YAM, Cramer M, Valkenburg VV, “Stops Walking when Talking” does not predict falls in Parkinson’s disease, Annals of Neurology. 48 (2000) 268. [DOI] [PubMed] [Google Scholar]
- [66].Lindenberger U, Marsiske M, Baltes PB, Memorizing while walking: increase in dual-task costs from young adulthood to old age, Psychology and Aging. 15 (2000) 417–436. [DOI] [PubMed] [Google Scholar]
- [67].Yogev-Seligmann G, Hausdorff JM, Giladi N, Do we always prioritize balance when walking? Towards an integrated model of task prioritization, Movement Disorders: Official Journal of the Movement Disorder Society. 27 (2012) 765–70. doi: 10.1002/mds.24963. [DOI] [PubMed] [Google Scholar]
- [68].Smulders K, Esselink RA, Weiss A, Kessels RP, Geurts AC, Bloem BR, Assessment of dual tasking has no clinical value for fall prediction in Parkinson’s disease, Journal of Neurology. 259 (2012) 1840–7. doi: 10.1007/s00415-012-6419-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Plotnik M, Giladi N, Dagan Y, Hausdorff JM, Postural instability and fall risk in Parkinson’s disease: impaired dual tasking, pacing, and bilateral coordination of gait during the “ON” medication state, Experimental Brain Research. 210 (2011) 529–38. doi: 10.1007/s00221-011-2551-0. [DOI] [PubMed] [Google Scholar]
- [70].Heinzel S, Maechtel M, Hasmann SE, Hobert MA, Heger T, Berg D, Maetzler W, Motor dual-tasking deficits predict falls in Parkinson’s disease: a prospective study, Parkinsonism & Related Disorders. 26 (2016) 73–7. doi: 10.1016/j.parkreldis.2016.03.007. [DOI] [PubMed] [Google Scholar]
- [71].Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD, The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis, Cognitive Psychology. 41 (2000) 49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]


