Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic has had a dramatic impact on cancer diagnosis and treatment. Most patients newly diagnosed with digestive system cancer are aged 65 and over.
Methods
We performed a retrospective, observational, multicentre cohort study based on prospectively collected electronic health records. All adults aged 65 or over and having been newly treated for a digestive system cancer between January 2018 until August 2020 were enroled.
Results
Data on 7882 patients were analysed. The first COVID-19 lockdown period led to a 42.4% decrease in newly treated digestive system cancers, and the post-lockdown period was associated with a 17% decrease. The decrease in newly treated digestive system cancer did not differ as a function of age, sex, comorbidities, primary tumour site, and disease stage. The proportion of patients admitted to an emergency department increased during the lockdown period. We do not observe a higher 3-month mortality rate in 2020, relative to the corresponding calendar periods in 2018 and 2019.
Conclusion
To avoid a decrease in newly treated cancers during future lockdown periods, access to healthcare will have to be modified. Although 3-month mortality did not increase in any of the patient subgroups, the 2020 cohort must be followed up for long-term mortality.
Keywords: COVID-19, Lockdown, Older patients, Digestive cancer, Public health
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has had a dramatic impact on cancer diagnosis and treatment - especially during periods of lockdown [1]. It is expected that a longer diagnostic delay will result in an increase in the cancer death rate within the next 5 years; this is especially likely for colorectal cancer, given the dramatic decrease in endoscopic screening [2,3] and disruption of the faecal immunochemical testing programme [4]. As access to general practitioners (GPs) has been restricted, the number of routine referrals has fallen [5]. Moreover, the pressure placed on healthcare systems worldwide by the influx of COVID-19 patients has delayed cancer treatment [6]. In France, the first period of lockdown lasted from March 17th to May 10th, 2020. The Ile-de-France (Greater Paris) and Great East regions were those most affected by the first wave of the COVID-19 pandemic, with high levels of pressure on hospitals.
Most patients newly diagnosed with digestive system cancer are aged 65 and over. Older age is associated with a greater diagnostic delay, less accurate treatment [7], and less frequently enrolment in a clinical trial [8]. The first wave of the COVID-19 pandemic prompted the publication of new guidelines on modified treatment strategies for digestive system cancer in patients of all ages [9] and specifically in older patients [10].
We hypothesised that the effects of lockdown on cancer care and the short-term mortality rate in older patients differed when comparing the very oldest individuals (aged 80+) with younger individuals (aged 65–79). Hence, the primary objective of the present study was to evaluate the number of newly treated digestive system cancers in older patients as a function of age group, sex, primary tumour site, disease stage, and comorbidities. The study's secondary objective was to assess the effect of lockdown on the type of treatment and the 3-month mortality rate.
2. Methods
2.1. Design
We performed a retrospective observational multicentre cohort study of prospectively collected electronic health records (EHRs) in the Greater Paris Public Hospitals Group's data warehouse (Entrepot de Données de Santé de l'Assistance Publique Hôpitaux de Paris (AP-HP); Paris, France).
2.2. Setting
Thirty-nine hospitals in the Greater Paris area contribute data to the AP-HP Data Warehouse. Thirty of these 39 hospitals provide care to patients with digestive system cancers and so were included in the study.
2.3. Participants
The study cohort comprised all adults aged 65 or over hospitalised in one of the 30 participating hospitals between January 1st, 2018, and August 30th, 2020, and for whom a digestive system cancer was the main diagnosis or a related diagnosis. Digestive system cancers were coded according to the International Classification of Diseases, 10th Revision (ICD-10) Supplementary data Table S1): cancers of the colon, rectum, oesophagus, stomach, pancreas, small intestine, anus or biliary tract and hepatocellular carcinoma. Patients having already been hospitalised with an ICD-10 code for a digestive system cancer in the previous 2 years were not included. The inclusion date was defined as the date of the first recorded hospital consultation or admission with a digestive system cancer code. Most of the enroled patients had been newly diagnosed with cancer in one of the 30 participating AP-HP hospitals, and the remainder had been diagnosed elsewhere but were referred to the AP-HP for the first time. The AP-HP data warehouse could not distinguish between these two categories of patients. Hence, we used the term “newly treated digestive system cancer” to encompass these two categories.
2.4. Follow-up
Patients were followed up with regard to the first treatment modality (chemotherapy, radiotherapy, surgery, endoscopy, or interventional radiology) and overall survival 3 months after the inclusion date. The overall study period was divided into a pre-lockdown period (January 1st, 2020, to March, 16th, 2020), a lockdown period (March 17th, 2020, to May 10th, 2020), and a post-lockdown period (May 11th, 2020, to August 30th, 2020).
2.5. Data sources
The study data were EHRs collected prospectively in the AP-HP hospitals and then stored in the AP-HP data warehouse. Since 2013, the AP-HP data warehouse has integrated administrative and clinical data on more than 11 million patients consulting at or hospitalised in AP-HP hospitals. The database was frozen on March 19th, 2021. The study was approved by an institutional review board (reference: 00,011,591). The study database was registered with the French National Data Protection Commission (Commission nationale de l'informatique et des libertés (Paris, France); reference: CNIL 1,980,120).
2.6. Outcome
The primary outcome was a digestive system cancer newly treated in one of the participating hospitals (defined as the first mention of a corresponding ICD-10 code in the care pathway, and no mentions in the previous 2 years). The secondary outcomes were the type of treatment (chemotherapy, radiotherapy, surgery, endoscopy, or interventional radiology) and the 3-month mortality rate.
2.7. Variables
We analysed the time period (pre-lockdown, lockdown and post-lockdown), sex, age, comorbidities, the primary tumour site (colon, rectum, oesophagus, stomach, pancreas, biliary tract, small intestine, anus, hepatocellular carcinoma) and metastasis status. Corresponding calendar periods were defined in the two reference years (2018 and 2019). Five age groups were pre-specified: 65–69, 70–75, 75–79, 80–85, and 85 or over. For the survival analysis, the five age groups were pooled into three: 65–69, 70–79 and 80 or over. Six tumour groups were defined: oesophageal cancer, stomach cancer, colorectal cancer, pancreas cancer, biliary tract cancer, and hepatocellular carcinoma. Comorbidities were assessed using a modified Charlson Comorbidity Index (adapted for use with hospital administrative data [11]), and patients were categorised in quartiles.
2.8. Statistical analysis
The patients’ demographic characteristics, primary tumour site, disease stage, and comorbidities were described as the frequency (percentage), the median (range) or the median [interquartile range [IQR)] for each of the three periods of interest in 2018, 2019 and 2020. Likewise, the number of newly digestive system cancers was described for each the three periods in 2018, 2019 and 2020. We then calculated the absolute and relative reductions in the number of newly digestive system cancers in the 2020 cohort vs. the average for the 2018 + 2019 cohorts. The absolute reduction was defined as the monthly number of newly treated cases in 2020 minus the average monthly number of newly treated cases in 2018 + 2019. The relative reduction was defined as the ratio between the absolute monthly reduction and the average monthly number of newly treated cases in 2018 and 2019. Weekly time trends were displayed and modelled using segmented linear regression. Subgroup analyses were performed by sex, age, primary tumour site, stage, comorbidity score, and presentation at an emergency department. Based on medical procedure codes in the 3 months following the first mention of a newly treated digestive system cancer for a given patient, the type of treatment was classified as curative surgery, palliative surgery, endoscopic treatment, interventional radiology, chemo/radiotherapy, and best supportive care only (Supplementary Table S2). The type of treatment was then described for the three periods of interest in 2020 vs. (2018 + 2019).
The 3-month overall survival rate and survival curves were analysed according to the baseline characteristics and the year (2020 vs. 2018 and 2019) separately for patients newly treated in the pre-lockdown, lockdown and post-lockdown periods, respectively. Univariate and multivariate analysis were performed using a Cox proportional Hazards regression model. The interaction term between year and baseline variable was assessed as a guide to the risk of death in 2020, relative to the reference period (2018 + 2019). All tests were two-sided, and the threshold for statistical significance was set to p < 0.05. The statistical analyses were performed with Python software and R software (version 2.4.3, The R Project for Statistical Computing, Vienna, Austria).
3. Results
3.1. A reduction in newly treated digestive system cancers in older patients
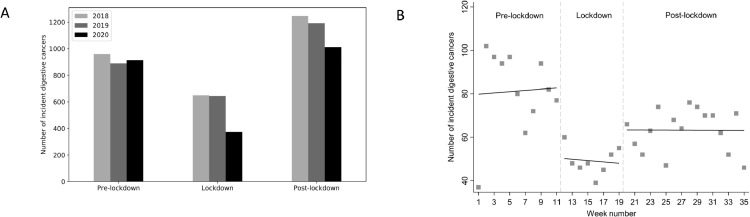
During the study period, a total of 7882 patients aged 65 and over (median (range) age: 74 (65–103); males: 60.8%) with newly treated digestive system cancer were included in the study. The lockdown period resulted in a dramatic reduction in newly treated digestive system cancers in older patients (Fig. 1 A). The number of newly treated digestive system cancer rose somewhat in the post-lockdown period but remained below the average value observed in 2018 and 2019 (Fig. 1B). The monthly absolute and relative reductions were respectively n = 9 and 2.5% (95% CI: 1.1; 4.7) in the period before lockdown, n = 155 and 42.4% (95% CI: 37.3; 47.7) during the lockdown period, and n = 56 and 17.0% (95% CI: 13.1; 21.5) in the post-lockdown period, compared with the corresponding periods in 2018 and 2019. Compared with the pre-lockdown period, there was a sharp decrease in cases (33 cases less; 95% CI: −58; −7; p = 0.014) at the start of the lockdown period (Fig. 1B). The trend was flat during the lockdown period. At the end of the lockdown, there was a sudden increase again but the number was lower than in the pre-lockdown period (20 cases less; 95% CI: −65; +26; p = 0.39).
Fig. 1.
The number of newly treated older patients with digestive system cancer during three time periods in 2020 vs. 2018 and 2019 (A), and the number of cases newly treated digestive system cancer patients in 2020 modelled by segmented linear regression (B).
3.2. The number of newly treated digestive system cancers according to age, sex, primary tumour site, tumour stage, comorbidities, and presentation at an emergency department
The distributions by age group, primary tumour site, sex and metastatic status for the periods of interest in 2018, 2019 and 2020 are summarized in Table 1 . The patients treated during the lockdown period did not differ from those treated during reference periods, except that the proportion of patients admitted with a digestive system cancer in an emergency department was higher during the lockdown period.
Table 1.
Characteristics of older patients with digestive system cancer, by period.
| Pre-lockdown period January 1st –March 16th (N = 2763) |
Overall p | Lockdown period March 17th –May 10th (N = 1668) |
Overall p | Post-lockdown period May 11th –August 30th (N = 3451) |
Overall p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2018 N = 960 |
2019 N = 889 |
2020 N = 914 |
2018 N = 650 |
2019 N = 645 |
2020 N = 373 |
2018 N = 1247 |
2019 N = 1192 |
2020 N = 1012 |
||||
| Age [IQR] | 74 [69–81] | 74 [69–81] | 74 [69–80] | 0.806 | 74 [69–81] | 74 [69–79] | 74 [69–80] | 0.502 | 74 [69–81] | 74 [70–81] | 74 [70–81] | 0.153 |
| Age, years | 0.787 | 0.234 | 0.502 | |||||||||
| 65–69 (%) | 257 (26.8) | 227 (25.5) | 239 (26.2) | 186 (28.6) | 178 (27.6) | 103 (27.6) | 341 (27.4) | 287 (24.1) | 239 (23.6) | |||
| 70–74 (%) | 229 (23.9) | 235 (26.4) | 251 (27.5) | 156 (24.0) | 164 (25.4) | 95 (25.5) | 319 (25.6) | 327 (27.4) | 277 (27.4) | |||
| 75–79 (%) | 186 (19.4) | 170 (19.1) | 163 (17.8) | 127 (19.5) | 142 (22.0) | 74 (19.8) | 234 (18.8) | 220 (18.5) | 189 (18.7) | |||
| 80–84 (%) | 142 (14.8) | 137 (15.4) | 135 (14.8) | 86 (13.2) | 92 (14.3) | 40 (10.7) | 169 (13.6) | 163 (13.7) | 154 (15.2) | |||
| ≥85 (%) | 146 (15.2) | 120 (13.5) | 126 (13.8) | 95 (14.6) | 69 (10.7) | 61 (16.4) | 184 (14.8) | 195 (16.4) | 153 (15.1) | |||
| Sex | 0.081 | 0.645 | 0.869 | |||||||||
| Male (%) | 601 (62.6) | 519 (58.4) | 576 (63.0) | 399 (61.4) | 403 (62.5) | 222 (59.5) | 740 (59.3) | 719 (60.3) | 609 (60.2) | |||
| Female (%) | 359 (37.4) | 370 (41.6) | 338 (37.0) | 251 (38.6) | 242 (37.5) | 151 (40.5) | 507 (40.7) | 473 (39.7) | 403 (39.8) | |||
| Primary tumour site | 0.502 | 0.397 | 0.587 | |||||||||
| Colon | 279 (29.1) | 249 (28.0) | 246 (26.9) | 166 (25.5) | 165 (25.6) | 96 (25.7) | 361 (29.0) | 309 (25.9) | 289 (28.6) | |||
| Rectum | 97 (10.1) | 83 (9.3) | 76 (8.3) | 57 (8.8) | 56 (8.7) | 39 (10.5) | 136 (10.9) | 132 (11.1) | 106 (10.5) | |||
| Oesophagus | 55 (5.7) | 46 (5.2) | 47 (5.1) | 33 (5.1) | 38 (5.9) | 14 (3.8) | 61 (4.9) | 65 (5.5) | 49 (4.8) | |||
| Stomach | 75 (7.8) | 71 (8.0) | 60 (6.6) | 59 (9.1) | 63 (9.8) | 27 (7.2) | 101 (8.1) | 91 (7.6) | 71 (7.0) | |||
| Pancreas | 195 (20.3) | 173 (19.5) | 205 (22.4) | 139 (21.4) | 123 (19.1) | 78 (20.9) | 256 (20.5) | 249 (20.9) | 210 (20.8) | |||
| Bile duct* | 71 (7.4) | 94 (10.6) | 84 (9.2) | 63 (9.7) | 58 (9.0) | 28 (7.5) | 123 (9.9) | 101 (8.5) | 100 (9.9) | |||
| Liver (HCC) | 151 (15.7) | 134 (15.1) | 162 (17.7) | 100 (15.4) | 108 (16.7) | 72 (19.3) | 165 (13.2) | 199 (16.7) | 139 (13.7) | |||
| Small intestine | 22 (2.3) | 20 (2.3) | 16 (1.8) | 12 (1.9) | 22 (3.4) | 6 (1.6) | 26 (2.1) | 31 (2.6) | 29 (2.9) | |||
| Anus | 15 (1.6) | 19 (2.1) | 18 (2.0) | 21 (3.2) | 12 (1.9) | 13 (3.5) | 18 (1.4) | 15 (1.3) | 19 (1.9) | |||
| Tumour stage | 0.339 | 0.203 | 0.493 | |||||||||
| Non-metastatic | 775 (80.7) | 711 (80.0) | 755 (82.6) | 524 (80.6) | 512 (79.4) | 313 (83.9) | 984 (78.9) | 957 (80.3) | 818 (80.8) | |||
| Metastatic | 185 (19.3) | 178 (20.0) | 159 (17.4) | 126 (19.4) | 133 (20.6) | 60 (16.1) | 263 (21.1) | 235 (19.7) | 194 (19.2) | |||
| Charlson Comorbidity Index | 0.871 | 0.007 | 0.700 | |||||||||
| 2 | 440 (45.8) | 428 (48.1) | 450 (49.2) | 290 (44.6) | 322 (49.9) | 176 (47.2) | 578 (46.4) | 550 (46.1) | 476 (47.0) | |||
| 3 | 51 (5.3) | 47 (5.3) | 47 (5.1) | 38 (5.8) | 30 (4.7) | 19 (5.1) | 59 (4.7) | 51 (4.3) | 58 (5.7) | |||
| 4–12 | 200 (20.8) | 174 (19.6) | 181 (19.8) | 137 (21.1) | 107 (16.6) | 96 (25.7) | 257 (20.6) | 262 (22.0) | 209 (20.7) | |||
| ≥13 | 269 (28.0) | 240 (27.0) | 236 (25.8) | 185 (28.5) | 186 (28.8) | 82 (22.0) | 353 (28.3) | 329 (27.6) | 269 (26.6) | |||
| Median Charlson Comorbidity Index | 3 [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13] | 3 [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13] | 3 [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13] | 0.146 | 3 [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13] | 3 [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13] | 3 [2], [3], [4], [5], [6], [7] | 0.323 | 3 [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13] | 3 [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13] | 3 [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13] | 0.670 |
| First treated in an emergency department | 0.029 | 0.001 | 0.093 | |||||||||
| No | 820 (85.4) | 755 (84.9) | 812 (88.8) | 541 (83.2) | 575 (89.2) | 305 (81.8) | 1044 (83.7) | 1007 (84.5) | 821 (81.1) | |||
| Yes | 140 (14.6) | 134 (15.1) | 102 (11.2) | 109 (16.8) | 70 (10.8) | 68 (18.2) | 203 (16.3) | 185 (15.5) | 191 (18.9) | |||
Intrahepatic, extrahepatic and gallbladder cancers.
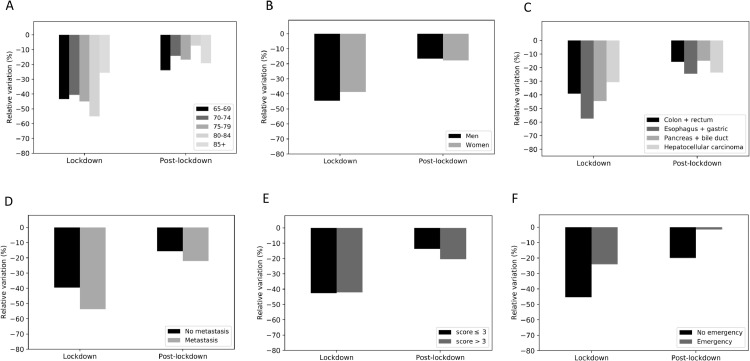
The absolute decreases in patient numbers as a function of the afore-mentioned characteristics are given in Table 2 . The relative reductions in the number of newly treated cancer patients during the lockdown period were greatest in the 80–84 age group (Fig. 2 A), for oesophagus and gastric cancer (Fig. 2C), for patients with metastasis (Fig. 2D), and for patients not treated in an emergency department (Fig. 2F).
Table 2.
The reduction in newly treated digestive system cancers in older patients during the lockdown and post-lockdown periods.
| Lockdown period |
Post-lockdown period |
|||||
|---|---|---|---|---|---|---|
| Mean number of patients in 2018-2019 | Number of patients in 2020 | Decrease in the number of patients in 2020, relative to the mean for 2018-2019 (n (%)). | Mean number of patients in 2018-2019 | Number of patients in 2020 | Decrease in the number of patients in 2020, relative to the mean for 2018-2019 (n (%)). | |
| Age | ||||||
| 65–69 | 182 | 103 | −79 (−43.4%) | 314 | 239 | −75 (−23.9%) |
| 70–74 | 160 | 95 | −65 (−40.6%) | 323 | 277 | −46 (−14.2%) |
| 75–79 | 134.5 | 74 | −60.5 (−45%) | 227 | 189 | −38 (−16.7%) |
| 80–84 | 89 | 40 | −49 (−55.1%) | 166 | 154 | −12 (−7.2%) |
| ≥85 | 82 | 61 | −21 (−25.6%) | 189.5 | 153 | −36.5 (−19.3%) |
| Sex | ||||||
| Male (%) | 401 | 222 | −179 (−44.6%) | 729.5 | 609 | −120.5 (−16.5%) |
| Female (%) | 246.5 | 151 | −95.5 (−38.7%) | 490 | 403 | −87 (−17.8%) |
| Primary tumour site | ||||||
| Colon | 165.5 | 96 | −69.5 (−42%) | 335 | 289 | −46 (−13.7%) |
| Rectum | 56.5 | 39 | −17.5 (−31%) | 134 | 106 | −28 (−20.9%) |
| Oesophagus | 35.5 | 14 | −21.5 (−60.6%) | 63 | 49 | −14 (−22.2%) |
| Stomach | 61 | 27 | −34 (−55.7%) | 96 | 71 | −25 (−26%) |
| Pancreas | 131 | 78 | −53 (−40.5%) | 252.5 | 210 | −42.5 (−16.8%) |
| Bile duct* | 60.5 | 28 | −32.5 (−53.7%) | 112 | 100 | −12 (−10.7%) |
| Liver (HCC) | 104 | 72 | −32 (−30.8%) | 182 | 139 | −43 (−23.6%) |
| Small intestine | 17 | 6 | −11 (−64.7%) | 28.5 | 29 | +0.5 (+1.8%) |
| Anus | 16.5 | 13 | −3.5 (−21.2%) | 16.5 | 19 | +2.5 (+15.2%) |
| Tumour stage | ||||||
| Non-metastatic | 518 | 313 | −205 (−39.6%) | 970.5 | 818 | −152.5 (−15.7%) |
| Metastatic | 129.5 | 60 | −69.5 (−53.7%) | 249 | 194 | −55 (−22.1%) |
| Charlson Comorbidity Index | ||||||
| 2 | 306 | 176 | −130 (−42.5%) | 564 | 476 | −88 (−15.6%) |
| 3 | 34 | 19 | −15 (−44.1%) | 55 | 58 | +3 (+5.5%) |
| 4–12 | 122 | 96 | −26 (−21.3%) | 259.5 | 209 | −50.5 (−19.5%) |
| ≥13 | 185.5 | 82 | −103.5 (−55.8%) | 341 | 269 | −72 (−21.1%) |
| First treated in an emergency department | ||||||
| No | 558 | 305 | −253 (−45.3%) | 1025.5 | 821 | −204.5 (−19.9%) |
| Yes | 89.5 | 68 | −21.5 (−24%) | 194 | 191 | −3 (−1.5%) |
Intrahepatic, extrahepatic and gallbladder cancers.
Fig. 2.
The reductions in newly treated older patients with digestive system cancer by time period in 2020 vs. 2018-2019, according to the age group (A), sex (B), tumour site (C), tumour stage (D), modified Charlson Comorbidity Index (E), and treated in an emergency department or not (F).
3.3. Treatment provided
The treatments provided during the first 3 months are reported in Table 3 . All treatment types were impacted during the lockdown period. Palliative surgery, endoscopic treatment and chemotherapy/radiotherapy were most impacted during the lockdown period, and curative surgery was most impacted during the post-lockdown period.
Table 3.
Treatments provided during the 3 months after the first hospital consultation or admission.
| Pre-lockdown period |
Lockdown period |
Post-lockdown period |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | 2018 N = 960 |
2019 N = 889 |
2020 N = 914 |
Change in the number of patients from the mean in 2018–2019 to 2020 (%) | 2018 N = 650 |
2019 N = 645 |
2020 N = 373 |
Evolution of number of patients from mean 2018–2019 to 2020 (%) | 2018 N = 1247 |
2019 N = 1192 |
2020 N = 1012 |
Change in the number of patients from the mean in 2018–2019 to 2020 (%) |
| Main treatment provided during the 3 months after the first attending | P = 0.163 | P = 0.778 | P = 0.334 | |||||||||
| Curative surgery | 265 (27.6) | 273 (30.7) | 253 (27.7) | −16 (−5.9%) | 184 (28.3) | 174 (27.0) | 110 (29.5) | −69 (−38,5%) | 365 (29.3) | 346 (29.0) | 264 (26.1) | −91.5 (−25.7%) |
| Palliative surgery | 20 (2.1) | 17 (1.9) | 18 (2.0) | −0.5 (−2,7%) | 15 (2.3) | 11 (1.7) | 5 (1.3) | −8 (−61.5%) | 31 (2.5) | 23 (1.9) | 29 (2.9) | 2 (+7,4%) |
| Endoscopic treatment | 49 (5.1) | 62 (7.0) | 55 (6.0) | −0.5 (−0.9%) | 54 (8.3) | 61 (9.5) | 26 (7.0) | −31.5 (−54.8%) | 112 (9.0) | 93 (7.8) | 90 (8.9) | −12.5 (−12.2%) |
| Interventional radiology | 64 (6.7) | 57 (6.4) | 67 (7.3) | 6.5 (+10,7%) | 44 (6.8) | 43 (6.7) | 29 (7.8) | −14.5 (−33.3%) | 71 (5.7) | 82 (6.9) | 72 (7.1) | −4.5 (−5.9%) |
| Chemotherapy/radiotherapy | 271 (28.2) | 229 (25.8) | 242 (26.5) | −8 (−1,6%) | 180 (27.7) | 176 (27.3) | 88 (23.6) | −90 (−50,6%) | 317 (25.4) | 269 (22.6) | 252 (24.9) | −41 (−14.0%) |
| Best supportive care only | 121 (12.6) | 85 (9.6) | 83 (9.1) | −20 (−9,7%) | 70 (10.8) | 81 (13.6) | 51 (13.7) | −24.5 (−32.4%) | 137 (11.0) | 159 (13.3) | 120 (11.9) | −28 (−18.9%) |
| No treatment recorded in the AP-HP hospital | 170 (17.7) | 166 (18.7) | 196 (21.4) | +28 (+8.3%) | 103 (15.9) | 99 (15.4) | 64 (17.2) | −37 (−36.6%) | 214 (17.2) | 220 (18.5) | 185 (18.3) | −32 (−14.7%) |
3.4. Overall survival
There were no significant changes in the 3-month overall survival rate amongst patients newly treated for cancer during the three periods in 2020, relative to the same calendar period in previous years (Fig. S1). Surprisingly, we observed significantly greater 3-month survival rates during the post-lockdown period for women, patients with metastatic cancer, and patients with a modified Charlson Comorbidity Index >3 (Table 4 ).
Table 4.
The 3-month survival rate amongst older patients with digestive system cancer, by period and year.
| Pre-lockdown perioda |
Lockdown periodb |
Post-lockdown periodc |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2020 | pd | 2018 | 2019 | 2020 | pd | 2018 | 2019 | 2020 | pd | |
| All patients | 81.1 | 81.9 | 81.2 | 0.89 | 78.9 | 81.1 | 81.7 | 0.57 | 82.0 | 79.2 | 82.8 | 0.06 |
| Age group | ||||||||||||
| 65–69 | 90.3 | 86.6 | 86.5 | 0.37 | 81.0 | 87.6 | 87.1 | 0.23 | 87.6 | 84.5 | 88.8 | 0.38 |
| 70–79 | 82.7 | 85.2 | 86.3 | 0.46 | 87.9 | 86.2 | 85.2 | 0.69 | 85.3 | 83.8 | 85.1 | 0.78 |
| ≥80 | 70.5 | 72.6 | 68.5 | 0.50 | 62.2 | 64.0 | 69.8 | 0.61 | 71.0 | 67.7 | 74.8 | 0.07 |
| Sex | ||||||||||||
| Male | 81.3 | 83.4 | 84.8 | 0.40 | 79.8 | 82.6 | 83.9 | 0.48 | 83.1 | 81.4 | 82.1 | 0.76 |
| Female | 80.9 | 79.8 | 74.5 | 0.12 | 77.4 | 78.4 | 78.4 | 0.96 | 80.3 | 75.8 | 83.9 | 0.011 |
| Primary tumour site | ||||||||||||
| Colon + rectum | 87.4 | 85.7 | 84.6 | 0.58 | 80.2 | 86.5 | 85.8 | 0.18 | 84.9 | 83.7 | 87.2 | 0.29 |
| Oesophagus + gastric | 79.1 | 80.7 | 85.5 | 0.65 | 75.4 | 80.5 | 75.0 | 0.81 | 82.7 | 81.0 | 74.7 | 0.24 |
| Pancreas + bile duct | 73.1 | 74.8 | 74.7 | 0.88 | 77.3 | 71.8 | 80.3 | 0.35 | 75.2 | 73.4 | 80.7 | 0.10 |
| Hepatocellular carcinoma | 77.9 | 85.1 | 83.1 | 0.33 | 77.2 | 85.5 | 78.7 | 0.31 | 84.3 | 76.5 | 85.1 | 0.06 |
| Small intestine | 95.2 | 95.0 | 81.0 | 0.25 | 100.0 | 85.4 | 83.3 | 0.38 | 95.8 | 89.4 | 76.7 | 0.08 |
| Anus | 86.7 | 83.9 | 87.1 | 0.96 | 90.5 | 74.1 | 92.3 | 0.36 | 94.1 | 72.2 | 68.1 | 0.13 |
| Tumour stage | ||||||||||||
| Non-metastatic | 87.1 | 87.5 | 86.7 | 0.86 | 83.6 | 87.2 | 85.3 | 0.30 | 88.3 | 87.0 | 88.1 | 0.57 |
| Metastatic | 55.5 | 58.8 | 56.3 | 0.80 | 59.2 | 57.3 | 63.5 | 0.83 | 58.2 | 47.8 | 61.0 | 0.021 |
| Charlson Comorbidity Index | ||||||||||||
| ≤3 | 91.3 | 89.2 | 88.6 | 0.30 | 85.3 | 88.5 | 88.5 | 0.49 | 90.7 | 89.2 | 89.1 | 0.58 |
| >3 | 70.6 | 73.5 | 72.9 | 0.64 | 72.5 | 72.4 | 74.9 | 0.92 | 72.9 | 69.3 | 76.2 | 0.048 |
Patients newly treated for a digestive system cancer between January 1st, 2020, and March 16th, 2020, and followed up for 3-month survival.
Patients newly treated for a digestive system cancer between March 16th, 2020 and May 10th, 2020, and followed up for 3-month survival.
Patients newly treated for a digestive system cancer between May 11th, 2020, and August 30th, 2020, and followed up for 3-month survival.
log-rank test.
Univariate analysis revealed that none of the subgroups of cancer patients newly treated during the lockdown period had a greater risk of 3-month mortality in 2020, relative to their counterparts in the same calendar period in 2018-2019 (Supplementary Tables S3 and S4). In a multivariate analysis, patients aged over 80, patients with pancreas or bile duct cancer, patients with a modified Charlson Comorbidity Index ≥3, and patients with best supportive care only had a lower 3-month mortality rate than their counterparts in the same calendar period in 2018-2019 (Fig. S2A).
For patients with cancers newly treated during the post-lockdown period, a univariate analysis revealed that none of the subgroups had an increased risk of 3-month mortality, compared with their counterparts in the same calendar period in 2018-2019 (Supplementary Tables S5 and S6). In a multivariate analysis, women, patients aged over 80, patients with pancreas or bile duct cancer, patients with a modified Charlson Comorbidity Index >3, and patients having undergone chemotherapy/radiotherapy had a higher 3-month survival rate than their counterparts in the same calendar period in 2018-2019 (Fig. S2B).
4. Discussion
During the COVID-19 pandemic, the first period of lockdown lead to a dramatic decrease (by 42.4%) in the number of newly treated digestive system cancers in older patients attending hospitals in the Greater Paris region. This type of decrease had also been observed (for patients of all ages) in a study of eastern France (−39%) [1] and in studies worldwide [12,13]. Our results are also in line with reports on the number of patients newly treated for cancer in our institution [14,15]. Furthermore, we observed a sustained, low number of newly treated older patients with digestive system cancer in the post-lockdown period (−17.0%), relative to 2018-2019. This finding showed that in the 3-month post-lockdown period, the healthcare system was not able to catch up with the delay in cancer treatment that had accumulated during lockdown. Hence, cancer care is likely to be delayed for a long time, which might lead to a lasting increase in the mortality rate [2,16]. The COVID-19 pandemic's indirect impact on deaths amongst cancer patients should be considered along with the direct impact due to SARS-CoV-2 infection [17,18].
The present study is the first to have evaluated the impact of lockdown and post-lockdown periods on care provision and early mortality in older patients with digestive system cancer. Surprisingly, the decrease in the number of newly treated cancer patients was larger in the 80–84 age class than in the 85+ age class. It must be borne in mind that the comorbidity burden was not associated with a greater reduction in the number of newly treated patients. This might be because patients with comorbidities are often already in a care pathway. Indeed, many patients being monitored for other reasons had remote consultations; this might have attenuated the drop-out rate.
The decrease in the number of newly treated patients was greatest for oesophageal and gastric cancer. Interestingly, a study in Italy also found that gastric cancer was the digestive tract cancer most strongly impacted by the COVID-19 pandemic [19]. Indeed, endoscopic screening for upper digestive symptoms was dramatically reduced during the lockdown period. The symptoms of upper gastrointestinal tract cancer are often not specific; prioritising endoscopy for cancer patients might be problematic but warrants evaluation. We found that hepatocellular carcinoma was one of the least impacted primary tumours. This might be explained by the fact that patients with cirrhosis are especially closely monitored. The decrease in the number of patients newly treated for hepatocellular carcinoma during lockdown (compared with 2019) was similar to the 27% decrease already reported for a part of the AP-HP network [20].
The number of patients newly treated in the emergency department decreased less than the number newly treated in other departments. However, this non-negligible (24%) decrease suggested that even access to primary care for patients with non-COVID-19 disease was impaired during the lockdown.
Relative decreases in curative surgery for colorectal cancer and neoadjuvant radiotherapy for rectal cancer during the COVID-19 pandemic have been reported [21]. Our results suggest that chemotherapy and endoscopic treatment were the most strongly impacted treatment modalities. It has been reported that levels of endoscopic treatment decreased dramatically during the lockdown period [22]. Oral chemotherapy has been recommended as a means of avoiding the need for hospital attendance during a lockdown period [9]. Unfortunately, the AP-HP data warehouse does not store data on oral chemotherapy prescribed to outpatients. Moreover, we did not have data on the intensity of chemotherapy (i.e. monotherapy, or two- or three-drug combination regimens). It is noteworthy that guidelines published at the end of 2020 recommended limiting changes in medical treatment for patients with a greater risk of developing a lethal form of COVID-19 [23]. Moreover, reassuring data on the risk of death of COVID-19 after colorectal surgery have been published recently [24]. Thus, it is important to continue considering standard treatments for older patients - even during the COVID-19 pandemic.
Interestingly, we did not observe a significant excess mortality rate amongst newly treated patients during the lockdown and post-lockdown periods. This might be due to a selection effect: patients who entered the healthcare system received similar levels of care and therefore had the same mortality risk. Given that the number of patients fell dramatically during the lockdown, people entering the healthcare system might even have received standard care. Moreover, part of the 3-month follow-up period was included in the post-lockdown period, when the healthcare system's activity returned to a more normal level.
Although the excess long-term mortality caused by diagnostic delay has been modelled [2], our study is the first to have looked for a direct, short-term effect of the pandemic and its associated measures on survival. In fact, we did not observe an increase in the short-term (3-month) mortality rate for newly treated cancer patients during the pandemic, although this variable probably did not capture all the consequences of lockdown on patient care. A long-term survey of the impact of disorganized patient management on cancer mortality is now needed. The overwhelming of the healthcare system probably degraded patient care. Lastly, we do not know how newly diagnosed cancer patients are treated outside our university hospital network.
Our study had several limitations. Although the AP-HP network accounts for a large proportion of the cancer care provided in the Greater Paris area, some patients might have been treated outside the network and thus would not have been documented in our study. The AP-HP hospitals were on the front line for treating COVID-19 patients and so were obliged to reduce their non-COVID-19 care activities. Indeed, a survey by the regional health agency in the Greater Paris area (Agence Régionale de Santé d'Ile-de-France) found overall decreases of 40% in April and 30% in May for digestive tract cancer surgery and 8% in April and 4% in May for cancer chemotherapy (unpublished data).
In conclusion, our study results evidenced a dramatic decrease in the number of older patients with newly treated digestive system cancer in a large hospital network during the first COVID-19 lockdown of 2020. The number of newly treated patients rose during the post-lockdown period but was still below the values for the corresponding periods in 2018 and 2019. With the exception of emergency department admissions, the patients’ clinical phenotype was not modified by the lockdown: all tumour sites, stages, age classes and comorbidities and both sexes were impacted to the same extent. Although a difference in short-time (3-month) mortality rate during lockdown was not found, longer-term follow-up is needed.
Conflict of interest
None declared.
Acknowledgments
Funding support
Assistance Publique Hôpitaux de Paris.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dld.2021.09.017.
Appendix. Supplementary materials
References
- 1.Brugel M., Carlier C., Essner C., Debreuve-Theresette A., Beck M.F., Merrouche Y., et al. Dramatic changes in oncology care pathways during the COVID-19 pandemic: the French ONCOCARE-COV study. Oncologist. 2021;26(2):e338–e341. doi: 10.1002/onco.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maringe C., Spicer J., Morris M., Purushotham A., Nolte E., Sullivan R., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023‑34. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer A., Drouin J., Zureik M., Weill A., Dray-Spira R. Colonoscopy in France during the COVID-19 pandemic. Int J Colorectal Dis. 2021;36(5):1073–1075. doi: 10.1007/s00384-020-03816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Jonge L., Worthington J., van Wifferen F., Iragorri N., Peterse E.F.P., Lew J.B., et al. Impact of the COVID-19 pandemic on faecal immunochemical test-based colorectal cancer screening programmes in Australia, Canada, and the Netherlands: a comparative modelling study. Lancet Gastroenterol Hepatol. 2021;6(4):304–314. doi: 10.1016/S2468-1253(21)00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones D., Neal R.D., Duffy S.R.G., Scott S.E., Whitaker K.L., Brain K. Impact of the COVID-19 pandemic on the symptomatic diagnosis of cancer: the view from primary care. Lancet Oncol. 2020;21(6):748‑50. doi: 10.1016/S1470-2045(20)30242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna T.P., King W.D., Thibodeau S., Jalink M., Paulin G.A., Harvey-Jones E., et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087. doi: 10.1136/bmj.m4087. 4 nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aparicio T., Pamoukdjian F., Quero L., Manfredi S., Wind P., Paillaud E. Colorectal cancer care in elderly patients: unsolved issues. Dig Liver Dis. 2016;48(10):1112‑8. doi: 10.1016/j.dld.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Canouï-Poitrine F., Lièvre A., Dayde F., Lopez-Trabada-Ataz D., Baumgaertner I., Dubreuil O., et al. Inclusion of older patients with cancer in clinical trials: the SAGE prospective multicenter cohort survey. Oncologist. 2019;24(12):e1351‑9. doi: 10.1634/theoncologist.2019-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Fiore F., Bouché O., Lepage C., Sefrioui D., Gangloff A., Schwarz L., et al. COVID-19 epidemic: proposed alternatives in the management of digestive cancers: a French intergroup clinical point of view (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR) Dig Liver. 2020;52(6):597‑603. doi: 10.1016/j.dld.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Battisti N.M.L., Mislang A.R., Cooper L., O'Donovan A., Audisio R.A., Cheung K.L., et al. Adapting care for older cancer patients during the COVID-19 pandemic: recommendations from the international society of geriatric oncology (SIOG) COVID-19 working group. J Geriatr Oncol. 2020;11(8):1190‑8. doi: 10.1016/j.jgo.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bannay A., Chaignot C., Blotière P.O., Basson M., Weill A., Ricordeau P., et al. The best use of the charlson comorbidity index with electronic health care database to predict mortality. Med Care. 2016;54(2):188‑94. doi: 10.1097/MLR.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 12.Patt D., Gordan L., Diaz M., Okon T., Grady L., Harmison M., et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform. 2020;4:1059‑71. doi: 10.1200/CCI.20.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinmohamed A.G., Visser O., Verhoeven R.H.A., Louwman M.W.J., van Nederveen F.H., Willems S.M., et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21(6):750‑1. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kempf E., Lamé G., Layese R., Priou S., Chatellier G., Chaieb H., et al. New cancer cases at the time of SARS-Cov2 pandemic and related public health policies: a persistent and concerning decrease long after the end of the national lockdown. Eur J Cancer. 2021;150:260‑7. doi: 10.1016/j.ejca.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Priou S., Lamé G., Chatellier G., Tournigand C., Kempf E. Effect of the COVID-19 pandemic on colorectal cancer care in France. Lancet Gastroenterol Hepatol. 2021;6(5):342‑3. doi: 10.1016/S2468-1253(21)00095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai A.G., Pasea L., Banerjee A., Hall G., Denaxas S., Chang W.H., et al. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open. 2020;10(11) doi: 10.1136/bmjopen-2020-043828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lièvre A., Turpin A., Ray-Coquard I., Le Malicot K., Thariat J., Ahle G., et al. Risk factors for coronavirus disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: a French nationwide cohort study (GCO-002 CACOVID-19) Eur J Cancer. 2020;141:62‑81. doi: 10.1016/j.ejca.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee L.Y., Cazier J.B., Angelis V., Arnold R., Bisht V., Campton N.A., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919‑26. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buscarini E., Benedetti A., Monica F., Pasquale L., Buttitta F., Cameletti M., et al. Changes in digestive cancer diagnosis during the SARS-CoV-2 pandemic in Italy: a nationwide survey. Dig Liver Dis. 2021;53(6):682‑8. doi: 10.1016/j.dld.2021.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Amaddeo G., Brustia R., Allaire M., Lequoy M., Hollande C., Regnault H., et al. Impact of COVID-19 on the management of hepatocellular carcinoma in a high-prevalence area. JHEP Rep Innov Hepatol. 2021;3(1) doi: 10.1016/j.jhepr.2020.100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris E.J.A., Goldacre R., Spata E., Mafham M., Finan P.J., Shelton J., et al. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: a population-based study. Lancet Gastroenterol Hepatol. 2021;6(3):199‑208. doi: 10.1016/S2468-1253(21)00005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutter M.D., Brookes M., Lee T.J., Rogers P., Sharp L. Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: a national endoscopy database analysis. Gut. 2021;70(3):537‑43. doi: 10.1136/gutjnl-2020-322179. [DOI] [PubMed] [Google Scholar]
- 23.Tougeron D., Michel P., Lièvre A., Ducreux M., Gaujoux S., Guiu B., et al. Management of digestive cancers during the COVID-19 second wave: a French intergroup point of view (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, SFR) Dig Liver Dis. 2021;53(3):306–308. doi: 10.1016/j.dld.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuech J.J., Manceau G., Ouaissi M., Denet C., Chau A., Kartheuser A., et al. Are colorectal cancer patients at risk for COVID-19 infection during the postoperative period? The COVID-GRECCAR study. Int J Colorectal Dis. 2021;36(3):611‑5. doi: 10.1007/s00384-021-03847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.