Abstract
PURPOSE
Standard-of-care treatment for metastatic hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2−) breast cancer includes endocrine therapy (ET) combined with a cyclin-dependent kinase 4/6 inhibitor (CDK4/6i). Optimal treatment after progression on CDK4/6i is unknown. The TRINITI-1 trial investigated ribociclib, a CDK4/6i which has recently demonstrated significant OS benefit in 2 phase III trials, in combination with everolimus and exemestane in patients with HR+, HER2− advanced breast cancer (ABC) after progression on a CDK4/6i.
METHODS
This multicenter, open-label, single-arm, phase I/II study included patients with locally advanced/metastatic HR+/HER2− BC. The primary endpoint was clinical benefit rate (CBR) at week 24 among patients with ET-refractory disease with progression on a CDK4/6i. Other endpoints included safety and biomarker analysis.
RESULTS
Of 104 patients enrolled (phases I and II), 96 had prior CDK4/6i. Recommended phase II doses (all once daily days 1–28 of 28-day cycle) were ribociclib 300 mg, everolimus 2.5 mg, and exemestane 25 mg (group 1) and ribociclib 200 mg, everolimus 5 mg, and exemestane 25 mg (group 2). CBR among 95 efficacy-evaluable patients (phases I and II) at week 24 was 41.1% (95% CI, 31.1%−51.6%), which met the primary endpoint (predetermined threshold: 10%). Common adverse events included neutropenia (69.2%) and stomatitis (40.4%). No new safety signals were observed; no grade 3/4 QTc prolongation was reported.
CONCLUSION
Preliminary TRINITI-1 safety and efficacy results support further investigation of CDK4/6 blockade and targeting of the PI3K/AKT/mTOR signaling pathway in patients with ET-refractory HR+/HER2− ABC after progression on a CDK4/6i.
Introduction
Although hormone receptor–positive (HR+) breast cancers are primarily driven by estrogen receptor signaling, additional signaling pathways may serve as an escape from treatment with endocrine therapy (ET).1,2 Aberrant activation of the cyclin D–cyclin-dependent kinase (CDK) 4/6–inhibitor of CDK4 (INK4)–retinoblastoma (RB) pathway (Supplemental Figure 1) can lead to unrestricted cell cycle progression as well as resistance to ET.1,3 The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mechanistic target of rapamycin (mTOR) pathway has also been widely implicated in breast cancer tumorigenesis and ET resistance.4–6
A standard of care for postmenopausal women with HR+/human epidermal growth factor receptor 2–negative (HER2−) advanced breast cancer (ABC) includes ET combined with a CDK4/6 inhibitor (CDK4/6i), usually given in the first-line setting.7,8 Although the addition of a CDK4/6i to ET has improved outcomes in patients with HR+/HER2– ABC, most patients will eventually experience disease progression due to de novo or acquired resistance.1,9–11 Optimal treatment after progression on a CDK4/6i remains unclear. Use of an mTOR inhibitor, or more recently, a PI3K inhibitor (for those whose tumors harbor mutated PIK3CA) may be considered.8,12–14 Numerous levels of crosstalk exist between the estrogen receptor (ER), CDK4/6, and PI3K/AKT/mTOR signaling pathways, suggesting a potential benefit in combining inhibitors of these pathways.1,4 However, a paucity of clinical data exists on the benefit of continued CDK4/6 blockade following disease progression on CDK4/6i therapy.8
In preclinical cell line and mouse xenograft studies, inhibition of the PI3K/AKT/mTOR signaling pathway blocked progression of ET/CDK4/6i-resistant tumors, although no benefit was observed with continuation of CDK4/6i.15–17 The phase III BOLERO-2 trial demonstrated that everolimus (EVE) + exemestane (EXE) resulted in significantly longer progression-free survival (PFS) than placebo + EXE among CDK4/6i-naive patients with ABC who were refractory to an aromatase inhibitor (AI).13,14 Subsequently, the potential benefit of EVE + ET in this patient population was also observed in phase II trials using other ET combinations: EVE + fulvestrant (PrECOG 0102) and EVE + tamoxifen (TAMRAD).18,19 However, none of these trials included patients who were previously treated with a CDK4/6i; thus, the activity of mTOR inhibition in CDK4/6i-resistant disease has not been well explored.
Ribociclib (RIB) is a selective, orally available CDK4/6i. Among patients with HR+/HER2– ABC, RIB in combination with ET has demonstrated a significant benefit (including a significant overall survival [OS] benefit in pre- and postmenopausal women) and an acceptable toxicity profile.20–24 Preliminary results from a phase Ib trial of RIB + EVE + EXE in postmenopausal women with ER+/HER2– ABC refractory to nonsteroidal AIs showed an acceptable toxicity profile and early signals of clinical activity.25,26
Triplet therapy with Ribociclib, AfINitor® and AI posT CDK 4/6 Inhibitor (TRINITI-1) is a phase I/II trial of RIB, EVE, and EXE in patients with ET-refractory HR+/HER2– ABC (NCT02732119). Here we present interim efficacy and exploratory biomarker results among patients with progression on a CDK4/6i as well as overall safety results from TRINITI-1.
Methods
Patients
Men and postmenopausal women aged ≥ 18 years with HR+/HER2– locally advanced/metastatic breast cancer not amenable to curative treatment by surgery or radiotherapy were eligible. Additional key inclusion criteria were disease progression on up to 3 lines of prior therapy for ABC including 1 to 3 lines of ET and ≤ 1 line of chemotherapy, measurable disease or lytic/mixed bone lesions, adequate bone marrow and organ function, and Eastern Cooperative Oncology Group performance status (ECOG PS) of ≤ 1. In phase II, patients were required to have progressed on a CDK4/6i after ≥ 4 months of therapy as the last prior treatment regimen. The selection of ≥ 4 months for secondary resistance was reflective of the inclusion of patients with multiple lines of prior treatment for ABC and the trend toward quicker progression on later lines of therapy. Patients with visceral crisis, central nervous system involvement < 4 weeks from completion of prior therapy, or clinically significant, uncontrolled heart disease or cardiac repolarization abnormalities were excluded.
Study Design
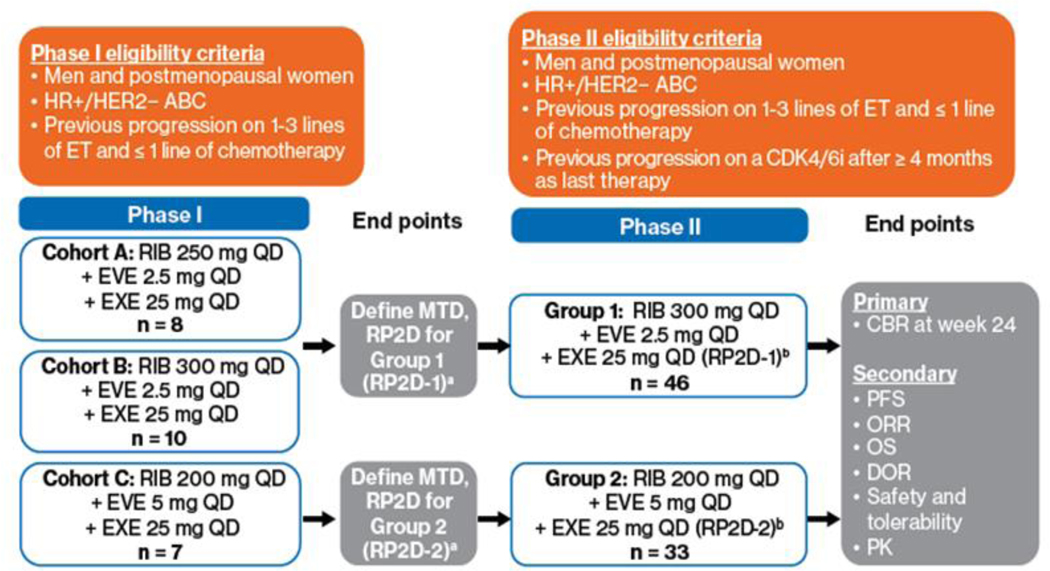
TRINITI-1 was a multicenter, open-label, phase I/II study (Supplemental Table 1). Phase I evaluated the maximum tolerated dose (MTD) of RIB, EVE, and EXE; phase II evaluated antitumor activity. All treatments were administered orally and were continuous, with no rest days (once daily on days 1–28 of a 28-day cycle). After optimal doses were determined in phase I, phase II was initiated (Figure 1). Treatment continued until disease progression, unacceptable toxicity, withdrawal of consent, loss to follow-up, or study termination.
Figure 1.
TRINITI-1 study design. a The MTD was defined as the highest combination drug dose not causing DLTs in > 33% of treated patients in the first treatment cycle. ABC, advanced breast cancer; CBR, clinical benefit rate; CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; DLT, dose-limiting toxicity; DOR, duration of response; ET, endocrine therapy; EVE, everolimus; EXE, exemestane; HER2−, human epidermal growth factor receptor 2 negative; HR+, hormone receptor positive; MTD, maximum tolerated dose; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PK, pharmacokinetics; QD, once daily; RIB, ribociclib; RP2D; recommended phase II dose.
In group 1 of phase I, patients were enrolled to treatment in cohort A (RIB 250 mg + EVE 2.5 mg + EXE 25 mg daily). If ≤ 33% of patients experienced a dose-limiting toxicity (DLT) in cohort A, enrollment proceeded to cohort B (RIB 300 mg + EVE 2.5 mg + EXE 25 mg daily). DLTs were defined as adverse events (AEs) or abnormal laboratory values unrelated to disease or disease progression with a reasonably possible relationship to the study medication(s) that occurred within the first 28 days of cycle 1 and met predefined criteria per the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) v4.03. In group 2, dose de-escalation (cohort C; RIB 200 mg + EVE 5 mg + EXE 25 mg daily) was explored. If DLTs were experienced, the treatment dose was reduced. In phase II, efficacy and safety were evaluated at the recommended phase II doses (RP2Ds) determined for groups 1 and 2 (RP2D1 and RP2D2).
Endpoints
The primary endpoint was clinical benefit rate (CBR), defined as the proportion of patients with complete response (CR), partial response, stable disease, or non-CR/nonprogressive disease, at week 24 (per Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1). The predefined primary endpoint threshold was > 10%, which was chosen as a conservative estimate of the percentage of patients post-CDK4/6i who might benefit from triplet therapy. Key secondary endpoints included PFS, OS, overall response rate, and safety outcomes. Safety outcome measures included percentages of patients with AEs, serious AEs, changes in hematology and chemistry values, vital signs, and electrocardiograms (ECGs). Exploratory endpoints included analysis of circulating tumor DNA (ctDNA) for gene mutations, including those relevant to the CDK4/RB pathway, ESR1, and other breast cancer resistance patterns.
Assessments
Efficacy
All patients enrolled in phase I or II whose disease progressed on prior CDK4/6i therapy and received ≥ 1 dose of the assigned combination of study drugs were included in efficacy analyses. Tumor response assessments were performed locally per RECIST 1.1 at screening, every 8 weeks starting from day 1 of study treatment for the first 12 months, and then every 12 weeks thereafter until disease progression.
Safety
The safety-evaluable population included all patients who enrolled in phase I or II, received ≥ 1 dose of any of the investigational treatment components, and had ≥ 1 valid postbaseline safety assessment. Safety was assessed at screening, continuously during treatment, and for 30 days after the last dose of study drug. AEs were assessed according to NCI CTCAE v4.03. A 12-lead standard ECG was performed at baseline, cycle (C) 1 day (D) 15, C2 D1 and D15, C3–6 D1, at every third cycle thereafter for patients with a QT interval corrected using Fridericia’s formula ≥ 481 ms at any time prior to cycle 7, and at the end of treatment. ECGs could also be performed at any time as clinically indicated.
Pharmacokinetics
Blood samples for pharmacokinetic (PK) analysis from all patients in phases I and II were collected on C1 D15, C2 D1 and D15, and C3 D1. Patients with prior CDK4/6i treatment within 30 days of starting study drug also provided samples on C1 D1.
Biomarkers
Patients enrolled in phase I or II whose disease progressed on prior CDK4/6i therapy and received ≥ 1 dose of the assigned combination of study drugs were included in the exploratory biomarker analyses. Blood for ctDNA analysis was collected (EDTA collection tube) at baseline, on D1 of C1, 3, 5, and 7, and at the end of study treatment. DNA was extracted from patient plasma (plasma extracted by double spin processing) using the QIAamp Circulating Nucleic Acid Kit (QIAGEN, Wetzlar, Germany). DNA libraries were constructed using the TruSeq Nano DNA Library Prep kit (Illumina, San Diego, CA). Coding regions were enriched by hybridization capture to a customized SureSelectXT (Agilent, Santa Clara, CA) 566-gene panel (Supplemental Table 2). Samples were sequenced on the HiSeq 2500 System (Illumina, San Diego, CA), aiming for an average target coverage depth of 1000×.
Statistical Analysis
The data cutoff for this interim analysis was October 24, 2018. For the primary endpoint, it was estimated (taking into account a dropout rate of 10%) that 66 patients would provide ≥ 80% power to test the null hypothesis that the CBR rate at 24 weeks was ≤ 10%, with an alternative hypothesis that this rate was > 10%. The CBR was calculated with an exact 95% Clopper-Pearson confidence interval (CI). The null hypothesis was to be rejected and a successful clinical benefit will be demonstrated if the lower limit of the 95% CI was greater than at least 0.10.
PFS and OS were analyzed using the Kaplan-Meier product-limit method. Similar analyses were performed for biomarkers identifying groups of patients by mutation type to evaluate their association with clinical outcomes (PFS, CBR). Clinical, safety, and biomarker data were also summarized. Summary statistics were provided and 95% CIs were also reported as appropriate. Inferential confirmatory statistics were not provided because of inadequate sizes of subgroups.
The study was conducted in accordance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Harmonized Tripartite Guidelines for Good Clinical Practice and the Declaration of Helsinki. The trial protocol and all amendments were approved by an institutional review board, independent ethics committee, or research ethics board. All patients provided written informed consent before enrollment. Safety, efficacy, and available PK data were monitored by an independent data monitoring committee.
Results
Patients
A total of 104 patients (all women) with ET-refractory disease were enrolled; 25 in phase I and 79 in phase II. Of those patients, 96 had prior CDK4/6i treatment. Among the patients with prior CDK4/6i treatment (n = 96), the median age at baseline was 58 years; the majority of patients were white (81.3%), with an ECOG PS of 0 (57.3%), and had a median of 1 prior line of ET (range, 1–4) (Table 1). At data cutoff, study treatment was ongoing in 14 of 96 patients (14.6%; Supplemental Table 3). The most frequent reason for discontinuation was progressive disease (PD) (56.3%). Median duration of exposure was 16.5 weeks.
Table 1.
Baseline Characteristics
| Totala (n = 96) | PIK3CA WT (n = 59) | PIK3CA Altered (n = 30) | ESR1 WT (n = 59) | ESR1 Altered (n = 30) | |
|---|---|---|---|---|---|
| Median age (range), years | 58 (32–83) | 59 (33–83) | 59 (32–83) | 59 (33–83) | 56 (32–70) |
|
| |||||
| Sex, n (%) | |||||
|
| |||||
| Female | 96 (100) | 59 (100) | 30 (100) | 59 (100) | 30 (100) |
|
| |||||
| Race, n (%) | |||||
|
| |||||
| White | 78 (81.3) | 51 (86.4) | 26 (86.7) | 51 (86.4) | 25 (83.3) |
|
| |||||
| Asian | 2 (2.1) | 1 (1.7) | 0 | 1 (1.7) | 0 |
|
| |||||
| Black | 4 (4.2) | 2 (3.4) | 1 (3.3) | 2 (3.4) | 2 (6.7) |
|
| |||||
| Other or unknown | 12 (12.5) | 5 (8.5) | 3 (10.0) | 5 (8.5) | 3 (10.0) |
|
| |||||
| ECOG PS, n (%) | |||||
|
| |||||
| 0 | 55 (57.3) | 37 (62.7) | 16 (53.3) | 37 (62.7) | 17 (56.7) |
|
| |||||
| 1 | 41 (42.7) | 22 (37.3) | 14 (46.7) | 22 (37.3) | 13 (43.3) |
|
| |||||
| Sites of metastases, n (%) b | |||||
|
| |||||
| Bone | 74 (77.1) | 48 (81.4) | 22 (73.3) | 45 (76.3) | 26 (86.7) |
|
| |||||
| Liver | 63 (65.6) | 40 (67.8) | 18 (60.0) | 37 (62.7) | 20 (66.7) |
|
| |||||
| Lung | 33 (34.4) | 19 (32.2) | 11 (36.7) | 20 (33.9) | 9 (30.0) |
|
| |||||
| Lymph nodes | 28 (29.2) | 19 (32.2) | 9 (30.0) | 18 (30.5) | 10 (33.3) |
|
| |||||
| Previous treatment c | |||||
|
| |||||
| Lines of treatment overall, median (range) | 1 (1–8) | 1 (1–8) | 1 (1–5) | 1 (1–5) | 1 (1–8) |
|
| |||||
| CDK4/6i, n (%) | 96 (100) | 59 (100) | 30 (100) | 59 (100) | 30 (100) |
|
| |||||
| Lines of treatment, median (range) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) |
| 1 prior line | 92 (95.8) | 56 (94.9) | 29 (96.7) | 57 (96.6) | 28 (93.3) |
| 2 prior lines | 4 (4.2) | 13 (5.1) | 1 (3.3) | 2 (3.4) | 2 (6.7) |
|
| |||||
| Median duration of CDK4/6i (range), mo | 18.0 (6.0–141.0) | 14.0 (4.0–29.4) | 10.0 (3.5–34.0) | 11.5 (3.5–34.0) | 14.0 (4.6–26.7) |
|
| |||||
| Prior CDK4/6i, nd | |||||
|
| |||||
| Ribociclib | 12 | 6 | 4 | 9 | 1 |
|
| |||||
| Palbociclib | 96 | 61 | 31 | 58 | 34 |
|
| |||||
| ET, n (%) | 96 (100) | 59 (100) | 30 (100) | 59 (100) | 30 (100) |
|
| |||||
| Lines of treatment, median (range) | 1 (1–4) | 1 (1–4) | 1 (1–3) | 1 (1–4) | 1 (1–3) |
| 1 prior line | 75 (78.1) | 46 (78.0) | 24 (80.0) | 48 (81.4) | 22 (73.3) |
| 2 prior lines | 17 (17.7) | 11 (18.6) | 4 (13.3) | 8 (13.6) | 7 (23.3) |
| ≥ 3 prior lines | 4 (4.1) | 2 (3.4) | 2 (6.7) | 3 (5.1) | 1 (3.3) |
|
| |||||
| Prior ET, n (%)d | |||||
|
| |||||
| Anastrozole | 17 (17.7) | 9 (15.3) | 7 (23.3) | 10 (16.9) | 5 (16.7) |
|
| |||||
| Letrozole | 69 (71.9) | 41 (69.5) | 23 (76.7) | 41 (69.5) | 23 (76.7) |
|
| |||||
| Exemestane | 3 (3.1) | 1 (1.7) | 2 (6.7) | 1 (1.7) | 2 (6.7) |
|
| |||||
| Fulvestrant | 37 (38.5) | 25 (42.4) | 8 (26.7) | 23 (39.0) | 10 (33.3) |
|
| |||||
| Chemotherapy, n (%) | 12 (12.5) | 6 (10.2) | 2 (6.7) | 6 (10.2) | 1 (3.3) |
|
| |||||
| 1 prior line | 12 (12.5) | 6 (10.2) | 2 (6.7) | 6 (10.2) | 1 (3.3) |
CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; ECOG PS, Eastern Cooperative Oncology Group performance status; ESR1, estrogen receptor 1; ET, endocrine therapy; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; WT, wild type.
Intent-to-treat population.
Select sites of metastases.
In advanced/metastatic setting.
Patients can be counted in multiple rows.
Dose Determination
In phase I, 18 and 7 patients were treated in groups 1 and 2, respectively, and the MTD was not reached in either group. RP2D1 was RIB 300 mg/day + EVE 2.5 mg/day + EXE 25 mg/day and RP2D2 was RIB 200 mg/day + EVE 5 mg/day + EXE 25 mg/day.
In a preliminary PK analysis (phase I; cohort A, n = 8; cohort B, n =10), a continuous combination regimen of RIB 250 to 300 mg/day, EVE 2.5 mg/day, and EXE 25 mg/day resulted in exposure that was consistent with that observed in a previous study of single-agent RIB 280 mg.27 The concentrations of RIB and its metabolite LEQ803 were not affected by EVE (Supplemental Figure 2A, B). A dose-dependent drug-drug interaction was observed between RIB and EVE during concurrent continuous dosing, resulting in an EVE plasma trough concentration that was 2- to 3-fold and 4- to 5-fold higher than expected in cohorts A and B, respectively, which allowed the use of a lower EVE dose to reach therapeutic range (Supplemental Figure 2C).28,29
Efficacy
A total of 95 patients progressed on prior CDK4/6i and were evaluable for efficacy (phase I, n = 17; phase II, n = 78; 1 patient in phase II group 2 did not meet criterion of prior CDK4/6i progression). At week 24, the CBR was 41.1% (95% CI, 31.1%−51.6%; Table 2). This exceeded the predetermined boundary of 10% and therefore met the primary endpoint. CBRs at week 24 among patients treated in phase II with RP2D1 vs RP2D2 were similar (44.2% [95% CI, 27.0%−56.8%] vs 37.9% [95% CI, 18.6%−53.2%]). The CBR among all patients in the study (n = 104) was comparable (Supplemental Table 4).
Table 2.
Best Overall Responsea
| Total Patients (n = 95) | |
|---|---|
| CBR at week 24, n (%) [95% CI] b | 39 (41.1) [31.1–51.6] |
| ORR, n (%) [95% CI] c | 8 (8.4) [3.7–15.9] |
| Best overall response, n (%) | |
| CR | 1 (1.1) |
| PR | 7 (7.4) |
| SD | 47 (49.5) |
| PD | 32 (33.7) |
| Non-CR/non-PD, n (%) | 3 (3.2) |
| DCR, n (%) [95% CI] d | 58 (61.1) [50.5–70.9] |
CBR, clinical benefit rate; CR, complete response; DCR, disease control rate; NCRNPD, non-CR, non-PD; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Local investigator assessment per Response Evaluation Criteria in Solid Tumors version 1.1. Patients with measurable disease at baseline: n = 75; patients with only nonmeasurable disease at baseline: n = 20. Five patients discontinued without postbaseline tumor evaluation.
CBR: patients with CR, PR, SD, or NCRNPD at week 24.
ORR: patients with CR or PR.
DCR: patients with CR, PR, SD, or NCRNPD at any time during the study.
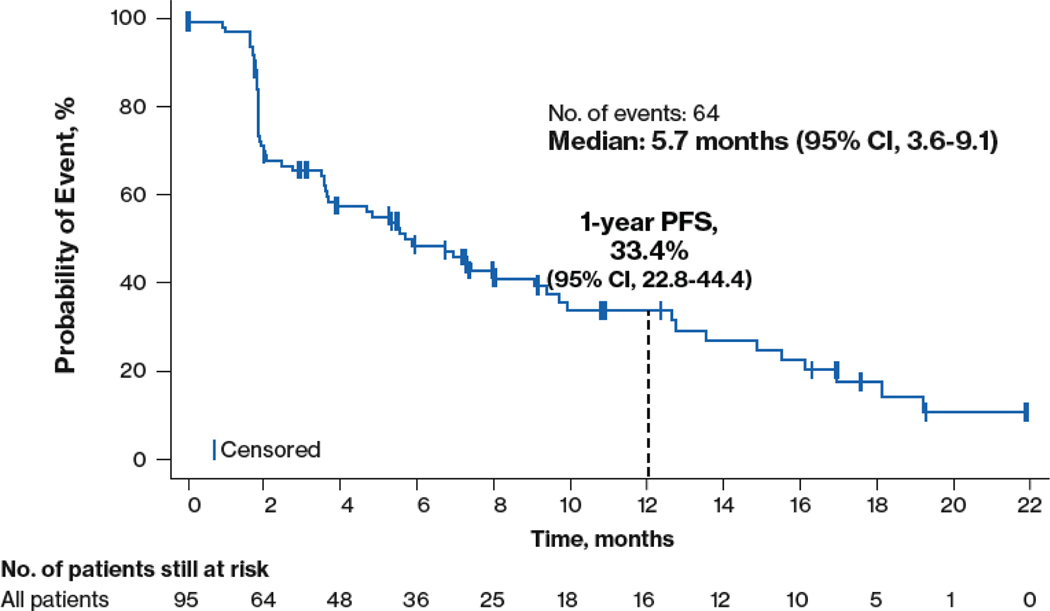
In the efficacy-evaluable population, the median follow-up was 3.1 months (mean, 5.4 months). The median PFS was 5.7 months (95% CI, 3.6–9.1 months) and the 1-year PFS rate was 33.4% (95% CI, 22.8%−44.4%; Figure 2). The median OS had not been reached at the time of this analysis. Nine patients remained on treatment for > 6 months, with 5 remaining on all 3 agents (Supplemental Figure 3).
Figure 2.
Progression-free survival among n = 95 efficacy-evaluable patients. PFS, progression-free survival.
Biomarkers
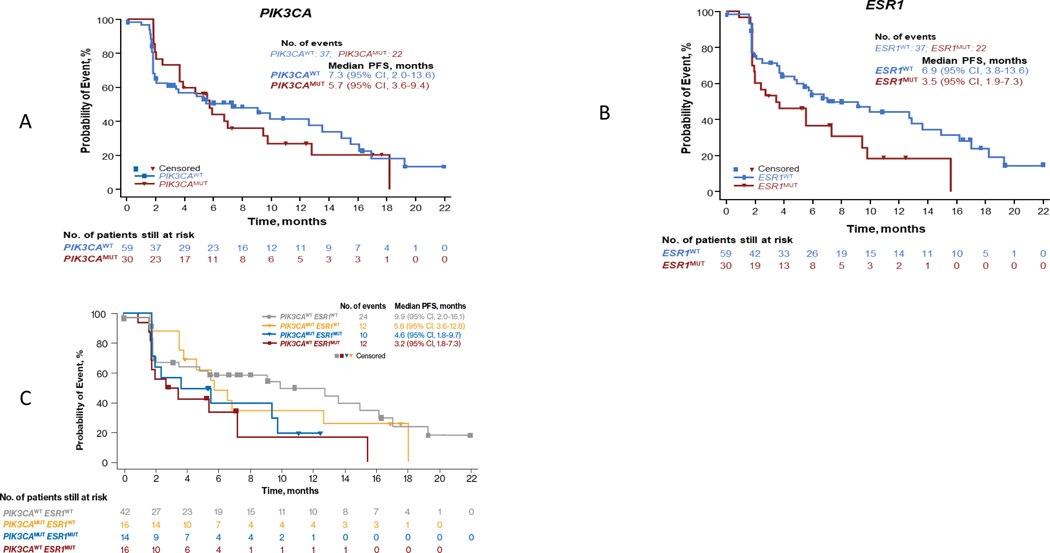
Of 95 efficacy-evaluable patients, 89 had a baseline ctDNA biomarker assessment. PIK3CA and ESR1 were the most common mutations at baseline, both occurring in 33.7% of patients (Supplemental Table 5). Baseline characteristics of patients with PIK3CA and ESR1 mutations were comparable to those of the overall population (Table 1). Concomitant PIK3CA and ESR1 mutations were found in 14 patients (15.7%; Supplemental Table 6). CBR at week 24 was 36.7% among patients with PIK3CA mutations and also 36.7% among those with ESR1 mutations (Supplemental Table 7). A trend of longer median PFS was found in patients with either wild-type (WT) PIK3CA or WT ESR1 at baseline compared with those who had a mutation in the respective gene (Figure 3A, B). Patients with both WT PIK3CA and WT ESR1 at baseline had a numerically longer median PFS than patients who had mutated PIK3CA and ESR1 or 1 mutated and 1 WT gene (PIK3CAWT/ESR1MUT or PIK3CAMUT/ESR1WT) (Figure 3C). Interestingly, patients with early PD (≤ 2 months) had a median of 3 ctDNA mutations, while those who did not (> 2 months) had a median of 4 (Supplemental Figure 4); further analysis is needed to understand the exact mutations in each group. The distributions between the 2 groups were similar, with the exception of 2 patients in the early PD group who had ≥ 10 mutations (all massively parallel sequencing results available as supplement file).
Figure 3.
Progression-free survival by baseline mutation of (A) PIK3CA, (B) ESR1, and (C) PIK3CA and/or ESR1. MUT, mutated; PFS, progression-free survival; WT, wild type.
Safety
The safety analysis included 104 patients. Among 5 patients who discontinued from only 1 agent of the triplet, 3 discontinued from EVE, 1 from RIB, and 1 from EXE. Ten deaths occurred (breast cancer, 7; AE, 1; infections/infestation, 1; pneumonia, 1). The most common hematologic (also most common overall) AE was neutropenia (all grades, 69.2%; grade 3/4, 51.0%) (Table 3). Additional common hematologic AEs or laboratory abnormalities included anemia (all grades, 28.8%; grade 3/4, 9.6%) and thrombocytopenia (all grades, 27.9%; grade 3/4, 1.0%). Grade 3/4 hypophosphatemia and hyperglycemia were observed in 5.8% and 6.7% of patients, respectively. Grade 3/4 gamma-glutamyltransferase, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) increases were observed in 1.9%, 1.0%, and 0% of patients, respectively.
Table 3.
Adverse Events Regardless of Study Drug Relationship
| Preferred Term, n (%) | All Grade | Grade 3/4 |
|---|---|---|
| Total | 104 (100) | 77 (74.0) |
| Hematologic AEs or laboratory abnormalities occurring at > 10% incidence | ||
| Neutropeniaa | 72 (69.2) | 53 (51.0) |
| Anemia | 30 (28.8) | 10 (9.6) |
| Thrombocytopenia | 29 (27.9) | 1 (1.0) |
| AST increased | 20 (19.2) | 1 (1.0) |
| Hypophosphatemia | 20 (19.2) | 6 (5.8) |
| Hyperglycemia | 19 (18.3) | 7 (6.7) |
| Hypokalemia | 16 (15.4) | 1 (1.0) |
| ALT increased | 15 (14.4) | 0 |
| GGT increased | 11 (10.6) | 2 (1.9) |
| Platelet count decreased | 11 (10.6) | 0 |
| Nonhematologic AEs occurring at > 10% incidence and grade 3/4 incidence of ≥ 1.5% | ||
| Stomatitis | 42 (40.4) | 3 (2.9) |
| Nausea | 35 (33.7) | 2 (1.9) |
| Diarrhea | 29 (27.9) | 2 (1.9) |
| Pyrexia | 19 (18.3) | 3 (2.9) |
| Pneumonitis | 15 (14.4) | 5 (4.8) |
| Dyspnea | 13 (12.5) | 4 (3.8) |
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferase.
Neutropenia or decreased neutrophil count.
The most common nonhematologic AE was stomatitis (all grades, 40.4%; grade 3/4, 2.9%). Patients were instructed to use dexamethasone alcohol-free mouthwash 3 times daily for 2 consecutive treatment cycles (56 days) as a prophylaxis for EVE-associated stomatitis; the median duration of patient adherence was 27.6 weeks. Nausea and diarrhea of all grades occurred in 33.7% (grade 3/4, 1.9%) and 27.9% (grade 3/4, 1.9%) of patients, respectively. No grade 3/4 QTc prolongation was reported. AEs were consistent with the individual safety profiles of RIB, EVE, and EXE.
Discussion
TRINITI-1 is the first trial to demonstrate the feasibility and tolerability of continuous triplet therapy with CDK4/6i + mTOR inhibitor + ET in patients with ET-refractory HR+/HER2– ABC after CDK4/6i progression. The CBR of 41.1% at week 24 exceeded the predefined threshold, meeting the primary endpoint of the study. The safety profile was acceptable; the most common AE was neutropenia, and there was no grade 3/4 QTc prolongation. Biomarker analyses demonstrated worsened outcomes in patients with mutations in PI3KCA or ESR1.
EVE may have counteracted compensatory aberrant activation of the PI3K/AKT/mTOR signaling pathway in patients resistant to ET. However, patients with mutations in PIK3CA and/or ESR1 at baseline had worse outcomes than those without. PIK3CA mutations are among the most common genetic alterations in HR+/HER2– breast cancer (≈ 40%) and may contribute to PI3K signaling pathway hyperactivation and resistance to ET.30–32 Blocking aberrant PI3K may shut down the PI3K signaling pathway in these patients.12 ESR1 mutations are also frequently observed in patients with ABC with prior ET and are known to confer resistance to aromatase inhibitor therapy.33,34 Additional data also suggest that alterations to ESR1 may contribute to tumor invasion and metastasis.35 Since TRINITI-1 was initiated, ctDNA analyses in other clinical trials studying fulvestrant in patients with ABC have been reported. In these analyses, treatment with fulvestrant (including in combination with a CDK4/6i) showed potential efficacy benefit in patients with ESR1-mutated tumors.36–38 Fulvestrant, rather than EXE (which was used in TRINITI-1), may be a more suitable option for these patients; however, it is unknown whether continuation of a CDK4/6i in combination with fulvestrant and EVE would be the optimal treatment. Consistent with the results of TRINITI-1, PIK3CA was the most common mutation in BOLERO-2 and the median PFS was shorter in patients with mutated PIK3CA.39 ESR1 mutations were also found to be associated with shorter median PFS.40 Together, these data suggest that tumor molecular alterations may potentially confer therapeutic resistance and that their presence at baseline may be associated with worsened outcomes. However, in TRINITI-1, no formal testing addressed interactions between biomarkers and outcomes, and treatment effect vs prognostic effect could not be separated. Additional biomarker-driven studies are needed to confirm these observations.
No new safety signals were observed in TRINITI-1. Overall, AEs were consistent with the known safety profiles of the individual components of the triplet therapy, although some differences should be noted. Incidences of both all-grade and grade 3/4 stomatitis were lower in TRINITI-1 than in the EVE arm of BOLERO-2 (all grade, 56%; grade 3/4, 8%), which is likely due to use of prophylactic dexamethasone mouthwash in the current study.14 In addition, the incidences of grade 3/4 AST and ALT increases were lower in TRINITI-1 than in the RIB arms of MONALEESA-2 or −3.21,22
TRINITI-1 also showed the potential for continuous and lower doses of RIB and EVE. The preliminary PK analysis demonstrated that continuous dosing of RIB resulted in exposure levels consistent with those in single-agent studies. Concurrent dosing of RIB with EVE increased dose-dependent exposure of EVE, allowing lower doses of EVE to be used. Results of TRINITI-1 showed that this dosing regimen used in triplet combination had acceptable safety and clinical benefit.
The single-arm study design of TRINITI-1 does not allow a definitive understanding of whether continuing CDK4/6 blockade is beneficial; it remains unclear whether continuing with a CDK4/6i in addition to EVE + endocrine therapy or EVE + endocrine therapy alone is the optimal treatment for these patients. However, an acceptable safety profile and preliminary efficacy results in TRINITI-1 provide support for further investigation in larger randomized, controlled trials. Additionally, targeting mTOR, a downstream component of the PI3K signaling pathway seems to be a reasonable treatment approach for progression on CDK4/6i, suggesting that other targets in the pathway may be promising as well. Additional studies are ongoing to evaluate treatment sequencing post-CDK4/6i + ET in HR+/HER2– ABC with or without continued CDK4/6 blockade, including the MAINTAIN trial of fulvestrant ± RIB (NCT02632045), the PACE trial of fulvestrant + palbociclib ± avelumab (NCT03147287), the PALMIRA trial of palbociclib + fulvestrant or letrozole (NCT03809988), and the BYLieve trial of alpelisib + fulvestrant or letrozole in PIK3CA-mutant HR+/HER2– ABC (NCT03056755).
Supplementary Material
Translational relevance.
Standard of care for patients with hormone receptor positive/ human epidermal growth factor receptor 2 negative (HR+/HER2−) advanced breast cancer includes endocrine therapy combined with a cyclin-dependent kinase 4/6 inhibitor (CDK4/6i). However, most patients will eventually experience disease progression, and optimal post-CDK4/6i treatment remains unclear. TRINITI-1 was a single-arm, open-label, multicenter, phase I/II study that tested ribociclib in combination with everolimus and exemestane in patients with HR+/HER2- advanced breast cancer and prior progression on a CDK4/6i. The study met its primary endpoint for efficacy and had an acceptable safety profile. These results suggest that continued CDK4/6 blockade with ribociclib and targeting of the PI3K/AKT/mTOR signaling pathway may be a promising approach in patients with HR+/HER2– advanced breast cancer who have progressed on a CDK4/6i. Additional studies of these combinations are warranted.
Acknowledgments
We thank the patients who participated in the trial and their families and caregivers; the members of the data monitoring committee; the staff members who assisted with the trial at each site; the team that supported the trial; Neilda Baron, Pooja Mathur, and Nicola Caria; and William Ho, MediTech Media, Ltd, for medical editorial assistance. Ribociclib was discovered by Novartis Institutes for BioMedical Research in collaboration with Astex Pharmaceuticals.
Conflicts of interest:
Aditya Bardia reports grants from Genentech, Novartis, Pfizer, Merck, Sanofi, Radius Health, Immunomedics, Mersana, Innocrin, grants and personal fees from Biothernostics Inc., personal fees from Pfizer, Novartis, Genentech, Merck, Radius Health, Immunomedics, Spectrum Pharma, Taiho, Sanofi, Daiichi Pharma, Puma.
Denise A. Yardley reports personal fees from Biotheranostics, Bristol Myers Squibb, Celgene, and NanoString Technologies, grants and personal fees from Daiichi Sankyo/Lilly and Eisai, personal fees and other from Genentech/Roche, grants, personal fees, and other from Novartis, and grants from Abbvie, AstraZeneca, Clovis Oncology, Immunomedics, InventisBio, Lilly, MedImmune, Medivation, Merck, Oncothyreon, Pfizer, Syndax, and Tesaro.
Lowell Hart has nothing to disclose.
Sara A. Hurvitz reports grants from Ambryx, Amgen, Bayer, Biomarin, Cascadian, Daiichi Sankyo, Dignitana, Genentech, GSK, Lilly, Macrogenics, Medivation, Merrimack, Novartis, OBI Pharma, Pfizer, Pieris, Puma, Roche, and Seattle Genetics, and other from Lilly, Novartis, and OBI Pharma.
Hope S. Rugo reports honoraria from Genomic Health, speakers bureau for Genomic Health, institutional research funding from Plexxikon, Macrogenics, OBI Pharma, Eisai, Pfizer, Novartis, Eli Lilly, GlaxoSmithKline, Genentech, Celsion, and Merck, and travel, accommodations, and expenses from Novartis, Roche/Genentech, OBI Pharma, Bayer, and Pfizer.
Cynthia Ma reports research funding from Pfizer, Tempus, Puma, consulting fees from Seattle Genetics, AstraZeneca, Eisai, Eli Lilly, Athenex, OncoSignal, Agendia.
Stacy Moulder reports support from Novartis for clinical trials (some used to support research effort/salary), and non-compensated advisor for Novartis.
Angela DeMichele reports honorarium from Pfizer, consulting fees from Context Therapeutics, Novartis, Calithera, and institutional research support from Novartis, Pfizer, Genentech, Calithera, Menarini.
Amy S. Clark reports institutional research support from Novartis and Lilly.
Amelia Zelnak reports consultant fees from Pfizer, Novartis.
Meghan Karuturi reports consulting for Pfizer.
Tara Sanft has nothing to disclose.
Sibel Blau reports honorarium for participation in advisory boards from Puma Biotechnology, Daiichi Sankyo, Novartis Ribo, and Adaptive Health, speaker honorarium from The American Journal of Managed Care, and Cardinal Health.
Das Purkayastha reports employment by, and stock ownership of Novartis.
References
- 1.Sammons SL, Topping DL, Blackwell KL. HR+, HER2- Advanced breast cancer and CDK4/6 Inhibitors: mode of action, clinical activity, and safety profiles. Curr Cancer Drug Targets. 2017;17(7):637–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Becerra R, Santos N, Diaz L, Camacho J. Mechanisms of resistance to endocrine therapy in breast cancer: focus on signaling pathways, miRNAs and genetically based resistance. Int J Mol Sci. 2012;14(1):108–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lukas J, Bartkova J, Bartek J. Convergence of mitogenic signalling cascades from diverse classes of receptors at the cyclin D-cyclin-dependent kinase-pRb-controlled G1 checkpoint. Mol Cell Biol. 1996;16(12):6917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortes J, Im SA, Holgado E, Perez-Garcia JM, Schmid P, Chavez-MacGregor M. The next era of treatment for hormone receptor-positive, HER2-negative advanced breast cancer: triplet combination-based endocrine therapies. Cancer Treat Rev. 2017;61:53–60. [DOI] [PubMed] [Google Scholar]
- 5.Mayer IA, Arteaga CL. The PI3K/AKT pathway as a target for cancer treatment. Annu Rev Med. 2016;67:11–28. [DOI] [PubMed] [Google Scholar]
- 6.Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol. 2011;29(33):4452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann Oncol. 2018;29(8):1634–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. V4.2020. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.Accessed July 15, 2020.
- 9.Portman N, Alexandrou S, Carson E, Wang S, Lim E, Caldon CE. Overcoming CDK4/6 inhibitor resistance in ER positive breast cancer. Endocr Relat Cancer. 2019;26:R15–30. [DOI] [PubMed] [Google Scholar]
- 10.AlFakeeh A, Brezden-Masley C. Overcoming endocrine resistance in hormone receptor-positive breast cancer. Curr Oncol. 2018;25(suppl 1):S18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andre F, Ciruelos E, Rubovszky G, Campne M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380(20):1929–40. [DOI] [PubMed] [Google Scholar]
- 13.Yardley DA, Noguchi S, Pritchard KI, Burris HA, Baselga J, Gnant M, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30(10):870–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baselga J, Campone M, Piccart M, Burris HA, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Brien NA, Conklin D, Luo T, Ayala R, Issakhanian S, Kalous O, et al. Anti-tumor activity of the PI3K/mTOR pathway inhibitors alpelisib (BYL719) and everolimus (RAD001) in xenograft models of acquired resistance to CDK-4/6 targeted therapy. Cancer Res. 2017;77(13 suppl) [abstract 4150]. [Google Scholar]
- 16.O’Brien NA, McDermott M, Conklin D, Gaither LA, Luo T, Ayala R, et al. Targeting activated PI3K/mTOR signaling overcomes acquired resistance to CDK4/6-based therapies in preclinical models of ER+ breast cancer. Cancer Res. 2019;79(13 suppl) [abstract 3825]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76(8):2301–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornblum N, Zhao F, Manola J, Klein P, Ramaswamy B, Brufsky A, et al. Randomized phase II trial of fulvestrant plus everolimus or placebo in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer resistant to aromatase inhibitor therapy: results of PrE0102. J Clin Oncol. 2018;36(16):1556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachelot T, Bourgier C, Cropet C, Ray-Coquard I, Ferrero J-M, Freyer G, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012;30(22):2718–24. [DOI] [PubMed] [Google Scholar]
- 20.Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904–15. [DOI] [PubMed] [Google Scholar]
- 21.Hortobagyi GN, Stemmer SM, Burris HA, Yap Y-S, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–48. [DOI] [PubMed] [Google Scholar]
- 22.Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–72. [DOI] [PubMed] [Google Scholar]
- 23.Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307–16. [DOI] [PubMed] [Google Scholar]
- 24.Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382(6):514–24. [DOI] [PubMed] [Google Scholar]
- 25.Bardia A, Modi S, Oliveira M, et al. Triplet therapy with ribociclib, everolimus, and exemestane in postmenopausal women with HR+/HER2– advanced breast cancer. Cancer Res. 2016;76(4 suppl) [abstract P6–13-01]. [Google Scholar]
- 26.Bardia A, Cortes J, Modi S, Campone M, Ma BB, Dirix L, et al. Triplet combination of endocrine therapy with the CDK4/6 inhibitor ribociclib, and the mTOR inhibitor everolimus, in HR+, HER2– ABC: results from the dose-expansion cohort. ABC4. 2017. [poster PO72]. [Google Scholar]
- 27.Novartis Pharmaceuticals Corporation. Data on file.
- 28.Franz DN, Lawson JA, Yapici Z, Brandt C, Kohrman MH, Wong M, et al. Everolimus dosing recommendations for tuberous sclerosis complex-associated refractory seizures. Epilepsia. 2018;59(6):1188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verheijen RB, Yu H, Schellens JHM, Beijnen JH, Steeghs N, Huitema ADR. Practical recommendations for therapeutic drug monitoring of kinase inhibitors in oncology. Clin Pharmacol Ther. 2017;102(5):765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukohara T. PI3K mutations in breast cancer: prognostic and therapeutic implications. Breast Cancer (Dove Med Press; ). 2015;7:111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mollon L, Aguilar A, Anderson E, Dean JL, Davis LE, Warholak TL, et al. A systematic literature review of the prevalence of PIK3CA mutations and mutation hotspots in HR+/HER2- metastatic breast cancer. Cancer Res. 2018;78(13 supplement) [abstract 1207]. [Google Scholar]
- 33.Angus L, Beije N, Jager A, Martens JW, Sleijfer S. ESR1 mutations: moving towards guiding treatment decision-making in metastatic breast cancer patients. Cancer Treat Rev. 2017;52:33–40. [DOI] [PubMed] [Google Scholar]
- 34.Fribbens C, Garcia Murillas I, Beaney M, Hrebien S, O’Leary B, Kilburn L, et al. Tracking evolution of aromatase inhibitor resistance with circulating tumour DNA analysis in metastatic breast cancer. Ann Oncol. 2018;29(1):145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lei JT, Gou X, Seker S, Ellis MJ. ESR1 alterations and metastasis in estrogen receptor positive breast cancer. J Cancer Metastasis Treat. 2019;5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fribbens C, O’Leary B, Kilburn L, et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2016;34(25):2961–8. [DOI] [PubMed] [Google Scholar]
- 37.Neven P, Petrakova K, Val Bianchi G, De la Cruz-Merino L, Jerusalem G, Sonke GS, et al. Biomarker analysis by baseline circulating tumor DNA alterations in the MONALEESA-3 study. Cancer Res. 2019;79(4 suppl):[abstract PD205]. [Google Scholar]
- 38.Tolaney SM, Toi M, Neven P, Sohn J, Grischke E, Llombart-Cussac A, et al. Clinical significance of PIK3CA and ESR1 mutations in ctDNA and FFPE samples from the MONARCH 2 study of abemaciclib plus fulvestrant. Cancer Res. 2019;79(13 suppl):[abstract 4458]. [Google Scholar]
- 39.Hortobagyi GN, Chen D, Piccart M, Rugo HS, Burris HA III, Pritchard KI, et al. Correlative analysis of genetic alterations and everolimus benefit in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from BOLERO-2. J Clin Oncol. 2016;34(5):419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chandarlapaty S, Chen D, He W, Sung P, Samoila A, You D, et al. Prevalence of ESR1 Mutations in cell-free DNA and outcomes in metastatic breast cancer: a secondary analysis of the BOLERO-2 clinical trial. JAMA Oncol. 2016;2(10):1310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dagogo-Jack I, Brannon AR, Ferris LA, Campbell CD, Lin JJ, Schultz KR, et al. Tracking the evolution of resistance to ALK tyrosine kinase inhibitors through Longitudinal analysis of circulating tumor DNA. JCO Precis Oncol. 2018;2:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.