Abstract
Objectives
Serum anti-dsDNA and anti-nucleosome IgGs have been proposed as signatures for SLE and LN in limited numbers of patients. We sought to show higher sensitivity and specificity of the same antibodies with the IgG2 isotype and included IgG2 antibodies vs specific intracellular antigens in the analysis.
Methods
A total of 1052 SLE patients with (n = 479) and without (n = 573) LN, recruited at different times from the beginning of symptoms, were included in the study. Patients with primary APS (PAPS, n = 24), RA (RA, n = 24) and UCTD (UCTD, n = 96) were analysed for comparison. Anti-nucleosome (dsDNA, Histone2A, Histone3), anti-intracellular antigens (ENO1), anti-annexin A1 and anti-C1q IgG2 were determined by non‐commercial techniques.
Results
The presence in the serum of the IgG2 panel was highly discriminatory for SLE/LN vs healthy subjects. Serum levels of anti-dsDNA and anti-C1q IgG2 were more sensitive than those of IgGs (Farr radioimmunoassay/commercial assays) in identifying SLE patients at low–medium increments. Of more importance, serum positivity for anti-ENO1 and anti-H2A IgG2 discriminated between LN and SLE (ROC T0–12 months), and high levels at T0–1 month were detected in 63% and 67%, respectively, of LN, vs 3% and 3%, respectively, of SLE patients; serum positivity for each of these was correlated with high SLEDAI values. Minor differences existed between LN/SLE and the other rheumatologic conditions.
Conclusion
Nephritogenic IgG2 antibodies represent a specific signature of SLE/LN, with a few overlaps with other rheumatologic conditions. High levels of anti-ENO1 and anti-H2A IgG2 correlated with SLE activity indexes and were discriminatory between SLE patients limited to the renal complication and other SLE patients.
Trial registration
The Zeus study was registered at https://clinicaltrials.gov, NCT02403115.
Keywords: LN, SLE, biomarkers, anti-ENO1 antibodies, anti-Histone 2A antibodies, anti-C1q antibodies
Rheumatology key messages
Our study demonstrated higher sensitivity of anti-dsDNA IgG2 than classical IgGs antibodies for marginal SLE diagnosis.
Positivity for the whole panel of circulating IgG2 antibodies is a specific signature of SLE/LN and distinguishes them from healthy people.
High anti-ENO1 and anti-H2A IgG2 serum levels represent the best discriminatory element for distinguishing between LN and SLE.
Introduction
SLE is one of the most frequent autoimmune conditions, with an incidence of 40–200 cases per 100 000 individuals [1]. Its presentation and clinical course are usually characterized by high variability and heterogeneity in severity of organ involvement, ranging from a rash or mild arthritis to severe target organ damage, such as in LN [2, 3]. Early detection of systemic complications, including LN, is fundamental for allowing a prompt start of treatment able to improve systemic and organ outcome for patients affected [4, 5]. The detection of organ autoantibodies able to integrate the classical markers of SLE activation (like complement components C3 and C4) represented a real challenge and has been a major focus of research activity, because antibodies are directly involved in the pathogenesis of the organ damage [3, 6–8]. Moreover, in clinical practice, detection of circulating autoantibodies offers considerable advantages, in terms of cost-effectiveness, non-invasivity and reproducibility for follow-up monitoring, when compared with other molecular diagnostic techniques, [9, 10].
Historically, the pathological and diagnostic role of anti-dsDNA and anti nucleosome IgG autoantibodies (including antibodies against specific histones) has been widely reported in the literature (for a review see [11]). Since their discovery in 1957, anti-dsDNA antibodies have been considered one of the most sensitive markers for the diagnosis of SLE [12]. Moreover, serum leels of anti-dsDNA antibodies have always represented a fundamental criterion for scores measuring SLE activity [13–15], including the SLEDAI score [16]. In addition, the predominant idea is that high levels of anti-dsDNA IgGs, and not just serum positivity, may predict SLE activity [17–20].
However, several technical issues (related to differences between the various assays commonly used for detection of serum anti-dsDNA antibodies) may limit the clinical significance of anti-dsDNA IgGs. In particular, the radioimmunoassay Farr test mainly detects high-avidity antibodies, but fails to distinguish between IgG and IgM; the Crithidia luciliae test, based on immunofluorescence, is highly specific, but not quantitative, for high-affinity antibodies; on the other hand, ELISAs allow a sensitive and a quantitative, but not specific, detection of these autoantibodies. Indeed, in previous studies on large cohorts of SLE patients, comparison of the various assays for testing serum anti-dsDNA antibodies [21–23] indicated significant variability in the results provided by the different assays, limiting valid comparison of the results of various studies.
In addition to the technical concerns, using detection of serum anti-dsDNA antibodies as a diagnostic tool for SLE presents further conceptual issues, which are still far from being completely resolved: the specific isotype of the antibodies, not considered in common commercial assays, may be important. According to previous findings [24, 25], IgG2 is the prevalent isotype of circulating anti-dsDNA and of anti-histone2/anti-histone3 antibodies [26]. This finding may lead to a better understanding of the pathogenesis of renal lesions, since IgG2 is the main isotype of the antibodies detected in renal and serum samples of patients with LN [24–28].
Materials and methods
Patients
In total, 1052 SLE/LN patients were included in the study; they were recruited within the framework of the nationwide collaborative Zeus study (https://clinicaltrials.gov study number: NCT02403115). The database and collected samples are located at the Giannina Gaslini Institute of Genoa (I) [29] (Table 1).
Table 1.
Clinical data relative to patients with LN and SLE, respectively
| LN | SLE | 0–1 month |
2–12 months |
13–24 months |
25–48 months |
49–96 months |
>96 months |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LN | SLE | LN | SLE | LN | SLE | LN | SLE | LN | SLE | LN | SLE | |||
| Patient | 479 | 573 | 168 | 31 | 38 | 36 | 32 | 32 | 30 | 50 | 69 | 85 | 142 | 339 |
| Sex M n (%) | 71 (15) | 51 (9) | 25 (15) | 3 (10) | 7 (18) | 8 (22) | 7 (22) | 7 (22) | 10 (30) | 5 (10) | 10 (14) | 5 (6) | 12 ( | 23 (7) |
| Sex F n (%) | 408 (85) | 522 (91) | 143 (85) | 28 (90) | 31 (82) | 28 (78) | 25 (78) | 25 (78) | 20 (70%) | 45 (90) | 59 (86) | 80 (94) | 130 | 316 (93) |
| Age,a year | 39 (28–49) | 42 (31–54) | 32 (22–43 | 24 (13–37) | 34 (23–47) | 29 (17–48) | 39 (28–54) | 39 (24–53) | 37 (32–44) | 28 (20–47) | 39 (28–48) | 36 (27–45) | 45 (38–54) | 47 (38–57) |
| anti-dsDNA ratio a | 1 (0.6–2.8) | 1 (0.5–2) | 17 (4–28) | 11 (3–106) | 1.8 (1–6.9) | 1.8 (0.6–14) | 0.6 (0.3–1.1) | 0.8 (0.3–1.2) | 1.9 (0.8–6.9) | 1.2 (0.5–5) | 1 (0.6–2.3) | 1.4 (0.7–2.1) | 1 (0.7–1.8) | 0.9 (0.5–1.8) |
| SLEDAI a | 7 (6–10) | 2 (0–5) | 10 (7–11) | 6 (5–8) | 9 (7–11) | 5 (2.5–6) | 6 (6–8) | 4 (2–7) | 8 (6–9) | 3 (2–5) | 6 (5–8) | 2 (0–5) | 6 (4–9) | 3 (1–5) |
| C3 a | 81 (60–101) | 91 (76–107) | 65 (46–85) | 61 (43–86) | 78 (54–95) | 85 (73–97) | 87 (62–109) | 88 (60–124) | 89 (66–110) | 80 (71–105) | 87 (70–116) | 96 (80–104) | 93 (77–107) | 93 (78–107) |
| C4 a | 13 (7–20) | 14 (10–19) | 9 (5–15) | 7 (5–13) | 15 (10–15) | 13 (9–18) | 13 (10–23) | 13 (7–22) | 17 (10–23) | 14 (8–22) | 16 (11–25) | 15 (10–19) | 16 (10–21) | 15 (11–20) |
| Histological class | ||||||||||||||
| I n (%) | 4 (0.8) | 00 | 00 | 1 (3) | 1 (3) | 1 (1) | 1 (1) | |||||||
| II | 23 (4.8) | 10 (6) | 1 (3) | 00 | 3 (10) | 4 (6) | 5 (4) | |||||||
| III | 71 (15) | 26 (15) | 5 (13) | 6 (19) | 3 (10) | 6 (9) | 25 (18) | |||||||
| IV | 170 (35) | 58 (35) | 14 (37) | 10 (31) | 14 (47) | 25 (36) | 49 (35) | |||||||
| V | 120 (25) | 60 (36) | 8 (21) | 7 (22) | 5 (17) | 17 (25) | 23 (16) | |||||||
| VI | 2 (0.4) | 0 | 00 | 00 | 1 (3) | 00 | 1 (1) | |||||||
| nd | ||||||||||||||
| Proteinuriaa g/die | 1 (0.3–2.8) | 0.1 (0.06–0.14) | 2.4 (1.2–4.3) | 0.11 (0.1–0.15) | 1.4 (0.8–2.9) | 0.1 (0.05–0.1) | 0.3 (0.1–1.2) | 0.1 (0.1–0.15) | 0.4 (0.2–0.6) | 0.1 (0.1–0.1) | 0.2 (0.1–0.6) | 0.1 (0.1–0.1) | 0.3 (0.1–0.8) | 0.1 (0.02–0.1) |
| eGFRa ml/min/1.73 m2 | 97 (69–116) | 103 (91–117) | 94 (66–117) | 126 (110–139) | 94 (83–122) | 100 (76–128) | 102 (86–109) | 116 (99–129) | 106 (73–116) | 105 (97–126) | 106 (82–123) | 109 (96–117) | 94 (63–108) | 101 (88–111) |
| Therapy | ||||||||||||||
| Steroids | 341 (63) | 363 (63) | 119 (71) | 14 (45) | 32 (84) | 10 (27) | 22 (69) | 6 (19) | 25 (83) | 40 (80) | 50 (72) | 59 (69) | 93 (65) | 234 (69) |
| CYC/CYA | 56 (12) | 70 (12) | 28 (17) | 00 | 4 (10) | 8 (22) | 1 (3) | 1 (3) | 4 (13) | 40 (80) | 12 (17) | 4 (5) | 7 (5) | 17 (5) |
| MMF/AZA | 176 (37) | 297 (52) | 24 (14) | 1 (3) | 17 (45) | 00 | 12 (9) | 4 (12) | 19 (63) | 21 (42) | 40 (58) | 24 (28) | 64 (45) | 247 (73) |
| Plaquenil | 264 (55) | 363 (63) | 57 (34) | 21 (68) | 20 (53) | 20 (56) | 21 (65) | 21 (66) | 20 (67) | 32 (64) | 44 (64) | 55 (65) | 102 (72) | 214 (63) |
Both groups were subdivided into six groups according to the time (months) elapsed between the onset of disease and recruitment.
Data are reported as median and interquartile range.
eGFR, estimated glomerular filtration rate; CSA, cyclosporin; CYA, cyclophosphamide; CYC, cyclophosphamide.
Inclusion criteria
Inclusion criteria were age between 18 and 55 years, any sex and the availability of informed consent. Diagnosis of SLE was made according to the ACR systemic lupus classification criteria as revised by the SLICC [15]. Serum autoimmunity was evaluated utilizing commercial assays; levels of anti-dsDNA antibodies were determined by different methodologies according to the assay used in the various hospitals: >50% of patients were studied by Farr radioimmunoassay (Farr assay, Kodak Clinical Diagnostics, Amersham, UK), and a smaller proportion was tested by the Crithidia luciliae indirect immunofluorescence test. To obtain an overall view of the status of the anti-dsDNA antibodies, the ratio of any level to the upper limit of normal was calculated (anti-dsDNA ratio). A non‐commercial assay was utilized for anti-C1q IgGs, as has already been described [27].
The cases of LN (stage I–VI according to WHO classification) were recruited from among the large cohort of patients with SLE who tested positive for haematuria, proteinuria, and/or worsening of renal function evaluated by the CPK-EPI formula. The histological diagnosis and classification of LN was based on typical renal lesions as analysed by immunofluorescence and classical histology staining. Further details on the staging and characterization of renal histological samples are reported in Part 2 of the study.
Exclusion criteria
Exclusion criteria were severe infections, malignancies, positivity for chronic hepatitis B virus (HBV) or hepatitis C virus (HCV), breast-feeding or pregnancy.
Other rheumatologic diseases
Patients with RA (RA, n = 24), UCTD (UCTD, n = 96) and primary APS (PAPS, n = 24) were consecutively enrolled in the Rheumatology Unit of ASST Spedali Civili of Brescia, Italy. Diagnoses were made according to the current classification criteria for these diseases [30–32].
Groups of controls
For the control groups, 182 healthy subjects were recruited from among the hospital staff.
Ethical approval
Before the initiation of the study, we obtained written approval of the protocol from the local Independent Ethics Committee (Comitato Etico Regione Liguria) on 24 October 2014 and from the Italian Drug Agency (Agenzia Italiana del Farmaco, AIFA). The study was registered at https://clinicaltrials.gov (study number: NCT02403115).
Antibody assays
Details on materials utilized in this study are reported in Supplementary Data S1, available at Rheumatology online.
ELISA assay
Levels of anti-ENO1, anti-AnnexinA1, anti-SOD, anti-C1q, anti-H2A and anti-H3 histones were determined utilizing non‐commercial ELISA assays. Briefly, each recombinant protein was coated overnight at 4°C in 96 MaxiSorp nunc immuno plate wells (ThermoFisher Scientific, MA, USA) and blocked with 3% w/v BSA in PBS. Serum samples (100 μl) diluted 1:50 in 3% w/v BSA and PBS-Tween 20 0.05% v/v (PBS-T) (with the exception of the anti-C1q assay, see below) was added; the sample was incubated for 4 h at room temperature and then at 4°C overnight. The amount of human recombinant protein utilized in the ELISA was 20 ng/well for ENO1, superoxide dismutase, histone H2A and H3, 5 ng/well for annexin A1 and 1 µg/well for complement C1q.
After three washes in PBS-T, HRP-conjugated rabbit anti-human IgG2 (Clone: HP6014, InVitrogen Corporation, CA, USA) diluted 1:2.000 in 1% w/v BSA and PBS-T was added; the sample was incubated at room temperature for 4 h. After three washes in PBS-T, colour reactions were developed in 100 μl of TMB Peroxidase EIA substrate kit (Bio-Rad, CA, USA) and stopped using 0.45 M H2SO4. Absorbance at 450 nm was read in an ELISA iMark microplate reader (Bio-Rad, CA, USA). Results were expressed as Relative Intensity value (RU/ml), given the lack of WHO international standards for each antibody [33].
In the assay for anti-C1q antibodies, serum samples were diluted 1:25 in 1% v/v FCS, PBS-T and 1 M NaCl, incubated at 37°C for 1 h and then processed following the indications above.
Dot-blot assay
Anti-dsDNA IgG2 serum levels were determined using a non‐commercial Dot-blot assay. dsDNA was loaded onto nitrocellulose membrane using Bio-Dot apparatus (Bio-Rad, CA, USA). After membrane blocking in 3% BSA in PBS, 100 μl of diluted serum (1:50) was added per well; the sample was incubated overnight at 4°C. After three washings with PBST, membranes were incubated 4 h with rabbit anti-human IgG2 (Clone: HP6014, InVitrogen Corporation, CA, USA) diluted 1:2.000 in PBST and 1% w/v BSA and then washed three times with PBS-T; chemiluminescence was used for detection. Images were acquired with the ChemiDoc Touch Imaging System (Bio-Rad, CA, USA).
Commercial assays for anti-dsDNA and anti-C1q
Anti-dsDNA IgGs levels were determined by the commercial Farr radioimmunoassay (Kodak Clinical Diagnostics); anti-C1q IgGs were determined by a commercial ELISA (Inova Diagnostic, Barcelona, Spain).
Normal limits
Normal limits for all the tests above were calculated from ROC curves; the cut-off represented the value that minimized the geometric distance between 100% sensitivity and 100% specificity on the ROC curves [34]. Our results confirmed what already has already been reported by Bruschi and colleagues [26].
High and low levels
Low levels corresponded to the values between the limit of normality and the median. High levels corresponded to levels higher than the median.
Statistics analysis
Datasets were compared using Mann–Whitney or Kruskal–Wallis tests for two or more unpaired samples, respectively. Comparisons between the percentage of positive vs negative results for patients with LN and patients with SLE for each antibody, and of high titres at each point of the cross-sectional study, were done using contingency 2 × 2 tables. Fisher’s exact test was used to determine the statistical significance.
Heat maps and volcano plots were utilized to highlight differences between the serum levels of each antibody in different groups of patients. In the heat maps, the serum antibody levels, after Z-score normalization, are presented with a pseudocolour scale, with red, white and blue indicating, respectively, positive, negative and equal levels; the dendogram (at the top left) displays the result of unsupervised hierarchical cluster analysis. Heat maps were also utilized to describe correlations between antibody levels and laboratory parameters. In this case, a correlogram based on Spearman’s coefficient represents (with a pseudocolour scale from red, +1, positive; to blue, –1, negative; and to white, 0, null), respectively, the correlation between two parameters.
Volcano plots were used to represent differences in the levels of each antibody between the different groups. The log2 of the difference in each auto-antibody level between two conditions (e.g. LN vs SLE) is shown on the x-axis and the –log10P-value of the difference obtained by a non-parametric Mann–Whitney U test is shown on the y-axis.
Test performance, in terms of ability of the test to identify true positive subjects (sensitivity) and ability of the test to identify true negative subjects (specificity), was evaluated for each parameter by the Receiving Operating Characteristic (ROC). The proportion of patients correctly identified by ROC is proportional to the area under the curve (AUC), where accuracy is absent for AUC = 0.5, poor for 0.5 <AUC≤ 0.7, moderate for 0.7 <AUC≤ 0.9, and high for 0.9 <AUC< 1. A test is perfect for AUC = 1. In the ROC curve, the Youden’s index [35] was utilized to identify the best cut-off value that maximized the difference between true positive subjects and false positive subjects. Results were considered significant at two-tailed P-values ≤ 0.05. All analyses were performed using software package R last version 4.0.3 available at the time of experiments.
Results
Clinical features of patient subgroups
In all, 1052 SLE/LN patients were included in the study; they were subdivided into six subgroups for SLE and six subgroups for LN on the basis of the time from diagnosis to recruitment (i.e. 0–1 month, 2–12 months, 13–24 months, 25–48 months, 49–96 months and >96 months). Major clinical features are reported in Table 1: sex prevalence, disease activity index, immunological and renal parameters and therapies are presented for each group included in the cross-sectional analysis.
New IgG2 antibodies: correlation with commercial assays
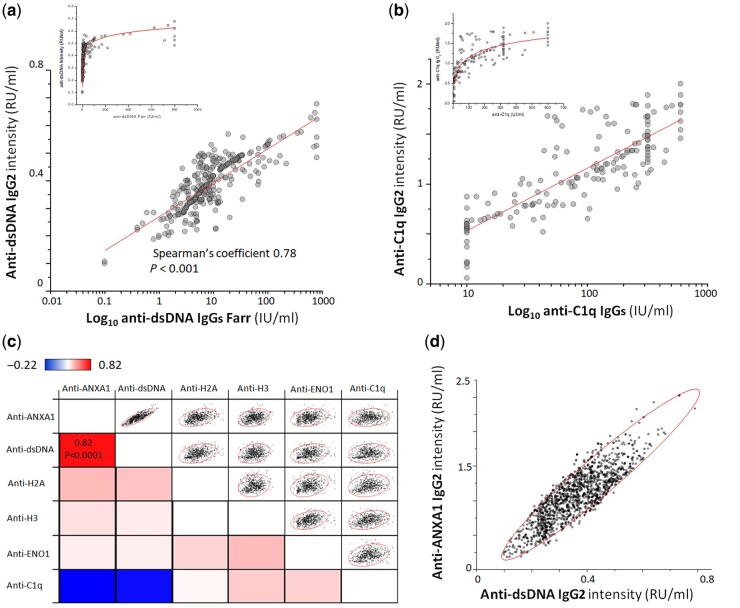
The new IgG2 panel here presented includes autoantibodies against classical nucleosome components, i.e. anti-DNA, anti-Histone2A, anti-Histone3, as well as autoantibodies against circulating proteins, i.e. anti-C1q and against an intracellular (podocyte) antigen, i.e. anti-ENO1; anti-ANXA1 antibodies are directed both against the circulating and the intracellular protein [36]. All autoantibodies were detected by home-made ELISA, except for levels of anti-dsDNA, which were determined by non‐commercial dot blot. Percentage of positivity in both LN and SLE patients was very high for all, except for anti-C1q (Supplementary Fig. S1, available at Rheumatology online). The correlation between serum anti-dsDNA and anti-C1q IgG2 levels and anti-dsDNA and anti-C1q IgGs, which are commonly used for the diagnosis of SLE, are reported in Fig. 1a. In both cases, the correlation between anti-dsDNA IgG2 (our assay) and anti-dsDNA IgGs (commercial Farr radioimmunoassay) resulted in an hyperbole graph, which became linear by applying a log10-scale to the values obtained from the commercial assay. This means that anti-dsDNA IgG2 tests are very sensitive up to an intensity of 0.5 RU/ml, over a range in which the Farr test results negative. The same occurred when we compared the assay for anti-C1q IgG2 (our assay) with the assay for anti-C1q IgGs (commercial assay), in which case the IgG2 test was more sensitive up to 1 RU/ml (Fig. 1b). Positivity limits and limits for high and low levels are reported in Supplementary Table S1, available at Rheumatology online.
Fig. 1.
Correlation between anti-dsDNA, anti-C1q IgG2 and IgGs levels (commercial kits)
(a) Correlation between circulating levels of anti-dsDNA IgG2 as determined by our assay and anti-dsDNA as determined by the Farr assay (Farr assay, Kodak Clinical Diagnostics) (levels on the x-xis are presented as log10 values); (b) correlation between circulating levels of anti-C1q IgG2 and anti-C1q IgGs, as determined by a non‐commercial assay [27] (levels on the x-axis are reported as log10 values); (c) heat map showing the correlation between the levels of each circulating IgG2 antibody, for all categories of patients considered together [the correlogram, based on Spearman’s coefficient, uses a pseudocolour scale (from red, +1, positive; to blue, –1, negative; and to white, 0, null), to represent the correlation between two antibodies]; (d) graphic representation of the correlation between anti-dsDNA and anti-Annexin A1 IgG2 serum levels (R = 0.82, P<0.0001).
Circulating antibody levels: cross-sectional analysis
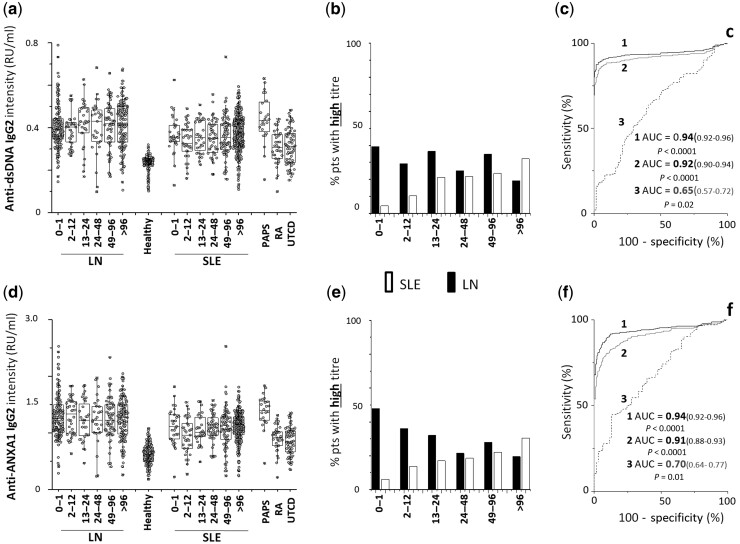
Serum levels of all components of the antibody panel, with the exception of anti-C1q antibodies, were significantly higher in patients with LN and patients with SLE than in healthy people (Figs 2–4). There were, however, peculiarities for each antibody, which are described below.
Fig. 2.
Circulating levels of anti-dsDNA and anti-Annexin A1 IgG2
(a) Circulating levels of anti-dsDNA and (d) of anti-Annexin A1 IgG2 in patients with LN (n = 479) and patients with SLE (n = 573) subdivided in six subgroups according to the time elapsed from the onset of the disease (0–1 months, 1–12 months, 12–24 months, 24–48 months, 48–96 months, >96 months); patients with RA (n = 24), PAPS (n = 24) and UCTD (n = 96) were included as representative of other rheumatologic conditions. (b, e) Percentage of patients with LN (black) and of patients with SLE (white) with high levels divided into subgroups according to the time from disease onset at recruitment; (c, f) ROC curves for difference in anti-dsDNA and anti-Annexin A1 IgG2 between patients with LN and healthy controls (1), patients with SLE and healthy controls (2) and patients with LNT0–12vs patients with SLET0–12 (3). In all cases, antibodies were of the IgG2 isotype, and levels were calculated as Relative Intensity value (RU/ml), given the absence of WHO international standards [33]. PAPS = primary APS.
Anti-dsDNA
IgG2 serum levels were much higher in LN/SLE patients compared with healthy people (AUC = 0.94, 0.92 respectively), with minimal differences between LN and SLE (AUC = 0.65 for T0–12m) (Fig. 2). The percentage of patients positive and negative at each time point of the cross-sectional study was similar for LN and SLE (Supplementary Table S2 and Supplementary Fig. S1 , available at Rheumatology online), whereas the percentage of patients with high levels of IgG2 was 39% in LN vs 4% in SLE at T 0–1 months and was markedly modified in those patients with longer periods of disease (Fig. 2a).
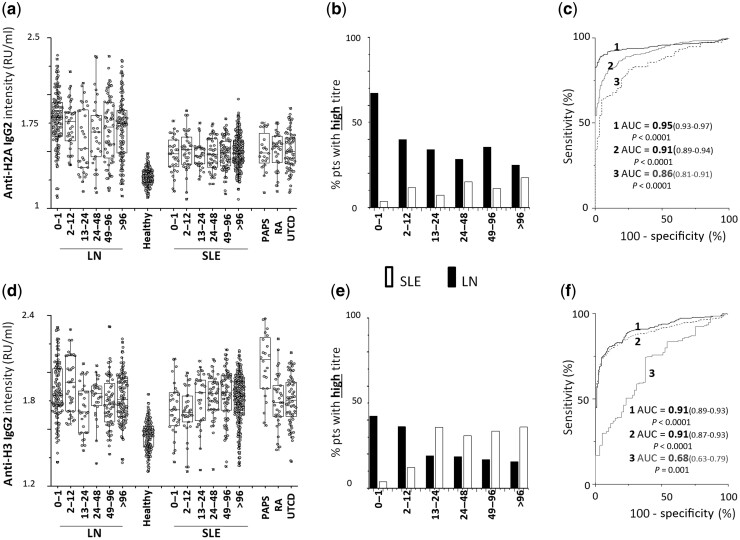
Anti-Histone2A
IgG2 serum levels were higher in LN/SLE patients than in healthy controls (AUC = 0.95, 0.91 respectively), with a significant difference between LN and SLE (AUC = 0.86 for T0–12months) (Fig. 3). The percentage of patients positive and negative at each time point of the cross-sectional study was higher in LN than in SLE (Supplementary Table S2 and Supplementary Fig. S1, available at Rheumatology online). The percentage of patients with high levels was much higher in LN (68%) than in SLE patients (2%) at T0–1months, and it steadily decreased in patients recruited after the onset of LN and who received specific therapies (Fig. 3b).
Fig. 3.
Circulating levels of anti-Histone 2 A and anti-Histone 3 IgG2
(a) Circulating levels of anti-Histone 2A and (d) anti-Histone 3 IgG2 in patients with LN (n = 479) and patients with SLE (n = 573) subdivided into six subgroups according to the time elapsed from the onset of the disease (0–1 months, 1–12 months, 12–24 months, 24–48 months, 48–96 months, >96 months); patients with RA (n = 24), PAPS (n = 24) and UCTD (n = 96) were included as representative of other rheumatologic conditions. (b, e) Percentage of LN (black) and of SLE (white) patients with high levels divided into subgroups according to the time from disease onset at recruitment; (c, f) ROC curves for difference in anti-Histone 2A and anti-Histone H3 IgG2 between patients with LN and healthy controls (1), patients with SLE and healthy controls (2) and patients with LNT0–12vs patients with SLET0–12 (3). In all cases, antibodies were of the IgG2 isotype and levels were calculated as Relative Intensity value (RU/ml), given the absence of WHO international standards [33].
Anti-Histone 3
IgG2 serum levels were overall higher in SLE/LN patients compared with healthy people (AUC = 0.91, 0.91, respectively). The difference between LN and SLE was low (AUC = 0,68 for T0–12months) (Fig. 3). The percentage of patients with high levels was remarkable at T0–1months, and increased in SLE from T13–24months (Fig. 3b).
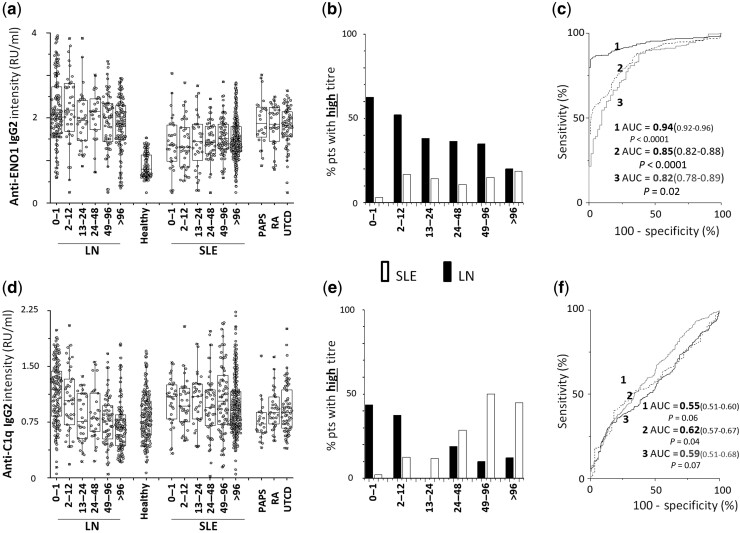
Anti- ENO1
IgG2 serum levels were higher in LN and, to a lesser extent, in SLE patients compared with healthy subjects (AUC = 0.94, 0.85, respectively); the difference between LN and SLE was significant (AUC = 0.82 forT0–12months) (Fig. 4). The percentage of patients positive and negative at each time point of the cross-sectional study was higher in LN than in SLE (Supplementary Table S2 and Supplementary Figure S1, available at Rheumatology online). The percentage of patients with high levels was much higher in LN (68%) than in SLE patients (2%) at T0–1month, and it steadily decreased in patients recruited after the onset of LN (Fig. 4b).
Fig. 4.
Circulating levels of anti-ENO1 and anti-C1q IgG2
(a) Circulating levels of anti-ENO1 IgG2 and (d) of anti-C1q IgG2 in patients with LN (n = 479) and SLE (n = 573) subdivided into six subgroups according to the time elapsed from the onset of the disease (0–1 months, 1–12 months , 12–24 months, 24–48 months, 48–96 months, >96 months); patients with RA (n = 24), PAPS (n = 24) and UCTD (n = 24) were included as representative of other rheumatologic conditions. (b, e) Percentage of LN (black) and of SLE (white) patients with high levels divided into subgroups according to the time from disease onset at recruitment, (c, f) ROC curves for difference in anti-dsDNA and anti-Annexin A1 between patients with LN and healthy controls (1), patients with SLE and healthy controls (2) and patients with LNT0–12 versus patients with SLET0–12 (3). In all cases, antibodies were of the IgG2 isotype and levels were calculated as Relative Intensity value (RU/ml), given the absence of WHO international standards [33].
Anti-ANXA1
IgG2 serum levels were higher in LN/SLE patients than in healthy controls (AUC = 0.94, 0.91, respectively); the difference between LN and SLE was moderate (AUC = 0.70 for T0–12months) (Fig. 2). The percentage of patients with high levels was 39% in LN vs 4% in SLE at T0–1month, and it was markedly modified in those patients with longer periods of disease (Fig. 2b).
Anti-C1q
Differences in anti-C1Q IgG2 serum levels in patients with LN/SLE compared with healthy controls were minimal, as was the difference between LN and SLE (AUC = 0.55, 0.62, 0.59, respectively) (Fig. 4). The percentage of patients with high levels of IgG2 was noticeably higher in LN (T0–1month and T2–12 months) compared with matched SLE groups, and then increased in the SLE (Fig. 4b).
Circulating antibodies: intra-panel correlations
Fig. 1c shows the level of correlations between all the antibodies of the panel. In almost all cases, serum levels of antibodies were not correlated with each other, although anti-dsDNA and anti-ANXA1 IgG2 were strongly correlated (Fig. 1d).
Other rheumatologic conditions
Supplementary Table S3, available at Rheumatology online, reports clinical parameters and laboratory data for PAPS, RA and UTCD patients. None of these patients presented clinical or laboratory renal involvement. In particular, none of the patients with PAPS fulfilled the classification criteria for SLE. Levels of all the antibodies of the panel were higher in PAPS, RA and UCTD when compared with healthy controls, excepted anti-C1q (Figs 2–4). Anti-H2A levels were higher in LN compared with PAPS, RA and UTCD (AUC = 0.78, 0.77 and 0.79); anti-ANXA1 and anti-dsDNA IgG2 were higher in LN compared with RA and UTCD (0.80 and 0.83 for the first antibody, and 0.77 and 0.76 for the latter antibody). Anti-H3 levels were higher in PAPS compared with LN and SLE (AUC = 0.76 and 0.80, respectively) (Supplementary Figs S2 and S3, available at Rheumatology online). An overview of how each antibody varied in the different pathologies is provided by the heat map presented in Supplementary Fig. S4, available at Rheumatology online, in which red and blue reflect high and low correlation, respectively, as calculated by Z scores.
Clinical associations
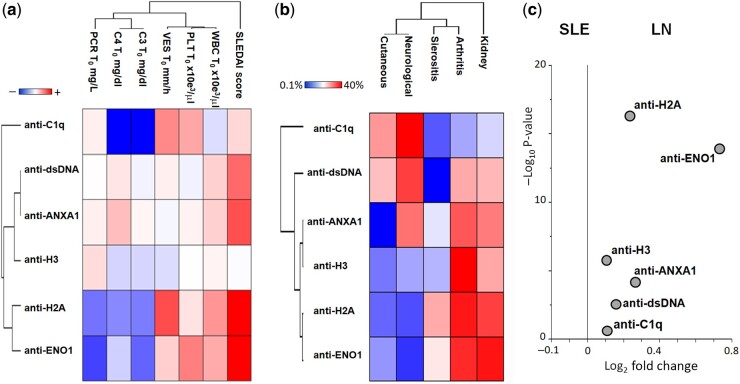
Fig. 5a shows a heat map that correlates (Spearman’s coefficient with Z-score modification) the circulating levels of each antibody with clinical and laboratory parameters: high anti-ENO1 and anti-H2A IgG2 corresponded to the highest values of the SLEDAI score (in red), and high anti-C1q IgG2 corresponded to the lowest C3 and C4 serum levels (in blue). Further associations, indicated by intermediate colours, were weak.
Fig. 5.
Clinical correlations and volcano plots
(a) Heat map showing the association between the levels of circulating IgG2 antibodies and the clinical and laboratory parameters in all categories of LN and SLE patients considered together [the correlogram, based on Spearman’s coefficient, uses a pseudocolour scale (from red, +1, positive; to blue, –1, negative; and to white, 0, null), to represent the correlation between two parameters]. Anti-ENO1 and anti-H2A IgG2 had the highest correlation with the SLEDAI index, and anti-C1q was correlated with low C3 and C4 levels (blue = inverse correlation). (b) Heat map showing the percentage incidence of target organ pathologies in all the categories of SLE and LN patients (0–1 months up to >96 months). Here the heat map indicates the correlation between two parameters (e.g. an antibody and an organ pathology) [again, the correlogram, based on Spearman’s coefficient, uses a pseudocolour scale (from red, +1, positive; to blue, –1, negative; and to white, 0, null)].
(c) Volcano plots based on fold change (Log2) and on P value (–Log10) of each antibody level identified SLE and LN patients who had experienced a renal flare in the previous 24 months. Anti-ENO1 and anti-H2A IgG2 were the antibodies with the most significant changes in LN patients.
The association between the positivity of each antibody with renal and extra-renal manifestations of SLE, presented in Fig. 5b, indicated a high percentage of kidney manifestations, followed by arthritis (except for anti-C1q and anti-dsDNA); sierositis and neurological manifestations were only occasionally observed. The volcano plot in Fig. 5c shows that anti-ENO1 and anti-H2A IgG2 levels were the most specific and sensitive markers of LN vs SLE (in particular in newly diagnosed patients of LN 0–24monthsvs SLE 0–24 months).
Discussion
The main result of the present cross-sectional analysis is that the antibody panel proposed was highly discriminatory between SLE/LN patients and healthy subjects. Areas under the curve (AUCs) were in all cases over 0.9, indicating high specificity. The association of some antibody levels with SLE flares is also clinically relevant, since they may provide the opportunity to personalize the therapeutic approach and prevent organ lesions.
We studied two large cohorts of patients affected by SLE and LN (in total 1052 patients with SLE/LN) stratified into different groups according to the time from disease onset. In these patients, we determined the serum levels of those antibodies of IgG2 isotype (i.e. anti-dsDNA, anti-H2/H3, anti-C1q, anti-αΕΝΟ and ANXA1) already shown to be predominant in the serum of patients with SLE/LN and in the glomeruli of patients with LN [26, 29, 37]. Based on the large number of patients enrolled in the study, and the technological advancements in the assays for the detection of antibodies, we suggest that the novel findings reported here should be considered in daily clinical practice.
Levels of circulating auto-antibodies were the main focus of our study because they are directly involved in the pathogenesis of any organ pathology linked with SLE. Previous studies have been mainly focused on antibodies against nucleosome components (i.e. anti-dsDNA, anti-histones) and, with reference to LN, on anti-C1q. Results for anti-dsDNA are variable, and largely dependent on antibody levels: only high titres of antibodies are, in fact, associated with SLE activity [17, 20]. The limited size of studies, the variability of technologies used for anti-dsDNA antibody detection and the lack of specificity of IgG isotypes, have been major limitations for most previous reports [11].
Before discussing the clinical relevance of our findings, two technical peculiarities of the new antibodies should be considered: first, there was a logarithmic correlation between circulating levels of anti-dsDNA IgG2 and anti-dsDNA IgGs as determined with the classical Farr radioimmunoassay in the serum of 500 SLE patients. The same logarithmic correlation was observed between anti-C1q IgG2 and anti-C1q IgGs as determined by commercial assays. This implies that significant changes in the IgG2 titres in the serum of SLE patients correspond to minimal variations in IgGs and means that evaluating antibodies with the IgG2 isotype offers a better chance to observe potential clinical associations. The second peculiarity is that, with the exception of anti-dsDNA and anti-AnxA1 IgG2, levels of the various circulating antibodies were not correlated with one another, indicating that the mechanism for their formation was not univocal. Anti-dsDNA and anti-AnxA1 IgG2 levels were directly correlated: as a possible but not definite explanation suggested by in vivo experiments, these two molecules may interact due to their opposite charge and form a macromolecular complex (DNA is negatively charged and Annexin A1 is cationic) [38, 39], and this could be recognized by the same autoantibody.
In addition to the main finding of the study (related to the high power of the IgG2 antibodies that constitute our panel to discriminate between SLE/LN patients and healthy controls), it is of note that anti-H2A, anti-ANXA1 and anti-dsDNA were also able to discriminate between SLE/LN and patients with different rheumatologic conditions, with AUCs ranging from 0.78 to 0.83. Finally, anti-H3 IgG2 levels were very high in patients with PAPS, representing a discriminatory element between these patients and healthy controls and, to a less extent, between these patients and those with SLE, LN and RA. The new findings for anti-H3 IgG2 require further confirmation in an additional larger cohort of patients with PAPS.
A second relevant new point, that is supported by the following findings, is that serum levels of anti−ΕΝΟ1 and anti-H2 IgG2 completely discriminate between LN and SLE: (i) at the beginning of the disease (T0–1month) and preceding the start of any therapy, the percentage of patients positive for both antibodies was much higher in LN than in SLE patients; (ii) limiting the time of observation to within the first year from disease onset (which includes acute renal flares and likely limits the effects of therapy), anti-ΕΝΟ1 and anti-H2 levels were higher in LN than in SLE (AUC 0.82 and 0.86 for anti-ENO1 and anti-H2, respectively); (iii) the percentages of patients with high levels of anti-αΕΝΟ and anti-H2 IgG2 at the beginning of symptoms (T0–1month) were 60% and 65%, respectively, compared with 3% of SLE patients, and 90% when combined; this difference was highly significant; (iv) the volcano plot that incorporates several elements (specificity, sensitivity) of the association between different antibodies levels and LN supports this important finding. More data about the potential use of anti-ΕΝΟ1 IgG2 as a biomarker of a specific LN subgroup are presented in the second part of this study, in which, prospective analysis provides crucial elements for the discussion (Bruschi et al. part 2). Variations in antibody levels between LN/SLE and other rheumatologic conditions were only partial, and there is a need to extend the study in larger cohorts of patients.
A third point to be discussed is the correlation between serum levels of anti−ΕΝΟ1 and anti-H2 IgG2 antibodies with the SLEDAI index of disease activity: this correlation, together with the higher AUC of ROC curves between LN and SLE of new onset and the correlation with arthritis, suggest that serum levels of these antibodies significantly reflect the disease activity. As an ancillary finding, an inverse correlation between anti-C1q IgG2 and C3/C4 serum levels has been observed that is supported by the increased fragmentation of complement components.
Our approach also has limitations, which are mainly related to the cross-sectional model and the relatively low number of SLE patients at the onset of the disease (in a phase before the start of therapies); to determine differences from other rheumatologic conditions, there is a need to extend the study to larger cohorts of patients.
In conclusion, the results of this first part of the study offer the opportunity to approach the broad field of SLE, LN and of other rheumatologic conditions in terms of personalized medicine in which newly discovered specific biomarkers of disease flares could be used for early diagnosis and prevention. A critical revision of classical parameters of lupus activity led to consider the inconsistency of those that, in the past, appeared as specific biomarkers and in particular of anti-dsDNA. Longitudinal studies will provide further data, enabling the consideration of new antibodies as specific and reliable biomarkers of LN (Bruschi et al. part 2).
Supplementary Material
Acknowledgements
Thanks to all the researchers in the Zeus study (doctors, nurses and laboratory personnel) and to all patients who agreed to be enrolled. Thanks to Miss Anna Capurro for reviewing grammar and English style.
G.M.G. and A.R. were the principal investigators of the study. They were involved in study design and coordination, patient recruitment, data management, supervision, manuscript writing and discussion; M.B. had key roles in laboratory analysis, proteomics, supervision, statistics and data management; G.C., A.P. and M.P. were involved in laboratory analysis; G.M., A.R.S., F.F., M.F., A.V., L.C., F.P., P.M., F.L., G.P., G.P., M.B., A.M., G.A.R., P.E., G.M., S.N., L.C., B.T., G.E., I.C., V.B., M.D., P.F., I.P., G.G., C.M., D.S., F.S., S.V., M.M. and A.T. were involved in patient recruitment, data management and discussions on the manuscript.
Funding: The Giannina Gaslini Institute (trial sponsor) provided logistic and financial support to the study through grants from the Ministry of Health (‘Ricerca corrente’ and ‘Cinque per mille of IRPEF-Finanziamento della ricerca sanitaria’). People working at the Zeuss project belong to the “Fondazione Malattie Renali del Bambino”, from which we acknowledge financial support. Grant ROL 9849 was received from Compagnia di San Paolo. P.C. is the recipient of the National Institutes of Health R01 grant AI132949.
Disclosure statement: The authors have declared no conflict of interest.
Data availability statement
Specific data are available on request. Please write to gmarcoghiggeri@gaslini.org.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1.Rahman A, Isenberg DA.. Systemic lupus erythematosus. N Engl J Med 2008;358:929–39. [DOI] [PubMed] [Google Scholar]
- 2.Hanly JG, O’Keeffe AG, Su L. et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford) 2016;55:252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagavant H, Fu SM.. Pathogenesis of kidney disease in systemic lupus erythematosus. Curr Opin Rheumatol 2009;21:489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houssiau FA, Vasconcelos C, D’Cruz D. et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum 2002;46:2121–31. [DOI] [PubMed] [Google Scholar]
- 5.Mahieu MA, Strand V, Simon LS, Lipsky PE, Ramsey-Goldman R.. A critical review of clinical trials in systemic lupus erythematosus. Lupus 2016;25:1122–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalaaji M, Mortensen E, Jorgensen L, Olsen R, Rekvig OP.. Nephritogenic lupus antibodies recognize glomerular basement membrane-associated chromatin fragments released from apoptotic intraglomerular cells. Am J Pathol 2006;168:1779–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramers C, Hylkema MN, van Bruggen MC. et al. Anti-nucleosome antibodies complexed to nucleosomal antigens show anti-DNA reactivity and bind to rat glomerular basement membrane in vivo. J Clin Invest 1994;94:568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramers K, Stemmer C, Monestier M. et al. Specificity of monoclonal anti-nucleosome auto-antibodies derived from lupus mice. J Autoimmun 1996;9:723–9. [DOI] [PubMed] [Google Scholar]
- 9.Illei GG, Tackey E, Lapteva L, Lipsky PE.. Biomarkers in systemic lupus erythematosus: II. Markers of disease activity. Arthritis Rheum 2004;50:2048–65. [DOI] [PubMed] [Google Scholar]
- 10.Seguier J, Jouve E, Bobot M. et al. Paradoxical association between blood modular interferon signatures and quality of life in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2020;59:1975–83. [DOI] [PubMed] [Google Scholar]
- 11.Ghiggeri GM, D’Alessandro M, Bartolomeo D. et al. An update on antibodies to necleosome components as biomarkers of sistemic lupus erythematosus and of lupus flares. Int J Mol Sci 2019;20:5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceppellini R, Polli E, Celada F.. A DNA-reacting factor in serum of a patient with lupus erythematosus diffusus. Proc Soc Exp Biol Med 1957;96:572–4. [DOI] [PubMed] [Google Scholar]
- 13.Touma Z, Urowitz MB, Ibanez D, Gladman DD.. SLEDAI-2K 10 days versus SLEDAI-2K 30 days in a longitudinal evaluation. Lupus 2011;20:67–70. [DOI] [PubMed] [Google Scholar]
- 14.Tan EM, Cohen AS, Fries JF. et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 15.Petri M, Orbai AM, Alarcon GS. et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aringer M, Costenbader K, Daikh D. et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis 2019;78:1151–9. [DOI] [PubMed] [Google Scholar]
- 17.Kavanaugh AF, Solomon DH, The American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Guidelines for immunologic laboratory testing in the rheumatic diseases: anti-DNA antibody tests. Arthritis Rheum 2002;47:546–55. [DOI] [PubMed] [Google Scholar]
- 18.Tron F, Letarte J, Roque-Antunes Barreira MC, Lesavre P.. Specific detection of circulating DNA: anti-DNA immune complexes in human systemic lupus erythematosus sera using murine monoclonal anti-DNA antibody. Clin Exp Immunol 1982;49:481–7. [PMC free article] [PubMed] [Google Scholar]
- 19.Swaak AJ, Aarden LA, Statius van Eps LW, Feltkamp TE.. Anti-dsDNA and complement profiles as prognostic guides in systemic lupus erythematosus. Arthritis Rheum 1979;22:226–35. [DOI] [PubMed] [Google Scholar]
- 20.Gensous N, Marti A, Barnetche T. et al. ; on behalf of the FHU ACRONIM. Predictive biological markers of systemic lupus erythematosus flares: a systematic literature review. Arthritis Res Ther 2017;19:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riboldi P, Gerosa M, Moroni G. et al. Anti-DNA antibodies: a diagnostic and prognostic tool for systemic lupus erythematosus? Autoimmunity 2005;38:39–45. [DOI] [PubMed] [Google Scholar]
- 22.Antico A, Platzgummer S, Bassetti D, et al. ; Study Group on Autoimmune Diseases of the Italian Society of Laboratory Medicine (SIMeL). Diagnosing systemic lupus erythematosus: new-generation immunoassays for measurement of anti-dsDNA antibodies are an effective alternative to the Farr technique and the Crithidia luciliae immunofluorescence test. Lupus 2010;19:906–12. [DOI] [PubMed] [Google Scholar]
- 23.Ghirardello A, Villalta D, Morozzi G. et al. Diagnostic accuracy of currently available anti-double-stranded DNA antibody assays. An Italian multicentre study. Clin Exp Rheumatol 2011;29:50–6. [PubMed] [Google Scholar]
- 24.Ravirajan CT, Rowse L, MacGowan JR, Isenberg DA.. An analysis of clinical disease activity and nephritis-associated serum autoantibody profiles in patients with systemic lupus erythematosus: a cross-sectional study. Rheumatology (Oxford) 2001;40:1405–12. [DOI] [PubMed] [Google Scholar]
- 25.Bijl M, Dijstelbloem HM, Oost WW. et al. IgG subclass distribution of autoantibodies differs between renal and extra-renal relapses in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2002;41:62–7. [DOI] [PubMed] [Google Scholar]
- 26.Bruschi M, Galetti M, Sinico RA. et al. Glomerular autoimmune multicomponents of human lupus nephritis in vivo (2): planted antigens. J Am Soc Nephrol 2015;26:1905–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moroni G, Radice A, Giammarresi G. et al. Are laboratory tests useful for monitoring the activity of lupus nephritis? A 6-year prospective study in a cohort of 228 patients with lupus nephritis. Ann Rheum Dis 2009;68:234–7. [DOI] [PubMed] [Google Scholar]
- 28.Sinico RA, Rimoldi L, Radice A. et al. Anti-C1q autoantibodies in lupus nephritis. Ann N Y Acad Sci 2009;1173:47–51. [DOI] [PubMed] [Google Scholar]
- 29.Bonanni A, Vaglio A, Bruschi M. et al. Multi-antibody composition in lupus nephritis: isotype and antigen specificity make the difference. Autoimmun Rev 2015;14:692–702. [DOI] [PubMed] [Google Scholar]
- 30.Miyakis S, Lockshin MD, Atsumi T. et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- 31.Aletaha D, Neogi T, Silman AJ. et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. [DOI] [PubMed] [Google Scholar]
- 32.Mosca M, Tani C, Vagnani S, Carli L, Bombardieri S.. The diagnosis and classification of undifferentiated connective tissue diseases. J Autoimmun 2014;48–49:50–2. [DOI] [PubMed] [Google Scholar]
- 33.Olesen H.Properties and units in the clinical laboratory sciences. I. Syntax and semantic rules IUPAC–IFCC recommendations 1995. Clin Chim Acta 1996;245:S5–21. [DOI] [PubMed] [Google Scholar]
- 34.Zweig MH, Campbell G.. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 1993;39:561–77. [PubMed] [Google Scholar]
- 35.Hughes G.Youden’s index and the weight of evidence revisited. Methods Inf Med 2015;54:576–7. [DOI] [PubMed] [Google Scholar]
- 36.Bruschi M, Petretto A, Vaglio A. et al. Annexin A1 and autoimmunity: from basic science to clinical applications. Int J Mol Sci 2018;19:1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruschi M, Sinico RA, Moroni G. et al. Glomerular autoimmune multicomponents of human lupus nephritis in vivo: alpha-enolase and annexin AI. J Am Soc Nephrol 2014;25:2483–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romay-Penabad Z, Montiel-Manzano MG, Shilagard T. et al. Annexin A2 is involved in antiphospholipid antibody-mediated pathogenic effects in vitro and in vivo. Blood 2009;114:3074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yung S, Cheung KF, Zhang Q, Chan TM.. Anti-dsDNA antibodies bind to mesangial annexin II in lupus nephritis. J Am Soc Nephrol 2010;21:1912–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Specific data are available on request. Please write to gmarcoghiggeri@gaslini.org.