Introduction
Multidrug-resistant tuberculosis (MDR-TB), poses a global threat to TB control programs, especially in developing countries (1). In 2019, among the 81 million people in Iran, there was an estimated TB incidence of 13 per 100,000 population (1). Estimated Iran MDR/ rifampicin (RIF)-resistant TB rates were 1.3% among new cases and 8.3% in retreatment cases (1). Patients with RIF-resistant TB, often seen as a proxy for MDR-TB, require treatment regimens that are longer, less effective, and less accessible than first-line regimens (2-8). The low numbers of well-equipped laboratories for drug susceptibility testing (DST) in Iran, make the diagnosis of RIF-resistance challenging in the country (6, 9, 10). As a result, RIF-resistant-TB, very often remains undetected, leading to further spread of drug-resistant TB and worse TB treatment outcomes (11-15). Given that RIF-resistant TB is among the major challenges for national TB control programs (NTP), identification of RIF-resistant TB resistance among Mycobacterium tuberculosis isolates could help us to better advance treatment achievement. Although some studies have investigated the prevalence of RIF-resistance in Iran, a comprehensive analysis has not yet been reported. In this study, we aimed to assess the frequency of RIF-resistance in M. tuberculosis isolates in Iran, using a systematic review and meta-analysis.
Materials and Methods
Search strategy
Pubmed/Medline, Embase, Web of Science, and Scopus from January 1, 1980, to January 1, 2020, were screened for English articles that contained the terms “tuberculosis”, “rifampicin”, and “Iran”. Details of strategies used in Pubmed/Medline are given in Table S1 in the Appendix. Articles in Persian were also searched in the Iranian databases (SID [www.sid.ir] and Magiran [www.Magiran.com]) with similar strategies and related Persian keywords. We performed a systematic review and meta-analysis of the literature following PRISMA guidelines (16).
Study selection
All articles identified by the initial search were reviewed independently by two reviewers (FB and MJN) for relevance, with disagreements mediated by a third author (AAIF). The same reviewers also double reviewed all full-text articles. Studies were selected for inclusion if they met the following criteria: 1) presented original data; 2) provided the primary data on the total number of patients with TB, as well as the number of those with RIF-resistance; and 3) used the standard phenotypic DST method as recommended by WHO/CDC (17, 18). Data from studies evaluating molecular drug susceptibility tests were also included if the results were verified by DNA sequencing. Studies with unrepresentative samples of the general population of TB as well as insufficient information about patients’ characteristics were excluded.
Data extraction
Two reviewers (FB and MJN) performed double data extraction and entry using Microsoft Excel. A third reviewer (AAIF) judged any discrepancies between the two reviewers. From each study, study location, design, age, year, the total number of TB patients, number of RIF-resistance, as well as, when available, status of HIV, and history of the previous TB among participants were extracted. All data were extracted and compiled using the MS Excel software package (Microsoft, Redmond, WA, USA).
In the text, the term “new cases” refers to patients with TB who have never received anti-TB drugs. The term “previously treated cases” or “history of treatment” is used to refer to patients who had previously received anti-TB drugs. “RIF mono resistance” was used to define the resistance to only RIF. “RIF any resistance” referred to resistance to any kind of RIF resistance regardless of mono-resistance or multi-drug resistance (resistance to at least isoniazid and rifampicin).
Quality assessment
Two authors (FB and MJN) applied the Joanna Briggs Institute quality assessment tool for cross-sectional studies to assess the risk of bias for each study. They independently evaluated the components of the scale as “Yes”, “No”, “Unclear” or “Not Applicable”. This was used to guide the overall rating for the quality of each study as “Good”, or “Poor”. In case of disagreement, a consensus opinion was reached.
Meta-analysis
Statistical analyses were performed with STATA (version 14, IC; Stata Corporation, College Station, TX, USA). The pooled frequency of RIF-resistance among patients with confirmed TB was assessed by the random-effects model. Heterogeneity across studies was estimated by calculating the I2 statistic. A P-value of less than 0.05 indicated that heterogeneity among the group of studies being analyzed was significant. To explore sources of studies’ heterogeneity, we did meta-regression and subgroup analysis. Publication bias was assessed statistically by using Begg’s tests (P<0.05 was considered indicative of statistically significant publication bias).
Results
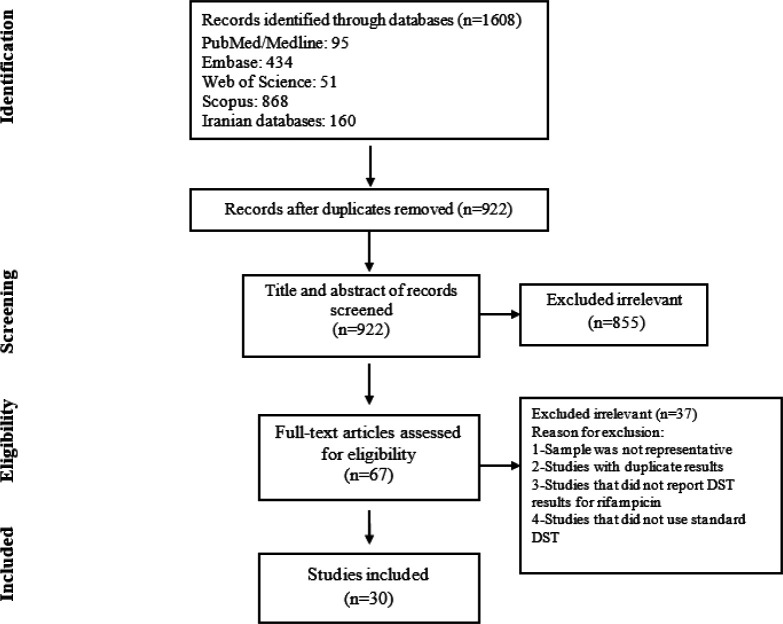
The results of the literature search are displayed in Figure 1. Our initial search yielded 1608 studies. Of these, 67 were referred for full-text assessment, and 30 cross-sectional studies met the inclusion criteria and were selected for inclusion in the qualitative synthesis and meta-analysis (10, 19-47). Table 1 provides information on each of the included studies. Studies were conducted in different regions of Iran: Tehran was the most frequently represented city with 13 studies. In all included studies, conventional DST was performed by the standard method according to the WHO or CDC guidelines. The sample size ranged from 31 to 1242 individuals enrolled per study. A total of 8215 patients with TB were included in the meta-analysis. Five studies reported RIF mono-resistance for a total of 3205 TB cases. Although we sought to extract data on HIV infection and previous TB treatment, most studies did not provide sufficient information. Data on previous TB treatment was provided by only five of the 30 included studies and HIV infection by one.
Figure 1.
Flow chart of study selection for inclusion in the systematic review and meta-analysis
Table 1.
Characteristics of the included studies investigating the frequency of RIF-resistance among patients with confirmed TB
| First author |
Published
time |
Enrollment
time |
Location | Mean age | Total No. of TB patients | Total No. of RIF-resistance | Type of patients | DST method |
|---|---|---|---|---|---|---|---|---|
| Amini | 2019 | 2015-2017 | Multicenter | Adult | 334 | 12 | New and retreatment case | WHO standard conventional DST |
| N Mansoori | 2018 | 2014-2015 | Golestan | 50 | 176 | 1 | New cases | WHO standard conventional DST |
| Sirous | 2018 | 2015-2017 | Ahvaz | NR | 487 | 11 | NR | WHO standard conventional DST |
| Sakhaee | 2017 | 2013-2016 | Tehran | NR | 395 | 2 | NR | CDC standard conventional DST |
| Darban-Sarokhalil | 2016 | NR | Tehran | Adult | 112 | 1 | New cases | WHO standard conventional DST |
| Sahebi | 2016 | 2011-2013 | Multicenter | 52 | 280 | 33 | New and retreatment case | WHO standard conventional DST |
| Zarei | 2016 | 2012-2014 | Shiraz | 48 | 199 | 30 | NR | WHO standard conventional DST |
| Badie | 2015 | NR | Ahvaz | Adult | 64 | 0 | NR | WHO standard conventional DST |
| Tavanaee Sani | 2015 | 2012-2013 | Mashhad | NR | 100 | 3 | New and retreatment case | WHO standard conventional DST |
| Imani Fooladi | 2014 | 2009-2011 | Tehran | Adult | 103 | 0 | NR | WHO standard conventional DST |
| Nasiri | 2014 | 2010-2012 | Multicenter | 45 | 252 | 15 | New cases | WHO standard conventional DST |
| Velayati | 2014 | 2010-2011 | Tehran | 47 | 1242 | NR | New and retreatment case | WHO standard conventional DST |
| Bahrami | 2013 | 2010-2012 | Tehran | Adult | 176 | 19 | NR | WHO standard conventional DST |
| Farazi | 2012 | 2005-2010 | Arak | 52 | 115 | 2 | New and retreatment case | WHO standard conventional DST |
| Marjani | 2012 | 2003-2008 | Tehran | 51 | 554 | 27 | New and retreatment case | WHO standard conventional DST |
| Yazdi | 2012 | 2009-2010 | Yazd | NR | 31 | 7 | New cases | WHO standard conventional DST |
| Hadizadeh | 2011 | 2006-2009 | Tehran | NR | 1027 | 118 | NR | WHO standard conventional DST |
| Livani | 2011 | 2009-2010 | Golestan | 54 | 148 | 5 | New and retreatment case | MGIT |
| Bahrmand2 | 2009 | 2005-2006 | Tehran | Adult | 286 | 41 | NR | CDC standard conventional DST |
| Shamaei | 2009 | 2000-2003 | Tehran | 45.4 | 548 | 120 | New and retreatment case | WHO standard conventional DST |
| Javid | 2009 | 2007-2008 | Golestan | NR | 45 | 6 | New cases | WHO standard conventional DST |
| Maleki | 2009 | 2007-2008 | Tabriz | NR | 103 | 0 | NR | WHO standard conventional DST |
| Farivar | 2006 | 2001-2003 | Zahedan | Adult | 84 | 47 | New and retreatment case | WHO standard conventional DST |
| Khosravi | 2006 | NR | Ahvaz | Adult | 80 | 6 | NR | WHO standard conventional DST |
| Namaei | 2006 | 2001-2002 | Mashhad | 56.6 | 105 | 0 | New cases | WHO standard conventional DST |
| Naderi | 2004 | 2001-2002 | Zahedan | NR | 84 | 47 | New and retreatment case | WHO standard conventional DST |
| Mansoori | 2003 | 1996-2000 | Tehran | 37 | 273 | 111 | New and retreatment case | WHO standard conventional DST |
| Heidarnejad | 2001 | NR | Tabriz | 44 | 155 | 1 | New and retreatment case | WHO standard conventional DST |
| Moniri | 2001 | 1998-2000 | Kashan | 75 | 94 | NR | NR | WHO standard conventional DST |
| Bahrmand1 | 2000 | 1998-1999 | Tehran | Adult | 563 | 25 | New | WHO standard conventional DST |
RIF: Rifampicin; TB: tuberculosis
Quality assessment
All included studies were rated as “Good” by both assessors, representing a low risk of bias.
Frequency of RIF-resistance among patients with TB
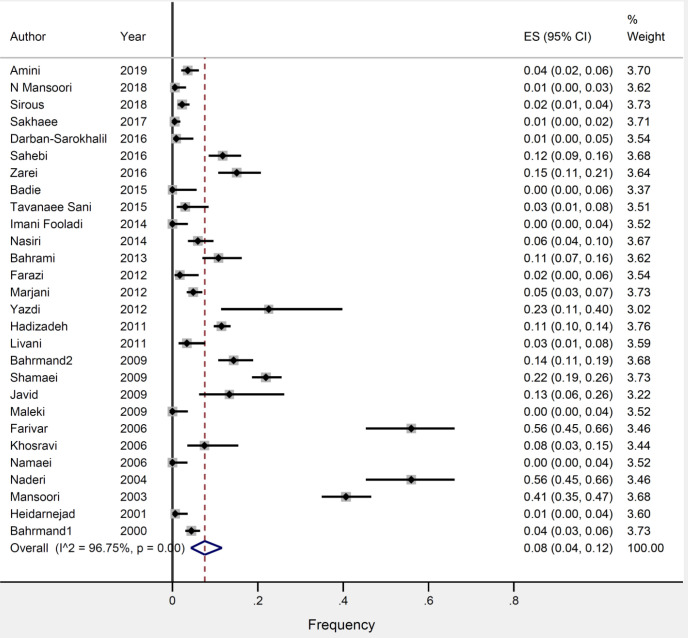
As shown in Figure 2, the overall frequency of RIF-resistance among all patients with TB was 8.0% (95% CI 4.0–12.0). We found a high degree of heterogeneity in the results across the included studies (I2=96%, P=0.00). Based on meta-regression, the number of RIF-resistances per study resulted in a significant source of heterogeneity in the current study (P-value= 0.03). As per Begg’s (P=0.1) test, there was no evidence of publication bias.
Figure 2.
Frequency of RIF-resistance among patients with confirmed TB
RIF: Rifampicin; TB: tuberculosis
Subgroup analysis
Table 2 shows the subgroup analysis of the studies based on the type of RIF-resistance, and history of TB treatment. RIF-resistance was significantly higher among previously treated patients compared to new patients (4% vs 36%).
Table 2.
Pooled frequency of RIF-resistance among subgroups of studies
| Subgroups | No. of study | Frequency (95 % CI) | Heterogeneity | |
|---|---|---|---|---|
| P -Value | I2 (%) | |||
| Type of RIF-resistance Any resistance Mono resistance |
28 (6879 TB cases) 5 (3205 TB cases) |
8.0 (4.0-12.0) 5.0 (0.0-12.0) |
0.00 0.00 |
96 100 |
| History of treatment New cases Previously treated cases |
10 (1904 TB cases) 5 (383 TB cases) |
4.0 (2.0-8.0) 36.0 (2.0-82.0) |
0.00 0.00 |
79 100 |
RIF: Rifampicin
Discussion
In the present study, the pooled frequency of RIF-resistant TB in all TB cases was found to be 8.0%. Our sub-group analysis also showed that 4.0% of newly diagnosed cases and 36.0% of previously-treated TB patients from different settings in Iran were RIF-resistant. The prevalence of RIF-resistant TB among new cases observed in this study is above the current WHO estimates of drug resistance for Iran (1). This suggests that the burden of RIF-resistance in new patients with TB may be underestimated and better programmatic strategies are needed.
Furthermore, several other studies reported quite a varied frequency of RIF-resistant TB in the different countries in the Middle East Region. The prevalence of RIF-resistant TB in this study compared to previous studies in Iraq (12.6%), Egypt (1.9%), Turkey (1%), Saudi Arabia (1%), and Kuwait (0.2%) (48). The variation of RIF-resistant-TB across the country might be related to geographical variation, study setting, differences in patient selection, sample size, method of diagnosis, and TB control practice.
Several countries in the world have adopted an algorithm placing Xpert MTB/RIF as the initial and diagnostic test for RIF-resistance (49-55). The results from the early programmatic implementation of Xpert MTB/RIF testing in nine countries indicated that testing with Xpert MTB/RIF can detect a large number of people with TB that routine services failed to detect (56). As more cases are rapidly detected and treated, there will be a reduction in transmission of primary drug resistance in the community. In Iran, due to limited resources, only a few TB laboratories use Xpert MTB/RIF for rapid diagnosis of TB and detection of drug resistance. Accordingly, in the current systematic review, all studies used conventional DST for investigating the drug-resistant pattern in patients infected with M. tuberculosis.
We also indicated that near half of previously-treated TB patients in the current study were resistant to RIF (Table 3). This indicates that in Iran there may be high rates of acquired resistance to RIF. Failure of the appropriate treatment of TB patients is among the most common causes of the occurrence of drug resistance. This could be from the supply or quality of the drugs, possible inadequate drug intake by patients, and deficient infection control in hospitals (57, 58). Our results suggest that NTP needs to strengthen the management of drug-resistant TB, and patients previously treated for TB should be prioritized in case findings.
This review has some limitations. Not all regions in Iran had reported RIF-resistant TB, as such these were considered not fully representative. Another limitation was that not all necessary information, such as age, sex, ethnicity, and HIV, could be obtained from all included studies. Therefore, relevant stratified analyses could not be performed to find out more details of the related risk factors.
Conclusion
Our study showed that the frequency of RIF-resistance among patients with TB was 8.0%. Programmatic implementation of rapid DST such as the Xpert MTB/RIF assay as a primary diagnostic test for persons suspected of having a RIF-resistant TB would be helpful for control of the drug resistance.
Acknowledgment
This study was jointly supported by Baqiyatallah University of Medical Sciences and Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Founding
This study was funded by a grant from National Institute for Medical Research Development (grant no: 976978).
Conflicts of Interest
None.
References
- 1.World Health Organization (WHO) Global Tuberculosis Report. 2020 [Google Scholar]
- 2.Nasiri M, Zamani S, Pormohammad A, Feizabadi M, Aslani H, Amin M, et al. The reliability of rifampicin resistance as a proxy for multidrug-resistant tuberculosis: a systematic review of studies from Iran. Eur J Clin Microbiol Infect Dis . 2018:1–6. doi: 10.1007/s10096-017-3079-4. [DOI] [PubMed] [Google Scholar]
- 3.Cox V, McKenna L, Acquah R, Reuter A, Wasserman S, Vambe D, et al. Clinical perspectives on treatment of rifampicin-resistant/multidrug-resistant TB. Int J Tuberc Lung Dis. 2020;24:1134–1144. doi: 10.5588/ijtld.20.0330. [DOI] [PubMed] [Google Scholar]
- 4.De Vos E, Scott L, Voss De Lima Y, Warren R, Stevens W, Hayes C, et al. Management of rifampicin-resistant TB: programme indicators and care cascade analysis in South Africa. Int J Tuberc Lung Dis. 2021;25:134–141. doi: 10.5588/ijtld.20.0598. [DOI] [PubMed] [Google Scholar]
- 5.Malenfant JH, Brewer TF. Rifampicin Mono-Resistant Tuberculosis—A Review of an Uncommon but Growing Challenge for Global Tuberculosis Control. Open Forum Infectious Diseases. Oxford University Press US: 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasiri MJ, Dabiri H, Darban-Sarokhalil D, Rezadehbashi M, Zamani S. Prevalence of drug-resistant tuberculosis in Iran: systematic review and meta-analysis. Am J Infect Control. 2014;42:1212–1218. doi: 10.1016/j.ajic.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Migliori GB, Tiberi S, Zumla A, Petersen E, Chakaya JM, Wejse C, et al. MDR/XDR-TB management of patients and contacts: Challenges facing the new decade. The. 2020 Clinical;92:S15–S25. doi: 10.1016/j.ijid.2020.01.042. [DOI] [PubMed] [Google Scholar]
- 8.Koch A, Cox H, Mizrahi V. Drug-resistant tuberculosis: challenges and opportunities for diagnosis and treatment. Curr Opin Pharmacol. 2018;42:7–15. doi: 10.1016/j.coph.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allahyartorkaman M, Mirsaeidi M, Hamzehloo G, Amini S, Zakiloo M, Nasiri MJ. Low diagnostic accuracy of Xpert MTB/RIF assay for extrapulmonary tuberculosis: A multicenter surveillance. Sci Rep. 2019;9:1–6. doi: 10.1038/s41598-019-55112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amini S, Hoffner S, Torkaman MRA, Hamzehloo G, Nasiri MJ, Salehi M, et al. Direct drug susceptibility testing of Mycobacterium tuberculosis using the proportional method: A multicenter study. J Glob Antimicrob Resist. 2019;17:242–244. doi: 10.1016/j.jgar.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Ismail NA, Mvusi L, Nanoo A, Dreyer A, Omar SV, Babatunde S, et al. Prevalence of drug-resistant tuberculosis and imputed burden in South Africa: a national and sub-national cross-sectional survey. Lancet Infect Dis. 2018;18:779–787. doi: 10.1016/S1473-3099(18)30222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phu PT, Vinh DN, Son VT, Hanh NT, Lan NH, Van Vinh T, et al. Risk factors for poor treatment outcomes of 2266 multidrug-resistant tuberculosis cases in Ho Chi Minh City: a retrospective study. BMC Infect Dis. 2020;20:1–10. doi: 10.1186/s12879-020-4887-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samuels JP, Sood A, Campbell JR, Khan FA, Johnston JC. Comorbidities and treatment outcomes in multidrug resistant tuberculosis: a systematic review and meta-analysis. Sci Rep. 2018;8:1–13. doi: 10.1038/s41598-018-23344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javaid A, Ullah I, Masud H, Basit A, Ahmad W, Butt Z, et al. Predictors of poor treatment outcomes in multidrug-resistant tuberculosis patients: A retrospective cohort study. Clin Microbiol Infect. 2018;24:612–617. doi: 10.1016/j.cmi.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Atif M, Bashir A, Ahmad N, Fatima RK, Saba S, Scahill S. Predictors of unsuccessful interim treatment outcomes of multidrug resistant tuberculosis patients. BMC Infect Dis. 2017;17:1–12. doi: 10.1186/s12879-017-2746-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 17.Organization WH. Guidelines for surveillance of drug resistance in tuberculosis. Geneva. World Health Organization; 2009. [Google Scholar]
- 18.Kent PT. Public health mycobacteriology: a guide for the level III laboratory: US Department of Health and Human Services, Public Health Service, Centers. 1985. [Google Scholar]
- 19.Mansoori N, Douraghi M, Rajabloo AA, Taziki M, Yaseri M, Vaziri F. Mycobacterium tuberculosis Complex Drug Resistance in a High Tuberculosis Incidence Area from the WHO Eastern Mediterranean Region. J Pharm Pharm Sci. 2018;20:428–434. doi: 10.18433/J3J64H. [DOI] [PubMed] [Google Scholar]
- 20.Sirous M, Khosravi AD, Tabandeh MR, Salmanzadeh S, Ahmadkhosravi N, Amini S. Molecular detection of rifampin, isoniazid, and ofloxacin resistance in Iranian isolates of Mycobacterium tuberculosis by high-resolution melting analysis. Infect Drug Resist. 2018;11:1819. doi: 10.2147/IDR.S178831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakhaee F, Ghazanfari M, Ebrahimzadeh N, Vaziri F, Jamnani FR, Davari M, et al. A comparative study of phenotypic and genotypic first-and second-line drug resistance testing of Mycobacterium tuberculosis. Biologicals. 2017;49:33–38. doi: 10.1016/j.biologicals.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Darban-Sarokhalil D, Nasiri MJ, Fooladi AA, Heidarieh P, Feizabadi MM. Rapid detection of Rifampicin-and Isoniazid-Resistant Mycobacterium tuberculosis using TaqMan Allelic Discrimination. Osong Public Health Res Perspect. 2016;7:127–130. doi: 10.1016/j.phrp.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahebi L, Ansarin K, Mohajeri P, Khalili M, Monfaredan A, Farajnia S, et al. Patterns of drug resistance among tuberculosis patients in west and Northwestern Iran. Open Respir Med J. 2016;10 doi: 10.2174/1874306401610010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zarei Z, Emami A, Moghadami M, Kashkooli GS, Pirbonyeh N. Molecular characterization of isoniazid and rifampicin target genes in multi-drug resistant Mycobacterium tuberculosis isolates from southwest of Iran. Gene Rep. 2017;6:19–25. [Google Scholar]
- 25.Badie F, Arshadi M, Mohsenpoor M, Gharibvand SS. Drug resistance pattern of Mycobacterium tuberculosis isolates from patients referred to TB reference laboratory in Ahvaz. Osong Public Health Res Perspect. 2016;7:32–35. doi: 10.1016/j.phrp.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tavanaee Sani A, Shakiba A, Salehi M, Taghanaki B, Reza H, Ayati Fard SF, et al. Epidemiological characterization of drug resistance among Mycobacterium tuberculosis isolated from patients in northeast of Iran during 2012-2013. Biomed Res Int. 2015:2015. doi: 10.1155/2015/747085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imani Fooladi AA, Babak F, Fazlollah MS, Nematollah JJ. Rapid detection of MDR–Mycobacterium tuberculosis using modified PCR-SSCP from clinical Specimens. Asian Pac J Trop Biomed. 2014;4:S165–S170. doi: 10.12980/APJTB.4.2014C1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nasiri MJ, Rezaei F, Zamani S, Darban-Sarokhalil D, Fooladi AAI, Shojaei H, et al. Drug resistance pattern of Mycobacterium tuberculosis isolates from patients of five provinces of Iran. Asian Pac J Trop Biomed. 2014;7:193–196. doi: 10.1016/S1995-7645(14)60019-5. [DOI] [PubMed] [Google Scholar]
- 29.Velayati AA, Farnia P, Mozafari M, Sheikholeslami MF, Karahrudi MA, Tabarsi P, et al. High prevelance of rifampin-monoresistant tuberculosis: a retrospective analysis among Iranian pulmonary tuberculosis patients. Am J Trop Med Hyg. 2014;90:99–105. doi: 10.4269/ajtmh.13-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bahrami S, Bahrmand AR, Safarpour E, Masoumi M, Saifi M. Detection of ethambutol-resistant associated mutations in Mycobacterium tuberculosis isolates from Iran using multiplex allele-specific PCR. J med microbiol infect dis. 2013;1:41–45. [Google Scholar]
- 31.Farazi A, Sofian M, Zarrinfar N, Katebi F, Hoseini SD, Keshavarz R. Drug resistance pattern and associated risk factors of tuberculosis patients in the central province of Iran. Caspian J Intern Med. 2013;4:785. [PMC free article] [PubMed] [Google Scholar]
- 32.Marjani M, Baghaei P, Tabarsi P, Shamaei M, Mansouri D, Masjedi M, et al. Drug resistance pattern and outcome of treatment in recurrent episodes of tuberculosis. East Mediterr Health J. 2012:18. doi: 10.26719/2012.18.9.957. [DOI] [PubMed] [Google Scholar]
- 33.Sharifi Yazdi M, Jabbari H, Soltan D. Primary drug resistance patterns in newly diagnosed tuberculosis patients in Yazd, Southern Province of Iran. Afr J Biotechnol. 2012;11:702–706. [Google Scholar]
- 34.AR HT, Yari S, Karimi A, Fateh A, Saifi M, Jabarzadeh E, et al. Survey of extensively drug-resistant tuberculosis XDR-TB) in Iran-Tehran: A retrospective study. Afr J Microbiol Res. 2011;5:3795–3800. [Google Scholar]
- 35.Livani S, Mirinargesi M, Nemati-Shoja E, Rafiei S, Taziki M, Tabarraei A. Prevalence of Multidrug Resistant Mycobacterium tuberculosis by Mycobacteria growth indicator tube in Golestan province, North of Iran. Med Lab J. 2011;5:7–14. [Google Scholar]
- 36.Bahrmand AR, Titov LP, Tasbiti AH, Yari S, Graviss EA. High-level rifampin resistance correlates with multiple mutations in the rpoB gene of pulmonary tuberculosis isolates from the Afghanistan border of Iran. J Clin Microbiol. 2009;47:2744–2750. doi: 10.1128/JCM.r00548-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shamaei M, Marjani M, Chitsaz E, Kazempour M, Esmaeili M, Farnia P, et al. First-line anti-tuberculosis drug resistance patterns and trends at the national TB referral center in Iran—eight years of surveillance. Int J Infect Dis. 2009;13:e236–e240. doi: 10.1016/j.ijid.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 38.Javid S, Ghaemi E, Amirmozaffari N, Rafiee S, Moradi A, Dadgar T. Detection of Isoniazid and Rifampin Resistant Strain of Mycobacterium Tuberculosis Isolated from patients in Golestan province (North of Iran) Med Lab J. 2009;3:0–0. [Google Scholar]
- 39.Maleki M, Moadab SR. Drug resistance patterns of Mycobacterium tuberculosis in Tabriz, Iran [In Persian] Iranian J Med Microbiol. 2009;3:18–24. [Google Scholar]
- 40.Farivar TN, Naderi M, Mohagheghi Fard AH, Oskoui Oweisi H, Sharifi Moud B. Drug Resistance of Mycobacterium tuberculosis Strains Isolated from Patients with Pulmonary Tuberculosis in South Eastern of Iran. J Med Sci. 2006;6:275–278. [Google Scholar]
- 41.Khosravi AD, Dezfulian A, Alavi SM. Detection of Isoniazid and Rifampin resistant Mycobacterium tuberculosis isolated from tuberculosis patients using conventional method and PCR. PaK J Med Sci. 2006;22:47. [Google Scholar]
- 42.Namaei MH, Sadeghian A, Naderinasab M, Ziaee M. Prevalence of primary drug resistant Mycobacterium tuberculosis in Mashhad, Iran. Indian J Med Res. 2006;124:77. [PubMed] [Google Scholar]
- 43.Naderi M, Naserpour FT, Kouhpayeh H, Owisi Oskouie H. Drug resistance of isolated strains of Mycobacterium tuberculosis [In Persian] Esfahan Med J. 2004;21:40–43. [Google Scholar]
- 44.Mansouri S-D, Arami S, Mirabolhasani Z, Farnia P, Velayati A-A. The pattern of drug resistance among newly diagnosed and old cases of pulmonary tuberculosis in NRITLD. Arch Iran Med. 2003;6:255–260. [Google Scholar]
- 45.Heidarnejad H, Nagili B. Primary resistance of Mycobacterium tuberculosis to isoniazid, streptomycin, rifampin, and ethambutol in pulmonary tuberculosis. Arch Iran Med. 2001;4:1–4. [Google Scholar]
- 46.Moniri R, Rasa S, Mousavi G. A survey on type of mycobacterium and drug resistance rates of Mycobacterium tuberculosis strains in Kashan [In Persian] J Shahid Sadughi Med Sci. 2001;9:67–70. [Google Scholar]
- 47.Bahrmand A, Velayati A, Bakayev V. Treatment monitoring and prevalence of drug resistance in tuberculosis patients in Tehran. Int J Tuberc Lung Dis. 2000;4:544–549. [PubMed] [Google Scholar]
- 48.Ahmad S, Mokaddas E, Al-Mutairi NM. Prevalence of tuberculosis and multidrug resistant tuberculosis in the Middle East region. Expert Rev Anti Infect Ther. 2018;16:709–721. doi: 10.1080/14787210.2018.1519393. [DOI] [PubMed] [Google Scholar]
- 49.Steingart KR, Sohn H, Schiller I, Kloda LA, Boehme CC, Pai M, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev . 2013 doi: 10.1002/14651858.CD009593.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang K, Lu W, Wang J, Zhang K, Jia S, Li F, et al. Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert MTB/RIF assay: a meta-analysis. J Infect. 2012;64:580–588. doi: 10.1016/j.jinf.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 51.Zeka AN, Tasbakan S, Cavusoglu C. Evaluation of the GeneXpert MTB/RIF assay for rapid diagnosis of tuberculosis and detection of rifampin resistance in pulmonary and extrapulmonary specimens. J Clin Microbiol. 2011;49:4138–4141. doi: 10.1128/JCM.05434-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iram S, Zeenat A, Hussain S, Yusuf NW, Aslam M. Rapid diagnosis of tuberculosis using Xpert MTB/RIF assay-Report from a developing country. PaK J Med Sci. 2015;31:105. doi: 10.12669/pjms.311.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raizada N, Sachdeva K, Sreenivas A, Vadera B, Gupta R, Parmar M, et al. Feasibility of decentralised deployment of Xpert MTB/RIF test at lower level of health system in India. PLoS One. 2014;9:e89301. doi: 10.1371/journal.pone.0089301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zar HJ, Workman L, Isaacs W, Dheda K, Zemanay W, Nicol MP. Rapid diagnosis of pulmonary tuberculosis in African children in a primary care setting by use of Xpert MTB/RIF on respiratory specimens: a prospective study. Lancet Glob Health. 2013;1:e97–e104. doi: 10.1016/S2214-109X(13)70036-6. [DOI] [PubMed] [Google Scholar]
- 55.Theron G, Zijenah L, Chanda D, Clowes P, Rachow A, Lesosky M, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014;383:424–435. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 56.Creswell J, Codlin AJ, Andre E, Micek MA, Bedru A, Carter EJ, et al. Results from early programmatic implementation of Xpert MTB/RIF testing in nine countries. BMC Infect Dis. 2014;14:2. doi: 10.1186/1471-2334-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caminero J. Multidrug-resistant tuberculosis: epidemiology, risk factors and case finding [State of the art series. Drug-resistant tuberculosis. Edited by CY. Chiang. Number. 4;14:382–390. [PubMed] [Google Scholar]
- 58.Sharma SK, Mohan A. Multidrug-resistant tuberculosis: a menace that threatens to destabilize tuberculosis control. Chest. 2006;130:261–272. doi: 10.1378/chest.130.1.261. [DOI] [PubMed] [Google Scholar]