Abstract
Post-acute coronavirus disease 2019 (COVID-19) syndrome is a novel, poorly understood clinical entity with life-impacting ramifications. Patients with this syndrome, also known as "COVID-19 long-haulers," often present with nonspecific ailments involving more than one body system. The most common complaints include dyspnea, fatigue, brain fog, and chest pain. There currently is no single agreed-upon definition for post-acute COVID-19 syndrome, but most agree that criterion for this syndrome is the persistence of mental and physical health consequences after initial infection. Given the millions of acute infections in the United States over the course of the pandemic, perioperative providers will encounter these patients in clinical practice in growing numbers. Symptoms of the COVID-19 long-haulers should not be minimized, as these patients are at higher risk for postoperative respiratory complications and perioperative mortality for up to seven weeks after initial illness. Instead, a cautious multidisciplinary preoperative evaluation should be performed. Perioperative care should be viewed through the prism of best practices already in use, such as avoidance of benzodiazepines in patients with cognitive impairment and use of lung-protective ventilation. Recommendations especially relevant to the COVID-19 long-haulers include assessment of critical care myopathies and neuropathies to determine suitable neuromuscular blocking agents and reversal, preoperative workup of insidious cardiac or pulmonary pathologies in previously healthy patients, and, thorough medication review, particularly of anticoagulation regimens and chronic steroid use. In this article, the authors define the syndrome, synthesize the available scientific evidence, and make pragmatic suggestions regarding the perioperative clinical care of COVID-19 long-haulers.
Key Words: post-acute COVID-19 syndrome, long COVID, post-viral syndrome, perioperative considerations
Introduction
As the rate of new respiratory syndrome coronavirus 2 (SARS-CoV-2) infections continues to increase with the dominance of the delta variant, there are a growing number of reports describing a post-acute coronavirus disease 2019 (COVID-19) syndrome.1 Patients with this syndrome, colloquially known as “COVID-19 long-haulers,” present with a constellation of nonspecific symptoms of mild-to-life-threatening consequences.2 Given the millions of acute infections with SARS-CoV-2 around the globe, clinicians will encounter COVID-19 long-haulers in practice in the aftermath of the pandemic. There are no current recommendations that would aid anesthesiologists when caring for these patients because of the novelty and the current fragmentary understanding of post-acute COVID-19 syndrome (PACS). Thus, the authors gathered available data on PACS and applied it to the perioperative setting to define the syndrome, describe the available scientific evidence, and make pragmatic recommendations regarding approaches to clinical care of the COVID-19 long-haulers.
Methods
A literature review of the US National Library of Medicine Database and Excerpta Medica Database was performed from November 1, 2019 through July 31, 2021 for the medical subject headings and key words related to long-COVID-19, chronic COVID-19 syndrome, COVID-19 long-haulers, post-acute COVID-19 syndrome, and post-COVID-19 syndrome. The reference lists of all included citations were hand-searched to identify any additional relevant studies. Abstracts were screened for clinical studies and case reports reporting on adult patients (aged ≥18 years) at least 28 days after acute COVID-19 infection.
Summary of Study Characteristics
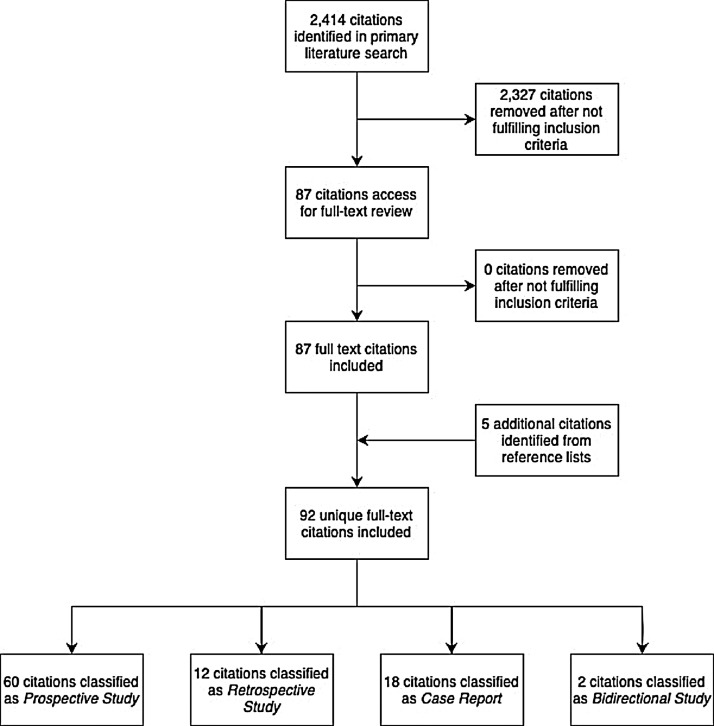
After the removal of duplicate citations, the literature search yielded a total of 2,414 unique citations. Of the potentially eligible abstracts identified, 2,327 did not satisfy the inclusion criteria based on title and abstract alone. The remaining 87 abstracts had their full-text citations retrieved for review. Five additional citations were identified after hand-searching the reference list of the included studies; therefore, the review ultimately encompassed the findings of 92 full-text citations. Specifically, these included 60 prospective studies,3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62 18 case reports,63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80 12 retrospective studies,81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92 and two bidirectional studies.93 , 94 There were no randomized controlled trials identified. A full-flow diagram for study inclusion can be seen in Figure 1; 40 publications reported on PACS prevalence, symptomatology, risk factors, and outcomes.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 , 23, 24, 25, 26 , 28, 29, 30, 31, 32, 33, 34, 35 , 63 , 81, 82, 83, 84, 85, 86 , 93 , 94 The remaining 52 articles addressed organ-specific sequelae of PACS, including 17 neuropsychiatric studies,36, 37, 38, 39, 40, 41, 42, 43, 44 , 64, 65, 66, 67, 68, 69 , 92 16 cardiovascular studies,45, 46, 47, 48, 49, 50, 51, 52, 53, 54 , 70, 71, 72, 73, 74 , 87 ten pulmonary studies,55, 56, 57, 58, 59, 60 , 75, 76, 77 , 88 three hematologic studies,62 , 80 , 91 three gastrointestinal studies,78 , 79 , 89 and three endocrine studies.27 , 61 , 90 The populations studied were heterogeneous, including patients who did not require hospitalization, those hospitalized for more severe illness, and those who required admission to the intensive care unit (ICU). Additionally, most studies evaluated patients one-six months after acute disease, with only two articles19 , 25 having study periods of 12 months.
Fig 1.
Study selection diagram.
Definition of Post-Acute COVID-19 Syndrome
Although there are no established criteria for PACS, most publications agree on a basic premise: the persistence of mental and physical health consequences after initial infection.95 However, the exact temporal course of long COVID currently is undetermined, and no single definition is universally employed. According to the Centers for Disease Control and Prevention, the criteria for PACS are met if symptoms persist for >four weeks.1 Meanwhile, the National Institute for Health and Care Excellence requires a 12-week period to make the diagnosis.96 At this time, studies that directly investigate the temporal course of PACS are missing. Moreover, while very sensitive, these definitions encompass a heterogeneous patient population of those recovered from asymptomatic or mild infection in addition to those who were severely ill.1 Notably, long-term consequences of a critical illness, also known as “postintensive care syndrome” (PICS), are now being considered as part of PACS.97 Given this ambiguity, subgroup analyses concentrating on severity of illness are needed, and as more data emerges, definitions are likely to evolve. For example, expanding PACS to include the subcategory of PICS for patients who required intensive care would add clarity to current terminology. Nevertheless, for the purpose of this article, the broad definition of PACS established by the Centers for Disease Control and Prevention is used, with a goal of making pragmatic suggestions regarding the perioperative clinical care of all patients with chronic sequelae of COVID-19.
Epidemiology
The reported prevalence of PACS among the collected reports ranged from roughly 20%61 to 95%.10 Fatigue and dyspnea were the most common symptoms, usually occurring in more than half of the studied population.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 , 23, 24, 25, 26 , 28, 29, 30, 31, 32, 33, 34, 35 , 63 , 81, 82, 83, 84, 85, 86 , 93 , 94 Other frequently reported complaints included smell and taste disturbances, cough, myalgias and arthralgias, sleep disturbances, anxiety, depression, post-traumatic stress disorder (PTSD), and cognitive impairment.2 Lastly, limited numbers of individuals reported palpitations, hair loss, diarrhea, and rashes.2
The authors found four studies that evaluated long-term mortality.19 , 31 , 85 , 86 An analysis of the US Veterans Affairs’ data estimated mortality at six months to be about eight per 1,000 nonhospitalized persons, and 29 per 1,000 hospitalized persons.85 Meanwhile, data from the UK’s National Health Service found a significantly higher mortality rate among previously hospitalized patients—12.3% at an average follow-up period of 140 days.86 This National Health Service data also showed a readmission rate of 29.4%, which was comparable to the 19.9% readmission rate at 60 days reported by a separate VA study.31 Additional studies validating these findings are needed to risk-stratify these patients more accurately.
Several reports revealed potential risk factors for developing chronic disease.7 , 98 Severity of acute illness is associated most strongly with long-term sequelae; thus, ICU survivors have the highest risk.6 Other factors that were associated with long-term sequelae included higher body mass index, preexisting respiratory disease, older age, female sex, and Black/Asian/minority ethnicities.33 Abnormal radiologic findings, reduced quality-of-life scores, and decreased pulmonary function on spirometry found on follow-up examination after acute disease also corresponded to an increased risk of developing PACS.16 Anesthesiologists should be vigilant when caring for patients with these risk factors and consider further preoperative workup when encountering patients with symptoms consistent with PACS.
Review of PACS and Its Perioperative Implications
Current reports suggest that COVID-19 long-haulers experience sequelae in multiple body systems, though the mechanisms of organ-specific dysfunction are unclear. Possible causes include virus-specific tissue damage,99 dysregulated immune and inflammatory response to the initial insult,100 and PICS.101 Although not elucidated fully, these have significant consequences on anesthetic care.
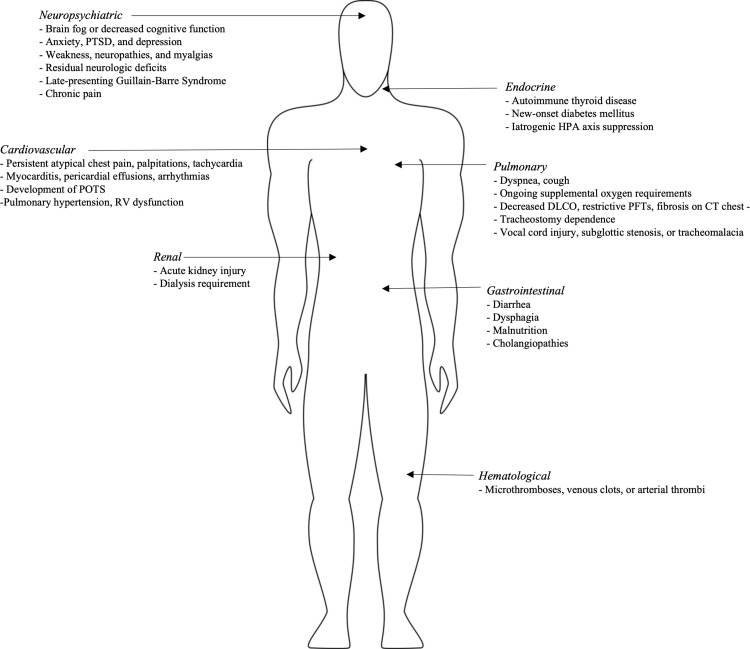
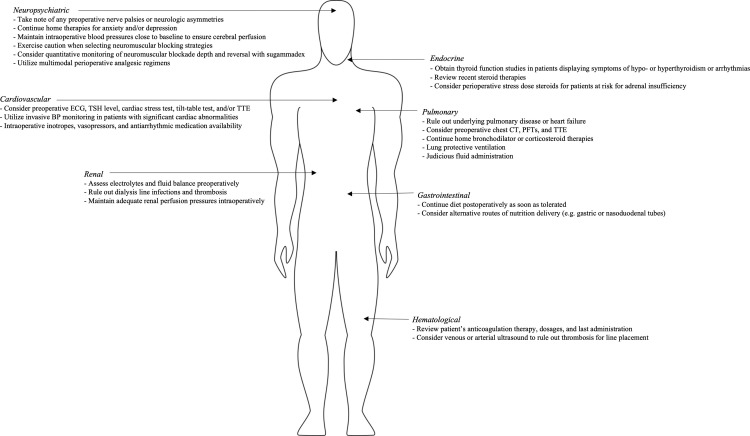
Given the novelty and scientific uncertainty, the overall approach to perioperative management should be pragmatic, with strict adherence to previously established standards of care. Preoperatively, symptoms should not be minimized but addressed in a multidisciplinary fashion. Deferring evaluation to the day of surgery should be strongly discouraged, except for urgent or emergent situations. As such, institutions need to develop preoperative evaluation and risk-assessment protocols for patients who previously had COVID-19.102 Until more specific scientific evidence emerges, care of COVID-19 long-haulers should be viewed through the prism of best practices already in use. Such practices are discussed below, categorizing them by organ systems. A summary of symptoms can be viewed in Figure 2 , and this review’s key recommendations in Figure 3 .
Fig 2.
Summary of common PACS symptoms.
Fig 3.
Summary of pragmatic perioperative recommendations. Abbreviations: PTSD, posttraumatic stress disorder; POTS, postural orthostatic tachycardia syndrome; RV, right ventricle; ECG, electrocardiogram; TSH, thyroid-stimulating hormone; TTE, transesophageal echocardiogram; CT, computed tomography; DLCO, diffusing capacity of carbon monoxide; PFTs, pulmonary function tests; HPA, hypothalamic-pituitary-adrenal.
Neuropsychiatric
Several neuropsychiatric sequelae of PACS that are relevant to anesthesiologists have been described in the emerging literature. These include cognitive dysfunction, anxiety, depression, PTSD, chronic pain, and complications of acute structural lesions. Dysfunction in reasoning, spatial planning, problem solving, and verbal fluency have been found to be the basis of what is commonly referred to as “brain fog.”103 Numerous reports of residual cognitive dysfunction have been published, estimating significant cognitive deficits in about one-third of recovered individuals who did not require hospitalization, and in as high as two-thirds of recovered individuals who required hospitalization for acute infection.39, 40, 41, 42 , 64 Although severity of initial disease was found to be a risk factor for cognitive problems, even patients with mild disease were more likely to develop cognitive deficits compared with matched controls.39 Among other psychiatric sequelae of PACS, anxiety and depression were reported to occur in 12%-to- 47% of patients, with higher rates in individuals requiring hospitalization.36 Symptoms consistent with PTSD also have been reported in roughly 10% of recovered patients, regardless of whether they were hospitalized or not.37 , 38 Additionally, recent articles also commented on chronic pain experienced by COVID-19 long-haulers.6 , 20 , 44 , 92 Myalgias, arthralgias, and headaches are among the most common complaints with quality-of-life implications, and 1 study noting the associated use of pain medications (including non-opioids).44 and quality-of-life implications of moderate-to-severe pain. Finally, many severely ill individuals suffered structural damage from ischemic or hemorrhagic strokes, anoxic brain injury, or seizures.104, 105, 106 Other serious neurologic sequelae stemmed from immune-mediated reactions, such as acute disseminated myelitis, encephalitis, vestibular neuritis, and late-presenting Guillain-Barré Syndrome.67, 68, 69 A follow-up study of patients developing neurologic manifestations during acute infection found that about nine in ten patients reported persisting deficits at six months, reflecting the pervasive and significant neurologic disability encountered in clinical practice.39
Neuropsychiatric sequelae of PACS have important implications on perioperative care. First, initial evaluation should consist of focused history and physical examination; noting baseline deficits, palsies, and asymmetries is especially important.107 , 108 Patients with anxiety/depression should have their therapies continued and offered preoperative premedication.109 However, due to an increased risk of postoperative delirium and cognitive dysfunction, benzodiazepine use is generally discouraged in individuals older than 65 and in patients experiencing cognitive dysfunction.110 Additionally, although low doses of ketamine are being used now as a therapy for PTSD, long-haulers with PTSD may not benefit from its use for sedation due to the risk of traumatic hallucinations with anesthetic doses.111 Moreover, the functionality of cerebral autoregulation is unknown, especially in individuals with anoxic brain injury, strokes, or dysautonomia, making cerebral perfusion pressure potentially more dependent on adequate blood pressure.112 Until more data are available, maintaining mean arterial pressure close to baseline is encouraged. Furthermore, neuromuscular blocking strategies should take into consideration risk factors for hyperkalemic response in individuals with history of strokes, immobility, and denervated muscles.113 Patients with critical illness neuropathy especially may benefit from quantitative monitoring of neuromuscular blockade depth, like acceleromyography, and reversal with sugammadex.114 Finally, for patients experiencing chronic pain, multimodal analgesia is paramount, with specific concentration on neuropathic modalities in individuals suffering from critical-illness neuropathy.115 Risk of opioid dependence in COVID-19 long-haulers has not been evaluated; however, individuals with PICS have been shown to be at increased risk and should be planned for accordingly.116 For this reason, these patients may be good candidates for regional or neuraxial anesthesia; nevertheless, individuals suffering from acquired peripheral or inflammatory neuropathies, such as critical-illness neuropathy or Guillain-Barré Syndrome, require particular caution and discussion with their anesthesiologist. The limited safety data on regional or neuraxial techniques in patients with neuropathies currently are equivocal. As such, the American Society of Regional Anesthesia recommends careful discussion of risks and benefits on a case-by-case basis.117
Cardiovascular
Anesthesiologists especially should be aware of the substantial cardiovascular disease experienced by COVID-19 survivors. Chest pain and palpitations are the most common subjective findings.2 Objectively, individuals recovering from acute infection have been found to be at risk for lasting vascular,45 , 70 pericardial,71 and myocardial inflammation.46 In studies of COVID-19 survivors evaluated with cardiac magnetic resonance, ongoing cardiac inflammation was present in anywhere from 0%-to-60% of participants.46, 47, 48, 49 These findings suggest that long-term sequelae, such as arrhythmias and heart failure, are possible even in seemingly healthy patients. This was substantiated recently by evaluations of resting heart rates after acute illness.50 , 51 In one of these studies, COVID-19-positive individuals experienced prolonged relative tachycardia that lasted on average 79 days after symptom onset; 13.7% of patients did not return to resting heart rate baseline until after 133 days.50 Those previously hospitalized appear to be at risk for even more severe sequelae. For example, a 140-day follow-up study in the United Kingdom found the risk of heart failure, arrhythmias, myocardial infarction, and stroke to be three times that of matched controls.72 Other complications have been reported as well. For instance, development of postural orthostatic tachycardia syndrome (POTS) and orthostatic intolerance without hemodynamic effects have been described in the literature.73 , 74 , 87 Finally, right ventricular dysfunction in response to fibrotic lung damage, pulmonary hypertension, and/or clot burden in people recovering from severe disease also recently has been reported.52, 53, 54 These studies consistently demonstrated an incidence of diastolic dysfunction of roughly 32%-to-55%, and an incidence of pulmonary hypertension of roughly 10%-to-35% after up to 12 weeks following acute disease.52, 53, 54
Given the significance of cardiac burden from COVID-19, perioperative cardiovascular challenges also should be anticipated. The most common symptoms of chest pain and palpitations could have a significant underlying cause. Given that myocardial damage, such as inflammation and fibrosis, have been found in these patients, palpitations can be a potential symptom of conduction abnormalities or ventricular dysfunction.118 Chest pain, although likely atypical in nature and related to respiratory sequelae, should not be minimized and should be investigated with focused history and physical examination for heart failure, coronary artery disease, and arrhythmias; obtaining an electrocardiogram, thyroid-stimulating hormone level, and transthoracic echocardiogram may be prudent, especially when other worrisome symptoms are present.119 Intraoperatively, patients with significant cardiac abnormalities require invasive monitoring and preparation of vasopressors, inotropes, and antiarrhythmic medications, depending on underlying pathology.120 A constellation of palpitations, chest pain, fatigue, and postural dizziness may be indicative of POTS.121 Patients with these symptoms should be evaluated further, preferably with a tilt-table test, or by a specialist in an autonomic specialty clinic. Due to adrenergic receptor hypersensitivity, patients with POTS can exhibit labile hemodynamics intraoperatively due to overcorrections of blood pressure and heart rate in response to reductions in blood pressure. Volume administration and careful titration of phenylephrine have been suggested as therapies of choice for hypotension.122 Lastly, right ventricular dysfunction should be of particular concern in individuals who had severe disease and continue to have respiratory dysfunction with other symptoms associated with heart failure.123 Echocardiographic evaluation is prudent, and intraoperative availability of invasive monitoring and inotropic support are paramount.124
Pulmonary
Respiratory sequelae are the second most common ailments reported by COVID long-haulers, with shortness of breath and cough being the most frequent. Numerically, shortness of breath has been noted in 8%-to-98% of patients, and cough in 10%-to-75%, with lower prevalence in patients with mild initial disease.2 Patients with severe dyspnea tended to exhibit abnormal performance on six-minute walk tests.55 These complaints and functional limitations have their basis in underlying lung pathology.56 Radiographically, multiple reports noted abnormal chest x-rays and computed tomography (CT) scans on three-sixmonth follow-ups, with ground-glass opacities and architectural distortion being the most common.54 , 57 , 75 , 76 These findings on CT scan also are correlated with abnormal pulmonary function test results. Functional evaluation of lung disease commonly revealed reduced diffusion capacity for carbon monoxide (DLCO), with a range between 21% and 50% of patients.57 , 58 , 88 Moreover, female sex and an ICU admission were found to be risk factors for DLCO <60% of predicted at four months.58 Other notable spirometry findings included restrictive flow patterns and impaired alveolar volume on spirometry.55 , 88 A study evaluating the incidence of interstitial lung disease after hospitalization found that 4.8% of patients developed interstitial lung disease,59 with an organizing pneumonia-like pattern similar to that seen in patients of another case series.77 Additionally, patients who required hospitalization for acute COVID-19 also experienced ongoing oxygen requirements, tracheostomy dependence, respiratory muscle weakness, fibrotic changes, and transplantation necessity.58 Unlike the cardiac toll of COVID-19, the severity of acute disease appears to be the main risk factor for development of these pulmonary complications.60
The perioperative pulmonary sequelae of COVID-19 should be addressed. Although COVID-19 survivors are at increased risk of developing postoperative respiratory failure, this risk decreases substantially ≥seven weeks after recovery from acute infection.125 Nevertheless, multidisciplinary preoperative optimization is of particular importance, as some patients will require a complete workup, including a CT scan of the chest, pulmonary function testing, or echocardiography.126 On the day of surgery, patients previously hospitalized may be using inhaled bronchodilators and corticosteroids, both of which should be continued.127 Intraoperatively, airway manipulation may be challenging in individuals previously tracheostomized, or intubated for extended periods.128 Vocal cord injuries, subglottic stenosis, and tracheomalacia all have been described.129, 130, 131, 132 Depending on the severity of respiratory/airway sequelae and type of surgery, use of supraglottic airway devices should be considered and offered to individuals on case-by-cases basis. Furthermore, given reports of significant fibrosis and bullae, lung-protective ventilation is encouraged strongly, with tidal volumes 4-to-8 mL/kg of predicted body weight and driving pressures <15 cm H2O.133 , 134 Additionally, judicious use of intravenous fluids should be a priority to limit expansion of extravascular lung water.135 , 136 Finally, given reports of diminished respiratory muscle strength; again, reversal of neuromuscular blockade with sugammadex should be considered.137
Hematologic
Intensivists quickly recognized thromboembolic disease as a frequent complication of acute COVID-19 disease. Microthrombosis, venous clots, and arterial thrombi frequently have been reported.138 The pulmonary vasculature appears to be especially prone to microthrombi, and while pulmonary embolism (PE) has been reported, the rate of PE varies widely in the literature.139 A meta-analysis of 102 studies stated a prevalence of deep vein thrombosis (DVT) and PE as roughly 15% and 8%, respectively, with higher rates in the critically ill population during acute infection.140 Long-term effects also have been described in a case series of four previously healthy, young individuals who developed arterial thromboses.80 However, the incidence of hematologic complications appeared to decrease with time after recovery. For example, at 30 days, the cumulative incidence of overall thrombosis and bleeding were only 2.5% and 3.7%, respectively.91 Likewise, an analysis of 146 previously hospitalized patients in Belgium, many of whom were on thromboprophylaxis, only revealed a single incidence of symptomatic DVT and PE at six weeks, and none at six months.62
Many of those who survived severe disease may be on oral anticoagulation therapy when they present for elective surgery, and this will require perioperative planning.141 Cessation of therapy should be addressed preoperatively to consider the risks and benefits; however, postoperatively, held medications promptly should be restarted.142 For acute warfarin reversal, prothrombin complex concentrates can be considered if intravascular volume is of concern;143 rivaroxaban and apixaban now can be reversed acutely with andexanet alpha;144 and dabigatran can be reversed with idarucizumab.145 For patients not on anticoagulation therapy, a preoperative D-dimer measurement and/or venous ultrasound for thrombosis should be considered.103 A D-dimer only has clinical utility if there is low suspicion for inflammation or malignancy, so prudent patient selection is necessary.146 If a deep-vein thrombosis is identified on ultrasound, anticoagulants such as heparin can be administered in addition to prophylactic compression stockings.146 As an additional consideration, ICU survivors may have thrombosed or injured arteries that were cannulated previously for monitoring, therefore intraoperative ultrasonographic examination before new instrumentation is advised.147
Renal
There is currently a scarcity of literature regarding the long-term consequences of PACS on kidney function; however, some inferences can be drawn from data following early recovery from COVID-19. Acute kidney disease was prevalent in hospitalized patients with acute COVID-19 infection, with 35% not recovering to their baseline renal function upon discharge.148 Moreover, one study found a greater rate of decline in glomerular filtration rate per year among recovered COVID-19 patients compared with a non-COVID-19 cohort.149 Electrolyte imbalances, volume status issues, dialysis line infections, and thrombosis all can be encountered and require attention.2 Intraoperatively, mean arterial pressure should target values ≥65 mmHg to ensure adequate renal perfusion.150
Gastrointestinal
From a gastrointestinal standpoint, 5%-to-10% of patients developed diarrhea after resolution of acute disease.5 , 93 However, newer data suggest that gastrointestinal sequelae may be more serious in some patients. For example, two case reports described four patients developing cholangiopathies in the months following acute disease infection, necessitating liver transplantation in one of these patients.78 , 79 Even among the noncritically ill, malnutrition in hospitalized patients is also a serious concern. For example, a report from Italy described nearly 50% of patients in a rehabilitation unit as malnourished after acute disease and 90% as having some degree of dysphagia.89 As such, patients recovering from severe acute disease who present for surgery may be malnourished, dysphagic, or in need of alternate mode of nutrition delivery, such as gastric or nasoduodenal tubes.151 Care should be taken to continue feeding as close to surgery as possible, especially when postpyloric tubes are present.152
Endocrine
The effect of COVID-19 on endocrine systems has not been investigated thoroughly; however, case reports of new-onset Hashimoto’s,153 Graves’ disease,154 and diabetes mellitus155 are emerging. A follow-up study of hospitalized patients noted a rate of new diabetes diagnosis of 29 per 1,000 on average at 140 days.90 Additionally, two studies evaluated long-term thyroid function after acute disease.27 , 61 Most patients who had abnormal thyroid function during acute hospitalization had normalized function on follow-up; however, 3%-to-5% developed antithyroid peroxidase antibodies, and <2 % developed subclinical thyrotoxicosis and hypothyroidism.61 It also is crucial to consider the iatrogenic effects of dexamethasone therapy on the hypothalamic-pituitary-adrenal axis.156 During the preoperative endocrinology workup, patients with symptoms worrisome for arrhythmias should have their thyroid function evaluated.157 Also, recent steroid therapy should be investigated, and perioperative stress doses administered in patients at risk for functional adrenal insufficiency.158
Timing of Surgery and Type of Anesthesia
Although the data are limited on when it is safe to schedule an elective surgery after acute infection, patients recovering from COVID-19 are at increased risk of a 30-day adjusted mortality when undergoing elective or emergent surgery.125 The latest international, multicenter, prospective cohort study found that risk of mortality returned to baseline ≥seven weeks after recovery from COVID-19 infection; however, patients with persisting symptoms continued to have increased mortality even after seven weeks.125 Patients at highest risk were those undergoing emergency surgery and those with an American Society of Anesthesiologists physical status of III-to-V presenting for surgery within six weeks of initial illness. In concert with these findings, the ASA and the Anesthesia Patient Safety Foundation recommend at least a 12-week delay before undergoing elective surgery for patients who experienced a critical illness, ten weeks for patients with diabetes/hospitalized patients/immunocompromised patients, six weeks for symptomatic patients not requiring hospitalization, and four weeks for asymptomatic individuals or those with mild, nonrespiratory symptoms.159 Nevertheless, the decision of when to proceed with a surgery must be reached by discussing the increased perioperative hazards with the potential health risks of delaying the procedure. Currently, no data exist regarding differences in outcomes between monitored anesthesia care versus general or neuraxial anesthesia in the COVID-19 long-hauler population. Therefore, until further analysis emerges, practitioners should exercise clinical prudence when devising an anesthetic plan for these patients.
Conclusion
Studies on PACS are just beginning to materialize. With time, bedside practitioners will possess more data in their armamentarium to make evidence-based decisions. Currently, it is of paramount importance to approach COVID-19 long-haulers in a multidisciplinary fashion. Symptoms should not be minimized, as life-threatening entities like pulmonary artery hypertension, severe lung fibrosis, and ventricular failure may be present. In the perioperative setting, practitioners should exemplify a pragmatic approach and use best practices from similar disease states to guide perioperative care.
Acknowledgments
Acknowledgements
The authors thank Ronald Harter, MD and Ravi Tripathi, MD for departmental support.
Conflict of Interest
None of the authors has any conflicts of interest to report.
Footnotes
No funding from the National Institutes of Health, Howard Hughes Medical Institute, or any other financial source was received for conducting this study.
References
- 1.July 14, 2021. July 14, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html Post-COVID conditions: Information for healthcare providers. Available at. Accessed. [Google Scholar]
- 2.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nature Med. 2021:1–15. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandal S, Barnett J, Brill SE, et al. Long-COVID’: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76:396–398. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen MS, Kristiansen MF, Hanusson KD, et al. Long COVID in the Faroe Islands–a longitudinal study among non-hospitalized patients. Clin Infect Dis. 2020:ciaa1792. doi: 10.1093/cid/ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno-Pérez O, Merino E, Leon-Ramirez J-M, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: A Mediterranean cohort study. J Infection. 2021;82:378–383. doi: 10.1016/j.jinf.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taboada M, Moreno E, Cariñena A, et al. Quality of life, functional status, and persistent symptoms after intensive care of COVID-19 patients. Br J Anaesth. 2021;126:e110–e113. doi: 10.1016/j.bja.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nature Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orrù G, Bertelloni D, Diolaiuti F, et al. Long-COVID Syndrome? A study on the persistence of neurological, psychological and physiological symptoms. Healthcare (Basel) 2021;9:575. doi: 10.3390/healthcare9050575. in Healthcare. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chopra N, Chowdhury M, Singh AK, et al. Clinical predictors of long COVID-19 and phenotypes of mild COVID-19 at a tertiary care centre in India. Drug Discov Ther. 2021;15:156–161. doi: 10.5582/ddt.2021.01014. [DOI] [PubMed] [Google Scholar]
- 10.Vaes AW, Goërtz YM, Van Herck M, et al. Recovery from COVID-19: A sprint or marathon? 6-month follow-up data from online long COVID-19 support group members. ERJ Open Res. 2021;7:00141–02021. doi: 10.1183/23120541.00141-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pujari S, Gaikwad S, Chitalikar A, et al. Long-coronavirus disease among people living with HIV in western India: An observational study. Immun Inflamm Dis. 2021;9:1037–1043. doi: 10.1002/iid3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson KB, Rao M, Bonilla H, et al. Patients with uncomplicated COVID-19 have long-term persistent symptoms and functional impairment similar to patients with severe COVID-19: A cautionary tale during a global pandemic. Clin Infect Dis. 2021;73:e826–e829. doi: 10.1093/cid/ciab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaber TAK, Ashish A, Unsworth A. Persistent post-covid symptoms in healthcare workers. Occup Med (Lond) 2021;71:144–146. doi: 10.1093/occmed/kqab043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor AK, Kingstone T, Briggs TA, et al. Reluctant pioneer’: A qualitative study of doctors’ experiences as patients with long COVID. Health Expectations. 2021 doi: 10.1111/hex.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanichkachorn G, Newcomb R, Cowl CT, et al. Post COVID-19 syndrome (long haul syndrome): Description of a multidisciplinary clinic at the mayo clinic and characteristics of the initial patient cohort. Mayo Clin Proc. 2021;96:1782–1791. doi: 10.1016/j.mayocp.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iqbal A, Iqbal K, Ali SA, et al. The COVID-19 sequelae: A cross-sectional evaluation of post-recovery symptoms and the need for rehabilitation of COVID-19 survivors. Cureus. 2021;13:e13080. doi: 10.7759/cureus.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Augustin M, Schommers P, Stecher M, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: A longitudinal prospective cohort study. Lancet Reg Health Eur. 2021;6 doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kayaaslan B, Eser F, Kalem AK, et al. Post-COVID syndrome: A single-center questionnaire study on 1007 participants recovered from COVID-19 [e-pub ahead of print] J Med Virol. 2021 doi: 10.1002/jmv.27198. Accessed 07/28/2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maestre-Muñiz MM, Arias Á, Mata-Vázquez E, et al. Long-term outcomes of patients with coronavirus disease 2019 at one year after hospital discharge. J Clin Med. 2021;10:2945. doi: 10.3390/jcm10132945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dennis A, Wamil M, Alberts J, et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashif A, Chaudhry M, Fayyaz T, et al. Follow-up of COVID-19 recovered patients with mild disease. Sci Rep. 2021;11:13414. doi: 10.1038/s41598-021-92717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lund LC, Hallas J, Nielsen H, et al. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: A Danish population-based cohort study. Lancet Infect Dis. 2021;21:1373–1382. doi: 10.1016/S1473-3099(21)00211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.González-Hermosillo JA, Martínez-López JP, Carrillo-Lampón SA, et al. Post-acute COVID-19 symptoms, a potential link with myalgic encephalomyelitis/chronic fatigue syndrome: A 6-month survey in a Mexican cohort. Brain Sci. 2021;11:760. doi: 10.3390/brainsci11060760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galván-Tejada CE, Herrera-García CF, Godina-González S, et al. Persistence of COVID-19 symptoms after recovery in Mexican population. Int J Environ Res Public Health. 2020;17:9367. doi: 10.3390/ijerph17249367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boscolo-Rizzo P, Guida F, Polesel J, et al. Sequelae in adults at 12 months after mild-to-moderate coronavirus disease 2019 (COVID-19) [e-pub ahead of print] Int Forum Allergy Rhinol. 2021 doi: 10.1002/alr.22832. Accessed 07/27/2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scherlinger M, Felten R, Gallais F, et al. Refining “long-COVID” by a prospective multimodal evaluation of patients with long-term symptoms attributed to SARS-CoV-2 infection. Infect Dis Therapy. 2021:1–17. doi: 10.1007/s40121-021-00484-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lui DT, Lee CH, Chow WS, et al. Insights from prospective follow-up of thyroid function and autoimmunity among Covid-19 survivors. J Endocrine Soc. 2021;5(Supplement_1):A840–A841. doi: 10.3803/EnM.2021.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen KJ, Vestergaard JM, Schlünssen V, et al. Day-by-day symptoms following positive and negative PCR tests for SARS-CoV-2 in non-hospitalised health-care workers: A 90-day follow-up study. Int J Infect Dis. 2021;108:382–390. doi: 10.1016/j.ijid.2021.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menges D, Ballouz T, Anagnostopoulos A, et al. Burden of post-COVID-19 syndrome and implications for healthcare service planning: A population-based cohort study. PloS One. 2021;16 doi: 10.1371/journal.pone.0254523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.COVID-ICU Groupon behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donnelly JP, Wang XQ, Iwashyna TJ, et al. Readmission and death after initial hospital discharge among patients with COVID-19 in a large multihospital system. JAMA. 2021;325:304–306. doi: 10.1001/jama.2020.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarthy CP, Murphy S, Jones-O'Connor M, et al. Early clinical and sociodemographic experience with patients hospitalized with COVID-19 at a large American healthcare system. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol. 2021;93:1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 34.Leth S, Gunst JD, Mathiasen V, et al. Persistent symptoms in patients recovering from COVID-19 in Denmark. Open Forum Infect Dis. 2021;8:ofab042. doi: 10.1093/ofid/ofab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seeßle J, Waterboer T, Hippchen T, et al. Persistent symptoms in adult patients one year after COVID-19: A prospective cohort study. Clin Infect Dis. 2021:ciab611. doi: 10.1093/cid/ciab611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naidu SB, Shah AJ, Saigal A, et al. The high mental health burden of “Long COVID” and its association with on-going physical and respiratory symptoms in all adults discharged from hospital. Eur Respir J. 2021;57 doi: 10.1183/13993003.04364-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarsitani L, Vassalini P, Koukopoulos A, et al. Post-traumatic stress disorder among COVID-19 survivors at 3-month follow-up after hospital discharge. J Gen Intern Med. 2021;36:1702–1707. doi: 10.1007/s11606-021-06731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Einvik G, Dammen T, Ghanima W, et al. Prevalence and risk factors for post-traumatic stress in hospitalized and non-hospitalized COVID-19 patients. Int J Environ Res Public Health. 2021;18:2079. doi: 10.3390/ijerph18042079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frontera JA, Yang D, Lewis A, et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J Neurol Sci. 2021;426 doi: 10.1016/j.jns.2021.117486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graham EL, Clark JR, Orban ZS, et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers. Ann Clin Translational Neurol. 2021;8:1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miskowiak K, Johnsen S, Sattler S, et al. Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables. Eur Neuropsychopharmacol. 2021;46:39–48. doi: 10.1016/j.euroneuro.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y-H, Wang Y-R, Wang Q-H, et al. Post-infection cognitive impairments in a cohort of elderly patients with COVID-19. Mol Neurodegener. 2021;16:1–10. doi: 10.1186/s13024-021-00469-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otte MS, Bork M-L, Zimmermann PH, et al. Persisting olfactory dysfunction improves in patients 6 months after COVID-19 disease. Acta Otolaryngol. 2021;141:626–629. doi: 10.1080/00016489.2021.1905178. [DOI] [PubMed] [Google Scholar]
- 44.Fernández-de-Las-Peñas C, Rodríguez-Jiménez J, Fuensalida-Novo S, et al. Myalgia as a symptom at hospital admission by severe acute respiratory syndrome coronavirus 2 infection is associated with persistent musculoskeletal pain as long-term post-COVID sequelae: A case-control study [e-pub ahead of print] Pain. 2021 doi: 10.1097/j.pain.0000000000002306. Accessed 07/29/2021. [DOI] [PubMed] [Google Scholar]
- 45.Sollini M, Ciccarelli M, Cecconi M, et al. Vasculitis changes in COVID-19 survivors with persistent symptoms: An [18 F] FDG-PET/CT study. Eur J Nuclear Med Mol Imaging. 2021;48:1460–1466. doi: 10.1007/s00259-020-05084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajpal S, Tong MS, Borchers J, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6:116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Starekova J, Bluemke DA, Bradham WS, et al. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. 2021;6:945–950. doi: 10.1001/jamacardio.2020.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Małek ŁA, Marczak M, Miłosz-Wieczorek B, et al. Cardiac involvement in consecutive elite athletes recovered from Covid-19: A magnetic resonance study. J Magn Reson Imaging. 2021;53:1723–1729. doi: 10.1002/jmri.27513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radin JM, Quer G, Ramos E, et al. Assessment of prolonged physiological and behavioral changes associated with COVID-19 infection. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.15959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barizien N, Le Guen M, Russel S, et al. Clinical characterization of dysautonomia in long COVID-19 patients. Sci Rep. 2021;11:1–7. doi: 10.1038/s41598-021-93546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tudoran C, Tudoran M, Pop GN, et al. Associations between the severity of the post-acute COVID-19 syndrome and echocardiographic abnormalities in previously healthy outpatients following infection with SARS-CoV-2. Biology. 2021;10:469. doi: 10.3390/biology10060469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bende F, Tudoran C, Sporea I, et al. A multidisciplinary approach to evaluate the presence of hepatic and cardiac abnormalities in patients with post-acute COVID-19 syndrome—A pilot study. J Clin Med. 2021;10:2507. doi: 10.3390/jcm10112507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sonnweber T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after COVID-19: An observational prospective multicentre trial. Eur Respir J. 2021;57 doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cortés-Telles A, López-Romero S, Figueroa-Hurtado E, et al. Pulmonary function and functional capacity in COVID-19 survivors with persistent dyspnoea. Respir Physiol Neurobiol. 2021;288 doi: 10.1016/j.resp.2021.103644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aparisi Á, Ybarra-Falcón C, García-Gómez M, et al. Exercise ventilatory inefficiency in post-COVID-19 syndrome: Insights from a prospective evaluation. J Clin Med. 2021;10:2591. doi: 10.3390/jcm10122591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Balbi M, Conti C, Imeri G, et al. Post-discharge chest CT findings and pulmonary function tests in severe COVID-19 patients. Eur J Radiol. 2021;138 doi: 10.1016/j.ejrad.2021.109676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Myall KJ, Mukherjee B, Castanheira AM, et al. Persistent post–COVID-19 interstitial lung disease. An observational study of corticosteroid treatment. Ann Am Thorac Soc. 2021;18:799–806. doi: 10.1513/AnnalsATS.202008-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Townsend L, Dowds J, O'Brien K, et al. Persistent poor health after COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc. 2021;18:997–1003. doi: 10.1513/AnnalsATS.202009-1175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lui DTW, Lee CH, Chow WS, et al. Long COVID in patients with mild to moderate disease: Do thyroid function and autoimmunity play a role? Endocr Pract. 2021;27:894–902. doi: 10.1016/j.eprac.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Engelen MM, Vandenbriele C, Balthazar T, et al. Venous thromboembolism in patients discharged after COVID-19 hospitalization. Semin Thromb Hemost. 2021;47:362–371. doi: 10.1055/s-0041-1727284. [DOI] [PubMed] [Google Scholar]
- 63.Leta V, Rodríguez-Violante M, Abundes A, et al. Parkinson's disease and post–COVID-19 syndrome: The Parkinson's long-COVID spectrum. Mov Disord. 2021;36:1287. doi: 10.1002/mds.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whiteside DM, Oleynick V, Holker E, et al. Neurocognitive deficits in severe COVID-19 infection: Case series and proposed model. Clin Neuropsychol. 2021;35:799–818. doi: 10.1080/13854046.2021.1874056. [DOI] [PubMed] [Google Scholar]
- 65.Park S, Majoka H, Sheikh A, et al. A presumed case of new-onset focal seizures as a delayed complication of COVID-19 infection. Epilepsy Behav Rep. 2021;16 doi: 10.1016/j.ebr.2021.100447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carroll E, Neumann H, Aguero-Rosenfeld ME, et al. Post–COVID-19 inflammatory syndrome manifesting as refractory status epilepticus. Epilepsia. 2020;61:e135–e139. doi: 10.1111/epi.16683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raahimi MM, Kane A, Moore CE, et al. Late onset of Guillain-Barré syndrome following SARS-CoV-2 infection: Part of ‘long COVID-19 syndrome’? BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2020-240178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abrams RM, Safavi F, Tuhrim S, et al. MRI negative myelopathy post mild SARS-CoV-2 infection: Vasculopathy or inflammatory myelitis? J Neurovirol. 2021:1–6. doi: 10.1007/s13365-021-00986-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kilinc D, van de Pasch S, Doets AY, et al. Guillain-Barré syndrome after SARS-CoV-2 infection. Eur J Neurol. 2020;27:1757–1758. doi: 10.1111/ene.14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lechien JR, Hervochon R, Hans S. Post-COVID-19 Kawasaki-like syndrome. Ear Nose Throat J. 2021 doi: 10.1177/01455613211006011. [DOI] [PubMed] [Google Scholar]
- 71.Rivera-Morales MD, Pell R, Rubero J, et al. Acute myopericarditis in the post COVID-19 recovery phase. Cureus. 2020;12:e11247. doi: 10.7759/cureus.11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong SW, Fan BE, Huang W, et al. ST-segment elevation myocardial infarction in post-COVID-19 patients: A case series. Ann Acad Med Singap. 2021;50:425–430. [PubMed] [Google Scholar]
- 73.Ishibashi Y, Yoneyama K, Tsuchida T, et al. Post-COVID-19 postural orthostatic tachycardia syndrome. Intern Med. 2021;60:2345. doi: 10.2169/internalmedicine.7626-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johansson M, Ståhlberg M, Runold M, et al. Long-haul post–COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: The Swedish experience. Case Rep. 2021;3:573–580. doi: 10.1016/j.jaccas.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heiss R, Grodzki DM, Horger W, et al. High-performance low field MRI enables visualization of persistent pulmonary damage after COVID-19. Magn Reson Imaging. 2021;76:49–51. doi: 10.1016/j.mri.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Susanto AD, Triyoga PA, Isbaniah F, et al. Lung fibrosis sequelae after recovery from COVID-19 infection. J Infect Dev Ctries. 2021;15:360–365. doi: 10.3855/jidc.13686. [DOI] [PubMed] [Google Scholar]
- 77.Vadász I, Husain-Syed F, Dorfmüller P, et al. Severe organising pneumonia following COVID-19. Thorax. 2021;76:201–204. doi: 10.1136/thoraxjnl-2020-216088. [DOI] [PubMed] [Google Scholar]
- 78.Roth NC, Kim A, Vitkovski T, et al. Post–COVID-19 cholangiopathy: A novel entity. Am Coll Gastroenterol. 2021;116:1077–1082. doi: 10.14309/ajg.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 79.Durazo FA, Nicholas AA, Mahaffey JJ, et al. Post–Covid-19 Cholangiopathy—A New Indication for Liver Transplantation: A Case Report. in Transplantation proceedings. Elsevier. 2021:1132–1137. doi: 10.1016/j.transproceed.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fan BE, Umapathi T, Chua K, et al. Delayed catastrophic thrombotic events in young and asymptomatic post COVID-19 patients. J Thromb Thrombolysis. 2021;51:971–977. doi: 10.1007/s11239-020-02332-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ogoina D, James HI, Ogoinja SZ. Post-discharge symptoms among hospitalized COVID-19 patients in Nigeria: A single-center study. Am J Tropical Med Hyg. 2021;105:731–736. doi: 10.4269/ajtmh.21-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Romero-Duarte Á, Rivera-Izquierdo M, de Alba IG-F, et al. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: The ANCOHVID multicentre 6-month follow-up study. BMC Med. 2021;19:1–13. doi: 10.1186/s12916-021-02003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng D, Calderwood C, Skyllberg E, et al. Clinical characteristics and outcomes of adult patients admitted with COVID-19 in East London: A retrospective cohort analysis. BMJ Open Respir Res. 2021;8 doi: 10.1136/bmjresp-2020-000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ganesh R, Ghosh AK, Nyman MA, et al. PROMIS scales for assessment of persistent post-COVID symptoms: A cross sectional study. J Prim Care Community Health. 2021;12 doi: 10.1177/21501327211030413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 86.Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: Retrospective cohort study. BMJ. 2021:372. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shouman K, Vanichkachorn G, Cheshire WP, et al. Autonomic dysfunction following COVID-19 infection: An early experience. Clin Auton Res. 2021;31:385–394. doi: 10.1007/s10286-021-00803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frija-Masson J, Debray M-P, Gilbert M, et al. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur Respir J. 2020;56 doi: 10.1183/13993003.01754-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brugliera L, Spina A, Castellazzi P, et al. Nutritional management of COVID-19 patients in a rehabilitation unit. Eur J Clinl Nutr. 2020;74:860–863. doi: 10.1038/s41430-020-0664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sathish T, Anton MC, Sivakumar T. New-onset diabetes in “long COVID. J Diabetes. 2021;13:693. doi: 10.1111/1753-0407.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patell R, Bogue T, Koshy A, et al. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136:1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Valent A, Dudoignon E, Ressaire Q, et al. Three-month quality of life in survivors of ARDS due to COVID-19: A preliminary report from a French academic centre. Anaesth Crit Care Pain Med. 2020;39:740. doi: 10.1016/j.accpm.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peghin M, Palese A, Venturini M, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. 2021;27:1507–1513. doi: 10.1016/j.cmi.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baig AM. Chronic COVID syndrome: Need for an appropriate medical terminology for long-COVID and COVID long-haulers. J Med Virol. 2021;93:2555–2556. doi: 10.1002/jmv.26624. [DOI] [PubMed] [Google Scholar]
- 96.National Institute for Health and Care Excellence. COVID-19 rapid guideline: Managing the long-term effects of COVID-19. 2020. [PubMed]
- 97.Hosey MM, Needham DM. Survivorship after COVID-19 ICU stay. Nat Rev Dis Primers. 2020;6:1–2. doi: 10.1038/s41572-020-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu Y, Hou B, Liu J, et al. Risk factors associated with long-term hospitalization in patients with COVID-19: A single-centered, retrospective study. Front Med (Lausanne) 2020;7:315. doi: 10.3389/fmed.2020.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Subbarao K, Mahanty S. Respiratory virus infections: Understanding COVID-19. Immunity. 2020;52:905–909. doi: 10.1016/j.immuni.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sinha P, Matthay MA, Calfee CS. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med. 2020;180:1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 101.Alefragkis D, Satolia K, Poulou G. Post intensive care syndrome prevention and impact of COVID 19. Progress Health Sci. 2021;11 [Google Scholar]
- 102.Bui N, Coetzer M, Schenning KJ, et al. Preparing previously COVID-19-positive patients for elective surgery: A framework for preoperative evaluation. Periop Med (Lond) 2021;10:1–4. doi: 10.1186/s13741-020-00172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hampshire A, Trender W, Chamberlain SR, et al. Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine. 2021;39 doi: 10.1016/j.eclinm.2021.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Needham EJ, Chou SH-Y, Coles AJ, et al. Neurological implications of COVID-19 infections. Neurocrit Care. 2020;32:667–671. doi: 10.1007/s12028-020-00978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Das G, Mukherjee N, Ghosh S. Neurological insights of COVID-19 pandemic. ACS Chem Neurosci. 2020;11:1206–1209. doi: 10.1021/acschemneuro.0c00201. [DOI] [PubMed] [Google Scholar]
- 106.Prasad K, AlOmar SY, Alqahtani SAM, et al. Brain disease network analysis to elucidate the neurological manifestations of COVID-19. Mol Neurobiol. 2021;58:1875–1893. doi: 10.1007/s12035-020-02266-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cooper S, Greene J. The clinical assessment of the patient with early dementia. J Neurol Neurosurg Psychiatry. 2005;76(suppl 5):v15–v24. doi: 10.1136/jnnp.2005.081133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shahrokhi M, Asuncion RMD. Neurologic exam. StatPearls. 2020 [PubMed] [Google Scholar]
- 109.Attri JP, Bala N, Chatrath V. Psychiatric patient and anaesthesia. Ind J Anaesth. 2012;56:8. doi: 10.4103/0019-5049.93337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gray SL, Dublin S, Yu O, et al. Benzodiazepine use and risk of incident dementia or cognitive decline: Prospective population based study. BMJ. 2016:352. doi: 10.1136/bmj.i90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schönenberg M, Reichwald U, Domes G, et al. Ketamine aggravates symptoms of acute stress disorder in a naturalistic sample of accident victims. J Psychopharmacol. 2008;22:493–497. doi: 10.1177/0269881107082481. [DOI] [PubMed] [Google Scholar]
- 112.Armstead WM. Cerebral blood flow autoregulation and dysautoregulation. Anesthesiol Clin. 2016;34:465–477. doi: 10.1016/j.anclin.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gulenay M, Mathai JK. Depolarizing neuromuscular blocking drugs. StatPearls. 2021 [PubMed] [Google Scholar]
- 114.Price DR, Mikkelsen ME, Umscheid CA, et al. Neuromuscular blocking agents and neuromuscular dysfunction acquired in critical illness: A systematic review and meta-analysis. Crit Care Med. 2016;44:2070–2078. doi: 10.1097/CCM.0000000000001839. [DOI] [PubMed] [Google Scholar]
- 115.Kopf A, Banzhaf A, Stein C. Perioperative management of the chronic pain patient. Best Pract Res Clin Anaesthesiol. 2005;19:59–76. [PubMed] [Google Scholar]
- 116.Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: An overview. J Translational Intern Med. 2017;5:90–92. doi: 10.1515/jtim-2016-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Neal JM, Barrington MJ, Brull R, et al. The second ASRA practice advisory on neurologic complications associated with regional anesthesia and pain medicine: Executive summary 2015. Reg Anesth Pain Med. 2015;40:401–430. doi: 10.1097/AAP.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 118.Thompson A, Balser J. Perioperative cardiac arrhythmias. Br J Anaesth. 2004;93:86–94. doi: 10.1093/bja/aeh166. [DOI] [PubMed] [Google Scholar]
- 119.Prakasa KR, Feldman LS. Perioperative chest pain/dyspnea. Periop Med (Lond) 2011:441–451. [Google Scholar]
- 120.Vincent J-L, Pelosi P, Pearse R, et al. Perioperative cardiovascular monitoring of high-risk patients: A consensus of 12. Crit Care. 2015;19:1–12. doi: 10.1186/s13054-015-0932-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Blitshteyn S, Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: A case series of 20 patients. Immunol Res. 2021;69:205–211. doi: 10.1007/s12026-021-09185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rabbitts JA, Groenewald CB, Jacob AK, et al. Postural orthostatic tachycardia syndrome and general anesthesia: A series of 13 cases. J Clin Anesth. 2011;23:384–392. doi: 10.1016/j.jclinane.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kopanczyk R, Al-Qudsi OH, Uribe A, et al. Right ventricular dysfunction in patients with coronavirus disease 2019 supported with extracorporeal membrane oxygenation [e-pub ahead of print. J Cardiothorac Vasc Anesth. 2021 doi: 10.1053/j.jvca.2021.05.019. Accessed 06/29/2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Forrest P. Anaesthesia and right ventricular failure. Anaesth Intensive Care. 2009;37:370–385. doi: 10.1177/0310057X0903700314. [DOI] [PubMed] [Google Scholar]
- 125.Collaborative; GlobalSurg Collaborative COVIDSurg. Timing of surgery following SARS-CoV-2 infection: An international prospective cohort study. Anaesthesia. 2021;76:748–758. doi: 10.1111/anae.15458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tisi GM. Preoperative evaluation of pulmonary function: Validity, indications, and benefits. Am Rev Respir Dis. 1979;119:293–310. doi: 10.1164/arrd.1979.119.2.293. [DOI] [PubMed] [Google Scholar]
- 127.Halpin DM, Singh D, Hadfield RM. Inhaled corticosteroids and COVID-19: A systematic review and clinical perspective. Eur Respir J. 2020;55 doi: 10.1183/13993003.01009-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Meister KD, Pandian V, Hillel AT, et al. Multidisciplinary safety recommendations after tracheostomy during covid-19 pandemic: State of the art review. Otolaryngol Head Neck Surg. 2021;164:984–1000. doi: 10.1177/0194599820961990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rouhani MJ, Clunie G, Thong G, et al. A prospective study of voice, swallow, and airway outcomes following tracheostomy for COVID-19. Laryngoscope. 2021;131:E1918–E1925. doi: 10.1002/lary.29346. [DOI] [PubMed] [Google Scholar]
- 130.Bertonz F, Robiolio E, Gervasio CF. Vocal cord ulcer following endotracheal intubation for mechanical ventilation in COVID-19 pneumonia: A case report from northern Italy. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.928126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mattioli F, Fermi M, Ghirelli M, et al. Tracheostomy in the COVID-19 pandemic. Eur Arch Otorhinolaryngol. 2020;277:2133–2135. doi: 10.1007/s00405-020-05982-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mattioli F, Marchioni A, Andreani A, et al. Post-intubation tracheal stenosis in COVID-19 patients. Eur Arch Otorhinolaryngol. 2021;278:847–848. doi: 10.1007/s00405-020-06394-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Güldner A, Kiss T, Serpa Neto A, et al. Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications: A comprehensive review of the role of tidal volume, positive end-expiratory pressure, and lung recruitment maneuvers. Anesthesiology. 2015;123:692–713. doi: 10.1097/ALN.0000000000000754. [DOI] [PubMed] [Google Scholar]
- 134.Xu W, Luo X, Wang H, et al. Pulmonary emphysema, bullae, and pneumothorax in COVID-19 pneumonia. Radiol Case Rep. 2021;16:995–998. doi: 10.1016/j.radcr.2021.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gan TJ, Soppitt A, Maroof M, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. J Am Soc Anesthesiol. 2002;97:820–826. doi: 10.1097/00000542-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 136.Della Rocca G, Vetrugno L, Tripi G, et al. Liberal or restricted fluid administration: Are we ready for a proposal of a restricted intraoperative approach? BMC Anesthesiol. 2014;14:1–8. doi: 10.1186/1471-2253-14-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Welch C, Greig C, Masud T, et al. COVID-19 and acute sarcopenia. Aging Dis. 2020;11:1345. doi: 10.14336/AD.2020.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in patients with COVID-19: Awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 140.Tan BK, Mainbourg S, Friggeri A, et al. Arterial and venous thromboembolism in COVID-19: A study-level meta-analysis. Thorax. 2021;76:970–979. doi: 10.1136/thoraxjnl-2020-215383. [DOI] [PubMed] [Google Scholar]
- 141.Kollias A, Kyriakoulis KG, Dimakakos E, et al. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: Emerging evidence and call for action. Br J Haematol. 2020;189:846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Douketis JD. Perioperative anticoagulation management in patients who are receiving oral anticoagulant therapy: A practical guide for clinicians. Thrombosis Res. 2002;108:3–13. doi: 10.1016/s0049-3848(02)00387-0. [DOI] [PubMed] [Google Scholar]
- 143.Leissinger CA, Blatt PM, Hoots WK, et al. Role of prothrombin complex concentrates in reversing warfarin anticoagulation: A review of the literature. Am J Hematol. 2008;83:137–143. doi: 10.1002/ajh.21046. [DOI] [PubMed] [Google Scholar]
- 144.Kaatz S, Bhansali H, Gibbs J, et al. Reversing factor Xa inhibitors–clinical utility of andexanet alfa. J Blood Med. 2017;8:141. doi: 10.2147/JBM.S121550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gómez-Outes A, Alcubilla P, Calvo-Rojas G, et al. Meta-analysis of reversal agents for severe bleeding associated with direct oral anticoagulants. J Am Coll Cardiol. 2021;77:2987–3001. doi: 10.1016/j.jacc.2021.04.061. [DOI] [PubMed] [Google Scholar]
- 146.Tateno Y, Harada K, Okamoto F, et al. Elective laparoscopic colectomy in a patient 3 weeks after coronavirus disease 2019 infection: A case report. J Med Case Rep. 2021;15:1–4. doi: 10.1186/s13256-021-02877-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vezzani A, Manca T, Vercelli A, et al. Ultrasonography as a guide during vascular access procedures and in the diagnosis of complications. J Ultrasound. 2013;16:161–170. doi: 10.1007/s40477-013-0046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chan L, Chaudhary K, Saha A, et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32:151–160. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nugent J, Aklilu A, Yamamoto Y, et al. Assessment of acute kidney injury and longitudinal kidney function after hospital discharge among patients with and without COVID-19. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Meng L, Yu W, Wang T, et al. Blood pressure targets in perioperative care: Provisional considerations based on a comprehensive literature review. Hypertension. 2018;72:806–817. doi: 10.1161/HYPERTENSIONAHA.118.11688. [DOI] [PubMed] [Google Scholar]
- 151.Frajkova Z, Tedla M, Tedlova E, et al. Postintubation dysphagia during COVID-19 outbreak–contemporary review. Dysphagia. 2020;35:549–557. doi: 10.1007/s00455-020-10139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Salvino RM, Dechicco RS, Seidner DL. Perioperative nutrition support: Who and how. Cleve Clin J Med. 2004;71:345–352. doi: 10.3949/ccjm.71.4.345. [DOI] [PubMed] [Google Scholar]
- 153.Tee LY, Hajanto S, Rosario BH. COVID-19 complicated by Hashimoto's thyroiditis. Singapore Med J. 2021;62:265. doi: 10.11622/smedj.2020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mateu-Salat M, Urgell E, Chico A. SARS-COV-2 as a trigger for autoimmune disease: Report of two cases of Graves’ disease after COVID-19. J Endocrinol Invest. 2020;43:1527–1528. doi: 10.1007/s40618-020-01366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rubino F, Amiel SA, Zimmet P, et al. New-onset diabetes in Covid-19. N Engl J Med. 2020;383:789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Reincke M, Allolio B, Würth G, et al. The hypothalamic-pituitary-adrenal axis in critical illness: Response to dexamethasone and corticotropin-releasing hormone. J Clin Endocrinol Metabolism. 1993;77:151–156. doi: 10.1210/jcem.77.1.8392081. [DOI] [PubMed] [Google Scholar]
- 157.Palace MR. Perioperative management of thyroid dysfunction. Health Serv Insights. 2017;10 doi: 10.1177/1178632916689677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yong SL, Coulthard P, Wrzosek A. Supplemental perioperative steroids for surgical patients with adrenal insufficiency. Cochrane Database Syst Rev. 2012;12 doi: 10.1002/14651858.CD005367.pub3. [DOI] [PubMed] [Google Scholar]
- 159.Mankarious M, Massand S, Potochny J. Considerations for elective surgery in the post-COVID-19 patient. Aesthetic Surg J. 2021;41:NP1347–NP1348. doi: 10.1093/asj/sjab214. [DOI] [PMC free article] [PubMed] [Google Scholar]