Supplemental Digital Content is available in the text.
Introduction:
Inadvertent perioperative hypothermia is common and associated with increased risk of perioperative complications. Adult data drives most guidelines for pediatric perioperative temperature management and does not consistently demonstrate effectiveness in children. This study aims to identify risk factors for hypothermia and determine the effectiveness of current interventions in the pediatric population.
Methods:
We carried out a prospective observational study in children undergoing anesthesia in our tertiary pediatric unit. We included 869 patients (<16 y) undergoing emergency/elective surgeries over 2 months. Our team recorded the incidence of hypothermia (tympanic core temperature < 36°C) within 15 minutes of arrival to the postanesthetic care unit. We collected data such as patient demographic, surgical, anesthetic (including monitoring and warming measures used), and operating theater (OT) temperature. We performed statistical analysis to identify risk factors associated with hypothermia.
Results:
Postoperative hypothermia incidence was 12.3% (107/869). The mean core temperature on arrival to the postanesthetic care unit was 36.6°C (+SD 0.45) in normothermic patients versus 35.6°C (+SD 0.34) in hypothermic patients. Multivariable analysis identified starting ambient OT temperature [odds ratio (OR) = 0.83, confidence interval (CI): 0.71–0.96; P = 0.010], intraoperative temperature monitoring (OR = 0.49, CI: 0.28–085; P = 0.011), use of occlusive dressing (OR = 0.35, CI: 0.15–0.85; P = 0.020), and active forced-air warming (OR = 0.42, CI: 0.25–0.70; P = 0.001) as factors independently protective of postoperative hypothermia. Hypothermia occurred less frequently with emergency vs. elective procedures (OR 0.43, CI: 0.21–0.91; P = 0.026).
Conclusions:
Routine core temperature monitoring and active forced-air warming are useful measures to prevent hypothermia. Additionally, occlusive covers and controlling ambient OT temperature are cost-effective and safe methods to reduce inadvertent hypothermia.
INTRODUCTION
Incidence of inadvertent perioperative hypothermia, defined as core body temperature < 36°C, has been reported at 20%–52% of surgical cases.1–3 Anesthesia impairs the body’s thermoregulatory control, leading to core-heat redistribution and heat loss to the environment—surgical exposure and evaporation of antiseptic fluids in the cold operating room further compound the problem.1 Pediatric patients have a higher risk of developing hypothermia4–6 due to poorer thermoregulatory capacity, limited subcutaneous fat, and increased heat loss from their relatively large head and surface area-to-body weight ratio.
In adults, perioperative hypothermia increases the risk of perioperative complications, including cardiac events,7 bleeding,8 and surgical site infections.9,10 Reduced drug metabolism owing to hypothermia prolongs recovery,11 while shivering and discomfort delays discharge.12 Adult data contribute to current studies and guidelines on perioperative hypothermia,13–15 which may not extrapolate directly to pediatric patients. Existing literature16 on this topic in children mostly reiterates similar concepts but does not rigorously study the effect of each intervention on children.
Our primary objective is to determine factors associated with inadvertent hypothermia in children undergoing anesthesia in this prospective observational study. Our secondary aim is to identify the most important protective measures that a pediatric anesthetic unit can implement easily. A concurrent survey of the anesthetists managing the cases during the study period aims to relate the awareness of temperature issues to prevention strategies.
METHODS
Our hospital is Singapore’s main tertiary women and children’s hospital with 8,000 pediatric surgeries each year. Surgical procedures range from day surgery cases (eg, bilateral myringotomy with tube insertion) to complex surgeries (eg, congenital cardiac, neurosurgeries, and scoliosis surgeries). This study received ethics approval from SingHealth Centralized Institutional Review Board (CIRB) and waived the need for informed consent. We included consecutive pediatric surgical patients younger than 16 years old (day surgery/inpatient and elective/emergency) undergoing general anesthesia, with or without regional anesthesia. Patients with pre-existing febrile illness, temperature dysregulation states (eg, traumatic brain injury, use of cardiac bypass), and patients admitted to the intensive care unit (ICU) postoperatively were excluded. Surgical patients admitted to ICU postoperatively did not pass through postanesthetic care unit (PACU) and would not have PACU temperature data.
A study number was assigned to each patient to maintain patient confidentiality. Intraoperative temperature monitoring and passive and/or active warming strategies were at the discretion of the consultant anesthetist, as no standard practice protocol existed in our unit at the time of the study. The anesthetist recorded all intraoperative data prospectively on a data collection form. The anesthetic nurse verified it. We recorded the methods of temperature monitoring, warming, or heat conservation techniques used. Options for intraoperative temperature monitoring methods included the measurement of tympanic, oral, nasal, esophageal, rectal, and skin temperatures. Options for active intraoperative warming devices included forced-air warming (ie, Bair Hugger Normothermia System, 3MTM, St. Paul, Minn.); underbody warming mattress using circulating water or heated coil; fluid warmer; heated humidifier; and radiant warmer devices. Options for passive warming devices included occlusive dressing, heat moisture exchanger, prewarmed fluids, blankets, and warm wash or lavage.
Data on ambient operating theater (OT) temperatures, objectively reflected on the OT thermostat panel, before and at the end of the surgery, were collected.
In addition to patient demographics, surgical details collected include nature, region, urgency, duration of surgery, and blood loss. We categorized operations into major surgery (defined as any open body cavity such as thoracotomy and laparotomy), versus minor surgery (defined as short surgeries that do not breach the body cavity, often day cases). Anesthetic data collected include the technique of anesthesia, the volume of crystalloids and blood products given, as well as the duration of anesthesia. Table 1c documents the use of various warming methods for both active and passive warming.
Table 1.
Patient Demographics in Normothermic and Hypothermic Patient Groups
| Variable | N Total (N = 869) | Normothermic, ≥36°C (N = 762) | Hypothermic, <36°C (N = 107) | P |
|---|---|---|---|---|
| Age (y), mean ± SD | 7.46 ± 4.42 (N = 868) | 7.50 ± 4.44 (N = 761) | 7.23 ± 4.30 (N = 107) | 0.562 |
| Age category, n (%) | ||||
| 0–<1 y | 61 (7.03) | 55 (7.23) | 6 (5.61) | 0.309 |
| 1–<8 y | 411 (47.4) | 353 (46.4) | 58 (54.2) | |
| 8–<16 y | 396 (45.6) | 353 (46.4) | 43 (40.2) | |
| Male gender, n (%) | 596 (69.7) | 528 (70.4) | 68 (64.8) | 0.257 |
| 259 (30.3) | 222 (29.6) | 37 (35.2) | ||
| Weight (kg) | 29.0 ± 19.1 | 29.4 ± 19.5 | 26.4 ± 15.6 | 0.408 |
| (N = 864) | (N = 757) | (N = 107) | ||
| BMI (kg/m2) | 18.2 ± 4.73 | 18.3 ± 4.78 | 17.7 ± 4.32 | 0.241 |
| (n = 742) | (n = 649) | (n = 93) | ||
| ASA, n (%) | ||||
| 1 | 587 (67.9) | 514 (67.8) | 73 (68.2) | 0.199 |
| 2 | 237 (27.4) | 212 (28.0) | 25 (23.4) | |
| 3 | 40 (4.62) | 31 (4.09) | 9 (8.41) | |
| 4 | 1 (0.12) | 1 (0.13) | 0 (0) |
Bold is for significant P value <0.05.
ASA, American Society of Anesthesiologists; BMI, body mass index.
We defined hypothermia as temperature < 36°C, measured using the infrared tympanic thermometer within 15 minutes on arrival in the PACU. Patients posttympanic surgery, or others on whom a tympanic thermometer cannot be used, have their temperature checked with a temporal artery infrared thermometer. The recovery nurse also documents any self-reported discomfort from the patient’s hypothermia and the type of warming measures employed (if any). The nurse records the presence and intensity of postanesthetic shivering (PAS) in the PACU, using the scale devised by Crossley and Mahajan17 (see Appendix 1, Supplemental Digital Content 1, http://links.lww.com/PQ9/A211).18
A member of the research team not involved in the preoperative care or intraoperative data collection checked all data forms collated in the PACU and traced any missing information on the data form from the anesthesia case notes. We excluded data forms with missing PACU data from the study.
We included a short 3-question survey at the end of the data form (see Appendix 3, Supplemental Digital Content 1, http://links.lww.com/PQ9/A211). We required the consultant anesthetists (n = 43) to answer the questions when they first encountered the form.
Statistical Analysis
Our team summarized continuous variables as mean (SD) or median (IQR) and categorical variables as frequency and percentage. We used a 2-sample t-test or a Wilcoxon rank-sum test on patient demographics, clinical, surgical, and anesthetic characteristics between normothermic and hypothermic patients. We deemed this as appropriate for measurable variables, depending on the tenability of the normality assumption. We used a chi-square test or Fisher’s exact test depending on whether or not expected cell counts met assumptions for a chi-square test. P values are unadjusted for multiple comparisons. We defer to the discretion of the reader to utilize any of several approaches available that can be applied to the reported P values, for example, Bonferroni, Holm–Bonferroni, or Sidak.
We identified patient and perioperative (surgical and anesthetic) variables associated with an inadvertent hypothermia risk using univariate and multivariable logistic regression analyses. The Tenfold cross-validation was performed on the fitted model. Statistical significance in univariate analysis was set at α = 0.15. As the aim was to develop a predictive model, we tolerated a higher type I error in deference to reducing the probability of type II error, namely failure to identify a legitimate predictor. We included variables significant at α < 0.15 in univariate analysis in a stepwise multivariable logistic regression analysis with significance levels to enter and stay of 0.05 and 0.10, respectively, to identify a parsimonious subset of independent predictors. To assess predictive accuracy, we performed receiver operating characteristic (ROC) analysis on both the noncross-validated probabilities from the final predictive model and the 10-fold cross-validated predicted probabilities.
RESULTS
We collected data from 869 patients over two months and present the data in Tables 1–3, showing patient demographics, anesthesia, and surgical characteristics, temperature management factors, respectively, in normothermic versus hypothermic groups.
Table 3.
Temperature Management Factors in Normothermic and Hypothermic Patient Groups
| Variable | N Total (N = 869) | Normothermic, ≥36°C (N = 762) | Hypothermic, <36°C (N = 107) | P |
|---|---|---|---|---|
| Use of prewarmed IV fluids, n (%) | 515 (59.3) | 465 (61.0) | 50 (46.7) | 0.006 |
| Prewarming, n (%) | 10 (1.16) | 9 (1.19) | 1 (0.96) | 1.00 |
| Starting OT temperature (°C) | 24.0 ± 1.66 | 24.0 ± 1.64 | 23.6 ± 1.70 | 0.010 |
| (N = 830) | (N = 729) | (N = 101) | ||
| Starting OT temperature, n (%) | ||||
| <21°C | 65 (7.48) | 54 (7.09) | 11 (10.3) | 0.056 |
| 21–<23°C | 175 (20.1) | 145 (19.0) | 30 (28.0) | |
| 23–<25°C | 400 (46.0) | 355 (46.6) | 45 (42.1) | |
| ≥25°C | 229 (26.4) | 208 (27.3) | 21 (19.6) | |
| Ending OT temperature (°C) | 23.65 ±1.60 | 23.70 ±1.57 | 23.30 ±1.73 | 0.030 |
| Intraoperative active warming, n (%) | ||||
| No | 138 (16.1) | 113 (15.1) | 25 (23.4) | 0.035 |
| Bair Hugger | 699 (81.7) | 622 (83.0) | 77 (72.0) | 0.008 |
| Fluid warmer† | 10 (1.17) | 9 (1.20) | 1 (0.93) | 1.00 |
| Humidifier | 11 (1.29) | 11 (1.47) | 0 (0) | 0.377 |
| Radiant warmer | 15 (1.75) | 13 (1.74) | 2 (1.87) | 1.00 |
| Circulating water mattress | 35 (4.09) | 28 (3.74) | 7 (6.54) | 0.188 |
| Heated coil underbody warmer | 6 (0.70) | 4 (0.54) | 2 (1.87) | 0.167 |
| Intraoperative passive warming, n (%) | ||||
| No | 15 (1.74) | 12 (1.59) | 3 (2.83) | 0.414 |
| Occlusive covers | 145 (16.8) | 138 (18.2) | 7 (6.60) | 0.002 |
| Heat-moisture exchanger | 702 (81.3) | 621 (82.0) | 81 (76.4) | 0.183 |
| Warm blankets | 702 (81.3) | 616 (81.4) | 86 (81.1) | 1.00 |
| Warm wash/lavage | 10 (1.16) | 9 (1.19) | 1 (0.94) | 1.00 |
| Intraoperative patient temperature monitoring, n (%) | 320 (37.0) | 296 (39.1) | 24 (22.4) | 0.001 |
| Intraoperative patient temperature monitor, n (%) | ||||
| Tympanic | 5 (1.58) | 4 (1.37) | 1 (4.17) | 0.296 |
| Oral/nasal | 220 (69.4) | 201 (68.6) | 19 (79.2) | |
| Esophageal | 17 (5.36) | 15 (5.12) | 2 (8.33) | |
| Rectal | 28 (8.83) | 28 (9.56) | 0 (0) | |
| Skin | 47 (14.8) | 45 (15.4) | 2 (8.33) | |
| PACU warming, n (%) | ||||
| None | 685 (81.7) | 667 (91.0) | 18 (17.1) | <0.001 |
| Bair Hugger | 144 (17.2) | 60 (8.19) | 84 (80.0) | |
| Others | 6 (0.72) | 4 (0.55) | 2 (1.90) | |
| Blanket | 3 (0.36) | 2 (0.27) | 1 (0.95) | |
| Shivering, n (%) | 45 (5.39) | 34 (4.64) | 11 (10.7) | 0.018 |
| Discomfort from cold, n (%) | 22 (2.65) | 15 (2.06) | 7 (6.86) | 0.012 |
| Temperature on arrival at PACU (within 15 mins) | 36.5 ± 0.55 | 36.6 ± 0.45 | 35.6 ± 0.34 | <0.001 |
Bold is for significant P value <0.05.
†Fluid warming devices, that is, Hotline Blood and Fluid Warmer, Smiths Medical, 3M Ranger Fluid Warming System.
Of the many variables measured, those with a significant difference between the hypothermic and normothermic groups are identified in bold in Tables 1–3. Of 869 pediatric patients, 107 (12.3%) patients experienced postoperative hypothermia. Mean ± SD core temperature on arrival at the PACU was 36.6 ± 0.45°C in the normothermic group versus 35.6 ± 0.34°C in the hypothermic group (P < 0.001).
Risk Factors and Interventions
There were significantly higher proportions of day-case surgical (61.7% versus 49.0%; P = 0.017), elective (90.7% versus 80.4%; P = 0.012) and minor (79.1% versus 64.9%; P = 0.004) cases of shorter anesthesia (53.1 versus 67.4 min; P = 0.009), and surgical duration (42.2 versus 55.0 min; P = 0.019), in the hypothermic compared to normothermic group (Table 2).
Table 2.
Patient Surgical and Anesthetic Characteristics in Normothermic and Hypothermic Patient Groups
| Variable | N Total (N = 869) | Normothermic, ≥36°C (N = 762) | Hypothermic, <36°C (N = 107) | P |
|---|---|---|---|---|
| Admission type, n (%) | ||||
| Day surgery | 439 (50.6) | 373 (49.0) | 66 (61.7) | 0.017 |
| Inpatient | 429 (49.4) | 388 (51.0) | 41 (38.3) | |
| Surgical type, n (%) | ||||
| Elective | 654 (81.7) | 566 (80.4) | 88 (90.7) | 0.012 |
| Emergency | 147 (18.4) | 138 (19.6) | 9 (9.28) | |
| Surgery, n (%) | ||||
| Minor | 572 (66.8) | 489 (64.9) | 83 (79.1) | 0.004 |
| Major | 286 (33.3) | 264 (35.1) | 22 (21.0) | |
| Surgery site, n (%) | ||||
| Head and neck | 342 (39.5) | 305 (40.2) | 37 (34.6) | 0.292 |
| Chest | 22 (2.54) | 17 (2.24) | 5 (4.67) | 0.177 |
| Abdomen | 120 (13.9) | 113 (14.9) | 7 (6.54) | 0.017 |
| Back | 7 (0.81) | 6 (0.79) | 1 (0.93) | 0.604 |
| Upper limb | 77 (8.89) | 69 (9.09) | 8 (7.48) | 0.717 |
| Lower limb | 78 (9.01) | 67 (8.83) | 11 (10.3) | 0.590 |
| Urology | 162 (3.34) | 134 (15.5) | 28 (3.23) | 0.034 |
| Duration of surgery (min) | 53.5 ±60.0 | 55.0 ± 60.6 | 42.2 ± 54.8 | 0.019 |
| (N = 853) | (N = 750) | (N = 103) | ||
| Blood loss (mL/kg), mean ± SD, median (IQR) | 0.37 ± 1.67 | 0.40 ± 1.72 | 0.19 ± 1.27 | 0.135 |
| 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0.022 † | |
| (N = 864) | (N = 757) | (N = 107) | ||
| Anesthesia technique, n (%) | ||||
| General anesthesia | 869 (100) | 762 (100) | 107 (100) | 0.603 |
| Central neuraxial block | 34 (4.07) | 30 (4.09) | 4 (3.88) | 1.00 |
| Peripheral neuraxial block | 171 (20.2) | 141 (19.0) | 30 (28.9) | 0.026 |
| Anesthesia time (min) | 65.7 ± 66.5 | 67.4 ± 67.0 | 53.1 ± 61.5 | 0.009 |
| (N = 852) | (N = 750) | (N = 102) | ||
| Blood used (mL/kg), mean ± SD, | 0.12 ± 1.26 | 0.12 ± 1.27 | 0.12 ± 1.24 | 0.994 |
| (N = 828) | (N = 726) | (N = 102) | ||
| Vol. warmed crystalloid (mL/kg), mean ± SD | 11.6 ± 13.9 | 11.7 ± 14.4 | 10.6 ± 7.24 | 0.383 |
| (n = 509) | (n = 461) | (n = 48) |
Bold is for significant P value <0.05.
†Wilcoxon rank-sum test.
Overall, >80% of patients had forced-air warming, although more in the normothermic group than in the hypothermic group (83% versus 72%; P < 0.01). Both groups used heat-moisture exchangers and warmed blankets > 80% of the time. However, occlusive covers (eg, plastic sheets) and warmed fluids were used less often in the hypothermic group (6.6% versus 18.2%; P < 0.01; 46.7% versus 61.0%; P = 0.006) compared to the normothermic group, respectively. Overall, only 37% of cases had continuous temperature monitoring intraoperatively. Children in the hypothermic group were monitored continuously less often than those in the normothermic group (22.4% versus 39.1%; P < 0.001, Table 3).
Table 4 presents variables selected for inclusion into the multivariable analysis based on perceived clinical importance and relevance among all variables significant at α = 0.15 in univariate analysis. These variables comprised the candidate predictors for inclusion in the stepwise multivariable analysis. Table 4 summarizes the univariate and multivariable analysis results with adjusted odds ratios (ORs), 95% confidence interval (CI), and P values. Variables selected by the stepwise algorithm are considered the best subset of predictors of inadvertent hypothermia among those analyzed.
Table 4.
Logistic Regression Analyses to Identify Independent Predictors of Inadvertent Hypothermia*
| Variable | Univariate Odds Ratio (95% CI) | P | Multivariable Adj. Odds Ratio 95% CI)* | P |
|---|---|---|---|---|
| Weight (kg) | 0.99 (0.98, 1.00) | 0.124 | 0.986 (0.972, 0.999) | 0.034 |
| Admission type | ||||
| Day surgery/inpatient | 0.60 (0.39, 0.90) | 0.015 | ||
| Surgical type | ||||
| Elective/emergency | 0.42 (0.21, 0.85) | 0.017 | 0.43 (0.21, 0.91) | 0.026 |
| Surgery | ||||
| Major/minor | 0.49 (0.30, 0.80) | 0.005 | ||
| Surgery site | ||||
| Chest (Y/N) | 2.14 (0.77, 5.92) | 0.143 | ||
| Abdomen (Y/N) | 0.40 (0.18, 0.88) | 0.023 | ||
| Anesthesia time (min) | 1.00 (0.99, 1.00) | 0.041 | ||
| Vol. warmed crystalloid (mL/kg) | 0.99 (0.96, 1.02) | 0.604 | ||
| Warmed fluids (Y/N) | 0.56 (0.37, 0.84) | 0.005 | ||
| Starting OT Temperature (°C) | 0.83 (0.73, 0.95) | 0.008 | 0.83 (0.71, 0.96) | 0.010 |
| Starting OT temperature (oC) | 0.060 | |||
| <21oC | 2.02 (0.92, 4.44) | 0.081 | ||
| 21–<23oC | 2.05 (1.13, 3.72) | 0.018 | ||
| 23–<25oC | 1.26 (0.73, 2.17) | 0.414 | ||
| ≥25oC | 1.00 | — | ||
| Intraoperative active warming | ||||
| Any form active warming (Y/N) | 0.58 (0.36, 0.95) | 0.031 | 0.42 (0.25, 0.70) | 0.001 |
| Bair Hugger (Y/N) | 0.52 (0.33, 0.83) | 0.006 | ||
| Intraoperative passive warming | ||||
| Occlusive dressing (Y/N) | 0.32 (0.13 0.70) | 0.004 | 0.35 (0.15, 0.85) | 0.020 |
| Intraoperative temperature monitoring (Y/N) | 0.45 (0.28, 0.73) | 0.001 | 0.49 (0.28, 0.85) | 0.011 |
*The multivariable analysis included only variables significant at P < 0.15 in univariate analysis, which are variables included in this table. Area under ROC curve (95% CI) for multivariable model was 0.71 (0.66, 0.76); 10-fold cross-validated area under ROC curve (95% CI) was 0.68 (0.63, 0.74).
The first variable selected by the stepwise algorithm associated with a significant reduction in risk of inadvertent hypothermia was intraoperative temperature monitoring [aOR = 0.49 (0.28, 0.85), P = 0.011]; the second was use of the Bair Hugger intraoperative active warming system [aOR = 0.42 (0.25, 0.70), P = 0.001]; third, higher OT temperature at the start of surgery [aOR = 0.83 (0.71, 0.96), P = 0.010]; fourth, elective versus emergency surgery [aOR = 0.43 (0.21, 0.91), P = 0.026]; fifth, passive warming using occlusive dressing [aOR = 0.35 (0.15, 0.85), P = 0.20]; and sixth, higher weight of the child [aOR = 0.986 (0.972, 0.999), P = 0.034]. Corresponding cumulative areas under the sequential ROC curves were 0.605, 0.645, 0.670, 0.688, 0.696, and 0.710. No variables were removed after having been entered into the model.
ROC Analysis
The fitted model for predicting the probability of inadvertent hypothermia (P) given a specific patient profile is given in Appendix 4, Supplemental Digital Content 1, http://links.lww.com/PQ9/A211. Two ROC curves were obtained: A noncross-validated ROC curve obtained directly from the fitted model and a second ROC curve resulting from 10-fold cross-validation (Fig. 1A). Area under the curve (AUC) (95% CI) for the noncross-validated ROC curve was 0.71 (0.66, 0.76) and for the cross-validated curve 0.68 (0.63, 0.74). Selecting a cutpoint for classifying patients as “low” versus “high” risk for inadvertent hypothermia was guided by both the Youden J-statistic (Fig. 1B) and clinical considerations of sensitivity and negative predictive value (Appendix 4, Supplemental Digital Content 1, http://links.lww.com/PQ9/A211). The “de-optimized,” cross-validated ROC curve (Fig. 1A) is expected to reflect predictive results when the model is applied to an applicable independent dataset. Figure 1B shows that the noncross-validated ROC curve achieves a definitive maximum at P = 0.08. In contrast, the cross-validated curve essentially plateaus over the range P = 0.07 to 0.15, with a slight upward trend, achieving the maximum at P = 0.13 (Fig. 1B). However, at P = 0.13, sensitivity is very low at 0.59, and the false-negative rate relatively high at FN = 0.08 (Appendix 5b, Supplemental Digital Content 1, http://links.lww.com/PQ9/A211). Hence, P = 0.07 was selected as a more clinically acceptable cutpoint, although not “statistically optimal,” having sensitivity and negative predictive value of 0.88 and 0.96, respectively; specificity and positive predictive value were 0.36 and 0.16, respectively. For comparison purposes reflecting on the cross-validated choice of P = 0.07, histograms for the noncross-validated ROC probabilities are presented in Figure 2A with associated ROC statistics in Appendix 5b, Supplemental Digital Content 1, http://links.lww.com/PQ9/A211. Hypothermia prevalence in our study cohort was 11.9%.
Fig. 1.
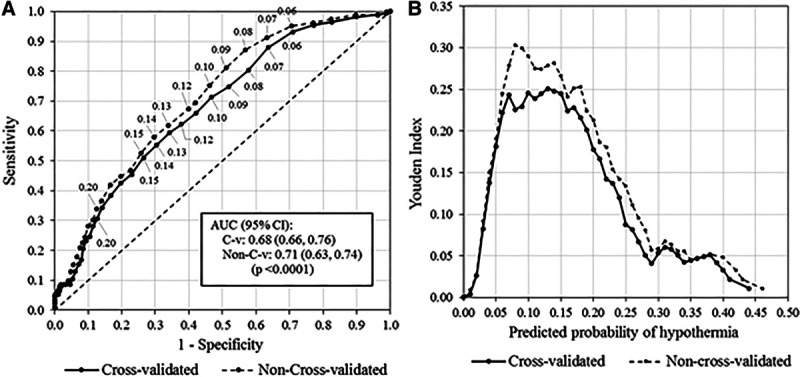
Noncross-validated and cross-validated and ROC curves, associated Youden J-Index plots, and predictive model variables and coefficients. A, ROC curves, noncross-validated and cross-validated. B, Youden plots, noncross-validated and cross-validated.
Fig. 2.
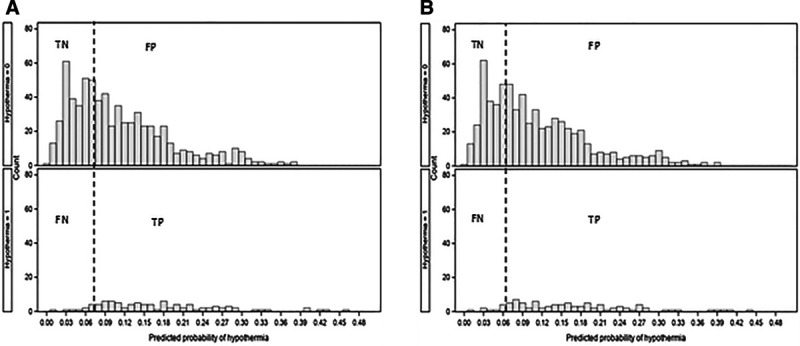
Histograms for non-cross-validated and cross-validated probabilities with cutpoints. A, Noncross-validated probabilities, clinically selected cutpoint, P = 0.08 (Sens = 0.87, Spec = 0.43, NPV = 0.96, PPV = 0.17). B, Cross-validated probabilities, clinically selected cutpoint, P = 0.07 (Sens = 0.88, Spec = 0.36, NPV = 0.96, PPV = 0.16). NPV, negative predictive value; PPV, positive predictive value.
Ambient OT Temperature
Our study shows that patients with higher starting OT temperatures had a lower risk of inadvertent hypothermia (OR = 0.83, 95% CI: 0.71–0.96; P = 0.010). Mean ± SD starting OT temperature was 24.0 ± 1.66oC. The mean ± SD starting OT temperature was significantly lower in the hypothermic group than in the normothermic group (23.6 ± 1.70°C vs 24.0 ± 1.64°C).
Appendix 2a, Supplemental Digital Content 1,http://links.lww.com/PQ9/A211, presents the association of OT starting temperature with age, and shows that higher OT starting temperature is associated with younger age (P = 0.013). Appendix 2b, Supplemental Digital Content 1, http://links.lww.com/PQ9/A211 shows that the proportionate use of active warming increased significantly with surgery duration (P < 0.0001), whereas the proportionate use of passive warming did not (P = 0.4676). Duration of surgery was ranked from low to high and then divided into 3 subgroups of equal sizes (tertiles) reflecting operations of shorter, medium, and longer duration.
Postanesthetic Care Unit
Significantly, more patients shivered (10.7% versus 4.6%; P = 0.018) and complained of discomfort from cold (6.9% versus 2.1%; P = 0.012) in the hypothermic compared to the normothermic group. As expected, significantly more patients in the hypothermic group received Bair Hugger active forced-air warming in PACU compared to the normothermic group. (80.0% versus 8.2%; P < 0.001).
Survey of Practice
Appendix 3, Supplemental Digital Content 1,http://links.lww.com/PQ9/A211, shows the survey results of anesthetists involved in our patient cohort’s care during the study. Out of the 43 anesthetists surveyed, 41 acknowledged the presence of guidelines, but only 23 used intraoperative monitoring for the case they were anesthetizing at the point of completing the survey.
DISCUSSION
Active Warming and Core Temperature Monitoring
This study showed that with our current standard of care, inadvertent hypothermia in our pediatric unit is lower than in other studies.1 We believe the rate of perioperative hypothermia varies due to different monitoring and temperature management practices across institutions. Although our hospital does not commonly practice preoperative warming (1.2%) and PACU warming (18.3%), 93.9% of patients received some form of active warming, and 98.3% received passive warming intraoperatively. Our study showed a higher proportion of minor, elective, day-case surgeries in the hypothermic group (Table 2). An explanation is shorter operations use less active warming (Appendix 2b, Supplemental Digital Content 1, http://links.lww.com/PQ9/A211). Active warming methods and continuous temperature monitoring were also used less frequently in minor cases compared to major cases (Table 5). Although not significant in the multivariable analysis owing to probable confounding with other active warming modalities, we believe that the use of warmed fluids, with a significant OR of 0.56 (95% CI: 0.37–0.84) in univariate analysis, may still be considered as an alternative form of active warming.
Table 5.
Temperature Management in Minor vs Major Surgeries
| Variable | N Total (N = 858) | Minor Surgeries (N = 572) | Major Surgeries (N = 286) | P |
|---|---|---|---|---|
| Starting OT temperature (oC) | 23.98 (1.65) N = 821 | 24.05 (1.67) N = 542 | 23.83 (1.62) N = 279 | 0.073 |
| Intraoperative active warming, n (%) | ||||
| No | 138 (16.27) | 103 (18.33) | 35 (12.24) | 0.024 |
| Bair Hugger | 690 (81.46) | 447 (79.68) | 243 (84.97) | 0.062 |
| Fluid warmer | 10 (1.18) | 4 (0.71) | 6 (2.10) | 0.096 |
| Humidifier | 11 (1.30) | 2 (0.36) | 9 (3.15) | 0.001 |
| Radiant warmer | 15 (1.77) | 9 (1.60) | 6 (2.10) | 0.592 |
| Circulating water mattress | 35 (4.13) | 24 (4.28) | 11 (3.85) | 0.856 |
| Heated coil underbody warmer | 6 (0.71) | 3 (0.54) | 3 (1.05) | 0.412 |
| Intraoperative passive warming, n (%) | ||||
| No | 14 (1.64) | 7 (1.23) | 7 (2.46) | 0.251 |
| Occlusive covers | 143 (16.74) | 84 (14.76) | 59 (20.70) | 0.032 |
| Heat-moisture exchanger | 698 (81.73) | 456 (80.14) | 242 (84.91) | 0.092 |
| Warm blankets | 696 (81.50) | 452 (79.44) | 244 (85.61) | 0.032 |
| Warm wash/lavage | 9 (1.05) | 5 (0.88) | 4 (1.40) | 0.491 |
| Intraoperative patient temperature monitoring, n (%) | 317 (37.12) | 153 (26.94) | 164 (57.34) | <0.001 |
Bold is for significant P value <0.05.
Of concern, intraoperative temperature monitoring was performed less often than active warming. This practice can be potentially harmful, leading to iatrogenic hyperthermia in pediatric patients. They tend to gain heat much faster from active warming due to their larger surface area-to-volume ratio.19 Moreover, initial heat loss from core-heat redistribution is less in neonates and infants with large head- or trunk–to-limbs ratios.18 Pediatric patients on prophylactic active forced-air warming should always have continuous core temperature monitoring.
The variability of intraoperative core temperature monitoring could be due to the lack of consensus for the modality of intraoperative core temperature monitoring.13 Temperature taken at the nasal pharynx was similar to tympanic, temporal, and axillary sites; however, tympanic and temporal temperatures were superior in detecting mild hypothermia.20 However, cost savings by the anesthetist is the more likely reason.21 It is probable that the surgeries, especially day-cases, have such high turnover that it is inconvenient to apply the monitoring probes intraoperatively. Moreover, there are no consensus guidelines on the indication for continuous monitoring in children in our country. Concurring with the literature,13–16 intraoperative temperature monitoring and active forced-air warming are protective factors against hypothermia. However, studies report that the feasibility of their routine use in actual practice is less consistent and sometimes impractical.22,23 As demonstrated in the short survey of 43 anesthetists, awareness of existing guidelines do not translate into clinical practice.
Ambient OT Temperature and Occlusive Covers
We found a higher ambient starting OT temperature and occlusive coverings to be independent protective factors against hypothermia. Although the temperature-conscious anesthetist is more likely to use a multitude of temperature control measures, these passive measures identified to be significant after adjustment for other active measures in the multivariate analysis, do seem to be useful on its own.
The adjustment of ambient OT temperature according to the patients’ age was apparent in our practice, as reflected in Appendix 2a, Supplemental Digital Content 1, http://links.lww.com/PQ9/A211. We postulate that age did not emerge as a significant risk factor for hypothermia because of preventive measures taken with this group of children, such as ensuring a higher starting OT temperature and other pre-emptive warming measures, especially in infants ≤ 1 year.
ROC Analysis
We provide the ROC analysis to validate our model in other institutions or develop a prediction tool of their own. It may provide insights as to which variables are good candidate predictors of inadvertent hypothermia. A value of AUC = 0.68 is not definitive evidence that hypothermia is predictable from the available variables. Nevertheless, it is clinically useful if cutpoints that give acceptable false positive (FP) and false-negative (FN) error rates are selected. At the Youden cutpoint of P = 0.07, an FN rate of 0.043 would provide a confident prognosis for children who were excluded as having a low risk of hypothermia.
Incidence and Risk Factors
Contrary to the literature, our study found that those undergoing emergency, major, and longer surgeries are at lower risk of becoming hypothermic. The discrepancy between our findings and the literature is probably due to awareness and increased warming strategies utilized in situations with a higher likelihood of hypothermia, thus decreasing hypothermia risk. Expectedly, smaller children are at a higher risk of hypothermia. With a significantly adjusted odds ratio of 0.99 for weight in the multivariable analysis, it interprets that for every kilogram increase in weight, the odds of hypothermia decreases by 1%. A 10-kg difference in weight represents a 10% decrease risk. Weight, which can be considered a surrogate of age, with a significance of only P = 0.124 in univariate analysis, was selected as an independent predictor of hypothermia in the multivariable analysis with P = 0.034. We can attribute this finding to be confounding with one or more of the original analysis variables that masked the effect of weight.
Limitation
Our incidence of postoperative hypothermia recorded may be inadequate as it denotes a point prevalence of hypothermia and likely underestimates the real incidence of perioperative hypothermia. As the American Society of Anesthesiologist recommends that “every patient receiving anesthesia shall have temperature monitored when clinically significant changes in body temperature are intended, anticipated or suspected,” frequent monitoring should ideally be performed in most if not all cases to detect and aid the prevention of hypothermia. We recommend a more rigorous prospective audit to use standardized continuous perioperative core temperature monitoring to better understand the percentage of perioperative duration that the child is hypothermic and its effects on short- and long-term outcomes.
CONCLUSIONS
Hypothermia is likely in the perioperative setting and can potentially be undiagnosed and untreated. If monitored, simple measures accessible to the operative team can prevent it.
There are few consensus guidelines for temperature management in the pediatric population. Even if guidelines exist, the routine use of intraoperative temperature monitoring and forced-air warming is challenging and expensive to implement.
Nonetheless, there is a need to find cost-effective measures that are simple to implement. From our results, occlusive covers such as plastic sheets and controlling ambient OT temperatures are safe yet straightforward and cost-efficient ways to minimize the risk of inadvertent hypothermia while striking a balance with the surgical team’s comfort. The authors recommend these to be a consideration in pediatric warming guidelines and protocols, especially in settings where the full suite of implementation is too expensive.22,23
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
ACKNOWLEDGMENTS
The authors acknowledge the assistance of the anesthetic nurses, Ms. Jaslin Nah and Ms. Zhaoli Wang, for their help with the study.
Supplementary Material
Footnotes
Published online September 25, 2020
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
To Cite: Lee SY, Wan SYK, Tay CL, Tan ZH, Wong I, Chua M, Allen JC. Perioperative Temperature Management in Children: What Matters? Pediatr Qual Saf 2020;5:e350.
REFERENCES
- 1.Pearce B, Christensen R, Voepel-Lewis T. Perioperative hypothermia in the pediatric population: prevalence, risk factors and outcomes. J Anesth 2010; 1:102. [Google Scholar]
- 2.Hart SR, Bordes B, Hart J, et al. Unintended perioperative hypothermia. Ochsner J. 2011; 11:259–270. [PMC free article] [PubMed] [Google Scholar]
- 3.Luis C, Moreno C, Silva A, Pascoa R, Abelha F. Inadvertent postoperative hypothermia at post-anesthesia care unit. Open J Anesthesiol 2012; 2:205, 213. [Google Scholar]
- 4.Galante D. Intraoperative hypothermia. Relation between general and regional anesthesia, upper- and lower-body warming: what strategies in pediatric anesthesia? Paediatr Anaesth. 2007; 17:821–823. [DOI] [PubMed] [Google Scholar]
- 5.Ohlson KB, Mohell N, Cannon B, et al. Thermogenesis in brown adipocytes is inhibited by volatile anesthetic agents. A factor contributing to hypothermia in infants? Anesthesiology. 1994; 81:176–183. [DOI] [PubMed] [Google Scholar]
- 6.Plattner O, Semsroth M, Sessler DI, et al. Lack of nonshivering thermogenesis in infants anesthetized with fentanyl and propofol. Anesthesiology. 1997; 86:772–777. [DOI] [PubMed] [Google Scholar]
- 7.Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events: a randomized clinical trial. J Am Med Assoc. 1997; 277:1127–1134. [PubMed] [Google Scholar]
- 8.Kurz A, Sessler DI, Lenhardt RA. Study of wound infections and temperature group: perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996; 334:1209–1215. [DOI] [PubMed] [Google Scholar]
- 9.Rajagopalan S, Mascha E, Na J, et al. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008; 108:71–77. [DOI] [PubMed] [Google Scholar]
- 10.Sessler DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001; 95:531–543. [DOI] [PubMed] [Google Scholar]
- 11.Leslie K, Sessler DI, Bjorksten AR, et al. Mild hypothermia alters propofol pharmacokinetics and increases the duration of action of atracurium. Anesth Analg. 1995; 80:1007–1014. [DOI] [PubMed] [Google Scholar]
- 12.Lenhardt R, Marker E, Goll V, et al. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology. 1997; 87:1318–1323. [DOI] [PubMed] [Google Scholar]
- 13.National Institute for Health and Care Excellence. Perioperative Hypothermia (Inadvertent): the Management of Inadvertent Perioperative Hypothermia in Adults. NICE Clinical Guideline 29. 2008London: National Institute for Health and Clinical Excellence; Available at www.nice.org.uk/CG065. Accessed October 7, 2018. [Google Scholar]
- 14.Hooper VD, Chard R, Clifford T, et al. ASPAN’s evidence-based clinical practice guideline for the promotion of perioperative normothermia 2010 (2nd edition). J Perianesth Nurs. 2010; 25:346–365. [DOI] [PubMed] [Google Scholar]
- 15.American Society of Anesthesiologists. An updated report by the American Society of Anesthesiologists Task Force on Postanesthesia Care. Anesthesiology. 2013; 118:1–17. [DOI] [PubMed] [Google Scholar]
- 16.Bajwa SJS, Swati . Perioperative hypothermia in pediatric patients: diagnosis, prevention and management. Anaesth Pain Intensive Care. 2014; 18:97–100. [Google Scholar]
- 17.Crossley AW, Mahajan RP. The intensity of postoperative shivering is unrelated to axillary temperature. Anaesthesia. 1994; 49:205–207. [DOI] [PubMed] [Google Scholar]
- 18.Luginbuehl I, Bissonette B, Davis PJ. Davis P, Cladis F. Thermoregulation: physiology and perioperative disturbances. In: Smith’s Anesthesia for Infants and Children. 2011. 8th ed. Philadelphia: Elsevier [Google Scholar]
- 19.Witt L, Dennhardt N, Eich C, et al. Prevention of intraoperative hypothermia in neonates and infants: results of a prospective multicenter observational study with a new forced-air warming system with increased warm air flow. Paediatr Anaesth. 2013; 23:469–474. [DOI] [PubMed] [Google Scholar]
- 20.Drake-Brockman TF, Hegarty M, Chambers NA, et al. Monitoring temperature in children undergoing anaesthesia: a comparison of methods. Anaesth Intensive Care. 2014; 42:315–320. [DOI] [PubMed] [Google Scholar]
- 21.Radauceanu DS, Dragnea D, Craig J. NICE guidelines for inadvertent peri-operative hypothermia. Anaesthesia. 2009; 64:1381–1382. [DOI] [PubMed] [Google Scholar]
- 22.Ross-Anderson DJ, Patel A. A NICE idea or a high price to pay? Local assessment of national guidelines. Anaesthesia. 2009; 64:330–331. [DOI] [PubMed] [Google Scholar]
- 23.Thwaites A, Willdridge D, Jinks A. NICE and warm: but is it necessary? Anaesthesia. 2010; 65:649–650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


