Abstract
STUDY QUESTION
What is the association of oral contraceptives (OCs) and tubal ligation (TL) with early natural menopause?
SUMMARY ANSWER
We did not observe an association of OC use with risk of early natural menopause; however, TL was associated with a modestly higher risk.
WHAT IS KNOWN ALREADY
OCs manipulate hormone levels, prevent ovulation, and may modify the rate of follicular atresia, while TL may disrupt the blood supply to the ovaries. These mechanisms may be associated with risk of early menopause, a condition associated with increased risk of cardiovascular disease and other adverse health outcomes.
STUDY DESIGN, SIZE, DURATION
We examined the association of OC use and TL with natural menopause before the age of 45 years in a population-based study within the prospective Nurses’ Health Study II (NHSII) cohort. Participants were followed from 1989 to 2017 and response rates were 85-90% for each cycle.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Participants included 106 633 NHSII members who were premenopausal and aged 25-42 years at baseline. Use, duration and type of OC, and TL were measured at baseline and every 2 years. Menopause status and age were assessed every 2 years. Follow-up continued until early menopause, age 45 years, hysterectomy, oophorectomy, death, cancer diagnosis, or loss to follow-up. We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% CIs adjusted for lifestyle, dietary, and reproductive factors.
MAIN RESULTS AND THE ROLE OF CHANCE
Over 1.6 million person-years, 2579 members of the analytic cohort experienced early natural menopause. In multivariable models, the duration, timing, and type of OC use were not associated with risk of early menopause. For example, compared with women who never used OCs, those reporting 120+ months of OC use had an HR for early menopause of 1.01 (95% CI, 0.87-1.17; P for trend=0.71). TL was associated with increased risk of early menopause (HR = 1.17, 95% CI, 1.06-1.28).
LIMITATIONS, REASONS FOR CAUTION
Our study population is homogenous with respect to race and ethnicity. Additional evaluation of these relations in more diverse populations is important.
WIDER IMPLICATIONS OF THE FINDINGS
To our knowledge, this is the largest study examining the association of OC use and TL with early natural menopause to date. While TL was associated with a modest higher risk of early menopause, our findings do not support any material hazard or benefit for the use of OCs.
STUDY FUNDING/COMPETING INTEREST(S)
The study was sponsored by UO1CA176726 and R01HD078517 from the National Institutes of Health and Department of Health and Human Services. The work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The authors have no competing interests to report.
TRIAL REGISTRATION NUMBER
N/A
Keywords: cohort, early menopause, epidemiology, menopause, natural menopause, oral contraceptives, ovarian function, prospective, reproductive factors, tubal ligation
Introduction
Early menopause, defined as the cessation of ovarian function before the age of 45 years, affects roughly 10% of women in Western populations (Shifren et al., 2014; Mishra et al., 2019). Current research suggests that women who experience early menopause are at increased risk for cardiovascular disease, cognitive decline, osteoporosis, and premature mortality (Shuster et al., 2010; Faubion et al., 2015).
Presently, 65% of American women of reproductive age (15–49 years) use some form of contraception, with oral contraceptives (OCs; 25.3%) and tubal ligation (TL; 21.8%) the most common (Kavanaugh and Jerman, 2018). OCs contain synthetic hormones and have been available for use in the USA since 1960 (Dhont, 2010). OCs prevent pregnancy by manipulating hormone levels, which in turn may inhibit ovarian follicle growth, prevent ovulation and, possibly, slow the rate of follicular atresia (Rivera et al., 1999; Birtch et al., 2005). OC use could plausibly delay menopause via these same mechanisms, thus, slowing the rate of decline in the ovarian follicle pool and lowering risk of early menopause (Gold, 2011; Seidman et al., 2015). The association of OC use with timing of menopause has been previously examined (Cramer et al., 1995; de Vries et al., 2001; Gold et al., 2001, 2013; Palmer et al., 2003; Sievert and Hautaniemi, 2003; Nagel et al., 2005; Ortega-Ceballos et al., 2006; Chang et al., 2007; Kaczmarek, 2007; Parazzini and Progetto Menopausa Italia Study Group, 2007; Dorjgochoo et al., 2008; Dratva et al., 2009; OlaOlorun and Lawoyin, 2009; Pokoradi et al., 2011; Nagata et al., 2012; Yasui et al., 2012; Stepaniak et al., 2013; Brand et al., 2015; Calvet et al., 2015; Dolleman et al., 2015; Zsakai et al., 2015; Lujan-Barroso et al., 2018). A recent systematic review and meta-analysis observed OC use to be associated with modestly later menopause timing (Roman Lay et al., 2020); however, the majority of studies reviewed were limited by cross-sectional and/or retrospective recall methods, which are subject to temporal biases. More heterogeneous results have been observed within prospective studies (de Vries et al., 2001; Nagel et al., 2005; Pokoradi et al., 2011; Nagata et al., 2012; Gold et al., 2013; Calvet et al., 2015; Dolleman et al., 2015; Lujan-Barroso et al., 2018). Though less susceptible to recall biases, results from longitudinal studies may be influenced by the inclusion of women in their later reproductive years who were prescribed OCs in response to menstrual irregularities, a common practice (Cho, 2018). Prospective studies that specifically account for age, timing, and duration of OC use are needed to fully understand the relation of OC use to menopause timing.
It is well established that TL is a preventive factor for ovarian cancer with one proposed mechanism for the protection being a disruption to the ovarian vascular blood supply resulting in diminished levels of sex steroid hormones produced by the ovaries (Hakverdi et al., 1994; Rice et al., 2012). As such, it has been hypothesized that the procedure may have negative implications for ovarian function and reserve, and lead to earlier menopause, yet few studies have examined the long-term association between TL and early menopause (Pokoradi et al., 2011; Nichols et al., 2013; Abi Tayeh et al., 2018; Ainsworth et al., 2019).
We therefore assessed the association of OC use and TL with early natural menopause in the prospective Nurses’ Health Study II (NHSII).
Materials and methods
Study population
The NHSII is a prospective cohort study established in June 1989 when 116 429 female registered nurses aged 25–42 years from 14 US states responded to a baseline questionnaire. At baseline, participants provided information regarding their medical history and health-related behaviors, such as use of OCs, menstrual and pregnancy history, and smoking status. Cohort members completed questionnaires every 2 years to identify new diagnoses of disease and update information on health-related behaviors. Questionnaire response rates have been 85% to 90% for each cycle (Bao, 2016).
Approvals
The study protocol was approved by the institutional review boards at Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health, and participants provided written informed consent.
Assessment of OC use and TL
History of OC use was self-reported at baseline, with the help of a color photo booklet of all brands and types of OCs marketed in the USA through 1989 (Bao et al., 2016). For each year of age from 13 to 42 years, participants reported each OC brand they used for 2 months or more and for 10 months or more. From 1991 to 2009, biennial questionnaires asked participants to indicate all forms of contraception used during the past 2 years, including the brand and type of the most-used OC, with the following response categories: 1 month or less, 2-4, 5-9, 10-14, 15-19, or 20-24 months. Within the NHSII cohort there are extensive procedures in place for integrating OC use data across questionnaire cycles. Carry forward and carry back methods of assigning data are used, as well as consideration of pregnancy and menopause status when assuming OC use and duration when a questionnaire, or specific responses to OC use questions, are missing. When it is not possible to make reliable assumptions, a missing indicator category is assigned for that specific 2-year questionnaire cycle.
TL was assessed every 2 years by asking participants if they currently used TL as a form of contraception. In 1993, participants reporting a history of TL were asked to indicate age at TL as follows: <25, 25-29, 30-34, 35-39, 40-44, 45+ years. After 1993, age at TL was derived from age at the biennial questionnaire. In 1997, participants were asked to report the type of their TL procedure as follows: Cautery/Coagulation, Ligation, Clip/ring/band, Other/don’t know.
Assessment of early menopause
Beginning in 1989, NHSII members were asked if their menstrual periods had ceased permanently with the following response options: “no: premenopausal,” “ yes: no menstrual periods,” “yes: had menopause but now have periods induced by hormones,” and “not sure.” Women reporting that their periods had ceased were then asked, “Age periods ceased?” with an open response field, and “For what reason did your periods cease?” with response options of surgery, radiation or chemotherapy, or natural. Women were also asked about use of hormone therapy (HT), including timing and type used. These questions were repeated on each biennial questionnaire. Age at menopause was defined as age at last menstrual period followed by 12 consecutive months of amenorrhea. For the women who reported being postmenopausal on one questionnaire and then subsequently reported being premenopausal, we defined age at menopause as age at which periods ceased for at least 12 months, followed by consistent reporting of cessation on at least three consecutive questionnaires.
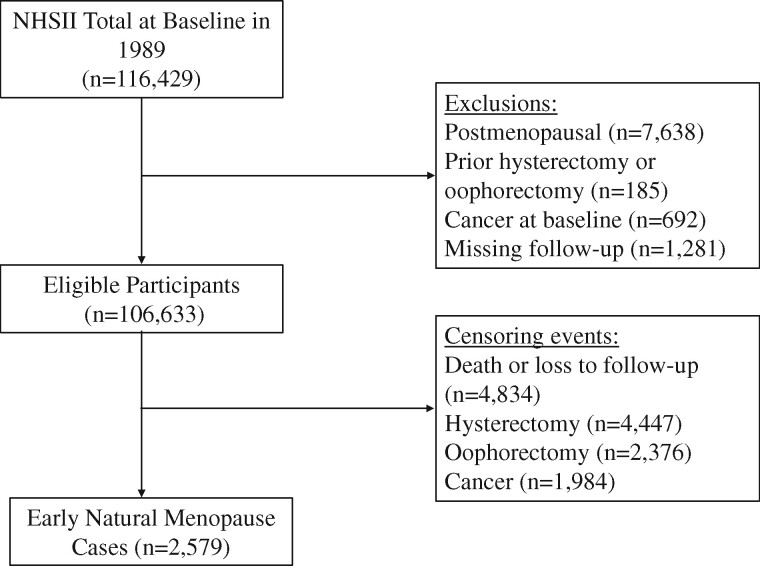
For the current analysis, exclusion criteria included not being premenopausal at baseline (n = 7638), prior hysterectomy or oophorectomy at baseline (n = 185), cancer at baseline (except for nonmelanoma skin cancer, n = 692), or not participating beyond 1989 (n = 1281), which resulted in a final sample of 106 633 participants (Fig. 1). We defined cases of early menopause as women who reported natural menopause at younger than 45 years.
Figure 1.
Flowchart of participant selection, from the Nurses’ Health Study II (NHSII), 1989–2017. Of the 116 429 participants at baseline in 1989, a total of 106 633 were included in the analytic sample and followed-up to 2017 for incident early natural menopause (n=2579). NHSII, Nurses’ Health Study II.
Covariates
Age at menarche, race/ethnicity, and height were reported in 1989. BMI was calculated using height reported at baseline and weight reported biennially. Age, pregnancy history, smoking status, and number of cigarettes smoked per day were assessed every 2 years. Cumulative breastfeeding was measured in 1993 and 1997 from all cohort members, and in 2003 from women reporting pregnancies after 1997. Infertility was assessed every 2 years to 2001 and every 4 years thereafter by asking women if they had ever tried to become pregnant for more than 1 year without success, and, if so, the cause (e.g., ovulatory disorder). Usual menstrual cycle length in 1993 was reported as ≤21, 21–25, 26–31, 32–39, 40–50, >50 days, or too irregular to estimate. Dietary factors, including amount of vitamin D from foods and supplements, and alcohol intake were assessed in 1991 and every 4 years thereafter via semi-quantitative food frequency questionnaires. Intake measures were adjusted for energy using the residual method (Willett, 1998).
Statistical analysis
Baseline characteristics were examined according to baseline measures of OC duration and TL history using age-adjusted generalized linear models. We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% CIs for the association of OC use with early menopause. Participants contributed follow-up time from the return of the baseline questionnaire in 1989 until: the onset of menopause; age 45 years; first report of a hysterectomy, bilateral or unilateral oophorectomy; cancer (not including nonmelanoma skin cancer); death; loss to follow-up; or the end of follow-up in May 2017, whichever came first. All models were stratified by age (in months) and questionnaire cycle. Linear trends were assessed and tested by modeling age at first OC use as a continuous variable and category medians for duration of OC use.
We modeled OC use in several ways. First, we updated OC use information every 2 years to classify never, past, or current use. We then evaluated associations by duration of OC use and age at first use, updating information through 2009. We investigated differences in associations by age by modeling current OC use (yes/no) at each age from 13 to 42 years. Finally, we compared association by OC formulation (ethinyl estradiol, mestranol, and first- and second-generation progestin).
We also examined the association of occurrence, age, and type of TL with early menopause controlling for duration of OC use. Linear trends were assessed by modeling category medians for age at TL.
To control for potential confounding, we created two multivariable models: model 1, adjusted for age; and model 2, adjusted for age, age at menarche, smoking status, alcohol intake, BMI, vitamin D from dairy foods, supplemental vitamin D, parity, cumulative total breastfeeding, infertility because of ovulatory disorder, and menstrual cycle length in 1993. Covariates were identified a priori from previous literature and associations with early menopause in the NHSII population (e.g., infertility because of ovulatory disorder and menstrual cycle length). Additional covariates were considered for inclusion in multivariable models (e.g., physical activity, race/ethnicity), but none were observed to substantively affect exposure estimates or model fit. All OC models were additionally adjusted for TL, models evaluating history of OC use and age at first use were adjusted for OC duration, and TL models were adjusted for OC duration. To account for potential bias due to missing OC data, we ran a sensitivity analysis among participants who had complete OC use and duration data for the entirety of their follow-up (n = 67 812). Lastly, to evaluate whether HT use affected associations, we conducted two sensitivity analyses, one adjusting for HT use and another censoring at first report of HT use.
Using the phreg procedure in SAS, we tested proportionality of hazards for each of our main exposures by including a time interaction variable in our fully adjusted models. All statistical analyses were conducted with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics by categories of OC duration for 106 633 premenopausal participants aged 25–42 years (mean [SD] age, 34.1 [4.6]) are shown in Table I. At baseline, participants who had longer duration of OC use had the highest levels of alcohol intake and smoking (past or current) and the lowest mean parity and cumulative duration of total breastfeeding. Women with the longest duration of OC use were youngest at first use (mean [SE] age 18.7 [0.03]). Baseline characteristics by history of TL are shown in Supplementary Table SI. Women who had a TL were older than women who did not have the procedure (37.0 versus 33.6) and had higher mean parity (2.1 versus 1.3).
Table I.
Age-standardized baseline characteristics of 106 633 participants by oral contraceptive duration, Nurses’ Health Study II, 1989–2017.
| No OC use | aOC use 1–23 mo | OC use 24–71 mo | OC use 72–119 mo | OC use ≥120 mo | |
|---|---|---|---|---|---|
| Characteristicb | (n = 18 169) | (n = 22 560) | (n = 36 273) | (n = 18 784) | (n = 8822) |
| Age, yc | 33.8 (4.6) | 34.4 (4.6) | 34.0 (4.8) | 33.7 (4.6) | 34.9 (3.9) |
| Smoking, pack-yearsd | 18.6 (1.27) | 17.4 (0.96) | 17.0 (0.73) | 18.8 (0.93) | 19.0 (1.30) |
| BMI | 24.4 (0.04) | 24.0 (0.03) | 24.0 (0.03) | 23.8 (0.04) | 23.7 (0.05) |
| Physical activity, MET-h/wk | 29.0 (0.51) | 27.9 (0.46) | 27.8 (0.36) | 28.6 (0.51) | 29.6 (0.74) |
| Calcium intake, mg/d | 1019 (3.5) | 1018 (3.2) | 1019 (2.5) | 1016 (3.5) | 1005 (5.1) |
| Vitamin D intake, IU/d | 398 (2.1) | 389 (1.9) | 384 (1.5) | 388 (2.1) | 397 (3.1) |
| Alcohol intake, g/d | 2.2 (0.04) | 2.6 (0.04) | 3.0 (0.03) | 3.6 (0.04) | 4.5 (0.06) |
| Non-Hispanic white, % | 91.2 | 93.1 | 95.5 | 95.3 | 94.3 |
| Household income ≥100 000, %e | 30.8 | 34.7 | 35.7 | 36.1 | 36.4 |
| Smoking status, % | |||||
| Past | 14.3 | 20.3 | 22.2 | 25.8 | 25.0 |
| Current | 8.6 | 11.8 | 12.8 | 16.0 | 19.0 |
| Age at menarche, y | 12.4 (0.01) | 12.4 (0.01) | 12.4 (0.01) | 12.4 (0.01) | 12.4 (0.02) |
| History of infertility because of ovulatory disorder, % | 3.3 | 5.5 | 5.6 | 4.3 | 2.4 |
| Parity | 1.3 (0.01) | 1.6 (0.01) | 1.5 (0.01) | 1.3 (0.01) | 0.92 (0.01) |
| Total breastfeeding duration, mof | 16.2 (0.14) | 14.1 (0.11) | 13.3 (0.09) | 10.8 (0.12) | 8.0 (0.20) |
| Age at first OC use, y | N/A | 21.9 (0.02) | 20.7 (0.02) | 19.5 (0.02) | 18.7 (0.03) |
| Tubal ligation, % | 10.4 | 16.6 | 18.5 | 17.8 | 11.3 |
MET-h/wk, metabolic equivalent of task hours/week; OC, oral contraceptive.
Women with missing duration of OC use at baseline (n = 2025) are not shown.
Values are age-adjusted mean ± SE unless otherwise indicated and all are significant at the P<0.05 level.
Values are expressed as unadjusted mean ± SD.
Among past or current smokers.
Assessed in 2001.
Among parous women.
Tests of proportionality of hazards did not suggest violation of assumptions (data not shown). During 1.6 million person-years of follow-up, 2579 women in the analytic cohort experienced early natural menopause. OC use and duration of use were not associated with risk of early menopause (Table II). In models adjusting only for age, past OC use was associated with a modestly higher risk of early menopause compared with never use (HR 1.12; 95% CI, 1.00–1.25), and longer duration of use was positively associated with risk (P for trend = 0.003). However, after adjustment for covariates, results were substantially attenuated. In multivariable-adjusted models, neither past nor current OC use was associated with early menopause (model 3: past use, HR, 1.01; 95% CI, 0.89-1.16; current use, HR, 0.87; 95% CI, 0.71-1.07). Duration of OC use was also no longer related to risk after adjustment; for example, compared with women who never used OCs, those reporting 120 or more months of OC use had an HR for early menopause of 1.01 (95% CI, 0.87-1.17; P for trend = 0.71). Attenuation appeared to be mostly due to the inclusion of smoking and menstrual cycle length in the models, with parity and breastfeeding also contributing slightly.
Table II.
Multivariable associations of oral contraceptive use with risk of early natural menopause, Nurses’ Health Study II, 1989–2017 (n = 106 633).
| Model 1b | Model 2c | Model 3d | |||
|---|---|---|---|---|---|
| OC use | Cases, Noa | Person-years | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| OC status | |||||
| Never | 356 | 233 916 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Past | 1991 | 1 131 633 | 1.12 (1.00–1.25) | 1.04 (0.92–1.16) | 1.01 (0.89–1.16) |
| Current | 206 | 174 442 | 1.00 (0.84–1.19) | 0.91 (0.76–1.08) | 0.87 (0.71–1.07) |
|
| |||||
| Duration of OC use, mo | |||||
| Never | 356 | 233 916 | 1 [Reference] | 1 [Reference] | |
| 1–23 | 491 | 294 823 | 1.05 (0.92–1.21) | 1.01 (0.88–1.16) | |
| 24–47 | 438 | 279 020 | 1.02 (0.89–1.17) | 0.98 (0.85–1.13) | |
| 48–71 | 365 | 217 012 | 1.13 (0.97–1.30) | 1.07 (0.92–1.24) | |
| 72–95 | 277 | 166 593 | 1.14 (0.97–1.33) | 1.04 (0.89–1.22) | |
| 96–119 | 196 | 117 531 | 1.16 (0.97–1.38) | 1.02 (0.85–1.21) | |
| 120+ | 383 | 218 983 | 1.20 (1.04–1.39) | 1.01 (0.87–1.17) | |
| P-value for trend | 0.003 | 0.71 | |||
|
| |||||
| Age at first OC use, y | |||||
| Never | 356 | 233 916 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 13–17 | 260 | 167 485 | 1.13 (0.97–1.33) | 0.95 (0.80–1.12) | 0.91 (0.76–1.10) |
| 18–19 | 614 | 381 893 | 1.06 (0.93–1.20) | 0.95 (0.83–1.09) | 0.92 (0.79–1.08) |
| 20–21 | 582 | 334 160 | 1.08 (0.95–1.23) | 1.01 (0.89–1.16) | 0.98 (0.84–1.15) |
| 22–23 | 318 | 200 483 | 1.06 (0.91–1.23) | 1.03 (0.88–1.20) | 1.00 (0.85–1.19) |
| 24–29 | 267 | 191 769 | 1.00 (0.85–1.18) | 0.96 (0.82–1.13) | 0.94 (0.80–1.12) |
| 30–34 | 61 | 30 604 | 1.27 (0.97–1.67) | 1.15 (0.87–1.51) | 1.13 (0.85–1.49) |
| ≥35 | 43 | 17 961 | 1.81 (1.31–2.48) | 1.66 (1.21–2.28) | 1.64 (1.19–2.26) |
| P-value for trend | 0.17 | 0.01 | 0.004 | ||
HR, hazard ratio; NA, not applicable.
Totals do not sum to 2579 cases because of missing data on OC use.
Age-adjusted.
Additionally adjusted for age at menarche (≤9, 10, 11, 12, 13, 14, 15, 16, ≥17 years), smoking (never, past 1–14, 15–24, 25+ years, current 1–14, 15–24, 25+ years), alcohol (0, 1 to <10, 10 to <30, ≥30 g/d), BMI (<18.5, 18.5–24.9, 25.0–29.9, ≥30 kg/m2), vitamin D from dairy (quintiles), vitamin D supplement use (0, >0 to <600, ≥600 IU/d), parity (0, 1, 2, 3, 4+), total cumulative breastfeeding (0 to <1, 1–3, >3–6, >6–12, >12–18, >18–24, >24–36, ≥36 months, parous but breastfeeding data missing), infertility because of ovulatory disorder (no, yes), menstrual cycle length in 1993 (≤25, 26–31, 32–39, ≥40 days), and tubal ligation (no, yes).
Additionally adjusted for OC duration (category medians).
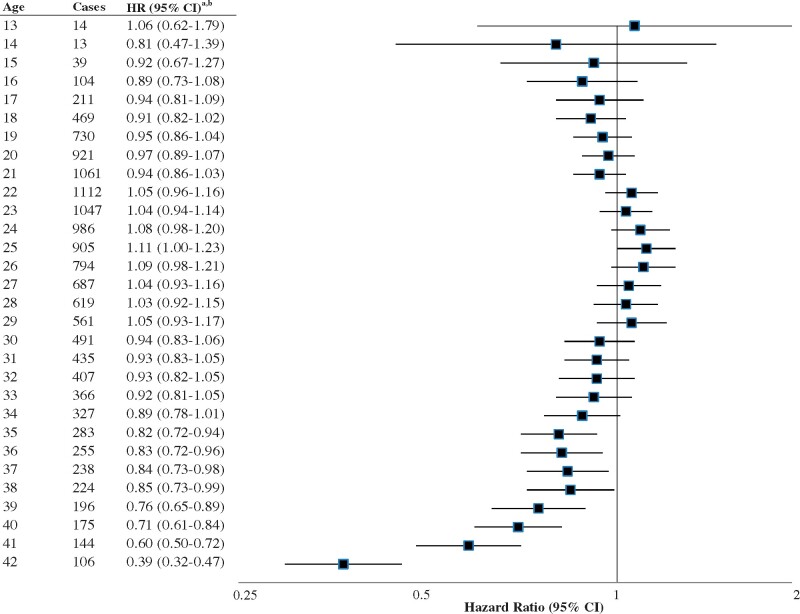
Risk of early menopause varied by age at use of OCs. In fully adjusted models (model 3), evaluating age at first use, we observed higher risk for early menopause among women who were 35 years or older when they began using OCs (HR, 1.64; 95% CI, 1.19-2.26; P for trend = 0.004), compared to women who never used OCs. Risk was not elevated for age at first use of OCs at younger ages. In our analysis of risk by current OC use by age (Fig. 2), use from age 13 years to age 34 years was not associated with early menopause. Beginning at age 35 years, OC use was associated with lower risk of early menopause.
Figure 2.
Associations of oral contraceptive use at ages 13–42 years with early natural menopause, NHSII, 1989–2017 (n = 106 633).aReference group for each Hazard Ratio (HR) is no oral contraceptive use at each age. bAdjusted for age at menarche (≤9, 10, 11, 12, 13, 14, 15, 16, ≥17 years), smoking (never, past 1–14, 15–24, 25+ years, current 1–14, 15–24, 25+ years), alcohol (0, 1 to <10, 10 to <30, ≥30 g/d), BMI (<18.5, 18.5–24.9, 25.0–29.9, ≥30 kg/m2), vitamin D from dairy (quintiles), vitamin D supplement use (0, >0 to <600, ≥600 IU/d), parity (0, 1, 2, 3, 4+), total cumulative breastfeeding, 0 to <1, 1–3, >3–6, >6–12, >12–18, >18–24, >24–36, ≥36 months, parous but breastfeeding data missing), infertility because of ovulatory disorder (no, yes), menstrual cycle length in 1993 (≤25, 26–31, 32–39, ≥40 days), tubal ligation (no, yes), and duration of oral contraceptive use (category medians).
In fully adjusted analyses, women who had a TL procedure experienced a 17% higher risk of early menopause compared to women who did not have the procedure (model 3, HR = 1.17; 95% CI, 1.06-1.28, Table III). Higher risk was noted for TL occurring between ages 25 and 39 years, but not for TL at younger or older ages. Based on the type of TL procedure reported by 15 600 participants, risk for early menopause was increased regardless of procedure type. Overall, the inclusion of covariates minimally affected the association of TL with early menopause, except for the inclusion of parity and breastfeeding, which caused estimates to be slightly raised.
Table III.
Multivariable associations of tubal ligation with risk of early natural menopause in the Nurses’ Health Study II, 1989–2017 (n = 106 633).
| Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|
| Tubal Ligation | Cases, No. | Person-years | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Tubal ligation | |||||
| No | 1885 | 1 252 815 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yes | 694 | 356 047 | 1.10 (1.01–1.20) | 1.17 (1.07–1.29) | 1.17 (1.06–1.28) |
|
| |||||
| Age at tubal ligation, y | |||||
| No | 1885 | 1 252 815 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| <25 | 14 | 8437 | 0.96 (0.56–1.62) | 0.88 (0.52–1.49) | 0.88 (0.52–1.50) |
| 25–29 | 127 | 62 624 | 1.21 (1.01–1.44) | 1.30 (1.08–1.57) | 1.30 (1.08–1.57) |
| 30–34 | 227 | 109 743 | 1.17 (1.02–1.35) | 1.30 (1.12–1.50) | 1.30 (1.13–1.50) |
| 35–39 | 142 | 62 179 | 1.18 (0.99–1.40) | 1.30 (1.10–1.56) | 1.31 (1.09–1.56) |
| ≥40 | 40 | 14 535 | 1.06 (0.78–1.46) | 1.09 (0.80–1.50) | 1.09 (0.79–1.49) |
| Age not specified | 144 | 98 528 | 0.91 (0.77–1.08) | 0.92 (0.76–1.10) | 0.91 (0.76–1.09) |
| P-value for trend | 0.83 | 0.97 | 0.99 | ||
|
| |||||
| Type of tubal ligationd | |||||
| No | 1885 | 1 235 664 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Cautery/Coagulation | 142 | 70 360 | 1.19 (1.00–1.41) | 1.22 (1.02–1.45) | 1.22 (1.02–1.45) |
| Ligation | 223 | 110 110 | 1.16 (1.01–1.33) | 1.22 (1.06–1.41) | 1.22 (1.05–1.41) |
| Clip/ring/band | 61 | 29 309 | 1.27 (0.98–1.63) | 1.25 (0.97–1.62) | 1.25 (0.96–1.61) |
| Other/don’t know | 99 | 66 071 | 0.89 (0.73–1.09) | 0.93 (0.76–1.15) | 0.93 (0.76–1.15) |
Adjusted for age.
Additionally adjusted for age at menarche (≤9, 10, 11, 12, 13, 14, 15, 16, ≥17 years), smoking (never, past 1–14, 15–24, 25+ years, current 1–14, 15–24, 25+ years), alcohol (0, 1 to <10, 10 to <30, ≥30 g/d), BMI (<18.5, 18.5–24.9, 25.0–29.9, ≥30 kg/m2), vitamin D from dairy (quintiles), vitamin D supplement use (0, >0 to <600, ≥600 IU/d), parity (0, 1, 2, 3, 4+), total breastfeeding (0 to <1, 1–3, >3–6, >6–12, >12–18, >18–24, >24–36, ≥36 months, parous but breastfeeding data missing), infertility because of ovulatory disorder (no, yes), and menstrual cycle length in 1993 (≤25, 26–31, 32–39, ≥40 days).
Additionally adjusted for OC duration (category medians).
20 153 women reported type of tubal ligation on the 1997 survey of which 15 600 were able to report exact type. Totals do not sum to 2579 cases because of missing data on type of tubal ligation.
In supplemental analyses, use of specific types of OC was modestly associated with risk of early menopause in age-adjusted models but results were attenuated and nonsignificant in fully adjusted models (Supplementary Table SII). Results from sensitivity analyses adjusting for HT use and censoring follow-up at first HT use were essentially identical to the main findings (Supplementary Table SIII). In analyses limited to women with a complete history of OC use and duration over the course of follow-up, results were also similar to the main findings (data not shown).
Discussion
To our knowledge, this is the largest study examining the association of OC use and TL with early natural menopause to date. Depending on the type of TL and a woman’s age at the time of the procedure, history of TL was associated with a modest but significant higher risk of early menopause. In contrast, our findings do not support any material hazard or benefit for the use of OCs, as history of OC use, duration of OC use and use prior to the age of 35 years were not associated with risk of early menopause.
Our results suggested a positive association between early menopause and OC use at older reproductive ages. While we do not know the reasons why our participants chose to use OCs, we hypothesize that the increased risk observed for women who began using OCs at age 35 years or older may be due to confounding by indication, as women experiencing menstrual irregularity prior to menopause may be prescribed OCs (Cho, 2018). Furthermore, our results are consistent with those observed by Lujan-Barroso et al. (2018) who found that women who began using OCs at age 25–30 years had a significantly lower risk of earlier menopause compared to women who were ≥31 years when they started using OCs.
To the best of our knowledge, only one other study has examined the association of OC use with risk of menopause prior to the age of 45 years, similar to our study (Yasui et al., 2012). In the Japan Nurses’ Health Study, no association of OC use and risk of early natural menopause was observed in a cross-sectional analysis of 24 152 pre- and postmenopausal women. A limited number of studies have prospectively examined the association of OC use with menopause timing and results have been conflicting (de Vries et al., 2001; Nagel et al., 2005; Pokoradi et al., 2011; Nagata et al., 2012; Gold et al., 2013; Dolleman et al., 2015; Lujan-Barroso et al., 2018). In a recent study conducted among 12 562 premenopausal women in the EPIC-Spain sub-cohort, a later age at natural menopause was observed for OC users compared to non-users in an age-adjusted model (HR for age at natural menopause = 0.85, 95% CI, 0.75-0.97) (Lujan-Barroso et al., 2018). A strength of the EPIC-Spain sub-cohort study was the exclusion of OC users >40 years of age, however, follow-up time was short (median = 3 years). A later age at natural menopause was also observed for OC users among 3302 premenopausal women in the Study of Women’s Health Across the Nation (SWAN) cohort (HR for age at final menstrual period = 0.85, 95% CI, 0.75-0.97; Gold et al., 2013). However, as women were 42–52 years of age at study enrollment when they reported their ever use of OCs, these findings may have been impacted by fairly recent use of OCs, as only women with no exogenous hormone use in the prior 3 months were excluded. When women use OCs in their later reproductive years, they may continue to have withdrawal bleeding even though they are in menopause, or conversely, they may have abnormal periods even though they are not in menopause (Cho, 2018). In our study, we observed lower risk of early menopause associated with current OC use at ages 35 years and older, with results strongest for current OC use at age 39 years and older. We hypothesize that the incidence of early menopause may have been masked by use of OCs at these later ages, thus delaying the detection of menopause until after the age of 45 years.
A small number of prior studies have accounted for changes in OC use patterns in the later reproductive years, which may be associated with varying risk as suggested by our age-specific analysis. Using data from a breast cancer screening program in Utrecht, Netherlands (DOM-3 cohort), de Vries et al. (2001) enrolled women who were most likely perimenopausal (41–53 years) but excluded women who used HT or OCs in the 4 years prior to their last menses to address the potential for reverse causality. After adjustment for parity, smoking, and other factors and when compared to women who never used OCs, only long-term OC use (≥11 years) was associated with an earlier age at menopause, but the magnitude of effect was small (HR for age at natural menopause = 1.13, 95% CI, 1.02-1.25).
Although studies examining hormonal changes after TL are limited and conflicting, several have observed decreased hormone levels following a TL procedure (Radwanska et al., 1982; Cattanach, 1985; Hakverdi et al., 1994; Özyer et al., 2012). Our findings of higher risk of early natural menopause for women reporting prior TL supports hypotheses positing that the effect of sterilization on ovarian vascular supply may have negative implications for ovarian function and reserve (Visvanathan and Wyshak, 2000). Similar to our findings, a prospective study of 5113 UK women found higher risk for menopause prior to the age of 49 years among participants reporting TL compared to those who did not (Odds Ratio = 1.38, 95% CI, 1.02-1.87) (Pokoradi et al., 2011). Conversely, TL was not associated with menopausal age among 50 314 women in the US Sister Study; however, the baseline sample included women aged 35-74 years and, for some women, both TL and menopause status were reported retrospectively (Nichols et al., 2013). We observed that women undergoing the TL procedure between the ages of 25 and 39 years had higher risk than women reporting TL at younger and older ages. Explanations for a potential difference in this association by age are unclear. We note that ovarian cancer studies have found stronger protective effects of TL procedures performed prior to the age of 35 years, but the mechanism is also unclear (Rice et al., 2012, 2014).
The relation of OC use and TL with early menopause could potentially be impacted by other reproductive factors. Pregnancy and breastfeeding are correlated with OC use and TL, and a previous study found both to be inversely associated with early menopause risk (Langton et al., 2020). We adjusted for parity and breastfeeding in our multivariable models, which show an independent association of TL (and no marked association of OC use) with early menopause, while results for TL are slightly attenuated in models not adjusting for these factors. Overall, these results suggest that the interrelations of reproductive factors are complex, and future studies should carefully consider how best to analyze these correlated factors.
Our study has several limitations. Although we relied on self-report of the onset of menopause, which might contribute to some misclassification (den Tonkelaar, 1997; Rodstrom et al., 2005), we collected menopause data prospectively. Furthermore, the reproducibility of self-reported menopause was assessed in the comparable Nurses’ Health Study (Colditz et al., 1987). Among 6591 women who were premenopausal at baseline and reported being postmenopausal 2 years later, 82% repeatedly recalled the same age at natural menopause, to within 1 year, on repeated questionnaires. While much of our OC use data were collected prospectively and we have much data on OC use prior to the perimenopausal period, information on use during the teens and early 20s was collected retrospectively at baseline, which could lead to some misclassification. However, the accuracy of retrospectively reported OC use has been previously validated in the NHSII cohort. Hunter et al. (1997) compared responses of a randomly selected sample of 215 participants with their data from a subsequent, detailed interview. Agreement for a history of ever having used OC’s was high between the two methods (exact agreement 99%) and reported durations of use were equivalent (mean duration 42.7 months by telephone interview versus 44.6 months by questionnaire, Spearman correlation=0.94, P=0.0001). Some of our analyses included variables with quite a few categorical levels, which may have resulted in low power and precision for some levels of exposure; as such, we emphasize caution in interpreting results. Our study population is homogenous with respect to race and ethnicity, yet we would expect that the physiological association between either OC use or TL and early menopause would not differ substantially by race/ethnicity. However, additional evaluation of these relations in more diverse populations is important (Richard-Davis and Wellons, 2013).
In this large, prospective cohort study that included over 106 000 premenopausal women we observed an increased risk of early natural menopause for women who reported having a TL between the ages of 25 and 39 years. Overall, our results do not suggest that the use, timing, and duration of OC is associated with risk of early natural menopause.
Data availability
This study uses data from the Nurses’ Health Study II and follows the data sharing policies established for the cohort.
Authors’ roles
Conception and design of study: C.R.L., A.C.P.S., J.E.M., and E.R.B.-J. Acquisition of data: C.R.L., J.E.M., and E.R.B.-J. Analysis and interpretation of data: C.R.L., B.W.W., A.C.P.S., L.L.S., S.E.H., J.E.M., B.A.R., and E.R.B.-J. Drafting the manuscript or revising it critically for important intellectual content: C.R.L., B.W.W., A.C.P.S., L.L.S., S.E.H., J.E.M., B.A.R., and E.R.B.-J. Final approval of the version to be published: C.R.L., B.W.W., A.C.P.S., L.L.S., S.E.H., J.E.M., B.A.R., and E.R.B.-J.
Funding
The study was sponsored by UO1CA176726 and R01HD078517 from the National Institutes of Health and Department of Health and Human Services. The work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Conflict of interest
The authors have no conflicts of interest to report.
Supplementary Material
References
- Abi Tayeh G, Naba T, Habib O, Attieh E, Mansour F, Kesrouani A, Maalouf S.. Tubal ligation and early menopause: a case-control study. Int J Gynaecol Obstet 2018;9:114–116. [Google Scholar]
- Ainsworth AJ, Baumgarten SC, Bakkum-Gamez JN, Vachon CM, Weaver AL, Laughlin-Tommaso SK.. Tubal ligation and age at natural menopause. Obstet Gynecol 2019;133:1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Bertoia ML, Lenart EB, Stampfer MJ, Willett WC, Speizer FE, Chavarro JE.. Origin, methods, and evolution of the three nurses’ health studies. Am J Public Health 2016;106:1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birtch RL, Baerwald AR, Olatunbosun OA, Pierson RA.. Ultrasound image attributes of human ovarian dominant follicles during natural and oral contraceptive cycles. Reprod Biol Endocrinol 2005;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand JS, Onland-Moret NC, Eijkemans MJ, Tjonneland A, Roswall N, Overvad K, Fagherazzi G, Clavel-Chapelon F, Dossus L, Lukanova A. et al. Diabetes and onset of natural menopause: results from the European Prospective Investigation into Cancer and Nutrition. Hum Reprod 2015;30:1491–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvet GA, Grinsztejn BG, Quintana MS, Derrico M, Jalil EM, Cytryn A, de Andrade AC, Moreira RI, Alves MR, Veloso Dos Santos VG. et al. Predictors of early menopause in HIV-infected women: a prospective cohort study. Am J Obstet Gynecol 2015;212:765.e1–765.e13. [DOI] [PubMed] [Google Scholar]
- Cattanach J.Oestrogen deficiency after tubal ligation. Lancet 1985;325:847–849. [DOI] [PubMed] [Google Scholar]
- Chang SH, Kim CS, Lee KS, Kim H, Yim SV, Lim YJ, Park SK.. Premenopausal factors influencing premature ovarian failure and early menopause. Maturitas 2007;58:19–30. [DOI] [PubMed] [Google Scholar]
- Cho MK.Use of combined oral contraceptives in perimenopausal women. Chonnam Med J 2018;54:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz GA, Stampfer MJ, Willett WC, Stason WB, Rosner B, Hennekens CH, Speizer FE.. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol 1987;126:319–325. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Xu H, Harlow BL.. Does “incessant” ovulation increase risk for early menopause? Am J Obstet Gynecol 1995;172:568–573. [DOI] [PubMed] [Google Scholar]
- de Vries E, den Tonkelaar I, van Noord PA, van der Schouw YT, Te Velde ER, Peeters PH.. Oral contraceptive use in relation to age at menopause in the DOM cohort. Hum Reprod 2001;16:1657–1662. [DOI] [PubMed] [Google Scholar]
- den Tonkelaar I. and reproducibility of self-reported age at menopause in women participating in the DOM-project. Maturitas 1997;27:117–123. [DOI] [PubMed] [Google Scholar]
- Dhont M.History of oral contraception. Eur J Contracept Reprod Health Care 2010;15:S12–8. [DOI] [PubMed] [Google Scholar]
- Dolleman M, Verschuren WM, Eijkemans MJ, Broekmans FJ, van der Schouw YT.. Added value of anti-Mullerian hormone in prediction of menopause: results from a large prospective cohort study. Hum Reprod 2015;30:1974–1981. [DOI] [PubMed] [Google Scholar]
- Dorjgochoo T, Kallianpur A, Gao YT, Cai H, Yang G, Li H, Zheng W, Shu XO.. Dietary and lifestyle predictors of age at natural menopause and reproductive span in the Shanghai Women’s Health Study. Menopause 2008;15:924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dratva J, Gómez Real F, Schindler C, Ackermann-Liebrich U, Gerbase MW, Probst-Hensch NM, Svanes C, Omenaas ER, Neukirch F, Wjst M. et al. Is age at menopause increasing across Europe? Results on age at menopause and determinants from two population-based studies. Menopause 2009;16:385–394. [DOI] [PubMed] [Google Scholar]
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA.. Long-term health consequences of premature or early menopause and considerations for management. Climacteric 2015;18:483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold EB.The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am 2011;38:425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, Skurnick J.. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol 2001;153:865–874. [DOI] [PubMed] [Google Scholar]
- Gold EB, Crawford SL, Avis NE, Crandall CJ, Matthews KA, Waetjen LE, Lee JS, Thurston R, Vuga M, Harlow SD.. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol 2013;178:70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakverdi AU, Taner CE, Erden AC, Satici O.. Changes in ovarian function after tubal sterilization. Adv Contracept 1994;10:51–56. [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Manson JE, Colditz GA, Chasan-Taber L, Troy L, Stampfer MJ, Speizer FE, Willett WC.. Reproducibility of oral contraceptive histories and validity of hormone composition reported in a cohort of US women. Contraception 1997;56:373–378. [DOI] [PubMed] [Google Scholar]
- Kaczmarek M.The timing of natural menopause in Poland and associated factors. Maturitas 2007;57:139–153. [DOI] [PubMed] [Google Scholar]
- Kavanaugh ML, Jerman J.. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception 2018;97:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton CR, Whitcomb BW, Purdue-Smithe AC, Sievert LL, Hankinson SE, Manson JE, Rosner BA, Bertone-Johnson ER.. Association of parity and breastfeeding with risk of early natural menopause. JAMA Netw Open 2020;3:e1919615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan-Barroso L, Gibert K, Obon-Santacana M, Chirlaque MD, Sanchez MJ, Larranaga N, Barricarte A, Quiros JR, Salamanca-Fernandez E, Colorado-Yohar S. et al. The influence of lifestyle, diet, and reproductive history on age at natural menopause in Spain: Analysis from the EPIC-Spain sub-cohort. Am J Hum Biol 2018;30:e23181. [DOI] [PubMed] [Google Scholar]
- Mishra GD, Chung HF, Cano A, Chedraui P, Goulis DG, Lopes P, Mueck A, Rees M, Senturk LM, Simoncini T. et al. EMAS position statement: Predictors of premature and early natural menopause. Maturitas 2019;123:82–88. [DOI] [PubMed] [Google Scholar]
- Nagata C, Wada K, Nakamura K, Tamai Y, Tsuji M, Shimizu H.. Associations of physical activity and diet with the onset of menopause in Japanese women. Menopause 2012;19:75–81. [DOI] [PubMed] [Google Scholar]
- Nagel G, Altenburg HP, Nieters A, Boffetta P, Linseisen J.. Reproductive and dietary determinants of the age at menopause in EPIC-Heidelberg. Maturitas 2005;52:337–347. [DOI] [PubMed] [Google Scholar]
- Nichols HB, Baird DD, DeRoo LA, Kissling GE, Sandler DP.. Tubal ligation in relation to menopausal symptoms and breast cancer risk. Br J Cancer 2013;109:1291–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OlaOlorun F, Lawoyin T.. Age at menopause and factors associated with attainment of menopause in an urban community in Ibadan. Nigeria. Climacteric 2009;12:352–363. [DOI] [PubMed] [Google Scholar]
- Ortega-Ceballos PA, Moran C, Blanco-Munoz J, Yunes-Diaz E, Castaneda-Iniguez MS, Salmeron J.. Reproductive and lifestyle factors associated with early menopause in Mexican women. Salud Publica Mex 2006;48:300–307. [DOI] [PubMed] [Google Scholar]
- Özyer Ş, Moraloğlu Ö, Gülerman C, Engin-Üstün Y, Uzunlar Ö, Karayalçın R, Uğur M.. Tubal sterilization during cesarean section or as an elective procedure? Effect on the ovarian reserve. Contraception 2012;86:488–493. [DOI] [PubMed] [Google Scholar]
- Palmer JR, Rosenberg L, Wise LA, Horton NJ, Adams-Campbell LL.. Onset of natural menopause in African American women. Am J Public Health 2003;93:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parazzini F, Progetto Menopausa Italia Study Group. Determinants of age at menopause in women attending menopause clinics in Italy. Maturitas 2007;56:280–287. [DOI] [PubMed] [Google Scholar]
- Pokoradi AJ, Iversen L, Hannaford PC.. Factors associated with age of onset and type of menopause in a cohort of UK women. Am J Obstet Gynecol 2011;205:34.e1–34.13. [DOI] [PubMed] [Google Scholar]
- Radwanska E, Headley SK, Dmowski P.. Evaluation of ovarian function after tubal sterilization. J Reprod Med 1982;27:376–384. [PubMed] [Google Scholar]
- Rice MS, Hankinson SE, Tworoger SS.. Tubal ligation, hysterectomy, unilateral oophorectomy, and risk of ovarian cancer in the Nurses’ Health Studies. Fertil Steril 2014;102:192–198.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice MS, Murphy MA, Tworoger SS.. Tubal ligation, hysterectomy and ovarian cancer: A meta-analysis. J Ovarian Res 2012;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard-Davis G, Wellons M.. Racial and ethnic differences in the physiology and clinical symptoms of menopause. Semin Reprod Med 2013;31:380–386. [DOI] [PubMed] [Google Scholar]
- Rivera R, Yacobson I, Grimes D.. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am J Obstet Gynecol 1999;181:1263–1269. [DOI] [PubMed] [Google Scholar]
- Rodstrom K, Bengtsson C, Lissner L, Bjorkelund C.. Reproducibility of self-reported menopause age at the 24-year follow-up of a population study of women in Goteborg. Sweden. Menopause 2005;12:275–280. [DOI] [PubMed] [Google Scholar]
- Roman Lay AA, do Nascimento CF, Horta BL, Dias Porto Chiavegatto Filho A.. Dias Porto Chiavegatto Filho A. Reproductive factors and age at natural menopause: A systematic review and meta-analysis. Maturitas 2020;131:57–64. [DOI] [PubMed] [Google Scholar]
- Seidman L, Kroll R, Howard B, Ricciotti N, Hsieh J, Weiss H.. Ovulatory effects of three oral contraceptive regimens: a randomized, open-label, descriptive trial. Contraception 2015;91:495–502. [DOI] [PubMed] [Google Scholar]
- Shifren JL, Gass ML, NAMS Recommendations for Clinical Care of Midlife Women Working Group. The North American Menopause Society recommendations for clinical care of midlife women. Menopause 2014;21:1038–1062. [DOI] [PubMed] [Google Scholar]
- Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA.. Premature menopause or early menopause: long-term health consequences. Maturitas 2010;65:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievert LL, Hautaniemi SI.. Age at menopause in Puebla. Mexico. Hum Biol 2003;75:205–226. [DOI] [PubMed] [Google Scholar]
- Stepaniak U, Szafraniec K, Kubinova R, Malyutina S, Peasey A, Pikhart H, Pająk A, Bobak M.. Age at natural menopause in three central and eastern European urban populations: the HAPIEE study. Maturitas 2013;75:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvanathan N, Wyshak G.. Tubal ligation, menstrual changes, and menopausal symptoms. J Womens Health Gend Based Med 2000;9:521–527. [DOI] [PubMed] [Google Scholar]
- Willett WC.Nutritional Epidemiology, 2nd edn. New York: Oxford University Press, 1998. [Google Scholar]
- Yasui T, Hayashi K, Mizunuma H, Kubota T, Aso T, Matsumura Y, Lee JS, Suzuki S.. Factors associated with premature ovarian failure, early menopause and earlier onset of menopause in Japanese women. Maturitas 2012;72:249–255. [DOI] [PubMed] [Google Scholar]
- Zsakai A, Mascie-Taylor N, Bodzsar EB.. Relationship between some indicators of reproductive history, body fatness and the menopausal transition in Hungarian women. J Physiol Anthropol 2015;34:35-015-0076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study uses data from the Nurses’ Health Study II and follows the data sharing policies established for the cohort.