Abstract
Genetic and biochemical evidence implicates chromatin structure in the silencing of the two quiescent mating-type loci near the telomeres of chromosome III in yeast. With high-resolution micrococcal nuclease mapping, we show that the HMRa locus has 12 precisely positioned nucleosomes spanning the distance between the E and I silencer elements. The nucleosomes are arranged in pairs with very short linkers; the pairs are separated from one another by longer linkers of ∼20 bp. Both the basic amino-terminal region of histone H4 and the silent information regulator protein Sir3p are necessary for the organized repressive chromatin structure of the silent locus. Compared to HMRa, only small differences in the availability of the TATA box are present for the promoter in the cassette at the active MATa locus. Features of the chromatin structure of this silent locus compared to the previously studied HMLα locus suggest differences in the mechanisms of silencing and may relate to donor selection during mating-type interconversion.
The silencing of the haploid mating-type loci is a critical requirement for the yeast life cycle (10). Mating types in the yeast Saccharomyces cerevisiae are defined by a set of genes expressed at the active MAT locus near the center of chromosome III. MATa and MATα differ by approximately 750-bp regions, designated Ya and Yα, respectively, which contain the promoters for genes encoding the master regulatory proteins that define the unique mating type of the cell. Strains with the MATa allele express the a1 and a2 genes, while strains with the MATα allele express the α1 and α2 genes. In addition to the active MAT locus, two almost identical HM loci are located near the telomeres of chromosome III. HMLα is near the left telomere, while HMRa resides near the right telomere. These loci are transcriptionally silent and make no direct contribution to mating type. Rather, they serve as donors during yeast mating-type interconversion, or switching (7).
The switching event is initiated by expression of the HO endonuclease. This enzyme recognizes and cleaves double-stranded DNA at a site at MAT. The break is repaired by replacing it with the Y region of one of the HM loci, usually that with information of the opposite mating type (18, 22, 27, 41). Interestingly, identical HO sites present at the silent loci are not recognized by the endonuclease. Thus, DNA at the silent mating-type loci is present in a unique state, in which it is invisible to the endonuclease and transcription machinery but completely competent to participate in a recombinational event.
Extensive genetic studies (20) led to a model in which proteins binding to cis-acting DNA elements flanking the loci, a number of interacting, non-DNA binding proteins, and histones cooperate to form a repressive, heterochromatin-like structure that packages DNA in a presumably inaccessible format. Heterochromatic condensation has been implicated in position effect variegation in Drosophila melanogaster (9) and X-chromosome inactivation (13) and gene imprinting (43) in mammalian cells. Silencing at the telomeres (23, 52) and mating-type loci in S. cerevisiae bears similarities to these phenomena in more complex eukaryotes, providing a tractable system for the study of chromatin structure and its involvement in epigenetic gene regulation.
Two cis-acting elements (E and I), termed silencers, that flank each of the HM loci have been found to be essential and important, respectively, for silencing (1, 2, 25). They confer repression which is independent of the sequence contained between the flanking regions (38) and, in some cases, of chromosomal location and orientation (2). Additionally, certain trans-acting factors are required for silencing. These include the family of silent information regulator proteins, Sir1p, Sir2p, Sir3p, and Sir4p (34, 35). Mutations in SIR2, SIR3, or SIR4 result in the complete derepression of HMLα and HMRa, while sir1 mutations only partially derepress the two loci (12, 34, 42). The suspected importance of chromatin in silencing became manifest with the demonstration that amino-terminal deletion mutations encoded by the gene for histone H4 that removed much of the conserved basic region led to the derepression of the HM loci (17). Certain point mutations leading to amino acid substitutions in the same region abolished the silencing of HMLα (26, 31). Two amino-terminal domains of H4 were found to be vital to silencing (31). The first is a basic region spanning amino acid residues 16 to 19 (16) while the second extends over the less basic residues 21 to 29 (15). In addition, H3 and H4 N-terminal regions interact with Sir3p and Sir4p (8).
Four silencers (HMR-E, HMR-I, HML-E, and HML-I) have been shown to confer repression to varying degrees when placed near genes on a plasmid. HMR-E, HML-E, and HML-I can all individually confer silencing on plasmids, but the deletion of both HML-E and HML-I is required to derepress HML in a chromosomal context (1, 5). It is important to note that a silencer element can be upstream and downstream of a controlled DNA sequence at the same time when it is present in a circular plasmid molecule. A mutation at HMR-E in the chromosome, however, is sufficient to relieve repression at HMR (2). Mutating HMR-I, on the other hand, does not result in derepression. This suggests a silencing hierarchy, with HMR-E as the strongest, followed by HML-E, HML-I, and finally HMR-I. The protein binding site composition of HMR and HML is distinctive as well. HML-E and HMR-I each contain two binding sites. Both silencers have an origin recognition complex (ORC) binding site in common, but they are unique because of the presence of a Rap1p binding site in HML-E and an Abf1p binding site in HMR-I. HML-I and HMR-E each contain binding sites for Abf1p, Rap1p, and ORC (10).
Further distinctions between HML and HMR were highlighted in studies involving histone H4. HML is affected by H4 amino-terminal substitutions regardless of chromosomal location. In contrast, HMR, while still repressed when at its natural location in an H4 point mutant, is progressively derepressed when the distance between HMR and the telomere is artificially increased (46). Different effects of mutations in a pair of genes of unknown function, SAS2 and SAS3, on the two silent loci have been documented (4, 33). Interpretation of the differences in HM loci behavior is complicated by the lack of knowledge of the promoter strength of α versus a, although the former appears more robust when at the MAT locus (36). Further, sterility as a result of the loss of silencing may be a complex phenomenon that is not linearly related to the level of transcription of the derepressed locus. Data which are cited but not shown led Thompson et al. (46) to conclude that transcription is absent at mating efficiencies of >0.1 (wild-type mating efficiency = 1.0), is at high levels when efficiencies are <10−4, and is intermediate at intermediate mating levels.
A high-resolution analysis of the chromatin organization of HMLα revealed the presence of a discontinuous array of well-positioned nucleosomes (51). These results provide a structural basis for the involvement of organized chromatin domains in transcriptional repression. Interestingly, the effects of sir mutations were confined to half of the silent locus. We have performed a comparative high-resolution chromatin analysis of HMRa and MATa in wild-type and sir3 and H4 histone mutant strains. Chromatin of HMR is organized as a continuous array of precisely positioned nucleosomes dependent on Sir3p and histone H4. The results are discussed in terms of the understanding of the mechanism of silencing at HMRa and the functional differences between HMLα and HMRa.
MATERIALS AND METHODS
S. cerevisiae strains, generously provided by J. E. Haber, were all derivatives of DBY745 (S288C): JKM108 (ho hmlΔ::ADE1 MATα HMRa ade1-112 lys5 leu2-3 ura3-52), JKM111 (ho hmlΔ::ADE1 MATa hmrΔ::ADE1 ade1-112 lys5 leu2-3 ura3-52) (MATa only), JKM115 (ho hmlΔ::ADE1 MATα hmrΔ::ADE1 ade1-112 lys5 leu2-3 ura3-52 trp1) (MATα only), PKY913 (from S. Roth) {ho MATα Δhhf1::HIS3 Δhhf2::LEU2 pUK613 [hhf2 del(4-28)] lys2 leu2 ura3 trp1 ade2 arg4 his3 thr4} (17), YKW05 (this study) (ho hmlΔ::ADE1 matΔ::URA3 HMRa ade1-112 lys5 leu2-3 ura3-52) (HMRa only); YSL101 (ho HMLα MATa HMRa ade1-112 lys5 leu2-3 trp1::hisG ura3-52 his3::hisG::URA3::hisG), and YSL102 (ho HMLα MATa HMRa ade1-112 lys5 leu2-3 trp1::hisG ura3-52 sir3::URA3).
Yeasts were grown in yeast extract-peptone-dextrose at 30°C to mid-log phase (optical density at 600 nm with 1-cm light path, ∼1). Nuclei were isolated and digested with micrococcal nuclease or DNase I (Worthington), and DNA was purified as described previously (37, 44, 51). Protein-free DNA control samples were obtained by digesting purified, previously undigested DNA with a 50-fold-lower concentration of enzyme or by digesting a DNA sample obtained by PCR amplification of yeast genomic DNA. A 3.0-kbp segment of DNA including HMRa was amplified with oligonucleotides p08 and q38 (see below) as primers. About 100 ng of the product was digested with 1 U of MNase per ml or 0.05 U of DNase I per ml at 37°C for 3 min in the presence of 36 μg of carrier DNA. After ethanol precipitation, DNA was resuspended in 50 μl of 0.1× Tris-EDTA.
Nuclease cleavage sites were located by a primer extension assay with 32P-, end-labeled oligonucleotide primers and Taq polymerase as previously described (39), with minor modifications (51). Oligonucleotides used as primers include the following, with coordinates being locations in the published sequence of S. cerevisiae chromosome III (30): p08, 290864 to 290890; p14, 291420 to 291442; p23, 292317 to 292342; p26, 292546 to 292583; p28, 292836 to 292860; p32, 293221 to 293240; q22, 292207 to 292181; q27.5, 292765 to 292738; q32, 293234 to 293214; q35, 293539 to 293512; and q38: 293902 to 293872.
RESULTS
While other methods have been used for inferring features of chromatin structure, both histone-DNA interactions and binding of regulatory proteins, the method of highest resolution for the identification of the location of nucleosomes in chromatin remains micrococcal nuclease digestion (40). The elucidation of chromatin structure entails the isolation of yeast nuclei, followed by partial nuclease digestion of chromatin. The location of nuclease cutting sites is mapped by single-strand radiolabeled primer extension with multicycle linear amplification, leading to DNA fragments which can be analyzed by gel electrophoresis and autoradiography. Nucleosomes can then be identified as regions of low nuclease susceptibility that are ∼150 bp in length and flanked by nuclease-hypersensitive sites. Primer extension analysis requires the use of yeast strains for which the primer sequence is unique, i.e., without sequence homology elsewhere in the genome. Therefore, the silent HMRa domain and the active MATa locus were mapped in otherwise wild-type strains in which HML and MAT or HML and HMR, respectively, were replaced. Comparison of the primer extension maps of these strains allows unambiguous assignment of chromatin organization at both HMRa and MATa.
Chromatin structure of the wild-type HMRa locus.
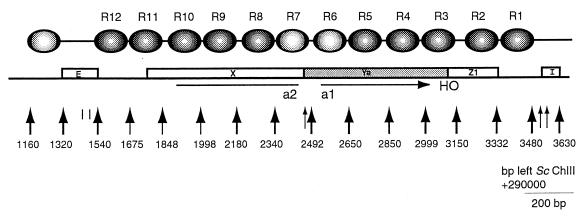
Figure 1 summarizes the chromatin organization of the HMRa locus that was inferred from the experimental data. The numbers of the map positions are the numbers of the corresponding sites in the sequence of chromosome III (30) minus 290,000. A highly organized domain consisting of 12 well-positioned nucleosomes spans a 1.94-kb region between the silencers HMR-E and HMR-I. For identifications given below, the nucleosomes are numbered R1 to R12, from the telomere-proximal end of the locus to the centromeric end. The cis-acting E element (bp 1320 to 1540) and I element (bp 3545 to 3630), which are sensitive to nuclease cleavage, flank this organized chromatin at HMRa.
FIG. 1.
Schematic representation of chromatin structure of the ∼2.5-kb HMRa region. The indicated map units plus 290,000 equate to the base pair positions of the published sequence of yeast chromosome III (30). Open boxes labeled E and I identify the silencer sequences. Boxes labeled X, Ya, and Z1 are regions of the mating-type locus. Large arrows indicate regions of micrococcal nuclease sensitivity, which serve to define nucleosomes. Darkly shaded ovals indicate precisely positioned nucleosomes, and the two lightly shaded ovals refer to less well defined nucleosomes. The horizontal arrow under the Ya region marks the open reading frame of the a1 gene. The line under the X region marks the open reading frame of the a2 gene.
The first strong nuclease cut site internal to the I element is at bp 3480, 65 bp proximal to the border of this silencer. This signals the beginning of the positioned nucleosome array which extends to the edge of the E element. Nucleosomes R1 to R4 are clearly seen in the map of the cutting in the Watson strand shown in Fig. 2, and the higher-molecular-weight, lower-resolution section of the sequencing gel suggests an even further extension of the organized pattern. The striking contrast of the nuclear chromatin digestion pattern with that of a protein-free DNA control dramatically demonstrates the highly organized chromatin structure present at HMRa. DNA which is associated with histones in randomly organized nucleosomes is expected to have a digestion pattern closely similar to that of the control, as any given sequence will randomly appear in a protected nucleosome region or a susceptible linker region.
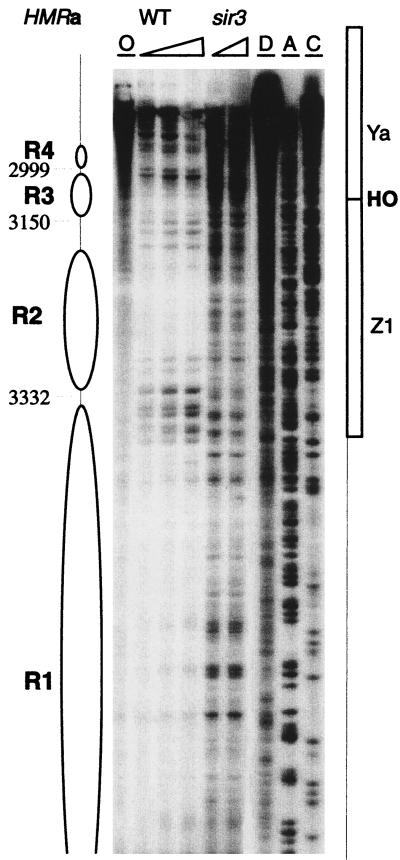
FIG. 2.
HMRa chromatin near the I silencer. Chromatin structure was mapped by primer extension analysis of micrococcal nuclease cleavage sites with primer q35. Wild-type (WT) and sir3 mutant strains are indicated. Triangles represent increasing concentrations of nuclease. Lane O, nuclease-free control; lane D, protein-free DNA subjected to nuclease cleavage; lanes A and C, sequencing reactions to facilitate the identification of locations in the map. The inferred positions of nucleosomes R1 to R4 are indicated. Four precisely positioned nucleosomes are located adjacent to the I silencer. Their structure in the sir3 mutants was disrupted, evidenced by the widespread nuclease accessibility.
Mapping of the Crick strand cutting pattern confirms the occurrence and locations of these four nucleosomes (Fig. 3). The hypersensitive site near the I element is apparent. There is no indication of an organized chromatin structure in the region to the right of the I silencer, towards the right telomere of chromosome III (data not shown). The contrast between chromatin and protein-free DNA for each of the four positioned nucleosomes that are clearly resolved in this structural map is again remarkable. In addition to the obstruction of nuclease cutting sites by histone-DNA interactions in the positioned nucleosomes, other features of the digestion pattern signify the precise organization of chromatin at HMRa. The nuclease susceptibilities of sites in the linker DNA are not identical to those of free DNA, demonstrating unique although undefined features of the architecture of the linker DNA between positioned nucleosomes in this chromatin domain (note particularly the R2-R3 linker for the Crick strand [Fig. 3]).
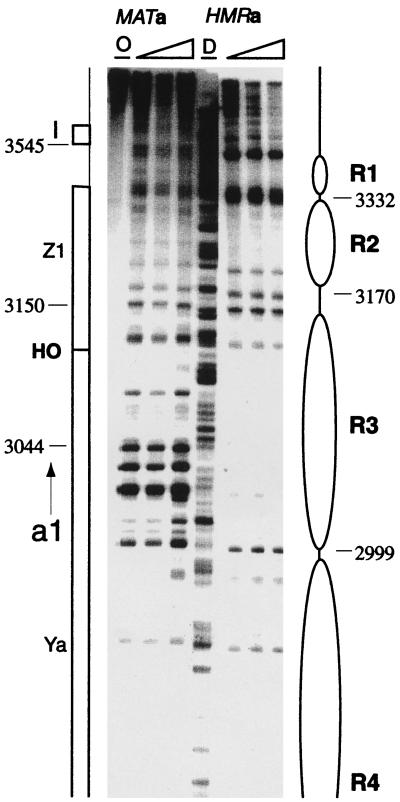
FIG. 3.
HMRa and MATa chromatin structure near the HO endonuclease recognition site at the Ya-Z1 border. Samples of the two loci were mapped with strains (HMRa only and MATa only) and primers that allow the specific detection of only one region. The chromosomal coordinates for the MAT locus are 93,633 bp less than the coordinates for the HMR locus. Extensive cleavage throughout the region of HMR occupied by nucleosome R3 was present at the active MAT locus.
To indicate the methods we used to infer chromatin structures from these nuclease cutting site susceptibility maps, we describe how the positions of nucleosomes R1 to R4 were assigned from the high-resolution maps of both DNA strands (Fig. 2 and 3). The first cut internal to I was at bp 3480. Since the nucleosome core particle contains 146 bp of DNA, the other edge of this nucleosome should have been at about bp 3330. A cutting site at bp 3332 was assigned as the left edge of R1. Cutting to the right of this site, internal to the assigned nucleosome position, was observed. We have seen a similar intrusion of micrococcal nuclease into positioned nucleosomes previously and discussed possible mechanisms (40). The next nucleosome, R2, must extend at least to bp ∼3180. A series of bands are cut between bp 3180 and 3150, suggesting a long linker region between R2 and R3. Assuming that the bp 3150 site is the right border of nucleosome R3, the left edge should be at bp ∼3000. In fact, a single sharp cutting site was present at bp 2999, indicating a close packing of nucleosomes R3 and R4. As expected, the left edge of R4 was marked by a strong cutting site at bp 2850.
Note that nucleosomes R1 and R2 are closely opposed with a very short linker, as are nucleosomes R3 and R4. Between these two pairs of closely packed dimeric nucleosomes is a relatively long linker of ∼30 bp. This motif of closely packed nucleosome dimers separated by a quantized linker of ∼20 to 30 bp is present for the entire HMRa locus and for the right portion of the HMLα domain (51).
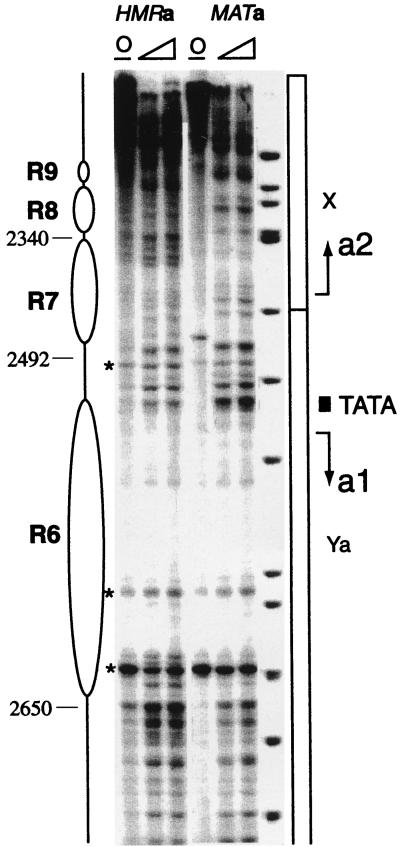
Nucleosomes R3 to R10 span all of the coding sequences of the a1 and a2 genes as well as the intergenic promoter for these divergently arranged genes. The expected regions of ∼140 bp in which micrococcal nuclease cleavage is inhibited are present for nucleosomes R5 and R9 (not shown) and R6 to R8 (Fig. 4), and these protected regions are flanked by sites which are highly sensitive to cutting by the nuclease, a characteristic feature of the linkers between nucleosome core particles. The level of internal cleavage by the nuclease within the positioned nucleosomes in this central segment of the HMRa domain is higher than those at either end of the locus; the significance of this observation remains to be established.
FIG. 4.
HMRa and MATa chromatin structure of the a1-a2 promoter region. For a description, see the legends to Fig. 2 and 3. The bands marked by an asterisk are primer extension stop artifacts, signified by their presence in a nuclease-free control sample (lanes O). At the promoter, the TATA box in the MAT sample was more accessible. The organized chromatin structure present at HMRa for the a2 gene is absent at the transcribed MATa locus.
Figure 5 shows micrococcal nuclease cutting site maps of the Crick strand to the right of the E silencer element. In contrast to the I silencer, the first major nuclease site is only about 25 bp from the edge of the E silencer element, at 1,540 map units. A nucleosome, R12, blocks cutting from there to a site at bp 1675. A neighboring, closely packed nucleosome, R11, blocks the nuclease up to a broad strong band of cutting at bp ∼1850. A site which is definitely cut within what we assigned to be nucleosome R11 remains an enigma. Nucleosome R10 and an indication of the location of R9 are present above the dimer pair of nucleosomes R12 and R11.
FIG. 5.
HMRa chromatin near the E silencer. Chromatin structure was mapped by primer extension analysis of micrococcal nuclease cleavage sites. Wild-type (WT) and sir3 mutant strains are indicated. Triangles represent increasing concentrations of nuclease. Lane O, a nuclease-free control; lane D, protein-free DNA subjected to nuclease cleavage. The inferred positions of nucleosomes R9 to R12 are indicated. Four precisely positioned nucleosomes are located adjacent to the I silencer. Their structure in the sir3 mutants was disrupted, evidenced by the widespread nuclease accessibility.
Chromatin structure of the MATa locus.
In contrast to the precisely positioned nucleosomes that span HMRa between the I and E silencers, the chromatin structure of most of the active MATa locus is randomly organized (data not shown). However, the organization of the promoter region at the active MATa locus is somewhat similar to the one mapped at HMRa (Fig. 4). Subtle differences include increased nuclease sensitivity of the linker region between R6 and R7, an additional cleavage site internal to nucleosome R7 of MAT at position 2460, and high accessibility of the a1 TATA box of MAT relative to that of HMRa. MATa displays cut sites primarily at the promoter of the a1 gene. The a2 promoter appears to be nuclease inaccessible. Overall, the active MATa promoter appears to be only slightly more accessible than that of the silent HMRa. Structural similarities of the promoter region between the active and silenced loci could reflect low transcriptional activity at MATa in a haploid strain (36), where the a1 and a2 gene products have no known role. Mata1p is transcribed in the diploid, where it complexes with Matα2p to form a repressor heterodimer that turns off haploid-specific genes (14). The chromatin structure of the a1 and a2 promoter region of MATa was examined in a diploid strain derived by mating the MATα-only and the MATa-only strains. The nuclease digestion pattern for this diploid was identical to that observed for MATa in the haploid strain (data not shown). It is possible that structural features of the DNA of the a genes could facilitate selective interactions with histones, leading to a similar chromatin organization for both the silenced and transcriptionally competent loci.
Away from the promoter, there is little similarity in the micrococcal nuclease digestion patterns for the two loci, one active and one silenced. The a2 coding region, occupied by nucleosomes R7 to R10 at silent HMRa, was digested extensively throughout MATa, and it lacks the hypersensitive sites which demarcate nucleosomes (Fig. 4). On the opposite side of the promoter, the chromatin structures for the silent and active loci also differ strikingly. With the exception of hypersensitive sites near bp 3150 (the R2 to R3 linker in wild-type cells), which are also cut at the MAT locus, the digestion pattern of the active locus lacks the distinctive features that characterize repressed chromatin at HMRa (Fig. 3). Specifically, in addition to missing positioned nucleosomes R4 to R6 (data not shown), which encompass the a1 gene, the MAT locus does not have nucleosomes in positions corresponding to R1 to R3 of HMRa.
Marked differences between HMRa and MATa around the HO endonuclease recognition site at the Ya-Z1 border can be noted (Fig. 3). The HO recognition site, present in both the active and the silent loci, is cleaved only at the active MAT locus to create a double-strand break, which induces mating-type switching via homologous recombination with the appropriate HM locus (7). Differences in the chromatin structures of the silent locus, where the HO site is inaccessible, and of the active MAT locus, where the HO site is cleaved, may be related to the differential susceptibility of identical sequences to the endonuclease. An area of nuclease protection spanning 151 bp, from bp 2999 to 3150, defines nucleosome R3 of HMRa. At MATa, on the other hand, nuclease sensitivity characterizes this region, with three strong cleavage sites at bp 3044, 3025, and 3016. Surprisingly, micrococcal nuclease did not cut the HO site at HMRa or at MATa. Also at MATa, cut sites flanked the HO site at bp 3120 and 3080. These sites were not cut at HMRa, which has one minor cut site at bp 3115. The protection of the HO site at MATa, a site which is hypersensitive at MATα (51), could reflect the binding of a specific protein to the HO site located at the Ya-Z1 intersection. Since sequences necessary for efficient HO cleavage are larger in vivo than the endonuclease cognate recognition site in vitro (28, 29), a protein could bind to the HO site at MATa specifically. Differences between the chromatin organization of the HO site at two susceptible sites in the two different cell types may offer a clue to distinctive features of mating-type interconversion in yeast.
Chromatin structure of HMRa in a sir3 mutant and histone H4 amino-terminal-deletion strains.
While the organized chromatin adjacent to the I silencer at HMLα in sir mutant strains was disrupted as expected, a surprising result was the persistence of organized chromatin structure near the E silencer of that silent locus in the mutant strains (51). To see if this is a general phenomenon shared by the silent loci, the chromatin organization of regions near the silencer elements of HMRa in sir3 mutant strains was analyzed. In the absence of Sir3p, the chromatin structure at HMRa near both silencers was disrupted. Compared to the closely packed dimer nucleosomes R1-R2 and R3-R4, which were separated in a wild-type strain by a long linker, the sir3 strain showed no evidence of a specific nucleosomal structure. Cutting by micrococcal nuclease occurred throughout the region, and the nuclease-hypersensitive sites, which demarcate linkers or the borders of nucleosomes, are absent (Fig. 2). The pattern of nuclear chromatin digestion in the sir3 strain is not identical to that of protein-free DNA, which suggests some differences in the structure of the nucleoprotein complex from free nucleic acid.
Similarly, abutting the E silencer element, the distinctive protection due to nucleosomes R11-R12 and R9-R10 is totally absent in the strain lacking Sir3p (Fig. 5). Hypersensitive sites at the edge of R12 (bp 1520 and 1530), between R11 and R12 (bp 1675), and the region around bp 1840 between the pairs are striking in the wild-type strain and are not present in the mutant strain. The E silencer itself appears to be more nuclease accessible in the sir3 strain than in the wild type; this would not have been predicted based on current ideas of which proteins physically interact with DNA at the silencer sequences. The entire HMRa locus responded to the absence of Sir3p, as did the right half of HMLα.
Nucleosomal organization in the highly structured chromatin format is also dependent on histone H4. The mapping of chromatin in a strain expressing an amino-terminal deletion (amino acids 4 to 28) of histone H4 shows that the nucleosomes were no longer well positioned across the HMR locus (data not shown). Specifically, the nuclease digestion patterns at HMRa of the H4 deletion sample around the promoter region and in the neighborhood of the HO endonuclease site are essentially identical to the patterns observed at MATa. In the remainder of the unsilenced locus outside of the transcription units, the histone tail deletion led to cutting patterns very similar to those seen for the sir3 mutant strain, indicating the disruption of the organized chromatin structure for the entire HMRa domain (data not shown).
DISCUSSION
Genetic studies have suggested that chromatin structure might be important for transcriptional repression at several sites in the yeast genome, including the silent mating-type loci and telomeres (6). Biochemical studies have confirmed that supposition for the mating-type loci (51; also this study) and have demonstrated the importance of highly organized chromatin structures in the repression of other regions of the yeast genome, specifically the recombination enhancer (50) and two genes regulated by the α2 repressor (32, 39, 48). A continuous array of positioned nucleosomes characterizes the structure at a number of these loci, such as the recombination enhancer, a-cell specific genes, and subtelomeric regions (19). Here, we report that the silent HMRa locus is also characterized by a continuously organized chromatin domain spanning its 1.9-kb length. This organization of a silenced HM locus contrasts with the nucleosomal arrays adjacent to the silencers of HML, which are punctuated by a nucleosome-free, nuclease-hypersensitive region at the intergenic central promoter (51).
An interesting pattern of nucleosomal organization seems to characterize all or a major portion of each of these repressed domains; nucleosomes occur as closely packed dimers with a linker of <5 bp. Micrococcal nuclease digestion of random DNA associated with histones in vitro yields a fragment ladder with a repeat of ∼150 bp (49), suggesting an energetically favorable situation for the association of core particles with a minimal linker length. In the X-ray structure of the nucleosome core particle (24), the basic amino-terminal region from amino acid K16 to N25 of histone H4 interacts with a highly acidic region derived from H2A and H2B on the face of the histone octamer disc of the neighboring core particle. The point at which the H4 tail emerges from the flat face of the octamer is two helical turns, or 90° from the pseudodyad of the core particle, and the location of the acidic patch is opposite the pseudodyad. Thus, the interaction of these two moieties would be geometrically favored in a closely packed dimer nucleosome in which the pseudodyads of adjacent core particles are displaced from one another by 90°. This interaction might provide some of the energy that favors the formation of closely packed nucleosomes.
Similarities of chromatin structure adjacent to the two HMR silencers are somewhat surprising, given the functional differences between HMR-E and HMR-I. HMR-E, by virtue of its ability to confer repression independently, is considered the strongest silencer, while HMR-I is not even required for silencing (2). This result may reflect the importance of ORC-Sir1p binding (47) and subsequent Sir1p-Sir3p–Sir4p interaction in the establishment of a repressive chromatin structure. In contrast, at HMLα, sir mutations led to the disruption of chromatin only near HML-I, while chromatin at HML-E appeared to be unaffected by the mutation (51). A correlation can be made between the presence of a binding site for Abf1p and the dependence of an adjacent organized chromatin structure on Sir3p; three silencers have both and HML-E has neither.
The structural similarities between the active and silenced a1 and a2 promoter regions are in contrast to the clear transcriptional signature at the α1 and α2 promoter observed at MATα and masked at the silent HMLα locus (51). These differences may be related in part to inherent properties of the loci. The level of expression may be modulated, since the functionality of the MATa gene products is limited compared to that of the α gene products (3, 14, 21, 45). Subtle differences in the chromatin structure of the promoters at HMRa and MATa may reflect lower levels of transcriptional activity of MATa. While overall levels of expression are low, recent data on the expression of a and α genes by the high-density oligonucleotide chip hybridization assay indicate higher levels of transcriptional activity for the α genes (36).
Different structural features distinguish HMR from HML. HMR is smaller than HML; sufficient DNA is present between the silencers to accommodate 12 nucleosomes at HMR and 22 nucleosomes at HML. The 12 positioned nucleosomes at HMR fill that space, but two arrays of 9 and 11 nucleosomes abut the silencers at HML. The number and combination of specific protein binding sites at the silencers are distinct and may relate to the interruption in the HML array. Rap1p has been shown to be important for silencing at HML (19). The presence of Rap1p binding sites at both silencers of HML and at the intergenic promoter may be critical for repressive chromatin structure at the HML locus. Possible loop formation via Rap1p interaction and karyoskeleton interactions with a repressive complex have been suggested as mechanisms for silencing at HML (11, 51). In contrast, at HMR, only a single Rap1p binding site is present at the centromere proximal silencer HMR-E.
Although they serve a common purpose in sheltering a copy of mating-type information in a location where it is not transcribable, a number of genetic studies have indicated both similarities and differences in the details of the mechanisms employed at the two HM loci. E and I silencers are present at both, but their repressive capabilities in ectopic locations differ. Common protein binding motifs are present in the silencers, but there are different combinations at various loci. The amino-terminal tails of H3 and H4 are involved in silencing, but mutations and deletions have different effects on the two loci in their native and/or alternative locations. Some of these differences may result from (or lead to) the similarities and differences in the chromatin structures of the two silenced domains, HMLα and HMRa, which we have described. The surprising differences in chromatin architecture for the two silent mating-type loci may be critical to the selection mechanism for the recombinational repair of an HO endonuclease-induced break of double-stranded DNA at the MAT locus that leads to mating-type interconversion.
ACKNOWLEDGMENTS
A. Ravindra and K. Weiss contributed equally to the study.
We thank members of our and the Workman laboratories for criticism and experimental guidance and Jim Haber for strains.
This work was supported by a grant from the National Institute of General Medical Sciences, National Institutes of Health, GM52311.
REFERENCES
- 1.Abraham J A, Nasmyth K, Strathern J, Klar A J S, Hicks J B. Regulation of mating-type information in yeast. Negative control requiring sequences both 5′ and 3′ to the regulated region. J Mol Biol. 1984;176:307–331. doi: 10.1016/0022-2836(84)90492-3. [DOI] [PubMed] [Google Scholar]
- 2.Brand A H, Breeden L L, Abraham J A, Sternglanz R, Nasmyth K. Characterization of a “silencer” in yeast: a DNA sequence with properties opposite to those of a transcriptional enhancer. Cell. 1985;41:41–48. doi: 10.1016/0092-8674(85)90059-5. [DOI] [PubMed] [Google Scholar]
- 3.Dranginis A M. Regulation of STA1 gene expression by MAT during the life cycle of Saccharomyces cerevisiae. Mol Cell Biol. 1989;9:3992–3998. doi: 10.1128/mcb.9.9.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehrenhofer-Murray A E, Rivier D H, Rine J. The role of Sas2, an acetyltransferase homologue of Saccharomyces cerevisiae, in silencing and ORC function. Genetics. 1997;145:923–934. doi: 10.1093/genetics/145.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldman J B, Hicks J B, Broach J R. Identification of sites required for repression of a silent mating type locus in yeast. J Mol Biol. 1984;178:815–834. doi: 10.1016/0022-2836(84)90313-9. [DOI] [PubMed] [Google Scholar]
- 6.Grunstein M. Yeast heterochromatin: regulation of its assembly and inheritance by histones. Cell. 1998;93:325–328. doi: 10.1016/s0092-8674(00)81160-5. [DOI] [PubMed] [Google Scholar]
- 7.Haber J E. Mating-type gene switching in Saccharomyces cerevisiae. Annu Rev Genet. 1998;32:561–599. doi: 10.1146/annurev.genet.32.1.561. [DOI] [PubMed] [Google Scholar]
- 8.Hecht A, Laroche T, Strahl-Bolsinger S, Gasser S M, Grunstein M. Histone H3 and H4 N-termini interact with SIR3 and SIR4 proteins: a molecular model for the formation of heterochromatin in yeast. Cell. 1995;80:583–592. doi: 10.1016/0092-8674(95)90512-x. [DOI] [PubMed] [Google Scholar]
- 9.Henikoff S. Position-effect variegation after 60 years. Trends Genet. 1990;6:422–426. doi: 10.1016/0168-9525(90)90304-o. [DOI] [PubMed] [Google Scholar]
- 10.Herskowitz I, Rine J, Strathern J. Mating-type determination and mating-type interconversion in Saccharomyces cerevisiae. In: Jones E W, Pringle J R, Broach J R, editors. The molecular and cellular biology of the yeast Saccharomyces. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 583–656. [Google Scholar]
- 11.Hofmann J F, Laroche T, Brand A H, Gasser S. RAP-1 factor is necessary for DNA loop formation in vitro at the silent mating type locus HML. Cell. 1989;57:725–737. doi: 10.1016/0092-8674(89)90788-5. [DOI] [PubMed] [Google Scholar]
- 12.Ivy J M, Klar A J S, Hicks J B. Cloning and characterization of four SIR genes of Saccharomyces cerevisiae. Mol Cell Biol. 1986;6:688–702. doi: 10.1128/mcb.6.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jamieson R V, Tam P P L, Gardiner-Garden M. X-chromosome activity: impact of imprinting and chromatin structure. Int J Dev Biol. 1996;40:1065–1080. [PubMed] [Google Scholar]
- 14.Jensen R E, Herskowitz I. Directionality and regulation of cassette substitution in yeast. Cold Spring Harbor Symp Quant Biol. 1984;49:97–104. doi: 10.1101/sqb.1984.049.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Johnson L M, Fisher-Adams G, Grunstein M. Identification of a non-basic domain in the histone H4 N-terminus required for repression of the yeast silent mating loci. EMBO J. 1992;11:2201–2209. doi: 10.1002/j.1460-2075.1992.tb05279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson L M, Kayne P S, Kahn E S, Grunstein M. Genetic evidence for an interaction between SIR3 and histone H4 in the repression of silent mating type loci in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1990;87:6286–6290. doi: 10.1073/pnas.87.16.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayne P S, Kim U, Han M, Mullen J R, Yoshizaki F, Grunstein M. Extremely conserved histone H4 N-terminus is dispensable for growth but essential for repressing the silent mating type loci in yeast. Cell. 1988;55:27–39. doi: 10.1016/0092-8674(88)90006-2. [DOI] [PubMed] [Google Scholar]
- 18.Klar A J S, Strathern J, Abraham J A. Involvement of double-strand chromosomal breaks for mating-type switching in Saccharomyces cerevisiae. Cold Spring Harbor Symp Quant Biol. 1984;49:77–88. doi: 10.1101/sqb.1984.049.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Kyrion G, Liu K, Liu C, Lustig A J. RAP1 and telomere structure regulate telomere position effects in Saccharomyces cerevisiae. Genes Dev. 1993;7:1146–1159. doi: 10.1101/gad.7.7a.1146. [DOI] [PubMed] [Google Scholar]
- 20.Laurenson P, Rine J. Silencers, silencing, and heritable transcriptional states. Microbiol Rev. 1992;56:543–560. doi: 10.1128/mr.56.4.543-560.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li T, Stark M R, Johnson A D, Wolberger C. Crystal structure of the MATa1/MAT α2 homeodomain heterodimer bound to DNA. Science. 1995;270:262–269. doi: 10.1126/science.270.5234.262. [DOI] [PubMed] [Google Scholar]
- 22.Loo S, Rine J. Silencers and domains of generalized repression. Science. 1994;264:1768–1771. doi: 10.1126/science.8209257. [DOI] [PubMed] [Google Scholar]
- 23.Lowell J E, Pillus L. Telomere tales: chromatin, telomerase and telomere function in Saccharomyces cerevisiae. Cell Mol Life Sci. 1998;54:32–49. doi: 10.1007/s000180050123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luger K, Mader A W, Richmond R K, Sargent D F, Richmond T J. Crystal structure of the nucleosome core particle at 2.8 angstrom resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 25.Mahoney D J, Broach J R. The HML mating-type cassette of Saccharomyces cerevisiae is regulated by two separate but functionally equivalent silencers. Mol Cell Biol. 1989;9:4621–4630. doi: 10.1128/mcb.9.11.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Megee P C, Morgan B A, Mittman B A, Smith M M. Genetic analysis of histone H4: essential role of lysines subject to reversible acetylation. Science. 1990;247:841–845. doi: 10.1126/science.2106160. [DOI] [PubMed] [Google Scholar]
- 27.Nasmyth K. Molecular genetics of yeast mating type. Annu Rev Genet. 1982;16:439–500. doi: 10.1146/annurev.ge.16.120182.002255. [DOI] [PubMed] [Google Scholar]
- 28.Nickoloff J A, Chen E Y, Heffron F. A 24-base-pair DNA sequence from the MAT locus stimulates intergenic recombination in yeast. Proc Natl Acad Sci USA. 1986;83:7831–7835. doi: 10.1073/pnas.83.20.7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nickoloff J A, Singer J D, Heffron F. In vivo analysis of the Saccharomyces cerevisiae HO nuclease recognition site by site-directed mutagenesis. Mol Cell Biol. 1990;10:1174–1179. doi: 10.1128/mcb.10.3.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliver S G, et al. The complete DNA sequence of yeast chromosome III. Nature. 1992;357:38–46. doi: 10.1038/357038a0. [DOI] [PubMed] [Google Scholar]
- 31.Park E-C, Szostak J W. Point mutations in the yeast histone H4 gene prevent silencing of the silent mating type locus HML. Mol Cell Biol. 1990;10:4932–4934. doi: 10.1128/mcb.10.9.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patterton H-G, Simpson R T. Nucleosomal location of the STE6 TATA box and Matα2p-mediated repression. Mol Cell Biol. 1994;14:4002–4010. doi: 10.1128/mcb.14.6.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reifsnyder C, Lowell J, Clarke A, Pillus L. Yeast SAS silencing genes and human genes associated with AML and HIV-1 Tat interactions are homologous with acetyltransferases. Nat Genet. 1997;14:42–49. doi: 10.1038/ng0996-42. [DOI] [PubMed] [Google Scholar]
- 34.Rine J, Herskowitz I. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics. 1987;116:9–22. doi: 10.1093/genetics/116.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rine J, Strathern J N, Hicks J B, Herskowitz I. A suppressor of mating-type locus mutations in Saccharomyces cerevisiae: evidence for and identification of cryptic mating-type loci. Genetics. 1979;93:877–901. doi: 10.1093/genetics/93.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roth F P, Hughes J D, Estep P W, Church G M. Finding DNA regulatory motifs within unaligned noncoding sequences clustered by whole-genome mRNA quantitation. Nat Biotechnol. 1998;16:939–945. doi: 10.1038/nbt1098-939. [DOI] [PubMed] [Google Scholar]
- 37.Roth S Y, Simpson R T. Yeast minichromosomes. Methods Cell Biol. 1992;35:289–314. [PubMed] [Google Scholar]
- 38.Schnell R, Rine J. A position effect on the expression of a tRNA gene mediated by the SIR genes in Saccharomyces cerevisiae. Mol Cell Biol. 1986;6:494–501. doi: 10.1128/mcb.6.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimizu M, Roth S Y, Szent-Gyorgyi C, Simpson R T. Nucleosomes are positioned with base pair precision adjacent to the α2 operator in Saccharomyces cerevisiae. EMBO J. 1991;10:3033–3041. doi: 10.1002/j.1460-2075.1991.tb07854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simpson R T. Chromatin structure and analysis of mechanisms of activators and repressors. Methods. 1998;15:283–294. doi: 10.1006/meth.1998.0632. [DOI] [PubMed] [Google Scholar]
- 41.Singh J, Klar A J S. Active genes in yeast display enhanced in vivo accessibility to foreign DNA methylases: a novel in vivo probe for chromatin structure of yeast. Genes Dev. 1992;6:186–196. doi: 10.1101/gad.6.2.186. [DOI] [PubMed] [Google Scholar]
- 42.Stone E M, Swanson M J, Romeo A M, Hicks J B, Sternglanz R. The SIR1 gene of Saccharomyces cerevisiae and its role as an extragenic suppressor of several mating-defective mutants. Mol Cell Biol. 1991;11:2253–2262. doi: 10.1128/mcb.11.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Surani M A. Imprinting and the initiation of gene silencing in the germ line. Cell. 1998;93:309–312. doi: 10.1016/s0092-8674(00)81156-3. [DOI] [PubMed] [Google Scholar]
- 44.Szent-Gyorgyi C, Isenberg I. The organization of oligonucleosomes in yeast. Nucleic Acids Res. 1983;11:3717–3736. doi: 10.1093/nar/11.11.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tatchell K, Nasmyth K, Hall B D, Astell C, Smith M. In vitro mutation analysis of the mating-type locus in yeast. Cell. 1981;27:25–35. doi: 10.1016/0092-8674(81)90357-3. [DOI] [PubMed] [Google Scholar]
- 46.Thompson J S, Johnson L M, Grunstein M. Specific repression of the yeast silent mating locus HMR by an adjacent telomere. Mol Cell Biol. 1994;14:446–455. doi: 10.1128/mcb.14.1.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Triolo T, Sternglanz R. Role of interactions between the origin recognition complex and SIR1 in transcriptional silencing. Nature. 1996;381:251–253. doi: 10.1038/381251a0. [DOI] [PubMed] [Google Scholar]
- 48.Tsukagoshi, Y., and R. T. Simpson. 1999. Unpublished data.
- 49.van Holde K E. Chromatin. New York, N.Y: Springer-Verlag; 1988. [Google Scholar]
- 50.Weiss K, Simpson R T. Cell type-specific chromatin organization of the region that governs directionality of yeast mating type switching. EMBO J. 1997;16:4352–4360. doi: 10.1093/emboj/16.14.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiss K, Simpson R T. High-resolution structural analysis of chromatin at specific loci: Saccharomyces cerevisiae silent mating type locus HMLα. Mol Cell Biol. 1998;18:5392–5403. doi: 10.1128/mcb.18.9.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zakian V A. Structure, function, and replication of Saccharomyces cerevisiae telomeres. Annu Rev Genet. 1996;30:141–172. doi: 10.1146/annurev.genet.30.1.141. [DOI] [PubMed] [Google Scholar]