Abstract
Background
As healthcare workers (HCWs) are at high risk for infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), they have priority for receipt of the coronavirus disease 2019 (COVID-19) vaccine. The inactivated SARS-CoV-2 vaccine has been used in Indonesia to induce an antibody response against SARS-CoV-2 infection in HCWs. However, information regarding the kinetics of antibodies induced by this vaccine remains scarce.
Objective
To investigate the magnitude and durability of antibodies against the spike (S) protein (anti-S) in fully-vaccinated HCWs using an electrochemiluminescence immunoassay.
Results
Seroconversion of anti-S antibodies was observed among 159 (99.4%) of 160 HCWs without prior SARS-CoV-2 infection 14 days after full-dose vaccination. The level of anti-S antibodies decreased significantly by day 42 post-vaccination compared with day 14 post-vaccination, but persisted for up to 98 days post-vaccination. In contrast, vaccinated HCWs with prior SARS-CoV-2 infection had significantly higher, stable levels of anti-S antibodies compared with vaccinated HCWs without prior SARS-CoV-2 infection.
Conclusion
The remarkable decline and lower level of anti-S antibodies among HCWs without prior SARS-CoV-2 infection may indicate the need for an additional booster dose of SARS-CoV-2 vaccine for protection against COVID-19. This study of antibody responses induced by the inactivated SARS-CoV-2 vaccine among HCWs may contribute to future policy decisions regarding vaccination.
Keywords: Antibody, Healthcare workers, SARS-CoV-2, Vaccination
Introduction
Healthcare workers (HCWs) are at risk for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection due to increased occupational exposure to SARS-CoV-2 (Nguyen et al., 2020). As well as being beneficial for the HCWs themselves, protecting HCWs from SARS-CoV-2 infection is important to prevent disease transmission in healthcare and community settings (Anonymous, 2020). In addition, protecting HCWs from coronavirus disease 2019 (COVID-19) is crucial for the preservation and protection of national healthcare systems (Anonymous, 2020). Therefore, HCWs have had priority for receipt of the COVID-19 vaccine since the first vaccine was approved and deployed in Indonesia (Ophinni et al., 2020).
CoronaVac, an inactivated SARS-CoV-2 and aluminium hydroxide adjuvanted vaccine (Sinovac Life Sciences, Beijing, China), was used in the initial COVID-19 vaccination programme in Indonesia. The vaccine elicited receptor-binding domain (RBD)-specific immunoglobulin G and neutralizing antibodies 14 days after the second dose, either on days 0 and 14 or on days 0 and 28 (Zhang et al., 2021). Antibody levels remained elevated 28 days after the last dose of vaccine (Zhang et al., 2021). However, the magnitude and durability of the humoral immune response beyond this time point have not been fully elucidated to date. A better understanding of the kinetics of antibodies to SARS-CoV-2 following vaccination with the inactivated SARS-CoV-2 vaccine is important to develop strategies to maximize the coverage and impact of the vaccine among HCWs.
This retrospective cohort study at Siloam Teaching Hospital, Indonesia enrolled 160 HCWs who had not been infected previously with SARS-CoV-2, based on the results of reverse-transcriptase polymerase chain reaction testing for total antibodies against the SARS-CoV-2 nucleocapsid protein (Elecsys anti-SARS-CoV-2, Roche Diagnostics, Basel, Switzerland) performed regularly by the hospital. This study also included 12 HCWs with documented molecular and/or serological evidence of SARS-CoV-2 infection. All participants had received two doses of 3 µg CoronaVac (Sinovac Life Sciences) intramuscularly 2 weeks apart.
The concentration of total antibodies against the RBD of the spike (S) protein (anti-S) was tested using an Elecsys anti-SARS-CoV-2 S electrochemiluminescence immunoassay with a Cobas e601 analyser (Roche Diagnostics), in accordance with the manufacturer's instructions. The linear measurement range of the assay was 0.4–250 U/mL. The cut-off value for a positive result proposed by the manufacturer is ≥0.8 U/mL. Serum samples for both groups were tested at four time points: 14, 42, 70 and 98 days after full-dose vaccination.
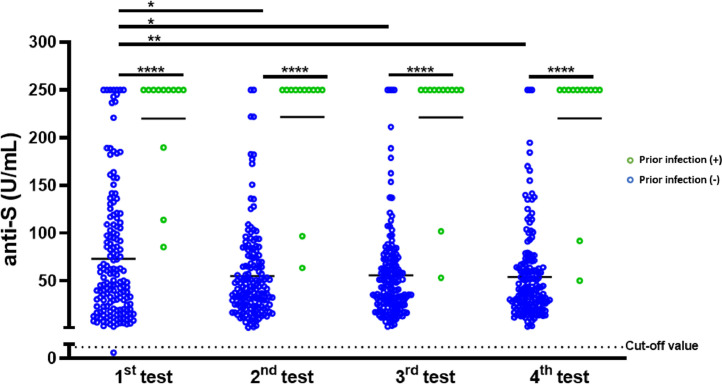
The mean age of the 160 HCWs (58 men and 102 women) without prior infection with SARS-CoV-2 was 28.1 years [standard deviation (SD) 5 years]. The mean age of the 12 HCWs (three men and nine women) with prior SARS-CoV-2 infection was 30.1 years (SD 4.9 years). The dynamic changes in anti-S antibody concentrations in the two groups are shown in Figure 1 . Seroconversion rates of anti-S antibodies were observed among 159 (99.4%) of 160 HCWs without prior SARS-CoV-2 infection 14 days after full-dose vaccination. The mean anti-S antibody level 14 days after full-dose vaccination was 73.2 U/mL (SD 68 U/mL), and this was significantly lower compared with the anti-S antibody level among HCWs with prior SARS-CoV-2 infection (mean 73.2 vs 219.9 U/mL; P<0.0001). Remarkable differences in anti-S antibody concentrations were also observed for different testing time points between vaccinated HCWs with prior SARS-CoV-2 infection and those without prior SARS-CoV-2 infection (P<0.0001).
Figure 1.
Serial measurement of the anti-S antibody response after vaccination with the inactivated severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) vaccine (CoronaVac). Titres of antibodies against the receptor-binding domain of the spike (S) protein (anti-S) among 160 healthcare workers (HCWs) without prior SARS-CoV-2 infection and 12 HCWs with prior SARS-CoV-2 infection were measured 14, 42, 70 and 98 days post-vaccination. The black dotted line represents the positivity cut-off value of the Elecsys anti-SARS-CoV-2 S quantitative electrochemiluminescence immunoassay. Statistical analysis was performed using the Mann–Whitney test and the Friedman test: *P<0.05, ⁎⁎P<0.01, ⁎⁎⁎⁎P<0.0001.
Compared with the first time point, the levels of anti-S antibodies among HCWs without prior SARS-CoV-2 infection were significantly lower at the second (mean 73.2 vs 55.2 U/mL; P<0.05), third (mean 73.2 vs 55.9 U/mL; P<0.05) and fourth (mean 73.2 vs 54.1 U/mL; P<0.01) time points. In contrast, the anti-S antibody concentration among HCWs with prior SARS-CoV-2 infection remained elevated at the different measurement time points. This result suggests that vaccination may boost the memory immune cells that develop after infection, resulting in a greater antibody response that is likely to be more protective and longer lasting against SARS-CoV-2 infection (Abbasi, 2021).
The presence of antibodies against the RBD of the S protein, a critical region for interaction with host cell receptors, is important due to its capacity to block virus entry, thus preventing infection and transmission (Jeyanathan et al., 2020). In addition, the level of antibodies against the RBD has been shown to correlate strongly with the level of protective neutralizing antibodies (Premkumar et al., 2020). Therefore, the measurement of antibodies against the RBD can be considered as a surrogate marker for the presence of anti-SARS-CoV-2 neutralizing antibodies (Premkumar et al., 2020; Resman Rus et al., 2021).
A positive correlation between anti-S antibodies detected using the Elecsys anti-S immunoassay and neutralizing antibodies measured by the SARS-CoV-2-specific virus neutralization test has been shown in post-COVID-19 patients (Resman Rus et al., 2021). In addition, another study showed that a cut-off value of 133 U/mL for the anti-S antibody level, as measured with the Elecsys anti-S assay, can be used to predict the presence of neutralizing antibodies after SARS-CoV-2 infection (Resman Rus et al., 2021). Given that the Elecsys anti-S immunoassay is a rapid high-throughput immunoassay (Resman Rus et al., 2021), it would be suitable to assess natural or vaccine-induced immunity in a large-scale screening. However, further investigation will be necessary to determine whether this cut-off value for anti-S antibodies is applicable in predicting the protective antibody response post-vaccination. Furthermore, compared with the the anti-S antibody level induced by the SARS-CoV-2 mRNA vaccine among healthy volunteers and measured by the Elecsys anti-S immunoassay (Bradley et al., 2021), the anti-S antibody level induced by the inactivated SARS-CoV-2 vaccine was lower, indicating that an additional booster dose of the vaccine may be necessary for HCWs.
Overall, although this study found detectable levels of anti-S antibodies among HCWs without prior SARS-CoV-2 infection for up to 98 days after full-dose vaccination, the level was significantly lower compared with HCWs with prior SARS-CoV-2 infection. It is uncertain whether this level of anti-S antibodies will be able to protect vaccinated HCWs against SARS-CoV-2 infection. Thus, further advanced techniques, such as the plaque reduction neutralization test, are needed to establish correlation between the protective anti-S antibody level induced by the inactivated SARS-CoV-2 vaccine and overall adaptive humoral and cellular immunity. Moreover, although molecular and serological criteria were used to exclude previous SARS-CoV-2 exposure in this group of uninfected HCWs, the possibility that some of the HCWs in this group had previous undetected infection in early 2020, before molecular testing was conducted regularly at the hospital, cannot be excluded. Antibodies against the nucleocapsid protein among undetected cases of infection at the start of the pandemic may have fallen below the detection level in the sampling baseline data; therefore, selection bias may have affected the study findings. In the current situation, where Indonesia is facing a significant surge in the number of active cases each day, and HCWs have been vaccinated with the inactivated SARS-CoV-2 vaccine, this study provides valuable information regarding future policy decisions for HCW vaccination programmes.
Acknowledgments
Conflict of interest statement
None declared.
Funding
None.
Ethical approval
This study was approved by the Research Ethics Committee of the Faculty of Medicine, Pelita Harapan University (No: 137/K-LKJ/ETIK/IV/2021).
Author contributions
Design of research studies: CC, RW and NL.
Data acquisition: CC.
Data analysis: CC, RW, NL and IS.
Interpretation of results: CC, RW, NL and IS.
Writing the manuscript: CC, RW, NL and IS.
References
- Abbasi J. Study suggests lasting immunity after COVID-19, with a big boost from vaccination. JAMA. 2021;326:376–377. doi: 10.1001/jama.2021.11717. [DOI] [PubMed] [Google Scholar]
- Anonymous. COVID-19: protecting health-care workers. Lancet. 2020;395:922. doi: 10.1016/S0140-6736(20)30644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley BT, Bryan A, Fink SL, Goecker EA, Roychoudhury P, Huang ML, et al. Anti-SARS-CoV-2 antibody levels measured by the AdviseDx SARS-CoV-2 assay are concordant with previously available serologic assays but are not fully predictive of sterilizing immunity. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.00989-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20:615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LH, Drew DA, Graham MS, Joshi AD, Guo CG, Ma W, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475–e483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophinni Y, Hasibuan AS, Widhani A, Maria S, Koesnoe S, Yunihastuti E, et al. COVID-19 vaccines: current status and implication for use in indonesia. Acta Med Indones. 2020;52:388–412. [PubMed] [Google Scholar]
- Premkumar L, Segovia-Chumbez B, Jadi R, Martinez DR, Raut R, Markmann A, et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol. 2020;5:eabc8413. doi: 10.1126/sciimmunol.abc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resman Rus K, Korva M, Knap N, Avsic Zupanc T, Poljak M. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J Clin Virol. 2021;139 doi: 10.1016/j.jcv.2021.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]