Abstract
OBJECTIVES:
Determine the incremental yield of next generation sequencing (predominantly exome sequencing (ES)) over quantitative fluorescence-polymerase chain reaction (QF-PCR) and chromosome microarray analysis (CMA)/karyotyping in; (i) all cases of prenatally diagnosed non-immune hydrops fetalis (NIHF); (ii) isolated NIHF; (iii) NIHF associated with additional structural anomalies and; (iv) NIHF according to severity (i.e., two cavities versus three or more cavities affected).
METHODS:
A prospective cohort study (from an extended group of the Prenatal Assessment of Genomes and Exomes (PAGE) study) of n=28 cases of prenatally diagnosed NIHF undergoing trio ES following a negative QFPCR and CMA/karyotype was combined with a systematic review of the literature. Electronic searches of relevant citations from MEDLINE, EMBASE and CINAHL and clinicaltrials.gov (January 2000 – October 2020) databases was performed. Studies included were those with: (i) ≥ n=2 cases of NIHF undergoing sequencing; (ii) testing initiated based on prenatal ultrasound-based phenotype and; (iii) a negative CMA/karyotype. PROSPERO Registration No. CRD42020221427.
RESULTS:
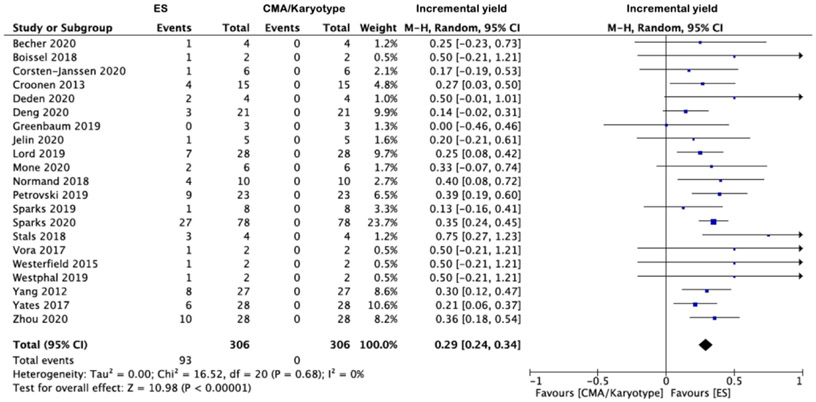
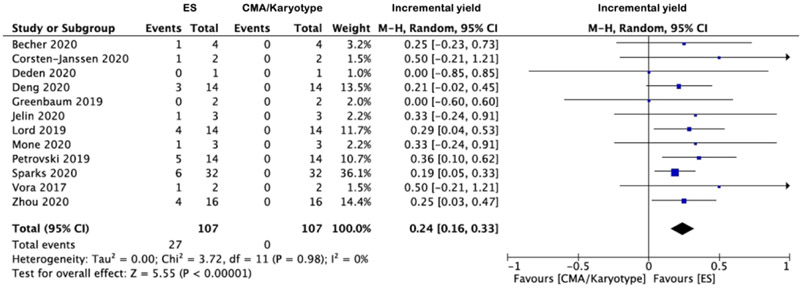
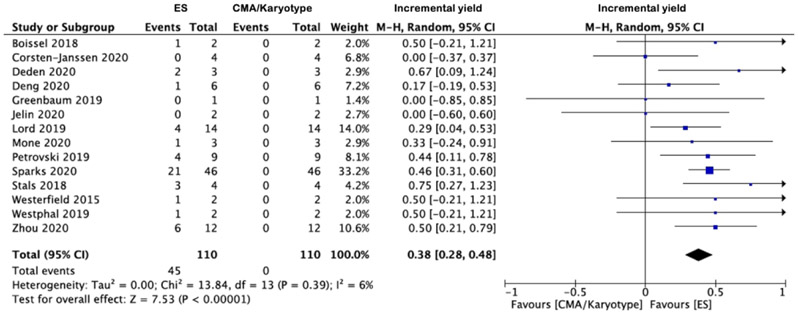
The PAGE cohort study noted the additional diagnostic yield of ES was 25.0% (n=7/28) for all NIHF, 21.4% (n=3/14) for isolated NIHF and 28.6% (n=4/14) for non-isolated NIHF. From the meta-analysis, the pooled incremental yields from n=21 studies (n=306 cases) were 29% (95% CI 24-34%, I2=0%, p<0.00001) in all NIHF, 24% (95% CI 16-33%, I2=0%, p<0.00001) in isolated NIHF and; 38% (95% CI 28%-48%, I2=6%, p<0.00001) in NIHF associated with additional anomalies. In the latter, congenital limb contractures were the most prevalent additional structural anomaly at 17.3% (n=19/110). Incremental yield did not differ significantly based upon hydrops severity. The commonest genetic disorders identified were RASopathies in 30.3% (n=27/89), most commonly due to PTPN11 variants in 44.4% (n=12) and the predominant inheritance pattern was autosomal dominant in monoallelic disease genes 57.3% (n=51/89), of which most were de novo 86.3% (n=44).
CONCLUSIONS:
Use of prenatal next generation sequencing in both isolated and non-isolated NIHF should be considered in developing clinical pathways. Given the wide range of potential syndromic diagnoses and heterogeneity in prenatal phenotypes of NIHF, exome or whole genome sequencing may prove to be a more appropriate testing approach than a targeted gene panel testing strategy.
Keywords: exome sequencing, fetus, hydrops, prenatal diagnosis, next generation sequencing, nonimmune hydrops fetalis
INTRODUCTION
Nonimmune hydrops fetalis (NIHF) is traditionally defined as fluid accumulation in two or more fetal body cavities (in cases not secondary to maternal red cell alloimmunization).1 It affects up to 1 in 1700 pregnancies, with associated high risks of perinatal morbidity and mortality.2 Excluding infection, fetal structural anomalies (FSAs) and complications of twin pregnancies, aneuploidy may explain a quarter of cases, with chromosome microarray (CMA) demonstrating a further abnormality of copy number variants (CNVs) in 6-14%.3,4 Despite this, the definitive diagnostic yield of CMA over standard G-banding karyotype is moderate and following exclusion of the aforementioned causes up to 50% of NIHF remains unexplained, with a significant proportion thought to be secondary to single gene variants.5 Over 170 genes have been identified as being associated with NIHF and until the recent revolution of next generation sequencing (NGS), testing for such conditions has relied upon targeted gene testing and enzyme assays.3,6 Single gene causes of NIHF are associated with significant risks of perinatal death or neurodevelopmental sequalae.2 Establishing a diagnostic aetiology prenatally is a vital step in facilitating informed decision making (for both parents and clinicians), considering options such as termination of pregnancy, planning neonatal care and addressing recurrence risks.2 The latter could theoretically be mitigated using novel technologies such as preimplantation genetic testing.7 While individual case cohort studies have assessed the diagnostic yield of exome sequencing (or an alternative sequencing approach) over Quantitative Fluorescent Polymerase Chain Reaction (QF-PCR) and CMA or karyotype in NIHF, they are heterogenous in relation to populations assessed and genetic platforms used.3 There is a need to integrate existing data on single gene disorders underlying NIHF given this heterogeneity. Hence, the aims of this study were to evaluate the incremental diagnostic yield of prenatal exome sequencing (ES) (or an alternative sequencing technology) in; (i) all NIHF; (ii) isolated NIHF; (iii) NIHF associated with fetal structural anomalies (FSAs) and; (iv) NIHF according to severity (i.e., two cavities versus three or more cavities affected).
METHODS
Extended Prenatal Assessment of Genomes and Exomes (PAGE) study Cohort
This included prospectively identified cases of prenatally confirmed NIHF from an extended cohort of the Prenatal Assessment of Genomes and Exomes (PAGE) Study.8 For the purposes of the FIND study, we defined NIHF as ultrasonographically prenatally confirmed pathological fluid accumulations in ≥two fetal cavities, where cases with aneuploidy, congenital infection, alloimmunization or and twin-twin transfusion syndrome had been excluded.1,2 The final PAGE cohort included n=850 fetuses (published cohort n=596) with trio ES performed in instances when an ultrasound-confirmed FSA was detected.8 Such cases were recruited between October 2014 and May 2018 across 34 fetal medicine centres in England and Scotland, with ES performed centrally at the Wellcome Trust Sanger Institute.8 PAGE eligibility criteria included: (i) prenatal detection of a FSA after 11-weeks’ gestation; (ii) availability of proband and parental DNA and; (iii) negative QF-PCR and CMA or karyotype testing. The PAGE study methodology has been published previously and utilized a standard ES approach with variant interpretation based on a targeted virtual 1628 gene panel for developmental disorders.8,9 Phenotypes of all cases were classified using Human Phenotype Ontology (HPO) terms,10 and those defined as Hydrops Fetalis HP:0001789 were selected and further analysed to determine if the criteria for NIHF for the purposes of the FIND study were met. Cases were further classified into ‘isolated’ and ‘associated with additional FSAs’ using the HPO approach to coding additional anomalies. Fetal phenotypes were described by fetal medicine specialists/sonographers and documented principally on Viewpoint® Version 5.6.16 (GE Healthcare). Variants were classified in accordance with the American College of Medical Genetics and Genomics (ACMG) guidelines as agreed by a clinical review panel and incidental findings (IFs) were not reported.11 Pathogenic and likely pathogenic variants explaining the fetal phenotype were confirmed using Sanger sequencing and results returned to parents after the end of pregnancy. Ethical approval was obtained from the Research Ethics Committees at the West Midlands – South Birmingham (ref: 13/WM/1219) and the Harrow - REC reference number 01/0095. Local Research and Development offices subsequently approved the study at each participating organisation.
Systematic review and meta-analysis
Information sources
This review was performed in a standardized fashion in line with recommended methods for systematic reviews and PRISMA guidance and was prospectively registered [PROSPERO No. CRD42020221427].12,13 The following databases were searched electronically for relevant citations, from January 2000 (ES was not an available technology prior to this) until October 2020: MEDLINE, EMBASE, CINAHL and clinicaltrials.gov. The search strategy consisted of relevant Medical Subject Headings (MeSH) terms, keywords and word variants for ‘exome sequencing’, ‘fetus’ and ‘abnormality’ were used with alternative terms encompassing ‘genome sequencing’, ‘exome’, fetal’, ‘prenatal’, ‘antenatal’, ‘defect’ and ‘anomaly’. Bibliographies of relevant articles were searched manually and experts in prenatal genomics were also contacted to identify further relevant studies. The search strategy is available from the corresponding author on request.
Study selection
The inclusion criteria for study selection were any prospective or retrospective cohort studies or case series which: (i) included two or more cases of NIHF undergoing ES (or an alternative sequencing strategy such as gene panels); (ii) initiated testing based on prenatal ultrasound-based phenotype; (iii) had a negative CMA/karyotype result and; (iv) results of genetic testing were known. Where ES was initiated postnatally, such cases were included if testing was based upon the prenatal phenotype and instances where sequential Sanger sequencing was utilised were also included. When studies were not specific to NIHF exclusively, data regarding such cases were extracted from the paper or via author request. All study abstracts were screened by two reviewers (F.M. and M.D.K.) and full manuscripts were subsequently reviewed when further information was required.
Data extraction and quality assessment
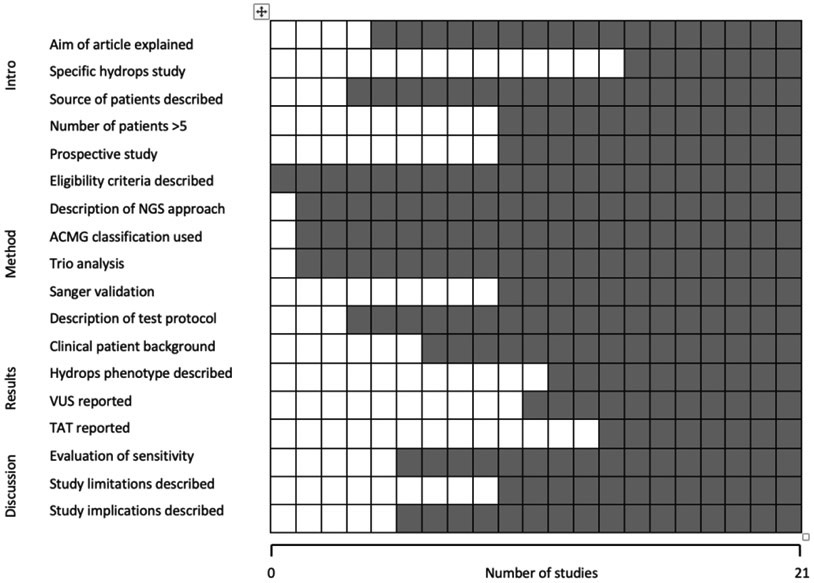
Both reviewers independently extracted data on study characteristics and outcome data using a proforma. Data extracted from studies, when obtainable, included: ultrasound phenotype, sequencing approach, reported variants, source of fetal DNA, turnaround time, fetal outcome, maternal age and gestational age at testing. Quality assessment was performed using modified Standards for Reporting of Diagnostic Accuracy (STARD) criteria.14. Criteria deemed most important to optimise accuracy were: (i) trio analysis; (ii) use of ACMG criteria for variant interpretation; (iii) Sanger sequencing validation and; (iv) description of the prenatal phenotype.
Data analysis
Descriptive tables were produced detailing study characteristics and outcomes. The incremental diagnostic yield, or risk difference, with 95% CI, of ES (or alternative sequencing strategy) over QF-PCR and CMA or karyotyping was calculated for each study and as a pooled value for: (i) all NIHF; (ii) isolated NIHF; (iii) NIHF associated with additional structural anomalies and; (iv) NIHF according to severity. Where reported, pooled values for variants of uncertain significance (VUS) and IFs was also determined. Risk differences from each study were pooled using a random effects model throughout to estimate incremental yield by a previously published method which facilitated calculation with adjustment for ‘zero’ values from negative QF-PCR and CMA or karyotype testing.9,15 Results were displayed in Forest Plots with corresponding 95% confidence intervals (CIs). Heterogeneity was assessed graphically within the forest plot and statistically using Higgins’ I2. Publication bias was assessed graphically using funnel plots. Statistical analysis was performed using RevMan version 5.3.4 (Review Manager, The Cochrane Collaboration, Copenhagen, Denmark) statistical software.
RESULTS
Extended PAGE cohort
Of the 850 cases of prenatal structural anomaly which underwent ES, there were n=28 (3.3%) cases that met the definition for NIHF. Of these 50% (n=14) were apparently isolated and 50% (n=14) were associated with additional FSAs. In the majority of cases (96.4%; n=27) the original genetic test was CMA, with the remainder being karyotype with most proband DNA originating from cultured amniocytes (50%; n=14). The diagnostic yield of ES overall in all NIHF was 25.0% (n=7/28) and was 21.4% (n=3/14) and 28.6% (n=4/14) in isolated NIHF and NIHF associated with additional FSA respectively. Where additional anomalies associated with pathogenic variants were present, there were most commonly congenital limb contractures due to arthrogryposis multiplex congenita (HP0002804) 75% (n=3/4). In instances where no pathogenic variant was obtained, the commonest additional anomalies were cardiac, genitourinary and thoracic in nature (each 50.0% (n=5/10)). One case of Noonan syndrome was initially not detected as pathogenic as it was filtered out of the bioinformatic pipeline due to inheritance from an apparently unaffected parent. Subsequently the pipeline was adjusted so that such variants were not filtered out even if inherited. The incidence of VUS was 7.1% (n=2/28). Pathogenic variants and VUS are described and outlined in supplementary tables S1 and S2.
Systematic review and meta-analysis
Where a study was suitable for inclusion but data were incomplete, the corresponding authors were contacted to request further data (n=5), regarding fetal phenotype, of which two responded and provided full datasets.16,17 One of these, the study from Columbia University Medical Centre, New York provided an extended dataset from the paper by Petrovski, et al. 2019.16 In addition, to the extended PAGE Study cohort8, there were a further n=20 studies which met the inclusion criteria as demonstrated in Figure 1.2,8, 16-34 Table 1 highlights the characteristics of included studies and Figure 2 shows the overall quality assessment.
Figure 1 –
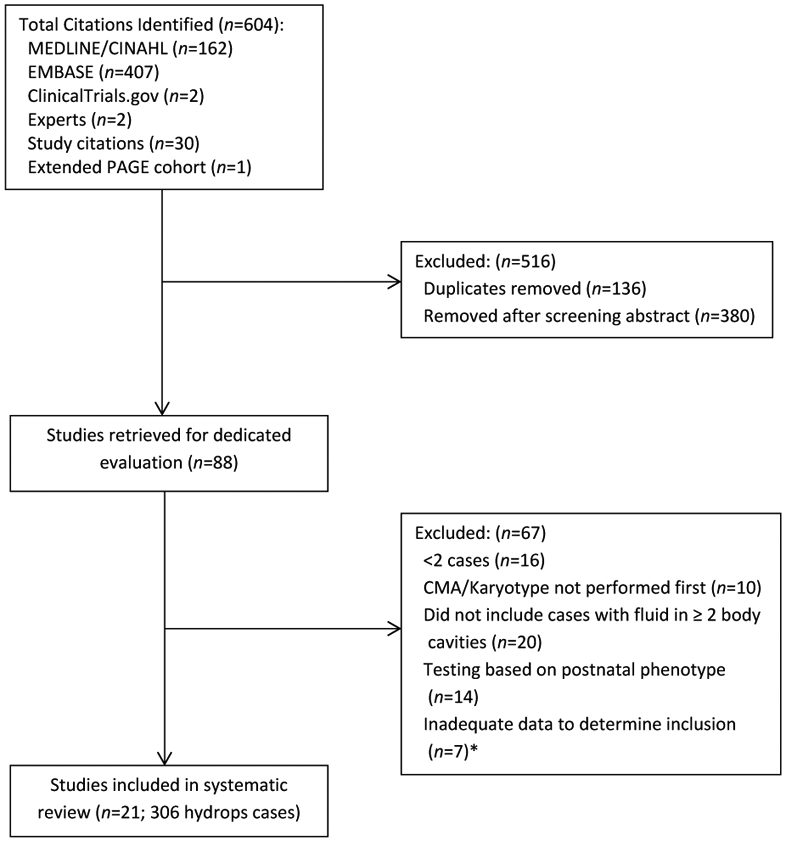
Flowchart demonstrating included studies *Corresponding author contacted to request additional information
Table 1-.
Characteristics of included studies [CE, clinical exome; FSA, fetal structural anomaly, NIHF, nonimmune hydrops fetalis; N/S, not-stated; WES, whole exome sequencing *coverage not stated]
| Study | Next Generation Sequencing Approach | Number of NIHF cases | ||
|---|---|---|---|---|
| All NIHF | Isolated NIHF |
NIHF and additional FSAs |
||
| Becher, et al.26 | WES Trio 103 × coverage Roche SeqCap EZ MedExome Plus capture + Illumina NextSeq 500 |
4 | 4 | 0 |
| Boissel et al.18 | WES Trio 110 × coverage Agilent capture + Illumina HiSeq 2000 or 2500 |
2 | 0 | 2 |
| Corsten-Janssen, et al.32 | WES Trio 20 × coverage Agilent capture + Illumina NextSeq500 |
6 | 2 | 4 |
| Croonen, et al.33* | Clinical Exome; Noonan Panel Illustra amplification. Sequencer not stated |
15 | N/S | N/S |
| Denden, et al.27 | WES Trio 200-300 × coverage Agilent capture + Illumina NextSeq500 |
4 | 1 | 3 |
| Deng, et al.19 | WES Trio 120 × coverage Agilent capture + Illumina HiSeq XTen or Novaseq 6000 |
21 | 14 | 6 |
| Jelin, et al.20 | WES Trio depth of coverage <10 removed Agilent capture + Illumina Hi-Seq 2500 |
5 | 3 | 2 |
| Greenbaum, et al.28 | WES Trio 100 × coverage Capture kit unknown + Illumina sequencing |
3 | 2 | 1 |
| Lord et al.8 | Trio WES Panel 1628 genes Agilent capture + Illumina Hi-Seq 2500 98.3% of the bait regions covered at a minimum depth of 5 × |
28 | 14 | 14 |
| Mone, et al.34 | Trio WES Panel 1628 genes Agilent capture + Illumina Hi-Seq 2500 98.3% of the bait regions covered at a minimum depth of 5 × |
6 | 3 | 3 |
| Normand et al.21 | WES Trio Coverage 150 × Roche NimbleGen capture Illumina Genome Analyzer IIx platform/HiSeq 2000 |
10 | N/S | N/S |
| Petrovski et al.16 | WES Trio Nimblegen SeqCap EZ capture + Illumina Hiseq 2500. Average read coverage 89.3 reads Bioinformatic signatures |
23 | 14 | 9 |
| Sparks, et al. 201929* | WES × 1 Clinical exome × 7 Details not specified |
8 | N/S | N/S |
| Sparks 2, et al. 20202* | WES Trio llumina HiSeq 2500 or Illumina NovaSeq 6000 |
78 | 32 | 46 |
| Stals et al.23 | WES Parents only 80 × coverage Agilent capture + Illumina HiSeq 2500 or NextSeq500. Only include het rare (MAF<0.001) variants in same gene in both parents |
4 | 0 | 4 |
| Vora et al.22* | CE and WES Trio Illumina Hi-Seq 2500 |
2 | 2 | 0 |
| Westerfield, et al.30 | WES Trio 130 × coverage Roche NimbleGen capture + Illumina Genome Analyzer IIx or HiSeq 2000 |
2 | 0 | 2 |
| Westphal et al.24 | WES Trio 20,000 genes 150 × coverage |
2 | 0 | 2 |
| Yang, et al.31* | Clinical exome; Lymphoedema panel Oligo 6.1 PCR amplification + ABI. PRISM 3000 DNA sequencer |
27 | N/S | N/S |
| Yates et al. 25 | WES Trio 140 × coverage Agilent capture + Illumina HiSeq 2000 or 2500 |
2 8 | N/S | N/S |
| Zhou, et al.17* | WES Trio in recurrent NIHF Agilent capture + Illumina HiSeq X Ten |
28 | 16 | 12 |
Figure 2 –
Quality assessment of 21 studies included in systematic review, using modified Standards for Reporting of Diagnostic Accuracy criteria. ACMG, American College of Medical Genetics and Genomics; NGS, next-generation Sequencing; TAT, turnaround time, VUS, variants of uncertain significance.  No
No  Yes
Yes
Systematic review outcomes
In total n=21 studies were included with a total of n=306 NIHF cases. Where stated (n=217), there were n=107 (49.3%) cases of apparently isolated NIHF (on prenatal detailed ultrasound) and n=110 (50.7%) cases associated with additional FSAs. The mean maternal age and gestation at testing was 30.9 (+/−3.5 SD) years and (21.9 +/−5.4 SD) weeks, respectively. Fetal DNA was obtained in the majority of cases via amniocentesis; 50.6% (n=121/239) with the initial test prior to ES performed; CMA; 84.0% (n=257) and the remainder G-banding karyotype. Where documented (n=12 studies), the median turnaround time for ES was 40 (range 7-140) days. Pregnancy outcome was available for (32.4%, 99/306 of cases (termination of pregnancy; n=79 (30.9%); in-utero demise; n=57 (22.3%) livebirth and; n=21 (8.1%) neonatal death). When reported, the pooled incremental yield for VUS and IFs was 19% (95% CI 6-22%, I2=62%, p=0.003) and 4% (95% CI −1-9%, I2=0%, p=0.09), respectively. Pathogenic variants and VUS are outlined in supplementary tables S1 and S2.
Systematic review pathogenic variants
The apparent incremental yields with ES (or an alternative sequencing strategy) in (i) all NIHF, (ii) isolated NIHF and (iii) NIHF associated with additional anomalies are demonstrated in Forest plots (Figures 3a-c) and were 29% (95% CI 24-34%, I2=0%, p<0.00001), 24% (95% CI 16-33%, I2=0%, p<0.00001) and, 38% (95% CI 28%-48%, I2=6%, p<0.00001) respectively. The corresponding funnel plots are displayed in supplementary figures S1-2. The commonest additional anomalies in the presence of pathogenic variants were those affecting the upper and/or lower limbs due to congenital contractures (HP:0002803); 17.3% (n=19/110). Where the NIHF phenotype was described, the incremental yield of pathogenic variants was not significantly greater where the hydrops was more severe (two cavities versus three or more cavities affected); 34% (95% CI 23-45%, I2=0%, p<0.00001) and 30% (95% CI 19-40%, I2=0%, p=0.003) respectively p=0.26. Where pathogenic variants were documented (n=89) (supplementary table 1) the commonest genetic disorders were (i) RASopathies 30.3% (n=27), primarily due to PTPN11 variants 44.4% (n=12/27); (ii) musculoskeletal disorders 14.6% (n=13), primarily due to RYR1 variants 46.2% (n=6/13) and; (iii) inborn errors of metabolism 12.4% (n=11), primarily due to GUSB variants 54.5% (n=6/11) The predominant inheritance pattern was autosomal dominant in monoallelic disease genes 57.3% (n=51), of which most were de novo 86.3% (n=44). Where the type of ES performed was stated [Table 1] (n=20 studies), the overall incremental yield did not differ significantly dependent on whether a panel or whole exome approach was used; 26% (95% CI 16-36%, I2=0%, p<0.00001) and 27% (95% CI 19-36%, I2=25%, p<0.00001) respectively.
Figure 3 -.
Forest plots showing incremental yield of exome sequencing (or an alternative sequencing strategy) over chromosomal microarray analysis/karyotyping in fetuses with prenatally detected non-immune hydrops fetalis (NIHF), overall (a) and in those with isolated NIHF (b) and NIHF with additional fetal structural anomalies (c). Only first author of each study is given. Refers to cases with a normal CMA result. CMA = chromosome microarray; M–H = Mantel–Haenszel.
DISCUSSION
This systematic review demonstrates substantial incremental yield with NGS (principally ES) over QF-PCR and CMA or karyotyping of 29% in cases of prenatally diagnosed NIHF. This yield was higher among cases with additional FSAs, but severity of NIHF did not demonstrate a significant difference in the incremental yield. In the majority of instances pathogenic variants were de novo in autosomal dominant disease genes, predominantly in those causative of RASopathies.
The findings of the final PAGE cohort and systematic review were broadly concordant, with a lower yield in the cohort study, which may be explained by the smaller case number as well as the unselected approach to case selection. The dominance of RASopathies and of de novo variants in autosomal dominant disease genes is expected and not mutually exclusive.2 Incremental yield was higher in instances where additional FSAs were present, predominantly so in cases of congenital arthrogryposis, which is intuitive as contractures are a common musculoskeletal phenotype of higher diagnostic yield with sequencing. Again this was unsurprising as contractures are seen commonly in the highest yielding musculoskeletal phenotype group.35 In contrast, isolated NIHF was seen commonly within the RASopathies; 47.8% (n=11/23). This is in keeping with the variable phenotype reported in the RASopathies and supports the use of prenatal ES in cases of isolated NIHF.36 There is phenotypic variability in cases with known RASopathy pathogenic variants, as well as in cases with pathogenic variants in other types of genetic diseases. This supports the use of ES or WGS, rather than a targeted or stepwise approach, in the investigation of NIHF.37 One must always respect the role of QF PCR or conventional karyotyping in NIHF, given the high incidence of aneuploidy.38 However, given the limited additional yield of CMA compared to karyotype and the ability of WGS to detect structural variants, it may be reasonable in the future as clinical and technical application of NGS technology includes validated CNV detection, to consider this as the second line test after QF-PCR or conventional karyotype.5 The list of novel causative genes in NIHF is constantly expanding, and with time the yield with prenatal NGS will likely improve as more genes are discovered and out understanding of the prenatal phenotype develops.2,37 This is supported by the high number of class III variants (VUS) identified within candidate genes from this study, high-lighted by the largest series in this study.2 Re-analysis and potential re-classification of VUS is currently underway for the PAGE cohort which may increase the diagnostic yield.
Due to the relatively high yield evident in isolated NIHF from this study (and individual papers in the literature) it was decided to include NIHF (from March 2021) as an indication for inclusion in the R21 pathway of the National Health Service (NHS) England National Genomic Test Directory for Rare and Inherited Disease.36,39 This (R21) pathway is a nationally (England presently) commissioned rapid prenatal ES service for fetuses with multiple, multisystem, major and selected isolated FSAs which is performed by two Genomic Laboratory Hubs in line with a set protocol.40 Inclusion of hydrops fetalis has been discussed as an inclusion phenotype and adopted in April 2021. Furthermore, the on-going Fetal Oedema and Lymphatic Disorder (FOLD) study is presently ongoing in the UK.41
Our study based its selection criteria upon the routine definition of what constitutes NIHF.1 It has been proposed that this definition be expanded to include pathological fluid accumulation in one or more fetal body cavity, inclusive of a large nuchal translucency (NT)[>3.5 mm] or cystic hygroma.2 This is being further explored but appears a reasonable argument given the large variability in NIHF phenotypes as well as their complex evolution and sometimes resolution seen in causative syndromes such as the RASopathies and is supportive by our finding that the mere presence of NIHF as opposed to its severity influence diagnostic yield with ES.2,42 Prenatal ES performed at the time of an isolated increased NT or pleural effusion for instance may be the only snapshot to obtaining a prenatal diagnosis and is indicative of the nature of evolving and resolving NIHF phenotypes. There are a need for studies which track the evolution of the phenotype and respective diagnostic yields with NGS. Despite this, prenatal ES offered in cases of isolated elevated NT appears to offer a modest increase in diagnostic yield over CMA at around 5-7%.2,42-44 It would appear to not just be the mere presence of the increased NT but its severity (≥5mm), persistence and association with additional anomalies that influence diagnostic yield with NGS.2,37,44
The strength of this systematic review lies in its novelty in concept, the robust methodology utilized as well as collaboration between experts of some of largest contemporary series in this area.2,8,16,17 The relatively small number of cases (n=306) represents the largest reviews of prenatal NIHF cases and did not appear to impact upon heterogeneity. Due to absence from the literature, no included studies used a WGS approach, hence the difference in yield between WGS and ES could not be assessed. This is likely to change in the coming years and will likely prove more beneficial due to its all-in-one ability to detect most chromosomal and genetic differences.7,39
In conclusion, the use of prenatal NGS in both isolated NIHF and NIHF associated with additional FSAs should be considered in developing clinical pathways. Given the vastness of syndromic categories and heterogeneity in prenatal phenotypes of NIHF, a whole exome or genome sequencing approach in combination with accurate prenatal phenotyping is likely a more appropriate tool than a targeted or stepwise single gene testing strategy in achieving an optimum diagnostic yield. The current definition of NIHF in assessing yield appears appropriate, although further studies assessing expansion of this definition are required to support this.
Supplementary Material
ACKNOWLEDGEMENTS
The PAGE study was supported by a Health Innovation Challenge from the UK Department of Health and Wellcome Trust (no. HICF-R7-396). We are grateful to Jane Fisher from Antenatal Results and Choices and to Michael Parker of The Ethox Centre, Nuffield Department of Population Health and Wellcome Centre for Ethics and Humanities for their valuable input into the study. We are also grateful to the members of the PAGE study clinical review panel. LSC an NIHR Senior Investigator and is partially funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at Great Ormond Street Hospital and ERM acknowledges support from NIHR Cambridge Biomedical Research Centre (an NIHR Senior Investigator Award). The University of Cambridge has received salary support with regard to ERM from the UK National Health Service (NHS) in the east of England through the Clinical Academic Reserve. The views expressed are those of the authors and not necessarily those of the NIHR, NHS or Department of Health.
Footnotes
CONFLICT OF INTEREST
RYE and JL report grants from the Health Innovation Challenge Fund during the conduct of the PAGE study. DJM reports grants for travel expenses from Congenica to attend educational symposia during the conduct of the PAGE study. MEH reports grants from the Wellcome Trust and the UK Government Department of Health during the conduct of the study and personal fees from Congenica, outside of the submitted work. MDK is a member of Illumina’s International Perinatal Advisory Group (but receives no payment for this) and is the Fetal Medicine Representative for the Central and South GLH. He is also the RCOG representative on the Joint Colleges Committee of Genomic and Genetic Medicine and the Royal College of Obstetricians and Gynaecologists Genomic Taskforce. ERM has received travel expenses, accommodation and consultant fees for participating in an Illumina International Advisory Group after completion of the PAGE study. MDK is funded through the Department of Health, Wellcome Trust and Health Innovation Challenge Fund (award number HICF-R7-396) for the PAGE and PAGE2 research studies complete August 2019. LSC was partially funded by the same group in relation to PAGE. RJW receives funding from Illumina and NIH for research. MN has been funded from Ultragenyx and the NIH for research relevant to included cases. All other authors declare no competing interests.
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/uog.23652
REFRENCES
- 1.Norton ME, Chauhan SP, Dashe JS. Society for Maternal- Fetal Medicine (SMFM) clinical guideline #7: nonimmune hydrops fetalis. Am J Obstet Gynecol 2015;212(2):127–139. [DOI] [PubMed] [Google Scholar]
- 2.Sparks TN, Lianoglou BR, Adami RR, Pluym ID, Holliman K, Duffy J, Downum SL, Patel S, Faubel A, Boe NM, Field NT, Murphy A, Laurent LC, Jolley J, Uy C, Slavotinek AM, Devine P, Hodoglugil U, Van Ziffle J, Sanders SJ, MacKenzie TC, Norton ME, University of California Fetal–Maternal Consortium; University of California, San Francisco Center for Maternal–Fetal Precision Medicine. Exome Sequencing for Prenatal Diagnosis in Nonimmune Hydrops Fetalis N Engl J Med 2020;383(18): 1746–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mardy AH, Chetty SP, Norton ME, Sparks TN. A system-based approach to the genetic etiologies of non-immune hydrops fetalis. Prenat Dian 2019;39:732–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ota S, Sahara J, Mabuchi A, Yamamoto R, Ishii K, Mitsuda N. Perinatal and one-year outcomes of non-immune hydrops fetalis by etiology and age at diagnosis. J Obstet Gynaecol Res 2016;42(4):385–91. [DOI] [PubMed] [Google Scholar]
- 5.Mardy AH, Rangwala N, Yessenia Hernandez-Cruz Y, Gosnell KA, Gonzalez JM, Norton ME, Sparks TN. Utility of chromosomal microarray for diagnosis in cases of nonimmune hydrops fetalis. Prenat Diagn 2020;40(4):492–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinn AM, Valcarcel BN, Makhamreh MM, Al-Kouatly HB, Berger SI. A systematic review of monogenic etiologies of nonimmune hydrops fetalis. Genet Med 2021;23(1):3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mone F, Quinlan-Jones E, Ewer AK, Kilby MD. Exome sequencing in the assessment of congenital malformations in the fetus and neonate. Arch Dis Child Fetal Neonatal Ed 2019;104(4):F452–F456. [DOI] [PubMed] [Google Scholar]
- 8.Lord J, McMullan DJ, Eberhardt RY, Rinck G, Hamilton SJ, Quinlan-Jones E, Prigmore E, Keelagher R, Best SK, Carey GK, Mellis R, Robart S, Berry IR, Chandler KE, Cilliers D, Cresswell L, Edwards SL, Gardiner C, Henderson A, Holden ST, Homfray T, Lester T, Lewis RA, Newbury-Ecob R, Prescott K, Quarrell OW, Ramsden SC, Roberts E, Tapon D, Tooley MJ, Vasudevan PC, Weber AP, Wellesley DG, Westwood P, White H, Parker M, Williams D, Jenkins L, Scott RH, Kilby MD, Chitty LS, Hurles ME, Maher ER; Prenatal Assessment of Genomes and Exomes Consortium. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): a cohort study. Lancet 2019; 393: 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mone F, Eberhardt RY, Morris RK, Hurles ME, McMullan DJ, Maher ER, Lord J, Chitty LS, Giordano JL, Wapner RJ, Kilby MD; CODE Study Collaborators. COngenital heart disease and the Diagnostic yield with Exome sequencing (CODE) study: prospective cohort study and systematic review. Ultrasound Obstet Gynecol. 2020;57(1):43–51 [DOI] [PubMed] [Google Scholar]
- 10.Köhler S, Gargano M, Matentzoglu N, Carmody LC, Lewis-Smith D, Vasilevsky NA, Danis D, Balagura G, Baynam G, Brower AM, Callahan TJ, Chute CG, Est JL, Galer PD, Ganesan S, Griese M, Haimel M, Pazmandi J, Hanauer M, Harris NL, Hartnett MJ, Hastreiter M, Hauck F, He Y, Jeske T, Kearney H, Kindle G, Klein C, Knoflach K, Krause R, Lagorce D, McMurry JA, Miller JA, Munoz-Torres MC, Peters RL, Rapp CK, Rath AM, Rind SA, Rosenberg AZ, Segal MM, Seidel MG, Smedley D, Talmy T, Thomas Y, Wiafe SA, Xian J, Yüksel Z, Helbig I, Mungall CJ, Haendel MA, Robinson PN. The Human Phenotype Ontology in 2021. Nucleic Acids Research 2021;49(D1):D1207–D1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17(5):405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, Mulrow C. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Plos Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012 [DOI] [PubMed] [Google Scholar]
- 14.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC; Standards for Reporting of Diagnostic Accuracy. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Standards for Reporting of Diagnostic Accuracy. Clin Chem 2003January;49(1):1–6. [DOI] [PubMed] [Google Scholar]
- 15.Jansen FA, Blumenfeld YJ, Fisher A, Cobben JM, Odibo AO, Borrell A, Haak MC. Array comparative genomic hybridization and fetal congenital heart defects; a systematic review and meta-analysis. Ultrasound Obstet Gynecol 2015;45(1):27–35 [DOI] [PubMed] [Google Scholar]
- 16.Petrovski S, Aggarwal V, Giordano JL, Stosic M, Wou K, Bier L, Spiegel E, Brennan K, Stong N, Jobanputra V, Ren Z, Zhu X, Mebane C, Nahum O, Wang Q, Kamalakaran S, Malone C, Anyane-Yeboa K, Miller R, Levy B, Goldstein DB, Wapner RJ. Whole-exome sequencing in the evaluation of fetal structural anomalies: a prospective cohort study. Lancet 2019; 393: 758–767 [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Zhou J, Wei X, Yang Y, Guo M, Deng L, Wang J, Sun L. Whole-exome sequencing for prenatal diagnosis of fetuses with recurrent nonimmune hydrops fetalis (NIHF). Prenat Diagn 2020;40(S1):3–21 [Google Scholar]
- 18.Boissel S, Fallet-Bianco C, Chitayat D, Kremer V, Nassif C, Rypens F, Delrue MA, Dal Soglio D, Oligny LL, Patey N, Flori E, Cloutier M, Dyment D, Campeau P, Karalis A, Nizard S, Fraser WD, Audibert F, Lemyre E, Rouleau GA, Hamdan FF, Kibar Z, Michaud JL. Genomic study of severe fetal anomalies and discovery of GREB1L mutations in renal agenesis. Genet Med 2018; 20: 745–753 [DOI] [PubMed] [Google Scholar]
- 19.Deng Q, Fu F, Yu Q, Li R, Li F, Wang D, Lei T, Yang X, Liao C. Nonimmune hydrops fetalis: genetic analysis and clinical outcome. Prenat Diagn 2020; 40(7): 803–12 [DOI] [PubMed] [Google Scholar]
- 20.Jelin AC, Sobreira N, Wohler E, Solomon B, Sparks T, Sagaser KG, Forster KR, Miller J, Witmer PD, Hamosh A, Valle D, Blakemore K. The utility of exome sequencing for fetal pleural effusions. Prenat Diagn 2020;40(5):590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Normand EA, Braxton A, Nassef S, Ward PA, Vetrini F, He W, Patel V, Qu C, Westerfield LE, Stover S, Dharmadhikari AV, Muzny DM, Gibbs RA, Dai H, Meng L, Wang X, Xiao R, Liu P, Bi W, Xia F, Walkiewicz M, Van den Veyver IB, Eng CM, Yang Y. Clinical exome sequencing for fetuses with ultrasound abnormalities and a suspected Mendelian disorder. Genome Med 2018; 10: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vora NL, Powell B, Brandt A, Strande N, Hardisty E, Gilmore K, Foreman AKM, Wilhelmsen K, Bizon C, Reilly J, Owen P, Powell CM, Skinner D, Rini C, Lyerly AD, Boggess KA, Weck K, Berg JS, Evans JP. Prenatal exome sequencing in anomalous fetuses: new opportunities and challenges. Genet Med 2017; 19: 1207–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stals KL, Wakeling M, Baptista J, Caswell R, Parrish A, Rankin J, Tysoe C, Jones G, Gunning AC, Lango Allen H, Bradley L, Brady AF, Carley H, Carmichael J, Castle B, Cilliers D, Cox H, Deshpande C, Dixit A, Eason J, Elmslie F, Fry AE, Fryer A, Holder M, Homfray T, Kivuva E, McKay V, Newbury-Ecob R, Parker M, Savarirayan R, Searle C, Shannon N, Shears D, Smithson S, Thomas E, Turnpenny PD, Varghese V, Vasudevan P, Wakeling E, Baple EL, Ellard S. Diagnosis of lethal or prenatal-onset autosomal recessive disorders by parental exome sequencing. Prenat Diagn 2018; 38: 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westphal DS, Leszinksi GS, Rieger-Fackeldey E, Graf E, Weirich G, Meitinger T, Ostermayer E, Oberhoffeer R, Wagner M. Lessons from exome sequencing in prenatally diagnosed heart defects: A basis for prenatal testing. Clin Genet 2019; 95: 582–589. [DOI] [PubMed] [Google Scholar]
- 25.Yates CL, Monaghan KG, Copenheaver D, Retterer K, Scuffins J, Kucera CR, Friedman B, Richard G, Juusola J. Whole-exome sequencing on deceased fetuses with ultrasound anomalies: expanding our knowledge of genetic disease during fetal development. Genet Med 2017; 19: 1171–1178 [DOI] [PubMed] [Google Scholar]
- 26.Becher N, Andreasen L, Sandager P, Lou S, Petersen OB, Christensen R, Vogel I. Implementation of exome sequencing in fetal diagnostics-Data and experiences from a tertiary center in Denmark. Acta Obstet Gynecol Scand 2020;99(6):783–790. [DOI] [PubMed] [Google Scholar]
- 27.Deden C, Neveling K, Zafeiropopoulou D, Gilissen C, Fundt R, Rinne T, de Leeuw N, Faas B, Gardeitchik T, Sallevelt SCEH, Paulussen A, Stevens SJC, Sikkel E, Elting MW, van Maarle MC, Diderich KEM, Corsten-Janssen N, Lichtenbelt KD, Lachmeijer G, Vissers LELM, Yntema HG, Nelen M, Feenstra I, van Zelst-Stams WAG. Rapid whole exome sequencing in pregnancies to identify the underlying genetic cause in fetuses with congenital anomalies detected by ultrasound imaging. Prenat Diagn 2020. ;40(8):972–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenbaum L, Pode-Shakked B, Eisenberg-Barzilai S, Dicastro-Keidar M, Bar-Ziv A, Goldstein N, Reznik-Wolf H, Poran H, Rigbi A, Barel O, Bertoli-Avella AM, Bauer P, Regev M, Raas-Rothschild A, Pras E, Berkenstadt M. Evaluation of Diagnostic Yield in Fetal Whole-Exome Sequencing: A Report on 45 Consecutive Families. Front Genet 2019;10:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sparks TN, Thao K, Lianoglou BR, Boe NM, Bruce KG, Datkhaeva I, Field NT, Fratto VM, Jolley J, Laurent LC, Mardy AH, Murphy AM, Ngan E, Rangwala N, Rottkamp CAM, Wilson L, Wu E, Uy CC, Lopez PV, Norton ME, University of California Fetal–Maternal Consortium (UCfC). Nonimmune hydrops fetalis: identifying the underlying genetic etiology. Genet Med 2019. ;21(6):1339–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westerfield LE, Stover SR, Mathur VS, Nassef SA, Carter TG, Yang Y, Eng CM, Van den Veyver IB. Reproductive genetic counseling challenges associated with diagnostic exome sequencing in a large academic private reproductive genetic counseling practice. Prenat Diagn 2015; 35: 1022–1029 [DOI] [PubMed] [Google Scholar]
- 31.Yang YS, Ma GC, Shih JC, Chen CP, Chou CH, Yeh KT, Kuo SJ, Chen TH, Hwu WL, Lee TH, Chen M. Experimental treatment of bilateral fetal chylothorax using in-utero pleurodesis. Ultrasound Obstet Gynecol 2012;39(1):56–62. [DOI] [PubMed] [Google Scholar]
- 32.Corsten-Janssen N, Bouman K, Diphoorn JCD, Scheper AJ, Kinds R, Mecky El, Breet H, Verheij JBGM, Suijkerbuijk R, Duin LK, Manten GTR, van Langen IM, Sijmons RH, Sikkema-Raddatz B, Westers H, van Diemen CC. A prospective study on rapid exome sequencing as a diagnostic test for multiple congenital anomalies on fetal ultrasound. Prenat Diagn 2020;40(10):1300–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Croonen EA, Nillesen WM, Stuurman KE, Oudesluijs G, van de Laar IMBM, Martens L, Ockeloen C, Mathijssen IB, Schepens M, Ruiterkamp-Versteeg M, Scheffer H, Faas BHW, van der Burgt I, Yntema HG. Prenatal diagnostic testing of the Noonan syndrome genes in fetuses with abnormal ultrasound findings. Eur J Hum Genet 2013;21(9):936–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mone F, Doyle S, Hamilton S, Allen S, Williams D, Kilby MD. VP33.06: Non-immune hydrops fetalis and diagnostic yield with prenatal-exome sequencing: a case series. Ultrasound Obstet Gynecol 2020;56(S1):195 [Google Scholar]
- 35.Pehlivan D, Bayram Y, Gunes N, Akdemir ZC, Shukla A, Bierhals T, Tabakci B, Sahin Y, Gezdirici A, Fatih JM, Gulec EY, Yesil G, Punetha J, Ocak Z, Grochowski CM, Karaca E, Albayrak HM, Radhakrishnan P, Erdem HB, Sahin I, Yildirim T, Bayhan IA, Bursali A, Muhsin Elmas M, Yuksel Z, Ozdemir O, Silan F, Yildiz O, Yesilbas O, Isikay S, Balta B, Gu S, Jhangiani SN, Doddapaneni H, Hu J, Muzny DM, Baylor-Hopkins Center for Mendelian Genomics; Boerwinkle E, Gibbs RA, Tsiakas K, Hempel M, Girisha KM, Gul D, Posey JE, Elcioglu NH, Tuysuz B, Lupski JR. The Genomics of Arthrogryposis, a Complex Trait: Candidate Genes and Further Evidence for Oligogenic Inheritance. Am J Hum Genet. 2019; 105(1): 132–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Genomic Test Directory. NHS England 2020/2021 available from: https://www.england.nhs.uk/publication/national-genomic-test-directories/ Accessed on 1st February 2021
- 37.Stuurman KE, Joosten M, van der Burgt I, Elting M, Yntema HG, Meijers-Heijboer H, Rinne T. Prenatal ultrasound findings of rasopathies in a cohort of 424 fetuses: update on genetic testing in the NGS era. J Med Genet 2019;56(10):654–61 [DOI] [PubMed] [Google Scholar]
- 38.Sileo FG, Kulkarni A, Branescu I, Homfray T, Dempsey E, Mansour S, B Thilaganathan B, Bhide A, Khalil A. Non-immune fetal hydrops: etiology and outcome according to gestational age at diagnosis. Ultrasound Obstet Gynecol. 2020September;56(3):416–421. [DOI] [PubMed] [Google Scholar]
- 39.Mone F, McMullan DJ, Williams D, Chitty LS, Maher ER, Kilby MD; Fetal Genomics Steering Group of the British Society for Genetic Medicine; the Royal College of Obstetricians and Gynaecologists. Evidence to Support the Clinical Utility of Prenatal Exome Sequencing in Evaluation of the Fetus with Congenital Malformations. Scientific Impact Paper No. 64. BJOG 2021; 10.1111/1471-0528.16616 [In press] [DOI] [PubMed] [Google Scholar]
- 40.NHS England. Rapid Exome Sequencing Service for Fetal Anomalies Testing. 2021. Accessed from https://labs.gosh.nhs.uk/media/1396340/rapid_prenatal_exome_sequencing_r21_faq_v1.pdf on 24th March 2021
- 41.Dempsey E. ISRCTN22076461 A study to improve our understanding of the genetic causes of swelling in babies before birth. ISRCTN registry available from; 10.1186/ISRCTN22076461 accessed on 8th January 2021. [DOI] [Google Scholar]
- 42.Achiron R, Heggesh J, Grisaru D, Goldman B, Lipitz S, Yagel S, Frydman M. Noonan syndrome: a cryptic condition in early gestation. Am J Med Genet 2000;92(3):159–65. [DOI] [PubMed] [Google Scholar]
- 43.Yang X, Huang LY, Pan M, Xu L, Zhen L, Han J, Li D. Exome sequencing improves genetic diagnosis of fetal increased nuchal translucency. Prenat Diagn 2020;40(11):1426–1431. [DOI] [PubMed] [Google Scholar]
- 44.Mellis R, Eberhardt R, Lord J, Quinlan Jones E, Rinck G, McMullan D, Maher E, Hurles M, Chitty L. Prenatal exome sequencing for isolated increased nuchal translucency: Should we be doing it? Prenat Diagn 2020;40:S1 19 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.