Abstract
Background
Transformation to small cell lung cancer (SCLC) is a resistance mechanism of epidermal growth factor receptor (EGFR) mutant lung adenocarcinoma (LADC) patients treated with EGFR tyrosine kinase inhibitors (TKIs). Here, we describe the clinical characteristics and prognosis of these patients and explore the treatment modes after transformation.
Methods
EGFR‐mutant LADC patients with SCLC transformation were retrospectively included in the study. Demographic and clinical data were collected. Survival outcomes and corresponding influential factors were analyzed.
Results
Twenty‐nine patients were included in the study. The median progression‐free survival (PFS) of patients who received first‐line EGFR‐TKIs was 13.1 months. The median time to SCLC transformation was 27.5 months. After transformation, the objective response rates of patients who received first‐line chemotherapy with or without EGFR‐TKIs were 43.8% and 37.5%, respectively. The median PFS of patients reveiving chemotherapy with EGFR‐TKIs was significantly longer than that of patients receiving chemotherapy without EGFR‐TKIs (5.2 vs. 3.0 months; HR, 0.19; 95% CI: 0.05–0.72; p = 0.014). However, there was no significant difference in median overall survival (OS) between patients who received chemotherapy with or without EGFR‐TKIs (14.8 vs. 13.0 months; p = 0.474). In the multivariate Cox proportional hazards regression analysis, both anti‐angiogenic treatment (HR, 0.04; 95% CI: 0.01–0.29; p = 0.001) and local radiotherapy (HR, 0.28; 95% CI: 0.08–0.97; p = 0.044) were significantly associated with better patient OS after transformation.
Conclusions
Compared with chemotherapy alone, the combination of chemotherapy and EGFR‐TKIs as first‐line treatment after SCLC transformation can benefit patients in PFS but not in OS. However, anti‐angiogenic therapies and local radiotherapy can significantly prolong OS after transformation.
Keywords: anti‐angiogenesis, epidermal growth factor receptor mutation, lung adenocarcinoma, small cell histological transformation, tyrosine kinase inhibitors
Compared with chemotherapy alone, the combination of chemotherapy and EGFR‐TKIs as first‐line treatment after SCLC transformation can benefit patients in PFS but not in OS. However, anti‐angiogenic therapies and local radiotherapy can significantly prolong the OS after transformation.
INTRODUCTION
Lung adenocarcinoma (LADC) patients with epidermal growth factor receptor (EGFR) mutations will inevitably develop resistance during treatment with EGFR tyrosine kinase inhibitors (TKIs). Although almost 60% of acquired resistance to first‐ or second‐generation EGFR‐TKIs have been reported to result from secondary T790M mutation,1 transformation to small cell lung cancer (SCLC) also accounts for about 3%–14% resistance to first‐ or second‐generation EGFR‐TKIs1, 2 and is seen in resistance to third‐generation EGFR‐TKIs as well.3, 4
Even though the mechanism of transformation from non‐small cell lung cancer (NSCLC) to SCLC has not previously been thoroughly investigated, several studies have attempted to explain the phenomenon. SCLC and adenocarcinoma are thought to originate from neuroendocrine cells and alveolar type II cells, respectively.5 However, experiments in mice have demonstrated that loss of Trp53 and Rb1 in alveolar type II cells could also effectively lead to the development of SCLC,6 indicating that some SCLCs and adenocarcinomas might have a shared ancestry.
Since SCLC is a more aggressive tumor than EGFR‐mutant LADC, early identification of the transformation to SCLC during LADC treatment is of great importance. Due to the significant role in SCLC transformation, inactivation of TP53 and Rb1 has also previously been demonstrated to have a strong predictive value during EGFR‐TKI treatment for LADC.7, 8 Unfortunately, TP53 and Rb1 status is not routinely evaluated in LADC patients before or during EGFR‐TKI treatment. Therefore, more clinical signs that indicate the probable SCLC transformation are needed to assist with early identification.
The optimal treatment choices for LADC patients who develop SCLC transformation remain unclear. Some cases have been reported to receive chemotherapy for SCLC alone after transformation,3, 4, 9, 10, 11, 12 while other cases have received a combination of chemotherapy and EGFR‐TKIs.4, 9, 13 The response status and progression‐free survival (PFS) varied from case to case. Two studies reported that the objective response rate (ORR) to platinum‐etoposide therapy was nearly 50% and the median PFS was about 3.5 months.14, 15 However, whether EGFR‐TKIs should be continued or not is controversial.
Here, we conducted a retrospective study on patients with transformation from EGFR‐mutant LADC to SCLC. The aim of our study was to describe the clinical and molecular characteristics and prognosis of these patients and to explore better treatment modes after transformation.
METHODS
Patients
We retrospectively reviewed patients with advanced EGFR‐mutant LADC who visited and received treatment in Cancer Hospital, Chinese Academy of Medical Sciences (Beijing, China) from January 1, 2011 to June 30, 2021. Patients who met the following criteria were included in this study: (1) diagnosis of histologically‐ or cytologically‐verified advanced LADC with sensitive EGFR mutations determined by polymerase chain reaction (PCR) or next‐generation sequencing (NGS); (2) diagnosis of SCLC transformation based on high‐quality tumor biopsies or well‐preserved cytological samples during LADC treatment. The study was approved by the Ethics Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (Approval no. 21/243‐2914).
Data collection and follow‐up
Demographic and clinical information were extracted from the medical history system and supplemented through follow‐up. Genomic profiles were also collected from patients with complete NGS results to analyze accompanying genomic alterations. Treatments and corresponding outcomes of both pre‐ and post transformation were collected. Tumor responses were evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 1.1). PFS of certain treatment was defined as the period from the initial date of the treatment to disease progression or death. Overall survival (OS) after transformation was defined as the period from the initial date of first‐line treatment after transformation to death or the date of last follow‐up. Time to SCLC transformation was defined as the period from the initial date of first‐line treatment for advanced disease to the confirmation date of histologically‐ or cytologically‐verified SCLC. The last follow‐up date was July 20, 2021.
Statistical analysis
PFS, OS and time to SCLC transformation were analyzed using Kaplan–Meier (K–M) analysis. Cox proportional hazards regression analysis was also used to explore corresponding influential factors. Two‐sided p values <0.05 were considered statistically significant. All analyses were performed with SPSS (version 23.0) and Rstudio (version 1.1.383).
RESULTS
Demographic and clinical characteristics before transformation
A total of 29 patients were included in this study, among whom 19 patients (65.5%) received third‐generation EGFR‐TKIs before transformation to SCLC. The demographic and clinical characteristics are summarized in Table 1. The majority of patients were female (65.5%) and non‐smokers (82.8%). The patients were relatively young with a median age of 56 years at diagnosis of advanced disease. Nearly half of the patients had already developed brain metastases before transformation to SCLC.
TABLE 1.
Demographic and clinical characteristics of EGFR‐mutant LADC patients
| Characteristics | All patients | Treatment of third generation TKIs before transformation | No third generation TKIs before transformation | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| N | 29 | 19 | 10 | |||
| Age | ||||||
| Median (years) | 56.0 | 56.0 | 57.0 | |||
| Range | 32.6–79.7 | 32.6–68.7 | 38.2–79.7 | |||
| Age distribution | ||||||
| ≥65 | 4 | 13.8% | 1 | 5.3% | 3 | 30.0% |
| <65 | 25 | 86.2% | 18 | 94.7% | 7 | 70.0% |
| Gender | ||||||
| Male | 10 | 34.5% | 6 | 31.6% | 4 | 40.0% |
| Female | 19 | 65.5% | 13 | 68.4% | 6 | 60.0% |
| Smoking history | ||||||
| Yes | 4 | 13.8% | 3 | 15.8% | 1 | 10.0% |
| No | 24 | 82.8% | 15 | 78.9% | 9 | 90.0% |
| Unknown | 1 | 3.4% | 1 | 5.3% | 0 | 0.0% |
| Brain metastasis before SCLC transformation | ||||||
| Yes | 12 | 41.4% | 8 | 42.1% | 4 | 40.0% |
| No | 17 | 58.6% | 11 | 57.9% | 6 | 60.0% |
| First‐line treatment | ||||||
| EGFR‐TKI | 19 | 65.5% | 14 | 73.7% | 5 | 50.0% |
| EGFR‐TKI + chemotherapy | 2 | 6.9% | 0 | 0.0% | 2 | 20.0% |
| Chemotherapy + EGFR‐TKI maintenance | 4 | 13.8% | 3 | 15.8% | 1 | 10.0% |
| Chemotherapy | 4 | 13.8% | 2 | 10.5% | 2 | 20.0% |
| Initial EGFR‐TKI treatment for advanced disease | ||||||
| First‐line | 21 | 72.4% | 14 | 73.7% | 7 | 70.0% |
| First‐line maintenance | 4 | 13.8% | 3 | 15.8% | 1 | 10.0% |
| Second‐line | 4 | 13.8% | 2 | 10.5% | 2 | 20.0% |
| Initial EGFR‐TKI treatment | ||||||
| Gefitinib | 13 | 44.8% | 7 | 36.8% | 6 | 60.0% |
| Erlotinib | 4 | 13.8% | 2 | 10.5% | 2 | 20.0% |
| Icotinib | 6 | 20.7% | 5 | 26.3% | 1 | 10.0% |
| Afatinib | 1 | 3.4% | 0 | 0.0% | 1 | 10.0% |
| Dacomtinib | 1 | 3.4% | 1 | 5.3% | 0 | 0.0% |
| Osimertinib | 4 | 13.8% | 4 | 21.1% | 0 | 0.0% |
| Types of EGFR‐TKIs used before SCLC transformation | ||||||
| 1 | 9 | 31.0% | 3 | 15.8% | 6 | 60.0% |
| 2 | 13 | 44.8% | 10 | 52.6% | 3 | 30.0% |
| 3 | 6 | 20.7% | 5 | 26.3% | 1 | 10.0% |
| 4 | 1 | 3.4% | 1 | 5.3% | 0 | 0.0% |
Abbreviations: EGFR, epidermal growth factor receptor; LADC, lung adenocarcinoma; SCLC, small cell lung cancer; TKIs, tyrosine kinase inhibitors.
Most patients (86.2%) received EGFR‐TKIs in the first‐line setting, including EGFR‐TKI maintenance and combination of EGFR‐TKI and chemotherapy. With only four (13.8%) patients receiving osimertinib as their initial EGFR‐TKI treatment, about 70% patients received at least two types of EGFR‐TKIs.
Molecular characteristics before and after transformation
Molecular tests were performed in all 29 patients before initial EGFR‐TKI treatment and most of these patients were found to harbor EGFR exon 19 deletions (55.2%) and exon 21 L858R mutations (31.0%) (Table 2). Eighteen patients received at least two molecular tests before transformation to SCLC and EGFR exon 20 T790M mutation was identified in 11 (61.1%) patients. Among 13 patients who received NGS tests, 12 harbored TP53 mutation; five harbored Rb1 mutation; three harbored PIK3CA mutation and one harbored PTEN mutation. All five patients who had Rb1 mutation also harbored a TP53 mutation.
TABLE 2.
Genomic features of EGFR‐mutant LADC patients transforming to SCLC
| Characteristics | Before transformation to SCLC | After transformation to SCLC | ||
|---|---|---|---|---|
| N | % | N | % | |
| EGFR mutations | 29 | 18 | ||
| Exon 19 deletion | 16 | 55.2% | 12 | 66.7% |
| Exon 21 L858R | 9 | 31.0% | 4 | 22.2% |
| Exon 19 deletion + exon 21 L858R | 2 | 6.9% | 0 | 0.0% |
| Exon 18 G719X | 1 | 3.4% | 1 | 5.6% |
| Exon 18 G719X + exon 20 S768I | 1 | 3.4% | 1 | 5.6% |
| EGFR exon 20 T790M mutationa | 18 | 18 | ||
| Yes | 11 | 61.1% | 4 | 22.2% |
| No | 7 | 38.9% | 14 | 77.8% |
| Accompanying mutationsb | 13 | 13 | ||
| TP53 | 12 | 92.3% | 13 | 100.0% |
| Rb1 | 5 | 38.5% | 6 | 46.2% |
| PIK3CA | 3 | 23.1% | 5 | 38.5% |
| PTEN | 1 | 7.7% | 3 | 23.1% |
Abbreviations: EGFR, epidermal growth factor receptor; LADC, lung adenocarcinoma; SCLC, small cell lung cancer.
Patients who received at least two molecular tests before transformation.
Patients who received next generation sequencing tests.
After transformation to SCLC, molecular tests were performed in 18 patients. All these patients harbored EGFR mutations. Among these patients, EGFR exon 20 T790M mutation was identified in four (22.2%) patients. Among 13 patients who received NGS tests after transformation, all patients harbored TP53 mutation; six harbored Rb1 mutation; five harbored PIK3CA mutation and three harbored PTEN mutation.
Treatment and efficacy before transformation
Among 19 patients treated with EGFR‐TKIs in the first line, four (21.1%) patients received third‐generation EGFR‐TKIs. The ORR of first‐line EGFR‐TKI treatment was 68.4% and the disease control rate (DCR) was 94.7%. The median PFS of first‐line EGFR‐TKI treatment was 13.1 months (95% confidence interval [CI]: 8.6–17.6 months] (Figure 1a). Among four patients who received EGFR‐TKIs as first‐line maintenance, the median PFS was 11.0 months (95% CI: 6.8–15.1 months).
FIGURE 1.
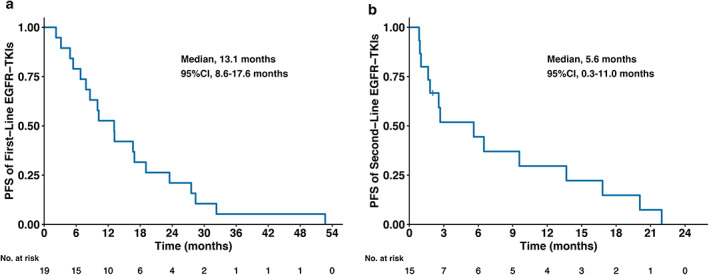
Progression‐free survival (PFS) of first‐ and second‐line epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) treatment before transformation to small cell lung cancer. (a) PFS of first‐line EGFR‐TKIs. (b) PFS of second‐line EGFR‐TKIs
A total of 15 patients were treated with second‐line EGFR‐TKIs, among whom eight (53.3%) received third‐generation EGFR‐TKIs. Apart from one patient that lacked a response status, the ORR of second‐line EGFR‐TKI treatment was 21.4% among the other 14 patients and the DCR was 64.3%. The median PFS of second‐line EGFR‐TKI treatment was 5.6 months (95% CI: 0.3–11.0 months) (Figure 1b).
Time to SCLC transformation and modes of progression
The median time to SCLC transformation was 27.5 months (95% CI: 15.6–39.5 months) (Figure 2a). When including gender, smoking history, EGFR mutation types, EGFR exon 20 T790M mutation status, treatment of third‐generation EGFR‐TKIs, response to initial EGFR‐TKIs and PFS of initial EGFR‐TKIs into univariate Cox proportional hazards regression analysis, only PFS of initial EGFR‐TKIs was associated with the time to SCLC transformation (Figure 2b). Compared to patients with PFS of initial EGFR‐TKIs ≤12 months, patients with PFS of initial EGFR‐TKIs >12 months experienced a significantly longer time to SCLC transformation (>12 vs. ≤12 months, 32.8 vs. 12.5 months; HR, 0.23; 95% CI: 0.09–0.56; p = 0.001) (Figure 2c).
FIGURE 2.
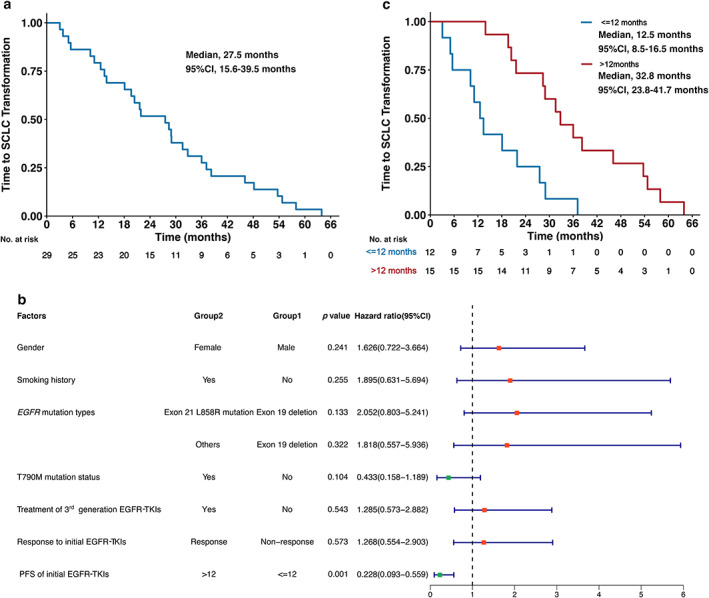
Time to small cell lung cancer (SCLC) transformation and corresponding influential factors. (a) Time to SCLC transformation of all patients. (b) Association between demographic and clinical factors and time to SCLC transformation analyzed by univariate cox proportional hazards regression analysis. (c) Time to SCLC transformation stratified by the PFS of initial EGFR‐TKIs
Detailed medical records or imaging examination results of the last disease evaluation before SCLC transformation in our hospital were available for 26 patients. Among these patients, 22 (84.6%) developed progression in multiple lesions at the point of SCLC transformation, one developed new lesions and only three experienced oligoprogression.
Treatment after SCLC transformation and clinical outcomes
Apart from one patient who died shortly after transformation to SCLC, the other 28 patients all received at least one treatment after transformation (Table 3). The median OS after transformation of these 28 patients was 14.8 months (95% CI: 12.9–16.6 months) (Figure 3a). The median PFS of first‐line treatment after transformation was 4.7 months (95% CI: 2.9–6.5 months) (Figure 3b). Among these 28 patients, 16 patients (57.1%) received chemotherapy for SCLC with EGFR‐TKIs in the first line, while eight patients (28.6%) received chemotherapy for SCLC without EGFR‐TKIs. Among these 24 patients, most (87.5%) were treated with etoposide‐based therapies. Among 16 patients continuing with EGFR TKIs, 12 patients (75.0%) received the same EGFR TKI as before transformation. The ORRs of chemotherapy with or without EGFR‐TKIs were 43.8% and 37.5%, respectively. The median PFS of chemotherapy with EGFR‐TKIs was significantly longer than that of chemotherapy without EGFR‐TKIs (5.2 vs. 3.0 months; HR, 0.19; 95% CI: 0.05–0.72; p = 0.014) (Figure 3c). However, there was no significant difference in median OS between chemotherapy with and without EGFR‐TKIs (14.8 vs. 13.0 months; p = 0.474) (Figure 3d).
TABLE 3.
Treatment after transformation to small cell lung cancer
| Treatment | N | % |
|---|---|---|
| Total patients | 28 | |
| Lines of treatment after transformation | ||
| 1 | 10 | 35.7% |
| 2 | 6 | 21.4% |
| ≥3 | 12 | 42.9% |
| First‐line treatment | ||
| EGFR‐TKI + chemotherapy | 16 | 57.1% |
| Chemotherapy | 8 | 28.6% |
| EGFR‐TKI + chemotherapy + anti‐angiogenesis | 1 | 3.6% |
| Chemotherapy + anti‐angiogenesis | 2 | 7.1% |
| EGFR‐TKI | 1 | 3.6% |
| First‐line chemotherapy | ||
| Etoposide + platinum | 21 | 77.8% |
| Etoposide | 2 | 7.4% |
| Irinotecan + platinum | 1 | 3.7% |
| Nab‐paclitaxel + platinum | 2 | 7.4% |
| Pemetrexed + platinum | 1 | 3.7% |
| Anti‐angiogenic treatment after transformation | ||
| Yes | 18 | 64.3% |
| No | 8 | 28.6% |
| Unknown | 2 | 7.1% |
| Initial line of anti‐angiogenic treatment | ||
| 1 | 6 | 33.3% |
| 2 | 6 | 33.3% |
| ≥3 | 6 | 33.3% |
| Anti‐angiogenic agents | ||
| Anlotinib | 15 | 83.3% |
| Bevacizumab | 6 | 33.3% |
| Apatinib | 2 | 11.1% |
| Regimens involving anti‐angiogenic treatment | ||
| Single‐agent anlotinib | 6 | 33.3% |
| Chemotherapy + anti‐angiogenesis | 5 | 27.8% |
| EGFR‐TKI + anti‐angiogenesis | 4 | 22.2% |
| Chemotherapy + EGFR‐TKI + anti‐angiogenesis | 6 | 33.3% |
| Immunotherapy + anti‐angiogenesis | 4 | 22.2% |
| Immunotherapy + chemotherapy + anti‐angiogenesis | 1 | 5.6% |
| Immunotherapy + EGFR‐TKIs + anti‐angiogenesis | 1 | 5.6% |
| Local radiotherapy | ||
| Yes | 13 | 46.4% |
| No | 15 | 53.6% |
| Reasons for local radiotherapy | ||
| Intracranial progression | 6 | 46.2% |
| Porgression of isolated metastasis | 4 | 30.8% |
| Progression of primary lung lesion | 2 | 15.4% |
| Consolidation radiotherapy of primary lung lesion | 3 | 23.1% |
| Bone metastasis | 3 | 23.1% |
FIGURE 3.
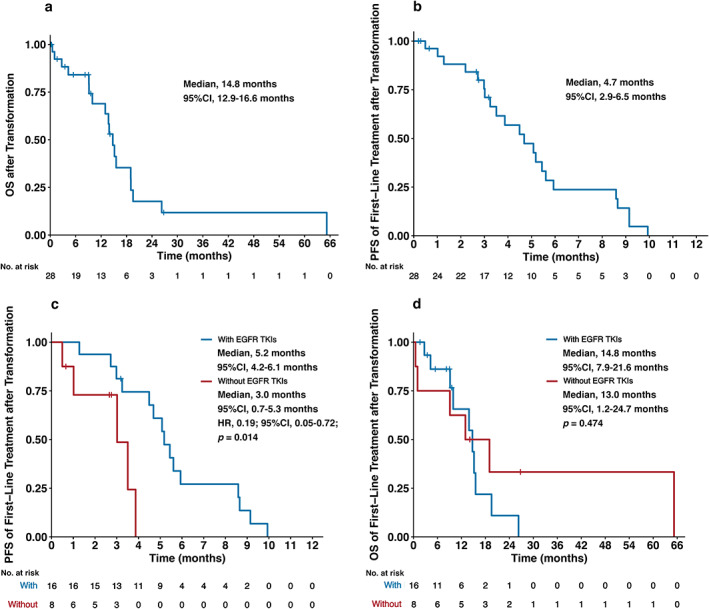
Clinical outcomes of treatment after transformation to small cell lung cancer (SCLC). (a) Overall survival (OS) after transformation to SCLC. (b) Progression‐free survival (PFS) of first‐line treatment after transformation to SCLC. (c) PFS of first‐line treatment after transformation stratified by chemotherapy with or without epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs). (d) OS of first‐line treatment after transformation stratified by chemotherapy with or without EGFR‐TKIs
In the 13 patients receiving molecular tests by NGS after transformation, we were unable to divide them with TP53 mutation status as all patients harbored the TP53 mutation. When dividing patients with Rb1 mutation status, the OS of patients without Rb1 mutation was longer than that of patients with Rb1 mutation, but the difference was not significant (19.5 vs. 9.1 months; p = 0.076).
Among 28 patients receiving treatment after transformation, anti‐angiogenic treatment was confirmed to be used in 18 patients (64.3%) and local radiotherapy was performed in 13 patients (46.4%) (Table 3).
Most patients (66.7%) started anti‐angiogenic treatment in the first‐ or second‐line after transformation. Anlotinib was the agent that used the most (83.3%). Among 18 patients receiving anti‐angiogenic agents, nine patients received anti‐angiogenic agents in more than one regimen. Anti‐angiogenic agents were mostly used in combination with chemotherapy, EGFR‐TKIs or both. Only six patients used single agent anlotinib treatment.
Among 13 patients who received local radiotherapy, five received local radiotherapy of more than one sites. Most patients received local radiotherapy for intracranial progression (46.2%) or progression of isolated distant metastasis (30.8%).
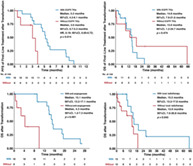
Compared to patients who did not receive anti‐angiogenic therapy, patients treated with anti‐angiogenic therapy experienced significantly longer OS after transformation (anti‐angiogenesis vs. non‐anti‐angiogenesis, 15.1 vs. 4.3 months; p < 0.001) (Figure 4a).
FIGURE 4.
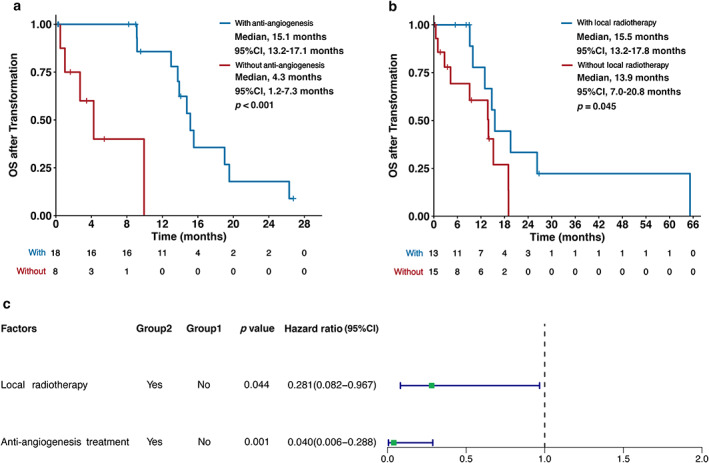
Overall survival (OS) after transformation to small cell lung cancer (SCLC) and corresponding influential factors. (a) OS after transformation stratified by anti‐angiogenic treatment. (b) OS after transformation stratified by local radiotherapy. (c) Association between treatment modes and OS after transformation analyzed by multivariate Cox proportional hazards regression analysis
Compared to patients who did not receive local radiotherapy, patients treated with local radiotherapy also obtained superior OS after transformation (local radiotherapy vs. non‐local radiotherapy, 15.5 vs. 13.9 months; p = 0.045) (Figure 4b).
When including anti‐angiogenic treatment and local radiotherapy into multivariate Cox proportional hazards regression analysis, both anti‐angiogenic treatment (HR, 0.04; 95% CI: 0.01–0.29; p = 0.001) and local radiotherapy (HR, 0.28; 95% CI: 0.08–0.97; p = 0.044) were still significantly associated with better OS after transformation (Figure 4c).
DISCUSSION
To the best of our knowledge, this is the first retrospective study to elaborate on treatment modes after transformation and explore the influential factors of survival outcomes of patients transforming to SCLC from EGFR‐mutant LADC. In addition, we also analyzed the clinical signs that may indicate SCLC transformation.
A previous study has4 reported that EGFR‐mutant patients may respond to a combination of etoposide‐based therapies and EGFR‐TKIs after transforming to SCLC. In our study, we also found that compared to chemotherapy for SCLC alone, a combination of chemotherapy and EGFR‐TKIs could improve the ORR and PFS in the first‐line setting after transformation to SCLC. However, this combination did not benefit those patients in OS. This may indicate that after transformation, even though there may be adenocarcinoma remaining that could benefit from treatment with EGFR‐TKIs, SCLC, the more invasive histological type, probably holds the dominant position. The OS of these patients may mainly depend on the efficacy of treatment for SCLC.
Even though the efficacy of anti‐angiogenic therapies for primary SCLC patients is controversial,16, 17, 18, 19, 20, 21 a previous study has suggested that anlotinib may be a therapeutic option for EGFR‐mutant patients transforming to SCLC, with an ORR of 66.7% and a median PFS of 6.2 months.15 Our study further identified the value of anti‐angiogenic treatment for these patients by revealing that patients treated with anti‐angiogenic therapies after transformation could obtain OS benefit. Most of the patients in our study were also treated with anlotinib. This may partly be because some patients progressed too rapidly before they had the opportunity to receive anti‐angiogenic therapies. However, in our study, most patients started anti‐angiogenic therapy in the first‐ or second‐line after transformation, which indicated that anti‐angiogenic therapies, in particular anlotinib, could be a favorable option for these patients in order to prolong survival.
A previous study demonstrated that for extensive stage SCLC patients responding to chemotherapy, thoracic radiotherapy in addition to prophylactic cranial irradiation could significantly improve 2‐year OS.22 Our study also evaluated the value of local radiotherapy for SCLC transformed from EGFR‐mutant LDAC. We found that patients who received local radiotherapy could have significantly prolonged OS after transformation compared to those who did not. This may be explained by the characteristics of SCLC as SCLC is also highly sensitive to radiotherapy.23 Local radiotherapy is therefore also recommended for EGFR‐mutant LADC patients with SCLC transformation.
Apart from exploring the treatment modes for these patients after transformation, we also attempted to identify the early signs that indicate SCLC transformation during clinical practice. Previous studies have suggested that EGFR/Rb1/TP53‐mutant lung cancers are strikingly at risk of transformation to SCLC,8, 24 and this was also indirectly verified by our study. In our study, among patients in whom NGS tests were performed before transformation, 38.4% (5/13) had concurrent TP53 and Rb1 mutations. This frequency was much higher than the frequency of Rb1/TP53‐mutant LADCs among all LADCs, which was 5% as reported.24, 25 We also found that 84.6% patients developed progression in multiple lesions at the time of transformation to SCLC and patients with PFS of initial EGFR‐TKIs ≤12 months experienced much shorter time to SCLC transformation. All these results suggest that rebiopsy would be needed to check if there was histological transformation for EGFR‐mutant LADC patients with concurrent TP53 and Rb1 mutations, if they experienced poor efficacy during the initial EGFR‐TKI treatment and developed progression in multiple lesions.
There are also some limitations in our study. First, due to its retrospective nature, recall bias may have been introduced into the study. In addition, with the limited number of patients in whom NGS tests were performed in our study, we were unable to explore and analyze further genomic and transcriptomic characteristics of these patients, and this requires further investigation of patient tissue samples. Additionally, even though we found that anti‐angiogenic therapies may bring OS benefit for transformed SCLC patients, the timing of initial anti‐angiogenic treatment after transformation and the influence of different anti‐angiogenic agents may need further prospective studies for confirmation.
In conclusion, compared with chemotherapy alone, the combination of chemotherapy and EGFR‐TKIs in the first‐line treatment after SCLC transformation can benefit patients in PFS but not in OS. Anti‐angiogenic therapies and local radiotherapy can significantly prolong the OS of patients after transformation. Therefore, after transforming to SCLC from EGFR‐mutant LADC, anti‐angiogenic therapies and local radiotherapy should be considered.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Wang S, Xie T, Hao X, Wang Y, Hu X, Wang L, et al. Comprehensive analysis of treatment modes and clinical outcomes of small cell lung cancer transformed from epidermal growth factor receptor mutant lung adenocarcinoma. Thorac Cancer. 2021;12:2585–2593. 10.1111/1759-7714.14144
Shouzheng Wang and Tongji Xie contributed equally and should be considered as co‐first authors.
Junling Li and Puyuan Xing contributed equally and should be considered as co‐corresponding authors.
Contributor Information
Junling Li, Email: lijunling@cicams.ac.cn.
Puyuan Xing, Email: xingpuyuan@cicams.ac.cn.
REFERENCES
- 1.Helena AY, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR‐TKI therapy in 155 patients with EGFR‐mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sequist LV, Waltman BA, Dias‐Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S, He Y, Liu J, Chen X, Yu J, Li W, et al. Third‐generation TKI resistance due to SCLC transformation: a case report and brief review. OncoTargets Ther. 2019;12:11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai L, Meng W, Wei J, Zhang X, Tan Z, Lu Y, et al. Transformation of NSCLC to SCLC after 1st‐and 3rd‐generation EGFR‐TKI resistance and response to EP regimen and erlotinib: 2 CARE‐compliant case reports. Medicine. 2021;100(10):e25046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non‐small‐cell lung cancer to small‐cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16(4):e165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutherland KD, Proost N, Brouns I, Adriaensen D, Song JY, Berns A. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell. 2011;19(6):754–64. [DOI] [PubMed] [Google Scholar]
- 7.Pros E, Saigi M, Alameda D, Gomez‐Mariano G, Martinez‐Delgado B, Alburquerque‐Bejar JJ, et al. Genome‐wide profiling of non‐smoking‐related lung cancer cells reveals common RB1 rearrangements associated with histopathologic transformation in EGFR‐mutant tumors. Ann Oncol. 2020;31(2):274–82. [DOI] [PubMed] [Google Scholar]
- 8.Lee JK, Lee J, Kim S, Kim S, Youk J, Park S, et al. Clonal history and genetic predictors of transformation into small‐cell carcinomas from lung adenocarcinomas. J Clin Oncol. 2017;35(26):3065–74. [DOI] [PubMed] [Google Scholar]
- 9.Yang H, Liu L, Zhou C, Xiong Y, Hu Y, Yang N, et al. The clinicopathologic of pulmonary adenocarcinoma transformation to small cell lung cancer. Medicine. 2019;98(12):e14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn S, Hwang SH, Han J, Choi YL, Lee SH, Ahn JS, et al. Transformation to small cell lung cancer of pulmonary adenocarcinoma: clinicopathologic analysis of six cases. J Pathol Transl Med. 2016;50(4):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roca E, Gurizzan C, Amoroso V, Vermi W, Ferrari V, Berruti A. Outcome of patients with lung adenocarcinoma with transformation to small‐cell lung cancer following tyrosine kinase inhibitors treatment: a systematic review and pooled analysis. Cancer Treatment Rev. 2017;59:117–22. [DOI] [PubMed] [Google Scholar]
- 12.Leonetti A, Minari R, Mazzaschi G, Gnetti L, La Monica S, Alfieri R, et al. Small cell lung cancer transformation as a resistance mechanism to osimertinib in epidermal growth factor receptor‐mutated lung adenocarcinoma: case report and literature review. Front Oncol. 2021;11:642190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mooradian MJ, Piotrowska Z, Drapkin BJ, Dias‐Santagata D, Marcoux N, Arnaoutakis K, et al. Clonal evolution and the role of serial liquid biopsies in a case of small‐cell lung cancer–transformed EGFR mutant non–small‐cell lung cancer. JCO Precis Oncol. 2017;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcoux N, Gettinger SN, O'Kane G, Arbour KC, Neal JW, Husain H, et al. EGFR‐mutant adenocarcinomas that transform to small‐cell lung cancer and other neuroendocrine carcinomas: clinical outcomes. J Clin Oncol. 2019;37(4):278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Xu C, Chen H, Jia J, Wang L, Feng H, et al. Genomic alterations and clinical outcomes in patients with lung adenocarcinoma with transformation to small cell lung cancer after treatment with EGFR tyrosine kinase inhibitors: a multicenter retrospective study. Lung Cancer. 2021;155:20–7. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Y, Wang Q, Li K, Shi J, Liu Y, Wu L, et al. Anlotinib vs placebo as third‐or further‐line treatment for patients with small cell lung cancer: a randomised, double‐blind, placebo‐controlled Phase 2 study. Br J Cancer. 2021;18:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horn L, Dahlberg SE, Sandler AB, Dowlati A, Moore DF, Murren JR, et al. Phase II study of cisplatin plus etoposide and bevacizumab for previously untreated, extensive‐stage small‐cell lung cancer: Eastern Cooperative Oncology Group Study E3501. J Clin Oncol. 2009;27(35):6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jalal S, Bedano P, Einhorn L, Bhatia S, Ansari R, Bechar N, et al. Paclitaxel plus bevacizumab in patients with chemosensitive relapsed small cell lung cancer: a safety, feasibility, and efficacy study from the Hoosier Oncology Group. J Thorac Oncol. 2010;5(12):2008–11. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Zeng J, Jin X, Yu X, Zhou G, Hong W. Apatinib for chemotherapy‐refractory extensive‐stage SCLC: a retrospective study. Cancer Chemother Pharmacol. 2019;83(6):1083–1090. [DOI] [PubMed] [Google Scholar]
- 20.Ready NE, Dudek AZ, Pang HH, Hodgson LD, Graziano SL, Green MR, et al. Cisplatin, irinotecan, and bevacizumab for untreated extensive‐stage small‐cell lung cancer: CALGB 30306, a phase II study. J Clin Oncol. 2011;29(33):4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spigel DR, Townley PM, Waterhouse DM, Fang L, Adiguzel I, Huang JE, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive‐stage small‐cell lung cancer: results from the SALUTE trial. J Clin Oncol. 2011;29(16):2215–22. [DOI] [PubMed] [Google Scholar]
- 22.Slotman BJ, van Tinteren H, Praag JO, Knegjens JL, El Sharouni SY, Hatton M, et al. Use of thoracic radiotherapy for extensive stage small‐cell lung cancer: a phase 3 randomised controlled trial. Lancet. 2015;385(9962):36–42. [DOI] [PubMed] [Google Scholar]
- 23.Jett JR, Schild SE, Kesler KA, Kalemkerian GP. Treatment of small cell lung cancer: diagnosis and management of lung cancer: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2013;143(5):e400S–19S. [DOI] [PubMed] [Google Scholar]
- 24.Offin M, Chan JM, Tenet M, Rizvi HA, Shen R, Riely GJ, et al. Concurrent RB1 and TP53 alterations define a subset of EGFR‐mutant lung cancers at risk for histologic transformation and inferior clinical outcomes. J Thorac Oncol. 2019;14(10):1784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collisson EA, Campbell J, Brooks A, Berger A, Lee W, Chmielecki J, et al. Comprehensive molecular profiling of lung adenocarcinoma: the cancer genome atlas research network. Nature. 2014;511(7511):543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


