Abstract
Objective
Sjögren syndrome in children is a poorly understood autoimmune disease. We aimed to describe the clinical and diagnostic features of children diagnosed with Sjögren syndrome and explore how the 2016 ACR/EULAR classification criteria apply to this population.
Methods
An international workgroup retrospectively collected cases of Sjögren syndrome diagnosed under 18 years of age from 23 centres across eight nations. We analysed patterns of symptoms, diagnostic workup, and applied the 2016 ACR/EULAR classification criteria.
Results
We identified 300 children with Sjögren syndrome. The majority of patients n = 232 (77%) did not meet 2016 ACR/EULAR classification criteria, but n = 110 (37%) did not have sufficient testing done to even possibly achieve the score necessary to meet criteria. Even among those children with all criteria items tested, only 36% met criteria. The most common non-sicca symptoms were arthralgia [n = 161 (54%)] and parotitis [n = 140 (47%)] with parotitis inversely correlating with age.
Conclusion
Sjögren syndrome in children can present at any age. Recurrent or persistent parotitis and arthralgias are common symptoms that should prompt clinicians to consider the possibility of Sjögren syndrome. The majority of children diagnosed with Sjögren syndromes did not meet 2016 ACR/EULAR classification criteria. Comprehensive diagnostic testing from the 2016 ACR/EULAR criteria are not universally performed. This may lead to under-recognition and emphasizes a need for further research including creation of paediatric-specific classification criteria.
Keywords: Sjögren Syndrome, childhood Sjögren syndrome, recurrent parotitis, pediatric rheumatology
Rheumatology key messages
Sjögren syndrome (SS) can occur in children at any age.
Younger children with SS are more likely to present with recurrent or persistent parotitis.
Most children diagnosed with SS do not fulfill the 2016 ACR/EULAR classification criteria.
Introduction
Sjögren syndrome (SS) is a chronic, systemic autoimmune disease characterized by immune-mediated attack on salivary and lacrimal glands resulting in the classic symptoms of dry mouth and dry eyes [1]. Dryness often prompts consideration of the diagnosis of SS, yet the presence of autoantibodies years before diagnosis suggests the autoimmune process is active long before presentation with sicca symptoms [2]. In children, clinical presentation with symptoms of dryness is infrequent. Children often seek medical attention for parotitis, but many present with nonspecific features such as arthralgia [3].
The first formal case of a child diagnosed with SS was published in the 1960s [4], but a prior case of keratoconjunctivitis sicca and recurrent salivary gland swelling in a 17-year-old girl published in the 1930s may represent the first published case in childhood [5]. Until recently, robust case series in children were lacking. A series of 26 children from the United States [3] and 61 Japanese children [6] reported a decreased prevalence of keratoconjuncitivitis sicca and xerostomia compared with the adult SS population. Parotitis was more prevalent in both of these populations and further substantiated in a literature review [7].
The 2016 ACR/EULAR Sjögren classification criteria (ACR/EULAR) [8] rely on a combination of histopathologic evidence of focal lymphocytic sialadenitis on minor salivary gland (MSG) biopsy, positive anti-SS-A/Ro antibodies, and evidence of glandular dysfunction with decreased tear or saliva production or positive ocular surface staining. Children may not have glandular dysfunction early in the disease [3, 7]. Furthermore, the traditional threshold for defining the presence of focal sialadenitis (focus score ≥1 focus/4 mm2) may not be appropriate for use in children [9].
Child-specific criteria have been suggested with sensitivity ranging from 76% to 85% when tested [3, 10–12]. A recent study of 67 children with SS exploring the diagnostic utility of salivary gland ultrasound (SGUS) noted that only 58% [13] met ACR/EULAR criteria [8]. Given the variability of disease presentation, there is critical need for additional research to improve understanding and awareness of this presumably underdiagnosed disease. We describe the features of SS in children in a multinational cohort and explore the validity of the ACR/EULAR criteria in a large paediatric population.
Materials and methods
Study design
A retrospective study was designed to collect patient data by members of the International Childhood Sjögren Syndrome Workgroup. This includes investigators from 23 institutions (13 in USA, three in Brazil, two in Spain, one each in Australia, Italy, Japan, Poland and Serbia). A minority of these patients (n = 27, 9%) have been included in previous reports [13–18]. A secure web-based database was constructed using REDCap [19] to electronically store the data. Each investigator contributed data from patients diagnosed with SS prior to age 18 years treated at their centre. Diagnosis was determined by expert opinion and not limited to children meeting specific criteria. Children with another autoimmune disease diagnosis were not excluded. Clinical symptoms were recorded as present or absent. Laboratory and diagnostic tests were recorded as performed or not. For performed tests, additional prompts to record results of the test were included. There was also a free text variable asking why the patient was diagnosed with SS or to elaborate on diagnostic evaluation. If a test result response was left blank when a test was indicated as being performed, or when discrepancy existed between binary responses and free text, we contacted the researcher who entered the data for clarification. We reconciled data discrepancies on all but one patient. The study was approved by institutional review boards at participating institutions.
Statistical analysis
Descriptive analyses were used to describe baseline characteristics between groups and reported as medians and interquartile range (IQR) after testing for normality. χ2 was used to compare proportions in categorical data between groups with and without parotitis and laboratory results. Logistic regression was used to evaluate symptoms, laboratory parameters and diagnostic test results, individually as independent variables, as predictors of meeting ACR/EULAR criteria and having a positive MSG biopsy (≥1 focus/4 mm2). Multivariate regression was performed with backward selection after identifying significant associations in univariate analysis. Binomial logistic regression was used to evaluate associations with age at diagnosis. Clinical symptoms, laboratory results, if a diagnostic test was performed, and having a positive MSG biopsy were individually assessed as dependent variables with age at diagnosis as an independent variable. Combinations of symptoms, laboratory and diagnostic test results were assessed as predictive tests. We only included patients who underwent the respective combinations of tests for each analysis. These were evaluated for sensitivity and specificity for detecting patients meeting ACR/EULAR criteria and having a positive MSG biopsy. The true disease prevalence is unknown. Assuming this is a rare disease, we used a prevalence range from 0.01% to 0.05%. Linear regression was used to examine the association between the number of diagnostic tests performed and age. We also applied the proposed paediatric SS criteria in our population [10]. P-values <0.05 were considered significant. Analyses were performed using Stata 16.0 (Stata Corp, College Station, TX, USA).
Results
In total, 300 cases of children diagnosed with SS were collected. The majority of patients were female with median age at diagnosis 12 years [IQR 9, 15] (Table 1). The minority of patients had a history of another autoimmune disease or disease with overlapping clinical features. Systemic lupus erythematosus was reported in 25 (8%), other connective tissue disease in 19 (6%), lymphoma in five (2%) and prior head and neck radiation reported in one patient (<1%).
Table 1.
Demographics and prevalence of signs and symptoms
| Characteristic | Total (n = 300) | Parotitis (n = 140, 47%) | No history of parotitis (n = 160, 53%) | P a |
|---|---|---|---|---|
| Age at diagnosis, median years (IQR) | 12 (9,15) | 11 (8, 13) | 14 (11.5, 16) | <0.001 |
| Age range | 1–17.8 | 1–17.5 | 5–17.8 | |
| Age at database entry, median years (IQR) | 17 (14,20) | 16 (13,19.3) | 17 (15,20.8) | 0.47 |
| Age range | 5–32 | 5–32 | 7.8–32 | |
| Female, n (%) | 250 (83%) | 110 (79%) | 140 (88%) | 0.04 |
| Anticholinergic use, n (%) | 0 (%) | 0 (%) | 0 (%) | N/A |
| Past medical history | n (%) | n (%) | n (%) | P a |
| Systemic lupus | 25 (8%) | 12 (9%) | 13 (8%) | 0.89 |
| Other connective tissue diseases | 19 (6%) | 6 (4%) | 13 (8%) | 0.17 |
| Lymphoma | 5 (2%) | 4 (3%) | 1 (<1%) | 0.13 |
| HIV/Sarcoidosis/IgG4-related disease/graft vs host | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
| Prior head and neck radiation | 1 (<1%) | 0 (0%) | 1 (<1%) | 0.35 |
| Symptoms | n (%) | n (%) | n (%) | P a |
| Sicca | ||||
| Dry eyes or dry mouth | 194 (65%) | 88 (63%) | 106 (66%) | 0.54 |
| Dry mouth | 156 (52%) | 74 (53%) | 82 (51%) | 0.78 |
| Dry eyes | 144 (48%) | 64 (46%) | 80 (50%) | 0.46 |
| Dry eyes and dry mouth | 106 (35%) | 50 (36%) | 56 (35%) | 0.90 |
| Non-sicca | ||||
| Arthralgia, n (%) | 161 (54%) | 59 (42%) | 102 (64%) | <0.001 |
| Recurrent or persistent parotitis | 140 (47%) | |||
| Arthritis | 71 (24%) | 27 (19%) | 44 (28%) | 0.10 |
| Lymphadenopathy | 54 (18%) | 33 (24%) | 21 (13%) | 0.02 |
| Cytopenia | 52 (17%) | 20 (14%) | 32 (20%) | 0.19 |
| Fevers | 34 (11%) | 19 (14%) | 15 (9%) | 0.25 |
| Cutaneous vasculitis | 27 (9%) | 12 (9%) | 15 (9%) | 0.81 |
| Weight loss | 26 (9%) | 8 (6%) | 18 (11%) | 0.09 |
| Pulmonary | 25 (8%) | 11 (8%) | 14 (9%) | 0.78 |
| Headache | 20 (7%) | 9 (6%) | 11 (7%) | 0.88 |
| Proteinuria | 18 (6%) | 8 (6%) | 10 (6%) | 0.85 |
| Peripheral neuropathy | 17 (6%) | 6(4%) | 11 (7%) | 0.33 |
| Vaginitis | 14 (5%) | 9 (6%) | 5 (3%) | 0.18 |
| Myositis | 9 (3%) | 3 (2%) | 6 (4%) | 0.42 |
| Other neurologic symptomsb | 7 (2%) | 3 (2%) | 4 (3%) | 0.84 |
| Interstitial nephritis | 6 (2%) | 1 (<1%) | 5 (3%) | 0.14 |
| Seizures | 5 (2%) | 4 (3 %) | 1 (<1%) | 0.13 |
| Transverse myelitis/neuro-myelitis optica spectrum | 5 (2%) | 0 (0%) | 5 (3%) | 0.04 |
| Renal tubular acidosis | 3 (1%) | 2 (1%) | 1 (<1%) | 0.49 |
P-value represents the significance of a χ2 analysis comparing patients with and without parotitis by each characteristic.
Includes CNS vasculitis, psychosis, anxiety, depression, fatigue, tic disorder.
IQR: interquartile range; HIV: human immunodeficiency virus.
Signs and symptoms
Most patients had sicca symptoms with 194 (65%) reporting dry eyes or mouth, but only 106 (35%) reported both. The most common non-sicca symptoms were arthralgia and parotitis, with other clinical symptoms reported in <25% of patients (Table 1). Demographics and clinical symptoms were stratified by history of parotitis to assess for variation between groups (Table 1) and also by report of sicca (Supplementary Table S1, available at Rheumatology online). Patients with parotitis were younger and less likely to be female (79% vs 88%, P =0.04). Patients with parotitis were less likely to report arthralgia and were more likely to have lymphadenopathy and transverse myelitis or neuro-myelitis-optica spectrum disease.
Laboratory testing
Diagnostic tests were not evenly implemented; however, laboratory testing was done in most patients (Table 2). The majority of patients were ANA positive and 217 (74%) had a positive anti- SSA/Ro. SSB/La was present in less than half of patients. Only nine patients (3%) had positive anti- SSB/La with negative anti-SSA/Ro and 66 (22%) had both negative anti-SSA/Ro and anti-SSB/La. More than half of the patients had an elevated rheumatoid factor and more than half were hypergammaglobulinemic. Laboratory parameters were stratified by history of parotitis to assess for variation between groups (Table 2). Patients with parotitis were more likely to have positive rheumatoid factor and less likely to be anti-SSA/Ro positive.
Table 2.
Laboratory evaluation of 300 children with Sjögren Syndromea
| Laboratory test | Patients who underwent testing, n (%) | Positive/ abnormal n (%)a | Parotitis, n (%) | No history of parotitis, n (%) | P b |
|---|---|---|---|---|---|
| ANA | 299 (99%) | 262 (88%) | 124 (89%) | 138 (86%) | 0.44 |
| SSA/Ro | 292 (97%) | 217 (74%) | 89 (67%) | 128 (81%) | 0.008 |
| SSB/La | 291 (97%) | 131 (45%) | 57 (43%) | 74 (47%) | 0.50 |
| Rheumatoid factor | 257 (86%) | 153 (60%) | 92 (71%) | 61 (48%) | <0.001 |
| Hypergammaglobulinemia | 230 (77%) | 125 (54%) | 58 (54%) | 67 (55%) | 0.99 |
| Anti-double stranded DNA or anti-Smith antibody | 275 (92%) | 35 (13%) | 14 (11%) | 21 (14%) | 0.55 |
| Neutropenia (<1500) or lymphopenia (<1000) | 300 (100%) | 32 (11%) | 13 (9%) | 19 (12%) | 0.47 |
| Thrombocytopenia | 300 (100%) | 16 (5%) | 6 (4%) | 10 (6%) | 0.45 |
| Immune mediated anaemiac | N/A | 8 (% unavailable) | 4 (3%) | 4 (3%) | 0.85 |
| Cryoglobulin | 29 (10%) | 1 (3%) | 1 (9%) | 0 (0%) | 0.19 |
The denominator used to calculate percentages of patients with positive test results is the number of patients tested in each group defined in that column.
P-value represents the significance of a χ2 analysis comparing patients with and without parotitis by each characteristic.
Number of patients undergoing testing for autoimmune anaemia unavailable. We asked if they had positive coombs testing or not. All patients had a complete blood count.
ANA: anti-nuclear antibody; SSA: anti-SS-A/Ro antibody; SSB: anti-SS-B/La antibody.
Diagnostic testing
Wide variation was seen in diagnostic testing of items from the ACR/EULAR criteria (Tables 2–3, Fig. 1). Almost all patients had SSA/Ro tested, but only 15% underwent unstimulated whole salivary flow (UWS). More than half of anti-SSA/Ro and MSG biopsy testing was abnormal, while fewer than half of the exocrine gland function tests were abnormal (Table 3). Among the 131 patients who underwent MSG biopsy, only four (3%) had a completely normal biopsy with focus score of 0 foci/4 mm2. Just over half had a focus score ≥1 focus/4 mm2 meeting the criteria established threshold of positivity. Twenty-six (20%) patients did not have a focus score reported as ≥1 focus/4 mm2 but did have inflammatory foci present (i.e. focus score >0 foci/4 mm2). Unfortunately, 24 (18%) biopsy reports noted inflammation but without sufficient description to determine whether they had true foci of mononuclear cells. Six biopsies (5%) did not list helpful information and one biopsy (<1%) reported MALT lymphoma.
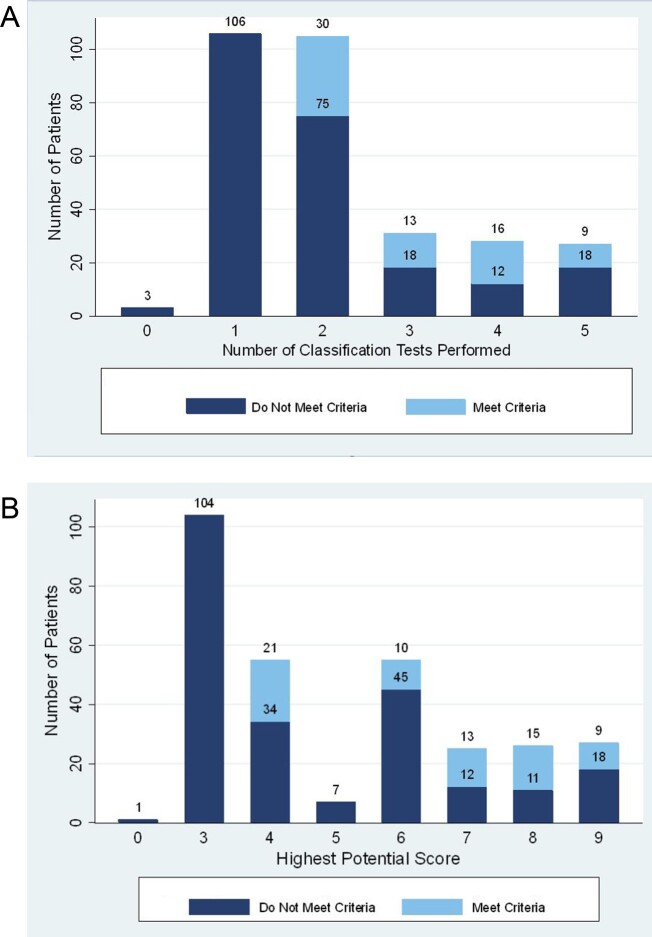
Fig. 1.
Distribution and proportion of children meeting ACR/EULAR criteria for Sjögren syndrome
(A) Distribution by number of tests performed. (B) Proportion based on diagnostic testing. The 2016 ACR/EULAR Criteria includes a labial salivary gland biopsy with focal lymphocytic sialadenitis and focus score ≥1 (3 points), Ant-SSA/Ro (3 points), ocular staining ≥5 in at least one eye (1 point), Schirmer ≤ 5 mm/5 min on at least one eye (1 point), and unstimulated whole saliva flow rate ≤0.1 ml/min (1 point). A score of 4 or higher is required to meet Sjögren syndrome classification. The figure highlights the highest score they could have achieved based on which criteria where tested.
Table 3.
Diagnostic testing and ACR/EULAR criteria
| Diagnostic test | Tested, n (%) | Positive/ abnormal, n (%) | Parotitis (n = 140, 47%) | No history of parotitis (n = 160, 53%) | Classification pointsa |
|---|---|---|---|---|---|
| ACR/EULAR criterion | |||||
| Anti-SSA/Ro antibody | 292 (97%) | 217 (74%) | 89/133 (67%) | 128/159 (81%) | 3 |
| Ocular surface staining score ≥5 (or van Bijsterveld score ≥4) on at least one eyeb | 54 (18%) | 10 (19%) | 4/42 (10%) | 6/12 (50%) | 1 |
| Schirmer ≤5 mm/5 min on at least one eye | 135 (45%) | 57 (42%) | 27/74 (36%) | 30/61 (49%) | 1 |
| Salivary gland biopsy with focal lymphocytic sialadenitis and focus score ≥1c | 131 (44%) | 70 (53%) | 39/80 (49%) | 31/51 (61%) | 3 |
| Unstimulated salivary flow rate ≤0.1 ml/min | 44 (15%) | 13 (30%) | 7/33 (21%) | 6/10 (60%) | 1 |
| Other diagnostic test | |||||
| Salivary gland ultrasound | 119 (40%) | 97 (82%) | 69/78 (88%) | 28/41(68%) | |
| Parotid sialography | 36 (12%) | 27 (75%) | 27/36 (75%) | 0 (0%) | |
| Renal biopsy | 7 (2%) | 7 (100%) | 1/1 (100%) | 6/6 (100%) |
The denominator for percent positive is the number of patients who underwent testing by each group defined in that column.
The 2016 ACR/EULAR Sjögren syndrome classification definition requires a score of 4 or higher to meet criteria.
In six patients, the charts state that they had a positive ocular surface staining score without the score listed.
The biopsy reports provided for 24 patients noted inflammation, but without sufficient description to determine whether they had true foci of mononuclear cells. Therefore, the data may be underreporting patients who truly had a focus score ≥1.
Additional testing beyond the ACR/EULAR criteria was common (Table 3). SGUS was performed in 119 (40%) patients with the majority being abnormal. Among patients who had an abnormal ultrasound, 69 (71%) reported parotitis, 45 (49%) had a positive SSA/Ro. The majority of 78 patients who reported a history of parotitis and underwent SGUS (n = 69, 88%) had abnormalities consistent with SS. Among 85 patients who underwent MSG biopsy and SGUS, sonography was consistent with SS in most patients with a focus score ≥1 focus/4 mm2 (n = 43, 80%) and with a focus score <1 and >0 foci/4 mm2 (n = 13, 76%). As an aggregate, 79% of the 71 children with a focus score >0 foci/4 mm2 had SGUS consistent with SS. A focus score of 0 foci/4 mm2 was reported in three patients who also had SGUS with two being abnormal despite reassuring histopathology. Parotid sialography was performed in 36 (12%) patients and was frequently abnormal.
Evaluation of 2016 ACR/EULAR classification criteria
In our cohort, only 68 patients (23%) met ACR/EULAR criteria [8] with a score of four or greater. The number of tests performed in each patient varied widely (Fig. 1a). A total of 190 patients (63%) underwent enough testing to have the potential to achieve a score of four or greater (Fig. 1b). In this subset of patients, only 68 (36%) achieved a score of four or more meeting classification criteria. Among patients meeting criteria, 44% underwent two tests, 19% underwent three tests, 24% underwent four tests, and 13% had five diagnostic tests from the classification criteria performed. Performing more testing was not associated with likelihood of meeting criteria (P =0.05) in children who had enough testing to potentially meet criteria.
In the 232 patients who did not meet criteria, 189 (81%) had classification score of three [positive SSA/Ro antibody in 157 (84%), positive MSG in 32 (17%)]. Thirty-one (10%) patients met no criteria and the diagnosis was made on a combination of recurrent parotitis, sicca symptoms, classic SGUS features, or abnormal MSG biopsy with <1 focus/4 mm2.
Logistic regression was performed to assess for clinical, laboratory and diagnostic predictors of meeting ACR/EULAR criteria and also for having a positive focus score (Table 4). Hypergammaglobulinemia, reporting dry eyes, persistent fevers, elevated rheumatoid factor, positive anti-SSA/Ro, and having both dry eyes and dry mouth were each independently associated with meeting ACR/EULAR criteria. Multivariate analysis upheld associations with persistent fevers, reporting dry eyes, and hypergammaglobulinemia with meeting ACR/EULAR criteria while controlling for age. Younger patients and those who denied having any sicca symptoms were less likely to meet criteria. Positive anti-SSA/Ro antibody was associated with meeting criteria, as expected, being one of the criteria items. Elevated anti-SSA/Ro and ANA were independently associated with a focus score of ≥1 focus/4 mm2. This was not upheld in multivariate analysis.
Table 4.
Predictors of meeting 2016 ACR/EULAR classification criteria and a positive salivary gland biopsy
| Predictors of meeting classification criteria | ||||
|---|---|---|---|---|
| Clinical feature | Univariate OR (95% CI) | P | Multivariate OR (95% CI)b | P |
| Age | 0.93 (0.87, 1.00) | 0.07 | 0.91 (0.82, 0.99) | 0.04 |
| Sex | 1.66 (0.73, 3.73) | 0.22 | ||
| Dry eyes | 2.85 (1.62, 5.06) | <0.001 | 3.40 (1.70, 6.77) | 0.001 |
| Dry mouth | 1.43 (0.83, 2.47) | 0.20 | ||
| Both sicca | 2.05 (1.18, 3.56) | 0.01 | ||
| No Sicca | 0.44 (0.23, 0.82) | 0.01 | ||
| Parotitis | 1.10 (0.64, 1.89) | 0.73 | ||
| Recurrent vaginitis | 1.39 (0.42, 4.57) | 0.59 | ||
| Persistent fever | 2.75 (1.30, 5.79) | 0.008 | 3.19 (1.25, 8.11) | 0.02 |
| Weight loss | 0.80 (0.29, 2.20) | 0.66 | ||
| Lymphadenopathy | 1.40 (0.72, 2.73) | 0.32 | ||
| Arthritis | 1.63 (0.89, 2.97) | 0.11 | ||
| Arthralgia | 0.96 (0.56, 1.66) | 0.89 | ||
| Cutaneous vasculitis | 1.49 (0.62, 3.58) | 0.37 | ||
| Pulmonary | 1.36 (0.54, 3.42) | 0.51 | ||
| Renal tubular acidosis | 1.72 (0.15, 19.22) | 0.66 | ||
| Proteinuria | 0.41 (0.09, 1.83) | 0.24 | ||
| Interstitial nephritis | N/A | N/A | ||
| Myositis | N/A | N/A | ||
| Peripheral neuropathy | 1.46 (0.49, 4.29) | 0.50 | ||
| Headaches | 0.17 (0.22,1.27) | 0.08 | ||
| Seizures | 0.85 (0.09, 7.74) | 0.89 | ||
| Transverse myelitis/neuro-myelitis optica | 2.31 (0.38, 14.13) | 0.36 | ||
| Other neurologic symptomc | 0.56 (0.07, 4.75) | 0.60 | ||
| Laboratory Test a | Univariate OR (95% CI) | P | Multivariate OR (95% CI)b | P |
| Any cytopenia | 1.32 (0.67, 2.62) | 0.42 | ||
| Neutropenia or lymphopenia | 1.15 (0.49, 2.70) | 0.74 | ||
| Autoimmune anaemia | 0.48 (0.06, 3.97) | 0.50 | ||
| Thrombocytopenia | 1.59 (0.53, 4.76) | 0.40 | ||
| Anti-nuclear antibody | 1.08 (0.47, 2.48) | 0.86 | ||
| Anti-SSA antibody | 3.20 (1.45, 7.06) | 0.004 | ||
| Anti-SSB antibody | 1.15 (0.67, 2.00) | 0.61 | ||
| Rheumatoid factor | 1.93 (1.04, 3.57) | 0.04 | ||
| Anti-dsDNA/Smith | 1.16 (0.52, 2.63) | 0.37 | ||
| Hypergammaglobulinemia | 3.45 (1.73, 6.90) | <0.001 | 3.69 (1.76, 7.72) | 0.001 |
| Diagnostic test a | Univariate OR (95% CI) | P | Multivariate OR (95% CI) | P |
| Salivary ultrasound | 0.47 (0.18, 1.25) | 0.13 | ||
| Sialography | 1.00 (0.16, 6.14) | 1.00 | ||
| Kidney biopsy | N/A | N/A | ||
| Predictors of a positive MSG biopsy Focus score | ||||
| Clinical feature | Univariate OR (95% CI) | P | ||
| Age | 0.93 (0.85, 1.02) | 0.12 | ||
| Sex | 1.30 (0.58, 2.92) | 0.52 | ||
| Dry eyes | 1.02 (0.52, 2.04) | 0.95 | ||
| Dry mouth | 0.97 (0.48, 1.96) | 0.94 | ||
| Both sicca | 0.70 (0.35, 1.41) | 0.32 | ||
| No sicca | 0.66 (0.31, 1.41) | 0.28 | ||
| Parotitis | 0.61 (0.30, 1.25) | 0.18 | ||
| Recurrent vaginitis | 0.41 (0.10, 1.72) | 0.22 | ||
| Persistent fever | 1.29 (0.46, 3.61) | 0.63 | ||
| Weight loss | 0.86 (0.21, 3.61) | 0.84 | ||
| Lymphadenopathy | 0.58 (0.25, 1.35) | 0.21 | ||
| Arthritis | 0.77 (0.33, 1.79) | 0.54 | ||
| Arthralgia | 1.48 (0.74, 2.96) | 0.26 | ||
| Cutaneous vasculitis | 0.27 (0.05, 1.39) | 0.12 | ||
| Pulmonary | 0.47 (0.13, 1.68) | 0.24 | ||
| Renal tubular acidosis | N/A | N/A | ||
| Proteinuria | 0.43 (0.04, 4.83) | 0.49 | ||
| Interstitial nephritis | N/A | N/A | ||
| Myositis | 0.87 (0.12, 6.35) | 0.89 | ||
| Peripheral neuropathy | 1.49 (0.34, 6.50) | 0.60 | ||
| Headaches | 0.23 (0.04, 1.14) | 0.07 | ||
| Seizures | 0.43 (0.04, 4.83) | 0.49 | ||
| Transverse myelitis/neuro-myelitis optica | 2.67 (0.27, 26.53) | 0.40 | ||
| Other neurologic symptomc | N/A | N/A | ||
| Laboratory test a | Univariate OR (95% CI) | P | ||
| Any cytopenia | 2.09 (0.68, 6.39) | 0.20 | ||
| Neutropenia/lymphopenia | 1.24 (0.37, 4.14) | 0.72 | ||
| Autoimmune anaemia | 0.87 (0.12, 6.35) | 0.89 | ||
| Thrombocytopenia | 2.69 (0.27, 26.53) | 0.40 | ||
| Anti-nuclear antibody | 0.37 (0.14, 0.97) | 0.04 | ||
| Anti-SSA antibody | 0.45 (0.22, 0.90) | 0.03 | ||
| Anti-SSB antibody | 0.72 (0.33, 1.56) | 0.40 | ||
| Rheumatoid factor | 0.89 (0.43, 1.81) | 0.74 | ||
| dsDNA/Smith | 0.78 (0.25, 2.48) | 0.67 | ||
| Hypergammaglobulinemia | 0.71 (0.33, 1.50) | 0.36 | ||
| Diagnostic test a | Univariate OR (95% CI) | P | ||
| Salivary ultrasound | 1.14 (0.39, 3.33) | 0.81 | ||
| Sialography | 0.8 (0.13, 5.07) | 0.81 | ||
| Kidney biopsy | N/A | N/A | ||
Diagnostic testing analysis limited to patients who underwent respective testing.
All multivariate models assessed controlled for age at diagnosis. Variables significant in univariate analysis and not reported in multivariate analysis dropped out of the final model as they were no longer statistically significant. Variables included in the multivariate model for meeting classification criteria includes age at diagnosis, presence of hypergammaglobulinemia, presence of persistent fevers, and report of dry eyes.
Includes CNS vasculitis, psychosis, anxiety, depression, fatigue, tic disorder.
Predictors of meeting classification criteria and a positive focus score
We assessed several combinations of clinical, laboratory and diagnostic test results as predictive tests both for meeting ACR/EULAR criteria or having a focus score of ≥1 focus/4 mm2.We did not identify any test that was both sensitive and specific for either outcome. Universally the test sets had low positive predictive values and high negative predictive values for both outcome measures when calculated with a prevalence range from 0.01–0.05% (Supplementary Table S2, available at Rheumatology online).
Age
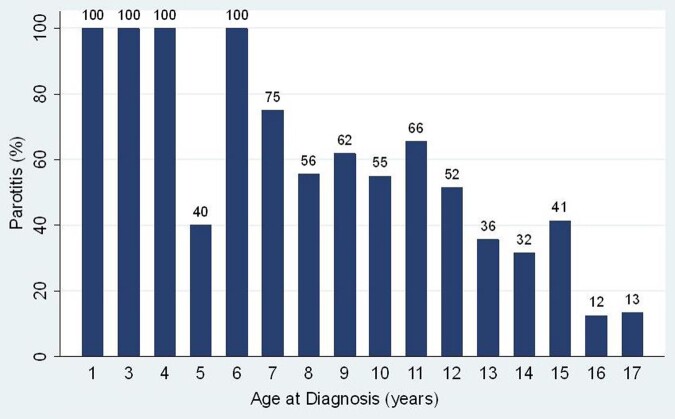
Median age at diagnosis for a patient reporting a history of parotitis was younger than those without reporting parotitis, 11 years [IQR 8, 13] compared with 14 years [IQR 11.5, 16]. Logistic regression revealed that younger age was significantly associated with reporting parotitis (OR=0.76, P <0.001 95% CI: 0.70, 0.82) (Fig. 2). Patient age was not associated with the number of tests performed, but younger patients were more likely to undergo MSG biopsy, OR 0.88, P <0.001 95% CI: 0.83, 0.94. Interestingly, the youngest age where a patient had all of the available diagnostic tests performed was three years. Age was not statistically associated with other presenting symptoms, laboratory abnormalities, or diagnostic tests.
Fig. 2.
Prevalence of parotitis by age
Percentage of patients reporting recurrent or persistent perotitis by age of Sjögren diagnosis.
Additional analyses
We performed a sensitivity analysis excluding patients with lupus or other connective tissue diseases from the assessment of the 2016 ACR/EULAR criteria, which did not alter our findings. We applied the proposed paediatric criteria [10] to our patient population. Only 166 (55%) children in our study achieved a score of four or higher satisfying the criteria. When excluding patients with underlying connective tissues diseases 144 of 257 children (56%) met criteria. As this was performed in a post-hoc fashion, we lacked data on all aspects of four of the twelve criteria (ocular: recurrent conjunctivitis without a source; systemic: hypokalemic paralysis or abdominal pain; biochemical: elevated serum amylase; and haematologic: high ESR).
Discussion
This robust multinational registry of children demonstrates that SS can occur at any age (Supplementary Fig. S1, available at Rheumatology online) and current adult classification criteria are not sensitive for the disease in children. SS is most commonly diagnosed in adults (peak diagnosis at ages 40–60) yet serologic evidence of the disease begins decades before diagnosis [2]. A study that surveyed adults with SS reported first symptoms of the disease during childhood or early adulthood (age <20 years) in 40% of individuals surveyed; however, symptoms were less disease-specific (such as arthralgia or myalgia) and sicca symptoms were more common later in life [20]. In children, SS commonly presents without complaints of dryness, but upon evaluation and further questioning the presence of dryness symptoms is not rare [3, 12, 13, 18]. When present, dryness should certainly prompt evaluation. Evidence of salivary gland inflammation, either clinically with parotitis or through imaging or histopathologic evaluation, is common and helpful in making the diagnosis. Despite this, up to half of children with SS may not have parotitis and a subset may not have sonographic salivary gland changes [3, 13].
In the absence of highly sensitive biomarkers, classification of SS in adults has relied on the development of rigorous criteria. While the specific criteria items have evolved, each set has included the main features specific to the disease (histopathologic evidence of exocrine gland inflammation, serologic evidence of autoimmunity, evidence of gland dysfunction or end-organ damage). For many children, a key barrier to diagnosis of SS is that these features may not be recognized as abnormal. However, upon questioning, we found that 65% of our patients did experience sicca symptoms. Recurrent or persistent parotitis, one of the more common reasons for referral to Rheumatology, is not included in current classification criteria. Similar to prior studies, 47% of our cohort reported parotitis, and, intriguingly, this was significantly associated with a younger age. Prior criteria designed for use in evaluating adults have not been adequate when retrospectively evaluated for use in classifying children with SS [3, 11, 12]. Proposed paediatric-specific criteria have a suboptimal sensitivity that we also found in our population [3, 10–12]. We performed this post-hoc and did not have all of the criteria available, likely resulting in a falsely low sensitivity.
The most recent classification criteria, 2016 ACR/EULAR [8], had not been evaluated for applicability in children at the time of our study conception. Our findings demonstrate a minority of children diagnosed with SS meet these criteria. However, it is clear from our study that when SS was being considered, few patients (<10%) underwent testing for all five criteria items. The majority were only tested for one or two. This may explain our lower percentage of children meeting criteria when compared with another recent study that found 58% of children met these criteria [13]. However, even when excluding children who could not possibly meet criteria (i.e. those who did not undergo sufficient testing to score ≥4), we still found just over one-third satisfied criteria. This was not due to lack of complete criteria item testing; only 33% (9 of 27) of children who had all five criteria items tested met criteria. In contrast, Hammenfors et al. [13], had a slightly higher proportion of children with all five criteria items tested (19%, 13 of 67) and a higher percentage of these children meeting criteria (77%, 10 of 13) (D. S. Hammenfors and M. V. Jonsson, personal communication). Of note, three children were included in both studies but none had all five criteria items tested. If we combine the cases from both international datasets, 40 children had all five criteria tested, and nearly half (49%, 19 of 40) met criteria. While the applicability of these criteria would be better evaluated prospectively, our paper suggests current adult classification criteria are not sensitive for diagnosing SS in children.
The question as to why adult criteria fail to identify children diagnosed with SS remains. The criteria were developed as classification rather than diagnostic criteria and are not intended to apply to all individuals with SS. Rather, they define a homogeneous population with definite disease for research studies resulting in high specificity with lower sensitivity [21, 22]. Failure to meet classification criteria does not negate disease presence. However, classification criteria are often used in clinical practice [23], and the ACR/EULAR classification criteria showed high sensitivity (>95%) for SS (based on expert opinion as gold standard) when applied retrospectively to a Japanese cohort and prospectively to a Dutch cohort [24, 25]. Together, these data suggest the classification criteria are reasonable to guide clinical workup as many adults with SS will indeed meet the criteria.
The high sensitivity of these criteria in adults along with the low proportion of children meeting criteria suggests other factors may contribute to poor sensitivity. One possibility is that the criteria rely too heavily on evidence of gland dysfunction, which requires time to develop and, thus, is less evident during childhood. This is supported by the decreased prevalence of dryness in children with SS compared with adults and the increase in sicca symptoms over time following diagnosis [3, 20, 26, 27]. In our study, when combining number of tests performed for gland function (Schirmer’s test, UWS) and ocular surface damage (OSS), only 80 (34%) of 233 individual tests were abnormal. In contrast, evidence of focal sialadenitis (focus score ≥1 focus/4 mm2) or positive anti-SSA/Ro antibodies occurred more frequently (287 of 423, 68%). Considering SS can be an indolent process, if we include children with any focal sialadenitis (focus score >0 foci/4 mm2), this proportion rises (313 of 423, 74%). This makes conceptual sense if the lymphocytic infiltration and production of autoantibodies are early features in the immunopathogenesis with subsequent gland dysfunction and end-organ damage. Similarly, if children represent an early stage in the development of SS, then the presence of any focal sialadenitis less than the currently defined cutoff may be sufficient to support diagnosis. A consideration can also be made for parotid biopsy, which may be more sensitive than MSG biopsy in children [28].
We explored clinical and laboratory predictors for meeting classification criteria and having positive MSG biopsy to aid clinicians in diagnosis. Hypergammaglobulinemia, persistent fevers and reporting dry eyes were associated with meeting criteria. Why these features were associated with criteria is not clear, but hypergammaglobulinemia and persistent fevers likely reflect a systemic inflammatory state. Only positive anti-SSA/Ro was predictive for positive MSG biopsy. Combinations of clinical, laboratory and diagnostic test results were neither sensitive nor specific for predicting meeting classification criteria (Supplementary Table S2, available at Rheumatology online).
In addition to relying on evidence of early inflammation or immunological features reliably detectable early in disease, additional criteria items are necessary to improve diagnostic sensitivity in children. SGUS is a non-invasive modality that is feasible in children and was associated with serologic positivity [13]. In adults, abnormal SGUS features were associated with positive MSG biopsy [29]. Further study is needed to clarify how specific these changes are for SS in children and what threshold yields sufficient discriminate validity to establish the diagnosis without biopsy. Because autoantibodies were detected decades before diagnosis in adults with SS [2] and the majority of children in our study have positive anti-SSA/Ro antibodies [3, 13, 27, 30], the combination of salivary gland inflammation (clinical parotitis, typical SGUS changes consistent with inflammation and histopathology) and positive autoantibody may be sufficient to diagnose SS in a child. Interestingly, in a small group of our children who reported parotitis and had a positive anti-SSA/Ro antibody, an abnormal SGUS, and any inflammation on their MSG, 9 out of 10 (90%) met ACR/EULAR criteria.
Developing paediatric-specific criteria is essential to identify children with SS prior to gland dysfunction. Such criteria may also be more applicable for diagnosing or classifying adults earlier in their course of disease and in diagnosing individuals lacking SS-specific serologies or those presenting with primarily extraglandular manifestations for whom the current criteria may be less sensitive [31].
Our study strengths include a large population of children diagnosed with SS from eight countries on five continents producing a robust generalizable dataset. A consequence of the observational nature is missing data, though this is not unique to our study. Open communication with investigators allowed for clarity when discrepancies were found in reported data. Also, as is common among rheumatic diseases, the lack of an objective standard for diagnosis of SS in children requires the use of expert opinion as our gold standard for inclusion. This also leads to the evaluation of children being performed in a non-standardized fashion. Lastly, there may be referral bias towards children with higher disease severity. Children with mild dryness may not seek medical attention resulting in a lower perceived prevalence of dryness and possibly the disease.
In summary, some children with SS meet classification criteria designed for use in adults with SS. Recognition of recurrent or persistent parotitis as a manifestation of the disease in children is paramount. Symptomatic exocrine gland dysfunction may not be apparent and its absence should not be falsely reassuring against the presence of disease. Diagnostic testing to evaluate for evidence of exocrine gland inflammation, serologic evidence for autoimmunity, and evidence of gland dysfunction or end-organ damage should be considered in the workup and ideally should be performed and scored by experienced specialists to ensure adequate testing and scoring. However, many children will not meet classification criteria designed for use in adults, and this should not preclude the diagnosis. Additional studies are needed to define child-specific normative values for these tests and additional criteria items should be considered and evaluated prospectively to define the best set of criteria items for classification of SS in children. Establishing paediatric-specific criteria is essential for use in future research studies as well as for the diagnosis of SS in children.
Supplementary Material
Acknowledgements
The authors wish to acknowledge CARRA and the ongoing Arthritis Foundation financial support of CARRA, Drs Daniel S Hammenfors and Malin V Jonsson (University of Bergen, Norway) for unpublished details from their published study.
Funding: No specific funding was received from any funding agency in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure section: The authors have declared no conflicts of interest.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1.Vivino FB, Bunya VY, Massaro-Giordano G. et al. Sjogren’s syndrome: an update on disease pathogenesis, clinical manifestations and treatment. Clinical Immunol 2019;203:81–121. [DOI] [PubMed] [Google Scholar]
- 2.Theander E, Jonsson R, Sjostrom B. et al. Prediction of Sjogren’s syndrome years before diagnosis and identification of patients with early onset and severe disease course by autoantibody profiling. Arthritis Rheumatol 2015;67:2427–36. [DOI] [PubMed] [Google Scholar]
- 3.Yokogawa N, Lieberman SM, Sherry DD, Vivino FB.. Features of childhood Sjogren’s syndrome in comparison to adult Sjogren’s syndrome: considerations in establishing child-specific diagnostic criteria. Clin Exp Rheumatol 2016;34:343–51. [PubMed] [Google Scholar]
- 4.O'Neill EM.Sjogren’s syndrome with onset at 10 years of age. Proc R Soc Med 1965;58:689–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rucker CW.KERATITIS SICCA: report of a case. Arch Ophthalmol 1938;19:584–5. [Google Scholar]
- 6.Tomiita M, Saito K, Kohno Y. et al. The clinical features of Sjogren’s syndrome in Japanese children. Acta Paediatr Jpn 1997;39:268–72. [DOI] [PubMed] [Google Scholar]
- 7.Virdee S, Greenan-Barrett J, Ciurtin C.. A systematic review of primary Sjogren’s syndrome in male and paediatric populations. Clin Rheumatol 2017;36:2225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiboski CH, Shiboski SC, Seror R. et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis 2017;76:9–16. [DOI] [PubMed] [Google Scholar]
- 9.Yokogawa N, Lieberman SM, Alawi F. et al. Comparison of labial minor salivary gland biopsies from childhood Sjogren syndrome and age-matched controls. J Rheumatol 2014;41:1178–82. [DOI] [PubMed] [Google Scholar]
- 10.Bartunkova J, Sediva A, Vencovsky J, Tesar V.. Primary Sjogren’s syndrome in children and adolescents: proposal for diagnostic criteria. Clin Exp Rheumatol 1999;17:381–6. [PubMed] [Google Scholar]
- 11.Schuetz C, Prieur AM, Quartier P.. Sicca syndrome and salivary gland infiltration in children with autoimmune disorders: when can we diagnose Sjogren syndrome? Clin Exp Rheumatol 2010;28:434–9. [PubMed] [Google Scholar]
- 12.Houghton K, Malleson P, Cabral D, Petty R, Tucker L.. Primary Sjogren’s syndrome in children and adolescents: are proposed diagnostic criteria applicable? J Rheumatol 2005;32:2225–32. [PubMed] [Google Scholar]
- 13.Hammenfors DS, Valim V, Bica B. et al. Juvenile Sjogren’s syndrome: clinical characteristics with focus on salivary gland ultrasonography. Arthritis Care Res 2020;72:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lieberman SM, Lu A, McGill MM.. Oral lesions as presenting feature of childhood Sjogren syndrome. Int J Pediatr Otorhinolaryngol 2018;113:303–4. [DOI] [PubMed] [Google Scholar]
- 15.Susic G, Stojanovic R, Milic V. et al. Juvenile Sjogren’s syndrome: case report. Srp Arh Celok Lek 2013;141:228–31. []. [DOI] [PubMed] [Google Scholar]
- 16.Schiffer BL, Stern SM, Park AH.. Sjogren’s syndrome in children with recurrent parotitis. Int J Pediatr Otorhinolaryngol 2020;129:109768. [DOI] [PubMed] [Google Scholar]
- 17.Hammett EK, Fernandez-Carbonell C, Crayne C. et al. Adolescent Sjogren’s syndrome presenting as psychosis: a case series. Pediatr Rheumatol Online J 2020;18:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saad Magalhães C, de Souza Medeiros PB, Oliveira-Sato J, Custódio-Domingues MA.. Clinical presentation and salivary gland histopathology of paediatric primary Sjogren’s syndrome. Clin Exp Rheumatol 2011;29:589–93. [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R. et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjerrum K, Prause JU.. Primary Sjogren’s syndrome: a subjective description of the disease. Clin Exp Rheumatol 1990;8:283–8. [PubMed] [Google Scholar]
- 21.Aggarwal R, Ringold S, Khanna D. et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res 2015;67:891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitali C, Del Papa N.. Classification and diagnostic criteria in Sjogren’s syndrome: a long-standing and still open controversy. Ann Rheum Dis 2017;76:1953–4. [DOI] [PubMed] [Google Scholar]
- 23.June RR, Aggarwal R.. The use and abuse of diagnostic/classification criteria. Best Pract Res Clin Rheumatol 2014;28:921–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuboi H, Hagiwara S, Asashima H. et al. Comparison of performance of the 2016 ACR-EULAR classification criteria for primary Sjogren’s syndrome with other sets of criteria in Japanese patients. Ann Rheum Dis 2017;76:1980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Nimwegen JF, van Ginkel MS, Arends S. et al. Validation of the ACR-EULAR criteria for primary Sjogren’s syndrome in a Dutch prospective diagnostic cohort. Rheumatology 2018;57:818–25. [DOI] [PubMed] [Google Scholar]
- 26.Cimaz R, Casadei A, Rose C. et al. Primary Sjogren syndrome in the paediatric age: a multicentre survey. Eur J Pediatr 2003;162:661–5. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi I, Okura Y, Ueki M. et al. Evaluation of systemic activity of pediatric primary Sjogren's syndrome by EULAR Sjogren's syndrome disease activity index (ESSDAI). Mod Rheumatol 2019;29:130–3. [DOI] [PubMed] [Google Scholar]
- 28.McGuirt WF, Whang C, Moreland W.. The role of parotid biopsy in the diagnosis of pediatric Sjogren syndrome. Arch Otolaryngol Head Neck Surg 2002;128:1279–81. [DOI] [PubMed] [Google Scholar]
- 29.Milic VD, Petrovic RR, Boricic IV. et al. Diagnostic value of salivary gland ultrasonographic scoring system in primary Sjogren’s syndrome: a comparison with scintigraphy and biopsy. J Rheumatol 2009;36:1495–500. [DOI] [PubMed] [Google Scholar]
- 30.Means C, Aldape MA, King E.. Pediatric primary Sjogren syndrome presenting with bilateral ranulas: a case report and systematic review of the literature. Int J Pediatr Otorhinolaryngol 2017;101:11–9. [DOI] [PubMed] [Google Scholar]
- 31.Tezcan ME, Kucuk H, Goker B.. American College of Rheumatology/European League Against Rheumatism Sjogren’s Syndrome Classification Criteria may not be adequate for extraglandular disease and necessitate defining “Seronegative Sjogren's Syndrome”: Comment on the Article by Shiboski et al. Arthritis Rheumatol 2017;69:1341–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.