Abstract
Blood cryptococcal antigen (CrAg) titers >160 are associated with concurrent subclinical cryptococcal meningitis (CM). When lumbar puncture (LP) is not immediately available in a CrAg screening program, semi-quantitative CrAg assays may provide risk stratification for CM. Two semi-quantitative assays (SQ [Immuno-Mycologics, Norman, OK, USA] and CryptoPS [Biosynex, Strasbourg, France]) were evaluated against a qualitative lateral flow assay (LFA) using 194 plasma samples from a cohort of HIV-seropositive individuals with CD4 counts <100 cells/μl. We compared SQ and CryptoPS results to titers for LFA-positive samples. Among patients with LP, we examined the association between semi-quantitative CrAg results and CM. We used a Cox proportional hazards model to determine the association between SQ score and mortality. Of 194 participants, 60 (31%) had positive LFA results, of whom 41 (68%) had a titer of ≤160 and 19 (32%) a titer >160. Fifty individuals with antigenemia had an LP; a clinically useful SQ score that identified all ten cases of subclinical CM was ≥3 (100% sensitivity, 55% specificity). Patients with an SQ score of 3 or 4 also had a 2.2-fold increased adjusted hazards of 6-month mortality (95% CI: 0.79–6.34; p = 0.13) versus those with score of <3. Nine of ten patients with subclinical CM had a strong-positive CryptoPS result versus 10/40 without subclinical CM (p < 0.001). Semi-quantitative assays offered a sensitive though not specific means of gauging the risk of concurrent CM in this patient population.
Lay summary
We evaluated two single-step laboratory tests that can quantify the amount of cryptococcal antigen in plasma of patients with advanced HIV disease and could thus gauge the risk of concurrent cryptococcal meningitis and subsequent mortality. These tests are not a substitute for a lumbar puncture.
Keywords: Cryptococcus, cryptococcal antigen, antigenemia, lateral flow assay, semi-quantitative, subclinical, cryptococcal meningitis
Introduction
HIV-associated cryptococcal meningitis (CM), caused by Cryptococcus neoformans in the vast majority of cases and less frequently by Cryptococcus gattii, accounts for an estimated 15% of AIDS-related deaths in sub-Saharan Africa.1 A lateral flow assay (LFA) (Immuno-Mycologics Inc. [IMMY], Norman, OK, USA) can detect cryptococcal antigen (CrAg) in plasma, serum, whole blood and cerebrospinal fluid (CSF) very accurately.2,3 Reflex laboratory CrAg screening using this LFA to test remnant blood samples with a CD4 + T-cell count of <100 cells/μl has been routinely performed across a national network of CD4 laboratories in South Africa since 2016.4,5 Patients with cryptococcal antigenemia are recommended to be routinely investigated for CM since up to 40% may have subclinical CM.5 Pre-emptive fluconazole therapy is offered to asymptomatic patients with no microbiological evidence of CM.6,7 Despite pre-emptive fluconazole, CrAg-positive patients have a 2- to 3-fold increased risk of mortality compared to CrAg-negative patients with similar CD4 counts, suggesting that this treatment regimen may be inadequate.8,9 Combination treatment regimens are being compared to fluconazole monotherapy for people with antigenemia in the ongoing EFFECT and ACACIA clinical trials.10,11 Furthermore, lumbar punctures for all CrAg-positive individuals are not possible in many low-resource settings in sub-Saharan Africa and the rate of refusal among asymptomatic patients is high.5,8,12 In addition, contraindications to lumbar puncture include significant coagulopathy and a suspected space-occupying lesion based on recurrent seizures or focal central nervous system signs. Therefore, an enhanced CrAg-screening strategy involving risk-stratification using antigen quantification needs to be evaluated. A CrAg LFA titer in blood can stratify the risk of subclinical CM prior to lumbar puncture. A blood titer of >160 had 88.2% sensitivity and 82.1% specificity to predict concurrent subclinical CM in a cohort of CrAg-screened adults with a CD4 count of <100 cells/μl.13 However, measuring CrAg titers using serial dilutions of CrAg-positive blood samples is costly and challenging to implement in high-volume pathology laboratories. Approximately 10 LFA strips were required for serial dilutions and an experienced technician took approximately 20 min to obtain a single CrAg titer result (unpublished data, National Institute for Communicable Diseases). Semi-quantitative CrAg assays could provide a simple single-step alternative, assisting clinicians to gauge the probability of subclinical CM among people with antigenemia prior to lumbar puncture. We evaluated the accuracy of the SQ (Immy) and CryptoPS assays (Biosynex, Strasbourg, France) compared to the currently-used LFA in stored samples from a cohort of CrAg-screened HIV-seropositive patients with a CD4 count of <100 cells/μl. We also determined the association of SQ assay results with subclinical CM and mortality.
Methods
A prospective cohort study was conducted at Helen Joseph and Tambo Memorial hospitals in Johannesburg, South Africa from June 2015 through to October 2017.9,13 HIV-seropositive adults ≥18 years of age with a CD4 count of <100 cells/μl and no symptoms or signs of CM, who were identified during routine reflex laboratory CrAg screening, were recruited. For each CrAg-positive participant, two CrAg-negative participants were concurrently enrolled with a similar CD4 count. Lumbar punctures were performed for a subset of asymptomatic CrAg-positive patients to investigate for subclinical CM at enrolment and all participants were followed up for 6 months. CrAg LFA titers were determined using ethylene diamine tetra acetic acid (EDTA)-plasma samples stored at −70°C. Concurrent subclinical CM was defined as a positive CSF India ink microscopy, CrAg test or culture for Cryptococcus at the time of study enrollment, when the patient was attending for the result of their screening CrAg test. The full details of the study methods, participants and relationship between titers, subclinical CM and mortality have been published elsewhere.13
For this sub-study, we retrieved stored EDTA-plasma samples from 201 study participants and re-tested 194 samples (60 CrAg-positive; 134 CrAg-negative) at an ISO 15189-accredited reference medical laboratory. Seven samples were excluded after they were visually assessed and found not suitable for re-testing due to a high lipid content, insufficient volume or high viscosity. All additional CrAg testing was performed as per the manufacturers’ instructions. Trained laboratory personnel (N.P.B. and I.R.) performing the SQ and CryptoPS assays were blinded to previously-recorded LFA qualitative and titer results. All samples were tested once with SQ and CryptoPS assays; repeat testing was later performed if the qualitative result yielded by the semi-quantitative assays was found to be different to those of the LFA. SQ testing was performed by mixing 40 μl of plasma and 1 drop of diluent in a test tube, inserting a test strip into the mixture and reading the results at 10 min. For SQ-positive specimens, the SQ interpretation card was used as an aid to scoring from 1 to 5 based on the presence or absence of T1 and T2 lines. For the CryptoPS assay, 20 μl of plasma and 3 drops of diluent were added to the sample well of the cassette and results were read after 10 min. The presence of a T1 line was interpreted as a positive result and both T1 and T2 lines recorded as a strong-positive result. All samples were also tested using a CrAg enzyme immunoassay (EIA) (Immy), for which results were obtained using an absorbance microplate reader at 450 and 630 nm wavelengths, BioTek EL808 (BioTek instruments, Inc., Winooski, VT). Samples with optical density (OD) readings of ≤0.265 were interpreted as negative, and positive if the OD reading was >0.265.
We calculated the sensitivity and specificity (with 95% confidence intervals [CI]) of qualitative results yielded by the SQ and CryptoPS assays compared to those of the LFA. We used the LFA as the primary reference method because this assay has been validated for use in the national CrAg screening program. We used EIA results to resolve discrepancies. We also calculated diagnostic likelihood odds ratios. For LFA-positive samples, we compared SQ scores and CryptoPS results to titers. For the sub-set of patients with CSF CrAg results, a receiver operating characteristic (ROC) curve was used to assess cut-off SQ scores for detecting subclinical CM. A chi-square test was used to assess the association between CryptoPS results and subclinical CM. We used a Cox proportional hazards model to determine the association between plasma SQ scores and mortality. A high SQ score was defined as 3 or 4 and a low score as 1 or 2. We plotted Kaplan-Meier survival curves over 6 months of follow-up for the cohort by SQ score category.
Ethics approval was obtained for the cohort study from the University of the Witwatersrand and the London School of Hygiene and Tropical Medicine. Additional ethics approval was granted by Cape Peninsula University of Technology to use the archived plasma samples to evaluate the SQ and CryptoPS assays.
Results
Of the 194 study participants included in this analysis, the routinely performed standard CrAg LFA results were positive for 60 and negative for 134. Of the 60 with antigenemia, 41 (68%) had a titer of ≤160 and 19 (32%) a titer >160.
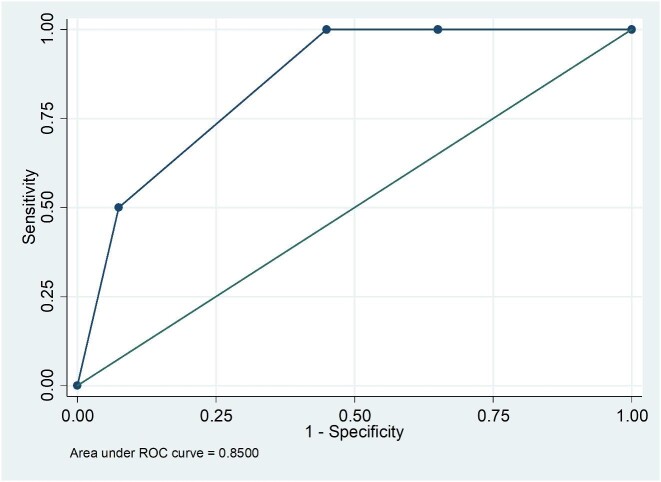
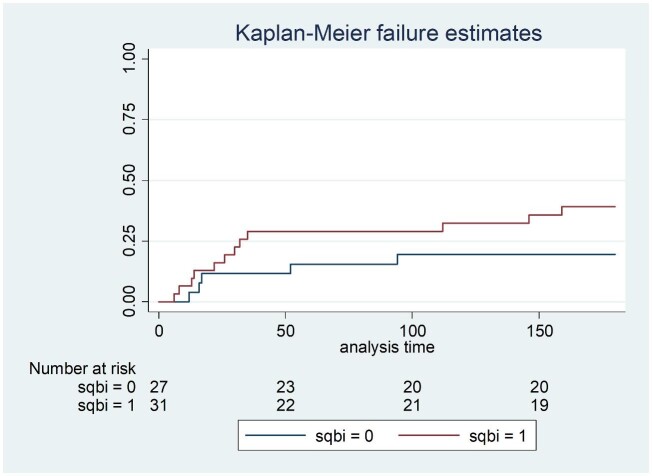
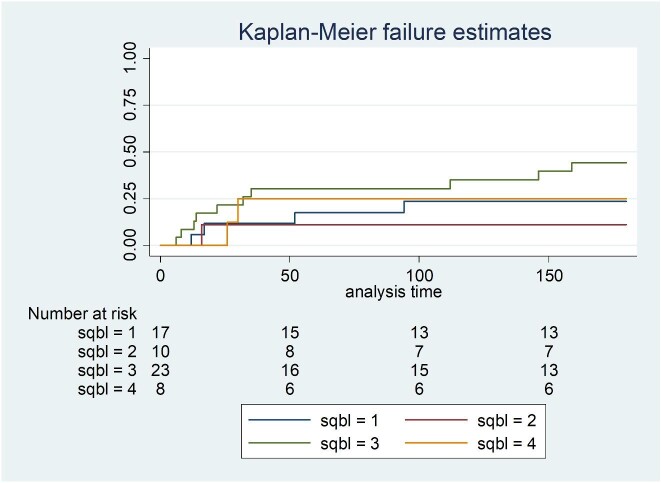
The sensitivity and specificity of qualitative results from the SQ assay was 98.3% (59/60 95% CI: 91.1–100) and 100% (134/134 95% CI: 95.97–100) compared to the LFA (Table 1). A single LFA-positive/SQ-negative sample had a CrAg titer of 20 and was also EIA-negative. Of 41 participants with a CrAg titer of ≤160, 40 had SQ scores of 1, 2 or 3 and 1 had a negative SQ result. Of the 19 with titers of >160, 18 had SQ scores of ≥3 and 1 had an SQ score of 2 (Fig. 1). Of 60 participants with antigenemia, 50 had a lumbar puncture performed (Table 2). Of 10 who were diagnosed with subclinical CM, 5 had titers ranging from 1280 to 163 840 and an SQ score of 4. The other 5 had titers that ranged from 80 to 327 680 with an SQ score of 3. Of the 40 without subclinical CM, 22 (55%) had an SQ score of <3 and 18 (45%) had a score of ≥3. The area under the ROC curve for the association between SQ score and subclinical CM was 0.85 (95% CI: 0.75–0.95) (Fig. 2). A cut-off SQ score of ≥3 had 100% sensitivity and 55% specificity (Table 3 and Fig. 2). Patients with a plasma SQ score of ≥3 also had a hazard ratio for death within 6 months of 2.20 (95% CI: 0.79–6.34; p = 0.13) compared to those with a low score, after adjusting for CD4 count (Figs 3 and 4).
Table 1.
Sensitivity and specificity of the SQ and CryptoPS assays for detection of CrAg in frozen-thawed plasma from HIV-seropositive patients with a CD4 count of <100 cells/μl compared to a reference lateral flow assay
| Statistic | SQ assay | 95% CI | CryptoPS assay | 95% CI |
|---|---|---|---|---|
| Sensitivity (%) | 98.33 | 91.06–99.99 | 90 | 79.49–96.24 |
| Specificity (%) | 100 | 97.28–100 | 94.1 | 89.5–97.9 |
| Positive likelihood ratio | >100 | 15.19 | 7.71–29.90 | |
| Negative likelihood ratio | 0.02 | 0.00–0.12 | 0.11 | 0.05–0.23 |
Note: Cryptococcal antigen (CrAg) enzyme immune-assay (EIA) results for the 194 participants were positive for 58 and negative for 136. Compared to the EIA, the sensitivity and specificity of the semi-quantitative (SQ) qualitative results were 100% (58/58) and 99% (135/136) while Crypto PS had 91.4% (53/58) sensitivity and 93.4% (127/136) specificity.
Figure 1.
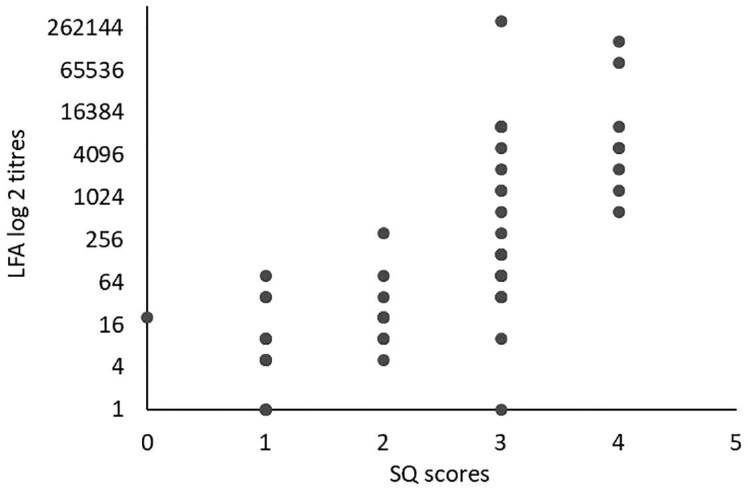
SQ assay scores compared to lateral flow assay (LFA) log2 titers from HIV-seropositive patients with a CD4 count of <100 cells/μl.
Table 2.
Association between SQ assay scores, lateral flow assay titers and subclinical cryptococcal meningitis (CM) for 50 patients with antigenemia and lumbar puncture results
| SQ assay score | Samples n = 50 | CrAg LFA titre ≤160 (n = 33) | CrAg LFA titre >160 (n = 17) | Subclinical CM (n = 10) | No subclinical CM (n = 40) |
|---|---|---|---|---|---|
| Negative | 0 | 0 | 0 | 0 | 0 |
| 1 | 14 (28%) | 14 (42%) | 0 | 0 | 14 (35%) |
| 2 | 8 (16%) | 7 (21%) | 1 (6%) | 0 | 8 (20%) |
| 3 | 20 (40%) | 12 (37%) | 8 (47%) | 5 (50%) | 15 (37%) |
| 4 | 8 (16%) | 0 | 8 (47%) | 5 (50%) | 3 (8%) |
Figure 2.
A receiver operating characteristic curve for plasma semi-quantitative (SQ) scores and subclinical cryptococcal meningitis among 50 asymptomatic cryptococcal-antigen positive patients.
Table 3.
Sensitivity and specificity of SQ assay score cut-offs from ≥1 to ≥4 for detecting subclinical cryptococcal meningitis among asymptomatic CrAg-positive patients with lumbar puncture results (n = 50)
| Cut point | Sensitivity % | Specificity | Positive likelihood ratio | Negative likelihood ratio |
|---|---|---|---|---|
| ≥1 | 100 | 0 | 1.0000 | |
| ≥2 | 100 | 35 | 1.5385 | 0.0000 |
| ≥3 | 100 | 55 | 2.2222 | 0.0000 |
| ≥4 | 50 | 92.5 | 6.6667 | 0.5405 |
| 5 | 0 | 100 | 1.0000 |
Receiver operating characteristic area = 0.85 (95% CI: 0.75–0.95).
Figure 3.
Kaplan-Meier survival estimates in 58 CrAg-positive patients by plasma SQ assay score category (0: score of ≥3 and 1: score of <3).
Figure 4.
Kaplan-Meier survival curve in 58 CrAg-positive patients by plasma SQ score category, adjusted for CD4 count (hazards ratio of mortality for SQ score of 2 versus 1: 0.45 (95% CI: 0.05–4.07); SQ score of 3 versus 1: 2.04 (95% CI: 0.63–6.61); SQ score of 4 versus 1: 1.00 (95% CI: 0.28–5.85)).
In contrast, the CryptoPS assay had 90% sensitivity (54/60; 95% CI: 79.49–96.24) and 94.8% specificity (127/134; 95% CI: 88.66–97.41) compared to LFA for qualitative results (Table 1). Thirteen samples had discordant LFA and CryptoPS qualitative results. Of the 6 CryptoPS-negative/LFA-positive samples, 3 had titers of 10, 1 had a titre of 5 and 2 had titers of <5. The EIA confirmed 5 of these samples as CrAg positive. The sixth sample was EIA-negative and had a titre of <5. Of the 7 CryptoPS-positive/LFA-negative samples, all sample dilutions were LFA negative and the EIA was negative. Of 41 participants with a positive LFA result and a titer of ≤160, 6 had negative CryptoPS results, 29 had positive results and 6 had strong positive results. Of the 19 with a positive LFA result and a titer of >160, 15 (78%) had strong positive CryptoPS results; the other 4 had positive CryptoPS results (Table 4). Nine of ten patients with subclinical CM had a strong-positive CryptoPS result versus 10/40 without subclinical CM (p<0.001). Compared to a lumbar puncture diagnosis of subclinical CM, the sensitivity and specificity of a strong-positive CryptoPS result was 90% (9/10; 95% CI: 55.5–99.7) and 75% (30/40; 95% CI: 58.8–87.3) respectively.
Table 4.
Association between CryptoPS, lateral flow assay titers and subclinical CM for 50 patients with antigenemia and lumbar puncture results.
| CryptoPS assay results | Samples (n = 50) | CrAg LFA titre ≤160 (n = 33) | CrAg LFA titre >160 (n = 17) | Subclinical CM (n = 10) | No subclinical CM (n = 40) |
|---|---|---|---|---|---|
| Negative | 5 (10%) | 5 (15%) | 0 | 0 | 5 (12%) |
| Positive | 26 (52%) | 22 (67%) | 4 (24%) | 1 (10%) | 25 (63%) |
| Strong positive | 19 (38%) | 6 (18%) | 13 (76%) | 9 (90%) | 10 (25%) |
Discussion
In this re-evaluation of stored plasma samples from a prospective cohort study, the SQ assay was simple and rapid to perform with identical testing steps to a single-strip qualitative LFA and an equivalent accuracy for CrAg detection in stored plasma. Using a cut-off score of ≥3, the SQ assay detected all cases of subclinical CM but had low specificity. A cut-off score of ≥4 reduced the sensitivity of this assay substantially but was much more specific for CM. For the purposes of risk stratification for subclinical CM among patients with antigenemia, a more sensitive cut-off (i.e., a score of ≥3) is preferable and specificity is a far less important consideration, given that lumbar puncture is now universally recommended. Although the CryptoPS assay did not perform well as a qualitative assay compared to the LFA, a strong-positive result was a relatively sensitive screening test for subclinical CM, though this was also not specific.
As previously reported, the SQ assay accurately detected CrAg compared to the LFA.14,15 In our study, only one low-titer sample had a negative SQ result. In contrast, the CryptoPS assay incorrectly classified a higher proportion (5/33; 15%) of low-titer samples as negative and also yielded 7 false-positive results. All 7 participants with false-positive CryptoPS results did not receive antifungal treatment and none progressed to CM during 6 months of follow-up. In an evaluation of the same assay in Botswana, a lower sensitivity (61%) but higher specificity (97%) was reported. In this study, 29 patients with false-positive CryptoPS results were followed up for 3 months and none developed CM.16
Since a blood CrAg titer cut-off of >160 had previously been identified as a threshold for subclinical CM,3,13 we evaluated the accuracy of the two semi-quantitative assays in categorizing plasma samples with a titer ≤160 and >160. All samples but one with an SQ score of <3 had a titer of <160. All samples with a score of >3 had a titer of >160. However, a score of 3 did not clearly distinguish between these two titer categories. Although a strong-positive CryptoPS result identified 76% of samples with a CrAg titer of >160, 18% of samples with a titer of ≤160 also had strong-positive results. In their evaluation, Tenforde et al. also reported a single LFA-negative plasma sample with a strong-positive CryptoPS result.16
We went on to investigate the relationship between plasma semi-quantitative assay results and subclinical CM. Semi-quantitative CrAg assays performed on blood are not a substitute for lumbar puncture which is universally recommended for all patients with a new diagnosis of antigenemia. However, such assays could be used to refine the pre-test probability of subclinical meningitis, especially at the primary healthcare level where patients need to be referred to hospital for a lumbar puncture or in settings where a high proportion of patients decline lumbar puncture. A score of ≥4 was highly specific for concurrent CM, though half of the cases in our study were missed at this threshold. A lower threshold (score ≥3) included all cases of CM and would thus be a more clinically useful risk prediction cut-off, despite also including 18/40 (45%) of cases without subclinical CM. Based on a sample of 189 screened patients, approximately one third of whom had lumbar punctures, the aforementioned Botswana study reported a strong association between plasma SQ score and central nervous system involvement at baseline (a composite endpoint of microbiologically confirmed CM and/or clinical signs of meningitis).14 Individuals with SQ scores of 2 or 3 were classified as being at moderate risk of central nervous system involvement and mortality, with a preliminary recommendation for more intensive evaluation including lumbar puncture. In our study, a strong-positive CryptoPS result was relatively sensitive and picked up 9 of 10 cases of subclinical CM but also 10/40 (25%) of those without CM. The lack of a clearer association between the semi-quantitative CrAg results and subclinical CM in our study might be explained by a smaller sample size since we restricted our analysis to patients who had a lumbar puncture. We found that CrAg-positive patients with an SQ score of ≥3 had a more than two-fold increase in mortality though the 95% CI spanned 1. In contrast to Jarvis et al, we did not find that the hazards of death increased with each step-wise increase in SQ score. Again, this may be related to a smaller sample size.
Compared to testing serial sample dilutions to obtain LFA titers, the SQ assay was a much less laborious method of obtaining a semi-quantitative CrAg result using a single test strip. SQ testing was easy to perform but reading the SQ test strip was more complex and the result interpretation card was needed to obtain an SQ score. For instance, for a CrAg-negative result, only a control line is positive with the LFA, whereas with the SQ assay, both the control and the T2 lines are positive. Moreover, grading of scores 1, 2 and 3 is determined by comparing the intensity of the T1 and T2 bands. Through an inter-laboratory comparison, we have identified reading and interpretation errors for the LFA in the national CrAg screening program and expect that the complexity of reading the SQ test strips would increase the proportion of erroneous readings. Using automated readers to read the SQ test strips could be considered to prevent such errors. Automated readers may also offer an advantage of interfacing CrAg results to the laboratory information system, and therefore reduce transcription errors. In contrast, the CryptoPS cassette design provided a simple method of semi-quantitative CrAg testing without using tubes. Results were available within 10 min and interpretation was very simple.
The strengths of this study included a prospective cohort design and enrolment of consecutive eligible CrAg-positive individuals. Patients with asymptomatic antigenemia were enrolled after excluding those with clear symptoms and signs of CM and a relatively large proportion of patients (25%) had a baseline lumbar puncture. The main limitation was a relatively small sample size. In addition, at the time that the cohort study was conducted, the South African guideline recommended that lumbar puncture should be considered for patients with cryptococcal antigenemia if this procedure was available. Ten of 60 CrAg-positive patients (17%) did not have a lumbar puncture. When we compared age, sex, CD4 count and proportion with a mild, non-persistent headache among those who had a lumbar puncture versus those who did not, we found no differences. We also tested stored rather than fresh plasma. To determine if freezing and thawing of plasma had an effect on SQ scores, fresh plasma samples that were tested using the SQ assay were stored in a −70°C freezer for >6 months. The samples were re-tested after 6 months and fresh and frozen-thawed plasma SQ results were compared. There was excellent result concordance between fresh plasma SQ scores and frozen-thawed SQ scores (unpublished data, NICD).
Conclusion
We found that two semi-quantitative assays offered a simple rapid means of estimating plasma CrAg concentrations. While not a substitute for lumbar puncture, using these semi-quantitative assays for risk stratification could be applied to patients with antigenemia for whom lumbar puncture is not immediately available, those who decline a lumbar puncture, even after careful counselling and those in whom lumbar puncture is contraindicated. The association between the semi-quantitative assay results and mortality was not clear in our study. These assays should not be implemented in routine screening programs until more prospective data have accumulated from diagnostic intervention studies.
Acknowledgements
The authors thank Immy and Biosynex for providing CrAg assays for research purposes. Data collection for the original cohort study was by: Neo Legare, Sr Matshediso Mkhwenezi and Siphiwe Kutta. The authors thank S. Hector for his expert advice and input throughout this project and Nqobile Ngoma for assistance with data cleaning and analysis.
Contributor Information
Nozuko P Blasich, National Institute for Communicable Diseases (Centre for Healthcare-Associated Infections, Antimicrobial Resistance and Mycoses), a Division of the National Health Laboratory Service, Sandringham 2131, Johannesburg, South Africa; Department of Biomedical Sciences, Faculty of Health and Wellness, Cape Peninsula University of Technology, 7530, Cape Town, South Africa.
Rachel M Wake, National Institute for Communicable Diseases (Centre for Healthcare-Associated Infections, Antimicrobial Resistance and Mycoses), a Division of the National Health Laboratory Service, Sandringham 2131, Johannesburg, South Africa; Institute of Infection & Immunity, St George's, University of London, SW17 0RE, London, United Kingdom.
Ivy Rukasha, National Institute for Communicable Diseases (Centre for Healthcare-Associated Infections, Antimicrobial Resistance and Mycoses), a Division of the National Health Laboratory Service, Sandringham 2131, Johannesburg, South Africa.
Yvonne Prince, Department of Biomedical Sciences, Faculty of Health and Wellness, Cape Peninsula University of Technology, 7530, Cape Town, South Africa.
Nelesh P Govender, National Institute for Communicable Diseases (Centre for Healthcare-Associated Infections, Antimicrobial Resistance and Mycoses), a Division of the National Health Laboratory Service, Sandringham 2131, Johannesburg, South Africa; School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, 2193, Johannesburg, South Africa; Division of Medical Microbiology, Faculty of Health Sciences, University of Cape Town, 7935, Cape Town, South Africa.
Author contributions
Conceptualization: R.W., N.P.G., N.P.B.
Laboratory testing: N.P.B., I.R.
Student supervision: N.P.G., Y.P.
Data analysis and interpretation: N.P.B., R.W., N.P.G.
Drafting and revising manuscript: N.P.B., N.P.G.
Manuscript review: All authors
Funding
R.W. received support from the National Institute for Health Research (ACF-2015-16-003); the St George's Hospital Research Charity; Sir Ratanji Dalal Research Scholarship and the Meningitis Research Foundation (1604.0). N.P.B., I.R. and N.P.G. were partly supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI118511. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of interest
No conflict of interest is declared.
References
- 1.Rajasingham R, Smith RM, Park BJet al. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect Dis. 2017; 17: 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulware DR, Rolfes MA, Rajasingham Ret al. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerg Infect Dis. 2014; 20: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajasingham R, Wake RM, Beyene T, Katende A, Letang E, Boulware DR.. Cryptococcal meningitis diagnostics and screening in the era of point-of-care laboratory testing. J Clin Microbiol. 2019; 57: e01238–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Govender NP, Glencross DK.. National coverage of reflex cryptococcal antigen screening: a milestone achievement in the care of persons with advanced HIV disease. S Afr Med J. 2018; 108: 534–535. [DOI] [PubMed] [Google Scholar]
- 5.Longley N, Jarvis JN, Meintjes Get al. Cryptococcal antigen screening in patients initiating ART in South Africa: a prospective cohort study. Clin Infect Dis. 2016; 62: 581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Govender NP, Meintjes G, Mangena Pet al. Southern African HIV Clinicians Society guideline for the prevention, diagnosis and management of cryptococcal disease among HIV-infected persons: 2019 update. Southern Afr J HIV Med. 2019; 20: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . Guidelines for the diagnosis, prevention, and management of cryptococcal disease in HIV-infected adults, adolescents and children, March 2018: Supplement to the 2016 consolidated guidelines of the use of antiretroviral drugs for treating and preventing hiv infection. Available at: https://www.who.int/hiv/pub/guidelines/cryptococcal-disease/en/ (Accessed 28 May 2021). [Google Scholar]
- 8.Mfinanga S, Chanda D, Kivuyo SLet al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: An open-label, randomised controlled trial. Lancet. 2015; 385: 2173–2182. [DOI] [PubMed] [Google Scholar]
- 9.Wake RM, Govender NP, Omar Tet al. Cryptococcal-related mortality despite fluconazole preemptive treatment in a cryptococcal antigen screen-and-treat program. Clin Infect Dis. 2020; 70: 1683–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Single Dose Liposomal Amphotericin for Asymptomatic Cryptococcal Antigenemia (ACACIA) . Available at: https://clinicaltrials.gov/ct2/show/NCT03945448 (Accessed 28 May 2021). [Google Scholar]
- 11.Treatment of cryptococcal antigen-positive patients identified through screening using fluconazole plus flucytosine vs fluconazole alone . Available at: 10.1186/ISRCTN30579828 (Accessed 28 May 2021). [DOI] [Google Scholar]
- 12.Thakur KT, Mateyo K, Hachaambwa Let al. Lumbar puncture refusal in sub-Saharan Africa: a call for further understanding and intervention. Neurology. 2015; 84: 1988–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wake RM, Britz E, Sriruttan Cet al. High cryptococcal antigen titers in blood are predictive of subclinical cryptococcal meningitis among human immunodeficiency virus-infected patients. Clin Infect Dis. 2018; 66: 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarvis JN, Tenforde MW, Lechiile Ket al. Evaluation of a novel semi-quantitative cryptococcal antigen lateral flow assay in patients with advanced HIV disease. J Clin Microbiol. 2020; 58: e00441–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skipper C, Tadeo K, Martyn Eet al. Evaluation of serum cryptococcal antigen testing using two novel semiquantitative lateral flow assays in persons with cryptococcal antigenemia. J Clin Microbiol. 2020; 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenforde MW, Boyer-Chammard T, Muthoga Cet al. Diagnostic accuracy of the Biosynex CryptoPS cryptococcal antigen semi-quantitative lateral flow assay in patients with advanced HIV disease. J Clin Microbiol. 2020; 59: e02307–20. [DOI] [PMC free article] [PubMed] [Google Scholar]