Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has had a severe impact on kidney transplantation (KT) programs worldwide.1 Since November 30, 2020, solid organ transplant recipients (SOTRs) and patients with end-stage renal disease have been prioritized for SARS-CoV-2 vaccination in France, using the BNT162b2 mRNA vaccine (Pfizer/BioNTech). Grupper et al2 found that 96% of 56 dialyzed patients developed anti-spike protein (anti-S) antibodies. In SOTRs, the seroconversion after 1 dose of mRNA vaccine is very low, varying between 3.8% and 15%.3-5 Boyarsky et al3 reported a better result after 2 doses of mRNA vaccine among a population of 658 SOTRs, in which 54% of patients developed anti-S antibodies. We decided to investigate the immediate effect of the induction treatment for KT on SARS-CoV-2 antibodies in vaccinated patients.
We report the evolution of anti-S antibody titers, quantified by the Alinity SARS-CoV-2 IgG II Quant assay (Abbott), in 9 KT recipients who were vaccinated before KT with the BNT162b2 mRNA vaccine (Pfizer/BioNTech). All patients provided informed consent to participate in this study. Seven patients had received 2 doses of vaccine, and 2 patients had received 1 dose. The mean age was 53.7 ± 11.7 y and 8 patients (89%) were male individuals. The mean delay between the last vaccine injection and transplantation was 20.1 ± 11.1 d. All patients received an induction treatment associating 500 mg of methylprednisolone and either antithymocyte globulin for 5 d (8 recipients of a cadaveric kidney) or basiliximab (1 recipient of a kidney from a living-donor). All patients received prednisone, mycophenolate mofetil, and tacrolimus during the entire duration of the study, except 1 patient who received 1 dose of belatacept before the second serology.
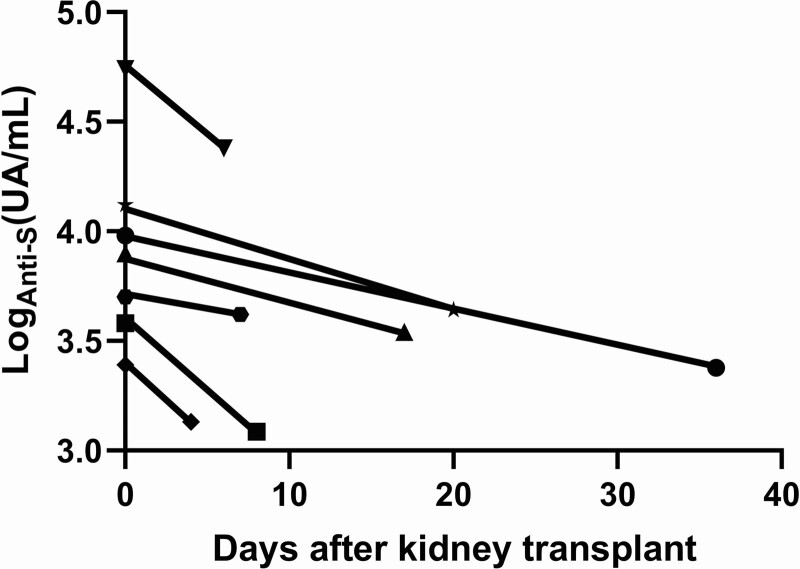
On the day of transplantation, no patients had SARS-CoV-2 antinucleocapsid antibody. All the patients who had received 2 vaccine doses had above 3.0 log (UA/mL) of anti-S antibodies (mean 3.6 ± 0.8 log [UA/mL]). In contrast, the 2 patients who had received only 1 dose of vaccine had lower antibody titers: 2.3 and 2.5 log (UA/mL), 18 and 22 d after vaccination, respectively. The evolution of anti-S levels after transplantation is shown in Figure 1. All patients who had received 2 doses of vaccine experienced a decrease in anti-S IgG titers. The mean decrease between the first and the second serology ([titer 1 – titer 2]/titer 1 × 100) was 55.0% ± 19.6% (P = 0.02 for the comparison of titers 1 with titers 2 by the Wilcoxon matched-pairs signed-rank test). The mean delay between the first and the second serology was 14.1 ± 11.7 d. The protective level of anti-S antibodies remains uncertain. However, none of the 7 patients who had received 2 doses of vaccine experienced a decrease below 3 log (UA/mL). As a comparison, hepatitis B virus anti-HbS antibodies measured on the same sera did not vary significantly (P = 0.2) and anti–varicella zoster virus antibodies decreased by 37.0% ± 47.4% (P = 0.05).
FIGURE 1.
Evolution of anti-S antibodies after induction therapy in patients who received 2 vaccine doses before transplantation. anti-S, anti-spike protein.
We did not explore the cellular response against SARS-CoV-2, which is also probably affected by the induction treatment. Our results tend to indicate that it is probably worth vaccinating waitlisted patients before KT with 2 doses of BNT162b2 mRNA vaccine.
Footnotes
The authors declare no funding or conflicts of interest.
I.M., P.G., M.C., N.A., B.B., D.B., and J.T.. participated in conception or design of the work. I.M., M.C., C.A., D.B., and J.T. participated in acquisition of data. I.M., D.B., and J.T. participated in analysis and/or interpretation of data. I.M., P.G., D.B., and J.T. participated in drafting the work or revising the article critically for important intellectual content. I.M., J.N., P.G, M.C., N.A., N.O., E.R., B.B., C.A., D.B., and J.T. approved the version of the article to be published.
REFERENCES
- 1.Ahn C, Amer H, Anglicheau D, et al. Global transplantation COVID report March 2020. Transplantation. 2020;104:1974–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grupper A, Sharon N, Finn T, et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2021;16:CJN.03500321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georgery H, Devresse A, Yombi J-C, et al. Very low immunization rate in kidney transplant recipients after one dose of the BNT162b2 vaccine: beware not to lower the guard! Transplantation. [Epub ahead of print. May 12, 2021]. doi:10.1097/TP.0000000000003818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi SG, Knight RJ, Graviss EA, et al. Kidney transplant recipients rarely show an early antibody response following the first COVID-19 vaccine administration. Transplantation. 2021;105:e72–e73. [DOI] [PubMed] [Google Scholar]