Abstract
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX) are enzymes that generate superoxide or hydrogen peroxide from molecular oxygen utilizing NADPH as an electron donor. There are seven enzymes in the NOX family: NOX1-5 and dual oxidase (DUOX) 1–2. NOX enzymes in humans play important roles in diverse biological functions and vary in expression from tissue to tissue. Importantly, NOX2 is involved in regulating many aspects of innate and adaptive immunity, including regulation of type I interferons, the inflammasome, phagocytosis, antigen processing and presentation, and cell signaling. DUOX1 and DUOX2 play important roles in innate immune defenses at epithelial barriers. This review discusses the role of NOX enzymes in normal physiological processes as well as in disease. NOX enzymes are important in autoimmune diseases like type 1 diabetes and have also been implicated in acute lung injury caused by infection with SARS-CoV-2. Targeting NOX enzymes directly or through scavenging free radicals may be useful therapies for autoimmunity and acute lung injury where oxidative stress contributes to pathology.
Keywords: NADPH Oxidase, NOX, Superoxide, Immunity, Autoimmunity, COVID-19, Acute lung injury
Abbreviations
- BCR
B Cell Receptor
- CGD
Chronic Granulomatous Disease
- COVID-19
Coronavirus Disease 2019
- DC
Dendritic Cell
- DPI
Diphenyleneiodonium
- DUOX
Dual Oxidase
- EGF
Epidermal Growth Factor
- EGFR
Epidermal Growth Factor Receptor
- ER
Endoplasmic Reticulum
- FAD
Flavin Adenine Dinucleotide
- fMLP
N-Formyl-Methionine-Leucyl-Phenylalanine
- G-MDSC
Granulocytic Myeloid-Derived Suppressor Cells
- G6PD
Glucose-6-phosphate dehydrogenase
- GILT
γ-Interferon-induced Lysosomal Thiol reductase
- IFN
Interferon
- IRF3
Interferon Regulatory Factor 3
- ISG
Interferon-Stimulated Gene
- MAVS
Mitochondrial Antiviral Signaling
- MPO
Myeloperoxidase
- NADH
Nicotinamide Adenine Dinucleotide
- NADPH
Nicotinamide Adenine Dinucleotide Phosphate
- NET
Neutrophil Extracellular Trap
- NLRP1
Nucleotide-binding oligomerization domain, Leucine rich Repeat, and Pyrin domain containing protein 1
- NLRP3
Nucleotide-binding oligomerization domain, Leucine rich Repeat, and Pyrin domain containing protein 3
- NOX
NADPH Oxidase
- PB1
Phox and Bem1
- Phox
Phagocytic Oxidase
- PKC
Protein Kinase C
- PMA
Phorbol 12-Myristate 13-Acetate
- PRR
Proline-Rich Region
- PTP1B
Protein-Tyrosine Phosphatase 1B
- PVPON
Poly(N-Vinylpyrrolidone)
- RA
Rheumatoid Arthritis
- ROS
Reactive Oxygen Species
- SARS
Severe Acute Respiratory Syndrome
- SLE
Systemic Lupus Erythematosus
- SOD
Superoxide Dismutase
- TCR
T Cell Receptor
- TLR
Toll-Like Receptor
- TNF
Tumor Necrosis Factor
- TPR
Tetratricopeptide Repeat
- VEGF
Vascular Endothelial Growth Factor
- VEGFR
Vascular Endothelial Growth Factor Receptor
- XOR
Xanthine Oxidoreductase
1. Introduction
Reactive oxygen species (ROS) play an important role in multiple cellular processes including metabolism, signaling, and immunity. Cellular ROS are commonly generated from superoxide which is derived from two main sources: the mitochondria via oxidative phosphorylation and through NADPH oxidase (NOX) enzymes [1]. Enzymes in the NADPH oxidase family produce superoxide during normal cellular processes, but also produce superoxide as part of a respiratory burst during phagocytosis [2]. Production of superoxide is a critical cellular process that is required for the generation of other ROS such as peroxynitrite, hydrogen peroxide, hypochlorite, and hydroxyl radicals (Fig. 1). Generation of ROS is necessary for a variety of cellular functions, which are impaired in the absence of superoxide [2]. This review will discuss the importance of NOX enzymes and related proteins in immunity to pathogens, autoimmunity, and inflammation.
Fig. 1.
Reactive oxygen species generated from NADPH oxidase-derived superoxide. NADPH oxidase enzymes convert molecular oxygen into superoxide anion (O2•−) using NADPH as an electron donor. Superoxide dismutase enzymes dismutate superoxide into hydrogen peroxide (H2O2), which can be converted into hydroxyl radicals (HO•) through the reduction of ferrous iron (Fe2+) to ferric iron (Fe3+) or hypochlorite (ClO−) by myeloperoxidase. Nitric oxide synthase using electrons from NADPH to oxidize arginine to produce citrulline and nitric oxide (NO). Nitric oxide (NO) reacts with superoxide anion (O2•−) to produce peroxynitrite (ONOO−).
1.1. Discovery of NOX enzymes
NOX enzymes were first discovered as the missing component in phagocytic cells like neutrophils in patients with chronic granulomatous disease (CGD) [3]. CGD is caused by any mutations that lead to deficiency in NOX2 activity [4]. CGD patients have an increased susceptibility to certain bacterial and fungal infections and often present with granulomas, not due to an obvious infection, which is where the name of the disorder is derived. Autoimmune diseases like systemic lupus erythematous (SLE) and rheumatoid arthritis (RA) are more common in patients with CGD and mouse models of NOX2 deficiency [5,6]. However, the cause of these aberrant immune responses is not completely understood [4,7].
It has long been known that ROS play an important role in diverse biological processes [8] and that ROS such as superoxide and hydrogen peroxide were produced in phagocytic leukocytes during phagocytosis [[9], [10], [11]]. The production of ROS during phagocytosis was proposed to be microbicidal [9], and it was later determined that this activity was dependent on NADH and NADPH oxidation [12,13]. Segal and colleagues determined that this respiratory burst was independent of mitochondrial-derived superoxide using spectroscopic analysis, which revealed a cytochrome b-like molecule that was present in fractionated phagosomes and separate from mitochondrial cytochrome b and endoplasmic reticulum (ER)-associated cytochrome P450 [14]. They also found that this cytochrome b peak was missing in patients with CGD [3]. The cytochrome b proteins of 91 and 22 kDa were biochemically isolated from granulocyte plasma membranes [15]. The genes coding for the 91 and 22 kDa proteins were mapped to the X chromosome and chromosome 16, respectively, and their gene products were subsequently cloned and characterized [[16], [17], [18], [19]]. The 91 kDa protein, also known as gp91phox or NOX2, is encoded by the CYBB gene (Fig. 2A). The 22 kDa or light chain of the cytochrome complex, also known as p22phox, is encoded by the CYBA gene. Since this initial discovery, there have been a total of five NOX enzymes and two dual oxidase (DUOX) enzymes discovered (Fig. 2A) with conserved features.
Fig. 2.
Protein domains of human NADPH oxidase enzymes 1–5 and dual oxidase enzymes 1–2. (A) Conserved domains of human NADPH oxidase enzymes. (B) Amino acid sequences of the conserved FAD binding site in human NADPH oxidase enzymes. (C) Amino acid sequences of the conserved NADPH binding region in human NADPH oxidase enzymes. A “*” indicates residues that are fully conserved, a “:” indicates residues that are strongly conserved, and a “.” indicates residues that are weakly conserved. The consensus sequence is in bold.
1.2. NOX enzyme complexes generate superoxide anion
The NOX enzyme complexes are so named because they utilize NADPH as an electron donor to generate superoxide from molecular oxygen [12,13]. The five NOX enzymes (NOX1-5) and two DUOX enzymes (DUOX1-2) each have six conserved transmembrane domains and a conserved C-terminal domain with FAD and NADPH binding sites (Fig. 2). The main catalytic units of NOX1-4 must form a dimer with the Superoxide-Generating NADPH Oxidase Light Chain Subunit (CYBA) for catalytic activity [20]. The activation of NOX1-3 also requires the activity of cytosolic factors for activation. DUOX1 and DUOX2 have an additional transmembrane domain called the peroxidase-like domain (Fig. 2A). NOX5, DUOX1, and DUOX2 also have EF hand domains that are involved in calcium signaling (Fig. 2A). After activation, the enzyme complex utilizes NADPH as an electron donor to convert molecular oxygen to superoxide (Eq. (1)).
| NADPH + 2O2 ↔ NADP+ + 2O2•− + H+ | (1) |
Superoxide can also be generated by xanthine oxidase activity of Xanthine Oxidoreductase (XOR) enzymes [21]. XOR is primarily localized to the cytoplasm, but can also be found in the peroxisomes and secreted extracellularly [22,23]. XOR-derived superoxide plays an important role in many physiological processes, which have recently been reviewed in Ref. [21], including commensal microbiome regulation, blood pressure regulation, and immunity. XOR- and NOX-derived superoxide can work cooperatively to maintain superoxide levels. For example, in response to sheer stress, endothelial cells produce superoxide through NOX and XOR pathways and XOR expression and activity is dependent on NOX activity [24]. While this review will focus on NOX-derived superoxide it is important to recognize the contribution of XOR-derived superoxide in physiological processes and disease.
After the generation of superoxide, other ROS can be generated. Peroxynitrite (ONOO−) is formed after superoxide reacts with nitric oxide (NO) [25]. Nitric oxide is a product of arginine metabolism by nitric oxide synthase which uses arginine as a nitrogen donor and NADPH as an electron donor to produce citrulline and NO [26,27]. Superoxide can also be converted to hydrogen peroxide by the superoxide dismutase enzymes (SOD), which are critical for maintaining the balance of ROS inside the cells (Fig. 1). There are three superoxide dismutase enzymes, SOD1, SOD2, and SOD3. SOD1 is primarily cytosolic and utilizes Cu2+ and Zn2+ ions to dismutate superoxide (Eq. (2)). SOD2 is localized to the mitochondria and utilizes Mn2+ to bind to superoxide products of oxidative phosphorylation and converts them to H2O2 (Eq. (2)). SOD3 is extracellular and generates H2O2 that can diffuse into cells through aquaporins [28,29].
| 2O2•− + 2H3O+ → O2 + H2O2 + 2H2O | (2) |
Following the generation of hydrogen peroxide by SOD enzymes, other ROS can be generated (Fig. 1). The enzyme myeloperoxidase (MPO) is responsible for hypochlorite (ClO−) formation by utilizing hydrogen peroxide as an oxygen donor and combining it with a chloride ion [30]. A spontaneous Fenton reaction with hydrogen peroxide and ferrous iron (Fe2+) results in the production of hydroxyl radicals (HO•) [31]. The specific role that each of these ROS play in cellular processes is beyond the scope of this review, but their dependence on superoxide generation highlights the key role of NOX enzymes in a variety of cellular processes.
2. Phagocytic NADPH oxidase 2 complex
The NOX2 complex is the prototypical and best-studied NOX enzyme complex. The NOX2 complex is comprised of two transmembrane proteins encoded by the CYBB and CYBA genes. The CYBB gene, located on the X chromosome, encodes for the cytochrome b-245 beta chain subunit also known as gp91phox [18]. The gp91phox heavy chain is initially translated in the ER where mannose side chains are co-translationally added to form a 65 kDa precursor glycoprotein [[32], [33], [34]]. The 65 kDa precursor is further glycosylated in the Golgi network to become the mature 91 kDa glycoprotein [15].
The CYBA gene, located on chromosome 16, encodes for the Superoxide-Generating NADPH Oxidase Light Chain Subunit also known as p22phox [35,36]. The p22phox protein has two transmembrane domains with an extracellular loop and its C-terminal and N-terminal ends on the cytosolic side of the membrane [37]. Mutations in p22phox that affect its function were discovered early on to be one cause of CGD [38]. p22phox has no catalytic activity by itself; however, it is necessary for stabilization of the heterodimer complex with gp91phox [37].
In the absence of p22phox, gp91phox is not able to exit the ER and move to the Golgi network and is degraded by the proteosome [39]. Therefore, individuals deficient for p22phox will also be deficient for gp91phox in phagocytic cells [18,40]. After gp91phox and p22phox are completely translated and gp91phox has been glycosylated into its final 91 kDa form, the heterodimer then moves to the plasma membrane. NOX2 enzyme complexes are also found on the phagosome membranes, secretory vesicles, and peroxidase-negative granules in neutrophils [41]. The C-terminal end of p22phox has a proline-rich region (PRR) that is important for binding of the cytosolic components of the enzyme complex [42].
2.1. NOX2 activation
The membrane-bound NOX2 components, gp91phox and p22phox, require the activity of the activator protein p47phox, the organizer protein p67phox, and the regulatory protein p40phox, which exist in a ternary complex in the cytoplasm [43]. Experiments attempting to create a cell-free NADPH oxidase system demonstrated that membrane components alone were insufficient to reconstitute NADPH oxidase activity and that some unknown cytosolic components were also required [[44], [45], [46], [47]]. The requirement for these cytosolic factors was confirmed in experiments where complementation with cytoplasmic fractions from phagocytic cells from certain patients with autosomal recessive forms of CGD could not restore oxidase activity [48,49].
NOX2 activation and assembly begins with a signaling event after stimulation of formylated peptide receptors, C5a receptor, Fc receptors, or stimulation through a pattern recognition receptor such as toll-like receptor 4 (TLR4) [[50], [51], [52], [53], [54]]. Stimulation with chemicals like concanavalin A or phorbol 12-myristate 13-acetate (PMA) also induces activation of NOX2 [52,55]. What these various signaling pathways have in common is the activation of protein kinase C (PKC) [56]. PKC begins the activation and assembly process by phosphorylating p47phox at serine residues 310 and 328 which causes a conformational change in p47phox that results in its activation and recruitment to the NOX2 complex on the plasma membrane [50]. p47phox recruits the other cytosolic components to the plasma membrane through multiple protein-protein interactions that are detailed in section 2.2.
2.2. Assembly of NOX2 complex
p47phox, the protein encoded by the NCF1 gene, was first purified from cellular fractions that produced a 47 kDa protein that was necessary to reconstitute a cell-free NADPH oxidase system [57,58]. The NCF1 gene was cloned and characterized a year later by two independent groups [59,60]. The NCF1 gene encodes for a 390 amino acid protein (Fig. 3A) that contains a Phox homology (PX) domain at its N-terminus that allows for p47phox to anchor to the plasma membrane through phosphatidylinositol 3,4-bisphosphate (PI(3,4)P2) binding [[61], [62], [63]]. p47phox also has two SH3 domains and a PRR that are required for protein-protein interactions with other members of the NADPH oxidase complex.
Fig. 3.
Protein domains of the NADPH oxidase-associated cytosolic proteins. (A) Protein domains of the organizing proteins p47phox and NOXO1. (B) Protein domains of the activating proteins p67phox and NOXA1. (C) Protein domains of the regulatory protein p40phox.
p47phox plays an important role in mediating protein-protein interactions required for activation and function of the NOX2 complex. p47phox binds directly to gp91phox and p22phox and also recruits p67phox to the plasma membrane to interact with the NOX2 enzyme complex. In its inactive state, the SH3 domains of p47phox are occluded by intramolecular interactions with the C-terminus of p47phox, an interaction that is undone by activators of oxidase activity [60,64,65]. After activation, p47phox is recruited to the membrane by p22phox via interactions between the SH3 domains of p47phox and the PRR of p22phox. This interaction is dependent on Ile152, Thr153, and Trp193 in p47phox and Pro152, Pro153, Pro156, and Arg158 in p22phox [60,64,66,67]. Indeed, patients with a Pro156Glu mutation on p22phox are unable to recruit p47phox and p67phox and are deficient in superoxide activity [60,68,69]. p47phox also binds to membrane-bound gp91phox with both of its SH3 domains required for this interaction with gp91phox [70]. Patients with an Asp500Gly mutation in gp91phox are unable to recruit p47phox to the membrane and are deficient in superoxide production [70]. p47phox is also responsible for recruiting p67phox to the NADPH oxidase complex on the membrane through interactions between the PRR of p47phox and the C-terminal SH3 on p67phox [65,68] as well as the interactions between the C-terminal SH3 domain of p47phox with the PRR of p67phox [71]. The binding of p47phox and p67phox is regulated by p40phox [38,72].
The p67phox protein, encoded by the NCF2 gene, was first purified as part of a cytoplasmic complex capable of complementing an inactive membrane-bound oxidase complex [73,74]. The NCF2 gene was subsequently cloned [[75], [76], [77]], and it was discovered that several mutations in this gene were also associated with CGD [78,79]. The NCF2 gene encodes for a 526 amino acid protein that has four tetratricopeptide repeat (TPR) motifs, two SH3 domains, and a Phox and Bem1 (PB1) domain (Fig. 3B). p67phox has two important roles in NOX2 enzyme activation: it recruits the Rac-GTP (RAC1 or RAC2) to the enzyme complex and it is responsible for electron transfer from NADPH to gp91phox [41].
p67phox is recruited to the membrane to interact with the NOX2 complex by p47phox. There are two main interactions between p47phox and p67phox. The first interaction is between the C-terminal SH3 domain of p67phox binding to the PRR of p47phox in a reverse orientation. This interaction is dependent on Asp16 in the C-terminal SH3 domain of p67phox [65,68,80] The second interaction is between the C-terminal SH3 domain of p47phox which directly binds to p67phox at its PRR that is on the N-terminal side of the SH3 domains [64]. The SH3 domains of p67phox do not bind to the PRR of p22phox, so p67phox must be recruited by p47phox and cannot directly interact with gp91phox and p22phox [81,82]. The two SH3 domains of p67phox are dispensable for oxidase activity in a cell-free system but are required in whole cells for superoxide production [60,79,80,83,84].
After p67phox is recruited to the membrane-bound components of the NOX2 enzyme complex, it is directly involved in the activation of the NOX enzyme complex. p67phox recruits the GTPase RAC2 through interactions with the TPR motifs on the N-terminal end of p67phox [85,86]. The Rac GTPase assembly with the NOX2 complex is absolutely required for its activity [87]. Ultimately, the activation domain of p67phox interacts with gp91phox and allows for the transfer of electrons from NADPH to the flavin center of gp91phox [88,89].
The third NADPH oxidase-associated factor is p40phox, which is encoded by the NCF4 gene. p40phox was first identified by Wientjes et al. (1993) and was shown to have an SH3 domain and an N-terminal domain with sequence similarity to the N-terminal domain of p47phox [81]. Like p67phox, p40phox also has a PB1 domain (Fig. 3C), which mediates its association with p67phox in the inactive cytoplasmic ternary complex [81,90,91]. The p40phox PB1 domain heterodimerizes with the PB1 domain of p67phox, an interaction that can be blocked with an antibody that binds the PB1 domain of p40phox [[92], [93], [94], [95]]. The SH3 domain on p40phox is not required for binding to p67phox and when p67phox is absent in patients with CGD, p40phox and Rac1 are not translocated from the cytosol to the membrane [68,91,96]. The PX domain from p40phox binds to phosphatidylinositol 3-phosphate found on phagosomal membranes [[97], [98], [99], [100], [101], [102]].
The exact role p40phox plays in the activation of the NOX2 enzyme complex is not entirely clear. p40phox is phosphorylated upon activation of NADPH oxidase by fMLP or PMA at amino acids Thr154 and Ser315 [103,104]. After activation, p40phox translocates to the membrane and disassociates from p67phox and p47phox [105]. p40phox has been shown to be a positive regulator of NOX2 activity [106,107]. However, it has also been proposed that p40phox negatively regulates NOX2 activity through its SH3 domain [108]. There is evidence that the SH3 domain of p40phox binds to the C-terminal PRR of p47phox at the same site as p67phox, thus preventing p67phox binding through competition [71].
3. Other NADPH oxidase family large transmembrane catalytic subunits
3.1. NADPH Oxidase 1 (NOX1)
This homologue of gp91phox was first cloned and characterized in 1999 by Suh et al. who demonstrated that it was highly expressed in the colon, but not in leukocytes [109,110]. Activation of NOX1, like that of NOX2, involves homologues of p47phox and p67phox known as NOX organizer 1 (NOXO1) and NOX activator 1 (NOXA1) [111,112]. NOXO1 has homologous SH3 and PX domains to those found in p47phox as well as the conserved PRR (Fig. 3A). NOXA1 also has protein domains homologous to those found in p67phox such as TPR, SH3, and PB1 domains (Fig. 3B). After an activating stimulus like PMA is administered to cells, NOXO1 is phosphorylated at Ser154 which is required for assembly with NOXA1 and subsequent interactions with p22phox [113]. Activation of the NOX1 complex also requires a Rac1 GTPase which is associated with NOXA1 [[114], [115], [116]]. Like NOX2, NOX1 must form a heterodimer with p22phox for activation and superoxide production [117].
Unlike NOX2, NOX1 is not expressed in immune cells, but still plays a role in immunity. NOX1 is primarily expressed in colon epithelial cells and is important for host defense, barrier function, and homeostasis of commensal bacteria [20]. Crosstalk between the commensal bacteria in the colon and NOX1 is important for epithelial homeostasis. Stimulation of formyl peptide receptors on epithelial cells by bacteria stimulates NOX1-dependent ROS production which promotes barrier maintenance through epithelial growth and repair [118,119]. Conversely, production of hydrogen peroxide from NOX1-derived superoxide helps to prevent overgrowth of commensal bacteria [120]. Interestingly, there are catalase-producing commensals like Escherichia coli as well as pathogenic bacteria like Citrobacter rodentium that can utilize NOX1-derived hydrogen peroxide to support cellular respiration in an otherwise anaerobic environment [121,122].
NOX1 has also been implicated in colon cancer due to its role in regulating cell proliferation and angiogenesis in the colonic epithelium [110,123,124]. Expression of NOX1 is regulated by the transcription factors GATA-6, HNF-1α, and CDX2. Expression of these transcription factors is higher in the distal colon than the proximal colon and correlates with NOX1 expression [125]. NOX1 is overexpressed in many epithelial and colon-related cancers as a direct result of k-Ras mutations that result in increased MEK/ERK signaling and activation of GATA-6 [126,127]. NOX1 overexpression in fibroblasts can promote tumorigenesis and angiogenesis through upregulation of VEGF and the VEGF receptors, VEGFR1 and VEGFR2 [124,127]. A novel inhibitor of NOX1, GKT771 has shown efficacy as a complementary treatment to anti-PD1 checkpoint inhibitor therapy in pre-clinical trials in mouse models of colon cancer [128].
3.2. NADPH Oxidase 3 (NOX3)
NADPH Oxidase 3 was identified as a protein with homology to NOX2 located on chromosome 6 [129]. NOX3 is expressed in fetal tissues, but has limited expression in adult tissues and is limited to the colon, testis, and inner ear [129,130]. Stimulation of cells with the PKC activator, PMA, leads to activation of NOX3 via p47phox and p67phox [131]. However, NOX3 also has activity in the absence of PKC stimulation through NOXO1 activity [132,133]. The PMA-independent activation of NOX3 is constitutive due to the interaction of NOX3 with p22phox [132]. Unlike NOX1 and NOX2, the constitutive activity of NOX3 does not require an activating or organizing protein [132]. However, when the activating or organizing proteins are present and activated, NOX3 activity is enhanced [132].
NOX3 is not known to play a role in immune cells or host defense. However, NOX3 activity is involved in the vestibular system in the inner ear [134]. Defects in NOX3 can result in a head-tilt in mice due to otoconia morphogenesis defects [130]. NOX3-derived superoxide has also been implicated in noise-induced and cisplatin-induced hearing loss [135]. NOX3 expression was shown to increase with cisplatin treatment, age, and noise insults in mice, which correlated to hearing loss [136]. It has been proposed that therapies targeting NOX3 in the inner ear could be used to prevent NOX3-induced hearing loss [135]. Proposed therapies include NOX3-specific siRNA delivery and small molecule inhibitors [[137], [138], [139]]. This would be beneficial as a preventative measure for patients undergoing cisplatin treatment for solid tumors.
NOX3 can also be activated in hepatocytes in response to insulin, which leads to the production of VEGF and the initiation of angiogenesis [140]. Hepatocytes stimulated with palmitate also produce ROS through NOX3, which leads to increased gluconeogenesis and reduced glycogen content [141]. It is thought that this may contribute to insulin resistance in obesity [141,142]. The mechanism has been revealed to be due to increased TNF production that stimulates hepatocytes through the JNK and p38MAPK pathways [129,143,144].
3.3. NADPH Oxidase 4 (NOX4)
NADPH Oxidase 4 was first characterized as a NOX enzyme that is expressed in the kidney with homology to NOX2 [145,146]. NOX4 is also unique compared to the previously discovered NOX enzymes in that it does not require association or activity from cytosolic factors for activation and organization like NOX1, NOX2, and NOX3 [145,[147], [148], [149], [150], [151]]. NOX4 has been associated with constitutive production of hydrogen peroxide rather than superoxide production [148,152]. It has been shown that when the extracellular loop between transmembrane domains five and six (E-loop) of NOX4 is deleted that NOX4 does in fact generate superoxide, which suggests that the E-loop may have dismutase activity that converts superoxide to hydrogen peroxide before it can be detected by current methods [143,148].
NOX4 was first discovered in the kidney, but is also highly expressed in pulmonary vasculature and endothelial cells and plays an important role in respiratory diseases such as pulmonary fibrosis, asthma, chronic obstructive pulmonary disease, pulmonary vascular diseases, and acute respiratory distress syndrome [153]. NOX4 has also been shown to be expressed in Jurkat T cells. Infection of Jurkat T cells with Entameoba histolytica was shown to induce cell death which was abrogated with siRNA knockdown of NOX4 [154]. However, this has not been shown in primary T cells. NOX4 expression is regulated by several different stimuli including oxygen levels [[155], [156], [157], [158]]. NOX4 expression is also stimulated by angiotensin II, glucose levels, hypoxia, or hyperoxia [[159], [160], [161], [162], [163], [164], [165], [166]]. This change in expression is driven by important transcription factors such as STAT1/STAT3, NRF2, HIF-1α, NFκB, Oct-1, SP3, SP1, c-JUN, and E2F [129,167].
3.4. NADPH Oxidase 5 (NOX5)
NADPH Oxidase 5 has an EF-Hand domain (calcium-binding) and is highly expressed in the adult testis, spleen, ovary, placenta, and pancreas and the fetal brain, heart, kidney, liver, lung, skeletal muscle, spleen, and thymus [129]. NOX5 is expressed at lower levels in the adult brain, heart, kidney, liver, lung, prostate, and small intestine [167]. NOX5 is responsible for ROS generation in human sperm [168]. Interestingly, NOX5 is not expressed universally in all mammalian species and is absent in rodents, which makes animal models for studying NOX5 difficult [167]. Unlike its homologues NOX1-4, NOX5 does not require an activating and organizing protein like p47phox or p67phox for activation and can be activated by calcium flux alone [117,169]. Knockout of p22phox or the introduction of mutations in p22phox that abrogate NOX1, NOX2, NOX3, or NOX4 activity does not affect NOX5 activity [170]. Activity of NOX5 is dependent on oligomerization of multiple NOX5 proteins, which bind to each other through the dehydrogenase domain [171]. Binding of phosphatidylinositol (4,5)-bisphosphate directs NOX5 to localize at the plasma membrane through interaction with the N-terminal polybasic region [172].
NOX5 can be activated by two different mechanisms: intracellular calcium flux and protein kinase C activation. The C-terminus of NOX5 contains a calmodulin-binding site that increases the sensitivity of NOX5 to calcium-mediated activation [173]. The binding of calcium to the EF-hand domains induces a conformational change in NOX5 which leads to its activation when intracellular calcium levels are high [174]. However, it has been noted that the calcium concentration needed for activation of NOX5 is extremely high and not likely physiological [175] and low levels of calcium-binding to NOX5 can work synergistically with PKC stimulation [176]. It has also been shown that in the presence of ROS that NOX5 is oxidized at cysteine and methionine residues in the Ca2+ binding domain thus inactivating NOX5 through a negative feedback mechanism [177,178]. NOX5 can also be activated by PKC-α stimulation [175] after phosphorylation of Thr512 and Ser516 on NOX5α [16,179].
3.5. Dual Oxidase 1/2 (DUOX1/2)
Two additional proteins with homology to NOX enzymes were discovered in the thyroid. These enzymes were called dual oxidase enzymes 1 and 2 (DUOX1 and DUOX2). Like NOX1-5, these enzymes have six transmembrane domains with a C-terminal domain containing an FAD and NADPH binding site. These enzymes can also convert molecular oxygen to hydrogen peroxide. However, DUOX1 and DUOX2 are more closely related to NOX5 due to the presence of calcium-regulated EF hand domains. DUOX-mediated hydrogen peroxide synthesis is induced transiently after calcium stimulation of epithelial cells [180]. Unlike NOX5, DUOX1 and DUOX2 have an additional transmembrane domain called the peroxidase-homology domain on its N-terminus. DUOX1 and DUOX2 require maturation factor proteins DUOXA1 and DUOXA2, respectively, in order to transition out of the ER to the Golgi [181].
The DUOX enzymes have roles in immune and non-immune physiological processes. DUOX1 and DUOX2 are both expressed in the thyroid gland and are involved in thyroid hormone synthesis. DUOX-derived hydrogen peroxide is utilized by thyroid peroxidase enzymes for the oxidation of iodide [182]. Nonsense and missense mutations in DUOX2 have been shown to result in hypothyroidism [183,184]. No mutations in the DUOX1 gene have been linked to hypothyroidism so it is unclear whether DUOX1 is required for thyroid hormone biosynthesis or whether it acts as a redundant mechanism for defective DUOX2 [185]. DUOX1 has been detected in bladder epithelial cells where it is thought to function in the sensing of bladder stretch [186]. DUOX enzymes have also been shown to be important for collagen crosslinking in the extracellular matrix in C. elegans [187].
DUOX1 is involved in immune cells like macrophages, T cells, and B cells. DUOX1 is expressed in alveolar macrophages where it is important for modulating phagocytic activity and cytokine secretion [188]. T cell receptor (TCR) signaling in CD4+ T cells induces expression of DUOX1 which promotes a positive feedback loop for TCR signaling. After TCR signaling, DUOX1-derived hydrogen peroxide inactivates SHP2, which promotes the phosphorylation of ZAP-70 and its subsequent association with LCK and the CD3ζ chain. Knockdown of DUOX1 in CD4+ T cells results in reduced phosphorylation of ZAP-70, activation of ERK1/2, and release of store-dependent calcium [189]. DUOX1 may also play a role in B cell receptor (BCR) signaling. DUOX1 expression is induced by BCR signaling in the presence of IL-4. One study showed that DUOX1-derived hydrogen peroxide negatively regulates B cell proliferation [190]. However, a second study, which used a DUOX1-and DUOX2-deficient mouse, showed that the DUOX enzymes were dispensable for BCR signaling [191]. Further work is necessary to fully understand the role of DUOX1 and DUOX2 in B cells.
More recently it has been appreciated that DUOX enzymes also play important roles in epithelial cells in the airway and gut. DUOX1 is expressed in epithelial cells in the trachea and bronchi and is associated with EGFR signaling after stimulation of TLRs to promote epithelial homeostasis and repair in response to microbial ligands [[192], [193], [194]]. DUOX2 is also expressed in the airway epithelium and is important for host antiviral (see section 4.3) and antibacterial immunity [[195], [196], [197]].
DUOX2 is also expressed in the tip of epithelial cells in the ileum and colon [198]. Expression of DUOX2 is stimulated by the microbiota through TLRs mediated by MyD88 and TRIF signaling pathways [198]. The role of DUOX in antibacterial host defense has been shown in several animal models including Drosophila, C. elegans, zebrafish, and mice, which require DUOX enzymes for protection from bacterial insults [[199], [200], [201], [202]]. In mice, DUOX-deficient mice were able to be colonized by H. felis, whereas control mice with intact DUOX were not [202].
4. NOX enzymes in immunity
4.1. Phagocytosis and pathogen clearance
NOX2-derived ROS play an important role in pathogen killing in neutrophils and macrophages (Fig. 4). Neutrophils and macrophages phagocytose bacteria and fungi which are then killed in the phagosome [203]. After activation, a respiratory burst occurs where NOX2 is activated and generates superoxide. The generation of superoxide inside the phagosomal lumen creates a change in electrical charge across the phagosomal membrane which can inhibit the further generation of superoxide by NOX2 [204]. This change in electrical charge is counteracted by Hv1 voltage-gated channels which allow for the simultaneous flow of protons into the phagosomal membrane [205]. In the absence of Hv1, NOX2 activity and superoxide production in the phagosome is severely limited [206].
Fig. 4.
NADPH oxidase-derived ROS regulate immunity. NOX-derived ROS regulate various aspects of immunity like phagocytosis, pathogen clearance, antigen processing, antigen presentation, type I interferon regulation, inflammasome regulation, and cell signaling.
The exact role of superoxide production in the phagosome is somewhat controversial. The dogma in the field is that NOX2-derived superoxide and its downstream products hydrogen peroxide and hypochlorite generated by myeloperoxidase (MPO) directly kill phagocytosed pathogens. However, recent evidence has suggested that proteases delivered to phagosomes by granules are primarily responsible for the microbicidal activity of phagosomes [207]. Indeed, mice deficient for cathepsin G or elastase were more susceptible to Staphylococcus aureus and Candida albicans infections respectively, despite intact NOX2 activity [207]. Further evidence to support this is the absence of patients identified with deficiencies in MPO that suffer from chronic bacterial infections like patients with CGD [208]. However, mice with MPO deficiencies do have increased susceptibility to infections by certain bacteria or fungi suggesting that MPO is important in some contexts [209].
The controversy surrounding the exact role of NOX2-derived superoxide and the subsequent activity of MPO in the phagosome is concerned with the pH of the phagosome. After the respiratory burst, the pH of the phagosome increases and becomes alkaline with a pH of approximately 9 [210,211]. This increase in pH is regulated by Hv1 voltage-gated channels and in their absence, the pH rises as high as 11 [210]. This alkaline pH is incompatible with hypochlorite generation by MPO which is optimal at a slightly acidic pH [212,213]. At an alkaline pH, MPO has SOD and catalase activity, which could convert superoxide into hydrogen peroxide and hydrogen peroxide into water [210,214,215]. This would suggest that the role of MPO in the phagosome is to dissipate the ROS generated by NOX2. While the high pH of the phagosome is incompatible with the halogenating activity of MPO, it is compatible with the maximal activity of proteases like elastase, cathepsin G, and proteinase 3 that are present in the phagocytic granules [210]. An increase in the pH and an influx of K+ are required for the activation of these microbicidal proteases and their release from the negatively charged proteoglycan matrix in the granules [207].
Levine and Segal have proposed that MPO has SOD and catalase activity at a pH of 9 in the phagosome, but in cases where a pathogen cannot be fully engulfed, and the pH is that of the extracellular environment, MPO generates hypochlorite, which assists in killing extracellular pathogens [208]. However, the recently developed rhodamine-based probe, R19-S, which has specificity for hypochlorite, has revealed hypochlorite present in phagosomes of isolated neutrophils infected with Staphylococcus aureus [216]. Further evidence for hypochlorite induction in the neutrophil phagosome comes from a recent study that demonstrated the induction of a chlorine-responsive transcription factor, RclR, in Escherichia coli after ingestion by neutrophils. The transcription factor was not induced when NOX2 or MPO was inhibited, suggesting that this was indeed due to hypochlorite production in the phagosome [217].
4.2. Macrophage polarization
NOX-derived ROS are important in driving macrophage polarization to a proinflammatory M1 macrophage phenotype and in their absence, anti-inflammatory M2 macrophage differentiation will prevail. In p47phox-deficient mice, a model for CGD, there is more skewing towards an M2 macrophage phenotype [218]. In the absence of NOX2, macrophages have attenuated STAT1 signaling and increased STAT3 signaling which promotes the expression of anti-inflammatory markers such as Arginase-1 [219]. Studies of Type 1 diabetes by our group (see section 5.2) have shown that NOD mice carrying the Ncf1m1J mutation, which results in a lack of p47phox activity, exhibit a skewed M2 macrophage phenotype that is partly responsible for delaying spontaneous T1D development [220].
In contrast, NOX4-and DUOX1-derived hydrogen peroxide promotes M2 macrophage polarization. Inhibition of NOX4 in murine bone marrow-derived macrophages results in M1 polarization due to reduced STAT6 activation and increased NFκB activity [221]. In certain disease contexts, NOX4 may be a potential therapeutic target to influence macrophage polarization. In pulmonary fibrosis after asbestos exposure, NOX4 expression in macrophages promotes profibrotic polarization of alveolar macrophages which are resistant to apoptosis [222,223]. In non-small cell lung cancer, expression of NOX4 in the tumor promotes recruitment and polarization of M2 macrophages, which is associated with tumor growth [224]. DUOX1 has also been shown to be expressed in macrophages [225,226]. DUOX1−/− macrophages tend to skew towards a proinflammatory M1 phenotype characterized by IFN-γ, CXCL9, CCL3, and CCL5 secretion. DUOX1−/− macrophages also have enhanced antitumor activity and promote the recruitment of IFN-γ+ tumor-infiltrating CD8+ T cells [188].
4.3. Antigen processing and presentation
NOX2-derived superoxide is important for pathogen killing in neutrophils and macrophages, but it also regulates antigen processing and presentation in dendritic cells (DCs) (Fig. 4). DCs differ from other phagocytic cells in that their primary function is to process antigens and present them to T cells rather than just destroying pathogens. NOX2 activation through PKC-δ promotes pinocytosis and antigen uptake in DCs through the SSH1-Cofilin pathway [227,228]. In addition to promoting antigen uptake, NOX2 plays a key role in antigen processing within the phagosome by modulating the pH and activity of proteolytic enzymes [229].
Proteolysis in the phagosome is necessary for generating antigens of the correct size for MHC loading. However, too much proteolysis will result in the complete destruction of peptides and poor antigen presentation [229]. Preventing the complete destruction of peptides for antigen presentation requires alkalinization of the phagosome, which is driven by NOX2 [230]. Indeed, NOX2-deficient DCs have more acidic phagosomes and increased antigen degradation [230]. Alkalinization of the phagosome is important for optimal activity of proteolytic enzymes which affects the types of antigens that can be presented to T cells [229].
DCs generally have less NOX2 activity in their phagosomes than neutrophils and macrophages, which helps to promote optimal proteolysis [231]. High levels of NOX2 activity result in inhibition of cysteine cathepsins and poor phagosomal proteolysis whereas a lack of NOX2 activity results in high levels of proteolysis and destruction of antigens [232]. High levels of NOX2 activity also result in decreased reduction of disulfide bonds by γ-interferon-inducible lysosomal thiol reductase (GILT), which is necessary for unfolding and linearizing peptides for antigen presentation [229,231]. GILT is a redox-sensitive reductase that is required for disulfide bond reduction and efficient processing of several model antigens [233]. GILT is also required for maintaining optimal proteolysis by cysteine cathepsins [234].
NOX2 activity is also important in promoting cross-presentation of antigens by CD8+ DCs [230]. Experimental inhibition of NOX2 by treatment with diphenyleneiodonium (DPI) results in the inhibition of phagosomal alkalinization and cross-presentation of model tumor antigens [235]. This phenotype is recapitulated in DCs from patients with CGD [235]. NOX2 is recruited to the endosomes through activity of the SNARE protein VAMP8 [236]. In addition to antigen preservation, NOX2 activity has also been shown to cause lipid peroxidation of endosomal membranes which promotes antigen release from the endosome to the cytosol for cross-presentation [237]. Cross-presentation has also been shown to require activity of Rac2 and not Rac1 for NOX2 activation [238].
4.4. Type I interferon regulation
NOX-generated ROS are also important in regulating type I interferons (IFNs) (Fig. 4). Patients with CGD as well as mice with nonfunctional NCF1 have an elevated type I IFN signature and are more prone to autoimmune manifestations [6]. In mice that are deficient for NCF1, STAT1-dependent gene transcription is increased, which may contribute to development of autoimmune SLE and RA [5,6]. In Listeria monocytogenes infection, a lack of NOX2-derived superoxide results in an exaggerated response to type I IFN signaling with increased expression of ISGs. In the case of Listeria, this results in the inability to control bacterial spread and mount an effective adaptive immune response [239]. However, this is dependent on the genetic background of mice since non-obese diabetic (NOD) mice have decreased type I IFN signaling, synthesis of ISGs, and a delay in autoimmune diabetes in the absence of NOX2-derived superoxide [240,241].
In viral infections, too much ROS can dampen type I IFN signaling enough to hinder the antiviral response. NOX-derived ROS are required for efficient viral sensing through the mitochondrial antiviral signaling protein (MAVS), and in their absence, MAVS expression is decreased and activation of IRF3 and ISGs is decreased [242]. In the absence of SOD2, ROS levels are elevated and the response to RNA viruses is deficient due to decreased type I IFN production [243]. ROS generation after IFN stimulation is negatively regulated by some ISGs like IFIT2 which can interact with p67phox to downregulate superoxide production [244].
DUOX1 and DUOX2 are required for an efficient antiviral response in airway epithelial cells after influenza A (IAV) infection [193,244]. IAV infection results in the upregulation of DUOX1 and DUOX2 in lung epithelial cells [246] and DUOX2-derived ROS are required for inducing the production of type I and III IFNs during IAV infection [247,248]. It has recently been demonstrated that DUOX1-derived hydrogen peroxide is important for innate immunity during IAV infection by inducing the expression of inflammatory cytokines, recruiting additional immune cells, and generating hypothiocyanite in conjunction with the lactoperoxidase enzyme [245]. DUOX2 expression in the lungs is driven by IFN-β and TNF which induces STAT2 and IRF9-dependent signaling pathways [249]. Expression of MDA5 and RIG-I, which is necessary for detecting IAV replication, is also dependent on DUOX2-derived ROS [250,251]. Inhibition of DUOX2 results in increased IAV replication in vivo and in vitro [248,250,251].
4.5. The inflammasome
NOX-derived ROS also play a role in regulating the inflammasome (Fig. 4). It has been demonstrated that NOX-derived ROS are necessary for activation of the NLRP3 inflammasome in response to extracellular ATP, silica, and asbestos [252]. Other studies have demonstrated the importance of NOX2-derived ROS for activation of the NLRP1 inflammasome [253,254] and NOX4-derived ROS for activation of the NLRP3 inflammasome [[255], [256], [257]]. The requirement for NOX4 in macrophages for inflammasome activation is specific to the NLRP3 inflammasome; NOX4 is not required for NLRC4, NLRP1, or AIM2 inflammasome activation [258]. Evidence shows that not only can ROS induce inflammasome activation, but that ROS generation is amplified by NLRP3 inflammasome activation as well [[259], [260], [261]]. However, there is also evidence that without NOX2-derived superoxide there is chronically elevated inflammasome activation, highlighting the complexity of interactions between NOX-derived ROS and the inflammasome [262]. Further complicating the relationship, it has been shown that caspase-1 may negatively regulate NOX2 [263].
There have been multiple studies that have linked NOX2-derived ROS and the inflammasome in disease. In chronic kidney disease, oxidative stress can lead to kidney damage due to activation of NOX2 and the NLRP3 inflammasome [264]. In nonalcoholic fatty liver disease in mice, lactate-producing bacteria in the gut can activate NOX2 which results in NLRP3 inflammasome activation and exacerbates disease [265]. Glucose-6-phosphate dehydrogenase (G6PD)-deficiency results in altered NADPH production. In human peripheral blood mononuclear cells with G6PD-deficiency, there is decreased superoxide production and defective inflammasome activation, which can be ameliorated by exogenous addition of hydrogen peroxide [266].
4.6. Cell signaling
Superoxide and hydrogen peroxide are pleiotropic signaling molecules that can affect a variety of cellular processes ranging from stress adaptation, the antioxidant response, the hypoxic response, and the inflammatory response (Fig. 4). A thorough examination of the role of ROS in cell signaling is beyond the scope of this review and has already been reviewed previously [1,267]. NOX-derived hydrogen peroxide can modulate signaling pathways by triggering redox switches through the oxidation of cysteine and methionine resides [268,269]. Redox switches can be used to promote signaling through a pathway by inactivating protein tyrosine phosphatases through the oxidation of conserved cysteine residues, thus maintaining levels of phosphorylated proteins [[270], [271], [272], [273]]. Redox switches can also direct the degradation of proteins by the proteasome. For example, oxidation of Met145 in calmodulin by peroxynitrite results in its degradation by the proteasome and downregulation of calcium signaling [268]. A large portion of cellular ROS is derived from superoxide produced by NOX enzymes. However, there are other sources of cellular ROS, such as mitochondrial-derived superoxide, which makes determining the specific contributions of NOX enzymes on signaling pathways more difficult.
The specific role of NOX enzymes in signaling pathways is not always simple to determine when there are multiple NOX enzymes involved such as in the well-characterized epidermal growth factor receptor (EGFR) pathway. Several NOX enzymes have been demonstrated to be involved in the regulation of EGFR signaling. After EGF stimulation, epithelial cells begin to produce ROS which is driven by NOX1 downstream of PI3K signaling [274]. EGF stimulation also activates the ERK pathway which acts to negatively regulate NOX1 activity through the phosphorylation of Ser282 in NOXA1 by ERK [275,276]. EGFR signaling transduction is also modulated by the oxidation of Cys797 in EGFR by hydrogen peroxide derived from NOX2 in A431 cells [277]. NOX4, located in the ER, is also involved in regulating EGFR trafficking through oxidation of PTP1B, which deactivates EGFR by dephosphorylation [278]. In the absence of NOX4, EGFR signaling is decreased due to increased PTP1B activity on EGFR after receptor endocytosis [277]. DUOX1 in the airway is also associated with EGFR signaling after stimulation of TLRs [[192], [193], [194]]. The role of different NOX enzymes in EGFR signaling highlights the key role that NOX enzymes play in cell signaling and the complex nature of their respective roles in these pathways.
5. NOX enzymes in inflammation and autoimmunity
5.1. Rheumatoid arthritis
Studies of NOX2-deficient mice have been used to determine the role of NOX2-derived ROS in autoimmune diseases. However, whether NOX2-derived ROS contribute to or protect from autoimmunity varies depending on the disease and the genetic background of the mice. B10.Q mice homozygous for a mutation in the Ncf1 gene (Ncf1m1J mutant), which results in aberrant splicing and a lack of NCF1 and NOX2 activity, have increased presentation of an autoantigen involved in collagen-induced arthritis. This is thought to be due to upregulation of GILT which facilitates disulfide bond-containing antigen processing [279]. It is worth noting that B10.Q mice are usually resistant to collagen-induced arthritis and have hyporesponsiveness to IL-12 due to a mutation in Tyk2 [280].
5.2. Type 1 diabetes
Previous work by our group has explored the role of NOX2-derived ROS in the context of Type 1 diabetes (T1D) using a mouse model with the Ncf1m1J mutation on the NOD mouse background (NOD.Ncf1m1J) [281]. NOD.Ncf1m1J mice are protected from spontaneous, adoptively transferred, and virus-accelerated diabetes [220]. An investigation into the mechanism of protection from T1D in these mice has revealed that NOD.Ncf1m1J mice have altered macrophage phenotypes. Macrophages from NOD.Ncf1m1J mice are skewed more towards an anti-inflammatory M2 phenotype compared to macrophages from NOD mice with intact NOX [281,282]. Macrophages from NOD.Ncf1m1J mice also have dysregulated signaling through TLRs and express significantly less proinflammatory cytokines such as TNF and IFN-β after stimulation with TLR ligands [281,282]. In contrast to the B10.Q mice, NOD mice are more prone to Th1 T cell responses and inflammation [283]. These findings suggest that the role of NOX2 in autoimmunity is also heavily dependent on the genetic background of the host.
The diverse biological functions that are regulated or modified by NOX-derived ROS make antioxidant-based therapies attractive for treating diseases associated with oxidative stress. Previous work by our group has investigated the use of a metalloporphyrin-based superoxide dismutase mimetic (SOD mimetic), which acts as a catalytic antioxidant, for the treatment of T1D. We have shown that spontaneous and adoptively transferred diabetes can be delayed in mice pretreated with the SOD mimetic [281]. We have also shown that treatment of macrophages with the SOD mimetic results in decreased TNF, IL-1β, and ROS production after treatment with inflammatory stimuli due to decreased DNA binding by redox-sensitive transcription factors like NFκB and SP1 [284].
Our group has also investigated the use of antioxidant-containing biomaterials to treat T1D. We have shown that microcapsules composed of poly(N-vinylpyrrolidone) (PVPON) and the antioxidant tannic acid can be used to deliver antigens in vivo to mice to promote antigen-specific tolerance [285]. The goal of this therapy would be to induce tolerance to autoantigens associated with T1D by dampening ROS, which results in antigen hyporesponsiveness [285]. We have also used PVPON and tannic acid-containing biomaterials to encapsulate islets for transplantation into diabetic recipients [286]. Encapsulation with the PVPON and tannic acid-containing biomaterial delays islet allograft and autoimmune-mediated rejection after transplantation into diabetic recipients [286].
6. NOX enzymes in SARS-CoV-2 infection and acute lung injury
NOX-derived ROS play important roles in viral infections and modulate aspects of the innate and adaptive immune responses to infection. DNA and RNA viruses can activate endosomal NOX2 via activation of PKC downstream of sensing by TLR7 or TLR9, which results in the production of hydrogen peroxide. The generation of endosomal hydrogen peroxide results in a suppressed antiviral response and a decrease in antibody production [287]. Studies in mouse models deficient in NOX2 have demonstrated that a lack of NOX2 results in skewing towards a Th1 response and increased production of IgG2c and IFN-γ [288]. Similarly, IgG2 levels were increased in human sera from CGD patients, which suggests a skewing towards Th1 responses [288]. Thus, viruses that can activate NOX2 will be able to dampen the antiviral response, favoring viral replication.
Recent evidence from the COVID-19 pandemic suggests that oxidative stress may be driving acute lung injury in patients with severe SARS-CoV-2 infection (Fig. 5) [289]. NOX2 activation is higher in COVID-19 patients compared to controls and higher in severe COVID-19 cases compared to non-severe cases [290]. Oxidative stress during SARS-CoV-2 infection may be due to activation of the NLRP3 inflammasome in infected cells [291]. It has been hypothesized that increased risk for oxidative stress and severe COVID-19 may be due to suppressed antioxidant responses through the NRF2 pathway, glutathione deficiency, or low levels of SOD3 expression in alveolar type II cells [[291], [292], [293]]. A recent study demonstrated an influx of NOX1 and NOX2 positive granulocytic-myeloid-derived suppressor cells (G-MDSCs) in the lungs of patients with severe COVID-19 complications. The study demonstrated that Arginase-1 positive G-MDSCs depleted l-arginine levels which resulted in impaired T cell and endothelial dysfunction [294]. However, the study did not conclusively demonstrate the role of NOX enzymes in these cells and whether NOX-derived ROS played a role in disease severity.
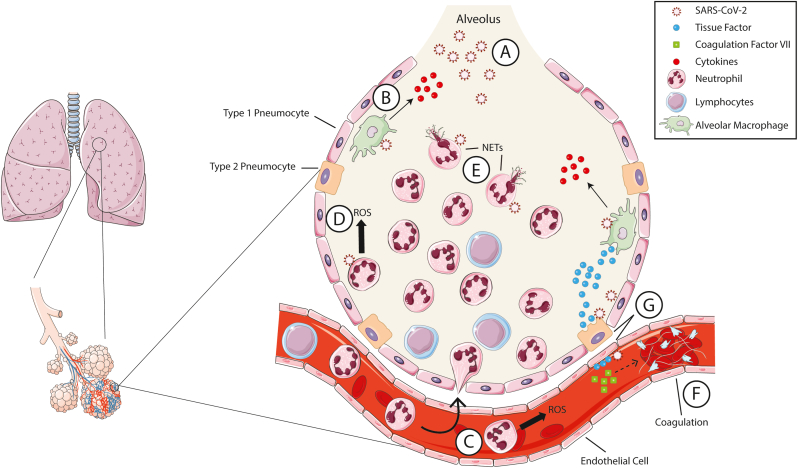
Fig. 5.
Acute lung injury during SARS-CoV-2 infection. (A) SARS-CoV-2 inhaled in the lung is first detected by (B) alveolar macrophages which produce proinflammatory cytokines and chemokines to recruit additional immune cells. (C) Neutrophils and lymphocytes are recruited to the lungs. (D) Severe COVID-19 cases are associated with a high neutrophil to lymphocyte ratio. NOX2 is activated in neutrophils which produce ROS in the alveoli driving lung damage. (E) SARS-CoV-2 can also activate NETosis and the release of neutrophil extracellular traps (NETs). (F) Platelet-fibrin thrombi are formed in the lungs causing further tissue damage. (G) Infected endothelial cells and type II pneumocytes in the lungs produce tissue factor which acts on coagulation factor VII to initiate clotting. Some images were modified from Servier Medical Art under a Creative Commons License.
During SARS-CoV-2 infection, activated neutrophils have been shown to be one of the main sources of ROS production in the lung tissue and a driver of lung tissue damage (Fig. 5A–D) [295,296]. Several studies have demonstrated that increased neutrophil to lymphocyte ratios correlate with more severe disease outcomes [297,298]. Post-mortem analysis of lung tissue of patients with severe COVID-19 showed evidence of neutrophil extracellular traps (NETs) which likely are contributing to lung tissue damage (Fig. 5E) [296]. In vitro experiments have demonstrated that SARS-CoV-2 can activate NETs in human neutrophils and that this correlates to increased production of ROS and IL-8 [299]. NETosis can also be induced through FcαRI engagement by IgA-virus immune complexes. Immune complexes made up of SARS-CoV-2 spike protein pseudotyped lentivirus purified IgA from COVID-19 convalescent patients were able to induce NETosis in vitro. NETosis was not seen when using purified serum IgA from COVID-19 naïve patients or when neutrophils were pretreated with the NOX inhibitor DPI [300].
Acute lung injury during COVID-19 also correlates with elevated levels of D-dimer and fibrinogen suggesting that thrombosis may be contributing to increased mortality in severe cases [297,298]. Indeed, severe COVID-19 cases and COVID-19 deaths have been linked to thrombotic complications like pulmonary embolism [301]. Analysis of post-mortem lung tissue has shown that COVID-19-related deaths appear to be correlated with increased platelet-fibrin thrombi and microangiopathy in the lung (Fig. 5F) [302,303]. NETs from activated neutrophils are likely directly contributing to thrombosis, but there is also evidence to suggest that endothelial cells may be involved [299].
Severe COVID-19 cases have been associated with endothelial cell activation which is present not only in the lungs but also in other vital organs like the heart, kidneys, and intestines [304]. Endothelial cells express the ACE2 receptor which is required for infection by SARS-CoV-2. One hypothesis is that infected endothelial cells produce tissue factor after activation of NOX2, which promotes clotting through interaction with coagulation factor VII (Fig. 5G) [305]. Escher and colleagues reported that treatment of a critically ill COVID-19 patient with anticoagulation therapy resulted in a positive outcome and hypothesize that endothelial cell activation may also be driving coagulation [306]. Studies of SARS-CoV that was responsible for the 2003 SARS epidemic have shown that oxidized phospholipids were found in the lungs of infected patients, which is associated with acute lung injury through promotion of tissue factor expression and initiation of clotting [307,308].
Therapies targeting ROS or NOX enzyme activation may be beneficial in acute lung injury. Given the role of NOX2-derived ROS as a driver of acute lung injury during COVID-19, therapies that target NOX2 enzymes or ROS may be beneficial in severe COVID-19 cases. Pasini and colleagues have extensively reviewed the subject and propose that studies should be performed to assess the use of ROS scavengers and NRF2 activators as potential COVID-19 therapeutics to be used alone or in conjunction with existing treatments [291]. It has also been proposed that supplementation of vitamin D may also have a positive effect on COVID-19 outcomes through its immunomodulatory effects including inducing downregulation of NOX2 [309]. However, vitamin D has also been shown to upregulate ACE2 which may facilitate viral replication [310]. Therefore, these proposed COVID-19 therapies need testing before their efficacy can be determined.
Targeting NOX enzymes in acute lung injury not caused by COVID-19 may also be beneficial. In acute lung injury caused by renal ischemia-reperfusion, treatment with dexmedetomidine reduces NOX4 activation in alveolar macrophages which correlates with decreased NLRP3 inflammasome activation [311]. Another recent study demonstrated that treatment with the NOX1/NOX4 inhibitor GKT137831 results in less severe acute lung injury after ischemia-reperfusion in mice by dampening the amount of proinflammatory cytokines produced in the lungs [312].
7. Perspectives & conclusions
7.1. Inhibitors of NOX enzymes
Given the role of NOX-derived ROS in various pathologies, specific inhibitors of NOX enzymes have been of interest. Numerous NOX inhibitors have been developed that are pan-NOX inhibitors or are specific to one NOX enzyme (reviewed in Ref. [313]). Inhibitors specific to NOX2 have been developed and shown efficacy in mice [313]. The peptide inhibitor (RKKRRQRRRCSTRIRRQL), called NOX2ds-tat, has been shown to prevent p47phox binding to the B-loop of NOX2 [314]. This inhibitor has been shown in a mouse model of ischemic retinopathy to block VEGF overexpression [315]. This inhibitor has also been successfully used to reduce vascular superoxide levels in response to angiotensin II [316]. These proof-of-concept studies demonstrate that NOX inhibitors may have therapeutic benefit for human diseases including COVID-19, type I diabetes, and cardiovascular disease.
7.2. Conclusions
NOX enzymes are an important group of enzymes that produce superoxide which is necessary for the generation of other ROS like hydrogen peroxide, peroxynitrite, and hydroxyl radicals. NOX-derived ROS function in diverse pathways in immunity including immunity to pathogens, generation of an adaptive immune response, cell signaling, and autoimmunity. Understanding the complex biology of NOX enzymes and their regulation will be important for treating a wide variety of diseases. NOX2 is the best-studied NOX enzyme, but more studies of the other NOX enzymes are being conducted to discover their role in health and disease. Future studies investigating the role of antioxidants or inhibitors of NOX enzymes could lead to the development of beneficial therapies for a wide variety of autoimmune and infectious diseases.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Katie Heath, Jessie Barra, and Samuel Blum for critically reading the manuscript.
This work was supported by an NIH/NIDDK R01 award (DK099550) (HMT), JDRF Award (2-SRA-2019-692-S-B) (HMT), NIH/NIDDK R01 award (DK127497) (HMT), NIH/NIDDK R01 award (DK126456) (HMT), and NIH/NIAID T32 Immunologic Diseases and Basic Immunology award (T32.AI007051) (JPT).
References
- 1.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents., Nature Reviews. MCB (Mol. Cell. Biol.) 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 2.Lambeth J.D. NOX enzymes and the biology of reactive oxygen., Nature Reviews. Immunology. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 3.Segal A.W., Jones O.T.G., Webster D., Allison A.C. ABSENCE OF A NEWLY DESCRIBED CYTOCHROME b FROM NEUTROPHILS OF PATIENTS WITH CHRONIC GRANULOMATOUS DISEASE. Lancet. 1978;312:446–449. doi: 10.1016/S0140-6736(78)91445-9. [DOI] [PubMed] [Google Scholar]
- 4.Stasia M.J., Li X.J. Genetics and immunopathology of chronic granulomatous disease. Semin. Immunopathol. 2008;30:209–235. doi: 10.1007/s00281-008-0121-8. [DOI] [PubMed] [Google Scholar]
- 5.Winter S., Hultqvist Hopkins M., Laulund F., Holmdahl R. A reduction in intracellular reactive oxygen species due to a mutation in NCF4 promotes autoimmune arthritis in mice., Antioxidants Redox Signal. 2016;25:983–996. doi: 10.1089/ars.2016.6675. [DOI] [PubMed] [Google Scholar]
- 6.Kelkka T., Kienhöfer D., Hoffmann M., Linja M., Wing K., Sareila O., Hultqvist M., Laajala E., Chen Z., Vasconcelos J., Neves E., Guedes M., Marques L., Krönke G., Helminen M., Kainulainen L., Olofsson P., Jalkanen S., Lahesmaa R., Souto-Carneiro M.M., Holmdahl R. Reactive oxygen species deficiency induces autoimmunity with type 1 interferon signature. Antioxidants Redox Signal. 2014;21:2231–2245. doi: 10.1089/ars.2013.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schäppi M.G., Jaquet V., Belli D.C., Krause K.H. Hyperinflammation in chronic granulomatous disease and anti-inflammatory role of the phagocyte NADPH oxidase. Semin. Immunopathol. 2008;30:255–271. doi: 10.1007/s00281-008-0119-2. [DOI] [PubMed] [Google Scholar]
- 8.Commoner B., Townsend J., Pake G.E. Free radicals in biological materials. Nature. 1954;174:689–691. doi: 10.1038/174689a0. [DOI] [PubMed] [Google Scholar]
- 9.Babior B.M., Kipnes R.S., Curnutte J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Invest. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drath D.B., Karnovsky M.L. Superoxide production by phagocytic leukocytes. J. Exp. Med. 1975;141:257–262. doi: 10.1084/jem.141.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyer G.Y.N., Islam M.F., Quastel J.H. Biochemical aspects of phagocytosis. Nature. 1961;192:535–541. doi: 10.1038/192535a0. [DOI] [Google Scholar]
- 12.Iyer G.Y., Questel J.H. NADPH and NADH oxidation by Guinea pig polymorphonuclear leucocytes. Can. J. Biochem. Physiol. 1963;41:427–434. http://www.ncbi.nlm.nih.gov/pubmed/13957122 [PubMed] [Google Scholar]
- 13.Rossi F., Zatti M. Biochemical aspects of phagocytosis in polymorphonuclear leucocytes. NADH and NADPH oxidation by the granules of resting and phagocytizing cells. Experientia. 1964;20:21–23. doi: 10.1007/BF02146019. [DOI] [PubMed] [Google Scholar]
- 14.Segal A.W., Jones O.T. Novel cytochrome b system in phagocytic vacuoles of human granulocytes. Nature. 1978;276:515–517. doi: 10.1038/276515a0. [DOI] [PubMed] [Google Scholar]
- 15.Parkos C.A., Allen R.A., Cochrane C.G., Jesaitis A.J. Purified cytochrome b from human granulocyte plasma membrane is comprised of two polypeptides with relative molecular weights of 91,000 and 22,000. J. Clin. Invest. 1987;80:732–742. doi: 10.1172/JCI113128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinauer M.C., Pierce E.A., Bruns G.A.P., Curnutte J.T., Orkin S.H. Human neutrophil cytochrome b light chain (p22-phox). Gene structure, chromosomal location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J. Clin. Invest. 1990;86:1729–1737. doi: 10.1172/JCI114898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Royer-Pokora B., Kunkel L.M., Monaco A.P., Goff S.C., Newburger P.E., Baehner R.L., Cole F.S., Curnutte J.T., Orkin S.H. Cloning the gene for an inherited human disorder–chronic granulomatous disease–on the basis of its chromosomal location. Nature. 1986;322:32–38. doi: 10.1038/322032a0. [DOI] [PubMed] [Google Scholar]
- 18.Dinauer M.C., Orkin S.H., Brown R., Jesaitis A.J., Parkos C.A. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. Nature. 1987;327:717–720. doi: 10.1038/327717a0. [DOI] [PubMed] [Google Scholar]
- 19.Teahan C., Rowe P., Parker P., Totty N., Segal A.W. The X-linked chronic granulomatous disease gene codes for the beta-chain of cytochrome b-245. Nature. 1987;327:720–721. doi: 10.1038/327720a0. [DOI] [PubMed] [Google Scholar]
- 20.Moghadam Z.M., Henneke P., Kolter J. From flies to men: ROS and the NADPH oxidase in phagocytes., frontiers in cell and developmental biology. 2021. 9,628991. [DOI] [PMC free article] [PubMed]
- 21.Bortolotti M., Polito L., Battelli M.G., Bolognesi A. Xanthine oxidoreductase: one enzyme for multiple physiological tasks. Redox Biology. 2021;41:101882. doi: 10.1016/j.redox.2021.101882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angermüller S., Bruder G., Völkl A., Wesch H., Fahimi H.D. Localization of xanthine oxidase in crystalline cores of peroxisomes. A cytochemical and biochemical study. Eur. J. Cell Biol. 1987;45:137–144. http://www.ncbi.nlm.nih.gov/pubmed/3443108 [PubMed] [Google Scholar]
- 23.Pritsos C.A. Cellular distribution, metabolism and regulation of the xanthine oxidoreductase enzyme system. Chem. Biol. Interact. 2000;129:195–208. doi: 10.1016/s0009-2797(00)00203-9. [DOI] [PubMed] [Google Scholar]
- 24.McNally J.S., Davis M.E., Giddens D.P., Saha A., Hwang J., Dikalov S., Jo H., Harrison D.G. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress., American Journal of Physiology. Heart and Circulatory Physiology. 2003;285:H2290–H2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- 25.Beckman J.S., Beckman T.W., Chen J., Marshall P.A., Freeman B.A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U. S. A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bredt D.S., Snyder S.H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc. Natl. Acad. Sci. U. S. A. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palacios M., Knowles R.G., Palmer R.M.J., Moncada S. Nitric oxide from L-arginine stimulates the soluble guanylate cyclase in adrenal glands. Biochem. Biophys. Res. Commun. 1989;165:802–809. doi: 10.1016/S0006-291X(89)80037-3. [DOI] [PubMed] [Google Scholar]
- 28.Bienert G.P., Møller A.L.B., Kristiansen K.A., Schulz A., Møller I.M., Schjoerring J.K., Jahn T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007;282:1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- 29.al Ghouleh I., Frazziano G., Rodriguez A.I., Csányi G., Maniar S., St Croix C.M., Kelley E.E., Egaña L.A., Song G.J., Bisello A., Lee Y.J., Pagano P.J. Aquaporin 1, Nox1, and Ask1 mediate oxidant-induced smooth muscle cell hypertrophy. Cardiovasc. Res. 2013;97:134–142. doi: 10.1093/cvr/cvs295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davies M.J. Myeloperoxidase: mechanisms, reactions and inhibition as a therapeutic strategy in inflammatory diseases. Pharmacology & Therapeutics. 2021;107685 doi: 10.1016/j.pharmthera.2020.107685. 218. [DOI] [PubMed] [Google Scholar]
- 31.Haschka D., Hoffmann A., Weiss G. Iron in immune cell function and host defense. Seminars in Cell & Developmental Biology. 2020 doi: 10.1016/j.semcdb.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Yu L., DeLeo F.R., Biberstine-Kinkade K.J., Renee J., Nauseef W.M., Dinauer M.C. Biosynthesis of flavocytochrome b558. gp91(phox) is synthesized as a 65-kDa precursor (p65) in the endoplasmic reticulum. J. Biol. Chem. 1999;274:4364–4369. doi: 10.1074/jbc.274.7.4364. [DOI] [PubMed] [Google Scholar]
- 33.Yu L., Zhen L., Dinauer M.C. Biosynthesis of the phagocyte NADPH oxidase cytochrome b558. Role of heme incorporation and heterodimer formation in maturation and stability of gp91phox and p22phox subunits. J. Biol. Chem. 1997;272:27288–27294. doi: 10.1074/jbc.272.43.27288. [DOI] [PubMed] [Google Scholar]
- 34.Porter C.D., Parkar M.H., Verhoeven A.J., Levinsky R.J., Collins M.K.L., Kinnon C. p22-phox-deficient chronic granulomatous disease: reconstitution by retrovirus-mediated expression and identification of a biosynthetic intermediate of gp91-phox. Blood. 1994;84:2767–2775. doi: 10.1182/blood.v84.8.2767.2767. [DOI] [PubMed] [Google Scholar]
- 35.de Boer M., de Klein A., Hossle J.P., Seger R., Corbeel L., Weening R.S., Roos D. Cytochrome b558-negative, autosomal recessive chronic granulomatous disease: two new mutations in the cytochrome b558 light chain of the NADPH oxidase (p22-phox) Am. J. Hum. Genet. 1992;51:1127–1135. https://pubmed.ncbi.nlm.nih.gov/1415254/ [PMC free article] [PubMed] [Google Scholar]
- 36.Dinauer M.C., Pierce E.A., Erickson R.W., Muhlebach T.J., Messner H., Orkin S.H., Seger R.A., Curnutte J.T. Point mutation in the cytoplasmic domain of the neutrophil p22-phox cytochrome b subunit is associated with a nonfunctional NADPH oxidase and chronic granulomatous disease. Proc. Natl. Acad. Sci. U. S. A. 1991;88:11231–11235. doi: 10.1073/pnas.88.24.11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Y., Marchal C.C., Casbon A.-J., Stull N., von Löhneysen K., Knaus U.G., Jesaitis A.J., McCormick S., Nauseef W.M., Dinauer M.C. Deletion mutagenesis of p22phox subunit of flavocytochrome b558: identification of regions critical for gp91phox maturation and NADPH oxidase activity. J. Biol. Chem. 2006;281:30336–30346. doi: 10.1074/jbc.M607191200. [DOI] [PubMed] [Google Scholar]
- 38.Volpp B.D., Nauseef W.M., Clark R.A. Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease., Science (New York, N.Y.) 1988. 242,1295,1297. [DOI] [PubMed]
- 39.DeLeo F.R., Burritt J.B., Yu L., Jesaitis A.J., Dinauer M.C., Nauseef W.M. Processing and maturation of flavocytochrome b558 include incorporation of heme as a prerequisite for heterodimer assembly. J. Biol. Chem. 2000;275:13986–13993. doi: 10.1074/jbc.275.18.13986. [DOI] [PubMed] [Google Scholar]
- 40.Parkos C.A., Dinauer M.C., Jesaitis A.J., Orkin S.H., Curnutte J.T. Absence of both the 91kD and 22kD subunits of human neutrophil cytochrome b in two genetic forms of chronic granulomatous disease. Blood. 1989;73:1416–1420. https://pubmed.ncbi.nlm.nih.gov/2713485/ [PubMed] [Google Scholar]
- 41.Nauseef W.M. In: Hydrogen Peroxide Metabolism in Health and Disease. first ed. Vissers M.C.M., Hampton M.B., Kettle A.J., editors. CRC Press; Boca Raton, FL: 2018. The neutrophil NADPH oxidase; pp. 237–279. [Google Scholar]
- 42.Dahan I., Issaeva I., Gorzalczany Y., Sigal N., Hirshberg M., Pick E. Mapping of functional domains in the p22(phox) subunit of flavocytochrome b(559) participating in the assembly of the NADPH oxidase complex by “peptide walking”. J. Biol. Chem. 2002;277:8421–8432. doi: 10.1074/jbc.M109778200. [DOI] [PubMed] [Google Scholar]
- 43.Nauseef W.M. The phagocyte NOX2 NADPH oxidase in microbial killing and cell signaling. Curr. Opin. Immunol. 2019;60:130–140. doi: 10.1016/j.coi.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bromberg Y., Pick E. Unsaturated fatty acids stimulate NADPH-dependent superoxide production by cell-free system derived from macrophages. Cell. Immunol. 1984;88:213–221. doi: 10.1016/0008-8749(84)90066-2. [DOI] [PubMed] [Google Scholar]
- 45.Heyneman R.A., Vercauteren R.E. Activation of a NADPH oxidase from horse polymorphonuclear leukocytes in a cell-free system. J. Leukoc. Biol. 1984;36:751–759. doi: 10.1002/jlb.36.6.751. [DOI] [PubMed] [Google Scholar]
- 46.McPhail L.C., Shirley P.S., Clayton C.C., Snyderman R. Activation of the respiratory burst enzyme from human neutrophils in a cell-free system. Evidence for a soluble cofactor. J. Clin. Invest. 1985;75:1735–1739. doi: 10.1172/JCI111884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curnutte J.T. Activation of human neutrophil nicotinamide adenine dinucleotide phosphate, reduced (triphosphopyridine nucleotide, reduced) oxidase by arachidonic acid in a cell-free system. J. Clin. Invest. 1985;75:1740–1743. doi: 10.1172/JCI111885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caldwell S.E., McCall C.E., Hendricks C.L., Leone P.A., Bass D.A., McPhail L.C. Coregulation of NADPH oxidase activation and phosphorylation of a 48-kD protein(s) by a cytosolic factor defective in autosomal recessive chronic granulomatous disease. J. Clin. Invest. 1988;81:1485–1496. doi: 10.1172/JCI113480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Curnutte J.T., Berkow R.L., Roberts R.L., Shurin S.B., Scott P.J. Chronic granulomatous disease due to a defect in the cytosolic factor required for nicotinamide adenine dinucleotide phosphate oxidase activation. J. Clin. Invest. 1988;81:606–610. doi: 10.1172/JCI113360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang J., Kleinberg M.E. Activation of the phagocyte NADPH oxidase protein p47(phox). Phosphorylation controls SH3 domain-dependent binding to p22(phox) J. Biol. Chem. 1999;274:19731–19737. doi: 10.1074/jbc.274.28.19731. [DOI] [PubMed] [Google Scholar]
- 51.McPhail L.C., Snyderman R. Activation of the respiratory burst enzyme in human polymorphonuclear leukocytes by chemoattractants and other soluble stimuli. Evidence that the same oxidase is activated by different transductional mechanisms. J. Clin. Invest. 1983;72:192–200. doi: 10.1172/JCI110957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McPhail L.C., Henson P.M., Johnston R.B. Respiratory burst enzyme in human neutrophils. Evidence for multiple mechanisms of activation. J. Clin. Invest. 1981;67:710–716. doi: 10.1172/JCI110087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simchowitz L., Spilberg I. Generation of superoxide radicals by human peripheral neutrophils activated by chemotactic factor. Evidence for the role of calcium. J. Lab. Clin. Med. 1979;93:583–593. doi: 10.5555/uri:pii:0022214379900076. [DOI] [PubMed] [Google Scholar]
- 54.Balce D.R., Rybicka J.M., Greene C.J., Ewanchuk B.W., Yates R.M. Ligation of FcγR alters phagosomal processing of protein via augmentation of NADPH oxidase activity., traffic (copenhagen. Denmark) 2016;17:786–802. doi: 10.1111/tra.12396. [DOI] [PubMed] [Google Scholar]
- 55.Bender J.G., McPhail L.C., van Epps D.E. Exposure of human neutrophils to chemotactic factors potentiates activation of the respiratory burst enzyme. Journal of Immunology. 1983;130:2316–2323. https://pubmed.ncbi.nlm.nih.gov/6300243/ (Baltimore, Md. : 1950) [PubMed] [Google Scholar]
- 56.Jiang F., Zhang Y., Dusting G.J. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol. Rev. 2011;63:218–242. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- 57.Lomax K.J., Leto T.L., Nunoi H., Gallin J.I., Malech H.L. Recombinant 47-kilodalton cytosol factor restores NADPH oxidase in chronic granulomatous disease., Science (New York, N.Y.) 1989. 245,409,412. [DOI] [PubMed]
- 58.Volpp B.D., Nauseef W.M., Donelson J.E., Moser D.R., Clark R.A. Cloning of the cDNA and functional expression of the 47-kilodalton cytosolic component of human neutrophil respiratory burst oxidase. Proc. Natl. Acad. Sci. U. S. A. 1989;86:7195–7199. doi: 10.1073/pnas.86.18.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sumimoto H., Kage Y., Nunoi H., Sasaki H., Nose T., Fukumaki Y., Ohno M., Minakami S., Takeshige K. Role of Src homology 3 domains in assembly and activation of the phagocyte NADPH oxidase. Proc. Natl. Acad. Sci. U. S. A. 1994;91:5345–5349. doi: 10.1073/pnas.91.12.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leto T.L., Adams A.G., de Mendez I. Assembly of the phagocyte NADPH oxidase: binding of Src homology 3 domains to proline-rich targets. Proc. Natl. Acad. Sci. U. S. A. 1994;91:10650–10654. doi: 10.1073/pnas.91.22.10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karathanassis D., Stahelin R. v, Bravo J., Perisic O., Pacold C.M., Cho W., Williams R.L. Binding of the PX domain of p47(phox) to phosphatidylinositol 3,4-bisphosphate and phosphatidic acid is masked by an intramolecular interaction. EMBO J. 2002;21:5057–5068. doi: 10.1093/emboj/cdf519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanai F., Liu H., Field S.J., Akbary H., Matsuo T., Brown G.E., Cantley L.C., Yaffe M.B. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K, Nat. Cell Biol. 2001;3:675–678. doi: 10.1038/35083070. [DOI] [PubMed] [Google Scholar]
- 63.Yaffe M.B. The p47phox PX domain: two heads are better than one! Structure. 2002;10:1288–1290. doi: 10.1016/S0969-2126(02)00860-2. [DOI] [PubMed] [Google Scholar]
- 64.Sumimoto H., Hata K., Mizuki K., Ito T., Kage Y., Sakaki Y., Fukumaki Y., Nakamura M., Takeshige K. Assembly and activation of the phagocyte NADPH oxidase. Specific interaction of the N-terminal Src homology 3 domain of p47phox with p22phox is required for activation of the NADPH oxidase. J. Biol. Chem. 1996;271:22152–22158. doi: 10.1074/jbc.271.36.22152. [DOI] [PubMed] [Google Scholar]
- 65.de Mendez I., Homayounpour N., Leto T.L. Specificity of p47phox SH3 domain interactions in NADPH oxidase assembly and activation. Mol. Cell Biol. 1997;17:2177–2185. doi: 10.1128/mcb.17.4.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Groemping Y., Lapouge K., Smerdon S.J., Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–355. doi: 10.1016/s0092-8674(03)00314-3. [DOI] [PubMed] [Google Scholar]
- 67.Taura M., Miyano K., Minakami R., Kamakura S., Takeya R., Sumimoto H. A region N-terminal to the tandem SH3 domain of p47phox plays a crucial role in the activation of the phagocyte NADPH oxidase. Biochem. J. 2009;419:329–338. doi: 10.1042/BJ20082028. [DOI] [PubMed] [Google Scholar]
- 68.de Mendez I., Adams A.G., Sokolic R.A., Malech H.L., Leto T.L. Multiple SH3 domain interactions regulate NADPH oxidase assembly in whole cells. EMBO J. 1996;15:1211–1220. doi: 10.1002/j.1460-2075.1996.tb00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leusen J.H., Bolscher B.G., Hilarius P.M., Weening R.S., Kaulfersch W., Seger R.A., Roos D., Verhoeven A.J. 156Pro-->Gln substitution in the light chain of cytochrome b558 of the human NADPH oxidase (p22-phox) leads to defective translocation of the cytosolic proteins p47-phox and p67-phox. J. Exp. Med. 1994;180:2329–2334. doi: 10.1084/jem.180.6.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leusen J.H.W., de Boer M., Bolscher B.G.J.M., Hilarius P.M., Weening R.S., Ochs H.D., Roos D., Verhoeven A.J. A point mutation in gp91-phox of cytochrome b558 of the human NADPH oxidase leading to defective translocation of the cytosolic proteins p47- phox and p67-phox. J. Clin. Invest. 1994;93:2120–2126. doi: 10.1172/jci117207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ito T., Nakamura R., Sumimoto H., Takeshige K., Sakaki Y. An SH3 domain-mediated interaction between the phagocyte NADPH oxidase factors p40phox and p47phox. FEBS (Fed. Eur. Biochem. Soc.) Lett. 1996;385:229–232. doi: 10.1016/0014-5793(96)00387-0. [DOI] [PubMed] [Google Scholar]
- 72.Nunoi H., Rotrosen D., Gallin J.I., Malech H.L. Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors., Science (New York, N.Y.) 1988. 242,1298,1301. [DOI] [PubMed]
- 73.Leto T.L., Lomax K.J., Volpp B.D., Nunoi H., Sechler J.M.G., Nauseef W.M., Clark R.A., Gallin J.I., Malech H.L. Cloning of a 67-kD neutrophil oxidase factor with similarity to a noncatalytic region of p60c-src. Science. 1990;248:727–730. doi: 10.1126/science.1692159. (New York, N.Y.) [DOI] [PubMed] [Google Scholar]
- 74.U. Francke, C.L. Hsieh, B.E. Foellmer, K.J. Lomax, H.L. Malech, T.L. Leto, Genes for two autosomal recessive forms of chronic granulomatous disease assigned to 1q25 (NCF2) and 7q11.23 (NCF1)., Am. J. Hum. Genet.. 47(1990) 483–492. https://pubmed.ncbi.nlm.nih.gov/2393022/(accessed February 4, 2021). [PMC free article] [PubMed]
- 75.Kenney R.T., Malech H.L., Epstein N.D., Roberts R.L., Leto T.L. Characterization of the p67phox gene: genomic organization and restriction fragment length polymorphism analysis for prenatal diagnosis in chronic granulomatous disease. Blood. 1993;82:3739–3744. doi: 10.1182/blood.v82.12.3739.bloodjournal82123739. [DOI] [PubMed] [Google Scholar]
- 76.Kenney R.T., Leto T.L. A HindIII polymorphism in the human NCF2 gene., nucleic acids research. 1990. 18,7193. [DOI] [PMC free article] [PubMed]
- 77.Noack D., Rae J., Cross A.R., Muñoz J., Salmen S., Mendoza J.A., Rossi N., Curnutte J.T., Heyworth P.G. Autosomal recessive chronic granulomatous disease caused by novel mutations in NCF-2, the gene encoding the p67-phox component of phagocyte NADPH oxidase. Hum. Genet. 1999;105:460–467. doi: 10.1007/s004390051131. [DOI] [PubMed] [Google Scholar]
- 78.de Mendez I., Garrett M.C., Adams A.G., Leto T.L. Role of p67-phox SH3 domains in assembly of the NADPH oxidase system. J. Biol. Chem. 1994;269:16326–16332. doi: 10.1016/S0021-9258(17)34011-5. [DOI] [PubMed] [Google Scholar]
- 79.Leusen J.H., Fluiter K., Hilarius P.M., Roos D., Verhoeven A.J., Bolscher B.G. Interactions between the cytosolic components p47phox and p67phox of the human neutrophil NADPH oxidase that are not required for activation in the cell-free system. J. Biol. Chem. 1995;270:11216–11221. doi: 10.1074/jbc.270.19.11216. [DOI] [PubMed] [Google Scholar]
- 80.Finan P., Koga H., Zvelebil M.J., Waterfield M.D., Kellie S. The C-terminal SH3 domain of p67phox binds its natural ligand in a reverse orientation. J. Mol. Biol. 1996;261:173–180. doi: 10.1006/jmbi.1996.0450. [DOI] [PubMed] [Google Scholar]
- 81.Wientjes F.B., Hsuan J.J., Totty N.F., Segal A.W. p40phox, a third cytosolic component of the activation complex of the NADPH oxidase to contain src homology 3 domains. the Biochemical Journal. 1993;296(Pt 3):557–561. doi: 10.1042/bj2960557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsunawaki S., Mizunari H., Nagata M., Tatsuzawa O., Kuratsuji T. A novel cytosolic component, p40phox, of respiratory burst oxidase associates with p67phox and is absent in patients with chronic granulomatous disease who lack p67phox, Biochem. Biophys. Res. Commun. 1994;199:1378–1387. doi: 10.1006/bbrc.1994.1383. [DOI] [PubMed] [Google Scholar]
- 83.Finan P., Shimizu Y., Gout I., Hsuan J., Truong O., Butcher C., Bennett P., Waterfield M.D., Kellie S. An SH3 domain and proline-rich sequence mediate an interaction between two components of the phagocyte NADPH oxidase complex. J. Biol. Chem. 1994;269:13752–13755. doi: 10.1016/S0021-9258(17)36710-8. [DOI] [PubMed] [Google Scholar]
- 84.Hata K., Takeshige K., Sumimoto H. Roles for proline-rich regions of p47phox and p67phox in the phagocyte NADPH oxidase activation in vitro. Biochem. Biophys. Res. Commun. 1997;241:226–231. doi: 10.1006/bbrc.1997.7807. [DOI] [PubMed] [Google Scholar]
- 85.Lapouge K., Smith S.J.M., Walker P.A., Gamblin S.J., Smerdon S.J., Rittinger K. Structure of the TPR domain of p67phox in complex with Rac.GTP. Mol. Cell. 2000;6:899–907. doi: 10.1016/s1097-2765(05)00091-2. [DOI] [PubMed] [Google Scholar]
- 86.Koga H., Terasawa H., Nunoi H., Takeshige K., Inagaki F., Sumimoto H. Tetratricopeptide repeat (TPR) motifs of p67(phox) participate in interaction with the small GTPase Rac and activation of the phagocyte NADPH oxidase. J. Biol. Chem. 1999;274:25051–25060. doi: 10.1074/jbc.274.35.25051. [DOI] [PubMed] [Google Scholar]
- 87.Groemping Y., Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem. J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Han C.H., Freeman J.L.R., Lee T., Motalebi S.A., Lambeth J.D. Regulation of the neutrophil respiratory burst oxidase. Identification of an activation domain in p67(phox) J. Biol. Chem. 1998;273:16663–16668. doi: 10.1074/jbc.273.27.16663. [DOI] [PubMed] [Google Scholar]
- 89.Nisimoto Y., Motalebi S., Han C.H., Lambeth J.D. The p67(phox) activation domain regulates electron flow from NADPH to flavin in flavocytochrome b(558)., J. Biol. Chem. 1999;274:22999–23005. doi: 10.1074/jbc.274.33.22999. [DOI] [PubMed] [Google Scholar]
- 90.Ito T., Matsui Y., Ago T., Ota K., Sumimoto H. Novel modular domain PB1 recognizes PC motif to mediate functional protein-protein interactions. EMBO J. 2001;20:3938–3946. doi: 10.1093/emboj/20.15.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dusi S., Donini M., Rossi F. Mechanisms of NADPH oxidase activation: translocation of p40phox, Rac1 and Rac2 from the cytosol to the membranes in human neutrophils lacking p47phox or p67phox. Biochem. J. 1996;314:409–412. doi: 10.1042/bj3140409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilson M.I., Gill D.J., Perisic O., Quinn M.T., Williams R.L. PB1 domain-mediated heterodimerization in NADPH oxidase and signaling complexes of atypical protein kinase C with Par6 and p62. Mol. Cell. 2003;12:39–50. doi: 10.1016/S1097-2765(03)00246-6. [DOI] [PubMed] [Google Scholar]
- 93.Tsunawaki S., Kagara S., Yoshikawa K., Yoshida L.S., Kuratsuji T., Namiki H. Involvement of p40phox in activation of phagocyte NADPH oxidase through association of its carboxyl-terminal, but not its amino-terminal, with p67phox. J. Exp. Med. 1996;184:893–902. doi: 10.1084/jem.184.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakamura R., Sumimoto H., Mizuki K., Hata K., Ago T., Kitajima S., Takeshige K., Sakaki Y., Ito T. The PC motif: a novel and evolutionarily conserved sequence involved in interaction between p40phox and p67phox, SH3 domain-containing cytosolic factors of the phagocyte NADPH oxidase., Eur. J. Biochem. 1998;251:583–589. doi: 10.1046/j.1432-1327.1998.2510583.x. [DOI] [PubMed] [Google Scholar]
- 95.Lapouge K., Smith S.J.M., Groemping Y., Rittinger K. Architecture of the p40-p47-p67phox complex in the resting state of the NADPH oxidase. A central role for p67phox. J. Biol. Chem. 2002;277:10121–10128. doi: 10.1074/jbc.M112065200. [DOI] [PubMed] [Google Scholar]
- 96.Wientjes F.B., Panayotou G., Reeves E., Segal A.W. Interactions between cytosolic components of the NADPH oxidase: p40phox interacts with both p67phox and p47phox. the Biochemical Journal. 1996;317(Pt 3):919–924. doi: 10.1042/bj3170919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bravo J., Karathanassis D., Pacold C.M., Pacold M.E., Ellson C.D., Anderson K.E., Butler P.J.G., Lavenir I., Perisic O., Hawkins P.T., Stephens L., Williams R.L. The crystal structure of the PX domain from p40phox bound to phosphatidylinositol 3-phosphate. Mol. Cell. 2001;8:829–839. doi: 10.1016/S1097-2765(01)00372-0. [DOI] [PubMed] [Google Scholar]
- 98.Suh C.-I., Stull N.D., Li X.J., Tian W., Price M.O., Grinstein S., Yaffe M.B., Atkinson S., Dinauer M.C. The phosphoinositide-binding protein p40phox activates the NADPH oxidase during FcgammaIIA receptor-induced phagocytosis. J. Exp. Med. 2006;203:1915–1925. doi: 10.1084/jem.20052085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tian W., Li X.J., Stull N.D., Ming W., Suh C.-I., Bissonnette S.A., Yaffe M.B., Grinstein S., Atkinson S.J., Dinauer M.C. Fc gamma R-stimulated activation of the NADPH oxidase: phosphoinositide-binding protein p40phox regulates NADPH oxidase activity after enzyme assembly on the phagosome. Blood. 2008;112:3867–3877. doi: 10.1182/blood-2007-11-126029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ellson C.D., Davidson K., Ferguson G.J., O'Connor R., Stephens L.R., Hawkins P.T. Neutrophils from p40phox-/- mice exhibit severe defects in NADPH oxidase regulation and oxidant-dependent bacterial killing. J. Exp. Med. 2006;203:1927–1937. doi: 10.1084/jem.20052069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ellson C., Davidson K., Anderson K., Stephens L.R., Hawkins P.T. PtdIns3P binding to the PX domain of p40phox is a physiological signal in NADPH oxidase activation. EMBO J. 2006;25:4468–4478. doi: 10.1038/sj.emboj.7601346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ellson C.D., Gobert-Gosse S., Anderson K.E., Davidson K., Erdjument-Bromage H., Tempst P., Thuring J.W., Cooper M.A., Lim Z.Y., Holmes A.B., Gaffney P.R.J., Coadwell J., Chilvers E.R., Hawkins P.T., Stephens L.R. PtdIns(3)P regulates the neutrophil oxidase complex by binding to the PX domain of p40(phox) Nat. Cell Biol. 2001;3:679–682. doi: 10.1038/35083076. [DOI] [PubMed] [Google Scholar]
- 103.Bouin A.P., Grandvaux N., Vignais P.v., Fuchs A. p40(phox) is phosphorylated on threonine 154 and serine 315 during activation of the phagocyte NADPH oxidase. Implication of a protein kinase c-type kinase in the phosphorylation process. J. Biol. Chem. 1998;273:30097–30103. doi: 10.1074/jbc.273.46.30097. [DOI] [PubMed] [Google Scholar]
- 104.Fuchs A., Bouin A.P., Rabilloud T., Vignais P. v. The 40-kDa component of the phagocyte NADPH oxidase (p40phox) is phosphorylated during activation in differentiated HL60 cells. Eur. J. Biochem. 1997;249:531–539. doi: 10.1111/j.1432-1033.1997.00531.x. [DOI] [PubMed] [Google Scholar]
- 105.Someya A., Nagaoka I., Nunoi H., Yamashita T. Translocation of Guinea pig p40-phox during activation of NADPH oxidase. Biochim. Biophys. Acta Bioenerg. 1996;1277:217–225. doi: 10.1016/S0005-2728(96)00099-0. [DOI] [PubMed] [Google Scholar]
- 106.Kuribayashi F., Nunoi H., Wakamatsu K., Tsunawaki S., Sato K., Ito T., Sumimoto H. The adaptor protein p40 phox as a positive regulator of the superoxide-producing phagocyte oxidase, EMBO J. 2002;21:6312–6320. doi: 10.1093/emboj/cdf642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cross A.R. p40(phox) Participates in the activation of NADPH oxidase by increasing the affinity of p47(phox) for flavocytochrome b(558) Biochem. J. 2000;349:113–117. doi: 10.1042/0264-6021:3490113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sathyamoorthy M., de Mendez I., Adams A.G., Leto T.L. p40(phox) down-regulates NADPH oxidase activity through interactions with its SH3 domain. J. Biol. Chem. 1997;272:9141–9146. doi: 10.1074/jbc.272.14.9141. [DOI] [PubMed] [Google Scholar]
- 109.Suh Y.A., Arnold R.S., Lassegue B., Shi J., Xu X., Sorescu D., Chung A.B., Griendling K.K., Lambeth J.D. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 110.Kikuchi H., Hikage M., Miyashita H., Fukumoto M. NADPH oxidase subunit, gp91(phox) homologue, preferentially expressed in human colon epithelial cells. Gene. 2000;254:237–243. doi: 10.1016/s0378-1119(00)00258-4. [DOI] [PubMed] [Google Scholar]
- 111.Takeya R., Ueno N., Kami K., Taura M., Kohjima M., Izaki T., Nunoi H., Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J. Biol. Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 112.Geiszt M., Lekstrom K., Witta J., Leto T.L. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J. Biol. Chem. 2003;278:20006–20012. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- 113.Debbabi M., Kroviarski Y., Bournier O., Gougerot-Pocidalo M.-A., El-Benna J., Dang P.M.-C. NOXO1 phosphorylation on serine 154 is critical for optimal NADPH oxidase 1 assembly and activation., FASEB Journal. Official Publication of the Federation of American Societies for Experimental Biology. 2013;27:1733–1748. doi: 10.1096/fj.12-216432. [DOI] [PubMed] [Google Scholar]
- 114.Cheng G., Diebold B.A., Hughes Y., Lambeth J.D. Nox1-dependent reactive oxygen generation is regulated by Rac1. J. Biol. Chem. 2006;281:17718–17726. doi: 10.1074/jbc.M512751200. [DOI] [PubMed] [Google Scholar]
- 115.Miyano K., Ueno N., Takeya R., Sumimoto H. Direct involvement of the small GTPase Rac in activation of the superoxide-producing NADPH oxidase Nox1. J. Biol. Chem. 2006;281:21857–21868. doi: 10.1074/jbc.M513665200. [DOI] [PubMed] [Google Scholar]
- 116.Ueyama T., Geiszt M., Leto T.L. Involvement of Rac1 in activation of multicomponent Nox1- and nox3-based NADPH oxidases. Mol. Cell Biol. 2006;26:2160–2174. doi: 10.1128/MCB.26.6.2160-2174.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kawahara T., Ritsick D., Cheng G., Lambeth J.D. Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J. Biol. Chem. 2005;280:31859–31869. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- 118.Leoni G., Alam A., Neumann P.-A., Lambeth J.D., Cheng G., McCoy J., Hilgarth R.S., Kundu K., Murthy N., Kusters D., Reutelingsperger C., Perretti M., Parkos C.A., Neish A.S., Nusrat A. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J. Clin. Invest. 2013;123:443–454. doi: 10.1172/JCI65831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Alam A., Leoni G., Wentworth C.C., Kwal J.M., Wu H., Ardita C.S., Swanson P.A., Lambeth J.D., Jones R.M., Nusrat A., Neish A.S. Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1. Mucosal Immunol. 2014;7:645–655. doi: 10.1038/mi.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Matziouridou C., Rocha S.D.C., Haabeth O.A., Rudi K., Carlsen H., Kielland A. iNOS- and NOX1-dependent ROS production maintains bacterial homeostasis in the ileum of mice. Mucosal Immunol. 2018;11:774–784. doi: 10.1038/mi.2017.106. [DOI] [PubMed] [Google Scholar]
- 121.Miller B.M., Liou M.J., Zhang L.F., Nguyen H., Litvak Y., Schorr E.-M., Jang K.K., Tiffany C.R., Butler B.P., Bäumler A.J. Anaerobic respiration of NOX1-derived hydrogen peroxide licenses bacterial growth at the colonic surface. Cell Host Microbe. 2020;28:789–797. doi: 10.1016/j.chom.2020.10.009. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chanin R.B., Winter M.G., Spiga L., Hughes E.R., Zhu W., Taylor S.J., Arenales A., Gillis C.C., Büttner L., Jimenez A.G., Smoot M.P., Santos R.L., Winter S.E. Epithelial-derived reactive oxygen species enable AppBCX-mediated aerobic respiration of Escherichia coli during intestinal inflammation. Cell Host Microbe. 2020;28:780–788. doi: 10.1016/j.chom.2020.09.005. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arnold R.S., Shi J., Murad E., Whalen A.M., Sun C.Q., Polavarapu R., Parthasarathy S., Petros J.A., Lambeth J.D. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc. Natl. Acad. Sci. U. S. A. 2001;98:5550–5555. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Arbiser J.L., Petros J., Klafter R., Govindajaran B., McLaughlin E.R., Brown L.F., Cohen C., Moses M., Kilroy S., Arnold R.S., Lambeth J.D. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc. Natl. Acad. Sci. U. S. A. 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Valente A.J., Zhou Q., Lu Z., He W., Qiang M., Ma W., Li G., Wang L., Banfi B., Steger K., Krause K.-H., Clark R.A., Li S. Regulation of NOX1 expression by GATA, HNF-1alpha, and Cdx transcription factors. Free Radical Biol. Med. 2008;44:430–443. doi: 10.1016/j.freeradbiomed.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 126.Adachi Y., Shibai Y., Mitsushita J., Shang W.H., Hirose K., Kamata T. Oncogenic Ras upregulates NADPH oxidase 1 gene expression through MEK-ERK-dependent phosphorylation of GATA-6. Oncogene. 2008;27:4921–4932. doi: 10.1038/onc.2008.133. [DOI] [PubMed] [Google Scholar]
- 127.Laurent E., McCoy J.W., Macina R.A., Liu W., Cheng G., Robine S., Papkoff J., Lambeth J.D. Nox1 is over-expressed in human colon cancers and correlates with activating mutations in K-Ras. Int. J. Cancer. 2008;123:100–107. doi: 10.1002/ijc.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stalin J., Garrido-Urbani S., Heitz F., Szyndralewiez C., Jemelin S., Coquoz O., Ruegg C., Imhof B.A. Inhibition of host NOX1 blocks tumor growth and enhances checkpoint inhibitor-based immunotherapy. Life Science Alliance. 2019;2 doi: 10.26508/lsa.201800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cheng G., Cao Z., Xu X., van Meir E.G., Lambeth J.D. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 130.R P., Ra B., F P., P W., Rj M., W J., U H., A M., A B., J L., A R., G S., Jc S., DE B. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004;18:486–491. doi: 10.1101/GAD.1172504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cheng G., Ritsick D., Lambeth J.D. Nox3 regulation by NOXO1, p47phox, and p67phox. J. Biol. Chem. 2004;279:34250–34255. doi: 10.1074/jbc.M400660200. [DOI] [PubMed] [Google Scholar]
- 132.Ueno N., Takeya R., Miyano K., Kikuchi H., Sumimoto H. The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: its regulation by oxidase organizers and activators. J. Biol. Chem. 2005;280:23328–23339. doi: 10.1074/jbc.M414548200. [DOI] [PubMed] [Google Scholar]
- 133.Nakano Y., Banfi B., Jesaitis A.J., Dinauer M.C., Allen L.-A.H., Nauseef W.M. Critical roles for p22phox in the structural maturation and subcellular targeting of Nox3. Biochem. J. 2007;403:97–108. doi: 10.1042/BJ20060819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bánfi B., Malgrange B., Knisz J., Steger K., Dubois-Dauphin M., Krause K.-H. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 135.Rousset F., Carnesecchi S., Senn P., Krause K.-H. NOX3-TARGETED therapies for inner ear pathologies. Curr. Pharmaceut. Des. 2015;21:5977–5987. doi: 10.2174/1381612821666151029112421. [DOI] [PubMed] [Google Scholar]
- 136.Mohri H., Ninoyu Y., Sakaguchi H., Hirano S., Saito N., Ueyama T. Nox3-derived superoxide in cochleae induces sensorineural hearing loss. J. Neurosci. 2021;41:4716–4731. doi: 10.1523/JNEUROSCI.2672-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shin Y.S., Song S.J., Kang S.U., Hwang H.S., Choi J.W., Lee B.H., Jung Y.-S., Kim C.-H. A novel synthetic compound, 3-amino-3-(4-fluoro-phenyl)-1H-quinoline-2,4-dione, inhibits cisplatin-induced hearing loss by the suppression of reactive oxygen species: in vitro and in vivo study. Neuroscience. 2013;232:1–12. doi: 10.1016/j.neuroscience.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 138.Rybak L.P., Mukherjea D., Jajoo S., Kaur T., Ramkumar V. siRNA-mediated knock-down of NOX3: therapy for hearing loss?, Cellular and Molecular Life Sciences. CMLS. 2012;69:2429–2434. doi: 10.1007/s00018-012-1016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Oishi N., Chen F.-Q., Zheng H.-W., Sha S.-H. Intra-tympanic delivery of short interfering RNA into the adult mouse cochlea. Hear. Res. 2013;296:36–41. doi: 10.1016/j.heares.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Carnesecchi S., Carpentier J.-L., Foti M., Szanto I. Insulin-induced vascular endothelial growth factor expression is mediated by the NADPH oxidase NOX3. Exp. Cell Res. 2006;312:3413–3424. doi: 10.1016/j.yexcr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 141.Gao D., Nong S., Huang X., Lu Y., Zhao H., Lin Y., Man Y., Wang S., Yang J., Li J. The effects of palmitate on hepatic insulin resistance are mediated by NADPH Oxidase 3-derived reactive oxygen species through JNK and p38MAPK pathways. J. Biol. Chem. 2010;285:29965–29973. doi: 10.1074/jbc.M110.128694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li L., He Q., Huang X., Man Y., Zhou Y., Wang S., Wang J., Li J. NOX3-derived reactive oxygen species promote TNF-alpha-induced reductions in hepatocyte glycogen levels via a JNK pathway. FEBS (Fed. Eur. Biochem. Soc.) Lett. 2010;584:995–1000. doi: 10.1016/j.febslet.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 143.Shiose A., Kuroda J., Tsuruya K., Hirai M., Hirakata H., Naito S., Hattori M., Sakaki Y., Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J. Biol. Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 144.Geiszt M., Kopp J.B., Várnai P., Leto T.L. Identification of renox, an NAD(P)H oxidase in kidney. Proc. Natl. Acad. Sci. U. S. A. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Martyn K.D., Frederick L.M., von Loehneysen K., Dinauer M.C., Knaus U.G. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell. Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 146.Serrander L., Cartier L., Bedard K., Banfi B., Lardy B., Plastre O., Sienkiewicz A., Fórró L., Schlegel W., Krause K.-H. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem. J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nisimoto Y., Jackson H.M., Ogawa H., Kawahara T., Lambeth J.D. Constitutive NADPH-dependent electron transferase activity of the Nox4 dehydrogenase domain. Biochemistry. 2010;49:2433–2442. doi: 10.1021/bi9022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nisimoto Y., Diebold B.A., Cosentino-Gomes D., Constentino-Gomes D., Lambeth J.D. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry. 2014;53:5111–5120. doi: 10.1021/bi500331y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ambasta R.K., Kumar P., Griendling K.K., Schmidt H.H.H.W., Busse R., Brandes R.P. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J. Biol. Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 150.Schröder K., Zhang M., Benkhoff S., Mieth A., Pliquett R., Kosowski J., Kruse C., Luedike P., Michaelis U.R., Weissmann N., Dimmeler S., Shah A.M., Brandes R.P. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 151.von Löhneysen K., Noack D., Hayes P., Friedman J.S., Knaus U.G. Constitutive NADPH oxidase 4 activity resides in the composition of the B-loop and the penultimate C terminus. J. Biol. Chem. 2012;287:8737–8745. doi: 10.1074/jbc.M111.332494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Takac I., Schröder K., Zhang L., Lardy B., Anilkumar N., Lambeth J.D., Shah A.M., Morel F., Brandes R.P. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li Z.-M., Xu S.-Y., Feng Y.-Z., Cheng Y.-R., Xiong J.-B., Zhou Y., Guan C.-X. The role of NOX4 in pulmonary diseases., J. Cell. Physiol. 2021;236:1628–1637. doi: 10.1002/jcp.30005. [DOI] [PubMed] [Google Scholar]
- 154.Lee Y.A., Kim K.A., Min A., Shin M.H. NOX4 activation is involved in ROS-dependent Jurkat T-cell death induced by Entamoeba histolytica. Parasite Immunol. 2019;41:e12670. doi: 10.1111/pim.12670. [DOI] [PubMed] [Google Scholar]
- 155.Ago T., Kuroda J., Pain J., Fu C., Li H., Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sedeek M., Callera G., Montezano A., Gutsol A., Heitz F., Szyndralewiez C., Page P., Kennedy C.R.J., Burns K.D., Touyz R.M., Hébert R.L. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy., American Journal of Physiology. Ren. Physiol. 2010;299:F1348–F1358. doi: 10.1152/ajprenal.00028.2010. [DOI] [PubMed] [Google Scholar]
- 157.Mittal M., Roth M., König P., Hofmann S., Dony E., Goyal P., Selbitz A.-C., Schermuly R.T., Ghofrani H.A., Kwapiszewska G., Kummer W., Klepetko W., Hoda M.A.R., Fink L., Hänze J., Seeger W., Grimminger F., Schmidt H.H.H.W., Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ. Res. 2007;101:258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 158.Pendyala S., Moitra J., Kalari S., Kleeberger S.R., Zhao Y., Reddy S.P., Garcia J.G.N., Natarajan V. Nrf2 regulates hyperoxia-induced Nox4 expression in human lung endothelium: identification of functional antioxidant response elements on the Nox4 promoter. Free Radical Biol. Med. 2011;50:1749–1759. doi: 10.1016/j.freeradbiomed.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang L., Sheppard O.R., Shah A.M., Brewer A.C. Positive regulation of the NADPH oxidase NOX4 promoter in vascular smooth muscle cells by E2F. Free Radic. Biol. Med. 2008;45:679–685. doi: 10.1016/j.freeradbiomed.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 160.Diebold I., Petry A., Hess J., Görlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol. Biol. Cell. 2010;21:2087–2096. doi: 10.1091/mbc.e09-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lu X., Bijli K.M., Ramirez A., Murphy T.C., Kleinhenz J., Hart C.M. Hypoxia downregulates PPARγ via an ERK1/2-NF-κB-Nox4-dependent mechanism in human pulmonary artery smooth muscle cells. Free Radical Biol. Med. 2013;63:151–160. doi: 10.1016/j.freeradbiomed.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lu X., Murphy T.C., Nanes M.S., Hart C.M. PPAR{gamma} regulates hypoxia-induced Nox4 expression in human pulmonary artery smooth muscle cells through NF-{kappa}B., American Journal of Physiology. Lung Cellular and Molecular Physiology. 2010;299:L559–L566. doi: 10.1152/ajplung.00090.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Goettsch C., Goettsch W., Brux M., Haschke C., Brunssen C., Muller G., Bornstein S.R., Duerrschmidt N., Wagner A.H., Morawietz H. Arterial flow reduces oxidative stress via an antioxidant response element and Oct-1 binding site within the NADPH oxidase 4 promoter in endothelial cells. Basic Res. Cardiol. 2011;106:551–561. doi: 10.1007/s00395-011-0170-3. [DOI] [PubMed] [Google Scholar]
- 164.Katsuyama M., Hirai H., Iwata K., Ibi M., Matsuno K., Matsumoto M., Yabe-Nishimura C. Sp3 transcription factor is crucial for transcriptional activation of the human NOX4 gene. FEBS J. 2011;278:964–972. doi: 10.1111/j.1742-4658.2011.08018.x. [DOI] [PubMed] [Google Scholar]
- 165.Siuda D., Zechner U., el Hajj N., Prawitt D., Langer D., Xia N., Horke S., Pautz A., Kleinert H., Förstermann U., Li H. Transcriptional regulation of Nox4 by histone deacetylases in human endothelial cells., Basic Research in Cardiology. 2012. 107,283. [DOI] [PubMed]
- 166.Manea A., Tanase L.I., Raicu M., Simionescu M. Jak/STAT signaling pathway regulates nox1 and nox4-based NADPH oxidase in human aortic smooth muscle cells., Arteriosclerosis. Thrombosis, and Vascular Biology. 2010;30:105–112. doi: 10.1161/ATVBAHA.109.193896. [DOI] [PubMed] [Google Scholar]
- 167.Bánfi B., Molnár G., Maturana A., Steger K., Hegedûs B., Demaurex N., Krause K.H. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 168.Bedard K., Jaquet V., Krause K.-H. NOX5: from basic biology to signaling and disease. Free Radical Biol. Med. 2012;52:725–734. doi: 10.1016/j.freeradbiomed.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 169.Prior K.-K., Leisegang M.S., Josipovic I., Löwe O., Shah A.M., Weissmann N., Schröder K., Brandes R.P. CRISPR/Cas9-mediated knockout of p22phox leads to loss of Nox1 and Nox4, but not Nox5 activity. Redox Biology. 2016;9:287–295. doi: 10.1016/j.redox.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kawahara T., Jackson H.M., Smith S.M.E., Simpson P.D., Lambeth J.D. Nox5 forms a functional oligomer mediated by self-association of its dehydrogenase domain. Biochemistry. 2011;50:2013–2025. doi: 10.1021/bi1020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kawahara T., Lambeth J.D. Phosphatidylinositol (4,5)-bisphosphate modulates Nox5 localization via an N-terminal polybasic region. Mol. Biol. Cell. 2008;19:4020–4031. doi: 10.1091/mbc.E07-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tirone F., Cox J.A. NADPH oxidase 5 (NOX5) interacts with and is regulated by calmodulin. FEBS (Fed. Eur. Biochem. Soc.) Lett. 2007;581:1202–1208. doi: 10.1016/j.febslet.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 173.Bánfi B., Tirone F., Durussel I., Knisz J., Moskwa P., Molnár G.Z., Krause K.H., Cox J.A. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5) J. Biol. Chem. 2004;279:18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 174.Fulton D.J.R. The molecular regulation and functional roles of NOX5., methods in molecular biology (clifton, N.J.) 2019. 1982,353,75. [DOI] [PubMed]
- 175.Jagnandan D., Church J.E., Banfi B., Stuehr D.J., Marrero M.B., Fulton D.J.R. Novel mechanism of activation of NADPH oxidase 5. calcium sensitization via phosphorylation. J. Biol. Chem. 2007;282:6494–6507. doi: 10.1074/jbc.M608966200. [DOI] [PubMed] [Google Scholar]
- 176.Petrushanko I.Y., Lobachev V.M., Kononikhin A.S., Makarov A.A., Devred F., Kovacic H., Kubatiev A.A., Tsvetkov P.O. Oxidation of Са2+-binding domain of NADPH oxidase 5 (NOX5): toward understanding the mechanism of inactivation of NOX5 by ROS. PLoS One. 2016;11:e0158726. doi: 10.1371/journal.pone.0158726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Serrander L., Jaquet V., Bedard K., Plastre O., Hartley O., Arnaudeau S., Demaurex N., Schlegel W., Krause K.-H. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie. 2007;89:1159–1167. doi: 10.1016/j.biochi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 178.Chen F., Yu Y., Haigh S., Johnson J., Lucas R., Stepp D.W., Fulton D.J.R. Regulation of NADPH oxidase 5 by protein kinase C isoforms. PLoS One. 2014;9:e88405. doi: 10.1371/journal.pone.0088405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bu-Ghanim H.N., Casimir C.M., Povey S., Segal A.W. The alpha subunit of cytochrome b-245 mapped to chromosome 16. Genomics. 1990;8:568–570. doi: 10.1016/0888-7543(90)90045-v. [DOI] [PubMed] [Google Scholar]
- 180.Conner G.E. Regulation of dual oxidase hydrogen peroxide synthesis results in an epithelial respiratory burst. Redox Biology. 2021;41:101931. doi: 10.1016/j.redox.2021.101931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Grasberger H., Refetoff S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J. Biol. Chem. 2006;281:18269–18272. doi: 10.1074/jbc.C600095200. [DOI] [PubMed] [Google Scholar]
- 182.Godlewska M., Banga P.J. Thyroid peroxidase as a dual active site enzyme: focus on biosynthesis, hormonogenesis and thyroid disorders of autoimmunity and cancer. Biochimie. 2019;160:34–45. doi: 10.1016/j.biochi.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 183.Moreno J.C., Bikker H., Kempers M.J.E., van Trotsenburg A.S.P., Baas F., de Vijlder J.J.M., Vulsma T., Ris-Stalpers C. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N. Engl. J. Med. 2002;347:95–102. doi: 10.1056/NEJMoa012752. [DOI] [PubMed] [Google Scholar]
- 184.Donkó Á., Morand S., Korzeniowska A., Boudreau H.E., Zana M., Hunyady L., Geiszt M., Leto T.L. Hypothyroidism-associated missense mutation impairs NADPH oxidase activity and intracellular trafficking of Duox2. Free Radical Biol. Med. 2014;73:190–200. doi: 10.1016/j.freeradbiomed.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Carvalho D.P., Dupuy C. Role of the NADPH oxidases DUOX and NOX4 in thyroid oxidative stress. European Thyroid Journal. 2013;2:160–167. doi: 10.1159/000354745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Donkó A., Ruisanchez E., Orient A., Enyedi B., Kapui R., Péterfi Z., de Deken X., Benyó Z., Geiszt M. Urothelial cells produce hydrogen peroxide through the activation of Duox1. Free Radical Biol. Med. 2010;49:2040–2048. doi: 10.1016/j.freeradbiomed.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 187.Edens W.A., Sharling L., Cheng G., Shapira R., Kinkade J.M., Lee T., Edens H.A., Tang X., Sullards C., Flaherty D.B., Benian G.M., Lambeth J.D. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J. Cell Biol. 2001;154:879–891. doi: 10.1083/jcb.200103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Meziani L., Gerbé de Thoré M., Hamon P., Bockel S., Louzada R.A., Clemenson C., Corre R., Liu W., Dupuy C., Mondini M., Deutsch E. Dual oxidase 1 limits the IFNγ-associated antitumor effect of macrophages. Journal for Immunotherapy of Cancer. 2020;8 doi: 10.1136/jitc-2020-000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kwon J., Shatynski K.E., Chen H., Morand S., de Deken X., Miot F., Leto T.L., Williams M.S. The nonphagocytic NADPH oxidase Duox1 mediates a positive feedback loop during T cell receptor signaling. Sci. Signal. 2010;3 doi: 10.1126/scisignal.2000976. ra59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sugamata R., Donko A., Murakami Y., Boudreau H.E., Qi C.-F., Kwon J., Leto T.L. Duox1 regulates primary B cell function under the influence of IL-4 through BCR-mediated generation of hydrogen peroxide. J. Immunol. 2019;202:428–440. doi: 10.4049/jimmunol.1601395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Feng Y.-Y., Tang M., Suzuki M., Gunasekara C., Anbe Y., Hiraoka Y., Liu J., Grasberger H., Ohkita M., Matsumura Y., Wang J.-Y., Tsubata T. Essential role of NADPH oxidase-dependent production of reactive oxygen species in maintenance of sustained B cell receptor signaling and B cell proliferation. J. Immunol. 2019;202:2546–2557. doi: 10.4049/jimmunol.1800443. [DOI] [PubMed] [Google Scholar]
- 192.Boots A.W., Hristova M., Kasahara D.I., Haenen G.R.M.M., Bast A., van der Vliet A. ATP-mediated activation of the NADPH oxidase DUOX1 mediates airway epithelial responses to bacterial stimuli. J. Biol. Chem. 2009;284:17858–17867. doi: 10.1074/jbc.M809761200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Koff J.L., Shao M.X.G., Kim S., Ueki I.F., Nadel J.A. Pseudomonas lipopolysaccharide accelerates wound repair via activation of a novel epithelial cell signaling cascade. Journal of Immunology. 2006;177:8693–8700. doi: 10.4049/jimmunol.177.12.8693. (Baltimore, MD: 1950) [DOI] [PubMed] [Google Scholar]
- 194.Koff J.L., Shao M.X.G., Ueki I.F., Nadel J.A. Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium., American Journal of Physiology. Lung Cellular and Molecular Physiology. 2008;294:L1068–L1075. doi: 10.1152/ajplung.00025.2008. [DOI] [PubMed] [Google Scholar]
- 195.Grandvaux N., Mariani M., Fink K. Lung epithelial NOX/DUOX and respiratory virus infections. Clin. Sci. (Lond.) 2015;128:337–347. doi: 10.1042/CS20140321. [DOI] [PubMed] [Google Scholar]
- 196.Geiszt M., Witta J., Baffi J., Lekstrom K., Leto T.L. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense., FASEB Journal. Official Publication of the Federation of American Societies for Experimental Biology. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 197.Joo J.-H., Ryu J.-H., Kim C.-H., Kim H.J., Suh M.-S., Kim J.-O., Chung S.Y., Lee S.N., Kim H.M., Bae Y.S., Yoon J.-H. Dual oxidase 2 is essential for the toll-like receptor 5-mediated inflammatory response in airway mucosa. Antioxidants Redox Signal. 2012;16:57–70. doi: 10.1089/ars.2011.3898. [DOI] [PubMed] [Google Scholar]
- 198.Sommer F., Bäckhed F. The gut microbiota engages different signaling pathways to induce Duox2 expression in the ileum and colon epithelium. Mucosal Immunol. 2015;8:372–379. doi: 10.1038/mi.2014.74. [DOI] [PubMed] [Google Scholar]
- 199.Ha E.-M., Oh C.-T., Bae Y.S., Lee W.-J. A direct role for dual oxidase in Drosophila gut immunity. Science (New York, N.Y.) 2005;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- 200.Chávez V., Mohri-Shiomi A., Garsin D.A. Ce-Duox1/BLI-3 generates reactive oxygen species as a protective innate immune mechanism in Caenorhabditis elegans. Infect. Immun. 2009;77:4983–4989. doi: 10.1128/IAI.00627-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Flores M.V., Crawford K.C., Pullin L.M., Hall C.J., Crosier K.E., Crosier P.S. Dual oxidase in the intestinal epithelium of zebrafish larvae has anti-bacterial properties. Biochem. Biophys. Res. Commun. 2010;400:164–168. doi: 10.1016/j.bbrc.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 202.Grasberger H., El-Zaatari M., Dang D.T., Merchant J.L. Dual oxidases control release of hydrogen peroxide by the gastric epithelium to prevent Helicobacter felis infection and inflammation in mice. Gastroenterology. 2013;145:1045–1054. doi: 10.1053/j.gastro.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nguyen G.T., Green E.R., Mecsas J. Neutrophils to the ROScue: mechanisms of NADPH oxidase activation and bacterial resistance. Frontiers in Cellular and Infection Microbiology. 2017;7 doi: 10.3389/fcimb.2017.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ramsey I.S., Ruchti E., Kaczmarek J.S., Clapham D.E. Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc. Natl. Acad. Sci. U. S. A. 2009;106:7642–7647. doi: 10.1073/pnas.0902761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Morgan D., Capasso M., Musset B., Cherny V. v, Ríos E., Dyer M.J.S., DeCoursey T.E. Voltage-gated proton channels maintain pH in human neutrophils during phagocytosis. Proc. Natl. Acad. Sci. U. S. A. 2009;106:18022–18027. doi: 10.1073/pnas.0905565106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Petheo G.L., Orient A., Baráth M., Kovács I., Réthi B., Lányi Á., Rajki A., Rajnavölgyi É., Geiszt M. Molecular and functional characterization of Hv1 proton channel in human granulocytes. PLoS One. 2010;5 doi: 10.1371/journal.pone.0014081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.E.P. Reeves, H. Lu, H.L. Jacobs, C.G.M. Messina, S. Bolsover, G. Gabella, E.O. Potma, A. Warley, J. Roes, A.W. Segal, Killing activity of neutrophils is mediated through activation of proteases by K+ flux., Nature. 416 (2002) 291–297. 10.1038/416291a. [DOI] [PubMed]
- 208.Ap L., Aw S. The NADPH oxidase and microbial killing by neutrophils, with a particular emphasis on the proposed antimicrobial role of myeloperoxidase within the phagocytic vacuole, in: myeloid cells in health and disease. American society of microbiology. 2016:599–613. doi: 10.1128/microbiolspec.mchd-0018-2015. [DOI] [PubMed] [Google Scholar]
- 209.Klebanoff S.J., Kettle A.J., Rosen H., Winterbourn C.C., Nauseef W.M. Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J. Leukoc. Biol. 2013;93:185–198. doi: 10.1189/jlb.0712349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Levine A.P., Duchen M.R., de Villiers S., Rich P.R., Segal A.W. Alkalinity of neutrophil phagocytic vacuoles is modulated by HVCN1 and has consequences for myeloperoxidase activity. PLoS One. 2015;10:e0125906. doi: 10.1371/journal.pone.0125906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Segal A.W., Geisow M., Garcia R., Harper A., Miller R. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH, Nature. 1981;290:406–409. doi: 10.1038/290406a0. [DOI] [PubMed] [Google Scholar]
- 212.Klebanoff S.J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J. Bacteriol. 1968;95:2131–2138. doi: 10.1128/JB.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Klebanoff S.J. Iodination of bacteria: a bactericidal mechanism. J. Exp. Med. 1967;126:1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kettle A.J., Winterbourn C.C. A kinetic analysis of the catalase activity of myeloperoxidase. Biochemistry. 2001;40:10204–10212. doi: 10.1021/bi010940b. [DOI] [PubMed] [Google Scholar]
- 215.Kettle A.J., Anderson R.F., Hampton M.B., Winterbourn C.C. Reactions of superoxide with myeloperoxidase. Biochemistry. 2007;46:4888–4897. doi: 10.1021/bi602587k. [DOI] [PubMed] [Google Scholar]
- 216.Albrett A.M., Ashby L. v, Dickerhof N., Kettle A.J., Winterbourn C.C. Heterogeneity of hypochlorous acid production in individual neutrophil phagosomes revealed by a rhodamine-based probe. J. Biol. Chem. 2018;293:15715–15724. doi: 10.1074/jbc.RA118.004789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Königstorfer A., Ashby L. v, Bollar G.E., Billiot C.E., Gray M.J., Jakob U., Hampton M.B., Winterbourn C.C. Induction of the reactive chlorine-responsive transcription factor RclR in Escherichia coli following ingestion by neutrophils. Pathogens and Disease. 2021;79 doi: 10.1093/femspd/ftaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yi L., Liu Q., Orandle M.S., Sadiq-Ali S., Koontz S.M., Choi U., Torres-Velez F.J., Jackson S.H. p47(phox) directs murine macrophage cell fate decisions. Am. J. Pathol. 2012;180:1049–1058. doi: 10.1016/j.ajpath.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Barrett J.P., Henry R.J., Villapol S., Stoica B.A., Kumar A., Burns M.P., Faden A.I., Loane D.J. NOX2 deficiency alters macrophage phenotype through an IL-10/STAT3 dependent mechanism: implications for traumatic brain injury. J. Neuroinflammation. 2017;14:65. doi: 10.1186/s12974-017-0843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Padgett L.E., Burg A.R., Lei W., Tse H.M. Loss of NADPH oxidase-derived superoxide skews macrophage phenotypes to delay type 1 diabetes. Diabetes. 2015;64:937–946. doi: 10.2337/db14-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Helfinger V., Palfi K., Weigert A., Schröder K. The NADPH oxidase Nox4 controls macrophage polarization in an NFκB-dependent manner. Oxidative Medicine and Cellular Longevity. 2019:3264858. doi: 10.1155/2019/3264858. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.He C., Larson Casey J.L., Davis D., Hanumanthu V.S., Longhini A.L.F., Thannickal V.J., Gu L., Carter A.B. NOX4 modulates macrophage phenotype and mitochondrial biogenesis in asbestosis. JCI Insight. 2019;4 doi: 10.1172/jci.insight.126551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Larson Casey J.L., Gu L., Kang J., Dhyani A., Carter A.B. NOX4 regulates macrophage apoptosis resistance to induce fibrotic progression. J. Biol. Chem. 2021;297:100810. doi: 10.1016/j.jbc.2021.100810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhang J., Li H., Wu Q., Chen Y., Deng Y., Yang Z., Zhang L., Liu B. Tumoral NOX4 recruits M2 tumor-associated macrophages via ROS/PI3K signaling-dependent various cytokine production to promote NSCLC growth. Redox Biology. 2019;22:101116. doi: 10.1016/j.redox.2019.101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lee Y., Kim S.-M., Jung E.-H., Park J., Lee J.W., Han I.-O. Lithium chloride promotes lipid accumulation through increased reactive oxygen species generation., Biochimica et Biophysica Acta. Molecular and Cell Biology of Lipids. 2020;1865:158552. doi: 10.1016/j.bbalip.2019.158552. [DOI] [PubMed] [Google Scholar]
- 226.Rada B., Park J.J., Sil P., Geiszt M., Leto T.L. NLRP3 inflammasome activation and interleukin-1β release in macrophages require calcium but are independent of calcium-activated NADPH oxidases., Inflammation Research. Official Journal of the European Histamine Research Society... [et Al.] 2014;63:821–830. doi: 10.1007/s00011-014-0756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Singla B., Ghoshal P., Lin H., Wei Q., Dong Z., Csányi G. Pkcδ-mediated Nox2 activation promotes fluid-phase pinocytosis of antigens by immature dendritic cells. Front. Immunol. 2018;9:537. doi: 10.3389/fimmu.2018.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Singla B., Lin H.-P., Ghoshal P., Cherian-Shaw M., Csányi G. PKCδ stimulates macropinocytosis via activation of SSH1-cofilin pathway. Cell. Signal. 2019;53:111–121. doi: 10.1016/j.cellsig.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ewanchuk B.W., Yates R.M. The phagosome and redox control of antigen processing. Free Radical Biol. Med. 2018;125:53–61. doi: 10.1016/j.freeradbiomed.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 230.Savina A., Jancic C., Hugues S., Guermonprez P., Vargas P., Moura I.C., Lennon-Duménil A.-M., Seabra M.C., Raposo G., Amigorena S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 231.Rybicka J.M., Balce D.R., Chaudhuri S., Allan E.R.O., Yates R.M. Phagosomal proteolysis in dendritic cells is modulated by NADPH oxidase in a pH-independent manner. EMBO J. 2012;31:932–944. doi: 10.1038/emboj.2011.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rybicka J.M., Balce D.R., Khan M.F., Krohn R.M., Yates R.M. NADPH oxidase activity controls phagosomal proteolysis in macrophages through modulation of the lumenal redox environment of phagosomes. Proc. Natl. Acad. Sci. U. S. A. 2010;107:10496–10501. doi: 10.1073/pnas.0914867107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Arunachalam B., Phan U.T., Geuze H.J., Cresswell P. Enzymatic reduction of disulfide bonds in lysosomes: characterization of a gamma-interferon-inducible lysosomal thiol reductase (GILT) Proc. Natl. Acad. Sci. U. S. A. 2000;97:745–750. doi: 10.1073/pnas.97.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Balce D.R., Allan E.R.O., McKenna N., Yates R.M. γ-Interferon-inducible lysosomal thiol reductase (GILT) maintains phagosomal proteolysis in alternatively activated macrophages. J. Biol. Chem. 2014;289:31891–31904. doi: 10.1074/jbc.M114.584391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mantegazza A.R., Savina A., Vermeulen M., Pérez L., Geffner J., Hermine O., Rosenzweig S.D., Faure F., Amigorena S. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood. 2008;112:4712–4722. doi: 10.1182/blood-2008-01-134791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dingjan I., Paardekooper L.M., Verboogen D.R.J., von Mollard G.F., ter Beest M., van den Bogaart G. VAMP8-mediated NOX2 recruitment to endosomes is necessary for antigen release. Eur. J. Cell Biol. 2017;96:705–714. doi: 10.1016/j.ejcb.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Dingjan I., Verboogen D.R.J., Paardekooper L.M., Revelo N.H., Sittig S.P., Visser L.J., von Mollard G.F., Henriet S.S.V., Figdor C.G., ter Beest M., van den Bogaart G. Lipid peroxidation causes endosomal antigen release for cross-presentation. Sci. Rep. 2016;6:22064. doi: 10.1038/srep22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Savina A., Peres A., Cebrian I., Carmo N., Moita C., Hacohen N., Moita L.F., Amigorena S. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8(+) dendritic cells. Immunity. 2009;30:544–555. doi: 10.1016/j.immuni.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 239.Rojas Márquez J.D., Li T., McCluggage A.R.R., Tan J.M.J., Muise A., Higgins D.E., Brumell J.H., Edge Cutting. NOX2 NADPH oxidase controls infection by an intracellular bacterial pathogen through limiting the type 1 IFN response. J. Immunol. 2021;206:323–328. doi: 10.4049/jimmunol.2000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Seleme M.C., Lei W., Burg A.R., Goh K.Y., Metz A., Steele C., Tse H.M. Dysregulated TLR3-dependent signaling and innate immune activation in superoxide-deficient macrophages from nonobese diabetic mice. Free Radic. Biol. Med. 2012;52:2047–2056. doi: 10.1016/j.freeradbiomed.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Burg A.R., Das S., Padgett L.E., Koenig Z.E., Tse H.M. Superoxide production by NADPH oxidase intensifies macrophage antiviral responses during diabetogenic coxsackievirus infection. J. Immunol. 2018;200:61–70. doi: 10.4049/jimmunol.1700478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Soucy-Faulkner A., Mukawera E., Fink K., Martel A., Jouan L., Nzengue Y., Lamarre D., vande Velde C., Grandvaux N. Requirement of NOX2 and reactive oxygen species for efficient RIG-I-mediated antiviral response through regulation of MAVS expression. PLoS Pathog. 2010;6:e1000930. doi: 10.1371/journal.ppat.1000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wang W., Jin Y., Zeng N., Ruan Q., Qian F. SOD2 facilitates the antiviral innate immune response by scavenging reactive oxygen species. Viral Immunol. 2017;30:582–589. doi: 10.1089/vim.2017.0043. [DOI] [PubMed] [Google Scholar]
- 244.Stawowczyk M., Naseem S., Montoya V., Baker D.P., Konopka J., Reich N.C. Pathogenic effects of IFIT2 and interferon-β during fatal systemic Candida albicans infection. mBio. 2018;9 doi: 10.1128/mBio.00365-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sarr D., Gingerich A.D., Asthiwi N.M., Almutairi F., Sautto G.A., Ecker J., Nagy T., Kilgore M.B., Chandler J.D., Ross T.M., Tripp R.A., Rada B. Dual oxidase 1 promotes antiviral innate immunity. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2017130118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kim H.J., Kim C.-H., Ryu J.-H., Kim M.-J., Park C.Y., Lee J.M., Holtzman M.J., Yoon J.-H. Reactive oxygen species induce antiviral innate immune response through IFN-λ regulation in human nasal epithelial cells. Am. J. Respir. Cell Mol. Biol. 2013;49:855–865. doi: 10.1165/rcmb.2013-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Strengert M., Jennings R., Davanture S., Hayes P., Gabriel G., Knaus U.G. Mucosal reactive oxygen species are required for antiviral response: role of Duox in influenza a virus infection. Antioxidants Redox Signal. 2014;20:2695–2709. doi: 10.1089/ars.2013.5353. [DOI] [PubMed] [Google Scholar]
- 248.Kim B.J., Cho S.W., Jeon Y.J., An S., Jo A., Lim J.H., Kim D.-Y., Won T.-B., Han D.H., Rhee C.-S., Kim H.J. Intranasal delivery of Duox2 DNA using cationic polymer can prevent acute influenza A viral infection in vivo lung. Appl. Microbiol. Biotechnol. 2018;102:105–115. doi: 10.1007/s00253-017-8512-1. [DOI] [PubMed] [Google Scholar]
- 249.Fink K., Martin L., Mukawera E., Chartier S., de Deken X., Brochiero E., Miot F., Grandvaux N. IFNβ/TNFα synergism induces a non-canonical STAT2/IRF9-dependent pathway triggering a novel DUOX2 NADPH oxidase-mediated airway antiviral response. Cell Res. 2013;23:673–690. doi: 10.1038/cr.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hong S.-N., Kim J.Y., Kim H., Kim D.-Y., Won T.-B., Han D.H., Rhee C.-S., Kim H.J. Duox2 is required for the transcription of pattern recognition receptors in acute viral lung infection: an interferon-independent regulatory mechanism. Antivir. Res. 2016;134:1–5. doi: 10.1016/j.antiviral.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 251.Kim H.J., Kim C.-H., Kim M.-J., Ryu J.-H., Seong S.Y., Kim S., Lim S.J., Holtzman M.J., Yoon J.-H. The induction of pattern-recognition receptor expression against influenza A virus through duox2-derived reactive oxygen species in nasal mucosa. Am. J. Respir. Cell Mol. Biol. 2015;53:525–535. doi: 10.1165/rcmb.2014-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Dostert C., Pétrilli V., van Bruggen R., Steele C., Mossman B.T., Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Xu T., Sun L., Shen X., Chen Y., Yin Y., Zhang J., Huang D., Li W., Li W. NADPH oxidase 2-mediated NLRP1 inflammasome activation involves in neuronal senescence in hippocampal neurons in vitro. Int. Immunopharm. 2019;69:60–70. doi: 10.1016/j.intimp.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 254.Sun L., Chen Y., Shen X., Xu T., Yin Y., Zhang H., Ding S., Zhao Y., Zhang Y., Guan Y., Li W. Inhibition of NOX2-NLRP1 signaling pathway protects against chronic glucocorticoids exposure-induced hippocampal neuronal damage. Int. Immunopharm. 2019;74 doi: 10.1016/j.intimp.2019.105721. [DOI] [PubMed] [Google Scholar]
- 255.Ko J., Kang H.J., Kim D.A., Ryu E.S., Yu M., Lee H., Lee H.K., Ryu H.M., Park S.H., Kim Y.L., Kang D.H. Paricalcitol attenuates TGF-b1-induced phenotype transition of human peritoneal mesothelial cells (HPMCs) via modulation of oxidative stress and NLRP3 inflammasome. FASEB (Fed. Am. Soc. Exp. Biol.) J. 2019;33:3035–3050. doi: 10.1096/fj.201800292RR. [DOI] [PubMed] [Google Scholar]
- 256.Liu G., Liu Q., Yan B., Zhu Z., Xu Y. USP7 inhibition alleviates H2O2-induced injury in chondrocytes via inhibiting NOX4/NLRP3 pathway. Front. Pharmacol. 2021;11 doi: 10.3389/fphar.2020.617270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Cai S.M., Yang R.Q., Li Y., Ning Z.W., Zhang L.L., Zhou G.S., Luo W., Li D.H., Chen Y., Pan M.X., Li X. Angiotensin-(1-7) improves liver fibrosis by regulating the NLRP3 inflammasome via redox balance modulation. Antioxidants Redox Signal. 2016;24:795–812. doi: 10.1089/ars.2015.6498. [DOI] [PubMed] [Google Scholar]
- 258.Moon J.-S., Nakahira K., Chung K.-P., DeNicola G.M., Koo M.J., Pabón M.A., Rooney K.T., Yoon J.-H., Ryter S.W., Stout-Delgado H., Choi A.M.K. NOX4-dependent fatty acid oxidation promotes NLRP3 inflammasome activation in macrophages. Nat. Med. 2016;22:1002–1012. doi: 10.1038/nm.4153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 259.Pfeiffer Z.A., Guerra A.N., Hill L.M., Gavala M.L., Prabhu U., Aga M., Hall D.J., Bertics P.J. Nucleotide receptor signaling in murine macrophages is linked to reactive oxygen species generation. Free Radic. Biol. Med. 2007;42:1506–1516. doi: 10.1016/j.freeradbiomed.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Noguchi T., Ishii K., Fukutomi H., Naguro I., Matsuzawa A., Takeda K., Ichijo H. Requirement of reactive oxygen species-dependent activation of ASK1-p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophage. J. Biol. Chem. 2008;283:7657–7665. doi: 10.1074/jbc.M708402200. [DOI] [PubMed] [Google Scholar]
- 261.Cruz C.M., Rinna A., Forman H.J., Ventura A.L.M., Persechini P.M., Ojcius D.M. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Meissner F., Seger R.A., Moshous D., Fischer A., Reichenbach J., Zychlinsky A. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood. 2010;116:1570–1573. doi: 10.1182/blood-2010-01-264218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sokolovska A., Becker C.E., Ip W.K.E., Rathinam V.A.K., Brudner M., Paquette N., Tanne A., Vanaja S.K., Moore K.J., Fitzgerald K.A., Lacy-Hulbert A., Stuart L.M. Activation of caspase-1 by the NLRP3 inflammasome regulates the NADPH oxidase NOX2 to control phagosome function. Nat. Immunol. 2013;14:543–553. doi: 10.1038/ni.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Conley S.M., Abais-Battad J.M., Yuan X., Zhang Q., Boini K.M., Li P.L. Contribution of guanine nucleotide exchange factor Vav2 to NLRP3 inflammasome activation in mouse podocytes during hyperhomocysteinemia. Free Radic. Biol. Med. 2017;106:236–244. doi: 10.1016/j.freeradbiomed.2017.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sarkar S., Kimono D., Albadrani M., Seth R.K., Busbee P., Alghetaa H., Porter D.E., Scott G.I., Brooks B., Nagarkatti M., Nagarkatti P., Chatterjee S. Environmental microcystin targets the microbiome and increases the risk of intestinal inflammatory pathology via NOX2 in underlying murine model of Nonalcoholic Fatty Liver Disease. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-45009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Yen W.-C., Wu Y.-H., Wu C.-C., Lin H.-R., Stern A., Chen S.-H., Shu J.-C., Tsun-Yee Chiu D. Impaired inflammasome activation and bacterial clearance in G6PD deficiency due to defective NOX/p38 MAPK/AP-1 redox signaling. Redox Biology. 2020;28:101363. doi: 10.1016/j.redox.2019.101363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Brown D.I., Griendling K.K. Nox proteins in signal transduction. Free Radical Biol. Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Bigelow D.J., Squier T.C. Redox modulation of cellular signaling and metabolism through reversible oxidation of methionine sensors in calcium regulatory proteins. Biochim. Biophys. Acta. 2005;1703:121–134. doi: 10.1016/j.bbapap.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 269.Groitl B., Jakob U. Thiol-based redox switches. Biochim. Biophys. Acta. 2014;1844:1335–1343. doi: 10.1016/j.bbapap.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Denu J.M., Tanner K.G. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 271.Kwon J., Lee S.-R., Yang K.-S., Ahn Y., Kim Y.J., Stadtman E.R., Rhee S.G. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl. Acad. Sci. U. S. A. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Meng T.-C., Fukada T., Tonks N.K. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 273.Leslie N.R., Bennett D., Lindsay Y.E., Stewart H., Gray A., Downes C.P. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Park H.S., Lee S.H., Park D., Lee J.S., Ryu S.H., Lee W.J., Rhee S.G., Bae Y.S. Sequential activation of phosphatidylinositol 3-kinase, beta Pix, Rac1, and Nox1 in growth factor-induced production of H2O2. Mol. Cell Biol. 2004;24:4384–4394. doi: 10.1128/mcb.24.10.4384-4394.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Oh H., Jung H.Y., Kim J., Bae Y.S. Phosphorylation of serine282 in NADPH oxidase activator 1 by Erk desensitizes EGF-induced ROS generation. Biochem. Biophys. Res. Commun. 2010;394:691–696. doi: 10.1016/j.bbrc.2010.03.053. [DOI] [PubMed] [Google Scholar]
- 276.Kroviarski Y., Debbabi M., Bachoual R., Périanin A., Gougerot-Pocidalo M.A., El-Benna J., Dang P.M.C. Phosphorylation of NADPH oxidase activator 1 (NOXA1) on serine 282 by MAP kinases and on serine 172 by protein kinase C and protein kinase a prevents NOX1 hyperactivation. FASEB (Fed. Am. Soc. Exp. Biol.) J. 2010;24:2077–2092. doi: 10.1096/fj.09-147629. [DOI] [PubMed] [Google Scholar]
- 277.Paulsen C.E., Truong T.H., Garcia F.J., Homann A., Gupta V., Leonard S.E., Carroll K.S. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat. Chem. Biol. 2011;8:57–64. doi: 10.1038/nchembio.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Chen K., Kirber M.T., Xiao H., Yang Y., Keaney J.F. Regulation of ROS signal transduction by NADPH oxidase 4 localization. JCB (J. Cell Biol.) 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Yang M., Haase C., Viljanen J., Xu B., Ge C., Kihlberg J., Holmdahl R. Cutting edge: processing of oxidized peptides in macrophages regulates T cell activation and development of autoimmune arthritis. J. Immunol. 2017;199:3937–3942. doi: 10.4049/jimmunol.1700774. [DOI] [PubMed] [Google Scholar]
- 280.Shaw M.H., Boyartchuk V., Wong S., Karaghiosoff M., Ragimbeau J., Pellegrini S., Muller M., Dietrich W.F., Yap G.S. A natural mutation in the Tyk2 pseudokinase domain underlies altered susceptibility of B10.Q/J mice to infection and autoimmunity. Proc. Natl. Acad. Sci. U. S. A. 2003;100:11594–11599. doi: 10.1073/pnas.1930781100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Piganelli J.D., Flores S.C., Cruz C., Koepp J., Batinic-Haberle I., Crapo J., Day B., Kachadourian R., Young R., Bradley B., Haskins K. A metalloporphyrin-based superoxide dismutase mimic inhibits adoptive transfer of autoimmune diabetes by a diabetogenic T-cell clone., Diabetes. 2002;51:347–355. doi: 10.2337/diabetes.51.2.347. [DOI] [PubMed] [Google Scholar]
- 282.Tse H.M., Milton M.J., Schreiner S., Profozich J.L., Trucco M., Piganelli J.D. Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. J. Immunol. 2007;178:908–917. doi: 10.4049/jimmunol.178.2.908. [DOI] [PubMed] [Google Scholar]
- 283.Anderson M.S., Bluestone J.A. The NOD mouse: a model of immune dysregulation. Annu. Rev. Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 284.Tse H.M., Milton M.J., Piganelli J.D. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: implication for their use in targeting oxidation-reduction reactions in innate immunity. Free Radical Biol. Med. 2004;36:233–247. doi: 10.1016/j.freeradbiomed.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 285.Feduska J.M., Kozlovskaya V., Alford A., Padgett L.E., Kharlampieva E., Tse H.M. Dampening antigen-specific T cell responses with antigens encapsulated in polyphenolic microcapsules. ImmunoHorizons. 2020;4:530–545. doi: 10.4049/immunohorizons.2000049. [DOI] [PubMed] [Google Scholar]
- 286.Barra J.M., Kozlovskaya V., Kharlampieva E., Tse H.M. Localized immunosuppression with tannic acid encapsulation delays islet allograft and autoimmune-mediated rejection. Diabetes. 2020;69:1948–1960. doi: 10.2337/db20-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.To E.E., Vlahos R., Luong R., Halls M.L., Reading P.C., King P.T., Chan C., Drummond G.R., Sobey C.G., Broughton B.R.S., Starkey M.R., van der Sluis R., Lewin S.R., Bozinovski S., O'Neill L.A.J., Quach T., Porter C.J.H., Brooks D.A., O'Leary J.J., Selemidis S. Endosomal NOX2 oxidase exacerbates virus pathogenicity and is a target for antiviral therapy. Nat. Commun. 2017;8:69. doi: 10.1038/s41467-017-00057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Cachat J., Deffert C., Alessandrini M., Roux-Lombard P., le Gouellec A., Stasia M.-J., Hugues S., Krause K.-H. Altered humoral immune responses and IgG subtypes in NOX2-deficient mice and patients: a key role for NOX2 in antigen-presenting cells. Front. Immunol. 2018;9:1555. doi: 10.3389/fimmu.2018.01555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Damiano S., Sozio C., la Rosa G., Santillo M. NOX-dependent signaling dysregulation in severe COVID-19: clues to effective treatments. Frontiers in Cellular and Infection Microbiology. 2020;10:608435. doi: 10.3389/fcimb.2020.608435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Violi F., Oliva A., Cangemi R., Ceccarelli G., Pignatelli P., Carnevale R., Cammisotto V., Lichtner M., Alessandri F., de Angelis M., Miele M.C., D'Ettorre G., Ruberto F., Venditti M., Pugliese F., Mastroianni C.M. Nox2 activation in covid-19. Redox Biology. 2020;36:101655. doi: 10.1016/j.redox.2020.101655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Fratta Pasini A.M., Stranieri C., Cominacini L., Mozzini C. Potential role of antioxidant and anti-inflammatory therapies to prevent severe SARS-cov-2 complications., antioxidants (basel, Switzerland) 2021. 10,1,22. [DOI] [PMC free article] [PubMed]
- 292.Polonikov A. Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients. ACS Infect. Dis. 2020;6:1558–1562. doi: 10.1021/acsinfecdis.0c00288. [DOI] [PubMed] [Google Scholar]
- 293.Abouhashem A.S., Singh K., Azzazy H.M.E., Sen C.K. Is low alveolar type II cell SOD3 in the lungs of elderly linked to the observed severity of COVID-19? Antioxidants Redox Signal. 2020;33:59–65. doi: 10.1089/ars.2020.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Dean M.J., Ochoa J.B., Sanchez-Pino M.D., Zabaleta J., Garai J., del Valle L., Wyczechowska D., Baiamonte L.B., Philbrook P., Majumder R., vander Heide R.S., Dunkenberger L., Thylur R.P., Nossaman B., Roberts W.M., Chapple A.G., Wu J., Hicks C., Collins J., Luke B., Johnson R., Koul H.K., Rees C.A., Morris C.R., Garcia-Diaz J., Ochoa A.C. Severe COVID-19 is characterized by an impaired type I interferon response and elevated levels of Arginase producing granulocytic myeloid derived suppressor cells., frontiers in Immunology. 2021. 12,695972. [DOI] [PMC free article] [PubMed]
- 295.Schönrich G., Raftery M.J., Samstag Y. Devilishly radical NETwork in COVID-19: oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Advances in Biological Regulation. 2020;77:100741. doi: 10.1016/j.jbior.2020.100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Laforge M., Elbim C., Frère C., Hémadi M., Massaad C., Nuss P., Benoliel J.-J., Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19., Nature Reviews. Immunology. 2020;20:515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Ye W., Chen G., Li X., Lan X., Ji C., Hou M., Zhang D., Zeng G., Wang Y., Xu C., Lu W., Cui R., Cai Y., Huang H., Yang L. Dynamic changes of D-dimer and neutrophil-lymphocyte count ratio as prognostic biomarkers in COVID-19. Respiratory Research. 2020;21:169. doi: 10.1186/s12931-020-01428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Fu J., Kong J., Wang W., Wu M., Yao L., Wang Z., Jin J., Wu D., Yu X. The clinical implication of dynamic neutrophil to lymphocyte ratio and D-dimer in COVID-19: a retrospective study in Suzhou China. Thromb. Res. 2020;192:3–8. doi: 10.1016/j.thromres.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Arcanjo A., Logullo J., Menezes C.C.B., de Souza Carvalho Giangiarulo T.C., dos Reis M.C., de Castro G.M.M., da Silva Fontes Y., Todeschini A.R., Freire-de-Lima L., Decoté-Ricardo D., Ferreira-Pereira A., Freire-de-Lima C.G., Barroso S.P.C., Takiya C., Conceição-Silva F., Savino W., Morrot A. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19) Scientific Reports. 2020;10:19630. doi: 10.1038/s41598-020-76781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Stacey H.D., Golubeva D., Posca A., Ang J.C., Novakowski K.E., Zahoor M.A., Kaushic C., Cairns E., Bowdish D.M.E., Mullarkey C.E., Miller M.S. IgA potentiates NETosis in response to viral infection. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2101497118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.v, Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Carsana L., Sonzogni A., Nasr A., Rossi R.S., Pellegrinelli A., Zerbi P., Rech R., Colombo R., Antinori S., Corbellino M., Galli M., Catena E., Tosoni A., Gianatti A., Nebuloni M. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study., the Lancet. Infectious Diseases. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet (London, England) 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.DiNicolantonio J.J., McCarty M. Thrombotic complications of COVID-19 may reflect an upregulation of endothelial tissue factor expression that is contingent on activation of endosomal NADPH oxidase. Open Heart. 2020;7:1337. doi: 10.1136/openhrt-2020-001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Escher R., Breakey N., Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thrombosis Research. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Imai Y., Kuba K., Neely G.G., Yaghubian-Malhami R., Perkmann T., van Loo G., Ermolaeva M., Veldhuizen R., Leung Y.H.C., Wang H., Liu H., Sun Y., Pasparakis M., Kopf M., Mech C., Bavari S., Peiris J.S.M., Slutsky A.S., Akira S., Hultqvist M., Holmdahl R., Nicholls J., Jiang C., Binder C.J., Penninger J.M. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Prinz N., Clemens N., Canisius A., Lackner K.J. Endosomal NADPH-oxidase is critical for induction of the tissue factor gene in monocytes and endothelial cells. Lessons from the antiphospholipid syndrome. Thromb. Haemostasis. 2013;109:525–531. doi: 10.1160/TH12-06-0421. [DOI] [PubMed] [Google Scholar]
- 309.Shiravi A.-A., Saadatkish M., Abdollahi Z., Miar P., Khanahmad H., Zeinalian M. Vitamin D can be effective on the prevention of COVID-19 complications: a narrative review on molecular aspects., International Journal for Vitamin and Nutrition Research. Internationale Zeitschrift Fur Vitamin- Und Ernahrungsforschung. Journal International de Vitaminologie et de Nutrition. 2020:1–13. doi: 10.1024/0300-9831/a000676. [DOI] [PubMed] [Google Scholar]
- 310.Shojaeefar E., Malih N., Rezaei N. The possible double-edged sword effects of vitamin D on COVID-19: a hypothesis. Cell Biol. Int. 2021;45:54–57. doi: 10.1002/cbin.11469. [DOI] [PubMed] [Google Scholar]
- 311.Chen Y., Huang Y., Xiong B., Luo H., Song X. Dexmedetomidine ameliorates renal ischemia reperfusion-mediated activation of the NLRP3 inflammasome in alveolar macrophages. Gene. 2020;758:144973. doi: 10.1016/j.gene.2020.144973. [DOI] [PubMed] [Google Scholar]
- 312.Cui Y., Wang Y., Li G., Ma W., Zhou X.S., Wang J., Liu B. The Nox1/Nox4 inhibitor attenuates acute lung injury induced by ischemia-reperfusion in mice, PLoS One. 2018;13:e0209444. doi: 10.1371/journal.pone.0209444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Chocry M., Leloup L. The NADPH oxidase family and its inhibitors. Antioxidants Redox Signal. 2020;33:332–353. doi: 10.1089/ars.2019.7915. [DOI] [PubMed] [Google Scholar]
- 314.Csányi G., Cifuentes-Pagano E., al Ghouleh I., Ranayhossaini D.J., Egaña L., Lopes L.R., Jackson H.M., Kelley E.E., Pagano P.J. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radical Biol. Med. 2011;51:1116–1125. doi: 10.1016/j.freeradbiomed.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Al-Shabrawey M., Bartoli M., El-Remessy A.B., Platt D.H., Matragoon S., Behzadian M.A., Caldwell R.W., Caldwell R.B. Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am. J. Pathol. 2005;167:599–607. doi: 10.1016/S0002-9440(10)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Rey F.E., Cifuentes M.E., Kiarash A., Quinn M.T., Pagano P.J. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ. Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]