Abstract
Objectives
Peripheral SpA (pSpA) is comprised of ReA, PsA, enteritis-associated arthritis and undifferentiated pSpA (upSpA). ReA and upSpA share T cell oligotypes and metabolomics in serum and SF. We investigated HLA-B27 subtypes and cytokines in serum and SF that were compared between ReA and upSpA.
Methods
ReA and upSpA were compared in two cohorts. In cohort I (44 ReA and 56 upSpA), HLA-B27 subtyping was carried out. In cohort II (17 ReA and 21 upSpA), serum and SF cytokines were compared using a multiplex cytokine bead assay (27 cytokines). A total of 28 healthy controls with similar age and sex to cohort II were included for comparison of serum cytokine levels.
Results
In cohort I, HLA-B27 was positive in 81.8% (36/44) of ReA and 85.71% (48/56) of upSpA patients. HLA-B27 typing was successful in 70 patients (30 ReA and 40 uSpA). HLA-B*2705 was the most common, followed by HLA-B*2704 and HLA-B*2707. Frequencies were the same between ReA and upSpA. In cohort II, 14 cytokines were detectable in the serum of patients. The levels of eight cytokines were higher than in the controls. The cytokine levels of ReA and upSpA were similar. Sixteen cytokines were detectable in the SF of patients. There was no statistical difference in the levels between ReA and upSpA. The cytokine profiles in sera and SF were also similar among HLA-B27-positive and negative patients.
Conclusion
ReA and upSpA have similar HLA-B27 subtype associations and similar cytokine profiles. They should be considered as a single entity during studies as well as clinical management.
Keywords: ReA, HLA-B27, cytokines, multibead cytokine assay, peripheral spondyloarthritis
Rheumatology key messages
Undifferentiated peripheral spondyloarthritis (upSpA) resembles ReA except for having a known preceding infection.
HLA-B27 subtypes are similar in ReA and upSpA (the most common subgroup being HLA-B*2705).
Cytokine profiles in serum and in SF are similar among ReA and upSpA patients.
Introduction
ReA is an oligoarthritis affecting predominantly large joints in the lower limbs, with variable other extra-articular manifestations, that occurs following a gastrointestinal or urogenital infection [1]. Clinicians often encounter a similar form of arthritis without the history (or documentation) of preceding diarrhoea or urogenital infection. Most of these are classified as ‘undifferentiated arthritis’, but this may include cases of seronegative RA as well [2]. However, the phenotype is different and resembles peripheral SpA (pSpA) rather than classical RA. For the lack of a standard term, we will be referring to this subgroup as ‘undifferentiated peripheral SpA’ (upSpA), a term that has been used by other authors [3]. This is separate from the previously used term ‘undifferentiated spondyloarthropathy’ (or ‘undifferentiated spondyloarthropathy’) that includes axial SpA [4] and has largely been superseded by the term ‘non-radiographic SpA’ as recommended by the Assessment of SpondyloArthritis International Society (ASAS) [5].
The ASAS has definitive criteria for pSpA. A portion of this pSpA is PsA, some are enteritis (IBD)-associated arthritis and some are ReA. The remaining are undifferentiated pSpA. These have been hypothesized to be a forme fruste of ReA [6]. Although it may be possible that a few of these might later evolve psoriasis skin lesions or IBD, the question remains how these should be treated. The evidence for treating upSpA as well as ReA is limited. Thus, to understand the pathogenesis or to plan clinical trials, it is imperative to know whether these two groups should be treated as separate or similar.
ReA is unique in the sense that it presents an interface between infection and arthritis. Understanding the pathogenesis may help us in understanding the genesis of different inflammatory arthritis and even to strategize how to prevent the chronicity of inflammatory arthritis. But ReA is an uncommon entity. Knowing if we can include or exclude upSpA patients along with ReA can be very helpful in this regard.
It has been previously shown that in about half of the patients with ReA with a definite history of preceding infections, no organism is isolated on culture, whereas ‘undifferentiated oligoarthritis’ without such a history reveals pathogenic bacteria on stool culture in ∼47% of cases [7]. It has been shown that the clinical profile and disease activity [3] and prevalence of HLA-B27 positivity are similar between ReA and upSpA [8]. Previous work from our centre has shown that patients with ReA and upSpA have CD8+ T cells that recognize Salmonella typhimurium outer membrane protein A (OMP-A) in their SF [9]. We have also previously shown that the metabolomic profile of sera and of SF is similar between ReA and upSpA [10], however, it is different from sera from patients with RA [11]. This seems to imply that both have similar immune-metabolic pathways involved.
Thus with similar aetiology (precipitating bacteria), clinical features, disease activity, HLA-B27 positivity, T cell response and metabolomics profile, ReA and upSpA seem to be similar entities. With this background we further explored the genetic associations and cytokine profiles of these two entities to strengthen or refute this hypothesis.
Methods
Two sets of experiments were carried out. In the first cohort (cohort I: 44 ReA and 56 upSpA), PCR for HLA-B27, sequencing for HLA-B27 subtypes and endoplasmic reticulum aminopeptidase 1 (ERAP1) polymorphism was studied, as both are known to have strong associations with the SpA spectrum of diseases. In the second cohort (cohort 2: 17 ReA and 21 upSpA), multiple cytokine levels were estimated in both serum and in SF using a multibead assay.
ReA was defined as patients who had at least two of three of the following features: asymmetric arthritis, oligoarthritis or lower limb predominant arthritis, which had developed within 4 weeks after an episode of diarrhoea or dysentery or urogenital infection [12]. As we had done previously, all patients meeting ASAS criteria for pSpA [5] were included after exclusion of patients having inflammatory back pain, restriction of back mobility or meeting criteria for ReA, PsA and IBD-associated arthritis. Patients with asymptomatic sacroiliitis on radiography or inflammatory backache in the past were not excluded (as these are part of the ASAS criteria for pSpA).
For cohort 2, patients (ReA or upSpA) were included only if they had at least one swollen large joint from which SF could be aspirated. Healthy controls with age and sex distributions similar to cohort II were included to compare serum cytokine levels. Blood samples (EDTA blood for DNA and plain blood for serum) and SF were collected from the knee joint before administration of intra-articular corticosteroid injection. Blood and SF was centrifuged (2000 rpm for 10 min) and stored at −20°C until analysis.
Informed consent was obtained from all participants in writing and the study was carried out as per the Declaration of Helsinki and its latest amendments. The two parts of the study were separately approved by the Sanjay Gandhi Postgraduate Institute of Medical Sciences Institutional Ethics Committee.
HLA-B27 typing by PCR (cohort I)
DNA was extracted from the stored EDTA samples by the salting out method [13]. The concentration and purity of DNA was determined by neon spectrophotometer [by measuring optical density (OD) at 260 and 280 nm]. DNA quality was checked on 0.8% agarose gel electrophoresis. HLA-B27 typing was done by PCR using the following B27-specific primers to amplify a 149 base pair region: B1, 5′-GCT ACG TGG ACG ACA CGC T-3′; B2: 5′-CTC GGT CAG TCT GTG CCT T-3′; B3: 5′-TCT CGG TAA GTC TGT GCC TT-3′. A conserved intronic region of HLA-DR of 796 bp was also amplified as an internal control using the following sets of primers: C1: 5′-TGC CAA GTG GAG CAC CCA A-3′; C2: 5′-GCA TCT TGC TCT GTG CAG AT-3′ (Sigma-Aldrich, St. Louis, MO, USA) [14]. The total volume for the PCR was 50 µl, containing 10 mM Tris-HCl, pH 8.3, 50 mM KCl, 1.5 mM MgCl2, 200 µM dNTP, 0.5 units Taq DNA polymerase (Bangalore Genei, Bangalore, India), 20 pM of each primer and 250–500 ng DNA. The PCR steps include denaturation at 96°C for 25 s, 70°C for 45 s and 72°C for 30 s; 21 cycles at 96°C for 25 s, 65°C for 45 s and 72°C for 30 s; 4 cycles each at 96°C for 25 s, 60°C for 45 s and 72°C for 30 s; 4 cycles each at 96°C for 25 s, 55°C for 1 min and 72°C 2 min and 1 cycle at 72°C for 10 min. The amplified PCR product was detected by agarose gel electrophoresis (1.5% agarose gel in 1X TBE containing 0.5 µg/ml ethidium bromide). The size of the HLA-B27-specific product was 149 bp and that of the internal control was 798 bp. Positive controls were also run with each set of samples.
HLA-B27 subtype analysis by sequencing (cohort I)
Subtype analysis was done in HLA-B27-positive patients by primers MW16 and MW09, which are used to amplify exon 2 and exon 3 of B27 to cover all known B27 subtypes. For amplification, the primers used were forward primer: 5′-CGCCGCGAGTCCGAGAGA-3′ and reverse primer 5′-GAGCCACTCCACGCACTC-3′ (Sigma-Aldrich) [15]. The PCR mixture contained 80 mM Tris-HCl, pH 9.0, 2 mM MgCl2, 20 mM (NH4)2SO4 (fermentas), 200 mΜ each dNTP (Invitrogen, Waltham, MA, USA), 0.5 units Taq DNA polymerase (Bangalore Genei), 20 pM of each primer (Sigma-Aldrich), 10% dimethylsulphoxide and 5 μl of genomic DNA at a concentration of 50–300 ng/µl was used as a template DNA. PCR conditions were similar to those for HLA-B27. The PCR product of HLA-B27 (exon-2, exon-3) was 670 bp and was detected by 2% agarose gel electrophoresis. After amplification of the desired gene, sequencing of PCR products was commercially carried out at Chromous Biotech (Bangalore, India). The PCR products were purified using Chromous DNA purification columns (Chromous Biotech) and amplified using forward primer (5 µmol). The BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) was used for sequencing the PCR. The purified product was then treated with Hi-Di Formamide to resuspend samples before electrokinetic injection in a capillary electrophoresis system (ABI 3500 XL Genetic Analyzer; Applied Biosystems). Each nucleotide is represented by one fluorochrome and the electropherogram shows different colour peaks that were analysed using BioEdit sequence alignment editor software. The sequences generated were matched with those available in the International ImMunoGeneTics project (HLA database; http://www.ebi.ac.uk/ipd/imgt/hla/) for assignment of subtypes [16].
ERAP1 polymorphism (cohort I)
Genotyping of ERAP1 was done by using commercial TaqMan-based RT-PCR (Applied Biosystems) for the two non-synonymous single-nucleotide polymorphisms rs27044(C/G) and rs30187(C/T) in the ERAP1 gene known to be associated with HLA-B27-positive SpA [17].
Multiplex cytokine/chemokine bead assays (cohort II)
A multiplex cytokine bead assay was performed using diluted serum supernatants and Milliplex MAP Human Cytokine/Chemokine Panel 1 Pre-mixed 27 Plex (Merck Millipore, Darmstadt, Germany) analysed with a Bio-Plex MAGPIX Multiplex Reader (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions. Both sera and SF were assayed by the Milliplex MAP Human Cytokine/Chemokine Panel 1 Pre-mixed 27 Plex to estimate levels of basic fibroblast growth factor (FGF), eotaxin, G-CSF, GM-CSF, IFN-γ, IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, inducible protein-10 (IP-10), monocyte chemotactic protein-1 (MCP-1/MCAF), macrophage inflammatory protein (MIP)-1α, MIP-1β, PDGF-BB, regulated on activation normal T cell expressed and secreted (RANTES), TNF and VEGF. The detectable limit for each cytokine was 10 pg/ml.
Statistical analysis
Data were analysed using SPSS (version 16.0; SPSS, Chicago, IL, USA) and R (version 3.6.3, via RStudio version 1.2.5; R Foundation for Statistical Computing, Vienna, Austria). Fisher’s exact test (two-sided) was used for comparison and correlation of allelic subtypes and clinical features between ReA and upSpA. The comparison of cytokines, chemokines and growth factors between ReA and upSpA and also between HLA-B27-positive vs negative patients’ serum and SF was done by using the Mann–Whitney U test. Correlation with clinical parameters, including disease activity score, was done using Spearman correlation. P-values <0.05 were considered significant. However, to account for multiple comparisons, Bonferroni corrections were carried out as appropriate.
Results
Clinical features
The clinical features of both the cohorts (I and II) are provided in Table 1. In cohort I, among 44 patients with ReA, 33 (75%) had a history of diarrhoea and 11 (25%) had a history of genitourinary infection. In cohort II, all 17 ReA cases had occurred after diarrhoea. The median disease duration in cohort I was 6 months [interquartile range (IQR) 2.5–24] while in cohort II it was 24 months (IQR 2–60).
Table 1.
Clinical features of the cohorts
| Characteristics | Cohort I (100 participants for HLA-B27 typing) | Cohort II (38 participants for cytokine multibead assay) | ||
|---|---|---|---|---|
| ReA | UpSpA | ReA | UpSpA | |
| Participants, n | 44 | 56 | 17 | 21 |
| Diarrhoea, n | 33 | 0 | 17 | 0 |
| Urogenital infection, n | 11 | 0 | 0 | 0 |
| Male:female ratio | 6.3:1 | 5.2:1 | 15:1 | 0.9:1 |
| Age, median (range), years | 24.5 (13–70) | 25 (17–58) | 26.5 (18–34) | 28.5 (15–44) |
| Disease duration, median (quartiles), months | 4 (2–24) | 6.5 (2.6–24) | 36 (12.5–54) | 12 (2–60) |
| Disease pattern, na | Episodic arthritis, 38 | Episodic arthritis, 33 | Episodic arthritis, 16 | Episodic arthritis, 19 |
| Chronic persistent arthritis, 5 | Chronic persistent arthritis, 23 | Chronic persistent arthritis, 1 | Chronic persistent arthritis, 2 | |
| Not defined = 1 | ||||
| HLA-B27, n | Positive, 36; negative, 8 | Positive, 48; negative, 8 | Positive, 14; negative, 3 | Positive, 14; negative, 6; NA, 1 |
Patients with first episode are included in episodic type.
NA: not available .
Cohort I: HLA-B27 prevalence and subtyping
In cohort I, HLA-B27 was positive in 81.8% (36/44) of ReA and 85.71% (48/56) of uSpA patients. Of these, subtyping was done in 70 (30 ReA and 40 upSpA) HLA-B27-positive patients. The distribution of B27 subtypes was similar in ReA and upSpA patients (Table 2).
Table 2.
Distribution of HLA-B27 subtypes between HLA-B27-positive patients with ReA and with upSpA
| HLA-B27 subtype | ReA, n | upSpA, n | χ2 test |
|---|---|---|---|
| B*2705 | 17 | 27 | P = 0.60 |
| B*2704 | 8 | 7 | |
| B*2707 | 5 | 6 |
ERAP1 rs27044(C/G) and rs30187(C/T) polymorphism
Minor allele frequencies of the rs27044(C/G) and rs30187(C/T) polymorphisms were similar in the HLA-B27-positive and negative groups (Supplementary Table 1, available at Rheumatology online). There was no association between the allele frequencies and disease susceptibility in HLA-B27-positive and negative patients (data not shown).
Cohort II: serum cytokine levels between patients and healthy controls
Serum TNF-α, IL-12 (p70), VEGF, IL-8, IP-10, MCP-1, MIP-1α and MIP-1β were higher in patients (ReA/upSpA) as compared with healthy controls. There was no difference in IL-1Ra, IL-6, IL-15, IFN-γ, eotaxin, IL-17, IL-10, G-CSF, FGF or RANTES levels.
Comparison of serum cytokine levels between ReA and upSpA
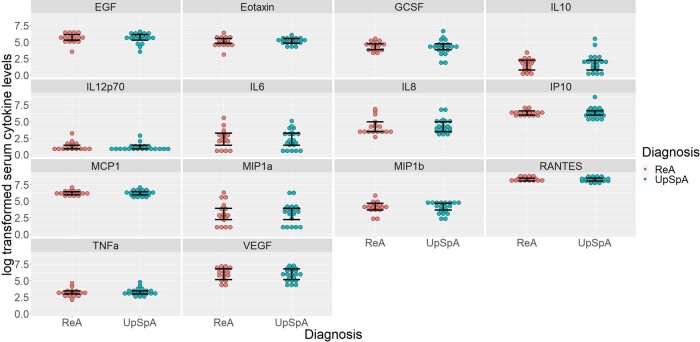
Levels of 14 cytokines were comparable between patients of ReA and upSpA (Fig. 1). The levels of the remaining 13 cytokines were not detectable in the serum of patients.
Fig. 1.
Log transformed serum cytokine levels in ReA and upSpA
Significance level P < 0.0036 after Bonferroni correction.
Comparison of SF cytokine levels between ReA and upSpA
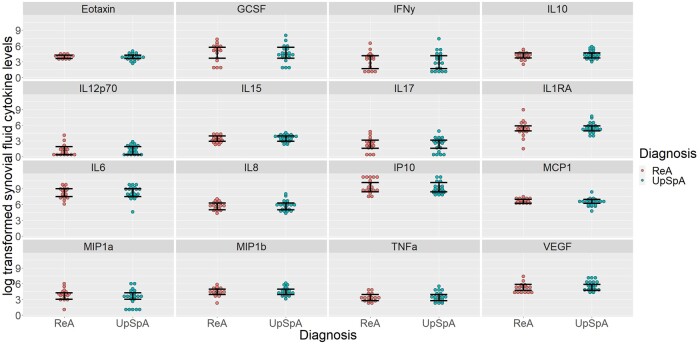
The chemokines eotaxin and RANTES and growth factors VEGF and basic FGF were not detectable in SF. IL-17, IL-15 and IFN-γ were detectable only in SF. In SF, 16 cytokines were detected, while the remaining 11 were not detected. There was no difference in the levels of different cytokines between ReA and upSpA (Fig. 2). Even the comparison of the ratios of serum to SF cytokine levels between ReA and upSpA revealed no statistically significant difference (data not shown).
Fig. 2.
Log transformed SF cytokine levels in ReA and upSpA
Significance level P < 0.0031 after Bonferroni correction.
Comparison between HLA-B27-positive and negative groups
Comparison of both serum and SF cytokines between HLA-B27-positive and HLA-B27-negative individuals revealed no difference (Supplementary Figs 2 and 3, available at Rheumatology online).
Discussion
The purpose behind this research was to check if ReA and upSpA had any differences in HLA-B27 subtypes or in the cytokine profiles in both serum and SF. These were explored in two different cohorts and no differences were found between these two groups.
In cohort I, HLA-B27 positivity was present in 82% of ReA and 86% of upSpA. Of the subtypes, B*2705 was the most common in both the groups (57% in ReA and 67.5% in upSpA). Although the prevalence of HLA-B27 and its various subtypes in SpA has been widely studied in the literature, such data for ReA or upSpA are limited. A southern Indian study of 28 ReA and 6 uSpA patients, HLA-B27 prevalence was 28.5% and 33.3%, respectively [18]. Other studies from northern India have reported prevalences of HLA-B27 similar to ours in ReA (83%) [19] and in ‘unclassifiable SpA’ (84%) [8].
Beyond validating these former studies that show similar prevalences of HLA-B27 in patients with ReA and upSpA, this study also establishes the similar HLA-B27 subtype distribution in these disease groups. In healthy controls from India, HLA-B*2705 is the predominant subtype (62–70% western India, 58% northern India) [20], followed by HLA-B*2704 (47% northern India, 10–14% western India) and HLA-B*2707 (13–28% western India, 6.8% northern India) [21–23]. HLA-B*2702 (1.4–2%) is a relatively uncommon subtype reported in these studies. HLA-B*2705 and 2704 are the only subtypes reported from southern India [18]. In most studies of AS in India, HLA-B*2705 (51–75%) is the most prevalent subtype [18, 22, 24]. At our centre, HLA-B27 subtypes in AS and in juvenile-onset enthesitis-related arthritis (ERA), again HLA-B*2705 was the most common in both adult AS (57%) and juvenile ERA (70%) patients [25]. However, a meta-analysis of studies from across the world showed the strongest association of AS with HLA-B*2702 and HLA-B*2704 [23].
Studies of HLA-B27 subtypes in ReA or uSpA (not upSpA, which has rarely been studied) are scarce in India as well as worldwide. In a study of 40 patients with HLA-B27-positive uSpA from Brazil, HLA-B*2705 was the most frequent allele (92.5% uSpA vs 77% control) followed by HLA-B*2702 (5% uSpA vs 12% control), whereas HLA-B*2704 was rare (2.5% uSpA vs 0% control) [26]. In another study of 17 HLA-B27-positive Reiter’s disease patients from Brazil, B*2705 was found in 65% and B*2702 in 35% [27]. In a study from northern India, HLA-B*2705 (73%) was the predominant subtype in uSpA and was significantly associated with females [23]. In a study from southern India, there were only two patients with uSpA; B*2705 and B*2704 was found in one patient each and in eight ReA patients and B*2705 and B*2704 were found in seven patients and one patient, respectively [18].
Although the first part of the study was based on convenient sampling and the sample size may seem small for genetic polymorphism studies, we were limited by the number of available patients. For ReA/upSpA, 100 is a sizeable number, and we have not been able to locate any genetic association study in ReA with a higher number of patients.
In cohort II, both serum cytokines as well as SF cytokines were explored, and there was no difference between the two groups. The role of chemokines and growth factors are not well described in the pathogenesis of ReA or upSpA. Our multibead assay helped us measure levels of chemokines like IL-8, MIP1-β, MCP-1 and IP-10. However, the chemokine eotaxin and growth factors like VEGF and basic FGF were not detectable in SF.
A comparison between acute and chronic ReA showed that chronic cases have lower levels of TNF [28]. Our centre previously reported increased levels of IL-17, IL-6, TGF-β and IFN-γ in the SF of patients with ReA/uSpA and a higher level of Th17-associated cytokines IL-17, IL-6, IL-1β and IL-21 in the SF of ReA/uSpA as compared with RA [29, 30]. The source for IL-17 and IFN-γ appear to be NK and γδ-T cells [31]. (It should be noted that these studies included uSpA, as per the ESSG criteria, and not the currently investigated upSpA. However, it is expected that most cases of uSpA and upSpA will overlap.) These studies compared a few cytokines in the SF of ReA patients with those in the SF of OA patients. In the current study, only serum cytokines were compared with those of healthy controls and we did not use controls for SF. TNF-α, but not IFN-γ, was higher in serum compared with healthy controls. Another study in Chlamydia-associated ReA showed lower levels of SF IFN-γ in HLA-B27-positive vs negative patients [32]. In our cohort, we could not find any difference between HLA-B27-positive and negative patients. This may be true, as the previous study [32] had some issues [33].
Our aim was to explore the similarities between ReA and upSpA. We have demonstrated that HLA-B27 associations and cytokine profiles in sera and SF are similar between ReA and upSpA, thus providing more evidence to link these two entities. The main contention against this is that upSpA may ‘differentiate’ into axial SpA, PsA or enteritis-associated arthritis. Nevertheless, ReA can also behave the same way. ReA often has an axial component and can evolve into AS [20, 34]. Chronic ReA may be difficult to differentiate clinically [35] or radiologically [36] from PsA.
The limitations of the current work include the lack of sample size calculation. However, due to the limited number of patients with these two disease entities, we will have to be satisfied with convenient samples. Moreover, in the cytokine study, it was a hypothesis-free approach looking at 27 different cytokines where sample size calculations cannot be done. One question may also arise as to why two different cohorts were used. For the second set of experiments, patients with at least one swollen large joint were required from which SF was available. Another limitation is that we did not included the effect of drugs on cytokine levels. Here again, most of the patients were newly diagnosed patients, meaning that they were drug naïve or were on NSAIDs only. Also, both ReA and upSpA are treated similarly under current standards of care, thus we expect the effects of any drug used would be more or less balanced between the two groups, allowing comparison of cytokine profiles. Ideally, a comparison with the other types of pSpA, namely PsA and enteritis-associated arthritis, could have highlighted the similarities and differences between these four groups better. However, the focus of the current study was limited to ReA and upSpA.
Thus we have shown that ReA and upSpA have similar HLA-B27 subtype associations and similar cytokine profiles. With previous studies showing similar clinical features, metabolomics of sera and SF and T cell responses (to Salmonella OMPs), it makes the case stronger to group these two together. Since both are uncommon diseases, it may be beneficial to consider them together to study pathogenesis and for clinical trials.
Supplementary Material
Acknowledgements
All authors contributed to the conception and design of the study, acquisition of data, analysis and interpretation of data, critical revision of the article for important intellectual content and final approval of the article for submission. J.R.P., S.A. and S.K. were responsible for drafting the article. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical approval was obtained from the Institutional Ethics Committee of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India.
Funding: Part of the work was funded by a grant from the Indian Rheumatology Association awarded to J.R.P. for HLA typing in ReA.
Disclosure statement: All authors declare no conflicts of interest.
Data availability statement
Data related to the study will be available from the corresponding author upon reasonable request.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1.Schmitt SK.Reactive arthritis. Infect Dis Clin North Am 2017;31:265–77. [DOI] [PubMed] [Google Scholar]
- 2.Wollenhaupt J, Zeidler H.. Undifferentiated arthritis and reactive arthritis. Curr Opin Rheumatol 1998;10:306–13. [DOI] [PubMed] [Google Scholar]
- 3.Malaviya AN, Agrawal N, Patil NS.. Clinical characteristics of peripheral spondyloarthritis without psoriasis, inflammatory enteropathy or preceding infection, from a single rheumatology clinic in northern India. Clin Rheumatol 2017;36:2613–8. [DOI] [PubMed] [Google Scholar]
- 4.Zochling J, Brandt J, Braun J.. The current concept of spondyloarthritis with special emphasis on undifferentiated spondyloarthritis. Rheumatology 2005;44:1483–91. [DOI] [PubMed] [Google Scholar]
- 5.Sieper J, Rudwaleit M, Baraliakos X. et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 2009;68:ii1–44. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal A, Misra R, Chandrasekhar S. et al. Is undifferentiated seronegative spondyloarthropathy a forme fruste of reactive arthritis? Br J Rheumatol 1997;36:1001–4. [DOI] [PubMed] [Google Scholar]
- 7.Fendler C, Laitko S, Sörensen H. et al. Frequency of triggering bacteria in patients with reactive arthritis and undifferentiated oligoarthritis and the relative importance of the tests used for diagnosis. Ann Rheum Dis 2001;60:337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prakash S, Mehra NK, Bhargava S, Malaviya AN.. HLA B27 related ‘unclassifiable’ seronegative spondyloarthropathies. Ann Rheum Dis 1983;42:640–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaurasia S, Shasany AK, Aggarwal A, Misra R.. Recombinant Salmonella typhimurium outer membrane protein A is recognized by synovial fluid CD8 cells and stimulates synovial fluid mononuclear cells to produce interleukin (IL)-17/IL-23 in patients with reactive arthritis and undifferentiated spondyloarthropathy. Clin Exp Immunol 2016;185:210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed S, Dubey D, Chowdhury A. et al. Nuclear magnetic resonance-based metabolomics reveals similar metabolomics profiles in undifferentiated peripheral spondyloarthritis and reactive arthritis. Int J Rheum Dis 2019;22:725–33. [DOI] [PubMed] [Google Scholar]
- 11.Dubey D, Kumar S, Chaurasia S. et al. NMR-based serum metabolomics revealed distinctive metabolic patterns in reactive arthritis compared with rheumatoid arthritis. J Proteome Res 2019;18:130–46. [DOI] [PubMed] [Google Scholar]
- 12.Braun J, Kingsley G, van der Heijde D, Sieper J.. On the difficulties of establishing a consensus on the definition of and diagnostic investigations for reactive arthritis. Results and discussion of a questionnaire prepared for the 4th International Workshop on Reactive Arthritis, Berlin, Germany, July 3–6, 1999. J Rheumatol 2000;27:2185–92. [PubMed] [Google Scholar]
- 13.Miller SA, Dykes DD, Polesky HF.. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988;16:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tonks S, Marsh SGE, Bunce M, Bodmer JG.. Molecular typing for HLA class I using ARMS-PCR: further developments following the 12th International Histocompatibility Workshop. Tissue Antigens 1999;53:175–83. [DOI] [PubMed] [Google Scholar]
- 15.Blasczyk R, Weber M, van Lessen A. et al. Discrimination of HLA-B27 alleles by group-specific amplification followed by solid-phase sequencing. Hum Immunol 1996;45:117–23. [DOI] [PubMed] [Google Scholar]
- 16.Robinson J, Halliwell JA, McWilliam H. et al. The IMGT/HLA database. Nucleic Acids Res 2013;41 (D1):D1222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherciu M, Popa LO, Bojinca M. et al. Functional variants of ERAP1 gene are associated with HLA-B27 positive spondyloarthritis. Tissue Antigens 2013;82:192–6. [DOI] [PubMed] [Google Scholar]
- 18.Thomas R, Philip J, Banerjee M.. Association of an extended haplotype of HLA class I alleles and their flanking microsatellites with spondyloarthropathies in South Indian patients. Hum Immunol 2006;67:318–23. [DOI] [PubMed] [Google Scholar]
- 19.Prakash S, Mehra NK, Bhargava S, Malaviya AN.. Reiter’s disease in northern India. A clinical and immunogenetic study. Rheumatol Int 1983;3:101–4. [DOI] [PubMed] [Google Scholar]
- 20.Kaarela K, Jäntti JK, Kotaniemi KM.. Similarity between chronic reactive arthritis and ankylosing spondylitis. A 32–35-year follow-up study. Clin Exp Rheumatol 2009;27:325–8. [PubMed] [Google Scholar]
- 21.Chhaya SU.HLA-B27 polymorphism in Mumbai, Western India. Tissue Antigens 2005;66:48–50. [DOI] [PubMed] [Google Scholar]
- 22.Chavan H, Samant R, Deshpande A, Mankeshwar R.. Correlation of HLA B27 subtypes with clinical features of ankylosing spondylitis. Int J Rheum Dis 2011;14:369–74. [DOI] [PubMed] [Google Scholar]
- 23.Kanga U, Mehra NK, Larrea CL. et al. Seronegative spondyloarthropathies and HLA-B27 subtypes: a study in Asian Indians. Clin Rheumatol 1996;15:13–8. [DOI] [PubMed] [Google Scholar]
- 24.López-Larrea C, Sujirachato K, Mehra NK. et al. HLA-B27 subtypes in Asian patients with ankylosing spondylitis. Evidence for new associations. Tissue Antigens 1995;45:169–76. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava R, Agnihotry S, Aggarwal R, Bajpai P, Aggarwal A.. HLA-B27 subtypes in enthesitis-related arthritis category of juvenile idiopathic arthritis and ankylosing spondylitis in northern India. Clin Exp Rheumatol 2015;33:931–5. [PubMed] [Google Scholar]
- 26.Sampaio-Barros PD, Conde RA, Donadi EA. et al. Undifferentiated spondyloarthropathies in Brazilians: importance of HLA-B27 and the B7-CREG alleles in characterization and disease progression. J Rheumatol 2003;30:2632–7. [PubMed] [Google Scholar]
- 27.Sampaio-Barros PD, Conde RA, Donadi EA. et al. Frequency of HLA-B27 and its alleles in patients with Reiter syndrome: comparison with the frequency in other spondyloarthropathies and a healthy control population. Rheumatol Int 2008;28:483–6. [DOI] [PubMed] [Google Scholar]
- 28.Butrimiene I, Jarmalaite S, Ranceva J. et al. Different cytokine profiles in patients with chronic and acute reactive arthritis. Rheumatology 2004;43:1300–4. [DOI] [PubMed] [Google Scholar]
- 29.Singh R, Aggarwal A, Misra R.. Th1/Th17 cytokine profiles in patients with reactive arthritis/undifferentiated spondyloarthropathy. J Rheumatol 2007;34:2285–90. [PubMed] [Google Scholar]
- 30.Singh AK, Misra R, Aggarwal A.. Th-17 associated cytokines in patients with reactive arthritis/undifferentiated spondyloarthropathy. Clin Rheumatol 2011;30:771–6. [DOI] [PubMed] [Google Scholar]
- 31.Chowdhury AC, Chaurasia S, Mishra SK, Aggarwal A, Misra R.. IL-17 and IFN-γ producing NK and γδ-T cells are preferentially expanded in synovial fluid of patients with reactive arthritis and undifferentiated spondyloarthritis. Clin Immunol 2017;183:207–12. [DOI] [PubMed] [Google Scholar]
- 32.Bas S, Kvien TK, Buchs N, Fulpius T, Gabay C.. Lower level of synovial fluid interferon-gamma in HLA-B27-positive than in HLA-B27-negative patients with Chlamydia trachomatis reactive arthritis. Rheumatology 2003;42:461–7. [DOI] [PubMed] [Google Scholar]
- 33.Cuchacovich R, Espinoza LR.. Lower level of synovial fluid interferon-γ in HLA-B27-positive than in HLA-B27-negative patients with Chlamydia trachomatis reactive arthritis. Rheumatology 2003;43:249–50. [DOI] [PubMed] [Google Scholar]
- 34.Misra R, Ahmed S, Chaudhury A. et al. THU0269 Development of ankylosing spondylitis in patients with reactive arthritis and peripheral spondyloarthropathy: hospital based study in north India. Ann Rheum Dis 2018;77(Suppl 2):353–353. [Google Scholar]
- 35.Engleman EP.Psoriatic arthritis and Reiter’s syndrome. Postgrad Med 1972;51:79–84. [DOI] [PubMed] [Google Scholar]
- 36.Helliwell PS, Hickling P, Wright V.. Do the radiological changes of classic ankylosing spondylitis differ from the changes found in the spondylitis associated with inflammatory bowel disease, psoriasis, and reactive arthritis? Ann Rheum Dis 1998;57:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data related to the study will be available from the corresponding author upon reasonable request.