Abstract
Background
Metastatic peritoneal carcinomatosis (MPC) is not common in patients with non-small cell lung cancer (NSCLC), and the clinical characteristics and treatment outcomes are still unclear.
Patients and Methods
We recruited 46 NSCLC patients with MPC at Keio University and affiliated hospitals (Keio Lung Oncology Group) between January 2011 and December 2017, then retrospectively investigated their clinical characteristics and the impact of treatment interventions on their survival.
Results
The profile of histological subtype was predominantly adenocarcinoma and 15 patients harbored driver oncogenes. Univariate and multivariate analysis demonstrated that performance status and the presence of a driver oncogene were significantly associated with the prolonged overall survival (OS). Regarding treatment, the median OS in the treatment group (9.3 months) was significantly longer than in the best supportive care group (1.3 months) (P < 0.0001).
Conclusion
The prognosis of MPC in NSCLC patients who receive only the best supportive care is poor, but therapeutic intervention may improve prognosis.
Keywords: metastatic peritoneal carcinomatosis, NSCLC, EGFR, malignant ascites, peritoneal nodule, intraperitoneal dissemination
Introduction
Lung cancer remains a deadly disease despite the recent development of more effective treatments.1 Morbidity and mortality in non-small lung cancer (NSCLC) is worsened by metastasis to extrapulmonary organs such as brain, bone and liver.2 Metastases not only shorten prognosis, but also compromise quality of life (QOL).3 In recent years, many treatment modalities for NSCLC have been developed, including molecularly targeted agents and immune checkpoint blockade, and these approaches have improved therapeutic options for patients with metastases. Recent trials have emphasized treatment strategies for NSCLC brain metastasis and intrathoracic dissemination, along with malignant pleural effusion.4–6 On the other hand, metastatic peritoneal carcinomatosis (MPC) is not a common NSCLC complication in daily practice.7 Since previous studies have reported that patients with MPC have worse QOL including various symptoms such as poor appetite, shortness of breath, nausea, abdominal pain, and general weakness,8 it is generally thought among oncologists that the prognosis of NSCLC with MPC is poor. Palliative therapy can relieve these symptoms, but adequate treatment strategies have not yet been developed. One of the reasons for disappointing outcomes with MPC may be that thoracic oncologists lack experience in treating peritoneal complications. However, at autopsy, metastasis to the peritoneum from lung cancer is not a rare finding.8 In addition, recent developments in medical diagnostics increase opportunities to detect MPC in NSCLC patients. In this multicenter study, we identified over 40 NSCLC patients with MPC and retrospectively investigated their clinical course. As a result, we clarified clinical characteristics of MPC which were associated with poor prognosis and evaluated the impact of therapeutic intervention on their prognosis.
Materials and Methods
NSCLC patients with MPC were recruited at Keio University and affiliated hospitals (Keio Lung Oncology Group) between January 2011 and December 2017.
MPC was defined as malignant ascites by cytologic pathology or a radiographic finding of peritoneal nodules ≥10 mm reflecting intraperitoneal dissemination. Eligible criteria were as follows: patients who were, (1) histologically or cytologically diagnosed with NSCLC, and (2) simultaneously or subsequently accompanied by MPC.
The following clinical data were collected from medical records: dates when lung cancer and MPC were diagnosed, age at the time of lung cancer diagnosis, sex, Eastern Cooperative Oncology Group (ECOG) performance status (PS), histology, smoking status, EGFR/ALK mutation status, treatment data, and survival data.
Statistical Analysis
Statistical analysis using Fisher’s exact test was performed to evaluate clinical characteristics. Kaplan–Meier method with log rank test was used to compare survival. Cox proportional hazards model was conducted for multivariate analysis of prognostic factors. P < 0.05 was considered statistically significant for all tests. Staging was according to the 8th edition of the Union for International Cancer Control TNM classification.
This study was approved by the ethical review board committee of Tachikawa Hospital, Keio University and affiliated hospitals for the use of an opt-out style covering patient data confidentiality, and conducted in accordance with the 1964 Declaration of Helsinki, as revised in 2013. The requirement to obtain informed consent was waived because of the retrospective design.
Results
Clinical Characteristics of the Study Participants
Baseline patient characteristics at MPC diagnosis are summarized in Table 1. In total, 46 patients met inclusion criteria and their median age was 66 years. Of them, 33 and 36 were male and current/former smokers, respectively (71.7%, 78.3%). The profile of histological subtype was predominantly adenocarcinoma (n = 40, 87.0%). Fifteen patients harbored driver oncogenes (32.6%): 14 EGFR mutations and 1 ALK fusion rearrangement. Twelve (26.1%) were diagnosed with MPC at the same time with the diagnosis of NSCLC, and 34 (73.9%) were subsequently diagnosed with MPC. Thirty-one were determined to have MPC based on radiographic findings (67.0%). Seventeen (37.0%) and 5 patients (11.0%) had malignant pleural effusion (MPE) and brain metastasis at MPC diagnosis, respectively. Ascites cytology tests successfully detected 4 EGFR mutation from 7 patients whose tumor specimen had previously harbored the mutation.
Table 1.
Patient Characteristic
| No. of Patients | |
|---|---|
| Total enrolled | 46 |
| Age (years) | |
| Median (range) | 66 (IQR 59–71) |
| Sex | |
| Male | 33 |
| Female | 13 |
| PS | |
| 0/1/2/3/4 | 5/17/10/11/3 |
| Histology | |
| Adenocarcinoma | 40 |
| Pleomorphic carcinoma | 1 |
| Adenosquamous | 1 |
| NSCLC-NOS | 4 |
| EGFR/ALK mutations | |
| Positive | 15 |
| Negative/unknown | 31 |
| Smoking Status | |
| Former or current | 36 |
| Never | 10 |
| Onset of MPC | |
| Initial | 12 |
| Late | 34 |
| Type of MPC | |
| Peritoneal nodules | 31 |
| Malignant ascites | 4 |
| Both | 11 |
| Ascites mutation status | |
| Driver oncogene positive | 4 |
| Driver oncogene negative | 3 |
| Not examined | 8 |
| Malignant pleural effusions | |
| Positive | 17 |
| Negative/unknown | 29 |
| Brain metastasis | |
| Positive | 5 |
| Negative | 41 |
Clinical Course of MPC
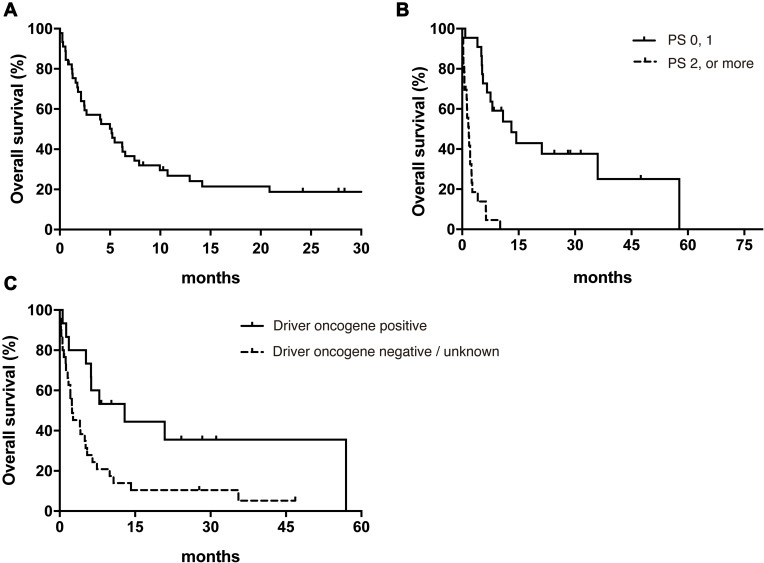
Median overall survival (OS) after MPC diagnosis was 5.2 months in all patients (95% Confidence interval 2.1–6.3) (Figure 1A). Log rank test revealed that good PS (0 or 1) and the presence of a driver oncogene were significantly associated with prolonged OS (Table 2). Median OS in the poor PS group (2 or more) was remarkably shorter than in the good PS group (1.8 months vs 13.1 months, hazard ratio, 11.2; 95% CI, 4.86–25.8) (Figure 1B). The presence or absence of a driver oncogene led to OS of 12.9 months and 2.5 months, respectively (hazard ratio, 0.41; 95% CI, 0.21–0.81) (Figure 1C). In addition, multivariate analysis demonstrated that PS and the presence of driver oncogene were significantly associated with prolonged OS (Table 3).
Figure 1.
Kaplan–Meier analysis of overall survival in all patients (A), in the good and poor PS groups (B) and in the driver oncogene positive and negative or unknown groups (C).
Abbreviation: PS, performance status.
Table 2.
The Univariate Analysis of Clinical Factors Associated with OS (Log Rank Test)
| Item | Category | N | Hazard Ratio (95% CI) | P-value |
|---|---|---|---|---|
| Age (years) | < 75 | 35 | Reference | |
| ≧75 | 11 | 1.33 (0.59–3.00) | p = 0.50 | |
| Sex | Male | 33 | Reference | |
| Female | 13 | 0.95 (0.47–1.93) | p = 0.90 | |
| Smoking history | Non-smoker | 10 | Reference | |
| Smoker | 36 | 1.57 (0.79–3.15) | p = 0.20 | |
| Driver oncogene | Negative/Unknown | 31 | Reference | |
| Positive | 15 | 0.41 (0.21–0.81) | p = 0.010 | |
| PS | 0, 1 | 22 | Reference | |
| 2, 3, 4 | 24 | 11.2 (4.86–25.8) | p < 0.001 | |
| Onset of MPC | Initial | 12 | Reference | |
| Late | 34 | 1.24 (0.60–2.60) | p = 0.57 | |
| Malignant ascites | No | 31 | Reference | |
| Yes | 15 | 0.63 (0.32–1.22) | p = 0.17 | |
| Previous treatment history | No | 14 | Reference | |
| Yes | 32 | 0.75 (0.28–1.98) | p = 0.90 | |
| Abdominal symptom | No | 24 | Reference | |
| Yes | 22 | 1.19 | p = 0.97 | |
| Brain metastasis | No | 41 | Reference | |
| Yes | 5 | 1.03 (0.68–1.39 | p = 0.89 | |
| Malignant pleural effusions | No | 29 | Reference | |
| Yes | 17 | 0.99 (0.51–1.93) | P = 0.97 |
Table 3.
The Multivariate Analysis of Clinical Factors Associated with OS (Cox Proportional Hazards Model)
| Item | Category | N | Hazard Ratio (95% CI) | P-value |
|---|---|---|---|---|
| Driver oncogene | Negative/Unknown | 31 | Reference | |
| Positive | 15 | 0.46 (0.21–0.99) | p = 0.049 | |
| PS | 0, 1 | 22 | Reference | |
| 2, 3, 4 | 24 | 7.84 (3.40–18.07) | p < 0.001 |
Therapeutic Outcomes
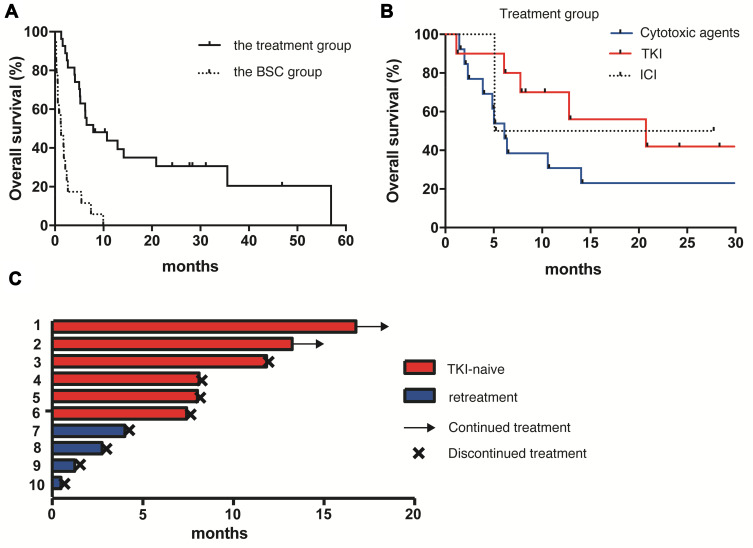
Next, we investigated the association between therapeutic interventions after the diagnosis of MPC and survival benefit. Twenty-five patients (54.3%) who received at least one line of anti-cancer treatment after MPC diagnosis were categorized as the treatment group, and the other 21 patients (45.7%) who were not administered any anti-cancer agent made up the best supportive care (BSC) group. The median OS in the treatment group (9.3 months, 95% CI 5.3–35.6 months) was significantly longer than that in the BSC group (1.3 months, 95% CI 0.33–2.4 months) (hazard ratio, 0.10; 95% CI, 0.042 to 0.25; P<0.0001) (Figure 2A). In the treatment group, cytotoxic agents, EGFR/ALK-tyrosine kinase inhibitors (EGFR/ALK-TKI), and immune-checkpoint inhibitors (ICI) were administered for 13, 10, and 2 patients, respectively. Three patients received bevacizumab with a cytotoxic agent or EGFR-TKI. The median OS in the groups treated with cytotoxic agent and EGFR/ALK-TKI was 6.2 and 20.9 months, respectively (hazard ratio, 0.10; 95% CI, 0.042 to 0.25; P<0.0001) (Figure 2B). In the EGFR/ALK-TKI group, 6 were TKI-naïve patients and the other 4 had previously received retreatment with a TKI (Figure 2C). All TKI-naïve patients were continuously administered TKI for more than 7 months, whereas no one in the TKI-retreatment group continued TKI beyond 4 months due to the worsening of PS or the disease progression.
Figure 2.
Kaplan–Meier analysis of overall survival in the treatment and the BSC groups (A), and in the groups treated with cytotoxic agents, TKI and ICI (B). Swimmer plot in the TKI-naïve and -retreatment groups (C).
Abbreviations: BSC, best supportive care; TKI, tyrosine kinase inhibitors; ICI, immune checkpoint inhibitors.
Discussion
Extrapulmonary metastasis in NSCLC is commonly observed and the optimal treatment strategies have been extensively investigated. In terms of MPC, especially in lung cancer patients, little is known about whether therapeutic interventions should be actively recommended and what type of treatment should be selected. Our retrospective multi-center study recruited 46 NSCLC patients with MPC, then clarified their clinical characteristics. We demonstrated that systemic drug therapy above and beyond BSC may result in prolonged survival in certain patient populations.
First, this study shows the profile of histological subtypes associated with MPC, predominantly adenocarcinoma. Besides, in patients with lung adenocarcinoma, MPE is common during treatment.9 Our results are in line with previous work by Su et al and Patil et al demonstrating that 25 of 30 lung cancer cases with MPC had adenocarcinoma8 and 26 of 33 non-squamous NSCLC patients with MPC had MPE,9 respectively.
Multivariate analysis revealed that the presence of a driver oncogene as well as better PS was associated with prolonged OS. A retrospective study investigating 60 NSCLC patients with MPC by Abbate et al had reported that overall survival in patients with EGFR mutations was better than in patients with wild type EGFR, likely as a result of receiving targeted agents more frequently.10 Our findings indicate that, in advanced lung cancer patients with MPC, histological subtype and genomic screening should be used to guide therapy. In this context, conducting ascites cytology with next-generation sequencing to detect genomic alterations may improve the prognosis of lung cancer patients with MPC.
Next, our study focused on how significantly therapeutic intervention affects prognosis. Median OS in the BSC group was less than 2 months and was as poor as that of NSCLC patients with symptomatic brain metastasis, considered one of the worst complications in advanced NSCLC.11 Our findings agree with previous studies reporting median OS in lung cancer patients with MPC of only 15 days to 3.5 months.8,12 This is likely due to the high morbidity associated with MPC, which critically affects prognosis. In contrast, overall survival in patients receiving active treatment was significantly improved when compared with that in the BSC group. Although peritoneal carcinomatosis from lung cancer may be one of the signs of the end of life, palliative systemic therapy should be considered since it appears to improve prognosis. The mechanisms underlying the efficacy of systemic anti-cancer therapy for MPC are not well understood. Here, drug penetrance and concentration in the peritoneal and pleural cavities are worth considering. Kobayashi et al reported that the concentration of paclitaxel intravenous administration reaches a sufficient concentration to suppress cancer cell growth in ascites of gastric cancer patients.13 This finding suggests that systemic cytotoxic agents may have the potential for anti-tumor activity by reaching the abdominal cavity. For small molecule inhibitors, there are several pharmacological studies investigating drug concentrations in pleural effusions, but no reports on concentrations in ascites.14,15 Since the median OS in the EGFR/ALK-TKI treatment group in our study was 20.9 months, comparable to previous studies investigating advanced lung cancer patients with metastasis, we suppose that TKIs reach sufficient intraperitoneal concentrations to treat MPC.15,16 However, this clinical benefit was limited in TKI-retreated patients, who showed only transient response (Figure 2C). In order to avoid missing this promising treatment opportunity, genomic screening should always be considered in metastatic NSCLC.
There are some limitations to the current study. First, it is a retrospective study recruiting a limited number of patients with MPC. Second, the number of patients administered novel therapeutic agents such as bevacizumab and ICI is too small to objectively evaluate treatment efficacy. Programmed cell death ligand-1 (PD-L1) tumor proportion score (TPS) was examined in 7 patients (2%), then resulting in positive (TPS was at least 1% or more) for 5 patients. Nassereddine et al recently reported high prevalence of MPC patients with positive PD-L1 and showed limited number of responses to ICI treatment.17 Third, the prevalence of MPC in NSCLC with driver oncogene other than EGFR and ALK alteration has not yet been evaluated in our study, which is now commonly investigated using next-generation sequencing technology. Conducting a prospective study would prove challenging since NSCLC MPC is a rare complication that is difficult to predict in advance. A detailed and longitudinal observational study throughout the course of NSCLC treatment at a high-volume center would be helpful.
Conclusion
In retrospective analysis of 46 patients, we clarified clinical characteristics of MPC, a rare complication with NSCLC. Better PS and the presence of a driver oncogene were significantly associated with prolonged overall survival. Although the overall prognosis of MPC remains poor, palliative therapeutic interventions including cytotoxic agents and small molecule inhibitors may show a survival benefit even after the diagnosis of MPC.
Acknowledgments
The authors thank Dr. Erik H Knelson for his intellectual review of the article.
Disclosure
Prof. Dr. Kenzo Soejima reports grants and/or personal fees from AstraZeneca, Nippon Boehringer Ingelheim, Taiho Pharmaceutical, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb Japan, MSD Oncology, Eli Lilly Japan, and Novartis Pharma, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Riihimaki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86:78–84. doi: 10.1016/j.lungcan.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 3.Sato T, Soejima K, Fujisawa D, et al. Prognostic understanding at diagnosis and associated factors in patients with advanced lung cancer and their caregivers. Oncologist. 2018;23:1218–1229. doi: 10.1634/theoncologist.2017-0329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rybarczyk-Kasiuchnicz A, Ramlau R, Stencel K, et al. Treatment of brain metastases of non-small cell lung carcinoma. Int J Mol Sci. 2021;22:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haas AR, Sterman DH, Musani AI. Malignant pleural effusions: management options with consideration of coding, billing, and a decision approach. Chest. 2007;132:1036–1041. doi: 10.1378/chest.06-1757 [DOI] [PubMed] [Google Scholar]
- 6.Morgensztern D, Waqar S, Subramanian J, et al. Prognostic impact of malignant pleural effusion at presentation in patients with metastatic non-small-cell lung cancer. J Thorac Oncol. 2012;7:1485–1489. doi: 10.1097/JTO.0b013e318267223a [DOI] [PubMed] [Google Scholar]
- 7.Hanane K, Salma B, Khadija B, et al. Peritoneal carcinomatosis, an unusual and only site of metastasis from lung adenocarcinoma. Pan Afr Med J. 2016;23:60. doi: 10.11604/pamj.2016.23.60.8910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su HT, Tsai CM, Perng RP. Peritoneal carcinomatosis in lung cancer. Respirology. 2008;13(3):465–467. doi: 10.1111/j.1440-1843.2008.01268.x [DOI] [PubMed] [Google Scholar]
- 9.Patil T, Aisner DL, Noonan SA, et al. Malignant pleural disease is highly associated with subsequent peritoneal metastasis in patients with stage IV non-small cell lung cancer independent of oncogene status. Lung Cancer. 2016;96:27–32. doi: 10.1016/j.lungcan.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 10.Abbate MI, Cortinovis DL, Tiseo M, et al. Peritoneal carcinomatosis in non-small-cell lung cancer: retrospective multicentric analysis and literature review. Future Oncol. 2019;15(9):989–994. doi: 10.2217/fon-2018-0469 [DOI] [PubMed] [Google Scholar]
- 11.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. doi: 10.1200/JCO.2011.38.0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satoh H, Ishikawa H, Yamashita YT, et al. Peritoneal carcinomatosis in lung cancer patients. Oncol Rep. 2001;8:1305–1307. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi M, Sakamoto J, Namikawa T, et al. Pharmacokinetic study of paclitaxel in malignant ascites from advanced gastric cancer patients. World J Gastroenterol. 2006;12:1412–1415. doi: 10.3748/wjg.v12.i9.1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashiwabara K, Fuji S, Tsumura S, et al. Prognosis of EGFR-mutant lung adenocarcinoma patients with malignant pleural effusion receiving first-line EGFR-TKI therapy without pleurodesis: a Single-institute Retrospective Study. Anticancer Res. 2020;40:1117–1121. doi: 10.21873/anticanres.14051 [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Lee OJ, Son SM, et al. EGFR mutation status in lung adenocarcinoma-associated malignant pleural effusion and efficacy of EGFR tyrosine kinase inhibitors. Cancer Res Treat. 2018;50:908–916. doi: 10.4143/crt.2017.378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, Phase 2 study. Lancet Oncol. 2014;15:1236–1244. doi: 10.1016/S1470-2045(14)70381-X [DOI] [PubMed] [Google Scholar]
- 17.Nassereddine H, Sannier A, Brosseau S, et al. Clinicopathological and Molecular Study of peritoneal carcinomatosis associated with non-small cell lung carcinoma. Pathol Oncol Res. 2020;26:2795–2800. doi: 10.1007/s12253-019-00713-1 [DOI] [PubMed] [Google Scholar]