Abstract
Patient-reported outcomes (PROs) are reports of a person’s health status that provide a global perspective of patient well-being. PROs can be classified into four primary domains: global, mental, physical, and social health. In this descriptive review, we focus on how PROs can be used in cardiac clinical trials, with an emphasis on cardiac surgical trials for patients with coronary heart disease and heart failure. We also highlight ongoing challenges and provide specific suggestions and novel opportunities to advance the field of cardiac clinical trials. Current challenges include the long-term measurement of PROs in clinical trials beyond one year, inconsistency in the choice of the outcome measures among studies, and the lack of measurement of PROs across multiple domains. Opportunities for advancement include measuring PROs using consumer health informatics tools, including returning information back to participants in formats that they can understand using visualization. Future opportunities include quantifying cohort-specific minimally clinically important differences for PROs.
Brief Summary
Patient-reported outcomes (PROs) are reports of a person’s health status that are directly measured by a patient. PROs are often primary or secondary outcomes within cardiovascular clinical trials because they provide a patient’s perspective on the consequences of an intervention on quality of life and symptoms. As such, it is important to design trials that collect PROs across multiple domains of health (global, mental, physical, and social).
Introduction
Patient-reported outcomes (PROs) are reports of a person’s health status that are directly measured by a patient without interpretation by a healthcare professional.1 PROs provide a global perspective of patient well-being, 2,3 and insight into multiple dimensions of health.4,5 PROs inform a holistic picture of where patients are on the disease trajectory during routine management recovery from a major clinical event, and before and after a cardiac intervention.6 Regulatory agencies (U.S. Food and Drug Administration) and international cardiac societies (European Society of Cardiology and the American Heart Association) recommend routine assessment of PROs in clinical trials.7,8 The International Society for Quality of Life Research (ISOQOL) has also published minimum standards for PRO outcome measures including: documentation of the conceptual and measurement model; evidence for reliability and validity; interpretability of scores; quality translation, and acceptable patient and investigator burden.9
The inclusion of PROs as endpoints in cardiac surgery clinical trials has grown over the last decade.10 PROs are now more commonly used in cardiac clinical trials as patient-centered endpoints to complement traditional clinical and health systems-centered outcomes.11 The growth in the inclusion of PROs has paralleled the survival benefits that many cardiac interventions provide for patients. For instance, coronary artery bypass grafting (CABG) has distinct survival benefits,12 so CABG studies are now measuring quality of life to optimize the years gained from the CABG surgery. In addition, patients want relief from mental and physical health symptoms that adversely impact their participation in activities of daily living and ability to participate in social interactions.13
In this descriptive review of the literature we focus primarily on cardiac surgical trials for patients with coronary heart disease and heart failure. We present a multi-domain approach using generic and cardiac-specific measures to advance cardiac clinical trials. We also discuss the ongoing challenges, and provide specific suggestions and novel opportunities to advance the field of cardiac clinical trials, such as quantifying cohort-specific and individually meaningful minimally clinically important differences for PROs.
Patient-reported outcomes
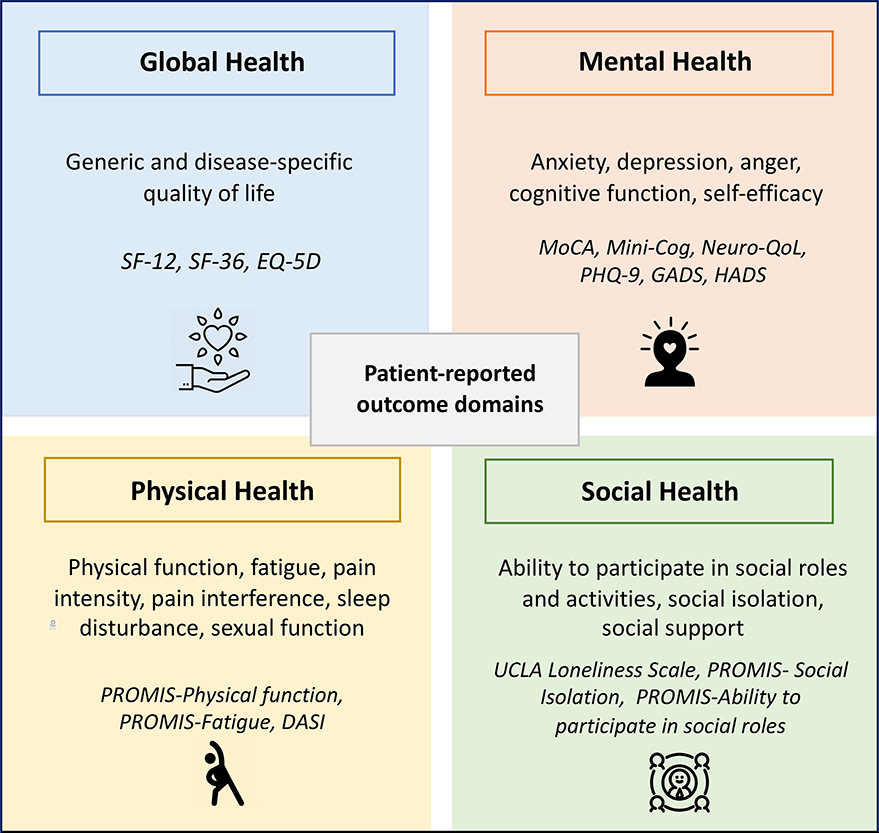
PROs fall into four primary domains: global, mental, physical, and social health (Figure 1). PROs can also be classified by whether they collect information that is generic to multiple conditions or specific to one disease. Generic PROs collect symptoms that are common among multiple chronic conditions, including fatigue, sleep impairment, decreased physical function, depression, and anxiety;14 whereas disease-specific PROs, focus on symptoms that are specific to a condition such as dyspnea or peripheral edema in heart failure, angina in coronary heart disease, and palpitations in atrial fibrillation.15
Figure 1.
Four patient-reported outcome domains
Abbreviations: DASI: Duke Activity Status Index, EQ-5D: Euro-Qol-5D, GADS: Generalized Anxiety Disorder
Assessment, HADS: Hospital Anxiety and Depression Scale, MoCA: Montreal Cognitive Assessment, Neuro-QoL:
Quality of Life in Neurological Disorders, PHQ-9: Patient Health Questionnaire-9, PROMIS: Patient Reported
Outcomes Measurement Information System, SF-12, SF-36: Short Form Health Survey 12-item, 36-item
Quality of life can be measured with disease-specific measures that are validated for specific conditions, or generic instruments that can be measured across multiple conditions. Quality of life is commonly measured using generic measures, such as the EuroQol (EQ-5D) or Short-Form Health Survey (SF-36), which includes both a mental and physical component score (Table 1). Many of the quality of life instruments were initially developed in English and then translated using forward and backward translation into multiple languages.
Table 1.
Summary of the common generic and disease-specific PROs measures in cardiac surgery trials
| Domain | Instrument | # of items | Time to complete | Score range | Dimensions |
|---|---|---|---|---|---|
| Generic quality of life | Short Form Health Survey (SF-12, SF-36) | 12, 36 | 2–5 min | 0–100*, mean (SD) | Physical functioning, role limitations due to physical problems, bodily pain, vitality, general health perception, social function, role limitations due to emotional problems, and mental health |
| Euro-Qol-5D (EQ-5D) | 5 | 1 min | Single index value: 0–1 Visual analog scale: 0–100* |
Mobility, self-care, usual activity, pain or discomfort, and anxiety or depression | |
| PROMIS-Global Health | 10 | 2 min | 0–55, t-score | Physical function, fatigue, pain, emotional distress, and social health), and perceptions of general health | |
| Disease-specific quality of life | Seattle Angina Questionnaire (SAQ) | 19 | 5 min | 0–100*, mean (SD) | Angina frequency, physical limitations, quality of life, angina stability, and treatment satisfaction |
| Kansas City Cardiomyopathy Questionnaire (KCCQ) | 23 | 5 min | 0–100*, mean (SD) | Physical limitations, symptoms (frequency, severity and recent change over time), social limitations, self-efficacy, and quality of life | |
| Minnesota Living with Heart Failure Questionnaire (MLHFQ) | 21 | 5 min | 0–105, mean (SD) | Physical and emotional |
higher score indicates better function
In 2004, the National Institutes of Health (NIH) developed a standardized way to measure generic PROs across patients with multiple comorbid conditions.4,5 The result was the Patient-Reported Outcomes Measurement Information System (PROMIS), a standardized series of validated PRO surveys measuring physical, mental, and social health.16 Researchers have used PROMIS in studies in numerous health disciplines to better understand multiple dimensions of research participants’ health.17 Specific benefits of the PROMIS measures are that they are freely available and have been translated and linguistically validated for use in multiple languages. Using PROMIS measures is especially useful among older adults with multiple chronic conditions. Since the instruments are disease-agnostic and designed to assess global domains of health, they can be compared between cardiac and other patient populations.
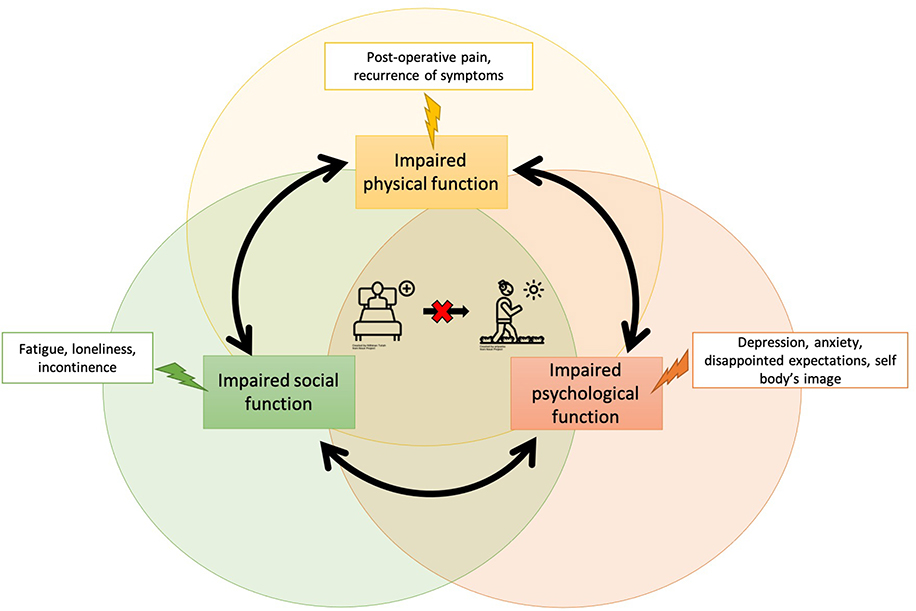
Overall, we suggest measuring PROs across all four domains (global, mental, physical, and social) using both generic and disease-specific outcome measures (Figure 2). Together these PROs can provide a patient’s unique perspective on the consequences of treatment or intervention in a clinical trial and specifically the impact that it has on symptoms and quality of life. When captured rigorously, PRO results can aid in decision-making, inform systematic reviews, meta-analysis, clinical guideline development and ultimately influence healthcare policy.
Figure 2.
Cycle of impaired physical, social, and psychological function
Relationship between PRO domains and outcomes in cardiac trials
Mental Health
Though mental health symptoms, such as depression and anxiety, put a substantial burden on patients and healthcare systems by reducing quality of life and increasing healthcare utilization, they are infrequently measured in cardiac clinical trials.18 The most common tools to measure mental health include: PROMIS- Depression and PROMIS-Anxiety,19 Patient Health Questionnaire-9 (PHQ-9),20 and the Hospital Anxiety and Depression Scale (HADS).21 PRO data may reveal psychological distress and require response from a healthcare professional, termed as a PRO alert. The intervention protocol should include ethical guidance for PRO alerts, and a PRO management plan to reduce co-intervention bias.22,23
Among patients with severe coronary artery disease, the prevalence of depression is high prior to CABG surgery.24,25 Depression is associated with poor medication adherence (including immunosuppressant medications after surgery),26 lower participation in cardiac rehabilitation,27 and other cardioprotective lifestyle behaviors.28 Conversely, there is a positive association between adherence to prescribed medication and mental quality of life.29 Mental health outcomes are important to measure in cardiac clinical trials as patients who are depressed after surgery are at higher risk of poor clinical outcomes and loss to follow-up.18
Patients’ mental health after cardiac surgical interventions are also affected by their body image perception.30 One cardiac clinical trial strategy is to evaluate minimally invasive versus conventional techniques, hypothesizing that smaller incisions may reduce post-surgical distress about body image.31,32 After cardiac surgery, a patient’s altered body image perception can put them at higher risk of depression and anxiety.33 Body disfigurement associated with scars at multiple surgical sites can significantly alter body image perception resulting in traumatic psychological effects, low self-esteem, and impair social interactions and functioning in both adult and pediatric populations.32,34 Specific body image outcome assessments include the Body Image Questionnaire (BIQ), Patient and Observer Scar Assessment Scale (POSAS) v2.0,31 and the Multidimensional Body-Self Relations Questionnaire (MBSRQ).35 In a study with CABG patients, a cardiac rehabilitation intervention was associated with better physical function and MBSRQ scores, indicating better body image postoperatively.33 Overall, prior to enrolling in a surgical cardiac clinical trial, there should transparent communication with patients about realistic expectations about the recovery process and postoperative body image.33
Physical function
The domain of physical function refers to the ability to do basic and instrumental activities of daily living,36 including routine activities such as walking, exercising, and playing sports. In cardiac clinical trials, there are multiple ways to measure physical function that are both generic and disease-specific for patients with multiple cardiac conditions. The most common PRO instruments used to measure physical function include: PROMIS-Short Form- Physical Function,37 SF-36 Physical Component Summary,38 and the Duke Activity Status Index (DASI).39 The compounding effect of distressing physical symptoms directly impacts mental health symptoms, and patients’ ability to participate in social activities (Figure 2).
Patients commonly consent to cardiac surgical trials with the goal of returning to normal levels of physical activity after recovering from surgery. Prior to surgery, many patients have deconditioned physical function, related to cardiac-specific symptoms such as angina on exertion, and increased levels of fatigue, which they hope cardiac surgery will improve.40 Angina relief is central to patient decision making around consenting to both percutaneous or surgical coronary interventions.41 In the hope of resolving angina, patients frequently overestimate the benefits of cardiac intervention and underestimate the risks (e.g. mortality, myocardial infarction, repeat revascularization, cerebrovascular events).42 On the other hand, some patients are asymptomatic before surgery, including patients with life-threatening anatomical conditions (e.g. aortic aneurysms). Postoperatively, they will experience limitations in physical function, but return to full functional capacity and quality of life is expected.
In the immediate postoperative period after CABG, physical function is severely impaired due to postoperative pain. The patient’s expectations of pain intensity and duration preoperatively are predictive of postoperative outcomes because those expectations impact all aspects of clinical management and rehabilitation.43,44 Importantly, changes in physical function after surgery are an important indicator of recovery, and poor physical functioning can be an early indicator of post-surgical complications. For example, in patients recovering from transcatheter aortic valve replacement, reduced gait speed was associated with almost a four-fold increased risk of mortality or rehospitalization, compared to patients with normal gait speed.45
In an effort to accelerate recovery time, surgical innovations over the last decade have been moving the field from conventional to minimally invasive access and robotic surgeries. The benefits of a smaller incision include faster and less painful recovery, a more rapid return to work,46 and normal functional activities. In one study that compared robotic versus conventional surgery they reported faster functional recovery (measured with DASI), quicker return to work (median, 33 vs 54 days), and improved quality of life (measured with SF-12) in the robotic intervention.47 Another example is the LImited Access aortic valve Replacement (LIAR) trial, which compares aortic valve replacement with minimally invasive access versus a full median sternotomy approach48 and uses the Kansas City Cardiomyopathy Questionnaire (KCCQ) as the primary study outcome. Overall, long-term limitations in physical function can put clinical trial participants at higher risk of poor outcomes which could affect the trial findings and conclusions.49
In advance of consenting patients to a cardiac surgery clinical trial, we suggest setting realistic expectations about the typical timeline for recovery and for resuming normal physical activities. After the postoperative recovery period, many patients experience psychological distress and anxiety over a perceived risk of potential cardiac complications. An area of anxiety for patients is when they can safely return to normal exertional activities, and to what degree after discharge from the hospital. Patients report wanting to be able to safely resume normal functional activities,50,51 including routine exercise, recreational sports, sexual activity,52 and any activity that includes increased degrees of exertion. Another example is with patients after an aortic dissection, some patients have a compromised perception of their cardiac function (e.g. fearing that their heart will suddenly stop), and are attuned to any potential body signals that could indicate postoperative complications.53 This subconscious anxiety can cause a profound sense of distress affecting both mental and physical well-being.53
Physical function and pediatric cardiac conditions
Cardiac disease impacts multiple dimensions of a child’s development, as such, PROs should be measured across the domains in pediatric patients to capture any adverse impacts on normal development. As length of survival among congenital heart disease patients increases, there has been a shift from survival to quality of life. PRO reporting among pediatric patients can either be self-administered, for patients typically over 8 years of age for PROMIS measures or by parents/guardians on behalf of children. The PedsQL 4.0 questionnaire was used in a multicenter, randomized clinical trial evaluating the efficacy of Ivabradine for reducing heart rate by >20% in children with dilated cardiomyopathy and symptomatic heart failure.54 Children aged 5–18 filled in the questionnaire themselves, while younger children’s questionnaires were reported by their parents. Patients in the Ivabradine arm showed a reduction in heart rate together with improvement in myocardial function and quality of life compared to children in the placebo group.54 Common instruments for measuring pediatric PROs include the PedsQL 4.0 questionnaire,55 and PROMIS Measures for Pediatric Self-Report, including Cognitive Function, Emotional Distress, Physical Activity, and Peer and Family Relationships.56
Children with congenital heart defects require multi-staged operations which can have long-term impacts not just on their physical and mental health,57 but also their social health.58,59 Reduced physical function, together with persistent symptoms and treatment needs are associated with school absences and ultimately could impact educational attainment.60,61 With pediatric patients, we suggest measuring PROs across all four domains because of the sensitive nature of child development and the need for early intervention if there are deficiencies in specific domains.
Social Health
The social health domain of PROs includes all aspects of being able to participate in social roles and activities with family, friends, and community members. Common ways to measure the domain of social health in clinical trials is by using specific PROMIS measures including: PROMIS- Ability to Participate in Social Roles and Activities, PROMIS- Satisfaction with Social Roles and Activities, and PROMIS- Social Isolation. Though the specific social activities differ, the ability to meaningfully participate in social roles is valued by patients across cultures, countries, and languages.62 Specific physical and mental health symptoms (e.g. pain, fatigue, incontinence, depression) that infringe on the ability to participate in important social roles and relationships are associated with detrimental impacts on patients’ overall quality of life. For instance, anxiety is common among participants with heart failure who are prescribed diuretics about engaging in normal social activities.63,64 Likewise, there is the potential for limitations to social health for patients who have advanced heart failure treated with left ventricular assist devices,65,66 by being tethered to an electric source,67,68 not being able to participate in water-related activities,69 and living with an external cable that can adversely impact patients’ self-image and body concept.70
For patients enrolling in cardiac surgical trials, their social health has frequently been impaired prior to surgery due to disease severity and limitations in physical function, which naturally leads to less participation in social activities and roles. A common pattern after surgery is for physical, mental, and social health to worsen in the first few weeks after discharge, then progressively recover over about 6-months.71 In a study that monitored physical recovery with the Sickness Impact Profile (ambulation, sleep-rest, body movement, and self-care), and social recovery (home management, social interaction, and recreational activities) after coronary revascularization surgery, patients with slower physical and social recovery were at higher risk of developing depression 1 week and 3 months after surgery.71 Physical limitations (e.g. pain at the surgical site, reduced exertional capacity, loss of appetite, and sleep disturbances) in the first four weeks after surgery were also associated with higher risk of depression and disruption in the recovery of social health.72 Cultural and geographical factors also have a significant impact on social health after surgery, especially in collectivistic cultures in which social interactions are fundamental to daily living.71
Increasingly, loneliness is having a detrimental effect on the health of older adults, and this has been exacerbated by COVID-19.73 The impact of loneliness on patients with severe cardiac conditions, including heart transplantation adversely impacts quality of life, and other clinical outcomes.74,75 Among 13,443 patients with cardiac diagnoses, there was a significant increase in 1-year all-cause mortality risk in patients who reported being lonely.76 Relevant to clinical trials, patients with higher levels of loneliness could be at more risk of loss to follow-up. We suggest measuring loneliness as part of the preoperative risk assessment using the UCLA Loneliness scale77 or PROMIS-Social Isolation scale78 as a potential explanatory factor for why some participants may have been lost to follow-up.
Use of PRO outcome measures in clinical trials
When PROs are included as primary or secondary trial endpoints, PRO-specific components are needed in clinical trial protocols, such as the SPIRIT-PRO Extension,79 to facilitate robust data collection, lower rates of avoidable missing data, and more informative data.80 The SPIRIT-PRO Extension includes eleven additions and five elaborations to their previous checklist for protocols to minimize missing data.79 The reporting of PRO data in publications should be done so using the (Consolidated Standards of Reporting Trials) CONSORT PRO extension.81
Coronary artery revascularization
Over the past decade, there has been a trend towards including generic PRO measures as secondary outcomes in randomized coronary revascularization trials.82,83 There is an inverse correlation between higher preoperative Society of Thoracic Surgeons risk scores and lower quality of life (measured with PROMIS Global Health).84 In four large CABG trials, “Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease” (FREEDOM), “Randomized On/Off Bypass” (ROOBY), “Synergy between PCI with Taxus and Cardiac Surgery” (SYNTAX), and “Evaluation of XIENCE versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization” (EXCEL), at least one PRO measure was included in each trial as a secondary outcome (Table 2).85–87 Among the listed cardiac surgery trials, the most common generic measure was the SF-36 or SF-12, and the most common disease-specific PRO was the Seattle Angina Questionnaire,86,88,89 with the EQ-5D also being intermittently measured.89–91 Most of the trials consistently reported improvement in PRO outcomes from the preoperative baseline to 1-year.85,86,89 The International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial, was an impactful coronary revascularization RCT, whose study hypothesis was neutral, but the pre-specified analysis focusing on angina frequency was positive in favor of an invasive strategy in patients with coronary ischemia.92 Together the findings across FREEDOM, EXCEL, ISCHEMIA are important for documenting the benefits of cardiac interventions on quality of life.
Table 2.
Select examples of generic and disease-specific PROs from patients with CHD and HF
| Author, date | Trial name | Comparators | Outcome Measures | Health Status Domain | Assessment Timepoints | Summary of Results |
|---|---|---|---|---|---|---|
| CABG | ||||||
| Abdallah, 2013 Magnuson, 2013 |
FREEDOM | CABG vs. PCI | EQ-5D | Generic | B, 1, 6, 12 months | CABG and PCI improved health status/QOL. PCI produced faster improvement, but health status improved with CABG at 6 months-2 year. Beyond 2 years, no consistent differences. |
| SAQ | Disease-specific | |||||
| Bishawi, 2013 | ROOBY | On-pump vs. Off-pump CABG | SF-36 | Generic | B, 3, 12 months | Both arms improved general and disease--specific QOL by 3 months through 1-year post-bypass. There were no differences in QOL between the two groups. |
| SAQ | Disease-specific | |||||
| Abdallah, 2017 | SYNTAX | CABG v. PCI | SF-36 | Generic | B, 1, 6, 12, 36, and 60 months | Greater improvement was reported in angina frequency and physical function (SAQ), and the role physical and emotional scales (SF-36) compared to PCI. |
| SAQ | Disease-specific | |||||
| Baron, 2017 | EXCEL | CABG vs. PCI | SF-12, EQ-5D, PHQ-8 | Generic | B, 1, 12, and 36 months | Both PCI and CABG patients showed significant improvements in disease- specific and generic health status through 36 months. |
| SAQ | Disease-specific | |||||
| Gaudino/Masterson Creber (ongoing trial) | ROMA: QOL | CABG (MAG vs. SAG) | PROMIS-29, SF-12, EQ-5D, PROMIS- PQ Neuro | Generic | B, first post-op visit, 6, 12, 24, 36, 48, 60 months | Trial recruitment is ongoing across the ROMA sites. PRO measures are assessed preoperatively, first-operative visit, 6 and 12 months, and 2, 3, and 4 years after CABG surgery. |
| SAQ | Disease-specific | |||||
| Heart Failure | ||||||
| Calvert, 2005 | CARE-HF | Usual care vs. device therapy | EQ-5D | Generic | B | Mean EQ-5D score was significantly lower in the CARE-HF population than in the general population. Increasing MLWHF score was associated with a decrease in EQ-5D score. |
| MLWHF | Disease-specific | |||||
| Xiong, 2012 | SADHART-CHF | Sertraline vs. placebo | SF-36 | Generic | B, 12 weeks | Depression remission in HF patients led to significantly higher improvements in SF-36 and KCCQ scores. |
| KCCQ | Disease-specific | |||||
| Bekelman, 2015 | PCDM | Usual care vs. PCDM | KCCQ | Disease-specific | B, 3, 6, 12 months | Significant improvement in the KCCQ overall summary scores in both groups after 1 year, but no significant difference between groups. |
| Solomon, 2019 | PARAGON-HF | Sacubitril-valsartan vs. valsartan for HF | KCCQ | Disease-specific | B, 8 months | More patients in sacubitril-valsartan group had improvements of 5+ points in the KCCQ compared to valsartan group. |
Abbreviations: B: baseline, EQ-5D: Euro-Qol-5D, GADS: Generalized Anxiety Disorder Assessment, HADS: Hospital Anxiety and Depression Scale, HF: heart failure, HFpEF: Heart failure with preserved ejection fraction, KCCQ: Kansas City Cardiomyopathy Questionnaire, MLWHF: Minnesota Living with Heart Failure questionnaire, MoCA: Montreal Cognitive Assessment, PCDM: Patient Centered Disease Management, PCI: Percutaneous Coronary Intervention, PHQ-9: Patient Health Questionnaire-9, POAF: postoperative atrial fibrillation, PROMIS: Patient Reported Outcomes Measurement Information System, PROMIS-PQ Neuro: Neuropathic Pain, PRO: patient-reported outcome, QOL: quality of life, SAQ: Seattle Angina Questionnaire, SF-12, SF-36: Short Form Health Survey 12-item, 36-item
PROMIS measures are also being used in coronary revascularization studies to measure PROs across multiple domains. In a newly designed international prospective randomized trial, “Randomized comparison of the clinical Outcome of single versus Multiple Arterial grafts: Quality of Life” (ROMA:QOL) trial (ClinicalTrials.gov #NCT03217006), PRO measures are being measured pre and post-surgery using both generic and disease-specific outcome measures across all four PRO domains. In the ROMA:QOL trial, the two CABG interventions being compared are multiple arterial grafts versus single arterial grafts. The hypothesis of this study is that patients in the multiple arterial graft arm will have longer-term benefits across all PROs compared to the single arterial graft arm. In order to capture the long-term differences between these two surgical techniques, PROs are being measured for a median of 4-years. Overall, we suggest that cardiac surgical trials include PROs across the domains that are most relevant to the specific intervention under evaluation. The ROMA:QOL study is the first global CABG trial to include a PRO as a primary outcome measure. This shift towards a PRO as primary study outcome reflects a broader trend towards asking research questions that focus on patient’s perspectives of their own health.
Heart failure trials
Traditionally, heart failure trials commonly include primary outcomes focused on mortality, hospitalizations, or composite adverse outcomes. Other common trial endpoints include physiologic parameters (e.g. NTproBNP, blood pressure) at specific time points. A systematic review of heart failure trials from 2005–2008 reported that only 16% of trials measured at least one PRO.93 The Prospective Comparison of ARNI [angiotensin receptor–neprilysin inhibitor] with ARB [angiotensin-receptor blockers] Global Outcomes in HF (PARAGON-HF) trial included 4,822 patients with heart failure with preserved ejection fraction. Though there was no difference in clinical outcomes between the valsartan vs sacubitril/valsartan arms, there was a trend towards better KCCQ values in the latter group. As this trend was statistically borderline, the implementation of other PROs could have provided more insights regarding treatment efficacy to support shared decision making with patients. Patients should be aware of the differences in risks to their health status between medications over time so that they can make informed decisions about which medications to take.
Challenges and Opportunities
Balancing measurement properties and burden
When designing a cardiac clinical trial and selecting PRO outcome assessments, it should be guided by the specific intervention and health condition being evaluated. The COnsensus-based Standards for the selection of health Measurement Instruments (COSMIN) database of systematic reviews of outcome measurement instruments has also published three checklists for designing a study (COSMIN Risk of Bias checklist for PROMs), reporting study measurements (COSMIN Risk of Bias tool to assess the quality of studies on reliability or measurement error of outcome measurement instruments), and to report missing data (COSMIN Study Design checklist).94–96 When selecting PRO measurements, the measurement properties of the instruments (reliability, measurement error, internal consistency, content validity, construct validity, criterion validity, cultural validity, responsiveness and interpretability) should be taken into consideration.97 Investigators need to balance instrument burden for patients, caregivers and research staff, with instrument measurement properties. Instruments that are too long, or the selection of too many instruments, can expose a study to a higher risk of missing data. Higher rates of missing PRO data can reduce power in a study, increase standard error, and weaken the effects of randomization.98 Further, data may be missing from the most vulnerable patients with the poorest outcomes so this can reinforce response bias.99 All trials including PRO outcomes should have strategies in place to address these risks.
Choosing global or disease-specific measurement outcomes
A resource for evaluating which outcome measures other researchers have used in similar disease populations includes the Core Outcome Measures in Effectiveness Trials (COMET) database.100 When the purpose is high-level symptom monitoring, generic global measures of quality of life, such as PROMIS short forms or Global Health could be used. Disease-specific measures are more sensitive to changes in quality of life than generic outcome assessments.101 We do suggest inclusion of the EQ-5D to facilitate cost-effectiveness analysis as the EQ-5D provides a measure of utility used to determine quality adjusted life years.102,103
Long-term assessment of PROs
A current limitation of most cardiac trials is the short duration of follow-up (typically six-months to 1 year), despite PROs being relevant for the duration of patients’ cardiovascular illness. While improved survival from a cardiac intervention is paramount, what also matters to patients is whether they will have sustained physical function and whether they can participate in the activities that they most enjoy.104 When patients are making decisions about participating in a cardiac clinical trial, they want to know whether it will impact how they feel not just three to six months after surgery, but if it will make a difference on their physical function and ability to participate in their social roles two, three and even five years after the intervention. Ultimately, patients prioritize feeling better so measuring PROs over the duration of trial follow-up, especially beyond 1-year, is suggested when resources are available.
Consumer health informatics tools for virtually measuring PROs in clinical trials
Consumer health informatics (CHI) tools are patient-facing digital tools that can be used by patients to directly report PROs. CHI tools can help to overcome barriers to accessing enrollment into cardiac clinical trials and can facilitate enrollment in the context of daily life without needing to attend an in-person visit. This is very timely, especially in the context of COVID-19 where many cardiac trials have been forced to pivot to virtual enrollment and follow-up.105,106 There are pragmatic barriers both to virtual enrollment and follow-up, especially among participants with low technology experience. For instance, some participants are unaware that they should check their spam filters for emails that have been automatically sent from a research data collection tool such as REDCap. Cardiac clinical trials, such as ROMA, which were designed with virtual follow-up have the advantage of fewer barriers to follow-up.
Using CHI tools can also help empower patients to report symptoms that are stigmatized or less frequently discussed with healthcare professionals.107 The inclusion of symptoms in a CHI tool normalizes the symptom and provides reassurance that the symptoms are experienced by other patients with the same condition. In a cardiac informatics intervention using the web-app, mi.Symptoms, 168 patients with heart failure were asked to report PROs across all four domains, including fatigue and pain interference with sexual satisfaction. Overall, 50.4% of patients reported fatigue-related impairment, and 38.6% reported pain-related impairment on sexual satisfaction.108 This information was important because participants also disclosed that impairment to sexual satisfaction was a reason why some had stopped taking some of their cardiac medications.107 The use of CHI tools can help overcome barriers and stigma around talking about sensitive topics such as sexual function or mental health.
Using CHI tools to measure PROs can also advance health equity109 in cardiac clinical trials by reducing barriers to access and by reaching a more diverse group of patients.110 For example, more patients can be reached by virtual recruitment and follow-up than in-person, and barriers to enrollment in a study, such as being able to attend an in-office visit, are reduced. In Table 3 specific CHI tools, including patient portals, smartphone applications, and two research platforms (REDCap and ClinvestiGator) are described with methods for collecting and sharing PRO data.109 These electronic tools are beneficial because they can send notifications (via email or text) to participants to complete their PRO assessments rather than having participants come in for a face-to-face in-person follow-up appointment.
Table 3.
Relevant CHI tools for measuring and reporting PROs in clinical trials
| CHI tool | Description | PRO data sharing |
|---|---|---|
| Patient portals | Secure and convenient online applications that allow patients to have access to their electronic health records | Healthcare professionals can send patients PRO surveys in advance of appointments and patients can input data |
| Smartphone applications | Program or software designed to be used on a mobile device | Passive (sensor data) and active PRO data collection including health summaries, patient education, diagnostic and treatment support |
| Research Electronic Data Capture (REDCap) | HIPAA-compliant web-based data entry system for building and managing online surveys and databases. For clinical visits, data can be harmonized across institutions to facilitate data storage and management | Any PRO measurement tools, including PROMIS. The REDCap system can interface using Fast Healthcare Interoperability Resources (FHIR) standards to electronic health records to pull pre-specified data |
| ClinvestiGator | HIPAA-compliant web-based data entry system for clinical trial data collection. Dashboards signal times for data collection, with emailed reminders to study staff | Any PRO measurement tools, including PROMIS |
Returning PROs research data to patients
Patients report that viewing their PROs is highly informative when monitored and reported over time.2 PROs provide insight into changes in current health status (i.e., symptoms, functioning, and quality of life).2,3,111 When PROs are measured on patients as part of cardiac clinical trials, they should be returned to patients to support them with ongoing disease management.109 Despite the value of PROs for the research community, policies and governance on the ownership of PRO data and whether it should be returned to research participants are in nascent stages. Moreover, associated ethical obligations have been largely unexplored. Overlooking these ethical obligations could erode public trust in participation in biomedical research broadly, including cardiac clinical trials.
Visualization of PROs
If PRO data is returned to participants in a clinical trial, the question is what format the data should be displayed in so that participants understand the data.112 Comprehension of the visualized health information is critical, as sharing raw results without interpretation can be both ineffective and make it difficult for the patient to understand their own information.113,114 Returning meaningful health information for patients across the continuum of health literacy, numeracy, and technology experience is an ongoing challenge. When written questionnaires are inaccessible, it may preclude patients’ engagement with PROs98,115 and cause intervention-generated inequity, a phenomenon where well-intentioned interventions worsen existing health disparities rather than reduce them.50–52
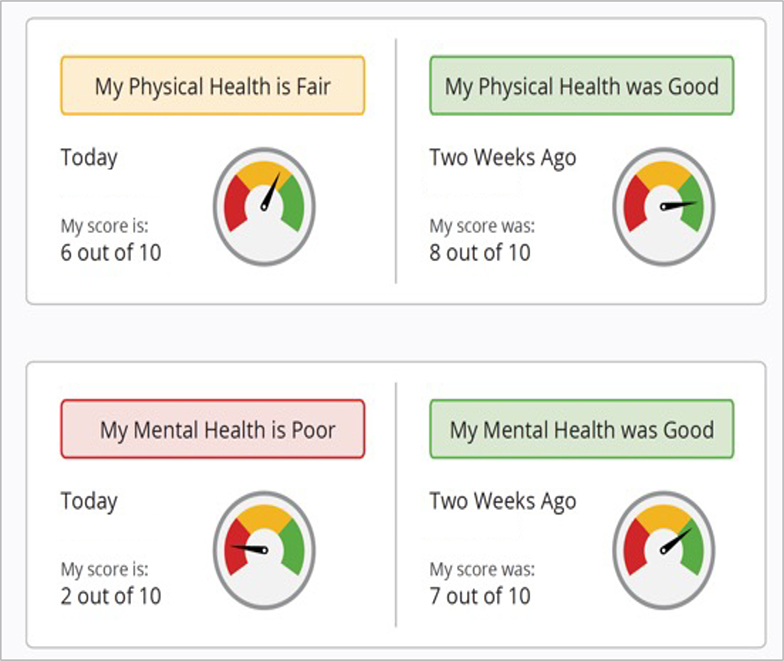
In a study of patients with heart failure, a web application was developed to collect and return PROs to patients.116,117,118 In order to enhance content comprehension the visualizations were tailored for older adults,118 and for those with low health literacy and numeracy.119 The web-app is now integrated with REDCap and can be used to collect multiple, brief PRO surveys, and display real-time, tailored symptom status visualizations. The health information is displayed as visual analogies to support varying levels of health literacy, graph literacy, and numeracy (Figure 3).112,120 In a cross-sectional survey conducted in 2020 among 300 adults over 50 years of age from the U.S,121 the vast majority of participants reported a strong desire to receive PROs back (79% agree/strongly agree), and in easily comprehensible formats (88% agree/strongly agree). It is becoming an expectation from patients for researchers to return PROs back to patients so that they can be used to support the management of their own health.
Figure 3.
Example of PRO visual analogy
Making clinical use of PROs by establishing minimal clinically important differences (MCIDs)
One reason why PROs are not routinely collected as part of cardiac clinical trials is that many healthcare professionals are unsure of how to meaningfully use them. A barrier to their routine use is that there are not established minimal clinically important differences (MCIDs), or the smallest amount a PRO must change for it to be clinically meaningful to a patient.122 Existing MCIDs are generic for cardiac populations (e.g. MCID for physical function 2.5–6.5, fatigue 2.5–6.5 or anxiety 3.0–8.0 after a stroke),123 but not specific for patients after a CABG surgery or patients with heart failure after a hospitalization. For patients after CABG, we would expect dramatic short-term fluctuations in health status that worsen after surgery but recover over time, compared to patients whose coronary heart disease is stable. Likewise, for patients with heart failure, we anticipate unique MCIDs for patients with heart failure who are stable in ambulatory care versus patients with heart failure who have been admitted to the hospital and are recovering from a hospitalization. Overall, we suggest that researchers invest in quantifying MCIDs within cohorts of cardiac clinical trials so that PROs can be clinically meaningful.
Limitations of measuring PROS in the context of clinical trials
There are a few limitations to acknowledge when measuring PROs in clinical trials. A long battery of outcome assessments can be time consuming for a participant to complete and repeat at follow-up time points. Low technology experience, education level, and cognitive impairments can also make it difficult for a participant to complete electronic PROs at multiple time points. While the PRO reporting tools are validated for use in multiple languages and across disease groups, the use of the tools is not standardized across clinical trials. As a result, comparisons across datasets and studies are hampered because of lack of standardization in the instruments used.
Conclusion
We propose using a multi-domain approach for the conceptualization and measurement of PROs, which incorporates best practices from COSMIN for the selection of instruments across multiple domains and includes both generic and disease-specific outcome assessments. We also support using both the SPIRIT-PRO Extension for the development of the protocol for measuring PROs and the CONSORT-PRO Extension for publishing PRO findings. Importantly, we also need to generate a more substantive body of evidence on the long-term impact of cardiac interventions. As such, long-term outcomes need to be assessed across cardiac clinical trials. Digital data collection for follow-up in cardiac trials can reduce the burden on research staff who historically have had to make phone calls or see patients in-person to collect follow-up information. CHI tools that keep protected health information secure, can help relieve the data collection burden from research staff and bridge barriers for patients coming into the office for initial enrollment or follow-up appointments. Digital data collection also allows for the return of PROs back to patients in a comprehensible format to support their own symptom management and engagement in long-term clinical trial follow-up. Future directions for the collection of PROs in cardiac clinical trials include standardizing MCIDs for specific cohorts of cardiac patients to support health care teams with understanding whether patients are in stable, recovering, or declining health status.
Acknowledgments:
Funding sources: Dr. Masterson Creber receives funding from the National Institute of Nursing Research (R00NR016275) and the National Heart, Lung, and Blood Institute (R01HL152021). Ms. Taylor receives funding from the National Institutes of Health (T32NR007969-19S1).
Footnotes
Disclosures: The authors have no conflicts to disclose.
References
- 1.Shuster JJ. Review: Cochrane handbook for systematic reviews for interventions, Version 5.1.0, published 3/2011. Higgins Julian P.T. and Green Sally, Editors. Res Synth Methods. 2011;2(2):126–130. [Google Scholar]
- 2.Lavallee DC, Chenok KE, Love RM, et al. Incorporating Patient-Reported Outcomes Into Health Care To Engage Patients And Enhance Care. Health Aff. 2016;35(4):575–582. [DOI] [PubMed] [Google Scholar]
- 3.Field J, Holmes MM, Newell D. PROMs data: can it be used to make decisions for individual patients? A narrative review. Patient Relat Outcome Meas. 2019;10:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moskowitz JT, Cheung EO, Snowberg KE, et al. Randomized controlled trial of a facilitated online positive emotion regulation intervention for dementia caregivers. Health Psychol. 2019;38(5):391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipton RB, Tepper SJ, Reuter U, et al. Erenumab in chronic migraine: Patient-reported outcomes in a randomized double-blind study. Neurology. 2019;92(19):e2250–e2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelkar AA, Spertus J, Pang P, et al. Utility of Patient-Reported Outcome Instruments in Heart Failure. JACC Heart Fail. 2016;4(3):165–175. [DOI] [PubMed] [Google Scholar]
- 7.Anker SD, Agewall S, Borggrefe M, et al. The importance of patient-reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J. 2014;35(30):2001–2009. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Food and Drug Administration. Center for Drug Evaluation and Research. CLINICAL OUTCOME ASSESSMENT COMPENDIUM. Published 2015.Accessed January 2021. https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM481225.pdf [Google Scholar]
- 9.Reeve BB, Wyrwich KW, Wu AW, et al. ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Qual Life Res. 2013;22(8):1889–1905. [DOI] [PubMed] [Google Scholar]
- 10.Vodicka E, Kim K, Devine B, Gnanasakthy A, Scoggins J, Patrick DL. Inclusion of Patient-Reported Outcome Measures In Registered Clinical Trials: Evidence From Clinicaltrials.Gov (2007–2013). Value in Health. 2015;18(3):A209–A210. doi: 10.1016/j.jval.2015.03.1214 [DOI] [PubMed] [Google Scholar]
- 11.Lewis EF, Claggett BL, McMurray JJV, et al. Health-Related Quality of Life Outcomes in PARADIGM-HF. Circ Heart Fail. 2017;10(8). doi: 10.1161/CIRCHEARTFAILURE.116.003430 [DOI] [PubMed] [Google Scholar]
- 12.Jones RH, Kesler K, Phillips HR 3rd, et al. Long-term survival benefits of coronary artery bypass grafting and percutaneous transluminal angioplasty in patients with coronary artery disease. J Thorac Cardiovasc Surg. 1996;111(5):1013–1025. [DOI] [PubMed] [Google Scholar]
- 13.MacMahon KMA, Lip GYH. Psychological factors in heart failure: a review of the literature. Arch Intern Med. 2002;162(5):509–516. [DOI] [PubMed] [Google Scholar]
- 14.Cella DF, Hahn EA, Jensen SE, et al. Patient-Reported Outcomes in Performance Measurement. Vol 97. RTI Press Research; Triangle Park, NC; 2015. [PubMed] [Google Scholar]
- 15.Algurén B, Coenen M, Malm D, et al. A scoping review and mapping exercise comparing the content of patient-reported outcome measures (PROMs) across heart disease-specific scales. J Patient Rep Outcomes. 2020;4(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bevans M, Ross A, Cella D. Patient-Reported Outcomes Measurement Information System (PROMIS): Efficient, standardized tools to measure self-reported health and quality of life. Nursing Outlook. 2014;62(5):339–345. doi: 10.1016/j.outlook.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumanian GA, Potter BK, Mioton LM, et al. Targeted Muscle Reinnervation Treats Neuroma and Phantom Pain in Major Limb Amputees: A Randomized Clinical Trial. Ann Surg. 2019;270(2):238–246. [DOI] [PubMed] [Google Scholar]
- 18.Ghoneim MM, O’Hara MW. Depression and postoperative complications: an overview. BMC Surg. 2016;16:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D. Item Banks for Measuring Emotional Distress From the Patient-Reported Outcomes Measurement Information System (PROMIS®): Depression, Anxiety, and Anger. Assessment. 2011;18(3):263–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snaith RP. The Hospital Anxiety And Depression Scale. Health Qual Life Outcomes. 2003;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyte D, Draper H, Calvert M. Patient-reported outcome alerts: ethical and logistical considerations in clinical trials. JAMA. 2013;310(12):1229–1230. [DOI] [PubMed] [Google Scholar]
- 23.Sackett DL. Clinician-trialist rounds: 5. Cointervention bias – how to diagnose it in their trial and prevent it in yours. Clinical Trials: Journal of the Society for Clinical Trials. 2011;8(4):440–442. doi: 10.1177/1740774511410995 [DOI] [PubMed] [Google Scholar]
- 24.Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the Heart and Soul Study. Arch Intern Med. 2005;165(21):2508–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tully PJ, Baker RA. Depression, anxiety, and cardiac morbidity outcomes after coronary artery bypass surgery: a contemporary and practical review. J Geriatr Cardiol. 2012;9(2):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terrie YC. Identifying and Managing Depression in Transplant Patients. US Pharm. Published online 2017.https://www.uspharmacist.com/article/identifying-and-managing-depression-in-transplant-patients [Google Scholar]
- 27.McGrady A, McGinnis R, Badenhop D, Bentle M, Rajput M. Effects of depression and anxiety on adherence to cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2009;29(6):358–364. [DOI] [PubMed] [Google Scholar]
- 28.Rao A, Zecchin R, Newton PJ, et al. The prevalence and impact of depression and anxiety in cardiac rehabilitation: A longitudinal cohort study. Eur J Prev Cardiol. 2020;27(5):478–489. [DOI] [PubMed] [Google Scholar]
- 29.Brocks Y, Zittermann A, Grisse D, et al. Adherence of Heart Transplant Recipients to Prescribed Medication and Recommended Lifestyle Habits. Prog Transplant. 2017;27(2):160–166. [DOI] [PubMed] [Google Scholar]
- 30.Pinto A, Faiz O, Davis R, Almoudaris A, Vincent C. Surgical complications and their impact on patients’ psychosocial well-being: a systematic review and meta-analysis. BMJ Open. 2016;6(2):e007224. doi: 10.1136/bmjopen-2014-007224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piarulli A, Chiariello GA, Bruno P, et al. Psychological Effects of Skin Incision Size in Minimally Invasive Valve Surgery Patients. Innovations. 2020;15(6):532–540. [DOI] [PubMed] [Google Scholar]
- 32.İyigün T, Kaya M, Gülbeyaz SÖ, et al. Patient body image, self-esteem, and cosmetic results of minimally invasive robotic cardiac surgery. Int J Surg. 2017;39:88–94. [DOI] [PubMed] [Google Scholar]
- 33.Adib-Hajbaghery M, Miranzadeh S, Tahmouresi M, Azizi-Fini I. Body image before and after coronary artery bypass graft surgery: comparison and its contributing factors. BMC Psychol. 2020;8(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kańtoch MJ, Eustace J, Collins-Nakai RL, Taylor DA, Bolsvert JA, Lysak PS. The significance of cardiac surgery scars in adult patients with congenital heart disease. Kardiol Pol. 2006;64(1):51–56; discussion 57–58. [PubMed] [Google Scholar]
- 35.Cash TF. Multidimensional Body-Self Relations Questionnaire. PsycTESTS Dataset. Published online 2018. doi: 10.1037/t08755-000 [DOI] [Google Scholar]
- 36.Garber CE, Greaney ML, Riebe D, Nigg CR, Burbank PA, Clark PG. Physical and mental health-related correlates of physical function in community dwelling older adults: a cross sectional study. BMC Geriatr. 2010;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patient-Reported Outcomes Measurement System- Physical Function. Health Measures; http://www.healthmeasures.net/images/PROMIS/manuals/PROMIS_Physical_Function_Scoring_Manual.pdf [Google Scholar]
- 38.Ware JE Jr. SF-36 Health Survey. The use of psychological testing for treatment planning and outcomes assessment, 2nd ed. 1507;2(1999):1227–1246. [Google Scholar]
- 39.Stokes JW, Wanderer JP, McEvoy MD. Significant discrepancies exist between clinician assessment and patient self-assessment of functional capacity by validated scoring tools during preoperative evaluation. Perioper Med (Lond). 2016;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charles EJ, Mehaffey JH, Hawkins RB, et al. Effect of Cardiac Surgery on One-Year Patient-Reported Outcomes: A Prospective Cohort Study. Ann Thorac Surg. Published online December 9, 2020. doi: 10.1016/j.athoracsur.2020.09.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibbons RJ, Chatterjee K, Daley J, et al. ACC/AHA/ACP--ASIM guidelines for the management of patients with chronic stable angina: executive summary and recommendations: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on Management of Patients with Chronic Stable Angina). Circulation. 1999;99(21):2829–2848. [DOI] [PubMed] [Google Scholar]
- 42.Lee JH, Chuu K, Spertus J, et al. Patients overestimate the potential benefits of elective percutaneous coronary intervention. Mo Med. 2012;109(1):79–84. [PMC free article] [PubMed] [Google Scholar]
- 43.Bayman EO, Parekh KR, Keech J, Larson N, Vander Weg M, Brennan TJ. Preoperative Patient Expectations of Postoperative Pain Are Associated with Moderate to Severe Acute Pain After VATS. Pain Med. 2019;20(3):543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borsody JM, Courtney M, Taylor K, Jairath N. Using self-efficacy to increase physical activity in patients with heart failure. Home Healthc Nurse. 1999;17(2):113–118. [DOI] [PubMed] [Google Scholar]
- 45.Goel K, O’Leary JM, Barker CM, et al. Clinical Implications of Physical Function and Resilience in Patients Undergoing Transcatheter Aortic Valve Replacement. J Am Heart Assoc. 2020;9(17):e017075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wyler von Ballmoos MC. Minimally invasive mitral valve surgery to maximally benefit patients-what is the key to success today and tomorrow? Ann Transl Med. 2020;8(5):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suri RM, Antiel RM, Burkhart HM, et al. Quality of life after early mitral valve repair using conventional and robotic approaches. Ann Thorac Surg. 2012;93(3):761–769. [DOI] [PubMed] [Google Scholar]
- 48.Klop IDG, van Putte BP, Kloppenburg GTL, Sprangers MAG, Nieuwkerk PT, Klein P. Comparing quality of life and postoperative pain after limited access and conventional aortic valve replacement: Design and rationale of the LImited access aortic valve replacement (LIAR) trial. Contemp Clin Trials Commun. 2021;21:100700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Neill D, Forman DE. The importance of physical function as a clinical outcome: Assessment and enhancement. Clin Cardiol. 2020;43(2):108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parker RD, Adams J. Activity restrictions and recovery after open chest surgery: understanding the patient’s perspective. Proc. 2008;21(4):421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chaturvedi RK, Blaise M, Verdon J, et al. Cardiac surgery in octogenarians: long-term survival, functional status, living arrangements, and leisure activities. Ann Thorac Surg. 2010;89(3):805–810. [DOI] [PubMed] [Google Scholar]
- 52.Rosman L, Cahill JM, McCammon SL, Sears SF. Sexual health concerns in patients with cardiovascular disease. Circulation. 2014;129(5):e313–e316. [DOI] [PubMed] [Google Scholar]
- 53.Jönsson M, Berg SK, Missel M, Palm P. Am I going to die now? Experiences of hospitalisation and subsequent life after being diagnosed with aortic dissection. Scand J Caring Sci. Published online September 23, 2020. doi: 10.1111/scs.12912 [DOI] [PubMed] [Google Scholar]
- 54.Bonnet D, Berger F, Jokinen E, Kantor PF, Daubeney PEF. Ivabradine in Children With Dilated Cardiomyopathy and Symptomatic Chronic Heart Failure. J Am Coll Cardiol. 2017;70(10):1262–1272. [DOI] [PubMed] [Google Scholar]
- 55.Varni JW, Burwinkle TM, Seid M. The PedsQLTM as a pediatric patient-reported outcome: reliability and validity of the PedsQLTM Measurement Model in 25,000 children. Expert Rev Pharmacoecon Outcomes Res. 2005;5(6):705–719. [DOI] [PubMed] [Google Scholar]
- 56.DeWalt DA, Gross HE, Gipson DS, et al. PROMIS(®) pediatric self-report scales distinguish subgroups of children within and across six common pediatric chronic health conditions. Qual Life Res. 2015;24(9):2195–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uzark K, Jones K, Slusher J, Limbers CA, Burwinkle TM, Varni JW. Quality of life in children with heart disease as perceived by children and parents. Pediatrics. 2008;121(5):e1060–e1067. [DOI] [PubMed] [Google Scholar]
- 58.Chen C, Rosenthal DN, Chen S, Dykes JC, Hollander SA, Almond CS. Clinician Perspectives on the Potential Utility of a Patient Reported Outcomes Measure for Pediatric Heart Failure. J Heart Lung Transplant. 2019;38(4):S203. [Google Scholar]
- 59.Knowles RL, Tadic V, Hogan A, et al. Self-Reported Health Experiences of Children Living with Congenital Heart Defects: Including Patient-Reported Outcomes in a National Cohort Study. PLoS One. 2016;11(8):e0159326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manrique-Garcia E, Sidorchuk A, Hallqvist J, Moradi T. Socioeconomic position and incidence of acute myocardial infarction: a meta-analysis. J Epidemiol Community Health. 2011;65(4):301–309. [DOI] [PubMed] [Google Scholar]
- 61.Khaing W, Vallibhakara SA, Attia J, McEvoy M, Thakkinstian A. Effects of education and income on cardiovascular outcomes: A systematic review and meta-analysis. Eur J Prev Cardiol. 2017;24(10):1032–1042. [DOI] [PubMed] [Google Scholar]
- 62.Umberson D, Montez JK. Social relationships and health: a flashpoint for health policy. J Health Soc Behav. 2010;51Suppl:S54–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Laan DM, van der Laan DM, Elders PJM, et al. The impact of cardiovascular medication use on patients’ daily lives: a cross-sectional study. International Journal of Clinical Pharmacy. 2018;40(2):412–420. doi: 10.1007/s11096-018-0601-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mohammed A, Mohammed MA, Moles RJ, Chen TF. Medication-related burden and patients’ lived experience with medicine: a systematic review and metasynthesis of qualitative studies. BMJ Open. 2016;6(2):e010035. doi: 10.1136/bmjopen-2015-010035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Modica M, Ferratini M, Torri A, et al. Quality of life and emotional distress early after left ventricular assist device implant: a mixed-method study. Artif Organs. 2015;39(3):220–227. [DOI] [PubMed] [Google Scholar]
- 66.Tosto C, Adamo L, Craddock H, et al. Relationship between device acceptance and patient-reported outcomes in Left Ventricular Assist Device (LVAD) recipients. Sci Rep. 2019;9(1):10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han J, Trumble DR. Cardiac Assist Devices: Early Concepts, Current Technologies, and Future Innovations. Bioengineering (Basel). 2019;6(1). doi: 10.3390/bioengineering6010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abshire M, Prichard R, Cajita M, DiGiacomo M, Dennison Himmelfarb C. Adaptation and coping in patients living with an LVAD: A metasynthesis. Heart Lung. 2016;45(5):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aburjania N, Sherazi S, Tchantchaleishvili V, Alexis JD, Hay CM. Stopping Conventional Showering Decreases Pseudomonas Infections in left Ventricular Assist Device Patients. The International Journal of Artificial Organs. 2017;40(6):282–285. doi: 10.5301/ijao.5000590 [DOI] [PubMed] [Google Scholar]
- 70.Brouwers C, Denollet J, Caliskan K, et al. Psychological distress in patients with a left ventricular assist device and their partners: an exploratory study. Eur J Cardiovasc Nurs. 2015;14(1):53–62. [DOI] [PubMed] [Google Scholar]
- 71.Lopez V, Sek Ying C, Poon C-Y, Wai Y. Physical, psychological and social recovery patterns after coronary artery bypass graft surgery: a prospective repeated measures questionnaire survey. Int J Nurs Stud. 2007;44(8):1304–1315. [DOI] [PubMed] [Google Scholar]
- 72.Moore SM. A comparison of women’s and men’s symptoms during home recovery after coronary artery bypass surgery. Heart & Lung. 1995;24(6):495–501. doi: 10.1016/s0147-9563(95)80027-1 [DOI] [PubMed] [Google Scholar]
- 73.Hwang T-J, Rabheru K, Peisah C, Reichman W, Ikeda M. Loneliness and social isolation during the COVID-19 pandemic. Int Psychogeriatr. 2020;32(10):1217–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Littlefield C, Abbey S, Fiducia D, et al. Quality of life following transplantation of the heart, liver, and lungs. Gen Hosp Psychiatry. 1996;18(6 Suppl):36S–47S. [DOI] [PubMed] [Google Scholar]
- 75.Tarabeih M, arit Bokek-Cohen Y’, Azuri P. Health-related quality of life of transplant recipients: a comparison between lung, kidney, heart, and liver recipients. Qual Life Res. 2020;29(6):1631–1639. [DOI] [PubMed] [Google Scholar]
- 76.Christensen AV, Juel K, Ekholm O, et al. Significantly increased risk of all-cause mortality among cardiac patients feeling lonely. Heart. 2020;106(2):140–146. [DOI] [PubMed] [Google Scholar]
- 77.Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66(1):20–40. [DOI] [PubMed] [Google Scholar]
- 78.Patient-Reported Outcomes Measurement Information System- Social Isolation. HealthMeasures; https://www.healthmeasures.net/images/PROMIS/manuals/PROMIS_Social_Isolation_Scoring_Manual.pdf [Google Scholar]
- 79.Calvert M, Kyte D, Mercieca-Bebber R, et al. Guidelines for Inclusion of Patient-Reported Outcomes in Clinical Trial Protocols: The SPIRIT-PRO Extension. JAMA. 2018;319(5):483–494. [DOI] [PubMed] [Google Scholar]
- 80.Kyte D, Retzer A, Ahmed K, et al. Systematic Evaluation of Patient-Reported Outcome Protocol Content and Reporting in Cancer Trials. J Natl Cancer Inst. 2019;111(11):1170–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Calvert M, Blazeby J, Altman DG, et al. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013;309(8):814–822. [DOI] [PubMed] [Google Scholar]
- 82.Takousi MG, Schmeer S, Manaras I, Olympios CD, Makos G, Troop NA. Health-Related Quality of Life after Coronary Revascularization: A systematic review with meta-analysis. Hellenic J Cardiol. Published online August 23, 2016. doi: 10.1016/j.hjc.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 83.Schmidt-RioValle J, Abu Ejheisheh M, Membrive-Jiménez MJ, et al. Quality of Life After Coronary Artery Bypass Surgery: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2020;17(22). doi: 10.3390/ijerph17228439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Charles EJ, Mehaffey JH, Hawkins RB, et al. Meaningful Patient-centered Outcomes 1 Year Following Cardiac Surgery. Ann Surg. Published online May 2, 2019. doi: 10.1097/SLA.0000000000003357 [DOI] [PubMed] [Google Scholar]
- 85.Abdallah MS, Wang K, Magnuson EA, et al. Quality of Life After Surgery or DES in Patients With 3-Vessel or Left Main Disease. Journal of the American College of Cardiology. 2017;69(16):2039–2050. doi: 10.1016/j.jacc.2017.02.031 [DOI] [PubMed] [Google Scholar]
- 86.Baron SJ, Chinnakondepalli K, Magnuson EA, et al. Quality-of-Life After Everolimus-Eluting Stents or Bypass Surgery for Left-Main Disease: Results From the EXCEL Trial. J Am Coll Cardiol. 2017;70(25):3113–3122. [DOI] [PubMed] [Google Scholar]
- 87.Bishawi M, Hattler B, Almassi GH, et al. Preoperative factors associated with worsening in health-related quality of life following coronary artery bypass grafting in the Randomized On/Off Bypass (ROOBY) trial. Am Heart J. 2018;198:33–38. [DOI] [PubMed] [Google Scholar]
- 88.Houlind K, Kjeldsen BJ, Madsen SN, et al. On-pump versus off-pump coronary artery bypass surgery in elderly patients: results from the Danish on-pump versus off-pump randomization study. Circulation. 2012;125(20):2431–2439. [DOI] [PubMed] [Google Scholar]
- 89.Magnuson EA, Farkouh ME, Fuster V, et al. Cost-effectiveness of percutaneous coronary intervention with drug eluting stents versus bypass surgery for patients with diabetes mellitus and multivessel coronary artery disease: results from the FREEDOM trial. Circulation. 2013;127(7):820–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abdallah MS, Wang K, Magnuson EA, et al. Quality of life after PCI vs CABG among patients with diabetes and multivessel coronary artery disease: a randomized clinical trial. JAMA. 2013;310(15):1581–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Calvert MJ, Freemantle N, Cleland JGF. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail. 2005;7(2):243–251. [DOI] [PubMed] [Google Scholar]
- 92.Spertus JA, Jones PG, Maron DJ, et al. Health Status after Invasive or Conservative Care in Coronary and Advanced Kidney Disease. N Engl J Med. 2020;382(17):1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rahimi K, Malhotra A, Banning AP, Jenkinson C. Outcome selection and role of patient reported outcomes in contemporary cardiovascular trials: systematic review. BMJ. 2010;341:c5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prinsen CAC, Mokkink LB, Bouter LM, et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27(5):1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mokkink LB, de Vet HCW, Prinsen CAC, et al. COSMIN Risk of Bias checklist for systematic reviews of Patient-Reported Outcome Measures. Qual Life Res. 2018;27(5):1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.COSMIN - Improving the selection of outcome measurement instruments. Published September 28, 2017.Accessed March 29, 2021. https://www.cosmin.nl/
- 97.Prinsen CAC, Vohra S, Rose MR, et al. How to select outcome measurement instruments for outcomes included in a “Core Outcome Set” – a practical guideline. Trials. 2016;17(1). doi: 10.1186/s13063-016-1555-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mercieca-Bebber R, Palmer MJ, Brundage M, Calvert M, Stockler MR, King MT. Design, implementation and reporting strategies to reduce the instance and impact of missing patient-reported outcome (PRO) data: a systematic review. BMJ Open. 2016;6(6):e010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fairclough DL, Peterson HF, Chang V. Why are missing quality of life data a problem in clinical trials of cancer therapy? Stat Med. 1998;17(5–7):667–677. [DOI] [PubMed] [Google Scholar]
- 100.COMET Initiative. Accessed March 29, 2021. https://www.cometinitiative.org/
- 101.Marshall Kate H, Yves D’Udekem, Sholler Gary F, et al. Health-Related Quality of Life in Children, Adolescents, and Adults With a Fontan Circulation: A Meta-Analysis. J Am Heart Assoc. 2020;9(6):e014172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goldsmith KA, Dyer MT, Buxton MJ, Sharples LD. Mapping of the EQ-5D index from clinical outcome measures and demographic variables in patients with coronary heart disease. Health and Quality of Life Outcomes. 2010;8(1):54. doi: 10.1186/1477-7525-8-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wailoo A, Alava MH, Pudney S, et al. An International Comparison of EQ-5D-5L and EQ-5D-3L for Use in Cost-Effectiveness Analysis. Value in Health. Published online 2021. doi: 10.1016/j.jval.2020.11.012 [DOI] [PubMed] [Google Scholar]
- 104.Perrotti A, Ecarnot F, Monaco F, et al. Quality of life 10 years after cardiac surgery in adults: a long-term follow-up study. Health Qual Life Outcomes. 2019;17(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gaudino M, Alexander JH, Bakaeen FG, et al. Randomized comparison of the clinical outcome of single versus multiple arterial grafts: the ROMA trial-rationale and study protocol. Eur J Cardiothorac Surg. 2017;52(6):1031–1040. [DOI] [PubMed] [Google Scholar]
- 106.Carvalho K, Gali B, LeBlanc J, Matzke LA, Watson PH. A Permission to Contact Platform Is an Efficient and Cost-Effective Enrollment Method for a Biobank to Create Study-Specific Research Cohorts. Biopreserv Biobank. Published online January 18, 2021. doi: 10.1089/bio.2020.0114 [DOI] [PubMed] [Google Scholar]
- 107.Tamargo J, Rosano G, Walther T, et al. Gender differences in the effects of cardiovascular drugs. European Heart Journal - Cardiovascular Pharmacotherapy. 2017;3(3):163–182. doi: 10.1093/ehjcvp/pvw042 [DOI] [PubMed] [Google Scholar]
- 108.Taylor BN, Reading Turchioe M, Goyal P, Masterson Creber RM. Using mHealth to Capture Patient-Reported Sexual Satisfaction in Heart Failure. Circulation. 2020;142(Suppl_3):A17004–A17004. [Google Scholar]
- 109.Bakken S, Marden S, Arteaga SS, et al. Behavioral Interventions Using Consumer Information Technology as Tools to Advance Health Equity. Am J Public Health. 2019;109(S1):S79–S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tirado M Role of mobile health in the care of culturally and linguistically diverse US populations. Perspect Health Inf Manag. 2011;8:1e. [PMC free article] [PubMed] [Google Scholar]
- 111.Weldring T, Smith SMS. Article Commentary: Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs). Health Serv Insights. 2013;6:HSI.S11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Turchioe MR, Grossman LV, Myers AC, Baik D, Goyal P, Masterson Creber RM. Visual analogies, not graphs, increase patients’ comprehension of changes in their health status. Journal of the American Medical Informatics Association. Published online 2020. doi: 10.1093/jamia/ocz217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rudin RS, Bates DW, MacRae C. Accelerating Innovation in Health IT. N Engl J Med. 2016;375(9):815–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Masterson Creber RM, Grossman LV, Ryan B, et al. Engaging hospitalized patients with personalized health information: a randomized trial of an inpatient portal. J Am Med Inform Assoc. 2019;26(2):115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Palmer MJ, Mercieca-Bebber R, King M, Calvert M, Richardson H, Brundage M. A systematic review and development of a classification framework for factors associated with missing patient-reported outcome data. Clin Trials. 2018;15(1):95–106. [DOI] [PubMed] [Google Scholar]
- 116.Baik D, Reading M, Jia H, Grossman LV, Masterson Creber R. Measuring health status and symptom burden using a web-based mHealth application in patients with heart failure. Eur J Cardiovasc Nurs. 2019;18(4):325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Masterson Creber R, Chen T, Wei C, Lee CS. Brief Report: Patient Activation Among Urban Hospitalized Patients With Heart Failure. J Card Fail. 2017;23(11):817–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grossman LV, Feiner SK, Mitchell EG, Masterson Creber RM. Leveraging Patient-Reported Outcomes Using Data Visualization. Appl Clin Inform. 2018;9(3):565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Creber RMM, Masterson Creber RM, Hickey KT, Maurer MS. Gerontechnologies for Older Patients with Heart Failure: What is the Role of Smartphones, Tablets, and Remote Monitoring Devices in Improving Symptom Monitoring and Self-Care Management? Current Cardiovascular Risk Reports. 2016;10(10). doi: 10.1007/s12170-016-0511-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Turchioe MR, Myers A, Isaac S, et al. A Systematic Review of Patient-Facing Visualizations of Personal Health Data. Applied Clinical Informatics. 2019;10(04):751–770. doi: 10.1055/s-0039-1697592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Palan S, Schitter C. Prolific.ac—A subject pool for online experiments. Journal of Behavioral and Experimental Finance. 2018;17:22–27. doi: 10.1016/j.jbef.2017.12.004 [DOI] [Google Scholar]
- 122.McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA. 2014;312(13):1342–1343. [DOI] [PubMed] [Google Scholar]
- 123.Gheorghiade M, De Luca L, Fonarow GC, Filippatos G, Metra M, Francis GS. Pathophysiologic targets in the early phase of acute heart failure syndromes. Am J Cardiol. 2005;96(6A):11G–17G. [DOI] [PubMed] [Google Scholar]