Abstract
Objectives:
Delays in the diagnosis and treatment of psoriatic arthritis (PsA) are common. These delays contribute to impairments in quality of life and joint damage. This study aims to calculate the incidence rate of PsA over time and identify clinical features that may be used for PsA prediction in psoriasis patients.
Methods:
The study population for PsA incidence analysis included 1128 participants enrolled in the Utah Psoriasis Initiative (UPI) between 2002 and 2014. Clinical evaluation and medical record review were performed to identify new cases of PsA after enrollment. For identifying psoriasis features associated with PsA, the population was restricted to 627 participants who did not have PsA before psoriasis phenotyping and had been followed up for subsequent PsA diagnosis. We conducted Cox proportional hazard regressions to estimate the hazard ratio (HR) of PsA associated with psoriasis characteristics and other health-related features.
Results:
PsA incidence rate increased for >60 years following psoriasis onset (trend p<0.0001). There was a significant association between PsA and induration severity in untreated lesions (p<0.001, HR=1.46), history of fingernail involvement (p<0.001, HR=2.38), pustular psoriasis (p<0.001, HR=3.32), fingernail involvement at enrollment (p<0.001, HR=2.04), and Koebner phenomenon (p<0.001, HR=1.90). Multivariate analysis yielded a model which included a history of fingernail involvement (p<0.001, HR=2.16) and untreated induration (p<0.001, HR=1.41).
Conclusion:
Risk of PsA increases steadily for >60 years following psoriasis onset. Patient-reported history of psoriasis characteristics has greater predictive power than physician measured features at enrollment visits. The characteristics identified in this study provide guidance for screening for PsA risk in psoriasis patients.
INTRODUCTION
Psoriasis is a chronic disease characterized by patches of raised, red, scaly skin. It is multifactorial, known to be both genetically and immunologically mediated. Psoriatic arthritis (PsA) occurs in up to 30% of psoriasis patients and is characterized by inflammation in joints, tendons, and ligaments.(1,2) The development of effective and consistent methods to screen for high-risk individuals and diagnose those in the early stages of PsA is critical to preserving the quality of life and mitigating irreversible joint damage in these populations.(3,4) Existing screening strategies for PsA in psoriasis patients are infrequently used in routine clinical practice because of challenges with feasibility and predictive performance.(5)
Previous research has identified patient characteristics associated with PsA, including psoriasis severity, obesity, psoriatic nail involvement, and exposure to physical trauma.(6–10) However, we are lacking consistent evidence elucidating which psoriasis severity measurements (induration, erythema, desquamation, investigator global assessment (IGA), body surface area affected with psoriasis (BSA), and the product of IGA and BSA (IGAxBSA)) have the best predictive value for subsequent onset of PsA in individuals with psoriasis. Although patient-reported worst-ever BSA has a reported association with PsA,(11) there has not been a comparison between the maximum BSA since psoriasis onset and the BSA measured at the time of study enrollment. Additionally, psoriasis severity measured at enrollment may be affected by treatment and therefore may not be ideal for subsequent PsA prediction. Investigation into the association between the erythema, desquamation, and induration severity of untreated psoriasis lesions and PsA onset has not been previously undertaken. Furthermore, a previous study showed that PsA risk does not dissipate for up to 20 years after the diagnosis of psoriasis,(8) but the risk in patients with psoriasis duration longer than 20 years remains unknown.
This study aimed to answer these questions and provide guidance for designing and applying effective screening strategies for PsA. We have also evaluated the predictive performance of a wide range of clinical features for PsA screening tool development.
METHODS
Patient Population.
The Utah Psoriasis Initiative (UPI) is a registry and tissue bank of 1379 psoriasis patients enrolled between 2002 and 2014. UPI participants were adult patients seen at the University of Utah Health who had been diagnosed with psoriasis by a dermatologist. Upon enrollment in the UPI, participants completed a questionnaire regarding their historical psoriasis characteristics, PsA diagnosis, and other comorbidities. A dermatologist reviewed and reconciled the questionnaire and conducted a detailed exam of psoriasis conditions. The University of Utah Institutional Review Board (IRB) has approved this study (IRB00010681).
Variables.
The outcome variable was PsA diagnosis. Patients were followed forward in time for PsA diagnosis after enrollment. UPI participants were invited to complete a rheumatologic study evaluation that included diagnostic classifications of PsA. Diagnostic codes indicative of PsA diagnosis were also collected from electronic medical record (EMR) data within the Electronic Data Warehouse (EDW) at the University of Utah Health and at the Intermountain Healthcare. Approximately 85% of the Utah population is represented in these EDWs.
Participants were classified into three categories with respect to their PsA status: Definitive PsA, Uncertain PsA, and No PsA. Definitive PsA classification required PsA diagnosis by a rheumatologist, defined by 1) patient-reported diagnosis of PsA from a rheumatologist, 2) PsA diagnosis reported by a UPI study rheumatologist after an evaluation with the patient, or 3) a diagnosis code for PsA or ankylosing spondylitis (AS) by a rheumatologist in an EMR (version 9 of the International Classification of Diseases, or ICD-9 codes 696.0 and 720.0; ICD-10 codes L40.5 and M45.9). Uncertain PsA are those not classified as Definitive PsA who also have 1) patient-reported PsA or AS diagnosis from a non-rheumatology provider, 2) a diagnosis code for PsA by a non-rheumatologist, 3) a diagnosis code for rheumatoid arthritis, or 4) an uncertain diagnosis reported by the study rheumatologist after a face-to-face evaluation. The study rheumatologist’s classification overrode previous reports if they were in contradiction. Patients who did not meet the criteria for Definitive PsA or Uncertain PsA were classified as No PsA, denoted by cutaneous-only psoriasis (PsC).
Exposure variables included psoriasis characteristics and additional health-related features such as comorbidities and body mass index. These variables included patient-reported features collected via questionnaires and investigator-reported features collected via a physician examination. All phenotypes were assessed at the time of enrollment. Patients also reported their psoriasis characteristics prior to enrollment. The questionnaire included the following questions about BSA: 1) “Please estimate the total percentage of your body surface area that is covered with psoriasis right now. The palm of your hand is approximately 1% of your body surface area. If you were to push all lesions together, how many palms would it take to cover your psoriasis?” and 2) “If you are currently being treated for your psoriasis, your body surface area is likely not at its worst. Using the above-described method, please estimate your body surface area affected by psoriasis when it was at its worst ever.”
For patient-reported erythema, desquamation, and induration, we asked patients to score the severity of their typical untreated lesions. Patients were provided with an induration card (provided by the National Psoriasis Foundation, Portland, Oregon, USA) and photographs as reference points for the possible scores they could give their typical untreated lesions. This is in contrast to the scores given to them by the physician at enrollment which may reflect response to treatment.
Statistical Analysis.
To calculate the PsA incidence rate since the first psoriasis symptom, we excluded patients with uncertain PsA status, missing psoriasis onset time, and PsA diagnosis prior to psoriasis onset. Person-years at risk were calculated from patient-reported onset date of the first psoriasis symptoms to the date of PsA diagnosis, or the date of the patient’s last clinical encounter documented in their medical records, whichever came first. The incidence rate of PsA was calculated as the number of PsA diagnoses divided by the number of person-years at risk. We used Poisson regression to model the increased risk of incident PsA over the course of the disease from psoriasis onset. This model included an effect for time since onset within 10-year categories and an offset for the time at risk within each category.(12)
To identify PsA-predicting psoriasis characteristics and health-related features assessed at UPI enrollment, we further excluded patients with a PsA diagnosis prior to enrollment, patients lacking follow-up data for PsA diagnosis after enrollment, and patients with survey responses with ≥50% missing data. Descriptive statistics of patient characteristics were reported as mean and standard deviation for quantitative variables and as number of observations and percentage for categorical variables. Rare (N<5) phenotypes were not described given uncertainty due to the small sample size. In total, 36 variables were analyzed. Raw comparisons between PsA and PsC were carried out by Kruskal-Wallis test for quantitative variables and chi-squared test for categorical variables.
We performed multiple imputation using the MICE package in R with 25 rounds of imputation with 20 iterations each. (13) This ensures valid inference if the data is missing-at-random (given the observed data, the data values and missingness pattern are independent). No variables assessed had ≥50% missingness. We did not perform imputation for IGAxBSA since this variable was calculated from IGA and BSA. (13)
We performed a univariate survival analysis using a Cox proportional hazards regression model to determine risk for PsA associated with each psoriatic phenotypic or health-related feature. We included sex as a covariate because of reported sex-related phenotypes of PsA(15) and an association in our dataset between sex and PsA. Duration of psoriasis at enrollment was treated as the left-truncation time. All tests were two-sided. Hazard ratios (HR) and 95% confidence intervals of PsA risk after psoriasis onset were calculated from multiple imputations using Rubin’s rule.(16–18) Using the Bonferroni method to control family-wise type I error, the significance threshold was 0.0014.(19) Using imputed data, multivariate analysis was performed by backward stepwise regression.
Sensitivity analyses were performed to explore (1) an analysis strategy without using multiple imputation; and (2) a relaxed classification of PsA status, i.e., including patients diagnosed with PsA by a non-rheumatologist in the Definitive PsA category, rather than the Uncertain PsA category.
All statistical analyses were performed in R version 3.6.0.
RESULTS
The UPI included 1379 participants. After excluding 232 patients with Uncertain PsA status, 8 with missing psoriasis onset time, and 11 with a PsA diagnosis prior to psoriasis onset, there were 1128 subjects for PsA incidence rate calculation. The annual PsA incidence increased from 0.0137 during the first 20 years to 0.0312 in >60 years after psoriasis onset (Figure 1). Test for trend of incidence rates yielded an estimate of the rate ratio of 1.028 for each year increase in psoriasis duration with a 95% confidence interval of [1.021–1.036] (p<0.0001).
Figure 1. Incidence of psoriatic arthritis following psoriasis onset.
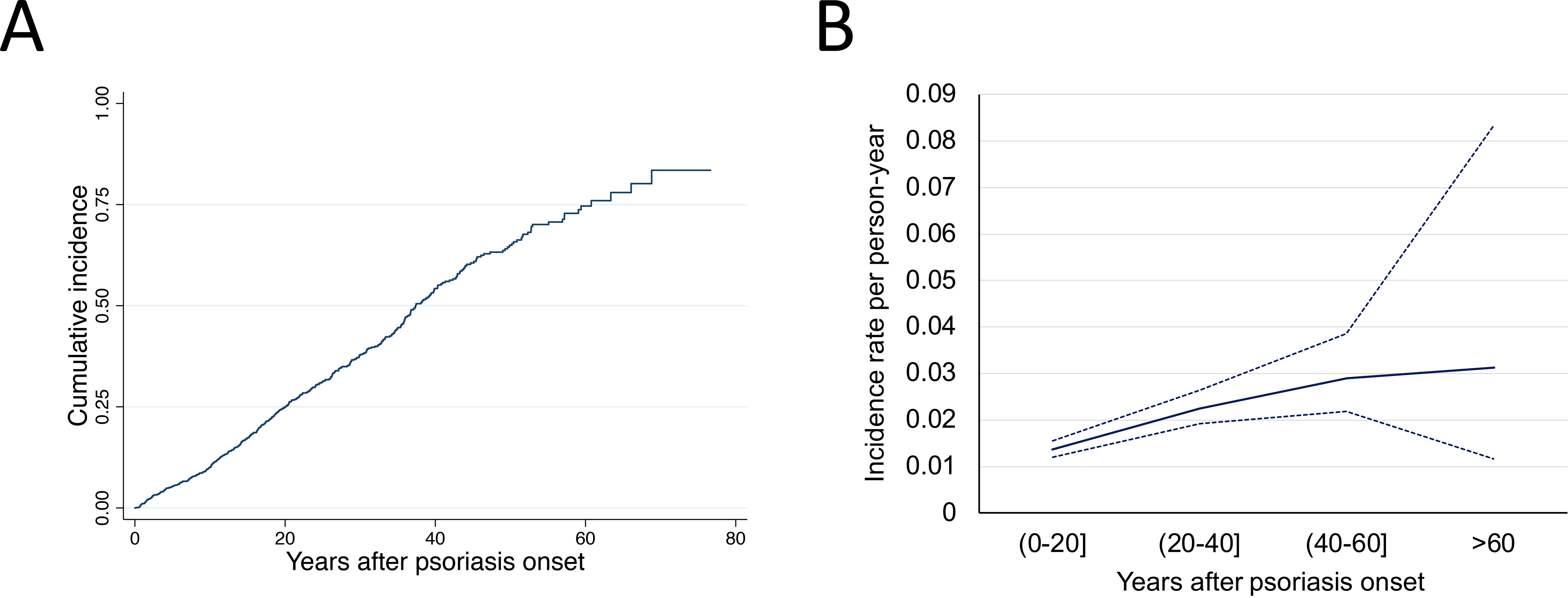
(A) Cumulative incidence of PsA; (B) psoriasis duration-dependent incidence rate of PsA. Solid blue line is incidence, dotted lines are 95% confidence intervals.
From the above 1128 psoriasis patients, we further excluded 148 patients with a PsA diagnosis prior to enrollment, 345 patients lacking follow-up data for assessing PsA diagnosis after enrollment, and 8 patients with survey responses containing ≥50% missingness, resulting in 627 individuals for PsA-predicting feature identification. Among these participants, 128 (20%) developed PsA after enrollment and had a PsA diagnosis from a rheumatologist, while 499 had PsC without PsA as of their most recent encounter in the medical system. PsC and PsA patients were comparable with regard to race, body mass index at age 18, psoriasis age of onset, duration of psoriasis at enrollment, follow-up time, and psoriasis severity at enrollment (Table 1). The mean and maximum follow-up times since enrollment were 7.7 and 16 years, respectively.
Table 1.
Summary statistics of study participants.
| Demographics | PsC (N=499) c | PsA (N=128) c | Missing (%) | P-value d | |
|---|---|---|---|---|---|
| Age at study entry | 45.8 (17.0) | 47.3 (12.9) | 0 (0.0) | 0.46 | |
| Male sex | 270 (54.1%) | 57 (44.5%) | 0 (0.0) | 0.07 | |
| Race | White | 447 (90.3%) | 108 (85.7%) | 6 (1.0) | 0.18 |
| Non-White | 48 (9.7%) | 18 (14.3%) | |||
| Follow-up time | 8.2 (5.0) | 6.0 (4.4) | 0 (0.0) | 0.39 | |
| Psoriasis age of onset | 28.1 (17.3) | 29.2 (14.9) | 0 (0.0) | 0.8 | |
| Years with psoriasis at study entry | 17.7 (14.4) | 18 (13.4) | 0 (0.0) | 0.47 | |
| normal | 380 (79.7%) | 101 (80.8%) | |||
| BMI at age 18 a | overweight | 75 (15.7%) | 18 (14.4%) | 25 (4.0) | 0.93 |
| obese | 22 (4.6%) | 6 (4.8%) | |||
| mild | 94 (19.4%) | 19 (15.2%) | |||
| Worst BSA b | moderate | 213 (43.9%) | 49 (39.2%) | 17 (2.7) | 0.17 |
| severe | 178 (36.7%) | 57 (45.6%) | |||
| IGA at enrollment | 1.95 (1.01) | 2.04 (0.99) | 16 (2.6) | 0.24 | |
| IGAxBSA at enrollment | 16.5 (33.0) | 20.4 (38.1) | 34 (5.4) | 0.52 | |
Abbreviations: PsC, cutaneous-only psoriasis; PsA, psoriatic arthritis; IGA, investigator global assessment; BSA, percent of body surface area affected with psoriasis; IGAxBSA, the product of IGA and BSA; BMI, body mass index.
Body mass index is separated as normal (<25), overweight (25–30), and obese (>30).
Body surface area affected with psoriasis is mild (<3%), moderate (3–10%), and severe (>10%).
No. (%) for categorical variables or mean (standard deviation) for continuous variables.
Chi-squared test for categorical variables or Kruskal-Wallis test for continuous variables.
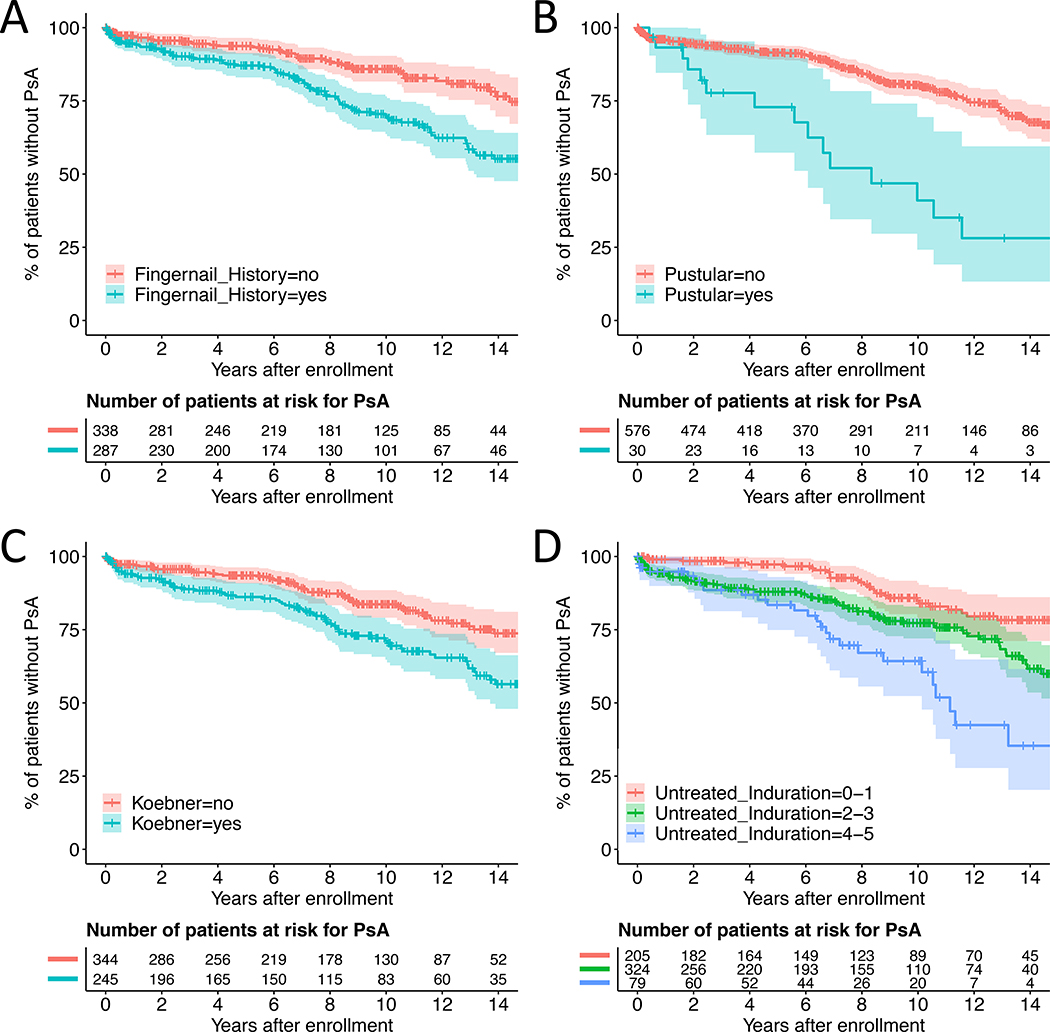
By Cox proportional hazards regression adjusted for sex, variables associated with a statistically significant increased risk of PsA include pustular psoriasis (hazard ratio [95% confidence interval] 3.32 [1.91–5.77]), a history of fingernail involvement (HR 2.38 [1.64–3.45]), fingernail involvement at time of enrollment (2.04 [1.40–2.95]), Koebner phenomenon (HR 1.90 [1.31–2.76]), and patient-reported untreated plaque induration (HR 1.46 [1.27–1.68]) (Figure 2 and Table 2). Patient-reported untreated erythema and desquamation were nominally significant (Table 2 and Figure S1). For induration, desquamation, and erythema, history of severity in untreated lesions associated with higher hazard ratios and lower p-values than the investigator-reported severity measured at enrollment (Table 2). The same was also observed for fingernail involvement (Table 2). Multivariate Cox regression yielded a model with history of fingernail psoriasis (HR 2.16 [1.49–3.15]) and patient-reported untreated induration (HR 1.41 [1.22–1.62]) (Table 3). Neither induration at enrollment nor fingernail psoriasis at enrollment was nominally significant in the multivariate analysis (data not shown).
Figure 2. Kaplan-Meier curves for PsA development stratified by psoriasis characteristics.
Panel (A) history of fingernail psoriasis, (B) pustular psoriasis, (C) Koebner phenomenon, (D) untreated induration. Induration was measured on a 0–5 scale, 0 being not present and 5 being the most severe. The x-axis is time in years from enrollment. The y-axis is the cumulative probability of not developing PsA.
Table 2.
Association between psoriasis characteristics and PsA risk.
| Phenotypes | Report by a | PsC (N=499) b | PsA (N=128) b | HR [95% CI] | P-value c | |
|---|---|---|---|---|---|---|
|
| ||||||
| Psoriasis phenotypes | ||||||
| History of fingernail psoriasis | P | 206 (41.4%) | 81 (63.8%) | 2.38 [1.64–3.45] | <0.001 | |
| Pustular psoriasis | I | 15 (3.1%) | 15 (12.0%) | 3.32 [1.91–5.77] | <0.001 | |
| Fingernail psoriasis | I | 213 (44.1%) | 77 (62.6%) | 2.04 [1.40–2.95] | <0.001 | |
| Koebner phenomenon | P | 178 (37.9%) | 67 (56.3%) | 1.90 [1.31–2.76] | <0.001 | |
| Palmoplantar pustular psoriasis | I | 9 (1.9%) | 8 (6.4%) | 3.30 [1.57–6.97] | 0.002 | |
| Generalized pustular psoriasis | I | 6 (1.2%) | 7 (5.6%) | 3.12 [1.42–6.85] | 0.005 | |
| Guttate psoriasis | I | 54 (11.2%) | 9 (7.3%) | 0.62 [0.31–1.22] | 0.17 | |
| Erythrodermic psoriasis | I | 4 (0.8%) | 3 (2.4%) | 1.96 [0.60–6.44] | 0.27 | |
| Inverse psoriasis | I | 132 (27.6%) | 39 (31.5%) | 1.23 [0.84–1.80] | 0.29 | |
| History of scalp psoriasis | P | 366 (73.5%) | 100 (79.4%) | 1.25 [0.81–1.93] | 0.31 | |
| Psoriasis age of onset | P | 28.1 (17.33) | 29.2 (14.94) | 1.05 [0.94–1.19] | 0.38 | |
| Palmoplantar psoriasis | I | 30 (6.2%) | 5 (4.0%) | 0.69 [0.28–1.71] | 0.42 | |
| Psoriasis severity | ||||||
|
| ||||||
| Untreated induration d | 0 | P | 35 (7.3%) | 4 (3.2%) | 1.46 [1.27–1.68] | <0.001 |
| 1 | 141 (29.3%) | 25 (19.8%) | ||||
| 2 | 165 (34.2%) | 40 (31.7%) | ||||
| 3 | 88 (18.3%) | 31 (24.6%) | ||||
| 4 | 37 (7.7%) | 18 (14.3%) | ||||
| 5 | 16 (3.3%) | 8 (6.3%) | ||||
|
| ||||||
| Untreated desquamation d | 0 | P | 3 (0.6%) | 0 (0.0%) | 1.22 [1.05–1.42] | 0.008 |
| 1 | 50 (10.4%) | 8 (6.4%) | ||||
| 2 | 117 (24.2%) | 31 (24.8%) | ||||
| 3 | 136 (28.2%) | 23 (18.4%) | ||||
| 4 | 120 (24.8%) | 37 (29.6%) | ||||
| 5 | 57 (11.8%) | 26 (20.8%) | ||||
|
| ||||||
| Untreated erythema d | 0 | P | 1 (0.2%) | 0 (0.0%) | 1.25 [1.06–1.48] | 0.009 |
| 1 | 26 (5.4%) | 5 (4.0%) | ||||
| 2 | 107 (22.2%) | 24 (19.0%) | ||||
| 3 | 178 (36.9%) | 33 (26.2%) | ||||
| 4 | 125 (25.9%) | 43 (34.1%) | ||||
| 5 | 46 (9.5%) | 21 (16.7%) | ||||
|
| ||||||
| Induration at enrollment d | 0 | I | 65 (13.7%) | 11 (8.7%) | 1.21 [1.02–1.44] | 0.03 |
| 1 | 193 (40.7%) | 50 (39.7%) | ||||
| 2 | 142 (30.0%) | 41 (32.5%) | ||||
| 3 | 48 (10.1%) | 19 (15.1%) | ||||
| 4 | 26 (5.5%) | 5 (4.0%) | ||||
| 5 | 0 (0.0%) | 0 (0.0%) | ||||
|
| ||||||
| Desquamation at enrollment d | 0 | I | 32 (6.8%) | 9 (7.2%) | 1.16 [0.99–1.37] | 0.07 |
| 1 | 164 (34.6%) | 39 (31.2%) | ||||
| 2 | 158 (33.3%) | 37 (29.6%) | ||||
| 3 | 77 (16.2%) | 33 (26.4%) | ||||
| 4 | 38 (8.0%) | 6 (4.8%) | ||||
| 5 | 5 (1.1%) | 1 (0.8%) | ||||
|
| ||||||
| Erythema at enrollment d | 0 | I | 22 (4.6%) | 4 (3.2%) | 1.14 [0.96–1.35] | 0.13 |
| 1 | 72 (15.2%) | 25 (19.8%) | ||||
| 2 | 179 (37.8%) | 37 (29.4%) | ||||
| 3 | 143 (30.2%) | 39 (31.0%) | ||||
| 4 | 52 (11.0%) | 19 (15.1%) | ||||
| 5 | 6 (1.3%) | 2 (1.6%) | ||||
|
| ||||||
| Worst-ever BSA | P | 17.9 (24.24) | 23 (27.57) | 1.00 [1.00–1.01] | 0.22 | |
| BSA at enrollment | I | 6.3 (9.41) | 8.2 (14.55) | 1.01 [1.00–1.02] | 0.09 | |
| IGA at enrollment | I | 2 (1.01) | 2 (0.99) | 1.15 [0.96–1.38] | 0.12 | |
| IGA×BSA at enrollment | I | 16.5 (33.04) | 20.4 (38.06) | 1.00 [1.00–1.01] | 0.22 | |
| Comorbidities | ||||||
| Depression | P | 96 (19.5%) | 42 (33.6%) | 1.78 [1.22–2.59] | 0.003 | |
| Inflammatory bowel disease | P | 4 (0.8%) | 5 (4.0%) | 3.51 [1.40–8.78] | 0.007 | |
| High blood pressure | P | 130 (26.2%) | 44 (35.2%) | 1.40 [0.97–2.02] | 0.07 | |
| Asthma | P | 55 (11.1%) | 20 (16.0%) | 1.47 [0.91–2.38] | 0.11 | |
| Ever smoked | P | 187 (37.7%) | 49 (39.2%) | 1.29 [0.90–1.85] | 0.17 | |
| Stroke | P | 13 (2.6%) | 1 (0.8%) | 0.30 [0.04–2.15] | 0.23 | |
| Eczema | P | 58 (11.8%) | 12 (9.5%) | 0.78 [0.43–1.42] | 0.42 | |
| Cancer | P | 33 (6.7%) | 8 (6.3%) | 1.21 [0.58–2.52] | 0.61 | |
| Skin cancer | P | 38 (7.7%) | 9 (7.2%) | 0.90 [0.45–1.80] | 0.77 | |
| Strep throat | P | 34 (6.9%) | 9 (7.3%) | 0.92 [0.46–1.81] | 0.8 | |
| High cholesterol | P | 102 (20.6%) | 27 (21.6%) | 0.96 [0.62–1.48] | 0.86 | |
| BMI at age 18 | P | 22.3 (4.10) | 22.2 (4.60) | 1.00 [0.96–1.05] | 0.86 | |
| Diabetes | P | 47 (9.5%) | 10 (8.0%) | 0.95 [0.50–1.82] | 0.89 | |
| Myocardial infarction | P | 18 (3.6%) | 4 (3.2%) | 1.01 [0.37–2.76] | 0.98 | |
Abbreviations: HR, hazard ratio; CI, confident interval; SD, standard deviation; PsC, cutaneous-only psoriasis; PsA, psoriatic arthritis; IGA, investigator global assessment; BSA, percent of body surface area affected with psoriasis; IGA×BSA, the product of IGA and BSA.
Reporter: P is by patient, I is by investigator.
Shown in the table are No. (%) or Mean (standard deviation).
Bolded p-values cross the threshold of 0.0014 for significance by Bonferroni correction.
The three components of the investigator global assessment (IGA) analysis (erythema, induration, and desquamation) are on a 0–5 scale with 0 representing phenotype absence and 5 being the most severe.
Table 3.
Multivariate Cox proportional hazards regression results
| Psoriasis Characteristic | Hazard Ratio [95% Confidence Interval] | p-value |
|---|---|---|
| Untreated induration | 1.41 [1.22–1.62] | <0.001 |
| History of fingernail psoriasis | 2.16 [1.49–3.15] | <0.001 |
| Female sex | 1.65 [1.15–2.37] | 0.006 |
We also performed a sensitivity analysis without imputing missing data which yielded concordant results (Table S1). These variables had similar hazard ratios in non-White individuals including Black, Native American, Latino, Asian, Oceania, and admixed (2.38 [0.95–5.93] for history of fingernail involvement and 2.29 [0.94–5.56] for untreated induration ≥3) but statistically non-significant p-values (0.06 for fingernail involvement at enrollment and 0.07 for untreated induration ≥3). Sensitivity analyses that additionally included PsA cases diagnosed by a non-rheumatologist demonstrated a multivariate result similar to our primary analyses (Table S2 and Table S3).
DISCUSSION
This time-to-event analysis revealed psoriasis characteristics associated with subsequent PsA onset. Various characteristics have been reported in previous studies to associate with PsA risk, including investigator-assessed characteristics measured at the time of a clinical visit (i.e. IGA, fingernail involvement, etc.).(1,20,21) We assessed both patient-reported and investigator-assessed variables and found that some of the strongest associations occurred with patient-reported variables. Specifically, we discovered an association with patient-reported untreated plaque induration severity. Compellingly, our data demonstrated that patient-reported untreated induration has a stronger association with PsA than induration assessed by providers at the time of enrollment. Similarly, a patient-reported history of fingernail involvement was found to be a superior predictor for PsA than provider-reported fingernail involvement at the time of enrollment. Select patient-reported disease features that account for the patient’s entire psoriatic history may be more predictive of future PsA onset than investigator-assessed outcomes that are limited to a snapshot of disease state at a single time point.
The large sample size and long follow-up period in this study enabled the discovery of associations with uncommon phenotypes (<5% in PsC) such as pustular psoriasis. The predictive association between pustular psoriasis and PsA risk has not been identified previously. With Koebner phenomenon, our results were similar to other studies that reported an association between patient-reported history of Koebner phenomenon and PsA risk.(23) Further investigation into the specific associations between these characteristics and PsA is necessary to further elucidate the risk they pose. In contrast to other studies, we did not find associations between PsA risk and inverse psoriasis or scalp psoriasis.(24,25)
Importantly, we demonstrated a steadily increasing risk of PsA onset for >60 years after psoriasis onset. This is consistent with results from another prospective psoriasis registry reporting a steady increase in PsA risk over time.(8) This is important for re-interpreting previous studies which defined PsC by a 10-year cutoff after psoriasis onset, and for appropriate design of future studies.(2,4,26)
Strengths of our study include the detailed psoriasis phenotyping data and a time-dependent analysis that evaluates development of PsA after the initial phenotyping, rather than reporting associations between phenotypes and prevalent PsA. The inclusive classification of PsA and PsC patients provides a comprehensive real-world approach to defining study populations. Our conclusions are strengthened by a strict correction for multiple testing by Bonferroni and additional stepwise multivariate regression analysis.
Limitations of our study include the retrospective nature of patient-reported variables as they may be subject to recall bias. The number of patients at risk for PsA after 60 years was small and may reflect selection bias as >75% of patients in the cohort had developed PsA (Figure 1a). Furthermore, ICD codes and patient-reported diagnoses of PsA are imperfect markers of PsA diagnosis. The study cohort includes a low proportion of non-White individuals. This may contribute to the lack of statistical significance when stratified by race and is prohibitive in undertaking higher resolution analyses with subsets from different racial groups. Additionally, multivariate model data should be interpreted cautiously. In particular, the selective inference reported for the multivariate model after variable selection by a low p-value cutoff is expected to be slightly optimistic (i.e. the "true" p-values are slightly greater than the reported p-values).
In conclusion, we demonstrate that PsA risk in psoriasis patients increases steadily for >60 years following psoriasis onset. Untreated plaque induration severity, pustular psoriasis, history of fingernail involvement, fingernail involvement at time of enrollment in this study, and Koebner phenomenon are significantly associated with an elevated risk for PsA. The predictive value of untreated plaque induration and history of fingernail involvement persisted through the multivariate analysis, suggesting that they are strong indicators of future development of PsA. These findings can assist researchers with developing PsA screening and referral strategies and can aid clinicians in assessing individuals’ risks for PsA.
Supplementary Material
ACKNOWLEDGEMENTS
This study is partially supported by the 2019 Discovery Research Grant and PsA Diagnostic Test Grant from the National Psoriasis Foundation, the 2018 Immunology, Inflammation, and Infectious Diseases (III) Initiative at the University of Utah, and Pfizer Inc. (grant numbers WI227108 and WI240276). The support and resources from the Center for High-Performance Computing at the University of Utah are gratefully acknowledged. The computational resources used were partially funded by the NIH Shared Instrumentation Grant 1S10OD021644–01A1. This project utilized REDCap at the University of Utah supported by the grant 8UL1TR000105 (formerly UL1RR025764) from NCATS/NIH. We thank the Pedigree and Population Resource of Huntsman Cancer Institute, University of Utah (funded in part by the Huntsman Cancer Foundation; grant P30 CA2014 from the National Cancer Institute, University of Utah; and the University of Utah’s program in Personalized Health and Center for Clinical and Translational Science) for its role in pulling electronic medical records from the Intermountain Healthcare Electronic Data Warehouse. Dr. Jian Ying at the Division of Epidemiology, University of Utah, has provided statistical guidance in this study.
Disclosure: The PERCH software, for which Bing-Jian Feng is the inventor, has been non-exclusively licensed to Ambry Genetics Corporation for their clinical genetic testing services and research. Dr. Feng also reports funding and sponsorship to his institution on his behalf from Pfizer Inc., Regeneron Genetics Center LLC., and Astra Zeneca. Sophie Belman and Courtney Carroll declare no potential conflict of interest.
REFERENCES
- 1.Alinaghi F, Calov M, Kristensen LE, Gladman DD, Coates LC, Jullien D, et al. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol 2019; [DOI] [PubMed] [Google Scholar]
- 2.Gladman DD. Clinical Features and Diagnostic Considerations in Psoriatic Arthritis. Rheum Dis Clin N Am 2015;41:569–79. [DOI] [PubMed] [Google Scholar]
- 3.Mcardle A, Flatley B, Pennington SR, Fitzgerald O. Early biomarkers of joint damage in rheumatoid and psoriatic arthritis. Arthritis Res Ther 2015;17:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iragorri N, Hazlewood G, Manns B, Danthurebandara V, Spackman E. Psoriatic arthritis screening: a systematic review and meta-analysis. Rheumatol Oxf Engl 2019;58:692–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karreman MC, Weel AEAM, van der Ven M, Vis M, Tchetverikov I, Nijsten TEC, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology Narnia; 2016;56:kew410. [DOI] [PubMed] [Google Scholar]
- 6.Egeberg A, Skov L, Zachariae C, Gislason G, Thyssen J, Mallbris L. Duration of Psoriatic Skin Disease as Risk Factor for Subsequent Onset of Psoriatic Arthritis. Acta Derm Venereol 2018;98:546–50. [DOI] [PubMed] [Google Scholar]
- 7.Rouzaud M, Sevrain M, Villani AP, Barnetche T, Paul C, Richard M-A, et al. Is there a psoriasis skin phenotype associated with psoriatic arthritis? Systematic literature review Conflicts of interest. JEADV 2014;2014:17–26. [DOI] [PubMed] [Google Scholar]
- 8.Eder L, Haddad A, Rosen CF, Lee K-A, Chandran V, Cook R, et al. The Incidence and Risk Factors for Psoriatic Arthritis in Patients With Psoriasis: A Prospective Cohort Study. Arthritis Rheumatol 2016;68:915–23. [DOI] [PubMed] [Google Scholar]
- 9.Soltani-Arabshahi R, Wong B, Feng B-J, Goldgar DE, Duffin KC, Krueger GG. Obesity in Early Adulthood as a Risk Factor for Psoriatic Arthritis. Arch Dermatol American Medical Association; 2010;146:721–6. [DOI] [PubMed] [Google Scholar]
- 10.Thorarensen SM, Lu N, Ogdie A, Gelfand JM, Choi HK, Love TJ. Physical trauma recorded in primary care is associated with the onset of psoriatic arthritis among patients with psoriasis. Ann Rheum Dis BMJ Publishing Group Ltd; 2017;76:521–5. [DOI] [PubMed] [Google Scholar]
- 11.Tey HL, Ee HL, Tan ASL, Theng TS, Wong SN, Khoo SW. Risk factors associated with having psoriatic arthritis in patients with cutaneous psoriasis. J Dermatol 2010;37:426–30. [DOI] [PubMed] [Google Scholar]
- 12.Hughes AO. Statistical Models in Epidemiology. David Clayton and Michael Hills, Oxford University Press, Oxford, 1993. No. of Pages: vii + 367. Price: £30. ISBN: 0-19-852221-5. [Google Scholar]; Stat Med 1995;14:104–5. [Google Scholar]
- 13.Van Buuren S, Groothuis-Oudshoorn K. Journal of Statistical Software mice: Multivariate Imputation by Chained Equations in R [Internet]. 2011. Available from: http://www.jstatsoft.org/
- 14.Rubin DB. Multiple Imputation after 18+ Years. J Am Stat Assoc Taylor & Francis; 1996;91:473–89. [Google Scholar]
- 15.Eder L, Chandran V, Gladman DD. Gender-related differences in patients with psoriatic arthritis. Int J Clin Rheumatol 2012;7:641–9. [Google Scholar]
- 16.Van Buuren S, Groothuis-Oudshoorn K. Journal of Statistical Software mice: Multivariate Imputation by Chained Equations in R. 2011. [Google Scholar]
- 17.van Ginkel JR, Kroonenberg PM. Analysis of Variance of Multiply Imputed Data. Multivar Behav Res Taylor & Francis Group; 2014;49:78–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubin DB, Wiley J, York N, Brisbane C, Singapore T. Multiple Imputation for Nonresponse in Surveys. 1987.
- 19.Bonferroni C. Teoria statistica delle classi e calcolo delle probabilita. Pubblicazioni R Ist Super Sci Econ E Commericiali Firenze 1936;8:3–62. [Google Scholar]
- 20.Busse K, Liao W. Which Psoriasis Patients Develop Psoriatic Arthritis? Psoriasis Forum Natl Psoriasis Found 2010;16:17. [PMC free article] [PubMed] [Google Scholar]
- 21.Ogdie A, Gelfand JM. Clinical Risk Factors for the Development of Psoriatic Arthritis Among Patients with Psoriasis: A Review of Available Evidence. Curr Rheumatol Rep NIH Public Access; 2015;17:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zabotti A, Tinazzi I, Aydin SZ, McGonagle D. From Psoriasis to Psoriatic Arthritis: Insights from Imaging on the Transition to Psoriatic Arthritis and Implications for Arthritis Prevention. Curr Rheumatol Rep 2020;22:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tinazzi I, McGonagle D, Aydin SZ, Chessa D, Marchetta A, Macchioni P. ‘Deep Koebner’ phenomenon of the flexor tendon-associated accessory pulleys as a novel factor in tenosynovitis and dactylitis in psoriatic arthritis. Ann Rheum Dis BMJ Publishing Group Ltd; 2018;77:922–5. [DOI] [PubMed] [Google Scholar]
- 24.Busse K, Liao W. Which Psoriasis Patients Develop Psoriatic Arthritis? Psoriasis Forum NIH Public Access; 2010;16:17–25. [PMC free article] [PubMed] [Google Scholar]
- 25.Ogdie A, Gelfand JM. Clinical Risk Factors for the Development of Psoriatic Arthritis Among Patients with Psoriasis: A Review of Available Evidence. Curr Rheumatol Rep 2015;17:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis BMJ Publishing Group Ltd; 2005;64:ii14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.