Abstract
Introduction
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are key complications of coronavirus disease 2019 (COVID-19). The study's primary outcome was assessing the utility of Wells DVT, Wells PE scores, and D-dimers in diagnosing DVT and PE. Secondary outcomes were the risk factors for the development of PE and DVT in COVID-19 patients.
Materials and methods
We compared COVID-19 patients with a positive and negative lower extremity (LE) duplex. A similar approach was made for patients who underwent imaging for PE.
Results
The prevalence of PE was 23.8% (26 out of 109 patients), and the prevalence of DVT was 33% (35 out of 106). A D-dimer of 500 ng/mL had a sensitivity of 95.6% and 93.7% for the diagnosis of PE and DVT, respectively. A Wells DVT score of 3 points had a specificity of 92.9% and sensitivity of 8.8% for DVT diagnosis in COVID-19. A Wells PE score of 4 had a specificity of 100% and a sensitivity of 20% for the diagnosis of PE. The combined approach of using a Wells DVT score of 3 in suspected DVT and a Wells PE score of 4 in suspected PE and D-dimers of 500 ng/ml has a sensitivity of 94.2% and 96.1%, respectively.
In the suspected DVT group, male gender (OR 3.88, 95% CI 1.55-9.7, P=0.004), lower body mass index (BMI) (OR 0.92, 95% CI 0.86-0.99, P=0.037), antiplatelet use (OR 0.19, 95% CI 0.04-0.88, P=0.035), systolic blood pressure ≤100 mmhg (OR 4.96, 95% CI 1.37-17.86, P=0.014), absolute lymphocytes ≤1 (OR 2.57, 95% CI 1.07-6.12, P=0.033), D-dimer ≥500 ng/ml (OR 6.42, 95% CI 1.40-29.38, P=0.016), blood urea nitrogen (BUN) ≥20 mg/dl (OR 2.33, 95% CI 1.00-5.41, P=0.048), and intubation (OR 3.32, 95% CI 1.26-8.78, P=0.015) were found to be statistically significant for DVT.
In the suspected PE group, history of cancer (OR 10.69, 95% CI 1.06-107.74, P=0.044), total WBC count (OR 1.07, 95% CI 0.95-1.21, P=0.032), platelets ≥ 400,000 (OR 5.13, 95% CI 1.79-14.68, P=0.002), D-dimer levels ≥ 500 ng/ml (OR 25.47, 95% CI 3.27-197.97, P=0.002), Wells PE score (OR 2.46, 95% CI 1.50-4.06, P<0.001), pulmonary embolism rule-out criteria (PERC) score (OR 1.79, 95% CI 1.05-3.05, P=0.054), and Sequential Organ Failure Assessment (SOFA) score (OR 1.91, 95% CI 1.16-3.12, P=0.002) were statistically significant.
Conclusions
The combined approach of using a Wells DVT score of 3 in suspected DVT and Wells PE score of 4 in suspected PE and D-dimers of 500 ng/ml may be used to diagnose PE and DVT in COVID-19. Venous thromboembolism (VTE) occurrence in COVID-19 is associated with non-traditional risk factors such as intubation and higher severity of systemic inflammation, and these patients may benefit from more aggressive testing for VTE.
Keywords: covid-19, deep vein thrombosis, pulmonary embolism, wells pe score, wells dvt score, d-dimers
Introduction
Coronavirus disease 2019 (COVID-19) is a pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). There is a broad spectrum of disease manifestations, including asymptomatic shedding, acute upper respiratory tract infection, multilobar pneumonia, acute respiratory distress syndrome, and death [1]. However, COVID-19 is also characterized by thrombotic complications such as pulmonary embolism (PE) and deep vein thrombosis (DVT) [2]. The pathogenesis of COVID-19-associated venous thromboembolism (VTE) involves hypercoagulability and endothelial damage, as shown by different studies [3-8]. Several studies have shown that the cumulative incidence of VTE in COVID-19 patients varies from 3.1% to as much as 40% [2,9-21].
VTE is screened based on a combination of Wells scores, D-dimers, and Clinical gestalt [22-25]. This approach enables clinicians to screen patients effectively and avoid unnecessary testing, as shown by previous studies [22-25]. The utility of this algorithm has not been robustly tested in the COVID-19 setting [26-28]. Therefore, we designed this study to validate the combined approach of using D-dimers and Wells scores to diagnose VTE in COVID-19 patients.
Materials and methods
Data collection
The charts of 1300 COVID-19 patients admitted from March 1, 2020, to December 1, 2020, were reviewed retrospectively. Patients who had imaging studies for DVT or PE within 90 days of COVID-19 illness were included. The patients with lower extremity (LE) duplex were included in the suspected DVT group, and patients with CT pulmonary angiogram (CT-PA) or V/Q scan were included in the suspected PE group. COVID-19 disease is diagnosed with active symptoms of COVID-19 and positive SARS-CoV-2 RT-PCR by nasopharyngeal swab.
Variables include demographics, risk factors for VTE such as ongoing malignancy, tobacco smoking, estrogen use, active pregnancy, recent surgery (within 12 weeks), previous history of DVT, history, physical exam, vitals, laboratory investigations, and outcomes. All the clinical variables, including Wells scores, were calculated based on the information in the charts on the day of imaging for VTE, and D-dimers were obtained within seven days prior to the day of imaging for VTE. There was high suspicion for VTE in COVID-19 patients in our institution, and hence clinicians obtained imaging for VTE based on clinical judgment even when D-dimer or Wells scores were low. Wells scores were calculated based on the information in the charts by three trained investigators.
The study's primary outcome was assessing the utility of Wells DVT, Wells PE scores, and D-dimers in diagnosing DVT and PE. Secondary outcomes were the risk factors for the development of PE and DVT in COVID-19 patients. We also included two serial D-dimers values within 10 days before VTE imaging to study the role D-dimer trend in diagnosing VTE.
Statistical analysis
A two-sample t-test was used to analyze normally distributed data, and the Wilcoxon rank-sum test was used to analyze non-normally distributed continuous data. Logistic regression was used to analyze categorical variables and to generate odds ratios for continuous variables. The multivariate analysis was done using penalized logistic regression as proposed by Firth. The final model was chosen based on the Akakai information criterion. Receiver operating curves, sensitivity, and specificity were calculated for D-dimer levels, Wells PE, and Wells DVT scores. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were presented as effective measures. Any variable with missing data of more than 10% was not included in the analysis. A p-value of less than or equal to 0.05 was considered significant. All data were analyzed with Stata/SE version 17 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC.).
Results
Prevalence of VTE
Out of the 1300 patients, 210 met the study criteria. Thus, 106 subjects were selected for the suspected DVT group, and 109 patients were selected for the suspected PE group. Seven patients were positive for both PE and DVT. These patients were included as cases in both the groups but not as a control in either group. In the suspected PE group, the prevalence of PE in the patients who underwent CT-PA or V/Q scan was 23.8% (26 out of 109 patients). On the other hand, the prevalence of DVT in patients who underwent duplex was 33% (35 out of 106).
Clinical features and risk factors of VTE
In the suspected DVT group, DVT was usually diagnosed within a few days after hospitalization (Median 4, IQR 1-10) and in the second week of COVID-19 illness (Median 11, IQR 4-22). In the suspected PE group, PE was usually diagnosed on the first day of presentation (Median 1, IQR 1-5) and in the second week of COVID-19 illness (Median 9, IQR 4-22). Neither the clinical features nor the classic risk factors for VTE were found to have a statistically significant association in both the DVT and PE subgroups. All the patients in our cohort received prophylactic anticoagulation on the day of admission. We also reported the subset of patients who were on any dose of prophylactic heparin or enoxaparin for at least five days before VTE testing (Tables 1-2).
Table 1. Key results on univariable analysis in suspected DVT group.
† Mean standard deviation for normally distributed data; ‡ Median (Interquartile range) for non-normally distributed data
Calf symptoms of DVT include unilateral or bilateral calf swelling, redness, or calf pain and prophylactic anticoagulation was mentioned only if patients were on any dose of prophylactic heparin or enoxaparin for at least five days prior to VTE testing. These criteria were chosen as almost all patients receive prophylactic anticoagulation unless patients have significant current bleeding. As most patients were tested for VTE during the initial days of hospitalization, they were not included as being prophylactically anticoagulated.
µ Labs were within one to seven days of being tested for VTE with most values being drawn one to three days prior to being tested for VTE.
DVT: deep vein thrombosis; SOFA: Sequential Organ Failure Assessment
| Variables | Duplex positive N=35 | Duplex negative N=71 | Odds ratio (95% CI) | P-value |
| Age, years | 63±14 † | 61±18 | 1.00(0.98-1.03) | 0.588 |
| Male sex, n (%) | 27(77%) | 33(46.4%) | 3.88(1.55-9.71) | 0.004 |
| Body mass index, kg/m2 | 27.9±4.1 | 30.6±6.9 | 0.92(0.86-0.99) | 0.037 |
| Calf symptoms of DVT | 6(17.1%) | 7(9.8%) | 1.89(0.58-6.12) | 0.288 |
| Bedbound | 5(14.2%) | 10(14%) | 1.01(0.31-3.24) | 0.978 |
| Active solid cancer | 0 | 2(2.8%) | ||
| Active hematologic cancer | 0 | 2(2.8%) | ||
| History of cancer | 1(2.8%) | 4(5.6%) | 0.49(0.05-4.58) | 0.534 |
| Past history of VTE | 1(2.8%) | 4(5.6%) | 0.49(0.05-4.58) | 0.534 |
| Full dose anticoagulation | 2(6.2%) | 5(7.3%) | 0.84(0.15-4.58) | 0.840 |
| Prophylactic anticoagulation >5 days & | 10(29.4%) | 25(35.2%) | 0.76(0.31-1.85) | 0.556 |
| Antiplatelet use | 2(5.7%) | 17(23.9%) | 0.19(0.04-0.88) | 0.035 |
| Systolic blood pressure, mmHg | 115(100-125) | 125(110-140) | 0.96(0.94-0.98) | 0.005 |
| Absolute lymphocytes, 109/L | 0.7(0.4-1.2) | 1(0.6-1.4) | 0.55(0.27-1.12) | 0.040 |
| D-dimer, ng/ml | 2937(1471-3680) | 721(396-1363) | 1.00(1.00-1.00) | P<0.001 |
| Blood urea nitrogen, mg/dl | 33(15-62) | 19(12-34) | 1.02(1.00-1.04) | 0.018 |
| Wells DVT Score | 0(1-2) | 0(1-2) | 1.36(0.91-2.05) | 0.150 |
| Wells DVT score=0 | 11(32.3%) | 29(40.8%) | ||
| Wells DVT score=1 | 6(17.6%) | 20(28.1%) | ||
| Wells DVT score=2 | 14(41.1%) | 17(23.9%) | ||
| Wells DVT score=3 | 3(8.8%) | 5(7%) | ||
| SOFA, n (%) | 2(1-2) | 1(1-2) | 1.11(0.90-1.36) | 0.051 |
| SOFA score=0 | 1(2.9%) | 8(11.2%) | ||
| SOFA score=1 | 15(44.1%) | 40(56.3%) | ||
| SOFA score=2 | 10(29.4%) | 10(14%) | ||
| SOFA score>2 | 8(23.5%) | 13(18.3%) | ||
| ICU, n (%) | 15(44%) | 21(29.5%) | 1.87(0.80-4.38) | 0.144 |
| Death, n (%) | 11(32.3%) | 21(29.5%) | 1.13(0.47-2.74) | 0.773 |
| Intubation, n (%) | 12(35.2%) | 10(14%) | 3.32(1.26-8.78) | 0.015 |
Table 2. Key results on univariable analysis for PE occurrence.
† Median (interquartile range) for non-normally distributed data
‡ Mean standard deviation for normally distributed data
! Calf symptoms of DVT include unilateral or bilateral calf swelling, redness, or calf pain
& Prophylactic anticoagulation is if patients were on any dose of prophylactic heparin or enoxaparin for at least five days prior to VTE testing. This criterion was chosen as almost all patients receive prophylactic anticoagulation unless patients have significant current bleeding. As most patients were tested for VTE during the initial days of hospitalization, they were not included as being prophylactically anticoagulated.
"Vitals include average vitals on the day of the imaging for VTE
µ Labs were within 1 to 7 days of being tested for VTE with most values being drawn 1 to 3 days prior to being tested for VTE
DVT: deep vein thrombosis; SOFA: Sequential Organ Failure Assessment; VTE: venous thromboembolism; PE: pulmonary embolism; PERC: pulmonary embolism rule-out criteria; CT-PA: CT pulmonary angiogram
| Variables | Case (CT-PA or V/Q positive) N=26 | Control (CT-PA negative) N=83 | OR (95% CI) | P-value |
| Age, years | 63 (47-78) † | 55 (44-70) | 1.01 (0.99-1.04) | 0.167 |
| Calf symptoms of DVT! | 2 (7.6%) | 5 (5.88%) | 1.64 (0.28-9.54) | 0.579 |
| History of cancer | 3 (11.5%) | 1 (1.1%) | 10.69 (1.06-107.74) | 0.044 |
| Past history of VTE | 1 (3.8%) | 3 (3.5%) | 1.06 (0.10-10.71) | 0.956 |
| Full dose anticoagulation | 2 (7.6%) | 7 (8.4%) | 0.88 (0.17-4.53) | 0.879 |
| Prophylactic anticoagulation & | 6 (23%) | 24 (28.2%) | 0.73 (0.26-2.06) | 0.562 |
| Antiplatelet use | 3 (11.5%) | 10 (11.7%) | 0.95 (0.24-3.75) | 0.944 |
| Heart rate " | 92±15 | 90±16 | 1.00 (0.97-1.03) | 0.714 |
| Oxygen saturation, % | 96±2 | 95±2 | 1.10 (0.89-1.35) | 0.370 |
| Temperature, F | 98.5 (98.2-99.5) | 99 (98.5-100) | 0.74 (0.49-1.11) | 0.028 |
| Rales | 1 (3.8%) | 19 (22.3%) | 0.13 (0.01-1.06) | 0.057 |
| Loud P2 | 1 (3.8%) | 0 | ||
| Total count, 109/L µ¶ | 8.55 (7.2-11.3) | 7.3 (5.7-8.9) | 1.07 (0.95-1.21) | 0.032 |
| Platelet, 109/L | 348 (203-414) | 255 (182-339) | 1.00 (1.00-1.00) | 0.027 |
| Absolute lymphocytes, 109/L | 1.22 (1-1.7) | 1.06 (0.7-1.44) | 1.11 (0.79-1.57) | 0.028 |
| D-dimer, ng/ml | 2247 (1711-3680) | 468 (267-865) | 1.00 (1.00-1.00) | <0.001 |
| Wells PE Score | 2 (1.5-3) | 1 (1-1.5) | 2.46 (1.50-4.06) | <0.001 |
| <2 | 9 (34.6%) | 70(84.3%) | ||
| 2-6 | 15 (57.6%) | 7 (8.4%) | ||
| >6 | 2 (7.6%) | 0 | ||
| PERC, n (%) | 1 (1-3) | 1 (1-1.5) | 1.79 (1.05-3.05) | 0.054 |
| 0 | 2 (7.6%) | 9 (10.8%) | ||
| 1 | 12 (46.1%) | 53 (63.8%) | ||
| >1 | 12 (46.1%) | 21 (25.3%) | ||
| SOFA, n (%) | 1 (1-2) | 1 (1-2) | 1.91 (1.16-3.12) | 0.002 |
| 0 | 1 (3.8%) | 19 (22.8%) | ||
| 1 | 12 (46.1%) | 47 (56.6%) | ||
| 2 | 8 (30.7%) | 31 (37.3%) | ||
| >2 | 5 (19.2%) | 4 (4.8%) | ||
| ICU, n (%) | 3 (11.5%) | 8 (9.4%) | 1.48 (0.35-6.20) | 0.592 |
| Death, n (%) | 2 (7.6%) | 5 (5.8%) | 1.71 (0.29-9.97) | 0.547 |
| Intubation, n (%) | 1 (3.8%) | 1 (1.1%) | 3.41 (0.20-56.68) | 0.391 |
Possible risk factors for DVT events
In the suspected DVT group, male gender (OR 3.88, 95% CI 1.55-9.7, P=0.004), lower BMI (OR 0.92, 95% CI 0.86-0.99, P=0.037), antiplatelet use (OR 0.19, 95% CI 0.04-0.88, P=0.035), systolic blood pressure 100 mmHg (OR 4.96, 95% CI 1.37-17.86, P=0.014), absolute lymphocytes 1 (OR 2.57, 95% CI 1.07-6.12, P=0.033), D-dimer 500 ng/ml (OR 6.42, 95% CI 1.40-29.38, P=0.016), BUN 20 mg/dl (OR 2.33, 95% CI 1.00-5.41, P=0.048), intubation (OR 3.32, 95% CI 1.26-8.78, P=0.015) were found to be statistically significant for DVT (Table 1). Wells DVT score was not significant (OR 1.36, 95% CI 0.91-2.05, P=0.150). On multivariate analysis using penalized logistic regression, the D-dimer levels (OR 1.00, 95% CI 1.00-1.00, P=0.003) and systolic blood pressure (OR 0.99, 95% CI 0.93-0.99, P=0.024) remained significant.
Possible risk factors for PE events
In the suspected PE group, history of cancer (OR 10.69, 95% CI 1.06-107.74, P=0.044), temperature 98.4F (OR 2.87, 95% CI 1.10-7.21, P=0.030), absence of rales (OR 0.13, 95% CI 0.01-1.06, P=0.057), total WBC count (OR 1.07, 95% CI 0.95-1.21, P=0.032), platelets 400,000 (OR 5.13, 95% CI 1.79-14.68, P=0.002), D-dimer levels 500 ng/ml (OR 25.47, 95% CI 3.27-197.97, P=0.002), Wells PE score (OR 2.46, 95% CI 1.50-4.06, P<0.001), PERC score (OR 1.79, 95% CI 1.05-3.05, P=0.054), and SOFA score (OR 1.91, 95% CI 1.16-3.12, P=0.002) were statistically significant (Table 2). On multivariate analysis using penalised logistic regression D-dimer levels (OR 1.00, 95% CI 1.00-1.00, P<0.001) and Wells PE score (OR 2.44, 95% CI 1.12-5.33, P=0.024) remained statistically significant.
Accuracy of D-dimers in VTE diagnosis
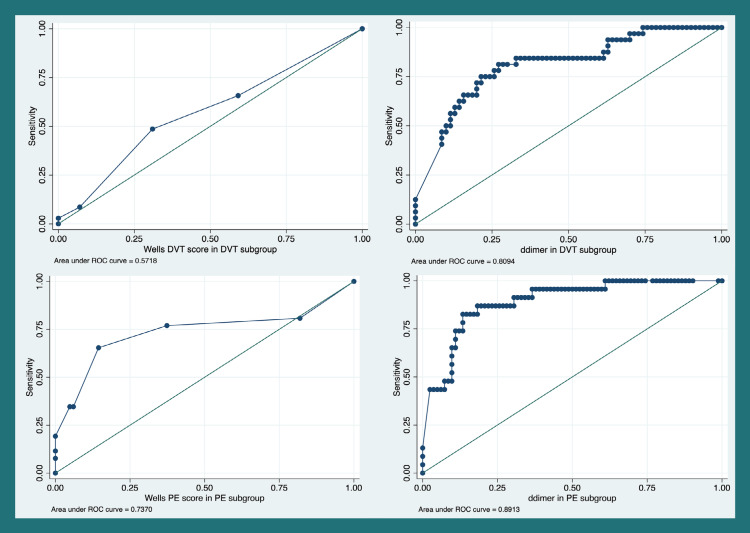
In the suspected PE group, receiver operating characteristic (ROC) analysis for D-Dimer and Wells PE score showed an area under the curve (AUC) of 0.89 and 0.73, respectively (Figure 1). A D-dimer cut-off of 1500 ng/ml had a sensitivity of 82.6% and a specificity of 85.4% for PE diagnosis. Assuming a disease prevalence of 10% (20-22), the negative predictive value for a D-dimer of 1500 ng/ml is 97.8%, and the positive predictive value is 38.5%. For a D-dimer of 500 ng/ml, the sensitivity is 95.6%, specificity is 53.6%, the negative predictive value is 99.1%, and the positive predictive value is 18.6%. In the suspected DVT group, ROC analysis for DVT using D-dimer levels and Wells DVT score showed an area under the curve (AUC) of 0.80 and 0.58, respectively. A D-dimer of 500 ng/ml has a sensitivity of 93.7 and specificity of 30% for DVT. Assuming a prevalence of 10%, the negative predictive value is 97.7% and the positive predictive value is 12.9% (Tables 3-4).
Table 3. Accuracy of Wells scores and D-dimer for prediction of PE.
PE: pulmonary embolism
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV(%) | |
| D-dimer of 500 ng/ml | 95.6 | 53.6 | 18.6 | 99.1 |
| D-dimer of 1500 ng/ml | 82.6 | 85.4 | 38.5 | 97.8 |
| Wells PE score of 4 | 20 | 100 | 100 | 91.8 |
| Wells PE score of 6 | 8 | 100 | 100 | 90.7 |
| Serial D-dimers | 94.1 | 32.5 | 13.4 | 98 |
Table 4. Accuracy of Wells scores and D-dimer for the prediction of DVT.
DVT: deep vein thrombosis; PPV: positive predictive value; NPV: negative predictive value
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
| D-dimer of 500 ng/ml | 93.7 | 30 | 12.9 | 97.7 |
| D-dimer of 1500 ng/ml | 75 | 77.1 | 26.7 | 96.5 |
| Wells DVT score of 2 | 50 | 69 | 15.2 | 92.5 |
| Wells DVT score of 3 | 8.8 | 92.9 | 12.2 | 90.1 |
| Serial D-dimers | 94.1 | 32.5 | 13.4 | 98 |
Figure 1. ROC analysis for Wells scores and D-dimers.
ROC: receiver operating characteristic
Accuracy of Wells scores for DVT events
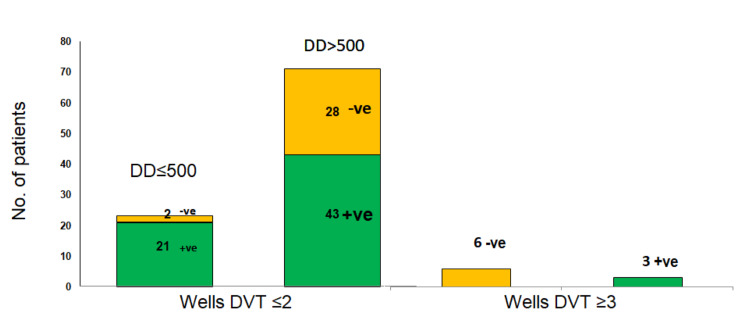
In the suspected DVT group, 6.6% of subjects had Wells DVT scores of 3 or above. In this group, the DVT prevalence was 28.5%. In the remaining patients with a Wells DVT score of 2 or less, the DVT prevalence was 8.6% in D-dimer 500 ng/ml and 43% in D-dimer 500 ng/ml. Notably, two patients with a D-dimer level of <500 ng/ml and a Wells DVT score of less than 2 had a positive LE duplex for DVT (Table 3, Figures 2-3). Overall the combined sensitivity of D-dimer of 500 ng/ml and Wells DVT score of 3 was 94.2%. A Wells DVT score of 2 has a sensitivity of 50% and specificity of 69% for DVT diagnosis. A Wells DVT score of 3 has a sensitivity of 8.8% and a specificity of 92.9% for DVT diagnosis.
Figure 2. Distribution of D-dimer and Wells DVT scores in COVID-19 DVT.
+ve represents DVT positive. -ve represents DVT negative.
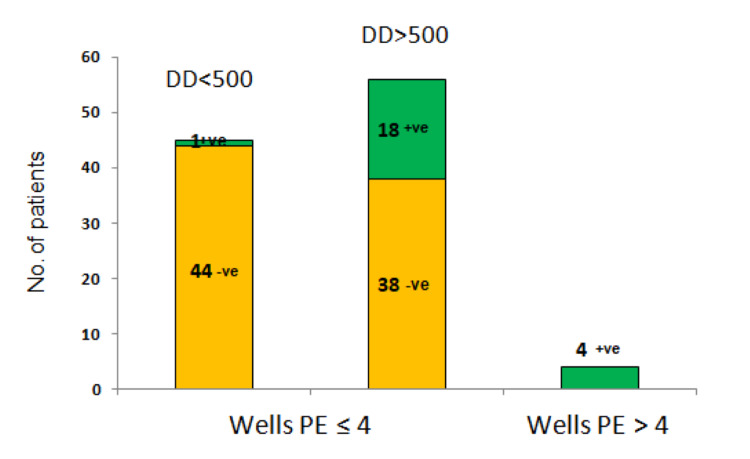
Figure 3. Distribution of Wells PE scores and D-dimers in COVID-19 PE.
+ve represents DVT positive. -ve represents DVT negative.
PE: pulmonary embolism; DVT: deep vein thrombosis
Accuracy of Wells scores for PE events
In the suspected PE group, only 4.5% of patients in the suspected PE group had a Wells PE score of 4 or above. However, in this group, the PE prevalence was 100%. In patients who had a Wells score of 4 or less, PE prevalence was 2.2% for D-dimer <500 ng/ml, 6.8% for D-Dimer in between 500-1500 ng/ml, and 60% when D-dimer was >1500 ng/ml, respectively. A Wells PE score of 4 has a sensitivity of 20% and specificity of 100% for PE diagnosis. A Wells PE score of 6 has a sensitivity of 8% and a specificity of 100% for PE diagnosis (Table 3).
Serial D-dimers for VTE diagnosis
Within 10 days before diagnostic imaging for VTE, serial D-dimer was available for 96 patients: 17 cases (DVT and PE positive) and 79 controls. Of the 17 cases, five were positive for PE, and 13 were positive for DVT. Up-trending D-dimer levels had a sensitivity of 94.1% and a specificity of 32.5%. Assuming a disease prevalence of 10%, the positive predictive value is 13.4%, and the negative predictive value is 98% (Table 3)
Discussion
Prevalence of VTE in COVID-19
Early literature suggested a 20% to 40% incidence of DVT and PE in COVID-19 [3,10-1]. However, several more extensive studies showed an incidence of 3 to 8% [12-23]. In our study, prevalence is 23.8% for PE and 33% for DVT, and the overall prevalence of VTE is 28.3% (61/215). However, our prevalence doesn't represent the true prevalence of VTE occurrence in COVID-19 as not all the 1300 patients had imaging for VTE.
Diagnostic challenges in COVID-19-associated DVT
Before the COVID-19 pandemic, Wells DVT scores and D-dimer levels were routinely used to determine the pre-test probability for DVT. The modified Wells DVT score includes clinical features and risk factors of DVT [24]. In patients with Wells DVT score 2, high-sensitivity D-dimer testing is recommended. If D-dimer is less than 500 ng/ml, DVT is considered to be ruled out, and no further testing is recommended. If the D-dimer level is more than 500 ng/ml, LE duplex should be done. Patients with a Wells DVT score of 3 should undergo a duplex without a D-Dimer test [22-25].
Wells DVT score was not significant in our study. So far, no studies have directly explored the utility of Wells DVT scores in the COVID-19 population. Most of the patients in our study did not have the typical symptomatology and risk factors present in the existent Wells DVT scoring. We believe that extensive prospective studies exploring other parameters in COVID-19 patients, such as intubation, SOFA scores, and severity of COVID-19, could potentially lead to a modified Wells DVT score that could be used for COVID-19 patients. A D-dimer level cut-off of 500 ng/ml has a good sensitivity of 93.7% and a specificity of 30%. Our findings align with the Wells DVT score algorithm, suggesting that patients with a Wells DVT score of 2 or less should get D-dimer levels, and if D-dimer levels are more than 500 ng/ml, duplex LE should be done.
In addition, similar to other studies, male sex, intubation, and higher BUN and SOFA scores are associated with DVT in COVID-19 [12,15,17,20]. These parameters reflect the severity of the disease, and hence clinicians should consider aggressive testing for DVT in this patient with severe COVID-19 disease.
Diagnostic challenges in COVID-19-associated PE
None of the classic clinical features or risk factors for PE were associated with PE occurrence in the PE group, likely because PE in COVID-19 was mostly clinically silent. In general, in patients with suspected PE, a modified Wells score, which classifies patients into low risk (Wells score<2), intermediate-risk (Wells score 2-6), and high risk (Wells score>6) categories, should be done. In patients with low-risk scores, the use of PERC is applied. If the PERC score is zero, no further testing is needed. If the PERC score is one or above, then D-dimer testing is recommended. D-dimer testing is also recommended in patients with intermediate wells PE scores. A D-dimer of more than 500 ng/ml is considered high risk for PE, and CT-PA or V/Q scan is recommended. Patients with high modified Wells scores should be assessed directly with CT-PA or V/Q scan [23-25].
In our subjects, only 11 out of 109 patients had zero scores on PERC. This was because two of the PERC variables, such as oxygen saturation less than 95% and tachycardia, were highly prevalent in our COVID-19 patients. Also, two patients who had zero scores on PERC were found to have PE. Therefore, the utility of PERC scores might be limited in COVID-19.
In our subjects, a D-dimer of 1500 had a high specificity. Hence, we strongly recommend testing for PE when the D-dimer value is above 1500. However, the existing D-dimer cut of 500 ng/ml has a higher sensitivity and should be used to screen for PE when Wells scores are less than 4. Overall, the combined approach of using Wells PE and D-dimer level of 500 ng/ml successfully predicted PE in all but one of our patients (25 out of 26) with a sensitivity of 96%.
Studies are conflicting about the usefulness of Wells PE scores and D-dimers in predicting PE in COVID-19 [26-28]. Our study supports the combined approach of using Wells PE score and D-dimer in COVID-19 patients. However, prospective and large-scale studies are needed to establish the usefulness in COVID-19 patients definitively.
Role of serial D-dimers
Although only half of our patients had serial D-dimers, our findings suggest that serial D-dimer levels may be predictive for VTE. If they are increasing, Imaging for VTE can be performed. On the other hand, if D-dimers decrease, duplex LE may not be necessary. However, clinicians should be aware of the low specificity of D-dimers and the different causes for elevations in D-dimers, such as systemic inflammatory response syndrome (SIRS) response, sepsis, and renal failure, which are common in COVID-19 patients.
Study limitations
The main limitation of our study is its retrospective nature. Wells scores are calculated based on information in the charts, which may have lead to measurement bias. Prospective studies in predicting VTE in the COVID-19 setting can be very challenging during a pandemic. Hence, the authors accept this as the first step to studying the utility of these scores in the COVID-19 setting. The prevalence in our study is not true prevalence, as patients were screened based on clinical suspicion, similar to most prior studies. Some patients received empiric anticoagulation over the suspicion of PE but were not included in this study, as they did not have diagnostic testing. The burden of missing variables is highest for serial D-dimers, as they were done in only half of the patients and should be interpreted cautiously.
Conclusions
A high wells DVT or PE score may be suggestive of PE or DVT, but such high scores occur less frequently in COVID-19-associated VTE as these VTEs are clinically silent and are not associated with the classic risk factors. Conversely, the clinical features of COVID-19, such as chest pain, hypoxia, and tachycardia, overlap with PE. This leads to erroneously giving 3 points on Wells PE scores. Thus, in our study, Wells PE scores appear to have more value than Wells DVT scores in COVID-19 patients.
Low D-dimer levels might be helpful to rule out VTE, and we recommend clinicians continue to use a D-dimer cut-off of 500 ng/dl in screening for DVT and PE. However, high D-dimers are more commonly seen in COVID-19 patients regardless of VTE. Serial D-dimers have the same issue, but they may be superior to a single D-dimer value. Serial D-dimers need to be evaluated in large prospective studies. Until more extensive studies are available, we recommend that clinicians continue to use the combined approach of Wells score and D-dimers but be aware of its limitations in the COVID-19 setting.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Saint Peter University Hospital issued approval 20:50. The institutional review board (IRB) of Saint Peter University Hospital approved the study, and the study was done under the IRB's guidelines (IRB number 20:50). Informed consent was waived by the institutional review board as the study was retrospective in nature
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Huang C, Wang Y, Li X, et al. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Klok FA, Kruip MJ, van der Meer NJ, et al. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.New findings on venous thrombogenesis. Byrnes JR, Wolberg AS. Hamostaseologie. 2017;37:25–35. doi: 10.5482/HAMO-16-09-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.SARS-CoV-2 pathophysiology and its clinical implications: an integrative overview of the pharmacotherapeutic management of COVID-19. Tsatsakis A, Calina D, Falzone L, et al. Food Chem Toxicol. 2020;146:111769. doi: 10.1016/j.fct.2020.111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. Panigada M, Bottino N, Tagliabue P, et al. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. Ackermann M, Verleden SE, Kuehnel M, et al. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coagulopathy and thromboembolic events in patients with SARS-CoV-2 infection: pathogenesis and management strategies. Allegra A, Innao V, Allegra AG, Musolino C. Ann Hematol. 2020;99:1953–1965. doi: 10.1007/s00277-020-04182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. Ranucci M, Ballotta A, Di Dedda U, et al. J Thromb Haemost. 2020;18:1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Klok FA, Kruip MJ, van der Meer NJ, et al. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: prevalence, risk factors, and outcome. Zhang L, Feng X, Zhang D, et al. Circulation. 2020;142:114–128. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 11.venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID-19) Nahum J, Morichau-Beauchant T, Daviaud F, Echegut P, Fichet J, Maillet JM, Thierry S. JAMA Netw Open. 2020;3:0. doi: 10.1001/jamanetworkopen.2020.10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.VTE in ICU patients with COVID-19. Moll M, Zon RL, Sylvester KW, et al. Chest. 2020;158:2130–2135. doi: 10.1016/j.chest.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Incidence of deep vein thrombosis among non-ICU patients hospitalized for COVID-19 despite pharmacological thromboprophylaxis. Santoliquido A, Porfidia A, Nesci A, et al. J Thromb Haemost. 2020;18:2358–2363. doi: 10.1111/jth.14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acute pulmonary embolism in non-hospitalized COVID-19 patients referred to CTPA by emergency department. Gervaise A, Bouzad C, Peroux E, Helissey C. Eur Radiol. 2020;30:6170–6177. doi: 10.1007/s00330-020-06977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. Cui S, Chen S, Li X, Liu S, Wang F. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Helms J, Tacquard C, Severac F, et al. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Incidence of venous thromboembolism in hospitalized patients with COVID-19. Middeldorp S, Coppens M, van Haaps TF, et al. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Lodigiani C, Iapichino G, Carenzo L, et al. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Fauvel C, Weizman O, Trimaille A, et al. Eur Heart J. 2020;41:3058–3068. doi: 10.1093/eurheartj/ehaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frequency of venous thromboembolism in 6513 patients with COVID-19: a retrospective study. Hill JB, Garcia D, Crowther M, Savage B, Peress S, Chang K, Deitelzweig S. Blood Adv. 2020;4:5373–5377. doi: 10.1182/bloodadvances.2020003083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acute pulmonary embolism and COVID-19. Poyiadji N, Cormier P, Patel PY, et al. Radiology. 2020;297:0–8. doi: 10.1148/radiol.2020201955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Comparison of clinical prediction scores for the diagnosis of deep vein thrombosis in unselected population of outpatients and inpatients. Sermsathanasawadi N, Suparatchatpun P, Pumpuang T, et al. Phlebology. 2015;30:469–474. doi: 10.1177/0268355514541981. [DOI] [PubMed] [Google Scholar]
- 23.Value of assessment of pre-test probability of deep-vein thrombosis in clinical management. Wells PS, Anderson DR, Bormanis J, et al. Lancet. 1997;350:1795–1798. doi: 10.1016/S0140-6736(97)08140-3. [DOI] [PubMed] [Google Scholar]
- 24.Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. Wells PS, Anderson DR, Rodger M, et al. N Engl J Med. 2003;349:1227–1235. doi: 10.1056/NEJMoa023153. [DOI] [PubMed] [Google Scholar]
- 25.Clinical decision rules for excluding pulmonary embolism: a meta-analysis. Lucassen W, Geersing GJ, Erkens PM, Reitsma JB, Moons KG, Büller H, van Weert HC. Ann Intern Med. 2011;155:448–460. doi: 10.7326/0003-4819-155-7-201110040-00007. [DOI] [PubMed] [Google Scholar]
- 26.Wells score to predict pulmonary embolism in patients with coronavirus disease 2019. Kirsch B, Aziz M, Kumar S, et al. Am J Med. 2021;134:688–690. doi: 10.1016/j.amjmed.2020.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Relationship of D-dimer and prediction of pulmonary embolism in hospitalized COVID-19 patients: a multicenter study. Nadeem I, Anwar A, Jordon L, Mahdi N, Rasool MU, Dakin J, Lok S. Future Microbiol. 2021;16:12. doi: 10.2217/fmb-2021-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells and Geneva decision rules to predict pulmonary embolism: can we use them in Covid-19 patients? Silva B, Jorge C, Rigueira J, et al. Eur Heart J Cardiovasc Imaging. 2021;22:0. [Google Scholar]