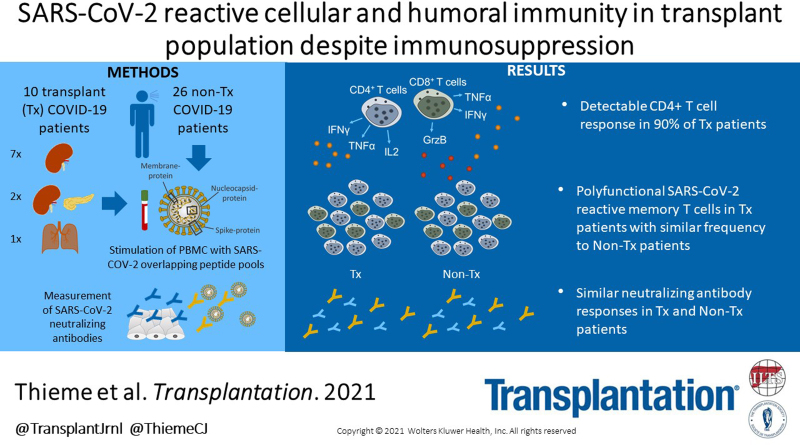
Supplemental Digital Content is available in the text.
Background.
The ability of transplant (Tx) patients to generate a protective antiviral response under immunosuppression is pivotal in COVID-19 infection. However, analysis of immunity against SARS-CoV-2 is currently lacking.
Methods.
Here, we analyzed T cell immunity directed against SARS-CoV-2 spike-, membrane-, and nucleocapsid-protein by flow cytometry and spike-specific neutralizing antibodies in 10 Tx in comparison to 26 nonimmunosuppressed (non-Tx) COVID-19 patients.
Results.
Tx patients (7 renal, 1 lung, and 2 combined pancreas-kidney Txs) were recruited in this study during the acute phase of COVID-19 with a median time after SARS-CoV-2-positivity of 3 and 4 d for non-Tx and Tx patients, respectively. Despite immunosuppression, we detected antiviral CD4+ T cell-response in 90% of Tx patients. SARS-CoV-2–reactive CD4+ T cells produced multiple proinflammatory cytokines, indicating their potential protective capacity. Neutralizing antibody titers did not differ between groups. SARS-CoV-2–reactive CD8+ T cells targeting membrane- and spike-protein were lower in Tx patients, albeit without statistical significance. However, frequencies of anti-nucleocapsid–protein-reactive, and anti-SARS-CoV-2 polyfunctional CD8+ T cells, were similar between patient cohorts. Tx patients showed features of a prematurely aged adaptive immune system, but equal frequencies of SARS-CoV-2–reactive memory T cells.
Conclusions.
In conclusion, a polyfunctional T cell immunity directed against SARS-CoV-2 proteins as well as neutralizing antibodies can be generated in Tx patients despite immunosuppression. In comparison to nonimmunosuppressed patients, no differences in humoral and cellular antiviral-immunity were found. Our data presenting the ability to generate SARS-CoV-2–specific immunity in immunosuppressed patients have implications for the handling of SARS-CoV-2–infected Tx patients and raise hopes for effective vaccination in this cohort.
INTRODUCTION
The emergence of the COVID-19 pandemic in late 2019 led to >1 million deaths attributed to COVID-19 as of November 2020. Since certain patient cohorts are at increased risk for critical courses, the identification and protection of such vulnerable groups are a major concern.1-3 Transplant (Tx)-patients were defined to be of high-risk by national health agencies early after the COVID-19 outbreak. This assumption is mainly based on the immunosuppressive treatment after Tx, and the subsequently higher susceptibility to infectious diseases including viral infections.4-6 In addition, Tx patients also suffer from higher rates of comorbidities compared to the general population.3,4,6,7
However, case series report diverse outcomes of immunosuppressed COVID-19 patients, with some suggesting an increased risk and others a decreased fatality rate compared to the general population.3,7-14 A reduction of COVID-19 symptoms and incidence of severe courses might be explained by the milder reaction of the suppressed immune system, therefore diminishing immunopathogenesis.8 In fact, immunosuppressive drugs used in transplantation, in particular the steroid dexamethasone, have been shown to improve the outcome of critically ill COVID-19 patients.15 Interestingly, studies revealed an inhibiting effect of the immunosuppressive drugs cyclosporine A and antimetabolites on coronavirus replication in vitro.16-18 On the other hand, the impairment of antiviral immunity by immunosuppressive medication is well documented, and community-acquired respiratory viruses pose a greater risk to Tx patients as compared to the general population.19-21
Therefore, there is a need to understand the SARS-CoV-2–reactive adaptive immunity in Tx recipients.6,9 Here, we provide data on the adaptive immune responses in SARS-CoV-2 infected Tx patients. We show that T cell and neutralizing antibody responses of Tx patients are similar to non-Tx patients, with polyfunctional and memory T cell reactivity. Thus, we suggest that Tx patients can mount a protective SARS-CoV-2–reactive adaptive immune response.
MATERIALS AND METHODS
Patient Samples
The study was approved by the ethical committee of the Ruhr-University Bochum (20-6886) and University Hospital Essen (20-9214-BO). Blood samples of 10 Tx patients and 26 non-Tx patients were collected after written informed consent was obtained. For reasons of limited patient material, resources, and the time that would be needed to generate a new cohort, the non-Tx patient control group was formed from patient samples already included in another study.22 In 8 Tx patients and 20 non-Tx patients, multiple blood samples (up to 5 visits) were collected, so that in total 27 Tx patient and 60 non-Tx patient samples were included in this study. In all but 1 patients, membrane- (M), nucleocapsid- (N), and spike- (S) protein reactive T cells were analyzed. In 1 patient, 2 of 3 blood samples could be analyzed for N- and S-protein reactive T cells only because of the limited amount of collected blood and lymphopenia. The clinical characteristics of the patients are presented in Table 1. COVID-19 severity was evaluated according to a guideline of the German Robert Koch Institute, as previously described.23 Of patients with multiple samples, the worst disease classification of this patient was reported. Samples of patients with moderate and severe COVID-19 were collected shortly after symptom-onset and positive SARS-CoV-2 PCR. Samples of critical COVID-19 patients were included at the time of ICU admission.
TABLE 1.
Clinical characteristics, COVID-19 treatment, and monitoring strategy of individual transplant patients
| Patient | A | B | C | D | E | F | G | H | I | J |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | 37 | 63 | 62 | 54 | 58 | 29 | 35 | 69 | 55 | 52 |
| Gender | Male | Female | Female | Male | Male | Female | Male | Male | Male | Female |
| Transplanted organs | Kidney | Kidney | Kidney | Kidney | Lung | Kidney | Kidney | Kidney-pancreas | Kidney | Kidney-pancreas |
| Transplant age (d) | 4420 | 7002 | 243 | 118 | NA | 3957 | 123 | 4657 | 1625 | 3191 |
| No. of previous transplants | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Donor type (living/deceased) | Living | Deceased | Deceased | Deceased | Deceased | Deceased | Deceased | Deceased | Deceased | Deceased |
| Initial IS | Tac (2.5 ng/mL)Pred (5 mg) | Tac (18.3 ng/mL)Pred (10 mg) | Tac (7.9 ng/mL)Pred (10 mg) | Tac (11.3 ng/mL)Pred (5 mg) | Unknown | Tac (5.8 ng/mL)Pred (5 mg) | Bela (350 mg)Pred (10 mg) | Tac (40 ng/mL) MMF (2 × 1g) | Everol (3.27 ng/mL)Pred (10 mg) | Tac (3.57 ng/mL)MMF (2 × 1g)Pred (5 mg) |
| IS during disease | Withdrawn | No changes | No changes | No changes | Unknown | Pred | HC | HC | HC | HC |
| COVID-19 severity | Moderate | Moderate | Severe | Moderate | Critical | Moderate | Moderate | Critical | Moderate | Moderate |
| COVID-19 treatment | Anticoag. | Anticoag. RDV and plasma therapy | Antib. anticoag. and plasma therapy | Anticoag. | Antib. | Anticoag. | Antib. and anticoag. | Antib. HCQ and anticoag. | Antib. HCQ and anticoag. | Antib. and anticoag. |
| Outcome (discharged/deceased) | Discharged | Discharged | Discharged | Discharged | Deceased | Discharged | Discharged | Discharged | Discharged | Discharged |
| ICU? (yes/no) | No | No | No | No | Yes | No | No | Yes | No | No |
| Invasive ventilation? (yes/no) | No | No | No | No | Yes | No | No | No | No | No |
| Hospitalization length (d) | 15 | 10 | 10 | 30 | 11 | 2 | 7 | 28 | 15 | 13 |
| AKI (yes/no) | No | Yes | No | Unknown | Yes | Unknown | No | Yes | Yes | Yes |
| SCr at admission (mg/dL) | HD | 2.17 | 1.06 | HD | 1.72 | 1.19 | 2.30 | 1.60 | 2.60 | 1.00 |
| SCr at discharge (mg/dL) | HD | 1.90 | 1.12 | HD | n.a. | 1.10 | 2.30 | 1.10 | 1.60 | 0.90 |
| First T cell measurement (d after first positive PCR) | 2 | 4 | 5 | 6 | 2 | 8 | 28 | -2 | 1 | 1 |
| No. of T cell measurements | 2 | 3 | 3 | 3 | 1 | 1 | 2 | 5 | 4 | 3 |
| Neutralizing antibody assay | Done | Done | Done | Done | Not done | Done | Done | Done | Done | Not done |
AKI, acute kidney injury; Antib., antibiotics; Anticoag., anticoagulants; Bela, belatacept; COVID-19, coronavirus disease 2019; Everol, everolimus; HC, hydrocortisone; HCQ, hydroxychloroquine; HD, hemodialysis; ICU, intensive care unit; MMF, mycophenolate mofetil; n.a., not applicable; NA, not available; PCR, polymerase chain reaction; Pred, prednisolone; RDV, remdesivir; SCr, serum creatinine; Tac, tacrolimus.
Phenotyping of Whole Blood Samples
Whole blood from EDTA collection tubes was stained for 10 min at room temperature in the dark with CD45-Alexa Fluor 488 (clone 2D1) (Biolegend [BL]), CD3-BV785 (clone OKT3) (BL), CD4-Alexa Fluor 700 (clone OKT4) (BL), CD8-V500 (clone RPA-T8) (Becton Dickinson), CD19-BV605 (clone HIB19) (BL), and CD56-PerCP-Cy5.5 (clone NCAM) (BL). VersaLyse (Beckman Coulter) was used for erythrocyte lysis for 15 min at room temperature in the dark. Samples were measured on a CytoFlex flow cytometer (Beckman-Coulter) after addition of propidium iodide (Thermo Fisher Scientific). Measured absolute counts were calculated as cells/nanoliter (nL).
SARS-CoV-2 Overlapping Peptide Pool Stimulation and Flow Cytometric Analysis of peripheral Blood Mononuclear Cell
Stimulation of peripheral blood mononuclear cell (PBMC) with SARS-CoV-2 M-, N-, and S-protein was performed as previously described.22 Briefly, PBMC were isolated from EDTA collection tubes (Sarstedt) and stored at −80 °C to allow analysis in batches. PBMCs were left resting overnight after thawing and afterward stimulated with SARS-CoV-2–PepTivator peptide-pools solved in water (Miltenyi Biotec). Staphylococcal-enterotoxin-B (Sigma-Aldrich) treated and untreated PBMCs were used as positive and negative control, respectively. After 2 h of stimulation, Brefeldin-A (Sigma-Aldrich) was added and the stimulation stopped after 16 h. Surface- and intracellular-staining for flow cytometry was performed using fixation and permeabilization (Thermo Fisher Scientific) and antibodies listed in Table S1 (SDC, http://links.lww.com/TP/C185). Samples were measured on a CytoFlex flow cytometer (Beckman-Coulter).
SARS-CoV-2 Neutralizing Antibody Measurement
Assessment of neutralizing antibodies was performed in 8 Tx patients (Table 1) and 20 non-Tx patients as previously described.22 In brief, complement factors in patient sera were inactivated by incubation at 56°C for 30 min. Quadruplicates of 2-fold serial dilutions of patient sera were incubated with a propagation-incompetent VSV*ΔG(FLuc)-pseudovirus-system bearing the SARS-CoV-2 S-protein in the envelope. Afterwards, Vero-E6-cells (1 × 104 cells/well) were infected with the pseudovirus in DMEM + 10% FBS (Life Technologies). Firefly-luciferase-reporter-activity was determined using a GloMax plate-reader (Promega) after addition of 25 µL of firefly luciferase ONE-Glo substrate (Promega) 18 h postinfection and the reciprocal antibody dilution causing 50% inhibition of the luciferase-reporter calculated.
Flow Cytometry Data Analysis
FlowJo version 10.7.1 (BD Biosciences) was used for analysis of flow cytometry data. Single stains and fluorescence-minus-1 controls were used for gating. Gates of each individual were adjusted according to the negative control. The gating strategy is presented in Figure S1 (SDC, http://links.lww.com/TP/C185). CD4+ T cells expressing CD154 and CD137 and CD8+ T cells expressing CD137 in combination with production of at least 1 of IL2/IL4/IFNγ/TNFα/GrzB were defined as reactive T cells. Unspecific activation in unstimulated controls was subtracted from stimulated samples to account for SARS-CoV-2–specific activation in the presented frequencies. Of patients with multiple samples, the mean value is presented. Negative values were set to 0. Stimulation index (SI) was calculated by dividing the measured T cell subset response by the respective negative control. If the negative control was 0, the minimum value across that subset was used for calculation. SI below 1 was set to 1. SI >3 was considered detectable response. Of patients with multiple samples, the maximum value is presented for analysis of detectable responses. Boolean gating of IL2, IL4, IFNγ, TNFα, and GrzB producing T cells in combination with CD154 for CD4+ and CD137 for CD8+ T cells was used to calculate polyfunctional T cells. Composition of polyfunctional T cells was analyzed by calculating the relative contribution of each subset to the total polyfunctional cells of each patient and then the mean contribution across all patients.
Statistical Analysis
Statistical analysis was performed using R, version 4.0.2,24 and GraphPad Prism v7, which was also used for graphical representation. Categorical variables are summarized as numbers and frequencies; quantitative variables are reported as median and interquartile range (IQR). Normal distribution was assessed using D’Agostino-Pearson omnibus normality test and parametric or nonparametric tests were then used accordingly. For the characterization of demographic, treatment, and clinical outcome, differences between groups were calculated using Fisher’s exact test for categorical variable and Mann-Whitney U test for quantitative variables. Characterization of absolute lymphocyte subset counts and immune responses of Tx and non-Tx patients was performed employing Mann-Whitney U test. Thereafter, bivariate-regression-analysis was performed with age and transplantation status as independent variables (without interactions) and was considered significant if a significant effect of transplantation status was found. Only differences significant for both tests are reported in this work; the P in the figures correspond to the Mann-Whitney U test. Ratio of memory cells among T cells and chronologic age was compared using unpaired t-test. P values <0.05 were considered significant; only significant P are reported.
RESULTS
Study Participants
Samples of 10 Tx patients and 26 nontransplant (non-Tx) patients were analyzed for this study. All patients were hospitalized and tested positive for SARS-CoV-2 infection. The median age of Tx patients was 55 (IQR 41–61) and significantly lower than that of non-Tx patients (median 69, IQR 58-82; P = 0.006) (Table S2, SDC, http://links.lww.com/TP/C185). Seven (70%), 1 (10%), and 2 (20%) of Tx patients and 6 (23%), 10 (38.5%), and 10 (38.5%) of non-Tx patients had moderate, severe, and critical COVID-19 severity, respectively (P = 0.043). Relatively more Tx patients were treated with anticoagulation as compared to non-Tx patients. There were no significant differences in the time between diagnosis and sample analysis between Tx and non-Tx patients (Table S2, SDC, http://links.lww.com/TP/C185). The clinical characteristics and details on the COVID-19 disease course of the individual Tx patients are listed in Table 1. Seven patients had a kidney Tx, 2 combined kidney-pancreas Tx, and 1 lung Tx. The immunosuppression of 2 patients remained unchanged during the COVID-19 treatment, 5 patients received glucocorticoid monotherapy and the immunosuppression of 2 patients was completely discontinued. Typically for COVID-19 patients,23,25 the majority of study participants were lymphopenic with low absolute counts of T cells, CD4+ and CD8+ T cell subsets, B cells, and NK cells without statistically significant differences between the Tx and non-Tx groups (Figure S2, SDC, http://links.lww.com/TP/C185).
Polyfunctional SARS-CoV-2–reactive T Cell Responses and Neutralizing Antibodies Do Not Differ in Tx Patients and Nonimmunosuppressed Patients
Specific and highly functional T cells play a pivotal role in viral control.26 Detection of SARS-CoV-2–specific T cells according to the expression of activation markers and cytokines after stimulation of PBMC with SARS-CoV-2 membrane- (M), nucleocapsid- (N), and spike- (S)-protein overlapping peptide pools has been described before by us and other groups (Figures S1 and S3, SDC, http://links.lww.com/TP/C185).22,27 In this study, we used this approach to compare the magnitude and functionality of cellular immunity in Tx COVID-19 patients to a general, non-Tx COVID-19 patient cohort.
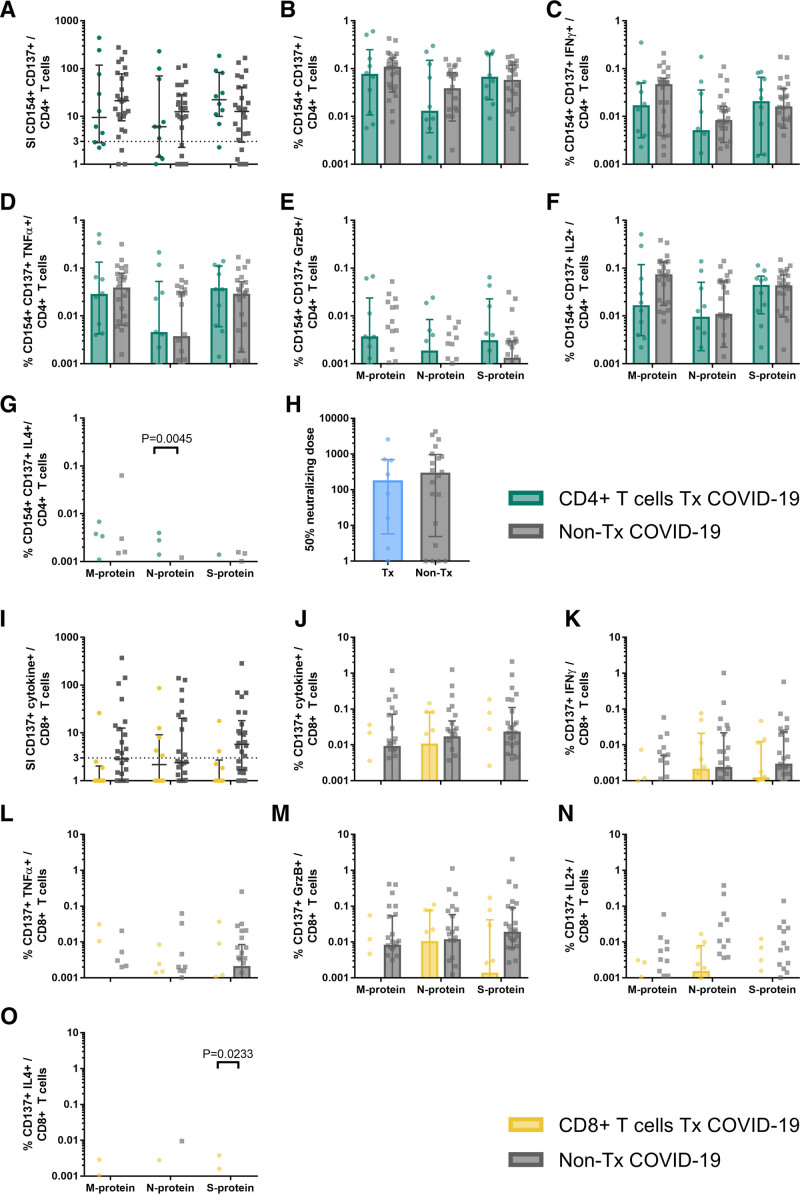
The frequencies of SARS-CoV-2–reactive CD4+ T cells were similar among Tx patients and non-Tx patients. The number of patients with detectable responses in both cohorts was between 70% and 90% after stimulation with M-, N-, and S-protein without significant differences between the patient groups (Figure 1A). In general, no significant differences were observed regarding the frequencies of cytokine-producing CD4+ T cells (Figure 1B–F). Only for IL4, we detected significantly higher frequencies of N-protein reactive CD4+ T cells in Tx patients (Figure 1G). However, the production of TH1-cytokines greatly exceeded the production of the TH2 cytokine IL4, and the difference in IL4 production between the cohorts could be clinically irrelevant.
FIGURE 1.
Characterization of SARS-CoV-2-reactive T cells in transplant (Tx) and non-Tx COVID-19 patients. PBMCs of 10 Tx and 26 non-Tx COVID-19 patients were stimulated overnight with overlapping peptide pools of SARS-CoV-2 membrane (M), nucleocapsid (N), and spike (S)-protein and analyzed by flow cytometry. A, Stimulation index (SI) of activation markers CD154 and CD137 expressing CD4+ T cells (SARS-CoV-2 specific CD4+ T cells). SI was calculated by dividing the measured T cell subset response by the respective response in the negative control. Values >3 are considered above detection limit. For patients with multiple samples, the maximal response was calculated. Scatter plot with line at median and interquartile range. B–G, Frequencies of SARS-CoV-2 specific CD4+ T cells (B) and SARS-CoV-2 specific CD4+ T cells expressing IFNγ (C), TNFα (D), GrzB (E), IL2 (F), or IL4 (G). Negative controls were subtracted from specifically stimulated samples to exclude unspecific activation. For patients with multiple samples, the mean response was calculated. Bars show median, error bars show interquartile range. H, SARS-CoV-2 spike neutralizing antibody dose (ND50) in patient sera of 8 Tx patients and 20 non-Tx patients. For patients with multiple samples, the maximal response was calculated. Scatter plot with line at median and interquartile range. I, Stimulation index (SI) of activation marker CD137 and at least 1 of the cytokines IFNγ, TNFα, IL2, IL4, or effector molecule GrB expressing CD8+ T cells (SARS-CoV-2 specific CD8+ T cells). SI was calculated by dividing the measured T cell subset response by the respective response in the negative control. Values >3 are considered detectable. For patients with multiple samples, the maximal response was calculated. Scatter plot with line at median and interquartile range. J–O, Frequencies of SARS-CoV-2 specific (CD137+ cytokine+) CD8+ T cells (B) and CD137+ CD8+ T cells expressing IFNγ (C), TNFα (D), GrzB (E), IL2 (F), or IL4 (G). Negative controls were subtracted from specifically stimulated samples to exclude unspecific activation. For patients with multiple samples, the mean response was calculated. Bars show median, error bars show interquartile range. Statistical comparison was done with Mann-Whitney U test and controlled by multivariate analysis for the influence of transplantation status and age. P < 0.05 was considered significant. PBMC, peripheral blood mononuclear cell.
Neutralizing antibodies are another arm of adaptive immunity crucial for antiviral defense. Like T cells, B cells are also susceptible to immunosuppressive therapy. Therefore, we compared the antibody-dependent capacity of SARS-CoV-2 neutralization between Tx and non-Tx patients. In accordance with the CD4+ T cells, which are required for the generation of effective humoral immunity,28 we observed that antibodies of Tx patients and non-Tx patients have a similar SARS-CoV-2 neutralizing activity (Figure 1H). Thus, the sera of Tx patients had equal inhibitory effects on the viral infectivity of susceptible cell culture cells as sera from non-Tx patients.
The number of patients with detectable SARS-CoV-2–specific CD8+ T cell responses in Tx patients was 1 (10%), 5 (50%), and 2 (20%) after stimulation with M-, N-, and S-protein, respectively. These numbers were lower than in the non-Tx patients cohort, in which 12 (46%), 10 (38%), and 17 (65%) showed detectable responses after stimulation with M-, N-, and S-protein, respectively (Figure 1I), without reaching statistical significance. Accordingly, the frequency of activated and IFNγ and GrzB producing SARS-CoV-2–specific CD8+ T cells was lower after stimulation with M- and S-protein, but not after stimulation with N-protein, and not reaching statistical significance (Figure 1J, K, and M). Similar to CD4+ T cells, there was a statistically significant difference in the frequency of S-protein reactive CD8+ T cells producing IL4. However, the very low frequencies of these cells, as well as of TNFα and IL2 producing CD8+ T cells, dissent a relevant role in this setting (Figure 1L, N, and O).
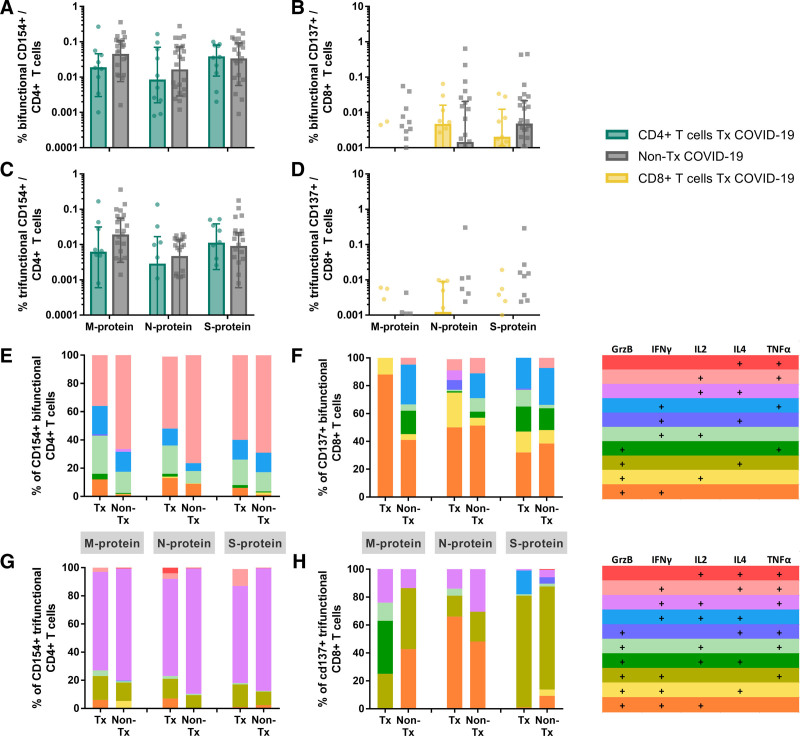
T cells producing multiple cytokines are correlates of effective viral control.26 Interestingly, polyfunctional CD4+ and CD8+ T cells were not diminished in patients receiving immunosuppression (Figure 2A–D). CD4+ bifunctional and trifunctional T cells produced mainly IL2, IFNγ, and TNFα (Figure 2E and G). CD8+ bifunctional and trifunctional T cells produced mainly GrzB in combination with IFNγ, IL2, or TNFα (Figure 2F and H).
FIGURE 2.
Composition of polyfunctional CD4+ and CD8+ T cells in transplant (Tx) and non-Tx COVID-19 patients. SARS-CoV-2 M-, N-, and S-protein reactive CD154+ CD4+ (A, C) and CD137+ CD8+ (B, D) polyfunctional T cells of 10 Tx and 26 non-Tx patients were analyzed by Boolean gating of production of IFNγ, TNFα, GrzB, IL2, and IL4. A–D, Bifunctional (A) and trifunctional (C) CD154+ CD4+ T cells and bifunctional (B) and trifunctional (D) CD137+ CD8+ T cells were calculated negative controls were subtracted from specifically stimulated samples to exclude unspecific activation. For patients with multiple samples, the mean response was calculated. Bars show median, error bars show interquartile range. Statistical comparison was done with Mann-Whitney U test and controlled by multivariate analysis for the influence of transplantation status and age. P < 0.05 was considered significant. E and F, Analysis of the relative contribution of individual cytokines and effector molecules to the pool of polyfunctional cells. Calculation was done for each patient and then the mean contribution across all patients was determined.
Tx Patients Develop SARS-CoV-2–specific Memory T Cells
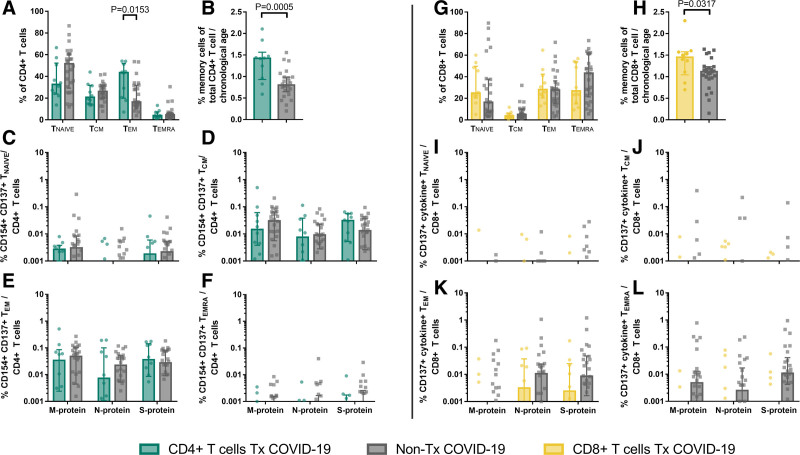
Memory T cells are hallmarks of adaptive immunity and convey long-term protection against pathogens. The diminished repertoire of naive T cells (TNAIVE) in elderly patients has been suggested as a factor contributing to critical COVID-19 course.29,30 Of interest, an expansion of CD4+ effector-memory T cells (TEM) and reduction of TNAIVE during end-stage renal disease and after transplantation has been described earlier.31-34 In line with these results, we observed higher frequencies of TEM and lower frequencies of TNAIVE in Tx patients as compared to non-Tx patients in CD4+ T cells (Figure 3A). This pattern was interestingly not observed in CD8+ T cells (Figure 3H). The immunologic age as defined by the memory T cell ratio among CD4+ T cells30,35 in comparison to the chronologic age of Tx patients was significantly higher than that of non-Tx patients (Figure 3B and H). However, the SARS-CoV-2–specific memory composition was nearly identical between both groups. Despite the overall smaller pool of TNAIVE that can progress into memory-phenotype T cells, there was no significant reduction of SARS-CoV-2–specific central-memory (TCM), TEM, and TEMRA in CD4+ (Figure 3C–F) and CD8+ T cells (Figure 3I–L). This finding demonstrates that Tx patients form SARS-CoV-2–specific memory cells early after infection, either by new formation from TNAIVE or by crossreactivity of existing memory T cells.
FIGURE 3.
Memory phenotypes of SARS-CoV-2-reactive T cells in transplant (Tx) and non-Tx COVID-19 patients. Stimulation of PBMC of 10 Tx patients and 26 non-Tx patients was performed overnight with SARS-CoV-2 membrane (M)-, nucleocapsid (N)-, and spike (S)-protein. Analysis of activation markers CD154 and CD137 in CD4+ T cells (SARS-CoV-2-reactive CD4+ T cells) and CD137 in combination with production of any of interferon γ, tumor necrosis factor α, granzyme B, interleukin (IL)-2, or IL4 (cytokine+) in CD8+ T cells (SARS-CoV-2-reactive CD8+ T cells) as well as C-C chemokine receptor type 7 (CCR7) and CD45-RA was performed using flow cytometry. A and G, Quantification of CD45RA+ CCR7+ (TNAIVE), CD45RA- CCR7+ (TCM), CD45RA− CCR7− (TEM), and CD45RA+ CCR7− (TEMRA) T cells among total CD4+ (A) and CD8+ (G) T cells. Bars show median, error bars show interquartile range. Statistical comparison was done with Mann-Whitney U test and controlled by multivariate analysis for the influence of transplantation status and age. B and H, Ratio of the proportion of memory T cells of the total CD4+ (B) and CD8+ (H) T cells and chronologic age. Bars show median, error bars show interquartile range. Statistical comparison was done with unpaired t-test. C–F and I–L, Frequencies of TNAIVE, TCM, TEM, and TEMRA among SARS-CoV-2-reactive CD4+ (C–F) and CD8+ (I–L) T cells. Negative controls were subtracted from specifically stimulated samples to exclude unspecific activation. Bars show median, error bars show interquartile range. Statistical comparison was done with Mann-Whitney U test and controlled by multivariate analysis for the influence of transplantation status and age. For patients with multiple samples, the mean response was calculated. P < 0.05 was considered significant.
SARS-CoV-2–reactive T Cells Are Detectable Already at Early Time Points After Symptom Onset
Since immunosuppressive medication in most study patients was reduced in follow-up of COVID-19, no final conclusion on the effect of immunosuppression can be drawn from samples obtained at later time points. To address the influence of immunosuppression on SARS-CoV-2–reactive immunity, we analyzed the frequencies of S-, N-, and M-protein reactive T cells in all patients at the first time point. This assured that the analyzed samples were obtained under immunosuppressive conditions. The time of study inclusion after positive SARS-CoV-2 PCR was in median 3 and 4 d for non-Tx and Tx patients, respectively (IQR 2–6 in Tx patients and 1–9 in non-Tx patients; no significant differences in Mann-Whitney U test). There was no statistically significant difference in COVID-19 severity in both groups at this time point (Table S2, SDC, http://links.lww.com/TP/C185). Surprisingly, frequencies of SARS-CoV-2–reactive CD4+ T cells were nearly identical between the Tx patient and the non-Tx patient cohort (Figure S4, SDC, http://links.lww.com/TP/C185). Overall CD8+ T cell responses towards SARS-CoV-2 peptides were again lower as compared to CD4+ T cell responses (Figure S5, SDC, http://links.lww.com/TP/C185). Although the frequency of S-protein-reactive CD8+ T cells in the non-Tx cohort exceeded the response in the Tx cohort, the difference did not reach statistical significance (Figure S5b and e, SDC, http://links.lww.com/TP/C185). There were no differences in the magnitude of M- and N-protein–reactive CD8+ T cells.
DISCUSSION
COVID-19 poses an especially severe risk to vulnerable patients, including the transplantation patient community. A main concern is the insufficient generation of SARS-CoV-2 directed adaptive immunity. The ability to generate efficient immune responses influences, among others, the risk assessment, treatment approaches, and vaccination strategies. The modification of immunosuppressive therapy comes at the cost of rejection risk and potentially reduced Tx survival.36 At the same time, the effectiveness of this measure in COVID-19 is not known,6,37,38 because it is unclear if Tx patients are able to mount an effective SARS-CoV-2 directed adaptive immune response after prolonged immunosuppression. With our study, we provide data on SARS-CoV-2–reactive humoral and cellular adaptive immunity in Tx patients early after diagnosis and in short-term follow-up.
Somewhat surprisingly, we did not observe strong differences in the formation of polyfunctional and memory SARS-CoV-2–reactive T cell responses between Tx and non-Tx patients. Following the data on cellular immunity, Tx patients showed similar titers of neutralizing antibodies as compared to non-Tx patients. As in other cohorts,22,27 we also saw that the SARS-CoV-2 CD4+ exceeded SARS-CoV-2–reactive CD8+ T cell immunity. Cytokine and effector molecule production was diminished in CD8+ T cells of Tx patients as compared to non-Tx patients after stimulation with M and S, but not N-protein. Since N-protein was a strong inducer of CD8+ T cell immunity in previous analyses,22 it is likely that also Tx patients mount a functional SARS-CoV-2–reactive CD8+ T cell as well as CD4+ T cell response.
Chronic organ failure and transplantation result in an aged immune system with a reduced repertoire of naive T cells and possible dependence on crossreactive memory T cell responses to new immunologic challenges.32-34,39 Interestingly, a recent study described a lower functional avidity and higher polyclonality of SARS-CoV-2–reactive T cells in hospitalized as compared to nonhospitalized patients, despite higher frequencies of SARS-CoV-2–reactive T cells.30 This was associated with a higher immunologic age defined as the ratio of memory cells among total CD4+ T cells of the more severely affected patients.30 Therefore, the higher immunologic age of Tx patients observed in our and previous cohorts,31-34 might convey a higher risk of critical COVID-19 as recently hypothesized by Bacher et al.30
Despite our encouraging results, one could speculate that the similar SARS-CoV-2–reactive T cell immunity detected in COVID-19 follow-up of Tx patients as compared to nonimmunosuppressed patients might be explained by the reduced or discontinued regimen of immunosuppression. To be able to demonstrate the effect of immunosuppression, we analyzed the SARS-CoV-2–reactive T cells at the time point of COVID-19 diagnosis. Here, we demonstrated that SARS-CoV-2–reactive T cells could be detected at early time points of COVID-19 in Tx patients with a similar magnitude as in the non-Tx cohort. This interpretation is supported by a recent study by Benotmane et al,40 in which the authors observed that humoral immune response in SARS-CoV-2 infected Tx patients was not significantly impaired. Similarly, Candon et al41 measured vigorous SARS-CoV-2 cellular and humoral immunity by enzyme-linked immune absorbent spot and ELISA, respectively, in renal Tx and hemodialysis patients. Therefore, although highly preliminary due to the low patient number, in line with previous data42-44 our study demonstrates a sufficient formation of antiviral response in Tx patients despite immunosuppressive medication.
Several limitations are important to consider regarding the interpretation of our findings. This study was not designed to answer the question of optimal therapy of SARS-CoV-2 infected Tx patients, and thus, the modification of treatment and sample collection was not done systematically. Furthermore, the low number of patients makes robust assumptions impossible. Tx patients were significantly younger than non-Tx patients, which we took account of by bivariate regression analysis. Lastly, the treatment modalities differed between the patients and additional immunomodulatory effects of other interventions cannot be excluded. Our results are likely not transferable to recently transplanted recipients that undergo induction therapy, and thus careful evaluation of transplantation activity in high-prevalence regions remains pivotal.38,45
Further studies are required to evaluate the role of individual immunosuppressive regimen on SARS-CoV-2–reactive immunity. Nevertheless, our data show an effective generation of neutralizing antibody and T cell responses towards SARS-CoV-2 in patients with a long history of immunosuppression and chronic disease early after COVID-19 diagnosis.
ACKNOWLEDGMENTS
We feel deep gratitude to the patients who donated their blood samples and clinical data for this project. We would like to acknowledge the excellent technical assistance as well as the expertise of the immune diagnostic laboratory (Sarah Skrzypczyk, Eva Kohut, Julia Kurek, and Jan Zapka) of the Center for Translational Medicine at Marien Hospital Herne. We thank Gert Zimmer for his support in providing VSV system.
Supplementary Material
Footnotes
This work was supported by grants from Mercator Foundation, the BMBF e:KID (01ZX1612A), and BMBF NoChro (FKZ 13GW0338B).
The authors declare no conflicts of interest.
C.J.T., M.A., T.R., O.W., P.S., R.V., U.S., T.H.W., and N.B. participated in research design. K.P., A.D., M.M.B., T.B., S.D., O.W., P.S., R.V., T.H.W., and N.B. participated in patient recruitment, collection of samples, and clinical data. C.J.T., M.A., K.P., A.D., F.S.S., B.H., M.J.K., T.L.M., S.P., E.S., and U.K. participated in the performance of the research. C.J.T., M.A., A.B.N., T.R., U.S., and N.B. participated in data analysis. C.J.T., K.P., A.B.N., T.R., and N.B. participated in writing of the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Mohamed IH, Chowdary PB, Shetty S, et al. Outcomes of renal transplant recipients with SARS-CoV-2 infection in the eye of the storm: a comparative study with waitlisted patients. Transplantation. 2021;105:115–120. [DOI] [PubMed] [Google Scholar]
- 2.Thieme CJ, Zgoura P, Todorova I, et al. COVID-19 associated risk score, behavior and symptom prevalence in German transplant recipients. Transplant Proc. 2020:S0041-1345(20)32897-9. doi: 10.1016/j.transproceed.2020.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domínguez-Gil B, Fernández-Ruiz M, Hernández D, et al. Organ donation and transplantation during the COVID-19 pandemic: a summary of the Spanish experience. Transplantation. 2021;105:29–36. [DOI] [PubMed] [Google Scholar]
- 4.Ritschl PV, Nevermann N, Wiering L, et al. Solid organ transplantation programs facing lack of empiric evidence in the COVID-19 pandemic: a By-proxy Society Recommendation Consensus approach. Am J Transplant. 2020;20:1826–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moal V, Zandotti C, Colson P. Emerging viral diseases in kidney transplant recipients. Rev Med Virol. 2013;23:50–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azzi Y, Bartash R, Scalea J, et al. COVID-19 and solid organ transplantation: a review article. Transplantation. 2021;105:37–55. [DOI] [PubMed] [Google Scholar]
- 7.Lai Q, Spoletini G, Bianco G, et al. SARS-CoV2 and immunosuppression: a double-edged sword. Transpl Infect Dis. 2020;22:e13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minotti C, Tirelli F, Barbieri E, et al. How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection? A systematic review. J Infect. 2020;81:e61–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babel N, Anft M, Blazquez-Navarro A, et al. Immune monitoring facilitates the clinical decision in multifocal COVID-19 of a pancreas-kidney transplant patient. Am J Transplant. 2020;20:3210–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akalin E, Azzi Y, Bartash R, et al. COVID-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20:1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Chen Y, Yuan Q, et al. Identification of kidney transplant recipients with coronavirus disease 2019. Eur Urol. 2020;77:742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mamode N, Ahmed Z, Jones G, et al. Mortality rates in transplant recipients and transplantation candidates in a high-prevalence COVID-19 environment. Transplantation. 2021;105:212–215. [DOI] [PubMed] [Google Scholar]
- 14.Ali T, Al-Ali A, Fajji L, et al. Coronavirus disease-19: disease severity and outcomes of solid organ transplant recipients: different spectrums of disease in different populations? Transplantation. 2021;105:121–127. [DOI] [PubMed] [Google Scholar]
- 15.The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19 — preliminary report. N Engl J Med. 2020;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfefferle S, Schöpf J, Kögl M, et al. The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. Plos Pathog. 2011;7:e1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato F, Matsuyama S, Kawase M, et al. Antiviral activities of mycophenolic acid and IMD-0354 against SARS-CoV-2. Microbiol Immunol. 2020;64:635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng KW, Cheng SC, Chen WY, et al. Thiopurine analogs and mycophenolic acid synergistically inhibit the papain-like protease of Middle East respiratory syndrome coronavirus. Antiviral Res. 2015;115:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fishman JA, Issa NC. Infection in organ transplantation: risk factors and evolving patterns of infection. Infect Dis Clin North Am. 2010;24:273–283. [DOI] [PubMed] [Google Scholar]
- 20.López-Medrano F, Aguado JM, Lizasoain M, et al. Clinical implications of respiratory virus infections in solid organ transplant recipients: a prospective study. Transplantation. 2007;84:851–856. [DOI] [PubMed] [Google Scholar]
- 21.Fischer SA. Emerging viruses in transplantation: there is more to infection after transplant than CMV and EBV. Transplantation. 2008;86:1327–1339. [DOI] [PubMed] [Google Scholar]
- 22.Thieme CJ, Anft M, Paniskaki K, et al. Robust T cell response toward spike, membrane, and nucleocapsid SARS-CoV-2 proteins is not associated with recovery in critical COVID-19 patients. Cell Rep Med. 2020;1:100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anft M, Paniskaki K, Blazquez-Navarro A, et al. COVID-19-induced ARDS is associated with decreased frequency of activated memory/effector T cells expressing CD11a+. Mol Ther. 2020;28:2691–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R-Core-Team. R: A language and environment for statistical computing. Available at https://www.r-project.org. Accessed August 26, 2020.
- 25.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sette A, Moutaftsi M, Moyron-Quiroz J, et al. Selective CD4+ T cell help for antibody responses to a large viral pathogen: deterministic linkage of specificities. Immunity. 2008;28:847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bacher P, Rosati E, Esser D, et al. Low-avidity CD4+ T cell responses to SARS-CoV-2 in unexposed individuals and humans with severe COVID-19. Immunity. 2020;53:1258–1271. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grigoryev YA, Kurian SM, Avnur Z, et al. Deconvoluting post-transplant immunity: cell subset-specific mapping reveals pathways for activation and expansion of memory T, monocytes and B cells. PLoS One. 2010;5:e13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Betjes MG, Langerak AW, van der Spek A, et al. Premature aging of circulating T cells in patients with end-stage renal disease. Kidney Int. 2011;80:208–217. [DOI] [PubMed] [Google Scholar]
- 33.Meijers RWJ, Litjens NHR, de Wit EA, et al. Uremia-associated immunological aging is stably imprinted in the T-cell system and not reversed by kidney transplantation. Transpl Int. 2014;27:1272–1284. [DOI] [PubMed] [Google Scholar]
- 34.Segundo DS, Fernández-Fresnedo G, Gago M, et al. Changes in the number of circulating T CM and T em subsets in renal transplantation: relationship with acute rejection and induction therapy. Kidney Int Suppl. 2011;1:31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirokawa K, Utsuyama M, Kikuchi Y, et al. Assessment of age-related decline of immunological function and possible methods for immunological restoration in elderly. In: Handbook on Immunosenescence. Springer; 2009:1547–1570. [Google Scholar]
- 36.Bae S, McAdams-DeMarco MA, Massie AB, et al. Early changes in kidney transplant immunosuppression regimens during the COVID-19 pandemic. Transplantation. 2021;105:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avery RK. COVID-19 therapeutics for solid organ transplant recipients; 6 months into the pandemic: where are we now? Transplantation. 2021;105:56–60. [DOI] [PubMed] [Google Scholar]
- 38.Weiss MJ, Lalani J, Patriquin-Stoner C, et al. Summary of international recommendations for donation and transplantation programs during the coronavirus disease pandemic. Transplantation. 2021;105:14–17. [DOI] [PubMed] [Google Scholar]
- 39.Woodland DL, Blackman MA. Immunity and age: living in the past? Trends Immunol. 2006;27:303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benotmane I, Gautier-Vargas G, Wendling MJ, et al. In-depth virological assessment of kidney transplant recipients with COVID-19. Am J Transplant. 2020;20:3162–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Candon S, Guerrot D, Drouot L, et al. T cell and antibody responses to SARS-CoV-2: experience from a French transplantation and hemodialysis center during the COVID-19 pandemic. Am J Transplant. 2021;21:854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rambal V, Müller K, Dang-Heine C, et al. Differential influenza H1N1-specific humoral and cellular response kinetics in kidney transplant patients. Med Microbiol Immunol. 2014;203:35–45. [DOI] [PubMed] [Google Scholar]
- 43.Bunde T, Kirchner A, Hoffmeister B, et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med. 2005;201:1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weist BJ, Wehler P, El Ahmad L, et al. A revised strategy for monitoring BKV-specific cellular immunity in kidney transplant patients. Kidney Int. 2015;88:1293–1303. [DOI] [PubMed] [Google Scholar]
- 45.Stock PG, Wall A, Gardner J, et al. ; TTS Ethics Committee. Ethical issues in the COVID era: doing the right thing depends on location, resources, and disease burden. Transplantation. 2020;104:1316–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.