Abstract
Background
Acute kidney injury (AKI) is increasingly being recognized after hepatectomy. This study aimed to identify factors predicting its occurrence and its impact on long-term outcome among patients with hepatocellular carcinoma (HCC).
Methods
This was a retrospective analysis of the incidence of AKI, factors predicting its occurrence, and its impact on patients undergoing hepatectomy between September 2007 and December 2018. A subgroup analysis included patients with histologically proven HCC.
Results
The incidence of AKI was 9.2 per cent in 930 patients. AKI was associated with increased mortality, morbidity, posthepatectomy liver failure (PHLF), and a longer hospital stay. On multivariable analysis, study period December 2013 to December 2018, diabetes mellitus, mean intraoperative BP below 72.1 mmHg, operative blood loss exceeding 377ml, high Model for End-Stage Liver Disease (MELD) score, and PHLF were predictive factors for AKI. Among 560 patients with HCC, hypertension, BP below 76.9 mmHg, blood loss greater than 378ml, MELD score, and PHLF were predictive factors. The 1-, 3-, and 5-year overall survival rates were 74.1, 59.2, and 51.6 per cent respectively for patients with AKI, and 91.8, 77.9, and 67.3 per cent for those without AKI. Corresponding 1-, 3-, and 5-year disease-free survival rates were 56.9, 42.3, and 35.4 per cent respectively in the AKI group, and 71.7, 54.5, and 46.2 per cent in the no-AKI group. AKI was an independent predictor of survival in multivariable analysis.
Conclusion
AKI is associated with longer hospital stay, and higher morbidity and mortality rates. It is also associated with shorter long-term survival among patients with HCC. To avoid AKI, control of blood loss and maintaining a reasonable BP (72–77 mmHg) during hepatectomy is important.
Acute kidney injury is associated with longer hospital stay, and higher morbidity and mortality rates. It is also associated with shorter long-term survival among patients with liver cancer. To avoid acute kidney injury, control of blood loss and maintaining a reasonable mean BP (72–77 mmHg) during hepatectomy is important.
Introduction
Partial hepatectomy is commonly indicated for various benign and malignant diseases of the liver and biliary tract. Although liver surgeons are mainly concerned with posthepatectomy liver failure (PHLF), acute kidney injury (AKI) is increasingly being recognized as an adverse event after hepatectomy1–3. AKI has been found to be strongly correlated with prolonged hospital stay, and increased morbidity and mortality4,5, but its impact on long-term outcomes for patients with malignant diseases remains uncertain. The reported incidence of AKI ranges from 3 to 21.6 per cent in the literature1–11. The wide variation is partly due to a non-standardized definition of AKI and partly because different patient populations were studied.
Various predictors for AKI after hepatectomy were identified by previous studies3–5,7,9,11. Preoperative factors included age, sex, BMI, diabetes mellitus (DM), hypertension, cardiovascular disease, underlying chronic kidney injury (CKI), haematocrit, biliary obstruction, raised alanine aminotransferase level, and Model for End-Stage Liver Disease (MELD) score12. Intraoperative factors included duration of operation, liver transection time, extent of liver resection, planned open procedure, Pringle’s manoeuvre, and intraoperative haemodynamic instability. Post-operative factors included PHLF, haemorrhage, sepsis, and serum urea level.
This study aimed to identify factors predicting the occurrence of AKI after hepatectomy and its clinical impact, as well as the impact on long-term outcome of patients with hepatocellular carcinoma (HCC).
Methods
Operative details, patient demographics, and histopathological findings from patients undergoing liver resection were all collected prospectively in a computer database at this centre. Long-term events, including recurrent disease, repeat treatment, and death were updated regularly in this database. For this study, patient data were extracted from the database to determine the incidence, consequence, and predictive factors for AKI after hepatectomy. Additional information concerning urine output, inotrope use, and adverse events during operation was retrieved from anaesthetic records. To minimize confounding factors resulting from different underlying histopathology, a subgroup analysis was undertaken of patients with histologically proven HCC, with emphasis on the impact of AKI on the long-term outcomes of HCC.
The Kidney Disease Improving Global Outcomes (KDIGO) criteria13 for the diagnosis of AKI were adopted in this study. According to KDIGO criteria, AKI was defined as an increase in serum creatinine level (sCr) by 26µmol/l or more within 48 h after surgery, or a 50 per cent increase above baseline sCr within 7 days after surgery, or urine output below 0.5 ml per kg per h for 6 h after surgery. The urine output criterion was not used in this study as a previous study10 showed that it could result in overestimation of AKI. Three stages of AKI according to the KDIGO were defined: stage 1, sCr increased to 1.5–1.9 times baseline or an increase in sCr of at least 26 µmol/l; stage 2, sCr increased to 2.0–2.9 times baseline; and stage 3, sCr increased to 3.0 times baseline or sCr 354 µmol/l or higher, or initiation of renal replacement therapy.
Baseline sCr was defined as the minimal value of sCr before surgery, usually the value taken on the day of admission for surgery. At baseline, patients were defined as having CKI when the estimated glomerular filtration rate (eGFR) was below 60 ml per min per 1.73 m2. The eGFR was calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation14.
Operative and anaesthetic technique
Hepatectomy was carried out by different approaches according to indication and feasibility, including open, laparoscopic and robotic techniques. Operations were performed by senior hepatobiliary surgeons or by junior specialists under supervision of the senior surgeons.
Open hepatectomy was carried out via a right subcostal incision with upward midline extension. In hemihepatectomy or extended hepatectomy, hilar dissection was performed, if possible, to ligate the hepatic artery and portal vein branch supplying the lobe of liver to be removed before parenchymal transection. For wedge resection or segmentectomy, intraparenchymal isolation and ligation of the vascular pedicle were done. Pringle’s manoeuvre was not applied routinely, except in patients who were recruited for another ongoing prospective study of this technique. It was achieved by encircling the hepaticoduodenal ligament and applying a vascular clamp in an intermittent manner: 15 min clamping followed by unclamping for 5 min until the end of transection. Liver transection was performed with an ultrasonic aspirator (Cavitron Ultrasonic Surgical Aspirator, CUSA™; ValleyLab, Boulder, CO, USA), and haemostasis was achieved by means of a saline-linked radiofrequency dissecting sealer (TissueLink™; TissueLink Medical, Dover, DE, USA), titanium clips, and ligatures. Major hepatic veins were divided with endovascular staplers (Tyco Healthcare, Norwalk, CT, USA).
For the laparoscopic approach, the liver was transected with a laparoscopic CUSA™ or LigaSureTM (Medtronic, Minneapolis, MN, USA) in combination with TissueLink™. Endovascular staplers were used to divide larger vascular pedicles. Robotic hepatectomy was performed with the da Vinci robot S or Xi system (Intuitive, Sunnyvale, CA, USA). Liver was transected with robotic Harmonic AceTM (Ethicon Endo-Surgery, Cincinatti, OH, USA) and TissueLink™. Pringle’s manoeuvre was not used in either laparoscopic or robotic hepatectomy.
To reduce blood loss, a low central venous pressure (CVP) anaesthesia technique was adopted for liver resection6. A central line was inserted for all procedures, except small-scale hepatectomy such as wedge resection. Other techniques to evaluate CVP by measuring inferior vena cava (IVC) diameter or IVC collapsibility were not used in this centre15,16. CVP was kept below 5 mmHg during liver transection if possible. This was achieved by restriction of intravenous fluid infusion during operation until liver transection had been completed. Intravenous infusion of glyceryl trinitrate (GTN) was occasionally used to bring down the CVP. In patients who developed low BP (roughly dropped more than 20 per cent of patient’s usual mean BP) after the use of GTN, infusion of phenylephrine could be used to raise the BP. Rapid fluid replacement was started once liver transection had been completed. Transfusion was given if patients developed haemodynamic instability owing to blood loss or when the haemoglobin level fell below 8 g/dl.
Outcomes measured
Liver transections were classified according to Brisbane 2000 terminology, and defined as major when three or more liver segments were removed17. Mean BP and CVP were captured during the liver transection phase, and the mean value was calculated for each patient. An intraoperative adverse event was defined as sustained systolic BP below 90 mmHg for more than 20 min9. Because phenylephrine was the main inotrope used if necessary during surgery, its use and dose were recorded. Total urine output and urine output per minute during operation were also recorded. Morbidity was reported after exclusion of AKI. Complications were graded according to the Clavien–Dindo classification18. PHLF was defined according to the 50–50 criteria on postoperative day 5 (serum bilirubin over 50 μmol/l and international normalized ratio more than 1.7)19. Operative mortality was defined as any patient death within 90 days after operation. Overall survival (OS) was calculated from time of hepatectomy to date of death or last available follow-up. Disease-free survival (DFS) was calculated from time of hepatectomy to date of diagnosis of first recurrence or last available follow-up.
Statistical analysis
Continuous data, expressed as mean(s.d.), were compared using the Student’s t test. Categorical data are expressed as number (percentage), with analysis using the χ2 test or Fisher’s exact test. Receiver operating characteristic (ROC) curves were used to identify cut-off points for BP, operative blood loss, and operating time. All areas under curve for these three variables were statistically significant (P < 0.050), ranging from 0.617 to 0.726. The cut-off points were calculated using the Youden index with satisfactory sensitivity, ranging from 0.603 to 0.858. Even though specificity was not good at the intraoperative BP cut-off point of 72.1 mmHg, the sensitivity was high (0.858). ROC curves and tables are shown in Fig. S1.
Univariate and multivariable analyses for the occurrence of AKI were carried out by logistic regression. Only variables considered to be clinical factors associated with AKI and variables with P < 0.100 were included. Survival data were summarized using the Kaplan–Meier method and compared by the log rank test. Cox regression was used in univariate and multivariable analysis of OS for patients with HCC.
The logistic regression and Cox regression models were selected by stepwise regression. The performance of the selected variables was evaluated using the Omnibus test at each step of stepwise regression. The P value of the test was less than 0.05 at each step. This indicated that the model had shown significant improvement. Therefore, the selected variables had good performance in the final model. The level of significance was set at 0.050 for all tests. Two-tailed tests were performed in all statistical testing. All statistical analysis was done using SPSS® version 25 (IBM, Armonk, NY, USA).
Results
Between September 2007 and December 2018, 930 patients underwent partial hepatectomy in this centre. Of these, 560 had histologically proven HCC (Table 1). The incidence of AKI was 9.2 per cent. The distribution of patients by grade of AKI was: 60 patients (69.8 per cent) in stage 1, 19 patients (22.0 per cent) in stage 2, and seven patients (8.2 per cent) in stage 3. Six patients needed renal replacement therapy as a result of AKI. Sixty patients (69.8 per cent) had complete recovery of renal function on discharge or follow-up. Of the remaining 26 patients, six died in hospital, seven needed long-term dialysis, and 13 patients had CKI without dialysis.
Table 1.
Demographics, operative characteristics, and postoperative outcomes of patients with or without acute kidney injury
| AKI | No AKI | P | Standardized difference (%) | |
|---|---|---|---|---|
| (n = 86) | (n = 844) | |||
| Age (years)* | 62.9(10.7) | 60.0(10.8) | 0.016# | 27.4 |
| Sex ratio (M : F) | 72 : 14 | 619 : 225 | 0.036 | 25.5 |
| BMI (kg/m2)* | 24.7(4.3) | 23.6(5.4) | 0.078# | 22.5 |
| ASA fitness grade | 0.082 | 29.1 | ||
| I | 4 (4.7) | 96 (11.4) | ||
| II | 64 (74.4) | 629 (74.5) | ||
| III | 17 (19.8) | 113 (13.4) | ||
| IV | 1 (1.2) | 6 (0.7) | ||
| Diabetes mellitus | 41 (47.7) | 216 (25.6) | < 0.001 | 47.1 |
| Hypertension | 53 (61.6) | 314 (37.2) | < 0.001 | 50.4 |
| Chronic kidney injury | 15 (17.4) | 63 (7.5) | < 0.001 | 30.6 |
| MELD score* | 8.5(3.6) | 7.4(1.7) | 0.004# | 40.9 |
| Surgical approach | 0.018 | 30.5 | ||
| Open | 80 (93.0) | 703 (83.3) | ||
| Laparoscopic or robotic | 6 (7.0) | 141 (16.7) | ||
| Intraoperative CVP (mmHg)* | 5.6(2.6) | 5.7(2.8) | 0.706# | −4.4 |
| Mean intraoperative BP (mmHg)* | 78.4(10.3) | 83.1(10.7) | < 0.001# | −44.2 |
| < 72.1 | 31 (36.0) | 120 (14.2) | < 0.001 | 52.0 |
| Intraoperative urine output (ml)*† | 400.9(257.1) | 366.7(280.6) | 0.352# | 12.7 |
| Intraoperative urine output (ml/min)*† | 1.20(0.74) | 1.41(1.03) | 0.093# | −19.4 |
| Phenylephrine use‡ | 49 (67.1) | 364 (47.3) | < 0.001 | 41.0 |
| Dose of phenylephrine used (μg)*‡ | 1538(2290) | 997(1674) | 0.115# | 27.0 |
| Intraoperative adverse event§ | 21 (29.2) | 132 (16.9) | 0.010 | 29.3 |
| Pringle manoeuvre | 7 (8.1) | 114 (13.5) | 0.159 | −17.3 |
| Duration of operation (min)* | 323.0(144.5) | 267.4(132.8) | 0.001# | 40.1 |
| > 266 | 54 (62.8) | 331 (39.2) | < 0.001 | 48.5 |
| Blood loss (ml)* | 1014(1400) | 459(545) | 0.001# | 52.2 |
| > 377 | 66 (76.7) | 331(39.2) | < 0.001 | 82.2 |
| Blood transfusion | 20 (23.3) | 82 (9.7) | < 0.001 | 37.1 |
| 90-day mortality | 9 (10.5) | 9 (1.1) | < 0.001 | 41.2 |
| Postoperative complications | 54 (62.8) | 210 (24.9) | < 0.001 | 82.7 |
| PHLF | 7 (8.1) | 10 (1.2) | < 0.001 | 33.4 |
| Duration of postoperative hospital stay (days)* | 15.5 (10.7) | 8.6 (7.2) | < 0.001# | 75.9 |
| Cirrhosis | 33 (38.4) | 282 (33.4) | 0.355 | 10.4 |
| Aetiology of cirrhosis | ||||
| Hepatitis B | 25 (29.1) | 237 (28.1) | 0.846 | 2.2 |
| Hepatitis C | 5 (5.8) | 22 (2.6) | 0.095 | 16.0 |
| Alcoholic liver disease | 2 (2.3) | 8 (0.9) | 0.235 | 11.2 |
| Non-alcoholic fatty liver disease | 1 (1.2) | 12 (1.4) | 1.000 | −1.8 |
| Autoimmune hepatitis | 0 (0) | 1 (0.1) | 1.000 | −4.5 |
| Wilson’s disease | 0 (0) | 1 (0.1) | 1.000 | −4.5 |
| Cryptogenic cirrhosis | 0 (0) | 1 (0.1) | 1.000 | −4.5 |
| Child–Pugh grade | 0.334 | −8.8 | ||
| A | 82 (95.3) | 818 (97.0) | ||
| B | 4 (4.7) | 25 (3.0) | ||
| Pathology | ||||
| Hepatocellular carcinoma | 58 (67.4) | 502 (59.5) | 0.151 | 16.4 |
| Metastasis | 9 (10.5) | 193 (22.9) | 0.008 | −33.7 |
| Colorectal liver metastasis | 8 (9.3) | 181 (21.4) | 0.008 | −34.1 |
| Nasopharyngeal carcinoma metastasis | 0 (0) | 4 (0.5) | 1.000 | −10.0 |
| Gastrointestinal stromal tumour metastasis | 0 (0) | 3 (0.4) | 1.000 | −9.0 |
| Pancreatic metastasis | 0 (0) | 2 (0.2) | 1.000 | −6.3 |
| Adrenal carcinoma metastasis | 1 (1.2) | 0 (0) | 0.092 | 15.6 |
| Ovarian carcinoma metastasis | 0 (0) | 1 (0.1) | 1.000 | −4.5 |
| Breast carcinoma metastasis | 0 (0) | 1 (0.1) | 1.000 | −4.5 |
| Melanoma metastasis | 0 (0) | 1 (0.1) | 1.000 | −4.5 |
| Cholangiocarcinoma | 5 (5.8) | 35 (4.1) | 0.407 | 7.8 |
| Recurrent pyogenic cholangitis | 1 (1.2) | 39 (4.6) | 0.167 | −20.4 |
| Carcinoma of gallbladder | 6 (7) | 16 (1.9) | 0.012 | 24.9 |
| Hepatocellular adenoma | 1 (1.2) | 8 (0.9) | 0.584 | 2.9 |
| Neuroendocrine tumour | 0 (0) | 7 (0.8) | 1.000 | −12.7 |
| Haemangioma | 0 (0) | 6 (0.7) | 1.000 | −11.9 |
| Others | 6 (7.0) | 38 (4.5) | 0.286 | 10.8 |
Values in parentheses are percentages unless indicated otherwise; *values are mean(s.d.). Missing values (acute kidney injury (AKI) and no AKI): †23 and 159, ‡13 and 74, §14 and 65. MELD, Model for End-Stage Liver Disease; CVP, central venous pressure; PHLF, posthepatectomy liver failure. ¶χ2 or Fisher’s exact test, except #Student’s t test.
Patient demographics, operative characteristics, and postoperative outcomes of patients with and without AKI are shown on Table 1. Patients with AKI were older, more likely to be men, had more DM, hypertension, and CKI, and a higher MELD score. During surgery, patients with AKI had lower BP, more open hepatectomy, longer duration of operation, more blood loss, more blood transfusion, more adverse events, and more patients needed phenylephrine. The cut-off points for BP, operating time, and blood loss were 72.1 mmHg, 266 min, and 377 ml respectively from ROC curves. AKI was associated with increased mortality, minor, major, and overall complications, as well as longer hospital stay. Patients with AKI also had significantly more PHLF (Table 1).
Logistic regression analysis was undertaken to determine predictors of AKI (Table 2). In multivariable analysis, only study period December 2013 to December 2018, DM, BP below 72.1 mmHg, blood loss exceeding 377 ml, high MELD score, and PHLF were predictive factors for AKI after hepatectomy.
Table 2.
Univariate and multivariable logistic regression analysis to determine predictive factors for acute kidney injury
| Univariate analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| Odds ratio | P | Odds ratio | P | |
| Study interval | ||||
| Sept 2007 to Nov 2013 | 0.56 (0.36, 0.89) | 0.01 | 0.48 (0.27, 0.86) | 0.02 |
| Dec 2013 to Dec 2018 | 1.00 (reference) | 1.00 (reference) | ||
| Age (per year) | 1.03 (1.01, 1.05) | 0.02 | ||
| Sex | ||||
| M | 1.87 (1.87, 3.38) | 0.04 | ||
| F | 1.00 (reference) | |||
| BMI (per kg/m2) | 1.02 (0.99, 1.06) | 0.13 | ||
| ASA fitness grade | ||||
| I–II | 0.62 (0.36, 1.08) | 0.09 | ||
| >III | 1.00 (reference) | |||
| Diabetes mellitus | 2.65 (1.69, 4.16) | < 0.01 | 2.36 (1.34, 4.17) | <0.01 |
| Hypertension | 2.71 (1.72, 4.28) | < 0.01 | ||
| Chronic kidney injury | 2.62 (1.42, 4.84) | < 0.01 | ||
| MELD score | 1.21(1.11, 1.32) | < 0.01 | 1.15 (1.01, 1.30) | 0.04 |
| Surgical approach | ||||
| Open | 2.67 (1.14, 6.25) | 0.02 | ||
| Laparoscopic/robotic | 1.00 (reference) | |||
| Pringle manoeuvre | 0.57 (0.26, 1.26) | 0.16 | ||
| Intraoperative CVP (per mmHg) | 0.99 (0.91, 1.07) | 0.71 | ||
| Mean intraoperative BP < 72.1 mmHg | 3.40 (2.10, 5.50) | < 0.01 | 2.08 (1.10, 3.91) | 0.02 |
| Duration of operation > 266 min | 2.62 (1.65, 4.14) | < 0.01 | ||
| Blood loss > 377 ml | 5.12 (3.04, 8.59) | < 0.01 | 5.22 (2.66, 10.24) | < 0.01 |
| PHLF | 7.39 (2.74, 19.95) | < 0.01 | 4.14 (1.24, 13.90) | 0.02 |
| Intraoperative urine output (per ml) | 1.00 (1.00, 1.00) | 0.35 | ||
| Intraoperative urine output (per ml/min) | 0.81 (0.59, 1.11) | 0.18 | ||
| Phenylephrine use | 2.28 (1.37, 3.79) | < 0.01 | ||
| Dose of phenylephrine used (per μg) | 1.00 (1.00, 1.00) | < 0.01 | ||
| Intraoperative adverse event | 2.02 (1.17, 3.47) | 0.01 | ||
Values in parentheses are 95 per cent confidence intervals. MELD, Model for End-Stage Liver Disease; CVP, central venous pressure; PHLF, posthepatectomy liver failure. Odds ratios for continuous variables are shown per unit.
In the subgroup of patients with HCC, 58 of 560 patients (10.3 per cent) developed AKI after surgery. Forty-two patients (72.4 per cent) had stage 1, 11 (20.0 per cent) stage 2, and five (8.6 per cent) had stage 3 AKI. All patients with stage 3 disease required renal replacement therapy; 38 patients (65.5 per cent) had normalized renal function on discharge or follow-up.
Patient demographics and operative characteristics of patients with HCC who did or did not have AKI are summarized in Table 3. Patients with AKI were older, had a higher BMI, more DM, hypertension, and CKI, and a higher ALP level and MELD score. During surgery, patients with AKI had lower BP, a longer operating time, more blood loss, more transfusion, and more patients needed phenylephrine. The cut-off points determined from ROC curves for BP, operating time, and blood loss were 76.9 mmHg, 266 min, and 378 ml respectively. Histopathological findings in the HCC cohort with and without AKI are shown in Table 4. In multivariable analysis, hypertension, BP less than 76.9 mmHg, blood loss exceeding 378ml, MELD score, and PHLF were predictive factors for AKI after hepatectomy in patients with HCC (Table 5).
Table 3.
Demographics and operative characteristics of patients with hepatocellular carcinoma with or without acute kidney injury
| AKI | No AKI | P ¶ | Standardized difference (%) | |
|---|---|---|---|---|
| (n = 58) | (n = 502) | |||
| Age (years)* | 63.1 (9.7) | 59.5 (9.9) | 0.009# | 36.8 |
| Sex (M : F) | 51 : 7 | 427 : 75 | 0.558 | 8.4 |
| BMI (kg/m2)* | 25.0 (4.2) | 23.8 (3.6) | 0.023# | 29.8 |
| ASA fitness grade | 0.738 | 21.1 | ||
| I | 3 (5.2) | 40 (8.0) | ||
| II | 45 (77.6) | 392 (78.1) | ||
| III | 10 (17.2) | 65 (12.9) | ||
| IV | 0 (0.0) | 5 (1.0) | ||
| Diabetes mellitus | 30 (51.7) | 150 (29.9) | 0.001 | 45.6 |
| Hypertension | 37 (63.8) | 191 (38.0) | < 0.001 | 53.3 |
| Chronic kidney injury | 12 (20.7) | 40 (8.0) | 0.002 | 36.9 |
| Alkaline phosphatase (IU/l)* | 110.2 (68.5) | 92.4 (61.0) | 0.039# | 27.3 |
| MELD score* | 8.6 (3.4) | 7.5 (1.8) | 0.024# | 38.8 |
| Cirrhosis | 32 (55.2) | 271 (54.0) | 0.863 | 2.4 |
| Aetiology of cirrhosis | ||||
| Hepatitis B | 25 (43.1) | 231 (46.0) | 0.673 | −5.8 |
| Hepatitis C | 5 (8.6) | 20 (4.0) | 0.166 | 19.0 |
| Alcoholic liver disease | 1 (1.7) | 7 (1.4) | 0.585 | 2.4 |
| Non-alcoholic fatty liver disease | 1 (1.7) | 10 (2.0) | 1.000 | −2.2 |
| Autoimmune hepatitis | 0 (0) | 1 (0.2) | 1.000 | −6.3 |
| Wilson’s disease | 0 (0) | 1 (0.2) | 1.000 | −6.3 |
| Cryptogenic cirrhosis | 0 (0) | 1 (0.2) | 1.000 | −6.3 |
| Child–Pugh grade | 0.418 | −11.1 | ||
| A | 55 (94.8) | 487 (97.0) | ||
| B | 3 (5.2) | 15 (3.0) | ||
| Surgical approach | ||||
| Open | 53 (91.4) | 415 (82.7) | ||
| Laparoscopic or robotic | 5 (8.6) | 87 (17.3) | 0.090 | 26.1 |
| Intraoperative CVP (mmHg)* | 5.6 (2.7) | 5.7 (2.7) | 0.916# | −1.5 |
| Mean intraoperative BP (mmHg)* | 77.7 (10.4) | 82.3 (10.4) | 0.001# | −44.4 |
| < 76.9 | 31 (53.4) | 153 (30.5) | < 0.001 | 47.9 |
| Intraoperative urine output (ml)*† | 336.9 (229.3) | 339.9 (256.5) | 0.941# | −1.2 |
| Intraoperative urine output (ml/min)*† | 1.24 (0.83) | 1.38 (1.05) | 0.371# | −14.8 |
| Phenylephrine use‡ | 34 (69.4) | 230 (50.1) | 0.010 | 40.1 |
| Dose of phenylephrine used (μg)*‡ | 1539 (2479) | 992 (1447) | 0.218# | 26.9 |
| Intraoperative adverse event§ | 14 (29.2) | 87 (18.8) | 0.086 | 24.5 |
| Pringle manoeuvre | 6 (10.3) | 85 (16.9) | 0.198 | −19.3 |
| Duration of operation (min)* | 276.9 (78.7) | 249.6 (84.3) | 0.019# | 33.5 |
| > 266 | 35 (60.3) | 175 (34.9) | < 0.001 | 52.8 |
| Blood loss (ml)* | 1072 (1604) | 486 (600) | 0.008# | 48.4 |
| > 378 | 45 (77.6) | 196 (39.0) | < 0.001 | 84.9 |
| Blood transfusion | 14 (24.1) | 45 (9.0) | < 0.001 | 41.7 |
Values in parentheses are percentages unless indicated otherwise; *values are mean(s.d.). Missing values (acute kidney injury (AKI) and no AKI): †15 and 96, ‡9 and 43, §10 and 39. MELD, Model for End-Stage Liver Disease; CVP, central venous pressure. ¶χ2 or Fisher’s exact test, except #Student’s t test.
Table 4.
Histopathological findings and operative outcomes of patients with hepatocellular carcinoma with or without acute kidney injury
| AKI | No AKI | P † | |
|---|---|---|---|
| (n = 58) | (n = 502) | ||
| Tumour size (cm)* | 5.9 (4.0) | 5.0 (3.7) | 0.068‡ |
| No. of tumours | |||
| 1 | 33 (56.9) | 384 (76.5) | 0.001 |
| 2 | 6 (10.3) | 43 (8.6) | 0.650 |
| 3 | 4 (6.9) | 11 (2.2) | 0.059 |
| ≥ 4 | 15 (25.9) | 64 (12.7) | 0.007 |
| Satellite lesion | 24 (41.4) | 98 (19.5) | < 0.001 |
| Vascular invasion | 22 (37.9) | 174 (34.7) | 0.621 |
| Differentiation | |||
| Well | 8 (14.3) | 58 (11.8) | 0.617 |
| Moderate | 43 (76.8) | 386 (78.6) | 0.639 |
| Poor | 5 (8.9) | 47 (9.6) | 0.853 |
| AJCC stage | |||
| I | 23 (39.7) | 278 (55.4) | 0.023 |
| II | 16 (27.6) | 131 (26.1) | 0.807 |
| III | 16 (27.6) | 91 (18.1) | 0.083 |
| IV | 3 (5.2) | 2 (0.4) | 0.009 |
| Resection margin (cm)* | 1.34(1.09) | 1.42 (1.16) | 0.627‡ |
| Clear margin | 58 (100) | 495 (98.6) | 1.000 |
| Child-Pugh grade | 0.418 | ||
| A | 55 (94.8) | 487 (97.0) | |
| B | 3 (5.2) | 15 (3.0) | |
| Cirrhosis | 32 (55.2) | 271 (54.0) | 0.863 |
| Ruptured HCC | 9 (10.3) | 30 (6.0) | 0.250 |
| 90-day mortality | 6 (10.3) | 5 (1.0) | 0.003 |
| Postoperative complications | 36 (62.1) | 122 (24.3) | < 0.001 |
| Clavien–Dindo grade of complication | |||
| I | 2 (3.4) | 42 (8.4) | 0.299 |
| II | 13 (22.4) | 41 (8.2) | < 0.001 |
| III | 12 (20.7) | 36 (7.2) | 0.002 |
| IV | 4 (6.9) | 1 (0.2) | < 0.001 |
| V | 5 (8.6) | 2 (0.4) | < 0.001 |
| PHLF | 5 (8.6) | 5 (1.0) | 0.002 |
| Duration of postoperative hospital stay (days)* | 13.3 (7.7) | 8.2 (5.5) | < 0.001‡ |
Values in parentheses are percentages unless indicated otherwise; *values are mean(s.d.). AKI, acute kidney injury; HCC, hepatocellular carcinoma; PHLF, posthepatectomy liver failure. †χ2 or Fisher’s exact test, except ‡Student’s t test.
Table 5.
Univariate and multivariable logistic regression analysis to determine predictive factors for acute kidney injury in patients with hepatocellular carcinoma
| Univariate analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| Odds ratio | P | Odds ratio | P | |
| Study period | ||||
| Sept 2007 to Nov 2013 | 0.58 (0.33, 1.01) | 0.05 | ||
| Dec 2013 to Dec 2018 | 1.00 (reference) | |||
| Age (per year) | 1.04 (1.01, 1.07) | 0.01 | ||
| Sex | ||||
| M | 1.28 (0.56, 2.93) | 0.56 | ||
| F | 1.00 (reference) | |||
| BMI (per kg/m2) | 1.08 (1.01, 1.16) | 0.03 | ||
| ASA fitness grade | ||||
| I–II | 0.78 (0.38, 1.61) | 0.50 | ||
| >II | 1.00 (reference) | |||
| Diabetes mellitus | 2.51 (1.45, 4.36) | < 0.01 | ||
| Hypertension | 2.87 (1.63, 5.05) | < 0.01 | 2.59 (1.33, 5.05) | 0.01 |
| Chronic kidney injury | 3.01 (1.48, 6.15) | < 0.01 | ||
| MELD score | 1.192 (1.08, 1.32) | < 0.01 | 1.151 (1.01, 1.31) | 0.04 |
| Surgical approach | ||||
| Open | 2.22 (0.86, 5.72) | 0.10 | ||
| Laparoscopic/robotic | 1.00 (reference) | |||
| Pringle manoeuvre | 0.57 (0.24 1.36) | 0.20 | ||
| Intraoperative CVP (per mmHg) | 1.00 (0.90, 1.10) | 0.92 | ||
| Mean intraoperative BP < 76.9 mmHg | 2.62 (1.51, 4.54) | < 0.01 | 2.24 (1.17 4.27) | 0.01 |
| Duration of operation > 266 min | 2.84 (1.63, 4.96 | < 0.01 | ||
| Blood loss > 378 ml | 5.40 (2.84, 10.28) | < 0.01 | 4.16 (2.05, 8.41) | < 0.01 |
| PHLF | 8.16(2.64, 25.19) | < 0.01 | 7.66 (1.73, 33.97) | 0.01 |
| Intraoperative urine output (per ml) | 1.00 (1.00, 1.00) | 0.94 | ||
| Intraoperative urine output (per ml/min) | 0.84(0.58, 1.22) | 0.84 | ||
| Phenylephrine use | 2.26 (1.20, 4.26) | 0.01 | ||
| Dose of phenylephrine used (per μg) | 1.00 (1.00, 1.00) | 0.01 | ||
| Intraoperative adverse event | 1.78 (0.92, 3.46) | 0.09 | ||
Values in parentheses are 95 per cent confidence intervals. MELD, Model for End-Stage Liver Disease; CVP, central venous pressure; PHLF, posthepatectomy liver failure. Odds ratios for continuous variables are shown per unit.
At a mean(s.d.) follow-up of 55.4(37.9) months in patients with HCC, there was no difference in tumour recurrence rate (AKI 51.7 per cent, no AKI 51.2 per cent). The 1-, 3-, and 5-year tumour recurrence rates were 31.0, 43.1, and 48.3 per cent respectively for the AKI group, and 26.9, 42.4, and 47.8 per cent for the group without AKI. There were significantly more patient deaths at each time point in the AKI group during follow-up (P = 0.010). The 1-, 3-, and 5-year mortality rates were 25.9, 39.7, and 44.8 per cent respectively among patients with AKI, and 8.2, 20.9, and 28.5 per cent in those without AKI. The 1-, 3-, and 5-year OS rates were 74.1, 59.2, and 51.6 per cent respectively in the AKI group, and 91.8, 77.9, and 67.3 per cent in the no-AKI group. The 1-, 3-, and 5-year DFS rates were 56.9, 42.3, and 35.4 per cent respectively among those with AKI, and 71.7, 54.5, and 46.2 per cent in the group without AKI. Both OS and DFS were significantly shorter in patients with AKI (P < 0.001 and P = 0.002 respectively) (Fig. 1). The differences remained significant when patients who died within 90 days of surgery were excluded from survival analysis (Fig. S2). Even in patients without HCC, both OS and DFS were significantly shorter among those with AKI (Fig. S3).
Fig. 1.
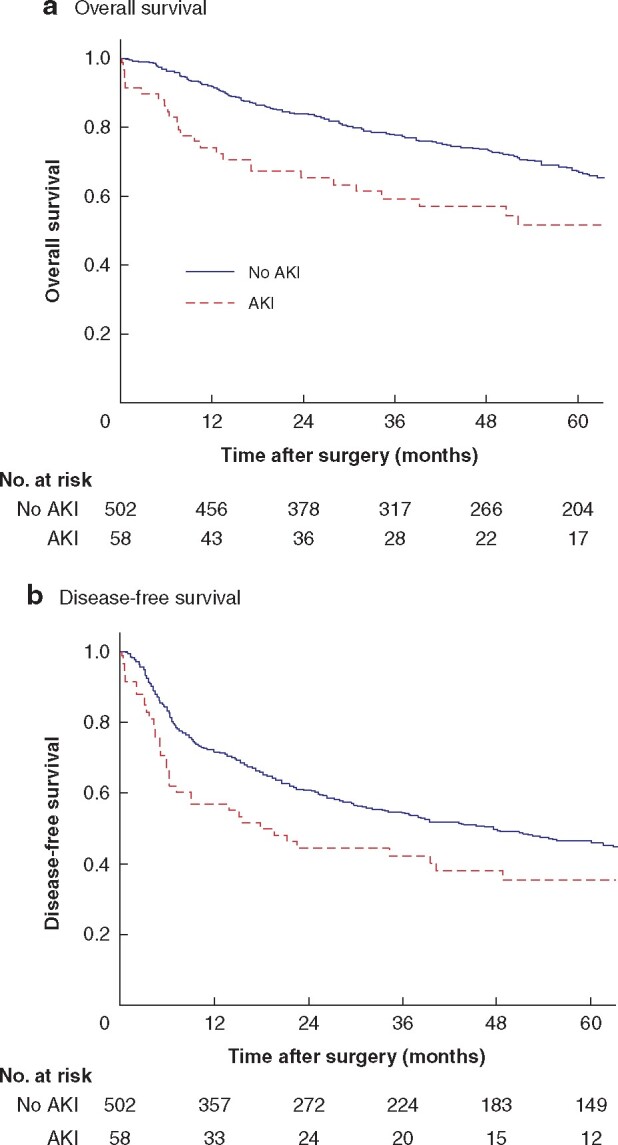
Overall and disease-free survival in patients with hepatocellular carcinoma with or without acute kidney injury
a Overall and b disease-free survival. AKI, acute kidney injury. aP < 0.001, bP = 0.002 (log rank test).
Univariate and multivariable analysis was undertaken to identify predictive factors affecting survival after hepatectomy in patients with HCC (Table 6). In multivariable analysis, AKI, PHLF, ASA fitness grade, cirrhosis, size of tumour, vascular invasion, and ruptured HCC were predictors of survival.
Table 6.
Univariate and multivariable Cox regression analysis to determine predictive factors affecting survival of patients with hepatocellular carcinoma after hepatectomy
| Univariate analysis |
Multivariable analysis* |
|||
|---|---|---|---|---|
| Hazard ratio | P | Hazard ratio | P | |
| AKI | 2.16 (1.50, 3.12) | < 0.01 | 2.10 (1.45, 3.05) | < 0.01 |
| PHLF | 5.54 (2.84, 10.82) | < 0.01 | 3.55 (1.77, 7.11) | < 0.01 |
| Age (per year) | 1.01(1.00, 1.02) | 0.23 | ||
| Sex | ||||
| M | 1.53 (0.99, 2.36) | 0.06 | ||
| F | 1.00 (reference) | |||
| BMI (per kg/m2) | 0.99 (0.95, 1.03) | 0.54 | ||
| ASA fitness grade | ||||
| I–II | 0.42 (0.30, 0.57) | < 0.01 | 0.64 (0.45, 0.92) | 0.01 |
| > II | 1.00 (reference) | 1.00 (reference) | ||
| Child-Pugh grade | ||||
| A | 0.36 (0.21, 0.63) | < 0.01 | ||
| B | 1.00 (reference) | |||
| MELD score | 1.10 (1.05, 1.15) | < 0.01 | ||
| Surgical approach | ||||
| Open | 1.58 (1.04, 2.40) | 0.03 | ||
| Laparoscopic/robotic | 1.00 (reference) | |||
| Hepatectomy | ||||
| Major | 1.17 (0.89, 1.53) | 0.26 | ||
| Minor | (reference) | |||
| Pringle manoeuvre | 0.79 (0.55, 1.15) | 0.23 | ||
| Duration of operation (per min) | 1.00 (1.00, 1.00) | <0.01 | ||
| Blood loss (per ml) | 1.00 (1.00, 1.00) | < 0.01 | ||
| Cirrhosis | 1.27 (0.96, 1.67) | 0.09 | 1.58 (1.16, 2.154) | <0.01 |
| Tumour size (per cm) | 1.12 (1.09, 1.15) | < 0.01 | 1.11 (1.07, 1.14) | < 0.01 |
| Multinodular tumours | 2.54 (1.922, 3.35) | < 0.01 | ||
| Vascular invasion | 2.86 (2.17, 3.78) | < 0.01 | 2.09 (1.60, 2.83) | < 0.01 |
| Differentiation | ||||
| Well | 0.64 (0.33, 1.22) | 0.17 | ||
| Moderate | 0.96 (060, 1.55) | 0.88 | ||
| Poor | (reference) | |||
| Clear margin | 0.57 (0.18, 1.79) | 0.34 | ||
| Ruptured HCC | 3.10 (2.07, 4.65) | < 0.01 | 1.66 (1.04, 2.65) | 0.03 |
Values in parentheses are 95 per cent confidence intervals. AKI, acute kidney injury; PHLF, posthepatectomy liver failure; MELD, Model for End-Stage Liver Disease; HCC, hepatocellular carcinoma. *Stepwise regression. Hazard ratios for continuous varables are shown per unit.
Discussion
According to this study, AKI occurred in almost one in ten patients who underwent hepatectomy. The incidence was similar that in other studies4,5,11 using KDIGO criteria for AKI, reported as 15–16 per cent, and up to 21.6 per cent after major hepatectomy. Although nearly 70 per cent of patients with AKI eventually had complete recovery of renal function, having AKI was associated with increased hospital stay, morbidity and mortality. In patients with AKI, the mortality rate was tenfold higher and the morbidity rate was more than double that in patients without AKI. Similar adverse short-term outcomes were seen in other studies3,4,7. The impact of AKI on mortality and morbidity was even more profound after major hepatectomy5. The incidence of AKI in patients with HCC was only slightly higher than that of the whole group (10.3 versus 9.2 per cent). This may have been because the patients with HCC were a selected group with well preserved liver function (at least 95 per cent had Child’s grade A disease), so their operative outcomes did not differ much from those of patients without HCC.
The cause of AKI after hepatectomy is multifactorial. Among others, extensive blood loss and PHLF have been recognized as the two most important factors1. Extensive blood loss induces renal hypoperfusion and is often associated with the deleterious renal effect of red cell transfusion20. PHLF is associated with distributive circulatory changes and subsequent hepatorenal syndrome19. Conversely, whether AKI is a predictor of PHLF deserves further study as it is well known that the two organs are closely related. The predictive factors identified in the present study are in concordance with previous findings. Both blood loss and PHLF were significant predictors. The MELD score is a useful and extensively validated tool for predicting PHLF. High MELD score was found to be a predictor for AKI in other studies4,9. Patients with DM were susceptible to renal hypoperfusion owing to associated nephropathy. Other studies3,11 also showed that DM was a predictor of AKI. Mean BP was an important factor as the kidneys were most at risk from pressure drop. Kidneys could maintain their blood flow in the BP range between 80 and 120 mmHg. If BP decreased to below 80 mmHg, there was a significant drop in GFR21. This is in concordance with the present finding that mean intraoperative BP below 72.1 mmHg was a predictor of AKI after hepatectomy. However, the cut-off point for blood loss in this study was 377 ml, whereas in previous studies1,22,23 blood loss of 1250 ml was found to be a cut-off point for major complications such as kidney injury. It was interesting to note that the study period December 2013 to December 2018 was a predictive factor for AKI. This was likely because more difficult cases were subjected to hepatectomy in the later period and more concomitant procedures were performed, such as synchronous colectomy for colorectal liver metastasis. Neither DM nor study period December 2013 to December 2018 remained as risk factors in the HCC subgroup. The authors believe these might not be strong predictors and their significance was lost compared with that of other stronger factors when smaller numbers of patients were entered into subgroup analysis.
Although CVP was not a predictive factor for AKI in the present analysis, in another study24 the AKI rate was significantly higher in a low-CVP group (less than 5mmHg) compared with a group with normal CVP (7–10 mmHg). On the other hand, low CVP was associated with reduced blood loss25. In the present study, the CVP value was similar in the AKI and no-AKI groups, but BP was significantly lower in the AKI group. This implied that, although the aim was to maintain a low CVP in both groups, the AKI group needed to be compromised with a lower BP, even with the use of phenylephrine.
Similar findings were obtained when the subgroup of patients with HCC was analysed. In multivariable analysis, only hypertension, BP below 76.9 mmHg, blood loss exceeding 378 ml, MELD score, and PHLF were predictive factors for AKI. All these factors can be optimized with proper patient selection, and appropriate operative and anaesthetic technique. More interesting is the long-term impact of AKI on patients with HCC. In contrast to a previous study4 which showed that AKI did not affect OS, AKI had negative impact on both OS and DFS in the present analysis. As the tumour recurrence rate did not differ between the two groups, patients with AKI might more frequently die from causes other than recurrent disease. The occurrence of AKI might make patients more susceptible to infective disease or organ failure. Another explanation is earlier recurrent disease in the AKI group owing to impaired host immunity. Further large-scale studies are needed to verify this finding.
The drawbacks of this study lie in its retrospective nature. There were missing data on intraoperative parameters such as urine output, inotrope use, and adverse events, as these were not collected prospectively. Besides, data on CVP readings were incomplete as not all patients undergoing hepatectomy had a CVP line inserted. BP during operation might have fluctuated considerably, and BP values recorded might not truly reflect some periods of profound hypotension which seriously affected renal perfusion. The use of a single sCr value rather than a mean of several readings to determine the occurrence of AKI and CKI was also less than satisfactory.
In summary, AKI is common after hepatectomy and is associated with longer hospital stay, and greater morbidity and mortality. AKI is also associated with poorer long-term survival among patients with HCC. Predictive factors for AKI, such as underlying hypertension, diabetes, and MELD score, are not reversible. To avoid AKI, control of blood loss and maintenance of a reasonable BP (around 72–77 mmHg) during hepatectomy is important. Although lowering CVP by fluid restriction is an effective measure to lower blood loss, low BP secondary to fluid restriction has the counter effect. The authors recommend maintaining the mean intraoperative BP at around 72– 77 mmHg even if the CVP cannot be lowered below 5 mmHg. Good collaboration between liver surgeons and anaesthetists in this regard cannot be overemphasized.
Supplementary Material
Acknowledgements
The authors thank P. Ip for assistance with data keeping, processing, and statistical analysis.
Disclosure. All authors have no conflict of interest to declare.
Supplementary material
Supplementary material is available at BJS Open online.
References
- 1. Saner F. Kidney failure following liver resection. Transplant Proc 2008;40:1221–1224. [DOI] [PubMed] [Google Scholar]
- 2. Armstrong T, Welsh FK, Wells J, Chandrakumaran K, John TG, Rees M.. The impact of pre-operative serum creatinine on short-term outcomes after liver resection. HPB (Oxford) 2009;11:622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Slankamenac K, Breitenstein S, Held U, Beck-Schimmer B, Puhan MA, Clavien PA.. Development and validation of a prediction score for postoperative acute renal failure following liver resection. Ann Surg 2009;250:720–728. [DOI] [PubMed] [Google Scholar]
- 4. Lim C, Audureau E, Salloum C, Levesque E, Lahat E, Merle JC. et al. Acute kidney injury following hepatectomy for hepatocellular carcinoma: incidence, risk factors and prognostic value. HPB (Oxford) 2016;18:540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garnier J, Faucher M, Marchese U, Meillat H, Mokart D, Ewald J. et al. Severe acute kidney injury following major liver resection without portal clamping: incidence, risk factors, and impact on short-term outcomes. HPB (Oxford) 2018;20:865–871. [DOI] [PubMed] [Google Scholar]
- 6. Melendez JA, Arslan V, Fischer ME, Wuest D, Jarnagin WR, Fong Y. et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg 1998;187:620–625. [DOI] [PubMed] [Google Scholar]
- 7. Tomozawa A, Ishikawa S, Shiota N, Cholvisudhi P, Makita K.. Perioperative risk factors for acute kidney injury after liver resection surgery: an historical cohort study. Can J Anaesth 2015;62:753–761. [DOI] [PubMed] [Google Scholar]
- 8. Correa-Gallego C, Berman A, Denis SC, Langdon-Embry L, O'Connor D, Arslan-Carlon V. et al. Renal function after low central venous pressure-assisted liver resection: assessment of 2116 cases. HPB (Oxford) 2015;17:258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bredt LC, Peres LAB.. Risk factors for acute kidney injury after partial hepatectomy. World J Hepatol 2017;9:815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bressan AK, James MT, Dixon E, Bathe OF, Sutherland FR, Ball CG.. Acute kidney injury following resection of hepatocellular carcinoma: prognostic value of the acute kidney injury network criteria. Can J Surg 2018;61:E11–E16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim M, Kiran RP, Li G.. Acute kidney injury after hepatectomy can be reasonably predicted after surgery. J Hepatobiliary Pancreat Sci 2019;26:144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL. et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464–470. [DOI] [PubMed] [Google Scholar]
- 13. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179–c184. [DOI] [PubMed] [Google Scholar]
- 14. Levey AS, Stevens LA, Schmid CH, Zhang Y(L), Castro AF, Feldman HI. et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ciozda W, Kedan I, Kehl DW, Zimmer R, Khandwalla R, Kimchi A.. The efficacy of sonographic measurement of inferior vena cava diameter as an estimate of central venous pressure. Cardiovasc Ultrasound 2016;14:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stawicki SP, Braslow BM, Panebianco NL, Kirkpatrick JN, Gracias VH, Hayden GE. et al. Intensivist use of hand-carried ultrasonography to measure IVC collapsibility in estimating intravascular volume status: correlations with CVP. J Am Coll Surg 2009;209:55–61. [DOI] [PubMed] [Google Scholar]
- 17. Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 systems. J Hepatobiliary Pancreat Surg 2005;12:351–355. [DOI] [PubMed] [Google Scholar]
- 18. Dindo D, Demartines N, Clavien PA.. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D. et al. The ‘50–50 criteria’ on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg 2005;242:824–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peres LA, Bredt LC, Cipriani RF.. Acute renal injury after partial hepatectomy. World J Hepatol 2016;8:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med 2007;357:797–805. [DOI] [PubMed] [Google Scholar]
- 22. Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S. et al. Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg 2002;236:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K. et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg 2003;138:1198–1206. [DOI] [PubMed] [Google Scholar]
- 24. Schroeder RA, Collins BH, Tuttle-Newhall E, Robertson K, Plotkin J, Johnson LB. et al. Intraoperative fluid management during orthotopic liver transplantation. J Cardiothorac Vasc Anesth 2004;18:438–441. [DOI] [PubMed] [Google Scholar]
- 25. Jones RM, Moulton CE, Hardy KJ.. Central venous pressure and its effect on blood loss during liver resection. Br J Surg 1998;85:1058–1060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


