Supplemental Digital Content is available in the text.
Keywords: acute kidney injury; RNA, small interfering; thoracic surgery
Background:
Acute kidney injury (AKI) affects up to 30% of patients undergoing cardiac surgery, leading to increased in-hospital and long-term morbidity and mortality. Teprasiran is a novel small interfering RNA that temporarily inhibits p53-mediated cell death that underlies AKI.
Methods:
This prospective, multicenter, double-blind, randomized, controlled phase 2 trial evaluated the efficacy and safety of a single 10 mg/kg dose of teprasiran versus placebo (1:1), in reducing the incidence, severity, and duration of AKI after cardiac surgery in high-risk patients. The primary end point was the proportion of patients who developed AKI determined by serum creatinine by postoperative day 5. Other end points included AKI severity and duration using various prespecified criteria. To inform future clinical development, a composite end point of major adverse kidney events at day 90, including death, renal replacement therapy, and ≥25% reduction of estimated glomerular filtration rate was assessed. Both serum creatinine and serum cystatin-C were used for estimated glomerular filtration rate assessments.
Results:
A total of 360 patients were randomly assigned in 41 centers; 341 dosed patients were 73±7.5 years of age (mean±SD), 72% were men, and median European System for Cardiac Operative Risk Evaluation score was 2.6%. Demographics and surgical parameters were similar between groups. AKI incidence was 37% for teprasiran- versus 50% for placebo-treated patients, a 12.8% absolute risk reduction, P=0.02; odds ratio, 0.58 (95% CI, 0.37–0.92). AKI severity and duration were also improved with teprasiran: 2.5% of teprasiran- versus 6.7% of placebo-treated patients had grade 3 AKI; 7% teprasiran- versus 13% placebo-treated patients had AKI lasting for 5 days. No significant difference was observed for the major adverse kidney events at day 90 composite in the overall population. No safety issues were identified with teprasiran treatment.
Conclusions:
The incidence, severity, and duration of early AKI in high-risk patients undergoing cardiac surgery were significantly reduced after teprasiran administration. A phase 3 study with a major adverse kidney event at day 90 primary outcome that has recently completed enrollment was designed on the basis of these findings (NCT03510897).
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02610283.
Clinical Perspective.
What Is New?
In patients undergoing cardiac surgery, the ischemia reperfusion process can lead to acute kidney injury. Teprasiran is a small interfering RNA that temporarily inhibits p53-mediated cell death and thus may allow injured renal tubule cells an opportunity for repair.
What Are the Clinical Implications?
Patients receiving teprasiran had a lower incidence of early acute kidney injury (37% for teprasiran versus 50% for placebo-treated patients, P=0.02; odds ratio, 0.58 [95% CI, 0.37–0.92]), and no safety issues were identified.
This study suggests a possible renal protective effect of teprasiran and forms the basis of a larger phase 3 study that has recently completed enrollment to explore the safety and efficacy of this agent to reduce major adverse kidney events at day 90 in this setting.
Acute kidney injury (AKI) affects up to 30% of all patients undergoing cardiac surgery1 despite recent advances in surgical techniques and postsurgical care. Known risk factors for postoperative AKI include procedure type, on-pump versus off-pump surgery,2 cardiopulmonary bypass (CPB) duration, comorbidities (eg, chronic kidney disease, congestive heart failure, diabetes), female sex, and advanced age.3,4
Patients who develop postoperative AKI demonstrate increased short- and long-term mortality and morbidity, including progression to chronic kidney disease.5–9 At present, the standard of care includes fluid management and avoiding nephrotoxic drugs in high-risk patients. However, no approved targeted therapies exist for the prevention or treatment of this life-threatening complication.3
Various underlying mechanisms contribute to the development of postoperative AKI that are mainly mediated by reduced renal perfusion and ischemia reperfusion injury (IRI). IRI, through the opening of mitochondrial permeability transition pores and oxidative stress, leads to cell injury and death. This mechanism of renal injury is typically seen with CPB.3
p53, a DNA-binding transcription factor, activates genes responsible for growth arrest or cell death after exposure to IRI.10 Activation of cell death versus growth arrest depends on the magnitude of p53 increase.11 The rationale for temporary inhibition of p53 to protect cells from IRI is that reducing p53 levels attenuates cell death and subsequent organ failure, switching the downstream pathway to a damage repair mode and allowing cells the opportunity to recover.12 In kidney IRI, p53 activation occurs primarily in proximal tubular epithelial cells.13,14 There is evidence suggesting that this activation is increased in aged kidneys.15 Animal studies indicate that p53 knockout specifically in proximal tubular epithelial cells is necessary and sufficient for injury attenuation.16 Although p53 has tumor suppressor functions, tumorigenesis was not demonstrated with brief inhibition, similar to that caused by teprasiran administration, in multiple animal models.17,18
RNA interference is a biological process in which small double-stranded RNA molecules inhibit gene expression by neutralizing targeted messenger RNA molecules. This process can be harnessed for therapeutic purposes using synthetic double-stranded RNA oligonucleotides called small interfering RNAs (siRNAs). After systemic administration, such compounds specifically accumulate in proximal tubular epithelial cells19 and, as such, appear tailor-made for local and temporary p53 inhibition for the prevention of IRI-induced AKI. Teprasiran is a synthetic, chemically stabilized small interfering RNA (siRNA) acting through RNA interference, which temporarily inhibits p53 expression for ≈48 to 72 hours. Teprasiran has a plasma residence time of <20 minutes. Previously published results in multiple preclinical models show that temporary p53 inhibition with siRNA reduces the risk of AKI and organ failure.19,20
Teprasiran has been under evaluation for the prevention of AKI and its consequences in high-risk patients undergoing cardiac surgery21 (NCT03510897) and for the prevention of delayed graft function in kidney transplant recipients from deceased donors (NCT00802347 and NCT02610296).
This phase 2 study was conducted to evaluate the safety and short-term efficacy of teprasiran in preventing AKI in high-risk patients undergoing cardiac surgery (NCT02610283). It also provided the basis for a larger phase 3 trial to meet regulatory approval requirements for an expanded safety data set and demonstration of efficacy in terms of long-term durable outcomes of renal function such as significant or prolonged deterioration of eGFR, need for renal replacement therapy, or death.
Methods
The authors declare that all supporting data have been provided within the article and its Data Supplement. The data, methods used in the analysis, and materials used to conduct the research will not be made available to any researcher for purposes of reproducing the results or replicating the procedure.
Study Design
This was a prospective, multicenter, double-blind, randomized, controlled phase 2 trial designed to evaluate the safety and efficacy of teprasiran in patients undergoing on-pump or off-pump cardiac surgery who were at moderate to high risk of developing AKI according to the presence of ≥1 risk factors.
The study was compliant with the Helsinki Declaration. Institutional ethical approval was obtained from each site and written informed consent was obtained from all participants before enrollment. An independent monitoring committee reviewed unblinded data to assess the safety and tolerability of treatment with teprasiran. No interim efficacy analyses were performed.
Patients
Men and women ≥45 years of age were enrolled if they were scheduled for an elective cardiac surgical procedure and had at least one of the following AKI risk factors: age >70 years, eGFR <60 mL·min–1·1.73 m–2, diabetes, proteinuria, or a history of congestive heart failure. Patients undergoing a single procedure, that is, isolated coronary artery bypass grafting (CABG) or single-valve surgery, needed to have ≥2 AKI risk factors to be eligible. Patients must have had stable renal function during the 4 weeks preceding study entry. Patients undergoing emergent surgeries, transcatheter aortic valve implantation, or transcatheter aortic valve replacement procedures, or those who were hemodynamically unstable before surgery were excluded. The complete list of eligibility criteria is included in the Data Supplement.
Patients were stratified by: eGFR (eGFR: 20–60 mL·min–1·1.73 m–2 and >60 mL·min–1·1.73 m–2), planned use of CPB (yes versus no), and circulatory arrest (yes versus no). A fixed block size of 4 was used within each stratum combination. Randomization to teprasiran 10 mg/kg or placebo occurred within the 24 hours preceding surgery in a 1:1 ratio. The study was designed to enroll ≈340 patients in North America and Germany. The sample size (n=170 per group) was based on the following assumptions: 50% of patients in the placebo group and 35% in the teprasiran group would develop AKI, with a type I error=0.05 (2-sided significance test), 80% power.
Trial Procedures
Study drug (teprasiran sodium 10 mg/kg; 25 mg/mL in phosphate-buffered saline, pH=7.0±0.5) or placebo (isotonic saline) was administered as a single intravenous bolus over 1 to 2 minutes at 4 hours±30 minutes after discontinuation of CPB or after the last coronary anastomosis for off-pump surgery (n=341). A baseline visit occurred within 24 hours before surgery, with daily follow-up visits up to day 7 (depending on the length of hospitalization); 3 additional follow-up visits occurred ≈30, 90, and 365 days after surgery (see Data Supplement for additional information).
Safety
Information about vital signs, laboratory values, and adverse events (AEs) were collected at prespecified visits as mentioned earlier through day 30; serious adverse events (SAEs) were collected through day 90. Vital status and malignancy status were obtained at 1 year. AEs were classified according to organ system using the Medical Dictionary for Regulatory Activities Version 19.1. An independent Data Monitoring Committee monitored safety during the study.
End Points and Statistical Analysis
The primary efficacy end point was the proportion of patients developing AKI as defined by the AKIN (Acute Kidney Injury Network) criteria through postoperative day 5 using sCr only.
For the primary end point analysis, the groups were compared by multivariable binary logistic regression analysis with treatment group and baseline stratification factors (eGFR, use of cardiopulmonary bypass, and circulatory arrest) included in the model. The null hypothesis of no treatment effect was tested at the ≤0.05 level (2-sided) using the Wald χ2 test on 1 degree of freedom. The analysis was prespecified to be performed on a modified intent-to-treat (mITT) population, defined as all patients who were randomly assigned, dosed with study drug (teprasiran or placebo), and who had a baseline sCr value available; the mITT-bsCr analysis set. No interim efficacy analyses were performed.
Secondary efficacy end points included severity and duration of AKI. Additional secondary end points included alternate definitions of AKI using other criteria (Risk, Injury, and Failure; and Loss, and End-stage kidney disease [RIFLE], Kidney Disease Improving Global Outcomes [KDIGO]), hospital and intensive care unit length of stay, and health status (EQ-5D). A composite end point of major adverse kidney events at 90 days (MAKE90) was included as a secondary end point to evaluate the effect of teprasiran on longer-term complications. MAKE90 was defined as the proportion of patients developing at least one of the following: death through day 90, need for renal replacement therapy through day 90, or ≥25% reduction in eGFR from baseline to 90 days after surgery. The eGFR component was analyzed separately using either sCr or sCys.
All dichotomous secondary outcomes were compared, similar to the primary outcome, using a multivariate binary logistic regression analysis with treatment group and the baseline stratification factors included in the model. Treatment comparisons for stages and duration of AKI were done by using the Wilcoxon nonparametric test.
Prespecified subgroup analyses were performed on the primary end point to determine whether the effect of treatment varied across stratification factors and other AKI prognostic indicators. Additional prespecified sensitivity analyses were done using the per-protocol and intention-to-treat populations and excluding patients with a rise in sCr of >0.3 mg/dL immediately before dosing.
Analyses were performed with the use of the SAS System, Version 9.4 (SAS Institute Inc).
Results
Demographics and Baseline Characteristics
From January 2016 to April 2017, a total of 360 patients were randomly assigned and 341 were dosed in 41 sites in North America and Germany (see the Data Supplement for list of sites and investigators). Among the 341 randomly assigned patients, 165 patients received teprasiran and 176 received placebo. A total of 24 (6.7%) patients were discontinued after dosing for various reasons (Figure 1).
Figure 1.
Patient disposition. Patients were assigned in a 1:1 ratio to the teprasiran or placebo groups. Of the 360 patients randomly assigned, 19 patients (6 assigned to placebo and 13 assigned to teprasiran) were discontinued before dosing because of events arising during or after the surgery (eg, death, physician decision, withdrawal by subject, or hemodynamic instability). Of those dosed, 93.2% of the placebo group and 92.7% of the teprasiran group completed the day 90 visit. *Two subjects (1 subject in each treatment group) died after study day 90. Both subjects experienced SAEs with fatal outcomes that began before study day 90.
Numbers of patients per analysis set are shown in Table I in the Data Supplement. The intention-to-treat (ITT) analysis set includes all patients who were randomly assigned and underwent surgery (n=360). The mITT analysis set includes all patients in the ITT analysis set who were dosed with the study drug (n=341). The mITT-bsCr analysis set includes all patients in the ITT analysis set who were dosed with study drug and had a baseline sCr result (n=322). mITT-bsCr was defined as the primary analysis population. Of those, 301 patients had a sCr value at baseline and at day 90. For those with no sCr value at day 90, values were imputed using last observation carried forward.
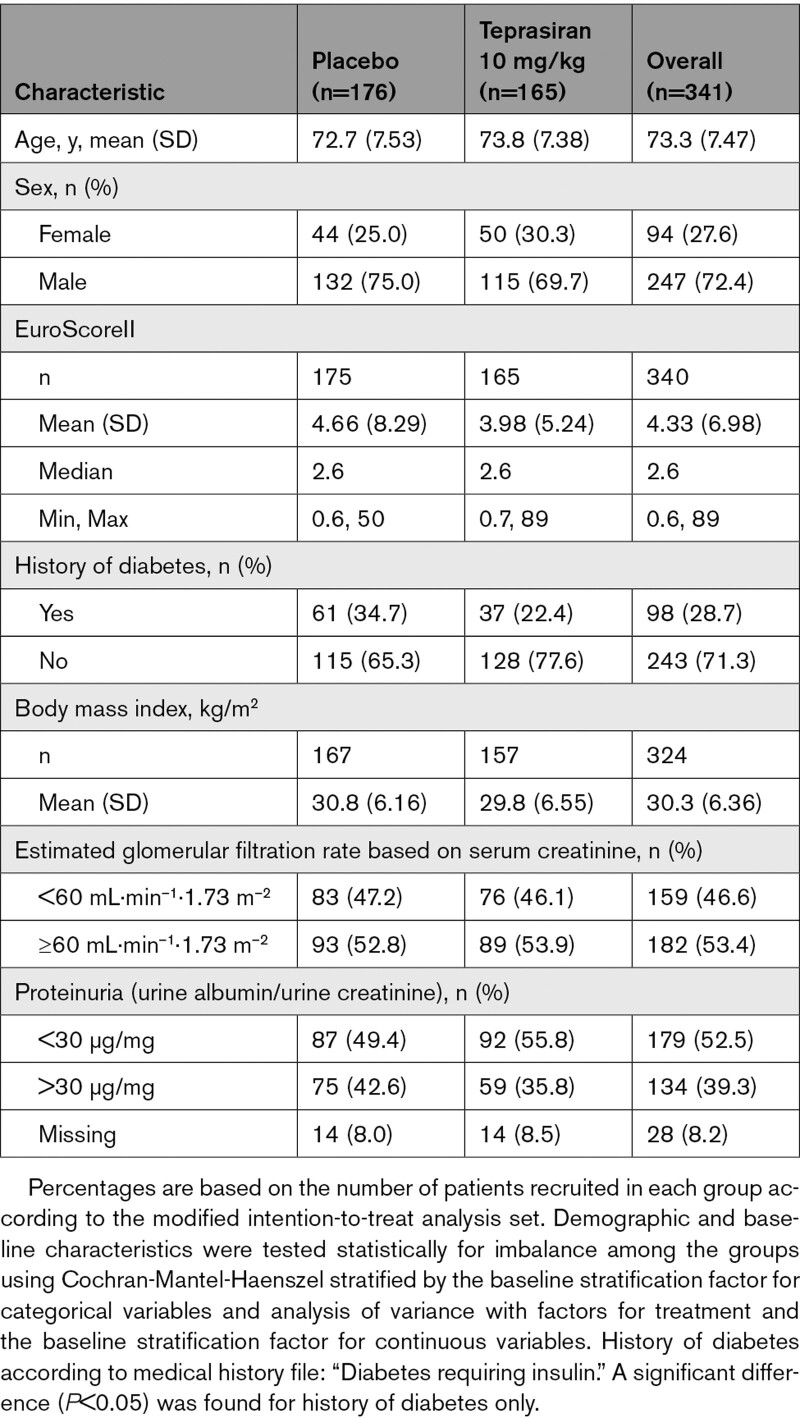
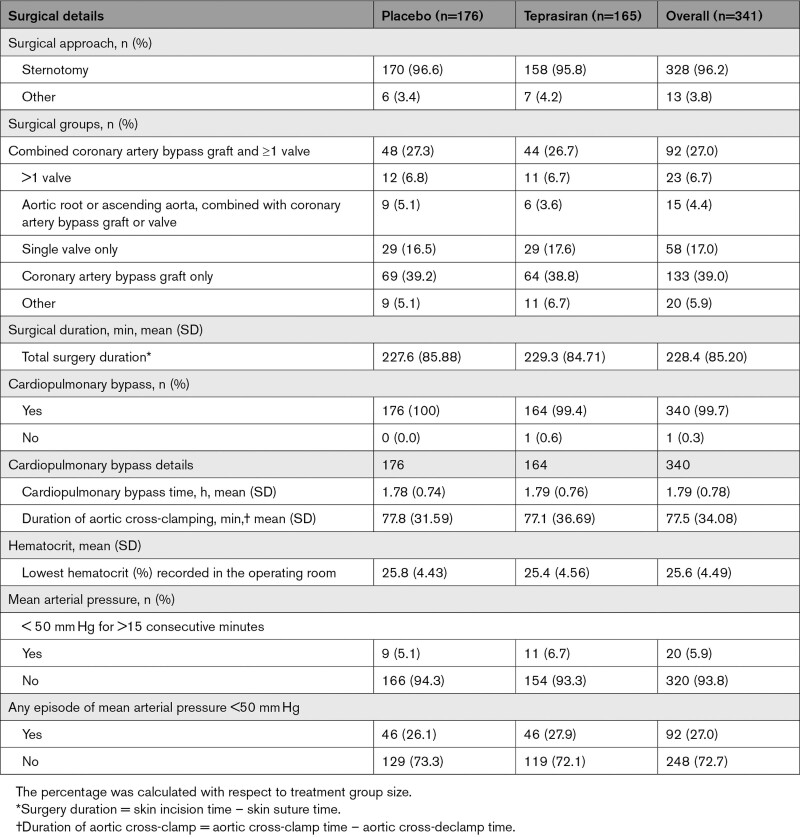
Baseline characteristics, demographics, and comorbidities were comparable among patients in the 2 treatment groups (Table 1). Dosed patients were predominantly men (72.4%) and White race (96.8%). Mean age was 73.3 years and most patients were obese with a mean body mass index of 30.3 kg/m2. Median Euroscore II value as reported by the study sites was 2.6%, 53% had evidence of preexisting renal dysfunction at study entry (defined as eGFRsCr < 60 mL·min–1·1.73 m–2) and 39% had proteinuria, with a urine albumin/urine creatinine ratio >30 μg/mg. The only noteworthy difference between treatment groups was the greater proportion of patients with diabetes in the placebo group (34.7% versus 22.4%). Details of the surgical procedure (Table 2) demonstrate a similar distribution of procedure type between the treatment groups. All but 1 patient underwent CPB, and the mean CPB duration was 1.79 hours. The majority of the operative procedures were CABG (39.2%) or CABG combined with ≥1 valve interventions (27.3%).
Table 1.
Demographic and Baseline Characteristics Summary by Treatment Group (Modified Intension-to-Treat Analysis Set)
Table 2.
Surgical Details Summary by Treatment Group (Modified Intention-To-Treat Analysis Set)
Primary Outcome
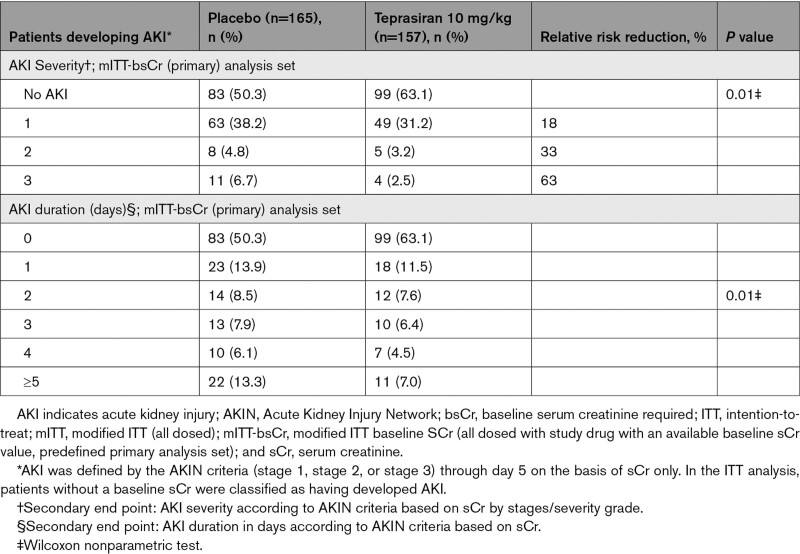
The number of patients in the primary analysis population who developed AKI per the AKIN criteria determined by sCr was 82 (49.7%) in the placebo group and 58 (36.9%) in the teprasiran group (absolute risk reduction, −12.8% [95% CI, −23.5 to −1.8]; odds ratio, 0.58 [95% CI, 0.37–0.92]; relative risk reduction, 25.8%; P=0.02; Tables 3 and 4).
Table 3.
Proportion of Patients Developing AKI by AKIN Criteria (Based on sCr) Through Day 5
Table 4.
Proportion of Patients Developing AKI, AKI Severity, and AKI Duration, by AKIN Criteria (Based on sCr) Through Day 5
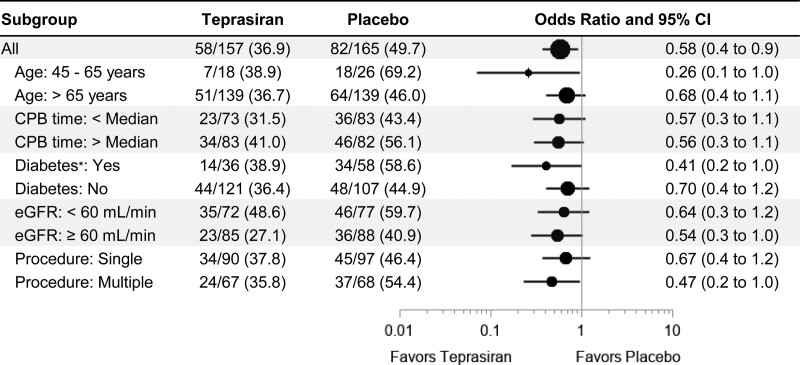
Sensitivity analyses, including ITT population and also excluding patients who developed AKI before dosing (with an increase of >0.3 mg/dL sCr between randomization and dosing), also yielded results that were consistent with the primary analysis (Tables 3 and 4). Teprasiran showed a consistent impact on AKI incidence across subgroups (Figure 2) with favorable results in multiple versus single procedures and patients with diabetes versus those without.
Figure 2.
Subgroup analysis of the primary end point (modified intention-to-treat–baseline serum creatinine required analysis set). The forest plot presents, for each subgroup, acute kidney injury odds ratio (OR) between treatments by a dot and its 95% CI by a horizontal line. The size of the dot varies proportionally with the total (Nteprasiran+Nplacebo) sample size of each subgroup. On the left side of the figure, the subgroup sample size by treatment is presented with the corresponding acute kidney injury rate. The column on the right lists the odds ratio and its 95% CI; the x-axis of the plot is in log scale. P values for odds ratio interaction between subgroups are as follows: age=0.17, CPB time=0.98, diabetes=0.34, eGFR=0.71, procedure=0.43. CPB indicates cardiopulmonary bypass; and eGFR, estimated glomerular filtration rate. *Diabetes requiring insulin treatment.
Secondary Outcomes
AKI severity according to AKIN criteria was also significantly reduced in teprasiran-treated patients, with a relative risk reduction of 18% (38.2% placebo versus 31.2% teprasiran), 33% (4.8% placebo versus 3.2% teprasiran), and 63% (6.7% placebo versus 2.5% teprasiran) for AKI stage 1, 2, and 3, respectively (Table 4). Similar reductions were demonstrated in AKI severity according to RIFLE and KDIGO criteria (Tables II and III in the Data Supplement). Moreover, teprasiran-treated patients had shorter AKI duration, with twice as many placebo-treated patients meeting the AKI criteria for ≥5 days (22 [13.3%] versus 11 [7%]; Table 4). Similar reductions in the incidence of prolonged AKI were shown by using both RIFLE and KDIGO criteria (Table IV in the Data Supplement).
Major Adverse Kidney Events at 90 Days
The incidence of MAKE90 was similar in teprasiran- versus placebo-treated patients when using eGFRsCr (19.3% versus 20.9% for placebo versus teprasiran respectively; odds ratio, 1.1 [95% CI, 0.6–1.9], P=0.71; Table V in the Data Supplement). Table V in the Data Supplement also includes components of the MAKE90 composite. The composite outcome when using eGFRsCys (43.9% versus 37% for placebo versus teprasiran, respectively; odds ratio, 0.8 [95% CI, 0.5–1.2], P=0.2065) is also shown in the Data Supplement, as are its individual components (Table VI in the Data Supplement). There was no difference in the proportion of deaths through day 90 (5.7% versus 4.8% for placebo versus teprasiran, respectively; odds ratio, 0.8 [95% CI, 0.3–2.2], P=0.72), or patients receiving renal replacement therapy (7.4% versus 4.8% for placebo versus teprasiran, respectively; odds ratio, 0.6 [95% CI, 0.3–1.6], P=0.34).
Safety
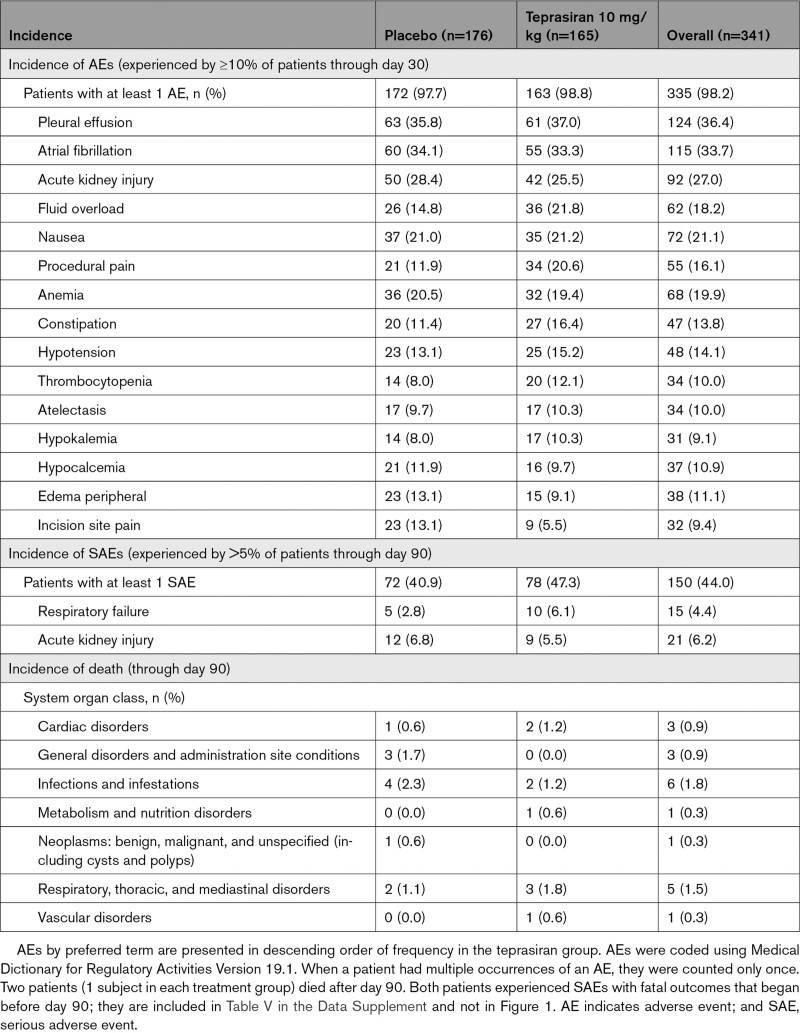
The frequency and types of AEs were similar for teprasiran- and placebo-treated patients. Overall, 335 patients (98.2%) experienced at least 1 AE. AEs experienced by ≥10% of patients in either group through day 30 are summarized in Table 5. The most common AEs were pleural effusion (36.4%) and atrial fibrillation (33.7%). Most events were assessed as unrelated to study drug by investigators; the most frequently reported AEs assessed as related were hypotension (1.2%), alanine aminotransferase increased (1.2%), and aspartate aminotransferase increased (0.9%). Analysis of aggregate data for related AEs suggested similar incidence between treatment groups.
Table 5.
Adverse Events and Deaths
The overall incidence of SAEs was similar between treatment groups, 47.3% for teprasiran and 40.9% for placebo. The most common SAEs, experienced by >5% in either group through day 90, were respiratory failure (4.4% overall, 6.1% in teprasiran-treated patients and 2.8% in placebo-treated patients, P=0.19) and AKI (6.2% overall, 5.5% in teprasiran-treated patients and 6.8% in placebo-treated patients).
Overall, 36 patients died through day 365. Of those, 20 patients died of a fatal SAE with a start date before day 90, 9 (5.5%) in the teprasiran group and 11 (6.3%) in the placebo group (P=0.72). The most frequently reported causes of death were infections and infestations (1.8%) and respiratory, thoracic, and mediastinal disorders (1.5%; Table 5). In addition, 15 patients died between day 90 and day 365, 4 in the teprasiran group and 11 in the placebo group, bringing the total number of deaths up to day 365 to 13 (7.9%) in the teprasiran group and 22 (12.5%) in the placebo group (P=0.17; Figure III in the Data Supplement). One subject died after randomization but before dosing.
Discussion
This study demonstrated a reduction in the incidence, severity, and duration of early postoperative AKI in high-risk patients undergoing on-pump cardiac surgery after a single dose of teprasiran. Teprasiran appeared to be well tolerated and no safety signal was detected. The reduction in AKI incidence was consistent across all prespecified subgroup analyses and reproducible when using alternative AKI definitions (ie, RIFLE and KDIGO). Of note, despite the observed baseline imbalance with a greater proportion of patients with diabetes in the placebo group (34.7% versus 22.4%; Table 1), the prespecified subgroup analysis indicated that teprasiran-treated subjects, with or without diabetes, had lower AKI rates (Figure 2).
The different observations for eGFRsCr and eGFRsCys in the overall study population undergoing cardiac surgery are in line with the published literature. The suggested explanation is that sCr is produced by muscle and can be confounded by numerous nonrenal factors that are prevalent in higher-risk patient populations like the one undergoing cardiac surgery in this study. Examples of such factors are: reduction of the creatinine pool attributable to the reduction of muscle mass (age- and disease-related), reduced intake of protein or meat, or water retention.22,23 Conversely, sCys is produced by all cells in the body and as such is not disproportionally affects by loss of muscle mass.22,24.
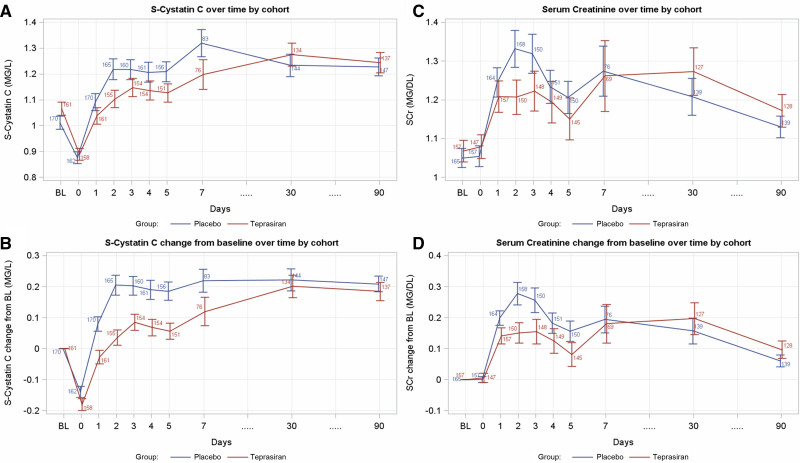
Previous publications suggest that eGFRsCys may be a better estimator of kidney function in populations of relevance to this study, such as those undergoing cardiac surgery,25,26 those with preexisting kidney dysfunction,27 and those with cardiorenal syndrome 2.28 eGFRsCys was demonstrated to be a good predictor of overall mortality in those patients undergoing elective CABG, in some cases better than eGFRsCr.29,30 The different dynamics over time of sCr and sCysC in this study (Figure 3) show that, although sCr levels seem to return to near baseline values at day 5 and remain almost entirely stable until day 90, sCysC values increase and remain elevated, potentially supporting the concept of sCysC being a much more sensitive marker of renal function in this specific population.
Figure 3.
Serum creatinine and serum cystatin C dynamics over time. Serum cystatin C values over time per cohort are presented as absolute (A) and change from baseline (BL; B). After a decrease in values on day 0 (day of surgery), an increase in serum cystatin C is observed in the acute postoperative period starting on day 1 in both treatment cohorts. The observed increase is maintained through day 90. Serum creatinine (sCr) values over time per cohort as absolute (C) and change from baseline (D). A rapid increase is observed on day 0 (day of surgery), reaching peak values on days 2 to 3 and decreasing to near presurgery values by day 5. sCr values at day 90 closely resemble baseline sCr values.
The tumor suppressor function of p53 may raise questions about a potential of increased risk for malignancy. However, tumorigenesis was not demonstrated with brief p53 inhibition, similar to that caused by teprasiran administration, in multiple animal models.17 After the intravenous injection of Cy3-labeled siRNAs targeting p53 in a rat model, rapid elimination of fluorescence signal from both total and cytosolic siRNA occurred, and there was no residual fluorescence detected in 24 hours in proximal tubular cells. These results were confirmed by using in situ hybridization. siRNA-mediated reduction of p53 mRNA levels was detected at 3 and 6 hours after siRNA administration and returned to baseline levels between 24 and 48 hours or between 6 and 24 hours after intravenous injection in the cortex and medulla, respectively.19 The temporary nature of p53 inhibition in the kidney is not expected to increase malignancy risk.12,17 In addition to these preclinical observations, malignancies have been monitored for up to 1 year in all clinical trials conducted to date with teprasiran. No specific malignancy signal has been identified so far in completed studies, with malignancies reported in 15 of 671 (2.2%) patients exposed to teprasiran versus 12 of 654 (1.8%) patients given placebo (data on file). Of note, many of these patients were kidney transplant recipients, concomitantly treated with immunosuppressants.
siRNAs are a novel therapeutic class with potential utility in various disease areas. The first siRNA to receive Food and Drug Administration approval in 2018 was for polyneuropathy caused by hereditary transthyretin-mediated amyloidosis. Additional siRNAs are being developed for diverse conditions. Inclisiran, a siRNA inhibiting hepatic synthesis of proprotein convertase subtilisin–kexin type 9, showed promising results in low-density lipoprotein reduction with a biannual administration regimen.31
To our knowledge, no other therapeutic intervention has demonstrated efficacy in treating or preventing AKI in a randomized, controlled trial setting. Because of the lack of robust and reproducible level 1 evidence from randomized, controlled trials, the standard of care is focused on closely monitoring patients, controlling recognized risk factors in the postsurgical setting, and avoiding nephrotoxic drugs.3
Several other preventive strategies showing early promise in small, noncontrolled, or retrospective studies, were not able to demonstrate reductions in postoperative AKI incidence in large, well-controlled studies or formal meta-analyses.3 These include preoperative statins,32 remote ischemic preconditioning,33 intravenous bicarbonate,34 fenoldopam,35 and the use of mannitol as the priming fluid for CPB.36 Although outcomes of clinical trials with the α-melanocyte–stimulating hormone analogue ABT-719 were initially encouraging, a phase 2 trial with end points and population similar to this study, failed to demonstrate treatment benefit on AKI or MAKE.37 The CiPRICS study (Ciclosporin to Protect Renal function in Cardiac Surgery) assessing the potential benefit of inhibiting the mitochondrial permeability transition pore opening during reperfusion by a single bolus of cyclosporine was not able to demonstrate differences between cyclosporin and placebo.38 Similarly, both Swaminathan et al39 and Himmelfarb et al40 reported negative results from interventional trials in a population similar to this study by using intra-arterial mesenchymal stem cells and THR-184, an antiapoptotic BMP 7 (bone morphogenetic protein 7) agonist, respectively.
The multiple tested and failed preventive strategies described here highlight postoperative AKI as an area of significant unmet medical need, with the potential to substantially affect future morbidity and mortality in high-risk patients.
This study has some limitations. First, 19 (5.6%) of patients randomly assigned and dosed (mITT) were unevaluable because of missing baseline creatinine values. However, in a sensitivity analysis counting patients with missing baseline creatinine values as treatment failures, the primary outcome of difference in AKI rates between treatment groups remained statistically significant. Second, AKI in this study was predominantly grade 1 with few grade 2 or 3 events. Thus, although the incidence of grade 2 and 3 AKI was numerically lower in the teprasiran group versus the placebo group by 33% and 63%, respectively, the study was not sufficiently powered to statistically conclude that teprasiran reduces the incidence of higher-grade AKI events. Similarly, this study was not adequately powered for the MAKE90 end point or mortality; future studies would be required to confirm whether or not teprasiran can improve these outcomes for it to receive regulatory approval. Third, the study included only 1 patient undergoing off-pump surgery. Because AKI rates, but not new renal failure requiring dialysis in the first 30 postoperative days, are higher for on-pump versus off-pump CABG,2 any extrapolation of the results to off-pump surgeries should be undertaken with caution. Fourth, although the rates of AEs, SAEs, and deaths were similar between groups, the sample size was too small to exclude an imbalance in rare safety events. Of note, in completed randomized, controlled studies using teprasiran in 921 patients undergoing kidney transplant to prevent delayed graft function, no safety signal was observed in up to 1 year of follow-up (data on file).
Conclusions
The best way to mitigate the long-term implications of AKI is to prevent it or treat it as early as possible. In this randomized, double-blind, placebo-controlled phase 2 clinical trial, teprasiran, a novel siRNA therapeutic targeting p53, was well tolerated and showed reductions in the incidence, severity, and duration of early AKI in high-risk, on-pump patients undergoing cardiac surgery. The results of this study suggest potential renal-protective effects of teprasiran and formed the basis of a larger phase 3 study (NCT03510897) that has recently completed enrollment and follow-up in >1000 high-risk, on-pump, patients undergoing cardiac surgery to explore the safety and efficacy of teprasiran to reduce MAKE90 in this setting.
Acknowledgments
Contributions acknowledged in study design and conduct from the following current and former Quark employees: R. Skaliter, S. Khan, U. Schwertschlag, A. I. Patel, J. Holman, A. Potts, E. K. Messersmith, K. Knowles, D. J. Odenheimer, D. Cafaro, E. Feinstein, N. Halevy, and the QRK209 study Investigators (complete list is in the Data Supplement).
Sources of Funding
The QRK209 study was funded by Quark Pharmaceuticals, Inc, Newark, CA. QRK209 was initiated, conducted, and analyzed by Quark. D. Rothenstein had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Disclosures
The investigators did not receive any honoraria for their work. M. Thielmann, C. D. Brown, and B. Molitoris are advisors to Quark. D. Rothenstein, S. Erlich, and E.C. Squiers are former Quark employees. The other authors report no conflicts.
Supplemental Materials
Study population and a complete list of inclusion/exclusion criteria
Schedule of Events
Data Supplement Figures I–III
Data Supplement Tables I–VI
List of Investigators, DMC membership, and Advisory Board membership
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AE
- adverse event
- AKI
- acute kidney injury
- AKIN
- Acute Kidney Injury Network
- bSCr
- baseline serum creatinine
- CABG
- coronary artery bypass grafting
- CPB
- cardio-pulmonary bypass
- eGFR
- estimated glomerular filtration rate
- EuroSCORE
- European System for Cardiac Operative Risk Evaluation
- IRI
- ischemia reperfusion injury
- ITT
- intention-to-treat analysis
- KDIGO
- Kidney Disease: Improving Global Outcomes
- MAKE
- major adverse kidney event
- mITT
- modified intention-to-treat analysis
- RIFLE
- risk, injury, failure, loss of kidney function, and end-stage kidney disease
- SAE
- severe adverse event
- sCr
- serum creatinine
- sCys
- serum cystatin C
- siRNA
- small interfering RNA
M. Thielmann, D. Coreville, and C.D. Mazer contributed equally.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.120.053029.
For Sources of Funding and Disclosures, see page 1143.
Contributor Information
Matthias Thielmann, Email: matthias.thielmann@uni-due.de.
David Corteville, Email: davidcorteville@gmail.com.
Gabor Szabo, Email: Gabor.Szabo@uk-halle.de.
Madhav Swaminathan, Email: madhav.swaminathan@duke.edu.
Andre Lamy, Email: lamya@mcmaster.ca.
Lukas J. Lehner, Email: lukas.lehner@charite.de.
Craig D. Brown, Email: cdbrown@nbnet.nb.ca.
Nicolas Noiseux, Email: noiseuxn@videotron.ca.
Mohamed G. Atta, Email: matta1@jhmi.edu.
Elizabeth C. Squiers, Email: bethsquiers@sbcglobal.net.
Shai Erlich, Email: serlich66@gmail.com.
Daniel Rothenstein, Email: RothensteinDaniel@gmail.com.
Bruce Molitoris, Email: bmolitor@iu.edu.
References
- 1.Huen SC, Parikh CR. Predicting acute kidney injury after cardiac surgery: a systematic review. Ann Thorac Surg. 2012;93:337–347. doi: 10.1016/j.athoracsur.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, Straka Z, Piegas LS, Akar AR, Jain AR, et al. ; CORONARY Investigators. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med. 2012;366:1489–1497. doi: 10.1056/NEJMoa1200388 [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017;13:697–711. doi: 10.1038/nrneph.2017.119 [DOI] [PubMed] [Google Scholar]
- 4.Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, Zarbock A. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–1561. doi: 10.1007/s00134-016-4670-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, Thottakkara P, Efron PA, Moore FA, Moldawer LL, Segal MS, Bihorac A. Cost and mortality associated with postoperative acute kidney injury. Ann Surg. 2015;261:1207–1214. doi: 10.1097/SLA.0000000000000732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozrazgat-Baslanti T, Thottakkara P, Huber M, Berg K, Gravenstein N, Tighe P, Lipori G, Segal MS, Hobson C, Bihorac A. Acute and chronic kidney disease and cardiovascular mortality after major surgery. Ann Surg. 2016;264:987–996. doi: 10.1097/SLA.0000000000001582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korenkevych D, Ozrazgat-Baslanti T, Thottakkara P, Hobson CE, Pardalos P, Momcilovic P, Bihorac A. The pattern of longitudinal change in serum creatinine and 90-day mortality after major surgery. Ann Surg. 2016;263:1219–1227. doi: 10.1097/SLA.0000000000001362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rimes-Stigare C, Ravn B, Awad A, Torlén K, Martling CR, Bottai M, Mårtensson J, Bell M. Creatinine- and cystatin c-based incidence of chronic kidney disease and acute kidney disease in AKI survivors. Crit Care Res Pract. 2018;2018:7698090. doi: 10.1155/2018/7698090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James MT, Bhatt M, Pannu N, Tonelli M. Long-term outcomes of acute kidney injury and strategies for improved care. Nat Rev Nephrol. 2020;16:193–205. doi: 10.1038/s41581-019-0247-z [DOI] [PubMed] [Google Scholar]
- 10.Ranjan A, Iwakuma T. Non-canonical cell death induced by p53. Int J Mol Sci. 2016;17:E2068. doi: 10.3390/ijms17122068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang L, Sheikh MS, Huang Y. Decision making by p53: life versus death. Mol Cell Pharmacol. 2010;2:69–77 [PMC free article] [PubMed] [Google Scholar]
- 12.Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737. doi: 10.1126/science.285.5434.1733 [DOI] [PubMed] [Google Scholar]
- 13.Tang C, Ma Z, Zhu J, Liu Z, Liu Y, Liu Y, Cai J, Dong Z. P53 in kidney injury and repair: mechanism and therapeutic potentials. Pharmacol Ther. 2019;195:5–12. doi: 10.1016/j.pharmthera.2018.10.013 [DOI] [PubMed] [Google Scholar]
- 14.McLaren BK, Zhang PL, Herrera GA. P53 protein is a reliable marker in identification of renal tubular injury. Appl Immunohistochem Mol Morphol. 2004;12:225–229. doi: 10.1097/00129039-200409000-00007 [DOI] [PubMed] [Google Scholar]
- 15.Kim MG, Yang J, Ko YS, Lee HY, Oh SW, Cho WY, Jo SK. Impact of aging on transition of acute kidney injury to chronic kidney disease. Sci Rep. 2019;9:18445. doi: 10.1038/s41598-019-54585-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang D, Liu Y, Wei Q, Huo Y, Liu K, Liu F, Dong Z. Tubular p53 regulates multiple genes to mediate AKI. J Am Soc Nephrol. 2014;25:2278–2289. doi: 10.1681/ASN.2013080902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature. 2006;443:214–217. doi: 10.1038/nature05077 [DOI] [PubMed] [Google Scholar]
- 18.Gudkov AV, Komarova EA. Pathologies associated with the p53 response. Cold Spring Harb Perspect Biol. 2010;2:a001180. doi: 10.1101/cshperspect.a001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E, Brafman A, Faerman A, Atkinson SJ, Thompson JD, et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol. 2009;20:1754–1764. doi: 10.1681/ASN.2008111204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imamura R, Isaka Y, Sandoval RM, Ori A, Adamsky S, Feinstein E, Molitoris BA, Takahara S. Intravital two-photon microscopy assessment of renal protection efficacy of siRNA for p53 in experimental rat kidney transplantation models. Cell Transplant. 2010;19:1659–1670. doi: 10.3727/096368910X516619 [DOI] [PubMed] [Google Scholar]
- 21.Demirjian S, Ailawadi G, Polinsky M, Bitran D, Silberman S, Shernan SK, Burnier M, Hamilton M, Squiers E, Erlich S, et al. Safety and tolerability study of an intravenously administered small interfering ribonucleic acid (siRNA) post on-pump cardiothoracic surgery in patients at risk of acute kidney injury. Kidney Int Rep. 2017;2:836–843. doi: 10.1016/j.ekir.2017.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Coresh J, Tighiouart H, Greene T, Inker LA. Strengths and limitations of estimated and measured GFR. Nat Rev Nephrol. 2019;15:784. doi: 10.1038/s41581-019-0213-9 [DOI] [PubMed] [Google Scholar]
- 23.Sandilands EA, Dhaun N, Dear JW, Webb DJ. Measurement of renal function in patients with chronic kidney disease. Br J Clin Pharmacol. 2013;76:504–515. doi: 10.1111/bcp.12198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Scholten BJ, Persson F, Svane MS, Hansen TW, Madsbad S, Rossing P. Effect of large weight reductions on measured and estimated kidney function. BMC Nephrol. 2017;18:52. doi: 10.1186/s12882-017-0474-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brondén B, Eyjolfsson A, Blomquist S, Dardashti A, Ederoth P, Bjursten H. Evaluation of cystatin C with iohexol clearance in cardiac surgery. Acta Anaesthesiol Scand. 2011;55:196–202. doi: 10.1111/j.1399-6576.2010.02361.x [DOI] [PubMed] [Google Scholar]
- 26.Zhu J, Yin R, Wu H, Yi J, Luo L, Dong G, Jing H. Cystatin C as a reliable marker of renal function following heart valve replacement surgery with cardiopulmonary bypass. Clin Chim Acta. 2006;374:116–121. doi: 10.1016/j.cca.2006.06.001 [DOI] [PubMed] [Google Scholar]
- 27.Jonsson AS, Flodin M, Hansson LO, Larsson A. Estimated glomerular filtration rate (eGFRCystC) from serum cystatin C shows strong agreement with iohexol clearance in patients with low GFR. Scand J Clin Lab Invest. 2007;67:801–809. doi: 10.1080/00365510701397538 [DOI] [PubMed] [Google Scholar]
- 28.Kervella D, Lemoine S, Sens F, Dubourg L, Sebbag L, Guebre-Egziabher F, Bonnefoy E, Juillard L. Cystatin C versus creatinine for GFR estimation in CKD due to heart failure. Am J Kidney Dis. 2017;69:321–323. doi: 10.1053/j.ajkd.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 29.Dardashti A, Nozohoor S, Grubb A, Bjursten H. Shrunken pore syndrome is associated with a sharp rise in mortality in patients undergoing elective coronary artery bypass grafting. Scand J Clin Lab Invest. 2016;76:74–81. doi: 10.3109/00365513.2015.1099724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mooney JF, Croal BL, Cassidy S, Lee VW, Chow CK, Cuthbertson BH, Hillis GS. Relative value of cystatin C and creatinine-based estimates of glomerular filtration rate in predicting long-term mortality after cardiac surgery: a cohort study. BMJ Open. 2019;9:e029379. doi: 10.1136/bmjopen-2019-029379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, Bisch JA, Richardson T, Jaros M, Wijngaard PLJ, et al. ; ORION-10 and ORION-11 Investigators. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382:1507–1519. doi: 10.1056/NEJMoa1912387 [DOI] [PubMed] [Google Scholar]
- 32.Billings FT, Hendricks PA, Schildcrout JS, Shi Y, Petracek MR, Byrne JG, Brown NJ. High-dose perioperative atorvastatin and acute kidney injury following cardiac surgery: a randomized clinical trial. JAMA. 2016;315:877–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menting TP, Wever KE, Hendriks EJ, Van der Vliet DJA, Rovers MM, Warle MC, Menting TP. Ischaemic preconditioning for the reduction of renal ischaemia reperfusion injury. Cochrane Database of Syst Rev. 2017;3:CD010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey M, McGuinness S, Haase M, Haase-Fielitz A, Parke R, Hodgson CL, Forbes A, Bagshaw SM, Bellomo R. Sodium bicarbonate and renal function after cardiac surgery: a prospectively planned individual patient meta-analysis. Anesthesiology. 2015;122:294–306. doi: 10.1097/ALN.0000000000000547 [DOI] [PubMed] [Google Scholar]
- 35.Bove T, Zangrillo A, Guarracino F, Alvaro G, Persi B, Maglioni E, Galdieri N, Comis M, Caramelli F, Pasero DC, et al. Effect of fenoldopam on use of renal replacement therapy among patients with acute kidney injury after cardiac surgery: a randomized clinical trial. JAMA. 2014;312:2244–2253. doi: 10.1001/jama.2014.13573 [DOI] [PubMed] [Google Scholar]
- 36.Bragadottir G, Redfors B, Ricksten SE. Mannitol increases renal blood flow and maintains filtration fraction and oxygenation in postoperative acute kidney injury: a prospective interventional study. Crit Care. 2012;16:R159. doi: 10.1186/cc11480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCullough PA, Bennett-Guerrero E, Chawla LS, Beaver T, Mehta RL, Molitoris BA, Eldred A, Ball G, Lee HJ, Houser MT, et al. ABT-719 for the prevention of acute kidney injury in patients undergoing high-risk cardiac surgery: a randomized phase 2b clinical trial. J Am Heart Assoc. 2016;5:e003549. doi: 10.1161/JAHA.116.003549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ederoth P, Grins E, Dardashti A, Brondén B, Metzsch C, Erdling A, Nozohoor S, Mokhtari A, Hansson MJ, Elmér E, et al. Ciclosporin to Protect Renal function In Cardiac Surgery (CiPRICS): a study protocol for a double-blind, randomised, placebo-controlled, proof-of-concept study. BMJ Open. 2016;6:e012299. doi: 10.1136/bmjopen-2016-012299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swaminathan M, Stafford-Smith M, Chertow GM, Warnock DG, Paragamian V, Brenner RM, Lellouche F, Fox-Robichaud A, Atta MG, Melby S, et al. ; ACT-AKI investigators. Allogeneic mesenchymal stem cells for treatment of AKI after cardiac surgery. J Am Soc Nephrol. 2018;29:260–267. doi: 10.1681/ASN.2016101150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Himmelfarb J, Chertow GM, McCullough PA, Mesana T, Shaw AD, Sundt TM, Brown C, Cortville D, Dagenais F, de Varennes B, et al. Perioperative THR-184 and AKI after cardiac surgery. J Am Soc Nephrol. 2018;29:670–679. doi: 10.1681/ASN.2017020217 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.