OBJECTIVES:
The pathophysiology of laryngopharyngeal reflux (LPR) remains incompletely understood. Proximal esophageal motor dysfunction may impair bolus clearance, increasing the risk of pharyngeal refluxate exposure. We aimed to evaluate the association of proximal esophageal contractility with objective reflux metrics.
METHODS:
We evaluated adults with LPR symptoms undergoing high-resolution manometry (HRM) and combined hypopharyngeal-esophageal multichannel intraluminal impedance-pH testing at a tertiary center between March 2018 and August 2019. Routine parameters per Chicago classification were obtained on HRM. Proximal esophageal contractility was evaluated using proximal contractile integral (PCI), which quantifies contractile pressure >20 mm Hg for the region spanning the distal margin of the upper esophageal sphincter and transition zone. Univariate (Kendall correlation and Student t test) and multivariable (general linear regression and logistic regression) analyses were performed.
RESULTS:
We enrolled 138 patients (66.7% women, mean age 57.1 years) in this study. Lower PCI was associated with an elevated risk of increased pharyngeal reflux (adjusted odds ratio 0.83 per 100 mm Hg-s-cm change in PCI, 95% confidence interval: 0.69–0.98), with a trend toward increased bolus exposure time and total reflux events, after multivariable adjustment. The relationship between PCI and pharyngeal reflux was strongest among participants without a primary motility disorder on HRM (adjusted odds ratio 0.63, 95% confidence interval: 0.42–0.85, P interaction = 0.04). Among continuously expressed reflux parameters, lower PCI was significantly associated with more distal acid reflux events (β = −0.0094, P = 0.03) and total reflux events (β = −0.0172, P = 0.05), after adjusting for confounders.
DISCUSSION:
Reduced proximal esophageal contractility as assessed by decreased PCI on HRM independently predicted increased pharyngeal reflux in patients with LPR symptoms, particularly among those without a coexisting motility disorder.
INTRODUCTION
Laryngopharyngeal reflux (LPR) is a heterogeneous condition caused by the retrograde flow of gastric contents into the upper aerodigestive tract (1–3). Both acid and nonacid (e.g., pepsin) exposure can contribute to LPR symptoms by direct and indirect mechanisms (4,5). These symptoms most commonly include chronic cough, dysphonia, globus sensation, mucus sensation, and throat clearing (6–8). A variety of mechanisms underlie the exposure of the laryngopharynx to pathologic reflux. There is a strong body of evidence that has linked esophageal hypomotility disorders with gastroesophageal reflux disease (GERD) (9–13). Several studies have similarly linked esophageal dysmotility with extraesophageal manifestations of gastric reflux, including LPR (14–18). These studies, however, have largely focused on the role of motor dysfunction of the distal esophagus in the pathogenesis of GERD and LPR.
Abnormalities of the proximal esophagus may play a distinct and underappreciated role in the development of true LPR events or its symptoms. The upper one-third of the esophagus is composed of striated skeletal muscle, in contrast to the smooth muscle of the distal esophagus. Peristalsis in the proximal esophagus is controlled exclusively by the central nervous system through vagal innervation (19). Aside from these functional differences, proximal esophagus motor function is the last mechanism against the escape of gastric contents past the upper esophageal sphincter (UES) and into the upper respiratory tract. The laryngopharynx lacks the effective stripping function of the esophagus and is less able to readily clear caustic materials. Thus, if reflux cannot be cleared by the upper esophagus, it may come into contact with highly sensitive laryngopharyngeal tissues for a longer period of time (20). Several small studies have identified abnormalities in proximal esophageal motor function among patients with symptoms of LPR (21,22). However, other results have been contradictory (23). Consequently, there remain few and discordant data about motility dysfunction in LPR, and its contribution to full-column esophagopharyngeal reflux (pharyngeal reflux) events is unclear.
In this study, we aimed to evaluate proximal esophageal contractility in a cohort of patients with LPR symptoms and examine its association with objective esophagopharyngeal reflux metrics.
MATERIALS AND METHODS
Study population
Adult patients with suspected LPR who were referred to a tertiary center for high-resolution manometry (HRM) and a 24-hour combined hypopharyngeal-esophageal multichannel intraluminal impedance (HEMII-pH) and dual pH testing from March 2018 to August 2019 were enrolled in this study. All patients were initially seen by an otolaryngologist with subspecialty training in laryngology. LPR was suspected by history and correlation with findings on laryngoscopic and/or stroboscopic examination, warranting referral for HEMII-pH and HRM testing. Patients with a history of esophageal or other foregut surgery, underlying pulmonary disease, sinus abnormalities, and laryngeal malignancies, were excluded. All patients completed HEMII-pH and HRM off acid-suppression medications for at least 7 days. This study was approved by the institutional review board of Mass General Brigham Healthcare, and informed consent was obtained from each subject before participation.
High-resolution manometry
Esophageal motor function was assessed with HRM. After an overnight fast, an HRM catheter (Diversatek Healthcare, Highlands Ranch, CO) consisting of 32 circumferential pressure sensors was introduced transnasally after application of topical anesthesia to the nasopharynx. The catheter was positioned so that the distal end was in the stomach and the proximal sensors were above the UES. Participants underwent a brief resting period to assess baseline esophageal parameters before performing ten 5-mL liquid swallows in the supine position.
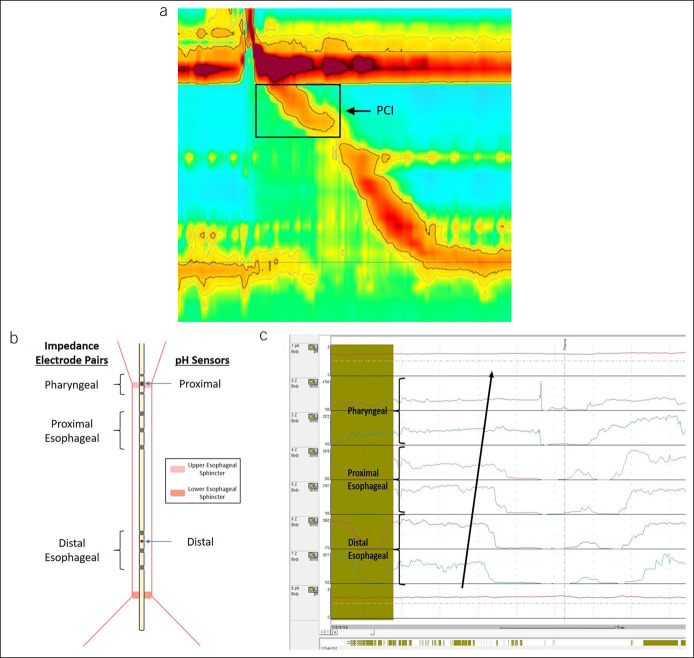
HRM data were analyzed using the Zvu software package. Standard manometric parameters were measured and categorized in accordance with the Chicago Classification v3.0 (24). In addition, proximal esophageal motor function was evaluated using the proximal contractile integral (PCI), which quantifies contractile pressure >20 mm Hg for the region spanning the distal margin of the UES and transition zone (Figure 1a). The median PCI across all swallows was subsequently calculated for each participant. To evaluate the UES, we recorded the UES pressure, which measures the basal pressure across the UES during the resting period. The presence and size of a hiatal hernia were additionally measured.
Figure 1.
(a) Measurement of the PCI on HRM. (b) The HEMII-pH catheter contains 2 pH sensors located within/above the upper esophageal sphincter and in the esophageal body and 6 impedance electrodes pairs, divided into pharyngeal (3 and 1 cm above UES and 1 cm below UES), proximal esophageal (1, 3 and 5 cm below UES), and distal esophageal (9, 11, and 13 cm below UES). (c) A full-column, pharyngeal reflux event on HEMII-pH, characterized by a ≥50% decrease in impedance, propagating from the distal esophageal to the pharyngeal electrode pairs in a retrograde fashion. HEMII-pH, hypopharyngeal-esophageal multichannel intraluminal impedance; HRM, high-resolution manometry; PCI, proximal contractile integral; UES, upper esophageal sphincter.
HEMII-pH testing
Reflux burden was assessed using HEMII-pH, as described in previous publications (25). In brief, the HEMII-pH catheter contains 2 pH sensors and 8 esophageal impedance electrodes located within/above the UES and in the esophageal body. The impedance electrode pairs are divided into pharyngeal (3 and 1 cm above UES and 1 cm below UES), proximal esophageal (1, 3, and 5 cm below UES), and distal esophageal (9, 11, and 13 cm below UES) (Figure 1b). A pharyngeal reflux event is characterized by at least 50% decrease in impedance, propagating in a retrograde fashion from the distal most electrode pairs to the pharyngeal impedance electrode pairs (25) (Figure 1c).
HEMII-pH tracings were manually analyzed with the assistance of a dedicated software package (BioView Analysis, version 5.6.3.0; Sandhill Scientific, Highlands Ranch, CO). Increased reflux burden was defined as ≥4% for acid exposure time (AET), ≥1.4% for bolus exposure time (BET), and ≥73 episodes/24 hours for total reflux episodes (26). Increased pharyngeal reflux was defined as >1 pharyngeal reflux events over 24 hours on HEMII-pH, as suggested by previous normative data based on measurements among healthy individuals (27–30).
Symptom measurements
Validated symptom and health-related quality of life instruments were prospectively collected at the time of esophageal function testing, including reflux symptom index (RSI), GERD questionnaire (GERDQ), and the 12-Item Short Form Health Survey. Symptom burden was also evaluated for the dominant esophageal symptoms (heartburn, belching, regurgitation, chest pain, liquid dysphagia, and solid dysphagia) and throat symptoms (cough and wheezing) using a validated survey described in previous publications (31). Each item was rated on a 5-point Likert scale for symptom frequency (from 0 = no symptoms to 4 = multiple daily episodes) and severity (from 0 = no symptoms to 4 = very severe symptoms). Dominant symptom intensity (DSI) was obtained by multiplying the frequency and severity of the dominant symptom identified by the patient. A separate DSI was generated for esophageal symptoms and throat symptoms.
Statistical analysis
Kendall tau rank correlation was computed, and general linear regression was performed to assess the relationship between proximal esophageal motor function and reflux burden. Student t test and multivariable logistic regression were used to assess dichotomized reflux burden parameters. Multivariable regression analyses were adjusted for age, sex, body mass index (BMI), smoking status (ever smoker vs never smoker), and percentages of ineffective swallows per Chicago Classification v3.0 (distal contractile integral [DCI] < 450 mm Hg-s-cm). We next performed stratified logistic regression analyses for the relationship between proximal contractility and pharyngeal reflux according to the presence of a hiatal hernia, UES pressure, and presence of a primary motility disorder on HRM. Interaction was assessed by evaluation of the cross-product of PCI and the stratification variable using the Wald test. For these analyses, the UES pressure was dichotomized at the median value across all participants. Odds ratios are expressed per 100 mm Hg-s-cm change in PCI. Statistical analyses were performed using R 3.6.0.
RESULTS
We enrolled 138 participants, among whom 92 (66.7%) were female. The mean age was 57.1 ± 16.0 years, and the BMI was 26 (23–30) kg/m2. The most common symptoms reported were throat clearing (n = 106, 89.1%), heartburn (n = 84, 71.2%), and globus sensation (n = 83, 70.3%). The mean RSI was 18.8 (SD = 10.2), and GERDQ was 7.7 (SD = 2.4). Other detailed demographic and clinical characteristics of the study cohort are summarized in Table 1.
Table 1.
Baseline demographic and clinical characteristics of the study population
| Demographics | |
| Age (yr) | 63 [46–69] |
| Female sex, n (%) | 92 (66.7) |
| BMI (kg/m2) | 26 [23–30] |
| Former or current smoker (%) | 45 (32.6) |
| Current regular alcohol use (%) | 63 (47.4) |
| Symptom burden | |
| Reflux Severity Index score | 17 [12–25] |
| GERDQ score | 7 [6–9] |
| Dominant throat symptom index | 4 [1–12] |
| Dominant esophageal symptom index | 3 [0–12] |
| HRQOL on the SF-12 | 52 [47–56] |
| Clinical testing | |
| UESP (mm Hg) | 126 [72–226] |
| Hiatal hernia on HRM (%) | 23 (16.7) |
| Motility disorder on HRM (%) | 59 (43.1) |
| IEM | 40 (29.0) |
| EGJ outflow obstruction | 11 (8.0) |
| Jackhammer | 5 (3.6) |
| Absent contractility | 2 (1.4) |
| Achalasia type I | 2 (1.4) |
| Distal AET | 0.9 [0.2–2.6] |
| Distal acid reflux events | 8 [3–18] |
| Proximal AET | 0 [0–0] |
| Proximal acid reflux events | 0 [0–0] |
| BET | 1.4 [0.8–2.4] |
| Total reflux events | 34 [21–49] |
| Proximal reflux events | 14 [8–25] |
| Pharyngeal reflux events | 1 [0–3] |
The median [IQR] is presented for continuous variables.
AET, acid exposure time; BET, bolus exposure time; BMI, body mass index; EGJ, esophagogastric junction; HRM, high-resolution manometry; HRQOL, health-related quality of life; IEM, ineffective esophageal motility; IQR, interquartile range; SF-12, 12-Item Short Form Health Survey; UESP, upper esophageal sphincter pressure.
PCI and HEMII-pH parameters
The median PCI across all participants was 287 mm Hg-s-cm (interquartile range: 137–466). PCI was significantly correlated with DCI (r = 0.34 P < 0.001). Significant inverse correlation was found between PCI and BET (τ = −0.180, P < 0.01) and total reflux episodes (τ = −0.120, P = 0.04) (Table 2). There was also a trend toward increased proximal reflux with lower PCI, although statistical significance was not reached (τ = −0.099, P = 0.09). On multivariable analyses, adjusting for age, sex, BMI, smoking status, and percentage of ineffective swallows, PCI remained inversely associated with distal acid reflux events (β = −0.0094, P = 0.03) and total reflux events (β = −0.0172, P = 0.05).
Table 2.
Correlation between PCI and reflux burden
| Reflux parameter | Τ | P value |
| pH testing parameters | ||
| Distal AET | −0.059 | 0.34 |
| Distal acid reflux events | −0.089 | 0.15 |
| Proximal AET | −0.018 | 0.80 |
| Proximal acid reflux events | −0.049 | 0.49 |
| Impedance testing parameters | ||
| BET | −0.180b | <0.01a |
| Total reflux events | −0.120b | 0.04a |
| Proximal reflux events | −0.099b | 0.09b |
| Pharyngeal reflux events | −0.098 | 0.13 |
Analysis of acid reflux exposure was conducted only among patients tested off proton pump inhibitors.
AET, acid exposure time; BET, bolus exposure time; PCI, proximal contractile integral.
P < 0.05.
P < 0.10.
We next examined the association between PCI and pathologic reflux expressed as a dichotomized outcome, using thresholds established by previous normative data. On univariate analyses, PCI was significantly lower among patients with increased pharyngeal reflux (272 vs 391 mm Hg-s-cm, P = 0.01), AET (281 vs 374 mm Hg-s-cm, P = 0.04), BET (281 vs 410 mm Hg-s-cm, P < 0.01), and total reflux episodes (206 vs 355 mm Hg-s-cm, P = 0.02), compared with patients with a normal reflux burden (Figure 2). On separate multivariable logistic regression models constructed for each reflux parameter and adjusting for potential confounders, each 100 mm Hg-s-cm reduction in PCI was associated with a 17% higher risk of increased pharyngeal reflux (adjusted odds ratio [aOR] 0.83, 95% confidence interval [CI]: 0.69–0.98, P = 0.04). There was a trend toward significance in the relationship between PCI and total reflux (aOR 0.65, 95% CI: 0.39–0.96, P = 0.06) and increased BET (aOR 0.88, 95% CI: 0.74–1.02, P < 0.10), but not increased AET (Table 3).
Figure 2.
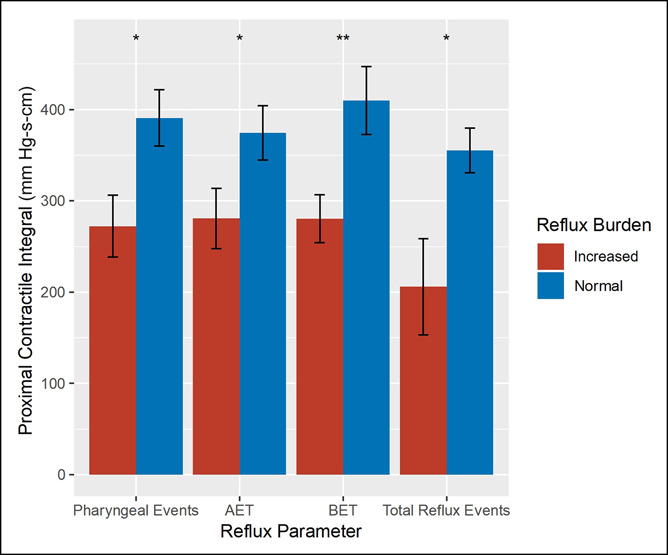
Association between PCI and dichotomized reflux parameters (normal vs increased). Overall, PCI was significantly lower among patients with increased pharyngeal reflux, acid exposure time, and bolus exposure time on HEMII-pH. *P < 0.05, **P < 0.01. AET, acid exposure; BET, bolus exposure time; HEMII-pH, hypopharyngeal-esophageal multichannel intraluminal impedance; PCI, proximal contractile integral.
Table 3.
Multivariate models predicting reflux exposure
| Reflux parameter | aOR (95% CI)a | P value |
| AET | 0.89 (0.74–1.05) | 0.19 |
| BET | 0.88 (0.74–1.02) | <0.10 |
| Pharyngeal events | 0.83 (0.69–0.98) | 0.04 |
| Total reflux episodes | 0.65 (0.39–0.96) | 0.06 |
All models were controlled for age, sex, BMI, smoking status, and percentage of ineffective swallows.
AET, acid exposure time; aOR, adjusted odds ratio; BET, bolus exposure time; BMI, body mass index; CI, confidence interval; PCI, proximal contractile integral.
ORs are expressed per 100 mm Hg-s-cm change in PCI.
Esophageal dysmotility and HEMII-pH parameters
Given the identified associations between proximal esophageal function and reflux parameters, we consequently sought to examine whether these parameters differed according to distal esophageal function, as defined by the Chicago Classification v3.0. Overall, 59 patients (43.1%) were found to have a motility disorder, with ineffective esophageal motility (IEM) being the most common (n = 39, 28.3%). Participants with minor disorders (e.g., IEM) were more likely to have increased proximal reflux compared with those with disorders of esophageal peristalsis or outflow obstruction (achalasia, esophagogastric junction outflow obstruction, Jackhammer esophagus, or absent contractility) (37/39 vs 15/20, pairwise = 0.03) or normal manometry (37/39 vs 65/79, pairwise = 0.05). Other measures of proximal and distal exposures, both acid and nonacid, did not seem to differ by Chicago Classification groupings. In addition to esophageal body motor function, we also assessed the UES barrier activities by obtaining its basal pressure and residual pressure with swallowing. Neither UES parameter demonstrated significant associations with any of the reflux measures on HEMII-pH.
Stratified analyses
Stratified analyses revealed that the association between PCI and increased pharyngeal reflux was most significant among participants without a coexisting motility disorder on HRM as defined by Chicago Classification v3.0 (aOR 0.63, 95% CI: 0.42–0.85), compared with those with abnormal HRM testing (aOR 0.99, 95% CI: 0.79–1.25; P interaction = 0.04; Figure 3). Risk estimates did not differ significantly by the presence of a hiatal hernia on HRM or UES pressure.
Figure 3.
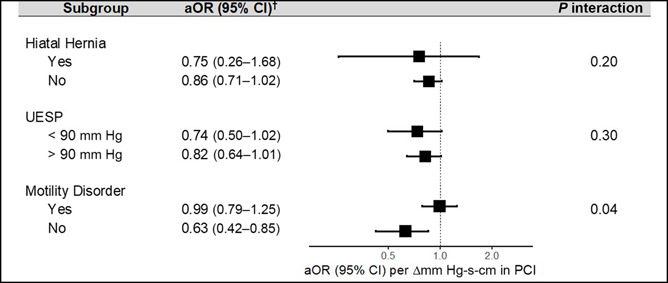
Association of PCI and increased pharyngeal reflux by various subgroups. All models controlled for age, sex, BMI, smoking status, and percentage of ineffective swallows, with the exception of the given stratification variable. †ORs are expressed per 100 mm Hg-s-cm change in PCI. aOR, adjusted OR; BMI, body mass index; CI, confidence interval; OR, odds ratio; PCI, proximal contractile integral; UESP, upper esophageal sphincter pressure.
Symptom assessment
There were no significant associations between PCI and measures of symptoms and severity, including RSI, GERDQ, DSI, and health-related quality of life on the 12-Item Short Form Health Survey (P > 0.05). Similarly, symptoms measured on these scales did not differ by pharyngeal reflux exposure measured on HEMII-pH (P > 0.05).
DISCUSSION
In our cohort of patients with LPR symptoms who were prospectively enrolled and systematically evaluated with HRM and HEMII-pH, we found that impaired contractility of the proximal esophagus was independently associated with increased pharyngeal reflux events. This observed risk of increased pharyngeal reflux associated with impaired proximal contractility was particularly elevated among patients without a primary esophageal motility disorder per Chicago classification on HRM. Together, our findings suggest a role for proximal esophageal contractile dysfunction in LPR which may be independent of distal esophageal motility.
Although the contribution of aberrant esophageal motor function, such as IEM and large peristaltic break, to typical GERD symptoms has been well established (9–13), less is known about the role of dysmotility in extraesophageal reflux syndromes, with previous studies reporting inconsistent results. In an early study of patients with LPR symptoms who underwent conventional esophageal manometry and pH monitoring, the presence of an esophageal motility disorder did not correlate with abnormal acid reflux (14). However, in a later study of patients with chronic cough, weak peristalsis in the distal esophagus and large peristaltic breaks on HRM were significantly associated with increased AET and decreased refluxate clearance, although the proximal esophageal and total reflux events were not different (15). Long peristaltic break was also found to correlate with cough as a presenting symptom in another study of patients undergoing both pH-impedance testing and HRM, although it was not associated with reflux burden, symptom association, or other symptom metrics (17). Notably, the presence of a long peristaltic break in this cohort of patients with cough predicted suboptimal symptom response to antireflux therapy (17), suggesting a possible protective role of intact esophageal peristalsis against extraesophageal reflux symptoms. We also previously reported that increased failed swallows in the distal esophagus independently correlated with increased RSI score among patients presenting with LPR symptoms (32). Therefore, our study contributes to the growing evidence of the possible role of esophageal dysmotility in the pathophysiology of LPR. It further adds to current literature by exploring the relationship between proximal esophageal function and pharyngeal reflux events because previous studies have largely focused on the more routinely measured distal esophageal parameters and standard GERD metrics.
A number of studies have evaluated and found increased prevalence of inappropriate or excessive relaxation of the UES among patients with LPR symptoms (18,23,33–38). The findings of this investigation suggest that, in addition to aberrant UES function, motor abnormalities of the proximal esophagus may also permit increased passage of gastric refluxate into the upper aerodigestive tract. Our findings also corroborate earlier evidence from smaller studies on proximal esophageal and UES functions in patients with laryngeal symptoms and globus sensation (21,22). Peng et al. (22) performed a small case-control study and found that the UES residual pressure was higher and the proximal esophageal contraction was lower among patients with globus sensation compared with controls. In the study by Passaretti et al. (21), patients with LPR symptoms and higher oropharyngeal acid exposure, defined by the Ryan score on oropharyngeal pH monitoring, had lower proximal esophageal contractions. Babei et al. (38) also found that patients with supraesophageal reflux disease often do not mount similar esophageal striated muscle activity compared with normal controls when the lower border of UES is exposed to simulated reflux events from intraesophageal infusion of liquid. Our study demonstrated an independent correlation between proximal esophageal function and objectively measured gastroesophageal reflux events that reached the oropharynx. Proximal esophageal contractile function impairment may, therefore, contribute to LPR symptoms through decreased bolus clearance that leads to a higher risk of retrograde flow of gastric contents to reach the oropharynx. The increased bolus retention that may result from proximal esophageal contractile impairment may also lead to increased intraluminal pressure, esophageal wall distention, and symptoms such as globus sensation.
The observed inverse correlation between PCI and pharyngeal reflux was independent of distal esophageal function. In particular, we found on stratified analysis that the effect of impaired proximal contractility on pharyngeal reflux was highest among subjects without a coexisting primary distal esophageal motility disorder. As previously discussed, there is growing evidence that hypomotility of the distal esophagus (14–18) and abnormal muscle interactions in the transition zone (15) may underlie the pathophysiology of LPR. In light of these findings, our study suggests an independent mechanism of pharyngeal reflux that occurs in the context of preserved distal motor function. This observation is also notable because proximal esophageal motor function is not currently incorporated into established diagnostic criteria for primary motility disorders for routine clinical care (39). Consequently, these results provide further evidence that proximal esophageal motility should be further explored and assessed as part of the comprehensive evaluation of patients with symptoms of the upper aerodigestive tract.
This study has several important strengths. We levered data across a number of clinical and testing parameters from a relatively large cohort of patients with LPR symptoms who underwent systematic evaluation with both HRM and HEMII-pH after a comprehensive laryngological evaluation. This multidisciplinary assessment helped to ensure proper patient selection. These data allowed us to adjust for several potential confounders, in contrast to many previous, smaller studies. The addition of ineffective swallows in our multivariable models permitted us to isolate the independent effect of proximal motility, rather than global esophageal motor dysfunction. This was especially important, given the observed correlation between PCI and DCI, which has also been previously reported (40), and the well-known relationship between distal esophageal hypomotility and GERD. The use of HEMII with pharyngeal impedance electrodes allowed for identification of full-column reflux events to the pharynx and objective assessment of reflux burden affecting the upper aerodigestive tract. Compared with conventional impedance-pH catheters, HEMII-pH has been shown to help detect LPR events with greater sensitivity and specificity (25), and the results may correlate better with clinical outcomes (41). Furthermore, in our study, a very few proximal acid reflux events were detected on the upper pH sensor compared with the higher number of proximal bolus reflux events on impedance monitoring (Table 1). This suggests that many proximal reflux events have pH > 4 on reaching the proximal esophagus or pharynx, supporting a potential advantage of HEMII-pH over pharyngeal/dual pH monitoring alone, as used in previous publications (3,42).
There are also several limitations to this study. Because of the cross-sectional nature of our data, we could not assess for temporal changes in reflux parameters and establish causation. It is possible that long-standing proximal reflux itself may lead to deterioration of motor function in the proximal esophagus. More likely, the relationship between proximal esophageal motility and pharyngeal/proximal reflux is bidirectional, with contractile impairment leading to increased bolus retention and reflux, which may, in turn, result in further decrease in contractility. The distal reflux parameters measured in our study using HEMII-pH may be less precise than those obtained on a traditional impedance-pH study, where placement of the catheter is referenced to the LES. Therefore, it is possible that some minor misclassifications may have occurred in the dichotomized analyses of these distal reflux parameters. However, a recent study of patients undergoing both HEMII-pH and traditional impedance-pH studies found no significant differences in the traditional reflux parameters obtained from both studies, thereby supporting the utility of these metrics on HEMII-pH (43). Our study population was also drawn from a tertiary referral center, which may limit the generalizability to other populations, particularly those with less severe symptoms and who present in the primary care setting. However, the patients with refractory symptoms referred to a tertiary center often represent the most challenging group that warrants more systematic, physiologic evaluations. Finally, the normative value for pathologic pharyngeal reflux or LPR has not been fully validated, although previous studies in healthy volunteers suggest >1 pharyngeal reflux events detected on HEMII-pH be considered abnormal, and treatment-based studies have shown clinical improvement in symptomatic LPR in subjects diagnosed based on these normative criteria (44).
In conclusion, we found that decreased proximal esophageal contractility independently correlated with increased pharyngeal reflux in patients with LPR symptoms. This association was stronger among patients with preserved distal contractile function. These findings suggest that impaired proximal contractile function may play a role in the pathophysiology of LPR, with decreased bolus/refluxate clearance leading to increased escape and exposure of refluxate to the laryngopharynx. Future studies are needed to better elucidate the precise mechanisms that contribute to the complex clinical presentation of LPR and to determine the longitudinal relationship between proximal esophageal function and LPR treatment outcome.
CONFLICTS OF INTEREST
Guarantor of the article: Walter W. Chan, MD, MPH.
Specific author contributions: D.R.S. and W.W.C. initiated study concepts and design; D.R.S., J.X.C., and R.L. contributed to acquisition of data; D.R.S. and W.W.C. performed analysis and interpretation of data; D.R.S. and W.W.C. drafted the article; D.R.S., J.X.C., R.L., T.L.C., and W.W.C. contributed to critical revision of the article for important intellectual content; W.W.C. provided administrative support and overall study supervision. All authors approved the final version of the article.
Financial support: None to report.
Potential competing interests: None to report.
Previous presentation: This study was selected for oral presentation during 2020 Digestive Disease Week; May, 2020 (originally scheduled in Chicago, IL that was changed to virtual due to COVID-19 pandemic)
Data availability statement: The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Study Highlights.
WHAT IS KNOWN
✓ The pathophysiology of laryngopharyngeal reflux remains incompletely understood.
✓ Motor dysfunction of the esophagus may impair bolus clearance, modulating the risk of pharyngeal refluxate exposure.
WHAT IS NEW HERE
✓ Reduced contractility of the proximal esophagus as assessed by decreased proximal contractile integral on high-resolution manometry is independently associated with increased pharyngeal reflux.
✓ The association between proximal contractile integral and pharyngeal reflux was strongest among those with preserved distal esophageal motor function.
Contributor Information
Daniel R. Sikavi, Email: dsikavi@mgh.harvard.edu.
Jennifer X. Cai, Email: jxcai@bwh.harvard.edu.
Ryan Leung, Email: ryle99@connect.hku.hk.
Thomas L. Carroll, Email: tcarroll@bwh.harvard.edu.
References
- 1.Ford CN. Evaluation and management of laryngopharyngeal reflux. JAMA 2005;294(12):1534–40. [DOI] [PubMed] [Google Scholar]
- 2.Koufman JA. Laryngopharyngeal reflux is different from classic gastroesophageal reflux disease. Ear Nose Throat J 2002;81(9 Suppl 2):7–9. [PubMed] [Google Scholar]
- 3.Pearson JP, Parikh S, Orlando RC, et al. Review article: Reflux and its consequences—The laryngeal, pulmonary and oesophageal manifestations. Conference held in conjunction with the 9th International Symposium on Human Pepsin (ISHP) Kingston-upon-Hull, UK, 21-23 April 2010. Aliment Pharmacol Ther 2011;33(Suppl 1):1–71. [DOI] [PubMed] [Google Scholar]
- 4.Wassenaar E, Johnston N, Merati A, et al. Pepsin detection in patients with laryngopharyngeal reflux before and after fundoplication. Surg Endosc 2011;25(12):3870–6. [DOI] [PubMed] [Google Scholar]
- 5.Lechien JR, Saussez S, Karkos PD. Laryngopharyngeal reflux disease: Clinical presentation, diagnosis and therapeutic challenges in 2018. Curr Opin Otolaryngol Head Neck Surg 2018;26(6):392–402. [DOI] [PubMed] [Google Scholar]
- 6.Book DT, Rhee JS, Toohill RJ, et al. Perspectives in laryngopharyngeal reflux: An international survey. Laryngoscope 2002;112(8 Pt 1):1399–406. [DOI] [PubMed] [Google Scholar]
- 7.Lechien JR, Akst LM, Hamdan AL, et al. Evaluation and management of laryngopharyngeal reflux disease: State of the art review. Otolaryngol Head Neck Surg 2019;160(5):762–82. [DOI] [PubMed] [Google Scholar]
- 8.Qua CS, Wong CH, Gopala K, et al. Gastro-oesophageal reflux disease in chronic laryngitis: Prevalence and response to acid-suppressive therapy. Aliment Pharmacol Ther 2007;25(3):287–95. [DOI] [PubMed] [Google Scholar]
- 9.Wu JC, Cheung CM, Wong VW, et al. Distinct clinical characteristics between patients with nonerosive reflux disease and those with reflux esophagitis. Clin Gastroenterol Hepatol 2007;5(6):690–5. [DOI] [PubMed] [Google Scholar]
- 10.Savarino E, Gemignani L, Pohl D, et al. Oesophageal motility and bolus transit abnormalities increase in parallel with the severity of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2011;34(4):476–86. [DOI] [PubMed] [Google Scholar]
- 11.Diener U, Patti MG, Molena D, et al. Esophageal dysmotility and gastroesophageal reflux disease. J Gastrointest Surg 2001;5(3):260–5. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Anggiansah A, Anggiansah R, et al. Effects of age on the gastroesophageal junction, esophageal motility, and reflux disease. Clin Gastroenterol Hepatol 2007;5(12):1392–8. [DOI] [PubMed] [Google Scholar]
- 13.Ribolsi M, Balestrieri P, Emerenziani S, et al. Weak peristalsis with large breaks is associated with higher acid exposure and delayed reflux clearance in the supine position in GERD patients. Am J Gastroenterol 2014;109(1):46–51. [DOI] [PubMed] [Google Scholar]
- 14.Knight RE, Wells JR, Parrish RS. Esophageal dysmotility as an important co-factor in extraesophageal manifestations of gastroesophageal reflux. Laryngoscope 2000;110(9):1462–6. [DOI] [PubMed] [Google Scholar]
- 15.Almansa C, Smith JA, Morris J, et al. Weak peristalsis with large breaks in chronic cough: Association with poor esophageal clearance. Neurogastroenterol Motil 2015;27(3):431–42. [DOI] [PubMed] [Google Scholar]
- 16.Kastelik JA, Redington AE, Aziz I, et al. Abnormal oesophageal motility in patients with chronic cough. Thorax 2003;58(8):699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett MC, Patel A, Sainani N, et al. Chronic cough is associated with long breaks in esophageal peristaltic integrity on high-resolution manometry. J Neurogastroenterol Motil 2018;24(3):387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vardar R, Sweis R, Anggiansah A, et al. Upper esophageal sphincter and esophageal motility in patients with chronic cough and reflux: Assessment by high-resolution manometry. Dis Esophagus 2013;26(3):219–25. [DOI] [PubMed] [Google Scholar]
- 19.Goyal RK, Chaudhury A. Physiology of normal esophageal motility. J Clin Gastroenterol 2008;42(5):610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston N, Bulmer D, Gill GA, et al. Cell biology of laryngeal epithelial defenses in health and disease: Further studies. Ann Otol Rhinol Laryngol 2003;112(6):481–91. [DOI] [PubMed] [Google Scholar]
- 21.Passaretti S, Mazzoleni G, Vailati C, et al. Oropharyngeal acid reflux and motility abnormalities of the proximal esophagus. World J Gastroenterol 2016;22(40):8991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng L, Patel A, Kushnir V, et al. Assessment of upper esophageal sphincter function on high-resolution manometry: Identification of predictors of globus symptoms. J Clin Gastroenterol 2015;49(2):95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi WS, Kim TW, Kim JH, et al. High-resolution manometry and globus: Comparison of globus, gastroesophageal reflux disease and normal controls using high-resolution manometry. J Neurogastroenterol Motil 2013;19(4):473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27(2):160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borges LF, Chan WW, Carroll TL. Dual pH probes without proximal esophageal and pharyngeal impedance may be deficient in diagnosing LPR. J Voice 2019;33(5):697–703. [DOI] [PubMed] [Google Scholar]
- 26.Shay S, Tutuian R, Sifrim D, et al. Twenty-four hour ambulatory simultaneous impedance and pH monitoring: A multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol 2004;99(6):1037–43. [DOI] [PubMed] [Google Scholar]
- 27.Wang AJ, Liang MJ, Jiang AY, et al. Gastroesophageal and laryngopharyngeal reflux detected by 24-hour combined impedance and pH monitoring in healthy Chinese volunteers. J Dig Dis 2011;12(3):173–80. [DOI] [PubMed] [Google Scholar]
- 28.Xiao YL, Liu FQ, Li J, et al. Gastroesophageal and laryngopharyngeal reflux profiles in patients with obstructive sleep apnea/hypopnea syndrome as determined by combined multichannel intraluminal impedance-pH monitoring. Neurogastroenterol Motil 2012;24(6):e258–65. [DOI] [PubMed] [Google Scholar]
- 29.Hoppo T, Sanz AF, Nason KS, et al. How much pharyngeal exposure is “normal”? Normative data for laryngopharyngeal reflux events using hypopharyngeal multichannel intraluminal impedance (HMII). J Gastrointest Surg 2012;16(1):16–24. discussion 24–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desjardin M, Roman S, des Varannes SB, et al. Pharyngeal pH alone is not reliable for the detection of pharyngeal reflux events: A study with oesophageal and pharyngeal pH-impedance monitoring. United Eur Gastroenterol J 2013;1(6):438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel A, Sayuk GS, Gyawali CP. Parameters on esophageal pH-impedance monitoring that predict outcomes of patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2015;13(5):884–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borges LF, Salgado S, Hathorn KE, et al. Failed swallows on high-resolution manometry independently correlates with severity of LPR symptoms. J Voice 2020. doi: 10.1016/j.jvoice.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Tokashiki R, Funato N, Suzuki M. Globus sensation and increased upper esophageal sphincter pressure with distal esophageal acid perfusion. Eur Arch Otorhinolaryngol 2010;267(5):737–41. [DOI] [PubMed] [Google Scholar]
- 34.Benjamin T, Zackria S, Lopez R, et al. Upper esophageal sphincter abnormalities and high-resolution esophageal manometry findings in patients with laryngopharyngeal reflux. Scand J Gastroenterol 2017;52(8):816–21. [DOI] [PubMed] [Google Scholar]
- 35.Nadaleto BF, Herbella FA, Pinna BR, et al. Upper esophageal sphincter motility in gastroesophageal reflux disease in the light of the high-resolution manometry. Dis Esophagus 2017;30(4):1–5. [DOI] [PubMed] [Google Scholar]
- 36.Szczesniak MM, Williams RB, Cook IJ. Mechanisms of esophago-pharyngeal acid regurgitation in human subjects. PLoS One 2011;6(7):e22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szczesniak MM, Williams RB, Brake HM, et al. Upregulation of the esophago-UES relaxation response: A possible pathophysiological mechanism in suspected reflux laryngitis. Neurogastroenterol Motil 2010;22(4):381–6, e89. [DOI] [PubMed] [Google Scholar]
- 38.Babaei A, Venu M, Naini SR, et al. Impaired upper esophageal sphincter reflexes in patients with supraesophageal reflux disease. Gastroenterology 2015;149(6):1381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahrilas P, Bredenoord A, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3. 0. Neurogastroenterol Motil 2015;27(2):160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosh SK, Pandolfino JE, Kwiatek MA, et al. Oesophageal peristaltic transition zone defects: Real but few and far between. Neurogastroenterol Motil 2008;20(12):1283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Worrell SG, DeMeester SR, Greene CL, et al. Pharyngeal pH monitoring better predicts a successful outcome for extraesophageal reflux symptoms after antireflux surgery. Surg Endosc 2013;27(11):4113–8. [DOI] [PubMed] [Google Scholar]
- 42.Tack J. Review article: The role of bile and pepsin in the pathophysiology and treatment of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2006;24(Suppl 2):10–6. [DOI] [PubMed] [Google Scholar]
- 43.Masui D, Fukahori S, Hashizume N, et al. Simultaneous evaluation of laryngopharyngeal reflux and swallowing function using hypopharyngeal multichannel intraluminal impedance measurements in neurologically impaired patients. J Neurogastroenterol Motil 2021;27(2):198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lechien JR, Bobin F, Muls V, et al. Validity and reliability of the reflux symptom score. Laryngoscope 2020;130(3):E98–107. [DOI] [PubMed] [Google Scholar]