Supplemental Digital Content is available in the text.
Background.
Few studies have analyzed differences in clinical presentation and outcomes in solid organ transplant (SOT) recipients with coronavirus disease 2019 (COVID-19) across different pandemic waves.
Methods.
In this multicenter, nationwide, prospective study, we compared demographics and clinical features, therapeutic management, and outcomes in SOT recipients diagnosed with COVID-19 in Spain before (first wave) or after (second wave) 13 July 2020.
Results.
Of 1634 SOT recipients, 690 (42.2%) and 944 (57.8%) were diagnosed during the first and second periods, respectively. Compared with the first wave, recipients in the second were younger (median: 63 y [interquartile range, IQR: 53–71] versus 59 y [IQR: 49–68]; P < 0.001) and less likely to receive anti-severe acute respiratory syndrome coronavirus 2 drugs (81.8% versus 8.1%; P < 0.001), with no differences in immunomodulatory therapies (46.8% versus 47.0%; P = 0.931). Adjustment of immunosuppression was less common during the second period (76.4% versus 53.6%; P < 0.001). Hospital admission (86.7% versus 58.1%; P < 0.001), occurrence of acute respiratory distress syndrome (34.1% versus 21.0%; P < 0.001), and case-fatality rate (25.8% versus 16.7%; P < 0.001) were lower in the second period. In multivariate analysis, acquiring COVID-19 during the first wave was associated with an increased risk of death (OR: 1.47; 95% confidence interval [CI], 1.12-1.93; P = 0.005), although this impact was lost in the subgroup of patients requiring hospital (OR: 0.97; 95% CI, 0.73-1.29; P = 0.873) or intensive care unit admission (OR: 0.65; 95% CI, 0.35-1.18; P = 0.157).
Conclusions.
We observed meaningful changes in demographics, therapeutic approaches, level of care, and outcomes between the first and second pandemic waves. However, outcomes have not improved in the more severe cases of posttransplant COVID-19.
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic has had a profound impact on national transplant programs across the world, with a variable decrease in donation and transplantation activities and a large number of solid organ transplant (SOT) recipients acquiring the infection.1-3
The first case of COVID-19 in Spain was reported at the end of January 2020, with an exponentially increasing number of cases that lead to a saturation of the healthcare system and an overwhelmed intensive care unit (ICU) capacity.4 Case-fatality rates were over 10% during that first wave. A second pandemic wave started by mid-July 2020. Although the cumulative incidence of COVID-19 increased over time, the overall case-fatality rate progressively decreased, reaching 2.3% by the end of 2020.5 This trend likely responded to the increased diagnostic capacity of the system, which now allows for also identifying asymptomatic or mildly symptomatic cases. In accordance with this notion, the proportion of younger patients was also higher during the second wave.6
Many different studies have confirmed the vulnerability of SOT recipients to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, with higher mortality compared to the general population.7,8 This has been the case in Spain, where we have previously reported a case-fatality rate of over 25% during the first surge of the pandemic.9 This poor outcome seems to be largely conditioned by the demographic profile and the burden of comorbidity in the SOT population, rather than by immunosuppression.10-12 As the diagnostic capacity has been expanded and evidence has been built about the management of COVID-19, the question is whether the outcomes of transplant patients diagnosed with SARS-CoV-2 infection have improved over time.
The aim of this study was to describe the experience with SOT recipients diagnosed with COVID-19 during 2020 in Spain, comparing the clinical presentation, therapeutic management and outcomes between the first wave and the second wave of the pandemic.
MATERIALS AND METHODS
Study Setting and Design
This is a multicenter, nationwide, prospective study focused on SOT recipients who were diagnosed with COVID-19 in Spain throughout the entire 2020 year. We included both cases confirmed by molecular methods (ie, real-time reverse transcription polymerase chain reaction [RT-PCR]) in nasopharyngeal swab or lower respiratory tract sample) and those in which the diagnosis was based on compatible clinical and radiologic features with positive anti-SARS-CoV-2 serology. We compared their baseline demographic and clinical features, the therapeutic approaches and management of immunosuppression, as well as their outcomes, according to the date of diagnosis of SARS-CoV-2 infection: before 13 July 2020 (first wave), and from 13 July to 31 December 2020 (second wave). This calendar date was used as the cutoff date since July 13 marked the lowest number of diagnoses of COVID-19 in the general population in Spain since the onset of the pandemic.4 The study was approved by the National Transplant Committee of the Inter-Regional Council of the Spanish National Healthcare System.
Management of COVID-19 in SOT Recipients
The therapeutic approach to posttransplant COVID-19—including the use of antiviral agents, immunomodulatory therapies, and supportive care—was in accordance with the national and local guidelines in place, which have evolved throughout the study period in Spain according to the scientific evidence available at each time.13 Of note, the use of protease inhibitors (ie, lopinavir/ritonavir) or hydroxychloroquine was no longer recommended from June-July 2020 onwards due to the negative results from clinical trials and the unfavorable safety profile.
Data Collection
Since the beginning of the pandemic, the Spanish Organización Nacional de Trasplantes (ONT) launched a centralized data collection on SOT and hematopoietic stem cell transplant recipients diagnosed with COVID-19. Transplant teams submitted a standardized notification form that included information on demographics, baseline clinical characteristics, date of transplantation, date of diagnosis of COVID-19, and information on whether the infection had been acquired in the hospital or in the community and on whether the case was suspected to be donor-derived. In case of a suspected donor-derived COVID-19, the Disease Transmission Advisory Committee (DTAC) tool was used to assess the likelihood that the SARS-CoV-2 infection actually derived from the donor.14 An additional follow-up form was filled in with details on therapeutic approaches, intermediate outcomes (hospital admission, admission to the ICU, use of invasive mechanical ventilation, acute distress respiratory syndrome [ARDS] defined according to the Berlin criteria,15 septic shock, or multiorgan failure), and final outcomes (resolution of the infection [ie, clinical improvement with or without virologic evidence of clearance] or death).
Statistical Analysis
Qualitative variables are described as absolute numbers and percentages, that have been calculated on the total number of cases with available information. Quantitative variables are presented as the median and interquartile range (IQR), after checking their skewed distribution. To compare the study groups, we used the χ2 test with the Fischer correction where applicable for qualitative variables, whereas quantitate variables were compared with the median test.
To identify factors associated with COVID-19-related mortality, univariate (χ2 test with the Fischer correction, where applicable) and multivariate analysis were performed. Variables were included in a logistic regression model on the basis of those showing a P ≤ 0.10 in the univariate analysis. Associations are expressed as adjusted odds ratios (OR) with 95% confidence intervals (95% CI). The period of diagnosis (first versus second wave) was a priori entered into the model as explanatory variable.
To take into account the possibility of reporting bias during the first wave of the pandemic compared with the second wave (which would have skewed the sample towards less severe cases during the latter period), we undertook a sensitivity analysis only focused on patients who required hospital admission. This intermediate outcome may serve as a proxy for similar disease severity across the 2 consecutive waves since no formal changes were introduced in clinical guidelines during the study period regarding the criteria for hospital admission among SOT recipients with COVID-19. In addition, we performed a second sensitivity analysis restricted to patients who were admitted to the ICU.
The statistical analysis was performed with SPSS version 25.0 (IBM Corp., Armonk, NY).
RESULTS
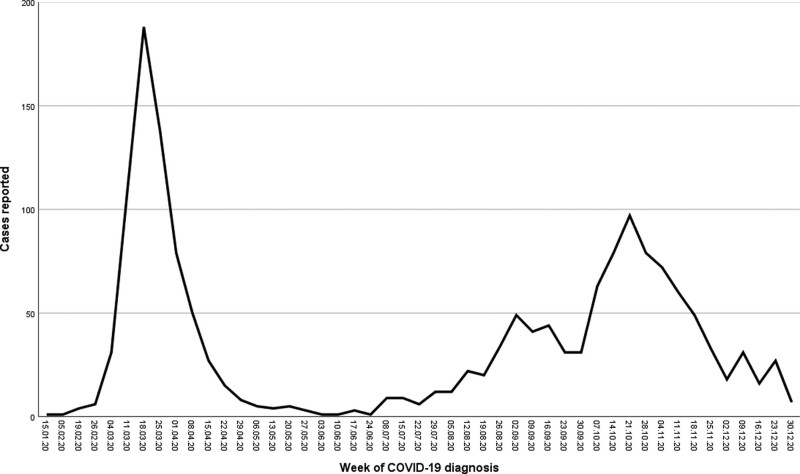
A total of 1634 SOT recipients diagnosed with COVID-19 between 20 February and 31 December 2020 were reported to ONT: 1063 kidney alone, 280 liver (including 14 combined liver–kidney), 149 heart (including 1 combined heart–kidney), 112 lungs, 27 pancreas (including 26 pancreas–kidney), and 3 mutivisceral transplant patients. Of those, 690 (42.2%) patients acquired the infection during the first wave and 944 (57.8%) during the second one. Figure 1 represents the evolution of notified cases by week of diagnosis, which mirrored those reported for the general population in Spain.4
FIGURE 1.
Evolution of the number of cases of COVID-19 in solid organ transplant recipients during 2020 in Spain by a week of diagnosis. COVID-19, coronavirus disease 2019.
Table 1 summarizes baseline features of the entire population and by period of diagnosis. Patient characteristics stratified by transplant type are provided in Supplementary material (Table S1, SDC, http://links.lww.com/TP/C261). Compared with recipients diagnosed with COVID-19 during the first wave, those diagnosed in the second wave were significantly younger. No statistically significant differences were observed in terms of sex, type of transplant, frequency of hospital-acquired COVID-19, and time since transplantation. There were significant differences between both groups in baseline immunosuppression. Recipients diagnosed with COVID-19 during the second wave were more frequently treated with calcineurin inhibitors and antimetabolites, and less frequently with mammalian target of rapamycin (mTOR) inhibitors. This difference in baseline immunosuppression was only evident, however, among kidney transplant recipients, but not in other SOT types (data not shown).
TABLE 1.
Baseline features, treatment, and outcomes of solid organ transplant recipients diagnosed with COVID-19 during 2020 in Spain. Global and by period of diagnosis
| GLOBAL (N = 1634) | First wave (date of diagnosis before 13 July, 2020) (N = 690) | Second wave (date of diagnosis after 13 July 2020) (N = 944) | P | |
|---|---|---|---|---|
| Baseline features | ||||
| Sex male, n (%) | 1075 (65.8%) | 459 (66.5%) | 616 (65.3%) | 0.594 |
| Age at diagnosis | ||||
| Years, median (IQR) | 61 (51–69) | 63 (53–71) | 59 (49–68) | <0.001 |
| >60 y, n (%) | 829 (50.7%) | 388 (56.2%) | 441 (46.7%) | <0.001 |
| Type of transplant | ||||
| Kidney | 1063 (65.1%) | 434 (62.9%) | 629 (66.6%) | |
| Liver | 280 17.1%) | 121 (17.5%) | 159 (16.8%) | |
| Heart | 149 (9.1%) | 66 (9.6%) | 83 (8.8%) | 0.110 |
| Lung | 112 (6.9%) | 60 (8.7%) | 52 (5.5%) | |
| Pancreas | 27 (1.7%) | 8 (1.2%) | 19 (2.0%) | |
| Multivisceral | 3 (0.2%) | 1 (0.1%) | 2 (0.2%) | |
| Hospital-acquired COVID-19, n (%) | 177 (10.9%) | 86 (12.6%) | 91 (9.7%) | 0.066 |
| Time since transplantation | ||||
| Months, median (IQR) | 71 (27–145) | 73 (27–151) | 70 (27–144) | 0.639 |
| Diagnosis within the first month, n (%) | 39 (2.4%) | 19 (2.8%) | 20 (2.1%) | 0.403 |
| Diagnosis beyond the first year, n (%) | 1417 (86.9%) | 594 (86.3%) | 823 (87.3%) | 0.580 |
| Baseline immunosuppression | N = 1627 | N = 689 | N = 938 | |
| Corticosteroids, n (%) | 1174 (72.2%) | 495 (71.8%) | 679 (72.4%) | 0.809 |
| Calcineurin inhibitor, n (%) | 1468 (90.2%) | 602 (87.4%) | 866 (92.3%) | 0.001 |
| Antimetabolite, n (%)a | 1209 (74.3%) | 492 (71.4%) | 717 (76.4%) | 0.022 |
| mTOR inhibitor, n (%) | 299 (18.4%) | 149 (21.6%) | 150 (16.0%) | 0.004 |
| Anti SARS-CoV-2 treatment | N = 1509 | N = 648 | N = 861 | |
| Any treatment, n (%) | 600 (39.8%) | 530 (81.8%) | 70 (8.1%) | <0.001 |
| Hydroxichloroquine, n (%) | 507 (33.6%) | 506 (78.1%) | 1 (0.1%) | <0.001 |
| Azithromycin, n (%) | 367 (24.3%) | 338 (52.2%) | 29 (3.4%) | <0.001 |
| Protease inhibitors, n (%) | 191 (12.7%) | 190 (29.3%) | 1 (0.1%) | <0.001 |
| Remdesivir, n (%) | 46 (3.0%) | 5 (0.8%) | 41 (4.8%) | <0.001 |
| Other antivirals, n (%)b | 8 (0.5%) | 4 (0.6%) | 4 (0.5%) | 0.731 |
| Interferon-β, n (%) | 26 (1.7%) | 26 (4.0%) | 0 (–) | <0.001 |
| Immunomodulatory treatment | N = 1510 | N = 648 | N = 862 | |
| Any treatment, n (%) | 708 (46.9%) | 303(46.8%) | 405 (47.0%) | 0.931 |
| Corticosteroids, n (%)c | 670 (44.4%) | 269(41.5%) | 401 (46.5%) | 0.053 |
| Tocilizumab, n (%) | 156 (10.3%) | 121(18.7%) | 35 (4.1%) | <0.001 |
| Anakinra, n (%) | 17 (1.1%) | 10(1.5%) | 7 (0.8%) | 0.183 |
| Outcomes | N = 1510 | N = 648 | N = 862 | |
| Hospital admission, n (%) | 1061 (70.4%) | 560(86.7%) | 501 (58.1%) | <0.001 |
| ICU admission, n (%) | 205 (13.8%) | 90(14.2%) | 115 (13.5%) | 0.709 |
| Invasive mechanical ventilation, n (%) | 155 (10.7%) | 64(10.8%) | 91 (10.7%) | 0.982 |
| ARDS, n (%) | 394 (26.6%) | 216(34.1%) | 178 (21.0%) | <0.001 |
| Septic shock, n (%) | 106 (7.2%) | 47(7.5%) | 59 (7.0%) | 0.730 |
| Multiorgan failure (%) | 151(10.2%) | 68(10.8%) | 83 (9.8%) | 0.556 |
| Death, n (%) | 311(20.6%) | 167(25.8%) | 144 (16.7%) | <0.001 |
aMainly mycophenolate.
bGancyclovir (n = 3), ribavirine (n = 2), oseltamivir (n = 2), raltegravir (n = 1).
cIncludes corticosteroid boluses, initiation of low-to-moderate doses or increase of baseline dose.
ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; ICU, intensive care unit; IQR, interquartile range; mTOR, mammalian target of rapamycin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Only 39 (2.4%) patients developed the infection during the first month after transplantation. These patients accounted for 0.9% of the 4095 SOT performed during the period extending from the date of the first diagnosis until 31 December 2020. In 4 cases, suspicion of donor-derived COVID-19 was raised. According to the DTAC algorithm and after a detailed investigation, the donor origin of the infection was excluded in all of them.
Table 1 also provides information on the management of the infection, both globally and by period of diagnosis. Repurposed drugs with supposed anti-SARS-CoV-2 activity were significantly less frequent during the second wave (81.8% versus 8.1%; P < 0.001). In detail, the use of hydroxychloroquine, azithromycin and protease inhibitors, quite frequent during the first wave, was almost abandoned beyond July 2020. On the contrary, the use of remdesivir significantly increased in the second wave, particularly among patients admitted to the hospital (8.2%) and among those who were admitted to the ICU (10.4%). With regards to immunomodulatory therapies, these were similarly administered in the 2 periods. The use of corticosteroids—either at low-to-intermediate doses or as boluses—increased during the second wave, this being more evident among patients admitted to the hospital (73.9%) and those admitted to the ICU (92.2%). On the contrary, the use of tozilizumab decreased throughout the second wave. Variations across the study period regarding the management of immunosuppression are displayed in Figure 2. Adjustment of baseline immunosuppression was significantly less common during the second wave for all types of immunosuppressive drugs.
FIGURE 2.
Management of baseline immunosuppression in solid organ transplant recipients diagnosed with COVID-19 according to the period of diagnosis. COVID-19, coronavirus disease 2019; mTOR, mammalian target of rapamycin.
At the time of submission of this report, follow-up information was available for 1510 of the 1634 cases reported (92.4%). Table 1 summarizes the outcomes of the entire series, and by the period of diagnosis. Of note, the need for hospital admission, the occurrence of ARDS and the case-fatality rate were significantly lower in the second wave. During the first wave, despite ARDS was developed by 216 (34.1%) patients, only 90 (14.0%) were admitted to the ICU. Corresponding percentages in the second wave were 21.0% and 13.5%, respectively. Within the group of hospitalized patients, 16.4% were admitted to the ICU during the first wave compared to 23.4% during the second (P = 0.004). The decrease in case-fatality rate during the second wave only reached statistical significance for kidney transplant recipients, evolving from 26.8% to 15.5% (P < 0.001). Differences in the corresponding rates in liver (20.4% versus 15.3%; P = 0.279), heart (19.7% versus 14.5%; P = 0.395), lung (40.0% versus 40.4%; P = 0.967), and pancreas (0% versus 7.1%; P = 1.000) recipients were not statistically significant. Of note, when the comparison was restricted to hospitalized patients, case-fatality rates did not decrease in the second compared with the first wave (28.9% versus 27.7%; P = 0.669). On the other hand, there was a nonsignificant trend toward a higher case-fatality rate among patients admitted to the ICU in the second wave (54.4% versus 66.1%; P = 0.090), with no differences in the subgroup who developed ARDS (63.0% versus 68.0%; P = 0.298).
Table 2 details the results of univariate and multivariate (logistic regression) analyses to identify factors associated with mortality in the entire series. Risk factors for COVID-19-related death were: lung versus other transplant types, recipient age >60 y, hospital-acquired versus community-acquired COVID-19, and diagnosis of COVID-19 during the first posttransplant year. Importantly, acquiring COVID-19 during the first wave was significantly associated with an increased risk of death as compared to the second wave (OR: 1.47; 95% CI, 1.12-1.93; P = 0.005)
TABLE 2.
Univariate and multivariate (logistic regression) models of factors associated with mortality in the overall cohort of solid organ transplant recipients diagnosed with COVID-19 during 2020 in Spain (N = 1489)
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Total cases | n (%) | P | P | OR (95% CI) | |
| Type of transplant | |||||
| Kidney | 954 | 193 (20.2%) | <0.001 | 0.065 | 1.41 (0.97-2.05) |
| Liver | 270 | 47 (17.4%) | Ref. | ||
| Heart | 149 | 25 (16.8%) | 0.871 | 1.04 (0.60-1.82) | |
| Lung | 112 | 45 (40.2%) | <0.001 | 4.21 (2.49-7.12) | |
| Pancreas | 21 | 1 (4.8%) | 0.485 | 0.47 (0.61-3.77) | |
| Age at diagnosis | |||||
| ≤60 y | 739 | 80 (10.8%) | <0.001 | Ref. | |
| >60 y | 770 | 231 (30.0%) | <0.001 | 3.38 (2.52-4.52) | |
| Sex | |||||
| Male | 991 | 211 (21.3%) | 0.365 | ||
| Female | 518 | 100 (19.3%) | |||
| Time since transplantation | |||||
| ≤1 y | 198 | 55 (27.8%) | 0.008 | 0.691 | 1.08 (0.72-1.61) |
| >1 y | 1308 | 256 (19.6%) | Ref. | ||
| Type of infection | |||||
| Community | 1337 | 238 (17.8%) | <0.001 | Ref. | |
| Hospital | 164 | 68 (42.1%) | <0.001 | 2.99 (2.04-4.39) | |
| Period of diagnosis | |||||
| First wave (before 13 July 2020) | 647 | 167 (25.8%) | <0.001 | 0.005 | 1.47 (1.12-1.93) |
| Second wave (after 13 July 2020) | 862 | 144 (16.7%) | Ref. | ||
| Baseline calcineurin inhibitor | |||||
| Yes | 1354 | 277 (20.5%) | 0.473 | ||
| No | 148 | 33 (23.0%) | |||
| Baseline antimetabolite | |||||
| Yes | 1117 | 233 (20.9%) | 0.802 | ||
| No | 385 | 78 (20.3%) | |||
| Baseline mTOR inhibitor | |||||
| Yes | 275 | 47 (17.1%) | 0.102 | Ref. | |
| No | 1227 | 264 (21.5%) | 0.095 | 1.36 (0.94-1.96) | |
CI, confidence interval; COVID-19, coronavirus disease 2019; OR, odds ratio; mTOR, mammalian target of rapamycin.
As previously detailed, we hypothesized that this protective effect on mortality of diagnosis during the second wave could be confounded by reporting bias, as it was likely that only the most severe cases of COVID-19 were diagnosed and reported during the first wave. Therefore, we performed a prespecified sensitivity analysis focused on the subgroup of SOT recipients who required hospital admission (Table 3). The same factors were identified as associated with COVID-19-related death, with the exception of the period of diagnosis, which lost statistical significance (OR: 0.97; 95% CI, 0.73-1.29; P = 0.873). Baseline features, treatment-related variables and outcomes in this subgroup of hospitalized SOT recipients are presented in Supplementary Material (Table S2, SDC, http://links.lww.com/TP/C261). The specific analysis of patients admitted to the ICU is displayed in Supplementary Material (Tables S3 and S4, SDC, http://links.lww.com/TP/C261). There was no significant association between the period of diagnosis and mortality in this subgroup of patients either (OR: 0.65; 95% CI, 0.35-1.18; P = 0.157).
TABLE 3.
Univariate and multivariate (logistic regression) models of factors associated with mortality in the subgroup of solid organ transplant recipients diagnosed with COVID-19 during 2020 in Spain who required hospital admission (N = 1050)
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Total cases | n (%) | P | P | OR(95% CI) | |
| Type of transplant | |||||
| Kidney | 696 | 187 (26.9%) | <0.001 | 0.650 | 1.09 (0.73-1.63) |
| Liver | 160 | 44 (27.5%) | Ref. | ||
| Heart | 97 | 25 (25.8%) | 0.894 | 0.96 (0.53-1.73) | |
| Lung | 96 | 45 (46.9%) | <0.001 | 3.15 (1.79-5.51) | |
| Pancreas | 12 | 0 (0.0%) | |||
| Age at diagnosis | |||||
| ≤60 y | 460 | 77 (16.7%) | <0.001 | Ref. | |
| >60 y | 601 | 224 (37.3%) | <0.001 | 2.90 (2.13-3.95) | |
| Sex | |||||
| Male | 719 | 206 (28.7%) | 0.768 | ||
| Female | 342 | 95 (27.8%) | |||
| Time since transplantion | |||||
| ≤1 y | 156 | 54 (34.6%) | 0.063 | 0.990 | 1.00 (0.66-1.51) |
| >1 y | 903 | 247 (27.4%) | Ref. | ||
| Type of infection | |||||
| Community | 912 | 232 (25.4%) | <0.001 | Ref. | |
| Hospital | 143 | 65 (45.5%) | <0.001 | 2.36 (1.58-3.53) | |
| Period of diagnosis | |||||
| First wave (before 13 July 2020) | 560 | 162 (28.9%) | 0.669 | 0.873 | 0.97 (0.73-1.29) |
| Second wave (after 13 July 2020) | 501 | 139 (27.7%) | Ref. | ||
| Baseline calcineurin inhibitor | |||||
| Yes | 952 | 269 (28.3%) | 0.632 | ||
| No | 105 | 32 (30.5%) | |||
| Baseline antimetabolite | |||||
| Yes | 790 | 225 (28.5%) | 0.996 | ||
| No | 267 | 76 (28.5%) | |||
| Baseline mTOR inhibitor | |||||
| Yes | 195 | 46 (23.6%) | 0.094 | Ref. | |
| No | 862 | 255 (29.6%) | 0.128 | 1.34 (0.91-1.96) | |
CI, confidence interval; COVID-19, coronavirus disease 2019; mTOR, mammalian target of rapamycin; OR, odds ratio.
DISCUSSION
To the best of our knowledge, this is the first nationwide study comparing the experience with recipients of all types of SOT who acquired COVID-19 during 2 consecutive waves of the pandemic. A previous Spanish report exclusively focused on kidney transplant recipients has been recently published, although it addressed different outcome variables and did not separately describe the particular evolution of recipients who required hospitalization or admission to the ICU.16
Not surprisingly, recipients diagnosed with SARS-CoV-2 infection during the second pandemic wave were younger. This finding mirrors the well-described demographic profile also observed in the general population in the second wave in Spain as well as in other countries,6 and it is likely explained by the increased diagnostic capacity and the ability to identify asymptomatic or mildly symptomatic cases, which are more frequent among the younger population.
Despite the measures implemented to ensure COVID-19-free hospital pathways, it was surprising that the percentage of hospital-acquired cases was similar across both pandemic waves. As previously described,9 we did not identify any confirmed case of donor-derived COVID-19. To our knowledge, only a documented case of transmission through lung transplantation has been reported in the literature.17 The donor tested negative for SARS-CoV-2 by RT-PCR in the nasopharyngeal swab but was retrospectively found to test positive in a bronchoalveolar lavage sample obtained during procurement. This experience led the authors to conclude that lung donors should be screened in samples from the lower respiratory tract, a recommendation that has been in place in Spain since the beginning of the pandemic.13
Our nationwide experience reveals important changes in the management of posttransplant COVID-19 over time. Many repurposed drugs used at the beginning of the pandemic on the grounds of apparent in vitro anti-SARS-CoV-2 activity have been proven ineffective in clinical trials, as it was the case of hydroxychloroquine, azithromycin and protease inhibitors.18-20 Consequently, their use was generally abandoned during the second wave. Remdesivir has been shown to shorten the time to recovery in adults hospitalized with respiratory tract infections.21 Consequently, remdesivir therapy increased over time, although its utilization was limited to barely 5% of patients in the second wave, with an expected higher use in patients admitted to the hospital and to the ICU. Drug shortages, stringent indication criteria established by the Spanish Ministry of Health,22 and concerns on the actual impact of this drug on mortality may explain such a low rate. In a controlled open-label trial, compared with usual care, dexamethasone resulted in a lower 28 d mortality among hospitalized patients who were receiving either invasive mechanical ventilation or oxygen alone.23 Hence, corticosteroid-based immunomodulation—measured as any increase in baseline dose or the initiation of therapy upon COVID-19 diagnosis—increased over time, particularly among hospitalized patients and those admitted to the ICU. Despite the promising results reported for anti-interleukin-6 agents in critically ill COVID-19 patients,24 tocilizumab was rarely used during the second wave, even in hospitalized patients. The negative results coming from initial clinical trials25 and the scarce evidence available for the specific SOT population26,27 may be reasons for this declining trend of use. Of note, monoclonal antibodies approved by the Food and Drug Administration for the treatment of COVID-19 (ie, bamlanivimab and etesevimab) are not yet available in Spain and hence were not used in our series. Although many changes in therapeutic management may be related to the enhanced capacity to detect asymptomatic or mild cases over time, these differences were also evident when the comparison was restricted to hospitalized patients and to those who required admission to the ICU, which suggests the impact in clinical practice of the acquired knowledge about the efficacy and safety of anti-SARS-CoV-2 therapies.28,29
Adjustment of baseline immunosuppression was significantly less frequent during the second wave. Once again, this finding may be partially explained by the evolving capacity to diagnose less severe cases. In addition, the marked decrease in the use of agents with potential for pharmacokinetic interactions (ie, protease inhibitors) with immunosuppressive drugs can also explain this observation. There may be some additional rationale behind these changes. Antimetabolites (mainly mycophenolate) remained the most commonly adjusted immunosuppressive drug during the second wave, with complete discontinuation in >50% of patients. In a Spanish registry study published in early August and focused upon liver transplant recipients, the baseline use of mycophenolate was identified as a risk factor for severe COVID-19.30 It has hence been proposed that most severe cases may benefit from the reduction or withdrawal of mycophenolate. This could explain why antimetabolite drugs were still commonly decreased or withdrawn during the second wave, whereas the proportion of patients with dose reduction or discontinuation for calcineurin or mTOR inhibitors markedly dropped as compared to the first period. In a recently published systematic review and meta-analysis, maintaining immunosuppression was concluded to be safe in SOT recipients with moderate or severe COVID-19, and continuing tacrolimus was suggested to be beneficial.31 Additional evidence to support concrete guidance on the management of immunosuppression in SOT recipients diagnosed with COVID-19 is needed.
A large number of studies have reported higher mortality rates in SOT recipients diagnosed with COVID-19 compared with the general population,7,8 as also confirmed in our series. However, these poor outcomes seem to be largely explained by the older age, higher number of comorbidities, and more common occurrence of renal failure in this group of patients.10-12 In fact, when the case-fatality rate in our series was adjusted by distribution of sex and age in the general population, it decreased from 21% to 12% (data not shown). Regardless of the underlying pathogenic mechanism, the vulnerability of SOT recipients to COVID-19 is evident and has led to give this group priority in the Spanish scheme for vaccination against SARS-CoV-2.32
Perhaps the most relevant finding of our study is the overall improvement in outcome variables observed beyond July 2020 compared with the first wave. The 15% case-fatality rate described during this second period for posttransplant COVID-19 likely constitutes a more realistic estimation than that reported earlier in the course of the pandemic.9 Overall, being diagnosed during the first period exerted a negative impact on the risk of mortality after multivariate adjustment, particularly for kidney transplant recipients. However, this is an apparent rather than a real improvement, since it seems confounded by the higher representation of mildly symptomatic cases during the second wave. Indeed, the case-fatality rate reported for the nontransplant population also dropped from >10% during the first months to 2% at the present point.5 In addition, ICU resources were scarce during the first wave, to the extent that the Spanish Society of Intensive Care (Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias [SEMICYUC]) issued guidance for professionals to allocate ICU resources to an exponentially increasing number of severely ill COVID-19 patients.33 Such shortage was evident during the first period of our study when the percentage of patients admitted to the ICU was disproportionally low given the incidence of ARDS. This discordance was not that evident during the second wave.
To determine whether there had been a real improvement in prognosis, we focused upon the subgroup of recipients who required hospitalization and upon that of recipients who required ICU admission who had, presumably, similar illness severity in the 2 waves. Unfortunately, the case-fatality rate did not decrease over time in these 2 subgroups of patients. This finding suggests that the outcome of severe posttransplant SARS-CoV-2 infection in SOT recipients has not meaningfully improved, despite our better knowledge of the disease and enhanced ICU care. On the other hand, we cannot rule out the effect of differences over time in the criteria applied for ICU admission. Indeed, restrictions in bed availability during the first wave might have favored the selection of recipients with better survival probabilities, as compared to the less stringent admission criteria in the second period. In line with previous reports from the US and other European countries,34,35 lung transplantation and older recipient age emerged as factors predictive of death. The negative impact of the occurrence of COVID-19 early after transplantation has been also specifically observed for the kidney transplant population.16 In addition, hospital-acquired infection exerted a negative impact, likely reflecting recipients with poorer graft function or higher comorbidity burden.
The present study benefits from its nationwide design, which allowed for assembling the largest cohort to date to compare clinical and therapeutic features of posttransplant COVID-19 throughout 2 consecutive pandemic waves. Nevertheless, no information was collected about underlying comorbidities, graft function, or analytical and radiographic findings. We also lacked granular data regarding dose adjustment of immunosuppressive agents or the corticosteroid regimen used as immunomodulatory therapy. Finally, although we attempted at capturing all posttransplant COVID-19 cases diagnosed in Spain as per ONT institutional mandate, the completeness and accuracy of the notification ultimately rely upon transplant teams.
In conclusion, we have observed meaningful changes in patient age, therapeutic approaches, level of care, and outcomes between the first and second pandemic waves among SOT recipients diagnosed with COVID-19 in Spain. Some of these differences, however, could be attributed to improvements in diagnostic capacities over time, since the case-fatality rate remained high in the more severe cases requiring hospital or ICU admission.
ACKNOWLEDGMENTS
Spanish group for the Study of COVID-19 in transplant recipients: Ingrid Auyanet (Hospital Univ. Insular de Gran Canaria), María Flor Nogueras (Hospital Univ. Virgen de las Nieves), Ana Roca (Complejo Hospitalario Univ. de Toledo), Pablo Castro, Concepción Díaz Aunión (Coordinación Autonómica de Trasplantes de Andalucía), José M. Sobrino (Hospital Univ. Virgen del Rocío), María Ángeles Rodríguez-Pérez (Hospital Univ. Virgen de la Macarena), Víctor Pérez-Beltrán, Teresa Pont, Joana Sellares (Hospital Univ. Vall d´Hebrón), Pilar Fraile (Complejo Hospitalario Univ. de Salamanca), José M. Vaquero (Hospital Univ. Reina Sofía), Laura Espinosa (Hospital Univ. La Paz), Raquel López-Vilella, David Ramos (Hospital Univ. La Fe), Gerardo Blanco, Román Hernández-Gallego (Hospital Univ. Infanta Cristina), María Pilar Pérez del Barrio (Hospital Univ. de Jaén), Carme Baliellas, Elena García Romero, Nicolás Manito, Nuria Montero (Hospital Univ. de Bellvitge), María Angeles Castel, Federico Oppenheimer (Hospital Clinic), Francisco Llamas, Inmaculada Lorenzo (Hospital Univ. de Albacete), Isabel María Saura (Hospital Univ. Virgen de la Arrixaca), Javier Paul (Hospital Univ. Miguel Servet), Victor M. Mora (Hospital Univ. Marqués de Valdecilla), Mikel Gastaca, Ane Mujika, Íñigo Yáñez, Sofía Zárraga (Hospital Univ. de Cruces), María Luisa González-Diéguez, José Luis Lambert (Hospital Univ. Central de Asturias), Sheila Cabello (Hospital de Son Espases), Sonia Mirabet (Hospital Univ. Santa Creu i Sant Pau), Yolanda Calzada (Hospital Sant Joan de Deu), Mercedes Cabello, Rocío González-Grande, Domingo Hernández, Verónica López (Hospital Regional de Málaga), Cristina Galeano, Rosa Martín-Mateos (Hospital Univ. Ramón y Cajal), Auxiliadora Mazuecos (Hospital Univ. Puerta del Mar), Ana Arias, Myriam Aguilar, Manuel Gómez-Bueno (Hospital Univ. Puerta de Hierro), Fernando Fernández-Girón (Hospital Juan Ramón Jiménez), Elena Burgos (Hospital Univ. Germans Trias i Pujol), Ana María Ramos (Fundación Jiménez Díaz), Cristina Canal (Fundación Puigvert), Asunción Sancho (Hospital Univ. Doctor Peset), María José Pérez-Sáez (Hospital del Mar), Amado Andrés, Alicia de Pablo, Juan F. Delgado, Esther González-Monte, Alberto Alejandro Marcacuzco, Carlos Andrés Quezada (Hospital Univ. 12 de Octubre), José Ignacio Herrero, Paloma Martín Moreno (Clínica Univ. de Navarra), María Lourdes Pérez Tamajón (Hospital Univ. de Canarias), María G. Crespo (Complejo Hospitalario Univ. A Coruña), OCATT.
Supplementary Material
Footnotes
M.F.-R. holds a research contract “Miguel Servet” (CP18/00073) from the Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation. The remaining authors have no disclosures.
E.C., M.F.-R., and B.D.-G. conceived and designed the study and drafted the first version of the article. E.C., M.P., R.H., and L.P. performed the quality control of the data and the statistical analysis. The rest of the authors contributed to the data required for the development of the study. All authors participated in the writing of the article and approved the final version of the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Domínguez-Gil B, Coll E, Fernández-Ruiz M, et al. COVID-19 in Spain: transplantation in the midst of the pandemic. Am J Transplant. 2020;20:2593–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loupy A, Aubert O, Reese PP, et al. Organ procurement and transplantation during the COVID-19 pandemic. Lancet. 2020;395:e95–e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Observatory Donation and Transplantation. WHO-ONT. Available at http://www.transplant-observatory.org/. Accessed June 2021.
- 4.Ministry of Health. Actualización n° 363. Enfermedad por el coronavirus (COVID-19). Available at https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_363_COVID-19.pdf. Accessed June 2021.
- 5.Fan G, Yang Z, Lin Q, et al. Decreased case fatality rate of COVID-19 in the second wave: a study in 53 countries or regions. Transbound Emerg Dis. 2021;68:213–215. [DOI] [PubMed] [Google Scholar]
- 6.Carlos III Health Institute. Informes COVID-19. Available at https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Paginas/InformesCOVID-19.aspx. Accessed June 2021.
- 7.Raja MA, Mendoza MA, Villavicencio A, et al. COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature. Transplant Rev (Orlando). 2021;35:100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahalingasivam V, Craik A, Tomlinson LA, et al. A systematic review of COVID-19 and kidney transplantation. Kidney Int Rep. 2021;6:24–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, et al. ; Spanish Group for the Study of COVID-19 in Transplant Recipients. COVID-19 in transplant recipients: the Spanish experience. Am J Transplant. 2021;21:1825–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linares L, Cofan F, Diekmann F, et al. ; Hospital Clínic COVID-19 research group. A propensity score-matched analysis of mortality in solid organ transplant patients with COVID-19 compared to non-solid organ transplant patients. PLoS One. 2021;16:e0247251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavarot N, Gueguen J, Bonnet G, et al. ; Critical COVID-19 France Investigators. COVID-19 severity in kidney transplant recipients is similar to nontransplant patients with similar comorbidities. Am J Transplant. 2021;21:1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadi YB, Naqvi SFZ, Kupec JT, et al. Outcomes of COVID-19 in solid organ transplant recipients: a propensity-matched analysis of a large research network. Transplantation. 2021;105:1365–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domínguez-Gil B, Fernández-Ruiz M, Hernández D, et al. Organ donation and transplantation during the COVID-19 pandemic: a summary of the Spanish experience. Transplantation. 2021;105:29–36. [DOI] [PubMed] [Google Scholar]
- 14.Ison MG, Hager J, Blumberg E, et al. Donor-derived disease transmission events in the United States: data reviewed by the OPTN/UNOS Disease Transmission Advisory Committee. Am J Transplant. 2009;9:1929–1935. [DOI] [PubMed] [Google Scholar]
- 15.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. [DOI] [PubMed] [Google Scholar]
- 16.Villanego F, Mazuecos A, Pérez-Flores IM, et al. Predictors of severe COVID-19 in kidney transplant recipients in the different epidemic waves: analysis of the Spanish Registry. Am J Transplant. 2021;21:2573–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaul DR, Valesano AL, Petrie JG, et al. Donor to recipient transmission of SARS-CoV-2 by lung transplantation despite negative donor upper respiratory tract testing. Am J Transplant. 2021; 21:2885–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.RECOVERY Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396:1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boulware DR, Pullen MF, Bangdiwala AS, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for COVID-19. N Engl J Med. 2020;383:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020;382:2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of COVID-19 – preliminary report. Reply. N Engl J Med. 2020;383:994. [DOI] [PubMed] [Google Scholar]
- 22.MINISTERIO DE SANIDAD. Protocolo farmacoclínico del uso de Remdesivir (veklury®) en el tratamiento de la enfermedad por covid-19 en el Sistema Nacional de Salud. Available at https://www.mscbs.gob.es/profesionales/farmacia/valtermed/docs/20200908_Protocolo_farmacoclinico_remdesivir2.pdf. Accessed June 2021.
- 23.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon AC, Mouncey PR, Al-Beidh F, et al. ; REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384:1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernández-Ruiz M, López-Medrano F, Aguado JM. Tocilizumab for the treatment of COVID-19. Expert Opin Biol Ther. 2021;21:431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trujillo H, Caravaca-Fontán F, Sevillano Á, et al. Tocilizumab use in kidney transplant patients with COVID-19. Clin Transplant. 2020;34:e14072. [DOI] [PubMed] [Google Scholar]
- 27.Pérez-Sáez MJ, Blasco M, Redondo-Pachón D, et al. ; Spanish Society of Nephrology COVID-19 Group. Use of tocilizumab in kidney transplant recipients with COVID-19. Am J Transplant. 2020;20:3182–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernández-Ruiz M, Aguado JM. Immunomodulatory therapies for COVID-19 in solid organ transplant recipients. Curr Transplant Rep. 2020;7:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avery RK. COVID-19 therapeutics for solid organ transplant recipients; 6 months into the pandemic: where are we now? Transplantation. 2021;105:56–60. [DOI] [PubMed] [Google Scholar]
- 30.Colmenero J, Rodríguez-Perálvarez M, Salcedo M, et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karruli A, Spiezia S, Boccia F, et al. Effect of immunosuppression maintenance in solid organ transplant recipients with COVID-19: systematic review and meta-analysis. Transpl Infect Dis. 2021:e13595. doi:10.1111/tid.13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Actualización 6. Estrategia de vacunación frente a COVID-19 en España. Available at https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/covid19/docs/COVID-19_Actualizacion6_EstrategiaVacunacion.pdf. Accessed June 2021.
- 33.Semicyuc.org. Recomendaciones éticas para la toma de decisiones en la situación excepcional de crisis por pandemia COVID-19 en las unidades de cuidados intensivos. Available at https://semicyuc.org/wp-content/uploads/2020/03/%C3%89tica_SEMICYUC-COVID-19.pdf. Accessed June 2021. [DOI] [PMC free article] [PubMed]
- 34.Kates OS, Haydel BM, Florman SS, et al. COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis. 2020:ciaa1097. doi:10.1093/cid/ciaa109732766815 [Google Scholar]
- 35.Belli LS, Fondevila C, Cortesi PA, et al. ; ELITA-ELTR COVID-19 Registry. Protective role of tacrolimus, deleterious role of age and comorbidities in liver transplant recipients with COVID-19: results from the ELITA/ELTR Multi-center European Study. Gastroenterology. 2021;160:1151–1163. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.