Abstract
Background:
Autologous platelet-rich plasma (PRP) is widely used for a variety of clinical applications. However, clinical outcome studies have not consistently shown positive effects. The composition of PRP differs based on many factors. An improved under-standing of factors influencing the composition of PRP is important for the optimization of PRP use.
Hypothesis:
Age and sex influence the PRP composition in healthy patients.
Study Design:
Controlled laboratory study.
Methods:
Blood from 39 healthy patients was collected at a standardized time and processed into leukocyte-poor PRP within 1 hour of collection using the same laboratory centrifuge protocol and frozen for later analysis. Eleven female and 10 male patients were ‘‘young’’ (aged 18–30 years), while 8 male and 10 female patients were ‘‘older’’ (aged 45–60 years). Thawed PRP samples were assessed for cytokine and growth factor levels using a multiplex assay and enzyme-linked immunosorbent assay. The platelet count and high-sensitivity C-reactive protein levels were measured. Two-way analysis of variance determined age- and sex-based differences.
Results:
Platelet and high-sensitivity C-reactive protein concentrations were similar in PRP between the groups (P = .234). Male patients had higher cytokine and growth factor levels in PRP compared with female patients for inflammatory cytokines such as interleukin–1 beta (IL-1β) (9.83 vs 7.71 pg/mL, respectively; P = .008) and tumor necrosis factor–alpha (TNF-α) (131.6 vs 110.5 pg/mL, respectively; P = .048); the anti-inflammatory IL-1 receptor antagonist protein (IRAP) (298.0 vs 218.0 pg/mL, respectively; P < .001); and growth factors such as fibroblast growth factor–basic (FGF-basic) (237.9 vs 194.0 pg/mL, respectively; P =.01), platelet-derived growth factor (PDGF-BB) (3296.2 vs 2579.3 pg/mL, respectively; P = .087), and transforming growth factor–beta 1 (TGF-β1) (118.8 vs 92.8 ng/mL, respectively; P = .002). Age-but not sex-related differences were observed for insulin-like growth factor–1 (IGF-1) (P < .001). Age and sex interaction terms were not significant. While mean differences were significant, there was also substantial intragroup variability.
Conclusion:
This study in healthy patients shows differences in the composition of PRP between men and women, with sex being a greater factor than age. There was also proteomic variability within the groups. These data support a personalized approach to PRP treatment and highlight the need for a greater understanding of the relationships between proteomic factors in PRP and clinical outcomes.
Clinical Relevance:
Variability in the proteomic profile of PRP may affect tissue and clinical responses to treatment. These data suggest that clinical studies should account for the composition of PRP used.
Keywords: platelet-rich plasma, cytokines, growth factors, sex differences
The clinical use of autologous platelet-rich plasma (PRP) in orthopaedics has exponentially increased in the past decade. First used in orthopaedic sports medicine to treat tendinopathy,39 PRP use has expanded to include a wide variety of pathological conditions such as muscle strain, augmentation of surgical repair, knee osteoarthritis,20,21,30,56 and osteomyelitis.46,72 The benefits of PRP are hypothesized to derive from the regenerative effects of growth factors released by platelets as well as anti-inflammatory effects that help tissue healing and relieve pain.23,37,51 Early in vitro and animal studies have reported improved effects on bone regeneration,29,44 chondrocyte proliferation,1 mesenchymal differentiation,70 extracellular matrix metabolism,2 and anabolic gene expression.61 Clinical outcome studies, how-ever, have not consistently shown positive effects.22,41
Several theories have been proposed to explain the wide variation in PRP outcomes. One area of focus has been variability in PRP produced using devices from different manufacturers in which wide disparities in the platelet count, white blood cell count, and some growth factors have been reported.7,11,17,49,66 However, equipment and protocols are not the only source of variation, and it has been suggested that even samples from the same patient pre-pared using the same system or at different times may yield different results.7,25 Given the autologous source of PRP, differences between patients are also likely to be a significant source of proteomic variation that may affect tissue responses and treatment efficacy.
Age and sex affect biology and may also be important variables that influence the composition of PRP. In a study evaluating the chondrogenic potential of human bone marrow–derived mesenchymal stem cells, age influenced chondroid differentiation in response to transforming growth factor–beta 1 (TGF-β1) in male participants but did not show a similar effect in female participants.53 Biological differences in healing potential and inflammation related to age and sex may be reflected in the biochemical composition of PRP. This study was therefore performed to test the hypothesis that the composition of PRP prepared from healthy patients differs by age and sex.
METHODS
Thirty-nine healthy patients (body mass index [BMI] <25 kg/m2; with no history of orthopaedic problems or surgery; and who were not taking medications in the non-steroidal anti-inflammatory class or anti-platelet class, aspirins, or any supplements for musculoskeletal health such as chondroitin sulfate) were recruited according to institutional review board–approved protocols and provided informed consent. Volunteers in a ‘‘young’’ group aged 18 to 30 years (10 male, 11 female) and an ‘‘older’’ group aged 45 to 60 years (8 male, 10 female) were recruited. The 15-year age gap between the groups was instituted in the study design to facilitate the observation of age-related differences. A 25 mL sample of whole blood was obtained by a licensed clinical phlebotomist using a standard venipuncture technique between 7 AM and 9 AM.
Preparation of PRP
Blood drawn into a vacutainer containing anticoagulant citrate dextrose solution (Becton Dickinson) was processed within 1 hour of collection at room temperature in a standard laboratory centrifuge (Beckman Coulter) according to a standard PRP preparation protocol established by the study hematologist to generate leukocyte-poor PRP.45 Briefly, the fresh whole blood was centrifuged at 180g for 10 minutes, after which the plasma fraction containing the platelets was carefully aspirated, avoiding the region immediately above the buffy coat. The resulting PRP was then subaliquoted into cryovials, taking care to gently invert the plasma tube periodically to allow the resuspension of platelets. The cryovials were stored at −80°C until analysis.
Hematological Analysis
Aliquots of whole blood were assessed for complete blood counts with differentials, high-sensitivity C-reactive protein levels, and hemoglobin A1c levels by the hospital clinical laboratory according to standard protocols. Platelet counts for the PRP samples were determined using a hemocytometer at 203 magnification using previously validated methods for counting platelets and white blood cells.34,35
Cytokine and Growth Factor Analysis
To assess the cytokine and growth factor composition of the PRP samples, frozen samples of PRP were thawed at room temperature for 30 minutes; thus, one freeze-thaw cycle was used to activate platelets.9 Thawed samples were used immediately and samples were briefly vortexed to ensure sample homogeneity before analysis. All samples were run on plates of the same lot number processed at the same time. Multiplex analysis of 27 cytokines was performed using the Bio-Plex Pro Human Cytokine 27-plex Assay (Bio-Rad Laboratories) run on a Luminex 200 System (Luminex Corp) according to the manufacturer’s instructions, with the exception that the proprietary Bio-Rad assay dilution buffer was modified to contain reagents that reduce the effect of heterophilic antibodies in multiplex immunoassays, as previously described.38,63 Data processing was performed with Bio-Plex Manager 5.0 Software (Bio-Rad Laboratories), and plasma concentrations (pg/mL) were interpolated from standard curves for each respective cytokine or chemokine. Blank wells were run concurrently and the resulting median fluorescence intensity subtracted from sample median fluorescence intensity values to ensure no background reactivity. Insulin-like growth factor–1 (IGF-1) and TGF-β1 levels were assessed by an enzyme-linked immunosorbent assay using the manufacturer’s protocols (R&D Systems). For all assays, samples were run in duplicate.
Statistical Analysis
Statistical analyses were performed using Stata SE version 13.0 for Mac (StataCorp). Demographic differences in age or BMI and differences in laboratory values (whole blood platelet or white blood cell count) with age and sex were assessed by 2-way factorial analysis of variance (ANOVA). Differences in the cytokine concentration between the sex and age groups were analyzed by 2-way factorial ANOVA with inter-action terms between age and sex. Although the Bio-Plex assay allows for the comprehensive analysis of 27 cytokines, further analysis was performed on selected cytokines of interest, and these proteins were divided into 3 groups: proinflammatory (interleukin–1 beta [IL-1β] and tumor necrosis factor–alpha [TNF-α]), anti-inflammatory (IL-1 receptor antagonist protein [IRAP]), and growth factors (platelet-derived growth factor [PDGF-BB], fibroblast growth factor– basic [FGF-basic], vascular endothelial growth factor [VEGF], TGF-β1, and IGF-1). Pearson correlation coefficients were calculated for platelet counts of whole blood or PRP compared with cytokine or growth factor levels for each age and sex group. To facilitate the comparison of variation patterns between cytokines, a ‘‘normalized’’ value was calculated in which each individual measurement was divided by the group mean to obtain a ratio. A patient who had a lower than average cytokine level would have a ratio <1.0 for that cytokine and >1.0 if he or she had an above average level. This allowed comparisons between cytokines or growth factors whose absolute levels were several orders of magnitude apart. These normalized values were used to calculate ratios between biomarker groups to assess their differences with age and sex between demographic groups using 2-way factorial ANOVA. Furthermore, hierarchical clustering was performed on the results according to similarities among cytokine levels using Cluster 3.0,16 and the results were visualized in the heat-map format using Java TreeView 1.1.3.60
RESULTS
PRP Preparation and Laboratory Data
The mean ages were 22.8 years (range, 18.5–26.8 years) for young male patients, 22.9 years (range, 18.8–27.6 years) for young female patients, 55.1 years (range, 47.0–58.7 years) for older male patients, and 52.3 years (range, 46.8–59.8 years) for older female patients (Table 1). The mean BMI was 23.4 kg/m2 in young male patients, 21.6 kg/m2 in young female patients, 24.7 kg/m2 in older male patients, and 24.9 kg/m2 in older female patients. Platelet counts in whole blood did not differ by age or sex (P = .234) (Table 1). The degree of platelet concentration from whole blood to PRP also did not differ significantly by age or sex (P = .313) (Table 1). White blood cells were not observed in any of the PRP samples. Demographic and laboratory data grouped by age and sex showed significance only by age in which the young group had a lower BMI (P = .01) and showed a trend toward lower high-sensitivity C-reactive protein values (P = .09). Interaction effects were not significant.
TABLE 1.
Human Subjects and PRP Characteristicsa
| Male |
Female |
|||
|---|---|---|---|---|
| Young (n = 10) | Older (n = 8) | Young (n = 11) | Older (n = 10) | |
| Age, y | 22.8 ± 3.2 | 55.1 ± 4.7 | 22.9 ± 3.6 | 52.3 ± 3.3 |
| Body mass index, kg/m2 | 23.4 ± 2.8 | 24.7 ± 2.5 | 21.6 ± 1.4 | 24.9 ± 3.0 |
| Whole blood platelet count, 103/uL | 216.4 ± 32.2 | 235.9 ± 64.2 | 219.8 ± 41.8 | 224.9 ± 35.8 |
| Whole blood white blood cell count, 109/L | 6.7 ± 2.0 | 6.1 ± 1.9 | 6.1 ± 1.3 | 5.1 ± 0.9 |
| PRP platelet count, 103/uL | 525.7 ± 352.3 | 372.8 ± 125.9 | 336.3 ± 90.5 | 386.9 ± 101.0 |
| PRP-fold platelet increase | 2.5 ± 1.8 | 1.7 ± 0.9 | 1.5 ± 0.4 | 1.8 ± 0.5 |
| High-sensitivity C-reactive protein, mg/L | 0.5 ± 0.3 | 1.8 ± 2.1 | 1.0 ± 1.3 | 1.2 ± 0.7 |
| Hemoglobin A1c, % | 4.8 ± 0.2 | 5.1 ± 0.1 | 5.0 ± 0.1 | 5.2 ± 0.3 |
Data are presented as mean ± SD. PRP, platelet-rich plasma.
Correlation Between Platelet Count and PRP Composition
Platelet counts obtained for whole blood samples before PRP preparation did not correlate with any growth factor or cytokine level at the .05 significance level. Platelet counts for PRP correlating with cytokines and growth factors of interest for young female patients were TGF-β1 (β = 0.736, P = .024) and for young male patients were the following: FGF-basic (β = −0.706, P = .023), TNF-a (β = −0.663, P = .037), and IGF-1 (β = 0.732, P = .016). Platelet counts for PRP from older female and older male patients showed no correlation with any of the cytokines or growth factors studied.
Differences in PRP Composition Among Individual Patients
Individual differences in cytokine and growth factor levels were observed within each age and sex group. A color heat-map representation of the distribution of cytokine and growth factors normalized to a Z score is shown in Figure 1. This heat map demonstrates hierarchical clustering and shows grouping of TGF-β1 and IGF-1 distinct from the cytokines. Furthermore, although older male patients consistently demonstrated, on average, higher cytokine levels, substantial intragroup variation can be seen. The 95% CI for cytokines across and within all groups is demonstrated in Table 2. To further illustrate the wide individual variation in cytokine levels, histogram plots and across-group means for selected cytokines and growth factors are demonstrated in Figure 2, showing a typical bell-curve distribution pattern. Within each group shown, there were no differences in subdivisions (eg, between young female and older female patients), and no interaction effects between age and sex were observed.
Figure 1.
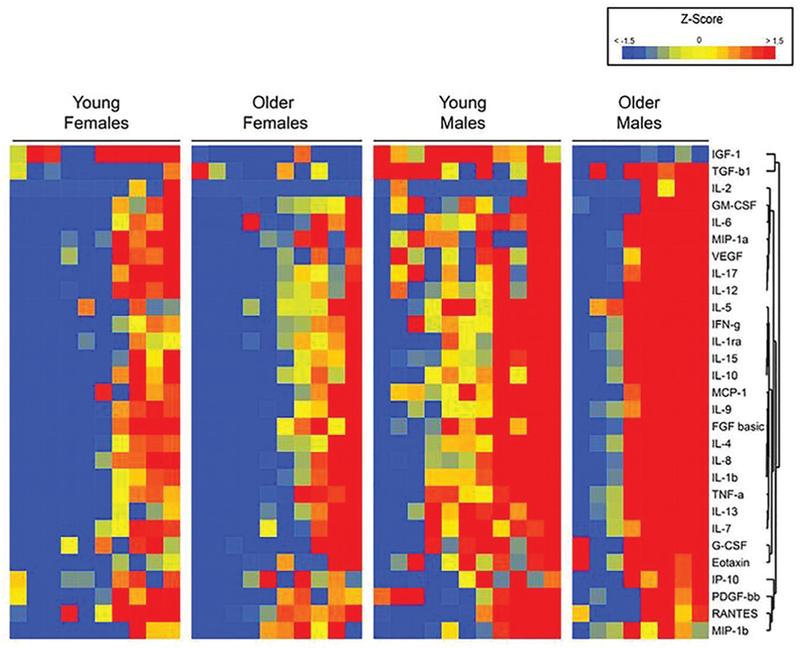
Heat-map comparison of cytokines and growth factors. This map demonstrates within- and across-group variations in cytokine and growth factor levels. Each individual molecule is normalized to the mean of the entire group and the color determined by the corresponding Z score, with low scores represented in blue and high scores represented in red. Note that there are patients with low (blue) and high (red) levels of cytokines in each group.
TABLE 2.
Age-and Sex-Related Differences in Selected Cytokines and Growth Factorsa
| Female |
Male |
Male to Female |
Young to Older |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Biomarker | Overall, Mean (95% CI) | Young, Mean (95% CI) | Older, Mean (95% CI) | Young, Mean (95% CI) | Older, Mean (95% CI) | Difference, % | P Value | Difference, % | P Value |
| IL-1 β, pg/mL | 8.74 (7.88–9.59) | 7.58 (6.41–8.75) | 7.82 (6.14–9.50) | 9.23 (7.65–10.82) | 10.64 (7.81–13.46) | 30.6 | .008 | –7.4 | .304 |
| IRAP, pg/mL | 257.02 (231.12–282.92) | 210.07 (179.68–240.45) | 226.12 (188.27–263.96) | 276.24 (225.64–326.85) | 327.91 (245.21–410.62) | 40.3 | <.001 | –10.9 | .137 |
| TNF-α, pg/mL | 120.78 (109.21–132.35) | 105.15 (89.51–120.79) | 115.84 (86.41–145.28) | 122.91 (102.41–143.41) | 143.56 (108.00–179.11) | 23.1 | .048 | –12.0 | .167 |
| FGF-basic, pg/mL | 215.43 (197.79–233.06) | 196.80 (164.96–228.64) | 191.21 (153.69–228.73) | 232.54 (199.94–265.14) | 245.44 (193.95–296.93) | 24.7 | .010 | 0.1 | .827 |
| PDGF-BB, pg/mL | 2928.58 (2522.50–3334.66) | 2775.86 (1867.39–3684.33) | 2382.78 (1860.61–2904.95) | 3364.96 (2513.39–4216.53) | 3201.70 (1848.13–4555.28) | 24.3 | .087 | 10.9 | .491 |
| VEGF, pg/mL | 125.04 (105.97–144.12) | 119.87 (72.04–167.71) | 97.07 (75.59–118.55) | 132.68 (105.85–159.51) | 155.97 (84.69–227.26) | 33.8 | .060 | 2.6 | .989 |
| TGF-β1, ng/mL | 106.18 (97.21–115.14) | 87.92 (76.40–99.43) | 97.70 (79.86–115.53) | 115.11 (98.43–131.79) | 123.99 (98.12–149.85) | 21.9 | .002 | –7.0 | .248 |
| IGF-1, ng/mL | 76.93 (67.22–86.63) | 97.04 (79.16–114.93) | 53.66 (42.03–65.29) | 98.19 (81.31–115.07) | 54.15 (43.96–64.34) | 6.8 | .903 | 44.8 | .000 |
FGF-basic, fibroblast growth factor–basic; IGF-1, insulin-like growth factor–1; IL-1β, interleukin–1 beta; IRAP, IL-1 receptor antagonist protein; PDGF-BB, platelet-derived growth factor; TGF-β 1, transforming growth factor–beta 1; TNF-α, tumor necrosis factor–alpha; VEGF, vascular endothelial growth factor.
Figure 2.
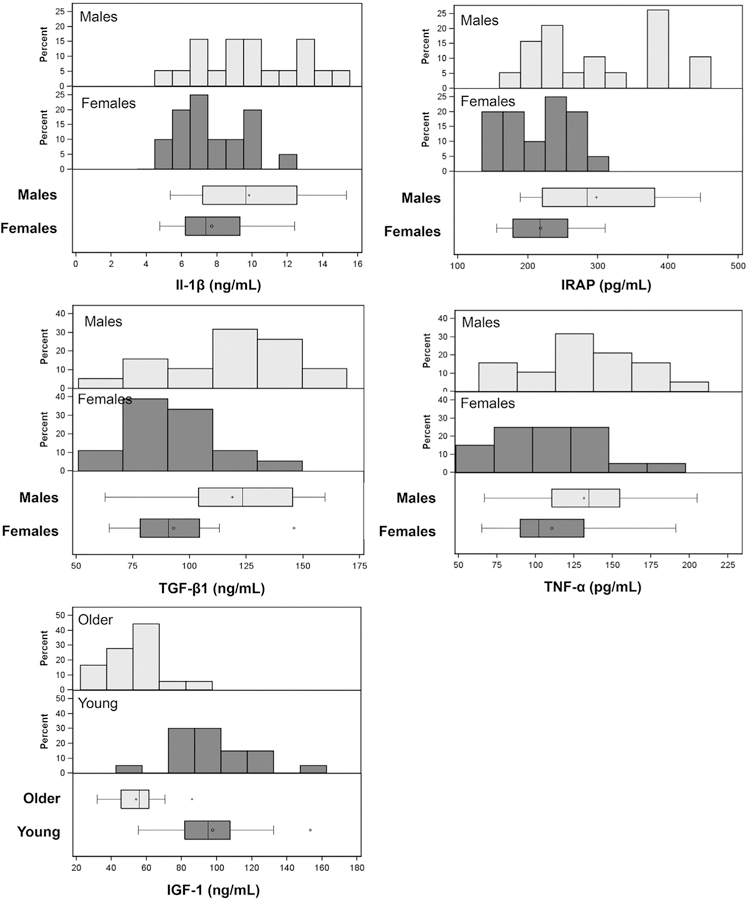
Cytokine and growth factor distributions of female compared with male patients. Histogram plots grouped by sex for interleukin–1 beta (IL-1β) (7.71 vs 9.83 pg/mL, respectively; P = .008), tumor necrosis factor–alpha (TNF-α) (110.5 vs 131.6 pg/mL, respectively; P = .048), IL-1 receptor antagonist protein (IRAP) (218.0 vs 298.0 pg/mL, respectively; P < .001), and transforming growth factor–beta 1 (TGF-β 1) (92.8 vs 118.8 ng/mL, respectively; P = .002) as well as insulin-like growth factor–1 (IGF-1) grouped by age (97.6 vs 53.8 ng/mL, for younger vs older; P < .001). Although an overall pattern of higher cytokine or growth factors is apparent in male compared with female patients, there is intragroup variation and overlap in the distributions.
Differences in PRP Composition Between Age and Sex Groups
Significant differences in PRP between male and female patients were observed in content for the majority of the cytokines studied (Figure 1) as well as the cytokines and growth factors selected for further analysis (Figure 2). For these proteins, differences between female and male patients were observed for the following proinflammatory cytokines: IL-1β (7.71 vs 9.83 pg/mL, respectively; P = .008) and TNF-a (110.5 vs 131.6 pg/mL, respectively; P = .048); for the anti-inflammatory cytokine IRAP (218.0 vs 298.0 pg/mL, respectively; P < .001); and for the following growth factors: FGF-basic (194.0 vs 237.9 pg/mL, respectively; P = .010) and TGF-β1 (92.8 vs 118.8 ng/mL, respectively; P = .002). There was a trend toward lower concentrations in female than male patients for PDGF-BB (2579.3 vs 3296.2 pg/mL, respectively; P = .087) and VEGF (108.4 vs 142.4 pg/mL, respectively; P = .060), while IGF-1 did not show significant differences (P = .903) between male and female patients (Table 2). Two-way ANOVA did not show significant differences between the young and older age groups for any of these cytokines and growth factors except for IGF-1 (97.6 vs 53.8 ng/mL, respectively; P < .001) (Table 2). The percentage increase in male compared with female patients for each biomarker that showed significant differences at the 5% alpha level were the following, in descending order: IRAP (40.3%), VEGF (33.8%), IL-1b (30.6%), FGF-basic (24.7%), PDGF-BB (24.3%), TNF-α (23.1%), and TGF-β1 (21.9%) (Table 2).
Young patients had a 44.8% greater mean IGF-1 level than older patients. There were no significant correlations between high-sensitivity C-reactive protein and cytokine levels. Furthermore, the interaction between age and sex was not significant for any of the cytokines (Figures 1 and 3).
Figure 3.
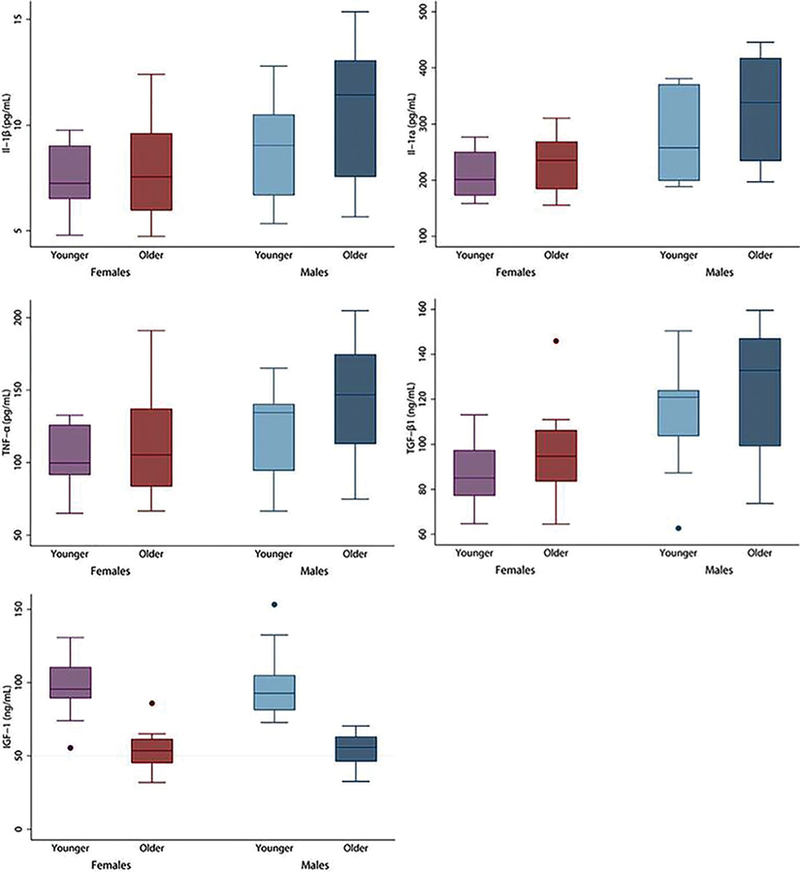
Cytokine and growth factor distributions of female compared with male patients by age group. Box plots are grouped visually by age and sex, with significant differences between females and males in: interleukin-1 beta (IL-1β) (7.71 vs 9.83 pg/mL, respectively; P = .008), tumor necrosis factor-alpha (TNF-α) (110.5 vs 131.6 pg/mL, respectively; P = .048), IL-1 receptor antagonist protein (IRAP) (218.0 vs 298.0 pg/mL, respectively; P < .001), transforming growth factor-beta 1 (TGF-β1) (92.8 vs 118.8 ng/mL, respectively; P = .002). Insulin-like growth factor-1 (IGF-1) showed significant differences between younger and older groups (97.6 vs 53.8 ng/mL, respectively; P < .001). Boxes demonstrate the median, 25th percentile, and 75th percentile. Upper and lower adjacent lines demonstrate upper and lower adjacent values, respectively. Outside values are shown by dots representing discrete data points.
Ratios Between Proinflammatory Mediators and Anti-inflammatory Mediators or Growth Factors
The ratio of anti-inflammatory (IRAP) and proinflammatory (IL-1β) cytokines was higher in male compared with female patients (1.036 vs 0.977, respectively; P = .051) (Figure 3), and there was no age-related difference (0.987 young vs 1.027 older, respectively; P = .28). The ratio of growth factors (PDGF-BB, FGF-basic, VEGF, TGF-β1, and IGF-1) to proinflammatory (IL-1β and TNF-α) cytokines was higher in the young group compared with the older group (1.12 vs 0.90, respectively; P = .018) (Figure 4) but not significantly different between male and female patients (1.04 vs 0.99, respectively; P = .78).
Figure 4.
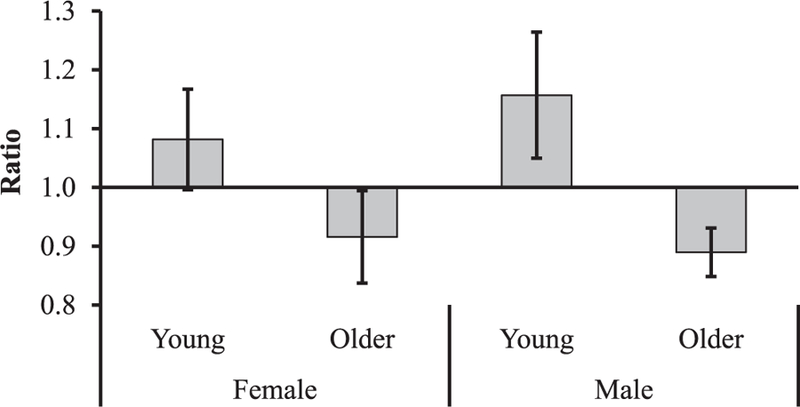
Ratio of growth factors to proinflammatory cytokines. The ratio of normalized values of growth factors (platelet-derived growth factor [PDGF-BB], fibroblast growth factor–basic [FGF-basic], vascular endothelial growth factor [VEGF], transforming growth factor–beta 1 [TGF-β1], and insulin-like growth factor–1 [IGF-1]) to proinflammatory (interleukin–1 beta [IL-1β] and tumor necrosis factor–alpha [TNF-α]) cytokines was higher in the young group compared with the older group (1.12 vs 0.90, respectively; P = .018) but not significantly different between male and female patients (1.04 vs 0.99, respectively; P = .78).
DISCUSSION
The results of this study show significant differences in the composition of PRP between healthy male and female patients. Specifically, PRP from male patients contained a higher level of cytokines such as IL-1β, IRAP, and TNF-α and growth factors such as PDGF-BB, VEGF, and TGF-β1 compared with female patients. Age-related differ-ences were less evident, with PRP from older patients showing a lower IGF-1 content. Furthermore, substantial within-group variability in the PRP composition was observed. The clinical laboratory test of high-sensitivity C-reactive protein did not correlate with PRP cytokine levels, suggesting that it is of low utility as a surrogate marker for levels of inflammatory mediators in PRP.
While some previous studies have shown differences between PRP prepared using different systems11,17,49,66 and other reports have suggested that individual differences in the PRP composition may contribute to variability in the PRP composition,7,25 prior studies typically have reported mean PRP values found in the study cohort.72 Thus, information on differences in the PRP composition between different people and different groups of people is lacking. This study specifically evaluated between-person differences in the PRP composition using a standard hematology protocol to generate leukocyte-poor PRP.45 The data further highlight that variability in the composition of PRP is high and that this variability complicates determining the mechanisms of action and clinical efficacy of PRP.
Despite the significant intergroup differences in the PRP composition, there was also significant intragroup variation among individual patients. These findings have high potential relevance to the clinical use of PRP. Unlike the administration of a medication or substance with known concentrations of an active ingredient or bioactive factor, there are no proteomic standards for PRP, which is minimally processed from whole blood. In addition to the previously shown variability in PRP depending on processing techniques and diurnal variations, we have further shown that sex appears to influence the composition of PRP in healthy patients. As PRP variability cannot be precisely controlled because of the minimally manipulated nature of the treatment, this study supports accounting for the composition of PRP in future basic and clinical studies of tissue and clinical effects.
As there are an exceptionally high number of variables related to PRP treatment, it would not be feasible to include every potential factor for evaluation. Thus, it would be important for a consensus panel of experts to develop basic guidelines based on the best available current literature. Platelet and white blood cell counts of the PRP prepared for administration as well as factors considered relevant to the disease being treated may be an appropriate reference point.
In this study, factors were selected for evaluation based on an interest in assessing PRP injections for the treatment of osteoarthritis in which leukocyte-poor preparations may reduce concerns for excessive proinflammatory activity.8 In addition to platelet and white blood cell levels, special emphasis was placed on studying the inflammatory mediators of IL-1β and TNF-α, the anti-inflammatory protein IRAP, and the cartilage anabolic growth factors TGF-β1 and IGF-1. The growth factors PDGF-BB, VEGF, and FGF-basic were additionally included because of their prominence in PRP and importance to tissue repair.
For example, the proinflammatory cytokine IL-1 β is a well-known instigator of cartilage damage and osteoarthritis and is used experimentally to induce an osteoarthritic state in animal models.77 In conjunction with other inflammatory markers such as IL-1β,27 TNF-α both potentiates cartilage damage and the development of osteophytes as well as prevents the migration of chondro-progenitors for cartilage healing5 and has been shown to play a role in potentiating pain responses.32,42,48 In this study, both of these cytokines showed significantly higher levels in male compared with female patients.
However, the observation of higher overall cytokine levels in male compared with female patients cannot be inter-preted in isolation, as the relationships are complex. In this study, IRAP levels were also significantly higher in male than female patients. IRAP is a competitive antagonist to the proinflammatory effects of IL-1 that works by binding to the IL-1 receptor.52,75,78 This is just one example of the checks and balances within the cytokine milieu that further complicates the attribution of PRP effects to one or a few factors.
The motivation behind the widespread clinical use of PRP includes the potential role of PDGFs to promote healing in musculoskeletal injuries. This study showed that TGF-β1 had significantly higher levels in the PRP of male patients compared with female patients, while the growth factor IGF-1 had significantly higher levels in the PRP of young patients compared with older ones. It has been shown that TGF-β1 promotes chondrocyte differentiation and cartilage health3,24,40,71 but may contribute to pathological conditions such as tendon healing15 and bone changes of osteoarthritis.18,76 Thus, the context and disease state for which PRP treatment is intended would necessarily affect desired levels of a particular growth factor.
For example, IGF-1 also shows positive effects on cartilage health and metabolism,6,14,26,50,68,79 particularly in the hypoxic, dynamic loading environment specific to chondro-cytes.36,73 In addition, IGF-1 has shown chondroprotective effects against inflammatory damage from cytokines such as IL-1β.43 However, diseased cartilage shows reduced sensitivity to the anabolic effects of IGF-1.13 This study demonstrated lower IGF-1 levels in older patients. Given the decreased sensitivity of aged, arthritic chondrocytes to IGF-1–mediated chondrogenesis,33,69 the reduction in IGF-1 levels with age further raises questions concerning whether this protein is relevant for the treatment of osteoarthritis, which is more commonly observed in older patients. This example illustrates the complexity in relating proteomic concentrations in PRP to tissue effects. Further research on the effect of age, sex, osteoarthritic disease state, and patient health on PRP composition and treatment effects is needed. This study showing the wide variation in PRP composition and patterns of PRP composition based on age and sex emphasizes the importance of a personalized approach in which the potential effects of PRP variation on tissue and clinical outcomes are accounted for. Despite the high number of variables and the complexity of evaluating PRP actions, the data from our study suggest that there may be either demographic or proteomic markers of likely responders and nonresponders to PRP treatment and that further study evaluating these markers against clinical outcomes is needed. While age alone did not readily account for proteomic differences in healthy patients, the ratio of growth factors to inflammatory cytokines was positive in the young group and negative in the older group and potentially suggestive of a higher regenerative capacity in the young. These ratios, while theoretical, illustrate the importance of pursuing a broader understanding of the numerous factors in PRP by evaluating the balance between anabolic and catabolic factors. Further exploration of these relationships is needed.
This work highlights another important area of sex-related differences in biology and the healing potential that deserves further study. Hormonal differences drive many sex-related differences between men and women. While several studies have shown an increase in inflammatory markers with menopause, this was not observed in our perimenopausal group of older female patients.54,62 The data from our study showing proteomic differences in PRP provide additional insight into the biological differences between the sexes and raise the question as to whether male and female patients may respond differently to PRP treatment. Male and female patients have been previously shown to demonstrate differences in innate healing responses, another component in the response to PRP treatment. Male patients and specimens have shown improved healing of mucosal tissue in human models19 as well as bone regeneration65 and ligament reconstruction28 in animal models. In addition to healing and regeneration, a potential analgesic effect of PRP may be also affected by sex-related factors such as the findings that women with osteoarthritis tend to exhibit more widespread pain and greater sensitivity to mechanical stimuli than men4 and tend to rate clinical pain higher for musculoskeletal conditions such as sprains and arthropathies.58
In addition to inherent differences among individual patients in the PRP composition, host factors may also affect responses to PRP treatment. For example, our study demonstrated that platelet counts for PRP from older female and older male patients showed no correlation with any of the cytokines or growth factors studied. This may relate to reduced platelet competency or a lower platelet activation potential in older patients. Host differences such as sex may indicate not only different cytokine levels, as demonstrated in this study, but also varying responses to these cytokines. Women tend to mount a more aggressive immunological response to inflammation and faster recovery of the preinflammatory state,10 and this may result in lower baseline cytokine levels. Lower cytokine concentrations in PRP, therefore, may prompt an equivalent response in women as it does in men. Furthermore, host age may affect the potential for regenerative responses. As previously mentioned, older, arthritic chondrocytes show a blunted response to IGF-1.33,69
Additionally, host behaviors such as diet and exercise may also affect responses to PRP content. One study demonstrated that dynamic compression mimicking exercise with C-type natriuretic peptide administration mitigated inflammatory chondrocyte damage by IL-1β55 and that male participants tend to exhibit greater decreases in inflammatory markers such as C-reactive protein in response to exercise.74 A recent study by Nazli et al47 showed decreased IGF-1–mediated proteoglycan production in mice on a high-fat diet compared with mice on a low-fat diet. Medications may also play a role. For example, dexamethasone was shown to be synergistic with increased IGF-1 levels in preventing cytokine-mediated chondrocyte damage in an in vitro model.31 This leads to a complex interplay of PRP composition and host factors, underscoring the importance of individual consideration in PRP clinical use.
Limitations of this study include that PRP was obtained only from healthy patients. As such, the findings may be more reflective of the PRP composition in those receiving treatment for athletic injuries, who are more likely to be healthy, than for those seeking relief of knee osteoarthritis, who may be older and have comorbidities such as obesity, diabetes, or heart conditions. Although this study standardized whole blood collection time to a single morning hour, there may also have been diurnal variations in personal habit that may have made this uniform collection time relatively early or late for different individual patients. Furthermore, this study utilized a previously described freeze-thaw method of obtaining platelet releasate for analysis,57,67 which may not reflect in vivo release kinetics in which differences in the ‘‘platelet activation potential’’ between individual patients may be another source of variability. The freeze-thaw method to obtain PRP releasate was chosen because activation with standard ‘‘activators’’ such as CaCl2 or thrombin results in fibrinogen cleavage and clot formation that is undesirable for clinical applications, such as intra-articular injections for knee osteoarthritis, and would also sequestrate growth factors and proteins in the clot.12,59 A single freeze-thaw cycle has long been used for this purpose.9 Recently, a study examining pooled platelet lysates has shown that multiple freeze-thaw cycles have differing effects on the concentrations of various growth factors and anti-inflammatory mediators, which highlights another potential source of variability.64 Finally, we used a standard PRP preparation protocol as defined in hematology45 as opposed to a commercial system. The results from this study were not intended to provide a standard or reference for commercial systems, for clinical treatment, or to model clinical platelet release kinetics and should not be interpreted in this fashion. Rather, the purpose of this study was to show differences between PRP from different individual patients and groups of patients processed and analyzed using the same techniques.
In summary, this study shows significant variation of PRP among individual patients and that this variation is influenced by age and sex. Combined with the known variability between PRP generated by different systems, these data highlight that future research needs to account for the composition of PRP used. Host factors may also affect responses to PRP treatment. Given the multitude of factors impacting PRP treatment, substantial additional basic and clinical research is needed to determine the mechanisms of action of PRP on different pathological processes, which components support or contradict these mechanisms, and whether there are groups or categories of patients that are more or less likely to benefit from PRP. Ultimately, it is likely that a personalized approach to the clinical use of PRP will be needed.
Acknowledgments
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was funded by the National Institutes of Health (R01 AR051963 [C.R.C.], I21 RX002045 [C.R.C.]), Stanford’s Medical Scholars Research Program (G.X.), and Stanford’s Department of Orthopaedic Surgery. N.L. declares ownership of stock or stock options in Abbott Laboratories.
REFERENCES
- 1.Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage 2006;14(12):1272–1280. [DOI] [PubMed] [Google Scholar]
- 2.Akeda K, An HS, Pichika R, et al. Platelet-rich plasma (PRP) stimulates the extracellular matrix metabolism of porcine nucleus pulposus and anulus fibrosus cells cultured in alginate beads. Spine (Phila Pa 1976) 2006;31(9):959–966. [DOI] [PubMed] [Google Scholar]
- 3.Andrades JA, Motaung SC, Jimenez-Palomo P, et al. Induction of superficial zone protein (SZP)/lubricin/PRG 4 in muscle-derived mesenchymal stem/progenitor cells by transforming growth factor-beta1 and bone morphogenetic protein-7. Arthritis Res Ther 2012;14(2): R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartley EJ, King CD, Sibille KT, et al. Enhanced pain sensitivity among individuals with symptomatic knee osteoarthritis: potential sex differences in central sensitization. Arthritis Care Res (Hoboken) 2016;68(4):472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bevill SLL, Briant PLL, Levenston MEE, Andriacchi TPP. Central and peripheral region tibial plateau chondrocytes respond differently to in vitro dynamic compression. Osteoarthritis Cartilage 2009;17: 980–987. [DOI] [PubMed] [Google Scholar]
- 6.Bos PK, van Osch GJ, Frenz DA, Verhaar JA, Verwoerd-Verhoef HL. Growth factor expression in cartilage wound healing: temporal and spatial immunolocalization in a rabbit auricular cartilage wound model. Osteoarthritis Cartilage 2001;9(4):382–389. [DOI] [PubMed] [Google Scholar]
- 7.Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy 2012;28(3): 429–439. [DOI] [PubMed] [Google Scholar]
- 8.Braun HJ, Kim HJ, Chu CR, Dragoo JL. The effect of platelet-rich plasma formulations and blood products on human synoviocytes: implications for intra-articular injury and therapy. Am J Sports Med 2014;42:1204–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnouf T, Strunk D, Koh MB, Schallmoser K. Human platelet lysate: replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 2016;76:371–387. [DOI] [PubMed] [Google Scholar]
- 10.Casimir GJ, Duchateau J. Gender differences in inflammatory processes could explain poorer prognosis for males. J Clin Microbiol 2011;49(1):478, author reply 478–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med 2011;39(2):266–271. [DOI] [PubMed] [Google Scholar]
- 12.Cavallo C, Filardo G, Mariani E, et al. Comparison of platelet-rich plasma formulations for cartilage healing: an in vitro study. J Bone Joint Surg Am 2014;96:423–429. [DOI] [PubMed] [Google Scholar]
- 13.Chu CR, Izzo NJ, Irrgang JJ, Ferretti M, Studer RK. Clinical diagnosis of potentially treatable early articular cartilage degeneration using optical coherence tomography. J Biomed Optics 2007;12:051703. [DOI] [PubMed] [Google Scholar]
- 14.Chubinskaya S, Hakimiyan A, Pacione C, et al. Synergistic effect of IGF-1 and OP-1 on matrix formation by normal and OA chondrocytes cultured in alginate beads. Osteoarthritis Cartilage 2007;15(4): 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies MR, Liu X, Lee L, et al. TGF-beta small molecule inhibitor SB431542 reduces rotator cuff muscle fibrosis and fatty infiltration by promoting fibro/adipogenic progenitor apoptosis. PLoS One 2016;11(5):e0155486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Hoon MJ, Imoto S, Nolan J, Miyano S. Open source clustering software. Bioinformatics 2004;20(9):1453–1454. [DOI] [PubMed] [Google Scholar]
- 17.Dragoo JL, Braun HJ, Durham JL, et al. Comparison of the acute inflammatory response of two commercial platelet-rich plasma systems in healthy rabbit tendons. Am J Sports Med 2012;40:1274–1281. [DOI] [PubMed] [Google Scholar]
- 18.Ehnert S, Baur J, Schmitt A, et al. TGF-β1 as possible link between loss of bone mineral density and chronic inflammation. PLoS One 2010;5:e14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engeland CG, Sabzehei B, Marucha PT. Sex hormones and mucosal wound healing. Brain Behav Immun 2009;23(5):629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filardo G, Kon E, Buda R, et al. Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2011; 19:528–535. [DOI] [PubMed] [Google Scholar]
- 21.Filardo G, Kon E, Di Matteo B, et al. Leukocyte-poor PRP application for the treatment of knee osteoarthritis. Joints 2013;1:112–120. [PMC free article] [PubMed] [Google Scholar]
- 22.Filardo G, Kon E, Roffi A, Di Matteo B, Merli ML, Marcacci M. Platelet-rich plasma: why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg Sports Traumatol Arthrosc 2015;23(9):2459–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res 2011; 469:2706–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukumoto T, Sperling JW, Sanyal A, et al. Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthritis Cartilage 2003;11(1):55–64. [DOI] [PubMed] [Google Scholar]
- 25.Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg 2013;21(12):739–748. [DOI] [PubMed] [Google Scholar]
- 26.Jenniskens YM, Koevoet W, de Bart AC, et al. Biochemical and functional modulation of the cartilage collagen network by IGF1, TGFbeta2 and FGF2. Osteoarthritis Cartilage 2006;14(11): 1136–1146. [DOI] [PubMed] [Google Scholar]
- 27.Joos H, Wildner A, Hogrefe C, Reichel H, Brenner RE. Interleukin-1 beta and tumor necrosis factor alpha inhibit migration activity of chondrogenic progenitor cells from non-fibrillated osteoarthritic cartilage. Arthritis Res Ther 2013;15(5):R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiapour AM, Fleming BC, Proffen BL, Murray MM. Sex influences the biomechanical outcomes of anterior cruciate ligament reconstruction in a preclinical large animal model. Am J Sports Med 2015; 43(7):1623–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitoh H, Kitakoji T, Tsuchiya H, et al. Transplantation of marrow-derived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis: a preliminary result of three cases. Bone 2004;35(4):892–898. [DOI] [PubMed] [Google Scholar]
- 30.Laver L, Marom N, Dnyanesh L, Mei-Dan O, Espregueira-Mendes J, Gobbi A. PRP for degenerative cartilage disease: a systematic review of clinical studies. Cartilage 2017;8(4):341–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Wang Y, Chubinskaya S, et al. Effects of insulin-like growth factor-1 and dexamethasone on cytokine-challenged cartilage: relevance to post-traumatic osteoarthritis. Osteoarthritis Cartilage 2015;23(2):266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lisowska B, Lisowski A, Siewruk K. Substance P and chronic pain in patients with chronic inflammation of connective tissue. PLoS One 2015;10(10):e0139206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loeser RF, Gandhi U, Long DL, Yin W, Chubinskaya S. Aging and oxidative stress reduce the response of human articular chondrocytes to insulin-like growth factor 1 and osteogenic protein 1. Arthritis Rheumatol 2014;66(8):2201–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lutz P, Dzik WH. Large-volume hemocytometer chamber for accurate counting of white cells (WBCs) in WBC-reduced platelets: validation and application for quality control of WBC-reduced platelets prepared by apheresis and filtration. Transfusion 1993;33(5):409–412. [DOI] [PubMed] [Google Scholar]
- 35.Masse M, Naegelen C, Pellegrini N, Segier JM, Marpaux N, Beaujean F. Validation of a simple method to count very low white cell concentrations in filtered red cells or platelets. Transfusion 1992;32(6): 565–571. [DOI] [PubMed] [Google Scholar]
- 36.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng 2003;9(4):597–611. [DOI] [PubMed] [Google Scholar]
- 37.McCarrel TM, Mall NA, Lee AS, Cole BJ, Butty DC, Fortier LA. Considerations for the use of platelet-rich plasma in orthopedics. Sports Med 2014;44:1025–1036. [DOI] [PubMed] [Google Scholar]
- 38.Mikuls TR, Padala PR, Sayles HR, et al. Prospective study of posttraumatic stress disorder and disease activity outcomes in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65(2):227–234. [DOI] [PubMed] [Google Scholar]
- 39.Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med 2006;34:1774–1778. [DOI] [PubMed] [Google Scholar]
- 40.Miyamoto C, Matsumoto T, Sakimura K, Shindo H. Osteogenic protein-1 with transforming growth factor-beta1: potent inducer of chondrogenesis of synovial mesenchymal stem cells in vitro. J Orthop Sci 2007;12(6):555–561. [DOI] [PubMed] [Google Scholar]
- 41.Mlynarek RA, Kuhn AW, Bedi A. Platelet-rich plasma (PRP) in orthopedic sports medicine. Am J Orthop (Belle Mead NJ) 2016; 45(5):290–326. [PubMed] [Google Scholar]
- 42.Moilanen LJ, Hamalainen M, Nummenmaa E, et al. Monosodium iodoacetate-induced inflammation and joint pain are reduced in TRPA1 deficient mice: potential role of TRPA1 in osteoarthritis. Osteoarthritis Cartilage 2015;23(11):2017–2026. [DOI] [PubMed] [Google Scholar]
- 43.Montaseri A, Busch F, Mobasheri A, et al. IGF-1 and PDGF-bb suppress IL-1beta-induced cartilage degradation through down-regulation of NF-kappaB signaling: involvement of Src/PI-3K/AKT pathway. PLoS One 2011;6(12):e28663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murray MM, Spindler KP, Devin C, et al. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res 2006;24(4):820–830. [DOI] [PubMed] [Google Scholar]
- 45.Nachman RL, Jaffe EA, Weksler BB. Immunoinhibition of ristocetin-induced platelet aggregation. J Clin Invest 1977;59(1):143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Namazi H Platelet-rich plasma as a novel therapeutic agent in osteomyelitis. Med Hypoth 2007;69:706. [DOI] [PubMed] [Google Scholar]
- 47.Nazli SA, Loeser RF, Chubinskaya S, Willey JS, Yammani RR. High fat-diet and saturated fatty acid palmitate inhibits IGF-1 function in chondrocytes. Osteoarthritis Cartilage 2017;25(9):1516–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neogi T, Guermazi A, Roemer F, et al. Association of joint inflammation with pain sensitization in knee osteoarthritis: the Multicenter Osteoarthritis Study. Arthritis Rheumatol 2016;68(3):654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oh JH, Kim W, Park KU, Roh YH. Comparison of the cellular composition and cytokine-release kinetics of various platelet-rich plasma preparations. Am J Sports Med 2015;43(12):3062–3070. [DOI] [PubMed] [Google Scholar]
- 50.O’Keefe RJ, Crabb ID, Puzas JE, Rosier RN. Effects of transforming growth factor-beta 1 and fibroblast growth factor on DNA synthesis in growth plate chondrocytes are enhanced by insulin-like growth factor-I. J Orthop Res 1994;12(3):299–310. [DOI] [PubMed] [Google Scholar]
- 51.O’Shaughnessey K, Matuska A, Hoeppner J, et al. Autologous protein solution prepared from the blood of osteoarthritic patients contains an enhanced profile of anti-inflammatory cytokines and anabolic growth factors. J Orthop Res 2014;32:1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Shaughnessey KM, Panitch A, Woodell-May JE. Blood-derived anti-inflammatory protein solution blocks the effect of IL-1beta on human macrophages in vitro. Inflamm Res 2011;60(10):929–936. [DOI] [PubMed] [Google Scholar]
- 53.Payne KA, Didiano DM, Chu CR. Donor sex and age influence the chondrogenic potential of human femoral bone marrow stem cells. Osteoarthritis Cartilage 2010;18:705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev 2002;23(1): 90–119. [DOI] [PubMed] [Google Scholar]
- 55.Ramachandran M, Achan P, Salter DM, Bader DL, Chowdhury TT. Biomechanical signals and the C-type natriuretic peptide counteract catabolic activities induced by IL-1beta in chondrocyte/agarose constructs. Arthritis Res Ther 2011;13(5):R145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med 2016;44(3): 792–800. [DOI] [PubMed] [Google Scholar]
- 57.Roffi A, Filardo G, Assirelli E, et al. Does platelet-rich plasma freezethawing influence growth factor release and their effects on chondro-cytes and synoviocytes? BioMed Res Int 2014;2014:692913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruau D, Liu LY, Clark JD, Angst MS, Butte AJ. Sex differences in reported pain across 11,000 patients captured in electronic medical records. J Pain 2012;13(3):228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rubio-Azpeitia E, Andia I. Partnership between platelet-rich plasma and mesenchymal stem cells: in vitro experience. Muscles Ligaments Tendons J 2014;4(1):52–62. [PMC free article] [PubMed] [Google Scholar]
- 60.Saldanha AJ. Java TreeView: extensible visualization of microarray data. Bioinformatics 2004;20(17):3246–3248. [DOI] [PubMed] [Google Scholar]
- 61.Schnabel LV, Mohammed HO, Miller BJ, et al. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res 2007;25(2):230–240. [DOI] [PubMed] [Google Scholar]
- 62.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev 2011;10(3):319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sokolove J, Bromberg R, Deane KD, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One 2012;7(5):e35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strandberg G, Sellberg F, Sommar P, et al. Standardizing the freeze-thaw preparation of growth factors from platelet lysate. Transfusion 2017;57(4):1058–1065. [DOI] [PubMed] [Google Scholar]
- 65.Strube P, Mehta M, Baerenwaldt A, et al. Sex-specific compromised bone healing in female rats might be associated with a decrease in mesenchymal stem cell quantity. Bone 2009;45(6):1065–1072. [DOI] [PubMed] [Google Scholar]
- 66.Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med 2011;39:2135–2140. [DOI] [PubMed] [Google Scholar]
- 67.Textor JA, Tablin F. Activation of equine platelet-rich plasma: comparison of methods and characterization of equine autologous thrombin. Vet Surg 2012;41(7):784–794. [DOI] [PubMed] [Google Scholar]
- 68.Trippel SB, Wroblewski J, Makower AM, Whelan MC, Schoenfeld D, Doctrow SR. Regulation of growth-plate chondrocytes by insulin-like growth-factor I and basic fibroblast growth factor. J Bone Joint Surg Am 1993;75(2):177–189. [DOI] [PubMed] [Google Scholar]
- 69.Verschure PJ, van Marle J, Joosten LA, van den Berg WB. Chondrocyte IGF-1 receptor expression and responsiveness to IGF-1 stimulation in mouse articular cartilage during various phases of experimentally induced arthritis. Ann Rheum Dis 1995;54(8):645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vogel JP, Szalay K, Geiger F, Kramer M, Richter W, Kasten P. Platelet-rich plasma improves expansion of human mesenchymal stem cells and retains differentiation capacity and in vivo bone formation in calcium phosphate ceramics. Platelets 2006;17(7):462–469. [DOI] [PubMed] [Google Scholar]
- 71.Waller KA, Zhang LX, Elsaid KA, Fleming BC, Warman ML, Jay GD. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proc Natl Acad Sci USA 2013;110:5852–5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wasterlain AS, Braun HJ, Dragoo JL. Contents and formulations of platelet-rich plasma. Oper Tech Orthop 2012;22(1):33–42. [Google Scholar]
- 73.Witt A, Salamon A, Boy D, et al. Gene expression analysis of growth factor receptors in human chondrocytes in monolayer and 3D pellet cultures. Int J Mol Med 2017;40(1):10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woods JA, Wilund KR, Martin SA, Kistler BM. Exercise, inflammation and aging. Aging Dis 2012;3(1):130–140. [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang P, Zhong ZH, Yu HT, Liu B. Exogenous expression of IL-1Ra and TGF-beta1 promotes in vivo repair in experimental rabbit osteoarthritis. Scand J Rheumatol 2015;44(5):404–411. [DOI] [PubMed] [Google Scholar]
- 76.Zhen G, Wen C, Jia X, et al. Inhibition of TGF-b signaling in subchondral bone mesenchymal stem cells attenuates osteoarthritis. Nature Med 2013;19:704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou PH, Liu SQ, Peng H. The effect of hyaluronic acid on IL-1beta-induced chondrocyte apoptosis in a rat model of osteoarthritis. J Orthop Res 2008;26(12):1643–1648. [DOI] [PubMed] [Google Scholar]
- 78.Zhou PH, Ma BL, Shi L, Xie T, Qiu B. Inhibition of interleukin-1beta-stimulated matrix metalloproteinases via the controlled release of interleukin-1Ra from chitosan microspheres in chondrocytes. Mol Med Rep 2015;11(1):555–560. [DOI] [PubMed] [Google Scholar]
- 79.Zhou Q, Li B, Zhao J, Pan W, Xu J, Chen S. IGF-I induces adipose derived mesenchymal cell chondrogenic differentiation in vitro and enhances chondrogenesis in vivo. In Vitro Cell Dev Biol Anim 2016;52(3):356–364. [DOI] [PubMed] [Google Scholar]


