Abstract
Background:
Multiple active vaccination approaches have proven ineffective in reducing the significant morbidity and mortality caused by respiratory syncytial virus (RSV) in infants and the elderly. A vaccine conferring a substantial and sustainable boost in neutralizing activity is likely required to protect against severe RSV disease.
Methods:
In a randomized open-label phase 1 clinical trial, the stabilized prefusion F vaccine DS-Cav1 was evaluated for dose, safety, tolerability, and immunogenicity in healthy adults at a single US site. Participants were randomized at a 1:1 ratio (with or without aluminum hydroxide per dose group) to receive escalating doses of 50 mcg, 150 mcg, or 500 mcg DS-Cav1 at weeks 0 and 12. A subset of subjects received only a single vaccination at week 0. The primary objectives evaluated the safety and tolerability at every dose level, and neutralizing activity and RSV F-binding antibodies were evaluated through week 44 as secondary and exploratory objectives. The trial is registered with ClinicalTrials.gov, NCT03049488, and is complete and no longer recruiting.
Findings:
Between February 2017 and November 2018, 244 participants were screened for eligibility and 95 were enrolled to receive DS-Cav1 at the 50 mcg (n=30), 150 (n=35), or 500 mcg (n=30) dose level. DS-Cav1 was safe and well-tolerated and no serious vaccine-associated adverse events deemed related to the vaccine were identified. DS-Cav1 vaccination elicited robust neutralizing activity and binding antibodies after a single dose. At week 44, F-specific antibody levels and neutralizing activity were significantly increased compared to baseline in all groups. While a higher vaccine dose or second immunization elicited a transient advantage compared to lower doses or a single immunization, neither significantly impacted long-term neutralization.
Interpretation:
DS-Cav1 vaccination elicited a robust boost in RSV F-specific antibodies and neutralizing activity that was sustained above baseline for at least ten months. There was no long-term impact of dose, number of vaccinations, or adjuvant on neutralizing activity, indicating that a single low-dose of pre-F immunization of antigen-experienced individuals may confer protection that extends through an entire RSV season.
Funding:
This work was supported with intramural funding from the National Institutes of Allergy and Infectious Diseases.
Trial Registration Number: NCT03049488
Introduction
Respiratory syncytial virus (RSV) is a leading cause of lower respiratory tract infection in infants and older adults. Globally, RSV causes an estimated 33·1 million acute lower respiratory tract infections and 3·2 million hospitalizations in children under five years of age annually.1 In older adults, RSV contributed to 14·5% of hospital admissions in industrialized countries and caused 8% of lower respiratory-related deaths in US hospitals.2,3 Despite decades of effort, there is no licensed vaccine for RSV, and vaccine development has encountered several obstacles. In the 1960s, administration of formalin-inactivated RSV to antigen-naïve children resulted in vaccine-enhanced respiratory disease upon subsequent RSV infection.4–7 There is no defined correlate of protection from severe disease, complicating efforts to evaluate vaccine efficacy. RSV F exists in two major conformations: the active prefusion (pre-F), and the inactive postfusion (post-F) conformation adopted after triggering and rearrangement. While several post-F-based vaccines have been shown to be immunogenic, they failed to demonstrate efficacy in phase 2 and 3 clinical trials.8 The DS-Cav1 subunit vaccine, which is stabilized in the pre-F conformation, maintains several neutralization-sensitive antigenic sites on the trimer apex and elicits unprecedented levels of neutralizing activity.9,10
A randomized, open-label dose-escalation phase 1 clinical trial was conducted to evaluate the safety and immunogenicity of two DS-Cav1 immunizations in healthy adults aged 18 to 50. We previously reported interim data from 40 subjects through 12 weeks following a single vaccination with 50 or 150 mcg of DS-Cav1 with or without alum, demonstrating that DS-Cav1 elicited robust neutralizing activity and antibodies targeting the pre-F exclusive antigenic sites and sites present on both conformations of the F protein.10 Here, we report the responses of all subjects enrolled in the trial through completion of the study at week 44, demonstrating that vaccination with DS-Cav1 is safe, well-tolerated, and capable of eliciting sustained, high-potency neutralizing activity against RSV.
Methods
Study Design and Participants
VRC 317 was a phase 1, open-label, randomized, domestic single site clinical trial to evaluate dose, safety, tolerability and immunogenicity of an investigational stabilized prefusion RSV F subunit protein vaccine, DS-Cav1, alone or with aluminum hydroxide adjuvant. Eligible participants were healthy adults aged 18 to 50 years. Participants were recruited from the Washington, D.C., metropolitan area. The trial was conducted by investigators at the Vaccine Research Center (VRC) of the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health (NIH) in Bethesda, MD, US. The study was reviewed and approved by the institutional review board at NIAID. The protocol was subsequently amended to give subjects the option to forgo the second immunization with DS-Cav1 and complete the scheduled study visits in order to further assess immunogenicity of a single vaccine dose. The Department of Health and Human Services Guidelines for the protection of human research subjects were followed. All participants provided written informed consent before enrollment. VRC investigators developed the vaccine, and the VRC Clinical Trials Program conducted the study.
Vaccine
The prefusion subunit vaccine VRC-RSVRGP084–00-VP (DS-Cav1) was manufactured under cGMP conditions at the VRC Pilot Plant, in contract with the Vaccine Clinical Material Program, Leidos Biomedical Research. DS-Cav1 is a sterile, aqueous, buffered solution that contains the RSV A2 fusion glycoprotein ectodomain assembled as a prefusion-stabilized trimer as described previously.10 The vaccine is supplied in single dose vials. The adjuvant is an aluminum hydroxide suspension (alum) with GMP-grade Alhydrogel 2% (CRODA, Frederikssund Denmark), in a sterile, pyrogen-free suspension at a 5 ± 1 mg/ml concentration. To prepare the adjuvanted dose, DS-Cav1 was contemporaneously mixed with the alum before administration.
Study Procedures
Study procedures were followed as previously described.10 Under the VRC 317 study protocol, participants were randomized at a 1:1 ratio (with or without alum per dose group) to receive escalating doses of 50 mcg, 150 mcg, or 500 mcg DS-Cav1. A single unmasked injection of the assigned DS-Cav1 vaccine was administered in the deltoid muscle by needle and syringe at weeks 0 and 12, except participants who declined the optional second dose. All second immunizations given at week 12 were administered at the same dose as the first. Subjects were observed for at least 60 minutes following vaccination. Prior to discharge from the clinic, vital signs were recorded, and the injection site was inspected for evidence of local reaction. Safety endpoints were obtained by subject reports, targeted physical exam and medical history review, and monitoring of hematological and chemical parameters. Subjects recorded solicited reactogenicity for 7 days after each vaccination. The solicited parameters included local events at the injection site of pain/tenderness, redness, and swelling, as well as systemic events of malaise, myalgia, headache, chills, nausea, and temperature. Adverse events were recorded through 28 days after each vaccination and were graded according to the Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials (modified from FDA Guidance, September 2007). Severe adverse events (SAEs) and new chronic medical conditions were recorded for the duration of the study (week 44). A nasopharyngeal PCR swab was collected if subjects experienced any symptoms of RSV infection while in study. All participants were followed through week 44.
Statistical Analysis
Methods for assessment of RSV neutralizing activity and F-specific antibody readouts are included in the supplementary appendix. Lognormal immunology assay results were log2 transformed for analysis, and all analyses were performed using R (Version 3·4·4) or GraphPad Prism (Version 7·0). For the primary endpoints, adverse event rates were summarized using proportions and Clopper-Pearson confidence intervals. Secondary analysis focused on the antigen-specific antibody response using paired t-tests to compare levels before and after doses, and linear regression to determine the significance of dose, adjuvant, and number of vaccinations in predicting the antibody level at week 4. Exploratory analysis repeated these investigations at weeks 16 and 44, supplemented by a longitudinal mixed model assuming random effects for participants, with fixed effects for dose, boosting, adjuvant and baseline covariates as appropriate. Exploratory analyses were not pre-specified and do not include adjustments for multiple comparisons, so we consider these results to be hypothesis generating, with those yielding p-values of <0·1 taken as the most promising. All pairwise correlations are based on Spearman’s correlation coefficient. Analyses were performed by intention to treat, with assessment of safety in all subjects that received at least one dose of DS-Cav1; secondary and exploratory immunogenicity analysis include all participants with available data at a given visit.
Sample Size
The sample size was limited for this phase 1 dose escalation study to a total of 95 subjects receiving a DS-Cav1 vaccine, with 20 subjects in the adjuvanted 150 mcg group and 15 subjects in all other arms. A total of 35 subjects are in the 150 mcg dose group, and the 50 mcg and 150 mcg dose group both include 30 subjects each. This was selected as adequate and reasonable for an initial review of the safety profile, with approximately 80% chance of seeing one or more adverse event in a dose group size of n=30 if the true rate is at last 5%. Immunogenicity analyses were secondary, but we estimated from previous data that the standard deviation of ELISA and neutralization results might be approximately 0·5 on a log10 scale, and if that held, then we would have at least 90% power to detect a difference between arms of 0·45 log10 with n=15 in each arm. Subjects were randomized 1:1 between arms within dose levels, using permuted block randomization. Participants were not randomized between doses, due to the dose-escalation design.
This study is registered with ClinicalTrials.gov, NCT03049488
Role of Funding Source
This work was supported with intramural funding from the National Institute of Allergy and Infectious Diseases. The funding source has no involvement with the study design, execution, or preparation of the manuscript. All authors full access to all of the data and the final responsibility to submit for publication.
Results
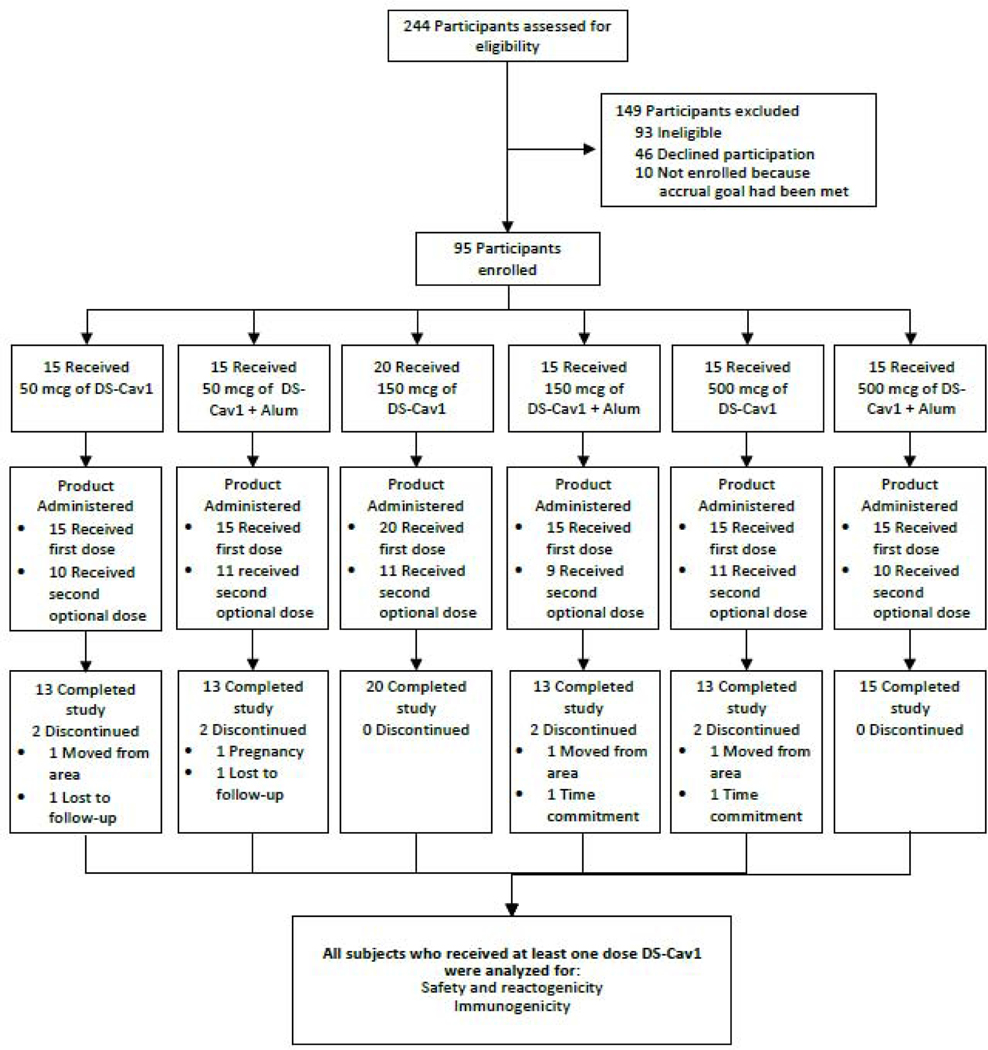
In total, 95 subjects were enrolled and vaccinated from February 21st, 2017 through November 29th, 2018, and the final study visit occurred October 3rd, 2019 (figure 1). The study population was composed of 49 women and 46 men, with ages ranging from 18 to 50 (mean age of 31) (table 1). Sixty-two subjects were vaccinated with DS-Cav1 at both weeks 0 and 12. The remaining 33 subjects received a single vaccination at week 0. In group 3 (150 mcg with no alum), five additional subjects were enrolled who agreed to undergo apheresis under protocol VRC 200 (A Multicenter Specimen Collection Protocol to Obtain Human Biological Samples for Research Studies, NCT00067054) for a total of 20 subjects in this group. Eighty-seven of the 95 subjects completed the study. Of the eight subjects who did not complete the protocol visits as scheduled, three moved from the area, two were lost to follow-up, two withdrew due to the required time commitment, and one became pregnant.
Figure 1.
Consort Diagram
Table 1.
Characteristics of the Participants at Enrollment.
| Characteristics | Group 1 50 mcg (N=15) | Group 2 50 mcg + Alum (N=15) | Group 3 150 mcg (N=20) | Group 4 150 mcg + Alum (N=15) | Group 5 500 mcg (N=15) | Group 6 500 mcg + Alum (N=15) | Overall (N=95) |
|---|---|---|---|---|---|---|---|
| Sex - no. (%) | |||||||
| Male | 6 (40) | 9 (60) | 11 (55) | 7 (46·7) | 6 (40) | 7 (46·7) | 46 (48·4) |
| Female | 9 (60) | 6 (40) | 9 (45) | 8 (53·3) | 9 (60) | 8 (53·3) | 49 (51·6) |
| Age - yr | 32·4 ± 5·8 | 32·9 ± 9·3 | 32·2 ± 9·6 | 31·8 ± 9·3 | 31·5 ± 8·5 | 27·6 ± 7·6 | 31·4 ± 8·5 |
| Race - no. (%) | |||||||
| Asian | 4 (26·7) | 1 (6·7) | 4 (20) | 0 (0) | 3 (20) | 3 (20) | 15 (15·8) |
| Black or African American | 1 (6·7) | 3 (20) | 1 (5) | 4 (26·7) | 0 (0) | 0 (0) | 9 (9·5) |
| White | 10 (66·7) | 11 (73·3) | 13 (65) | 9 (60) | 12 (80) | 10 (66·7) | 65 (68·4) |
| Multiracial | 0 (0) | 0 (0) | 2 (10) | 2 (13·3) | 0 (0) | 2 (13·3) | 6 (6·3) |
| Hispanic or Latino ethnic group - no. (%) | 0 (0) | 1 (6·7) | 2 (10) | 0 (0) | 4 (26·7) | 1 (6·7) | 8 (8·4) |
| Body-mass index | 26·0 ± 3·5 | 28·1 ± 6 | 26·7 ± 4·1 | 27 ± 4·2 | 25·8 ± 3·3 | 26·2 ± 3·5 | 26·6 ± 4·1 |
| College or higher educational level – no. (%) | 13 (86·7) | 13 (86·7) | 19 (95) | 14 (93·3) | 15 (100) | 15 (100) | 89 (93·7) |
When present, reactogenicity was usually mild to moderate and most commonly consisted of local injection site pain or swelling, or systemic headache, malaise, or myalgias. There was no effect of antigen dose, number of immunizations, or adjuvant with respect to local reactogenicity. There was a trend toward greater systemic reactogenicity after the first vaccine dose and in subjects who received alum adjuvant. (table S1, S2).
There were no SAEs reported. Eighteen adverse events (AEs) were deemed as possibly or probably related to the study product, and all resolved without residual effects. Mild related AEs included one episode each of headache, vivid dreams, leukocytosis, lymph node enlargement, elevated creatinine, lymphopenia, leukopenia, chest wall pain, and muscle spasm, three episodes of neutropenia and two episodes of dizziness. Moderate related AEs included three episodes of neutropenia and one episode of presyncope. There was no evidence of a dose, number of immunizations, or adjuvant effect with respect to AEs.
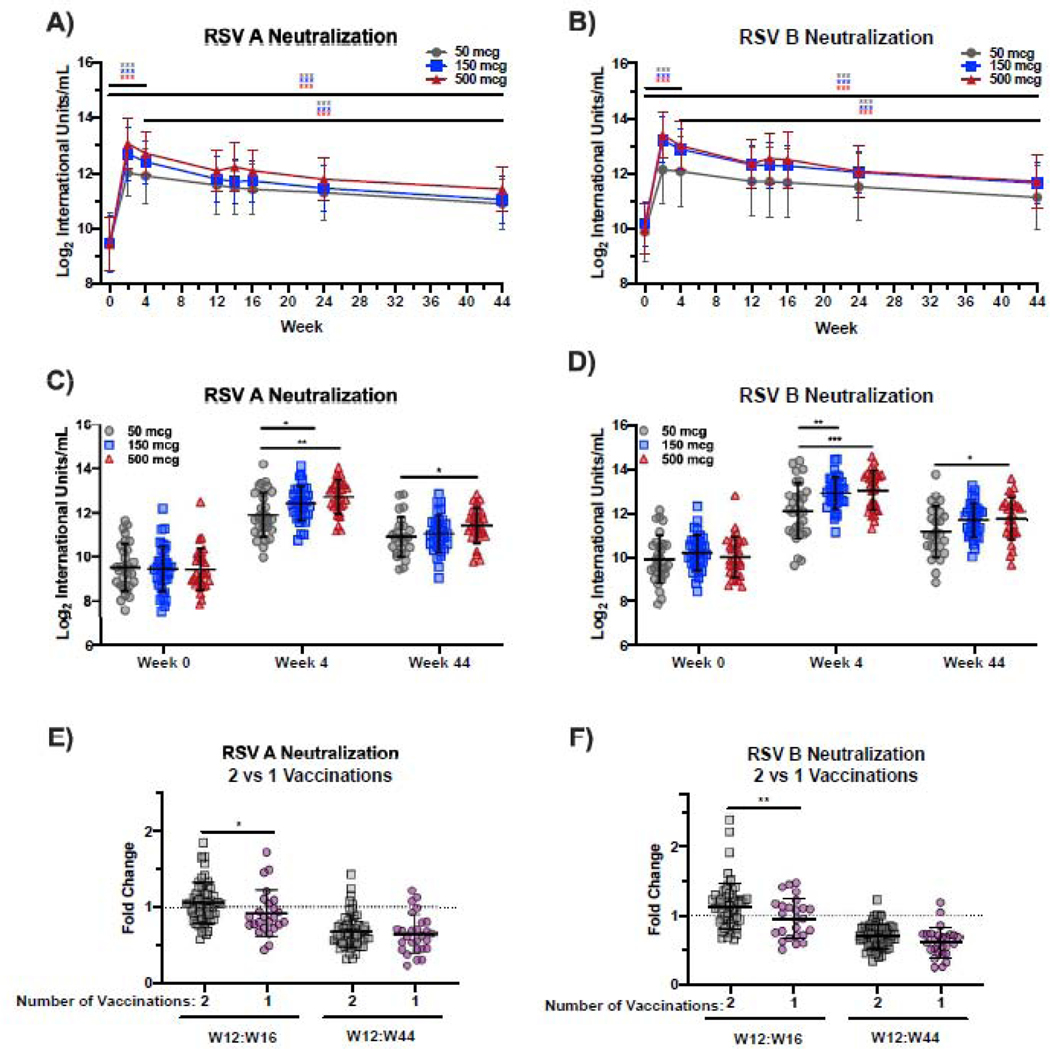
One case of RSV was diagnosed during the trial. The subject displayed symptoms shortly after receiving the first dose of vaccine and tested positive for RSV from a nasal swab on day 5. The secondary objective of the study was to measure the increase in neutralizing activity elicited by DS-Cav1 vaccination. At week four (W4) after the first vaccination, there was a robust increase in neutralizing activity to both RSV subtypes A and B in all dose groups (p<0·001, figure 2A, B, table S3, S4), with no effect of alum adjuvant within each dose (figure S1). RSV A neutralizing activity waned between weeks 4 and 44, but remained 3·1-, 3·8-, and 4·5-fold above baseline activity in the 50, 150, and 500 mcg groups, respectively (p<0·001); our modeling suggested that it would take more than a year after the second immunization for neutralizing activity to return to the level measured at baseline (table S4). A similar pattern was evident for RSV B neutralizing activity which remained 2·8-fold (50 mcg), 3·4-fold (150 mcg), and 3·7-fold (500 mcg) higher at W44 than neutralizing activity at baseline (p<0·001) (figure 2B, table S5). At W4, neutralizing activity against RSV A was marginally higher in the 150 mcg dose group compared to the 50 mcg dose group (p=0·016, mean fold-change 10·5 and 7·5, respectively) and highest in subjects immunized with 500 mcg of DS-Cav1 (mean fold-change 12·6, p=0·002 compared to the 50 mcg dose group) (figure 2C). By week 44 week (W44), there was no significant difference in RSV A neutralization between groups immunized with 50 mcg and 150 mcg, and only a marginal increase in RSV A neutralization for the 500 mcg dose compared to the 50 mcg dose group (p=0·016, figure 2C). At W4, RSV B neutralizing activity was significantly higher in 500 mcg (p<0·001) and 150 mcg dose recipients (p=0·003) compared to the 50 mcg dose recipients (mean fold-changes of 9·8, 8·4, and 6·4, respectively) (figure 2D). Similar to RSV A neutralizing activity, only the 500 mcg dose group had marginally higher RSV B neutralizing activity than the 50 mcg dose group at W44 (p=0·028, figure 2D). Neutralizing activity against RSV A was directed towards pre-F-exclusive sites as well sites shared by the pre-F and post-F protein (figure S2A, B).
Figure 2.
DS-Cav1 elicits potent and durable neutralizing activity through 44 weeks post-vaccination.
Panels A and B show neutralizing activity in the serum against a reporter RSV A2 virus (RSV A) and RSV B18537 virus (RSV B), respectively at weeks 0, 2, 4, 12, 14, 16, 24 and 44 for subjects immunized with 50, 150, or 500 mcg of DS-Cav1. Significance determined by Student’s t-test (at specific timepoints) without adjustment for multiple comparisons. Panels C and D show neutralizing activity in the serum against RSV A and RSV B, respectively, for each subject vaccinated with 50, 150, or 500 mcg of DS-Cav1 at baseline (W0) and weeks 4 and 44. Significance between dose groups determined by linear regression. Panel E and F show the fold-change in neutralizing activity against RSV A and B, respectively, between W12 and W16 in subjects that received 2 or 1 vaccinations and between W12 and W44 in these groups. Dotted line represents a fold-change of 1. Significance determined by linear regression without adjustment for multiple comparisons. Significance indicated as ***p<0·001, **p<0·01, *p<0·05.
To address whether the second immunization with DS-Cav1 at W12 significantly affected neutralizing activity, we compared the fold-change in neutralizing activity between W12 to W16 in subjects that received both immunizations with DS-Cav1 (n=59) to those that only received a single immunization (n=28). Neutralizing activity against RSV A and RSV B was marginally greater at W16 in subjects that received the second immunization (mean fold-change= 1·0 for RSV A, 0·82 for RSV B) at W12 compared to those that received only a single immunization (mean fold-change= 0·91, and 0·71 for RSV B, p=0·031 for RSV A and p=0·01 for RSV B) (figure 2E, F). By W44, there was no significant difference in neutralizing activity between subjects receiving two or one vaccinations, suggesting that durable neutralizing activity is not affected by a second immunization with DS-Cav1 (figure 2E, F).
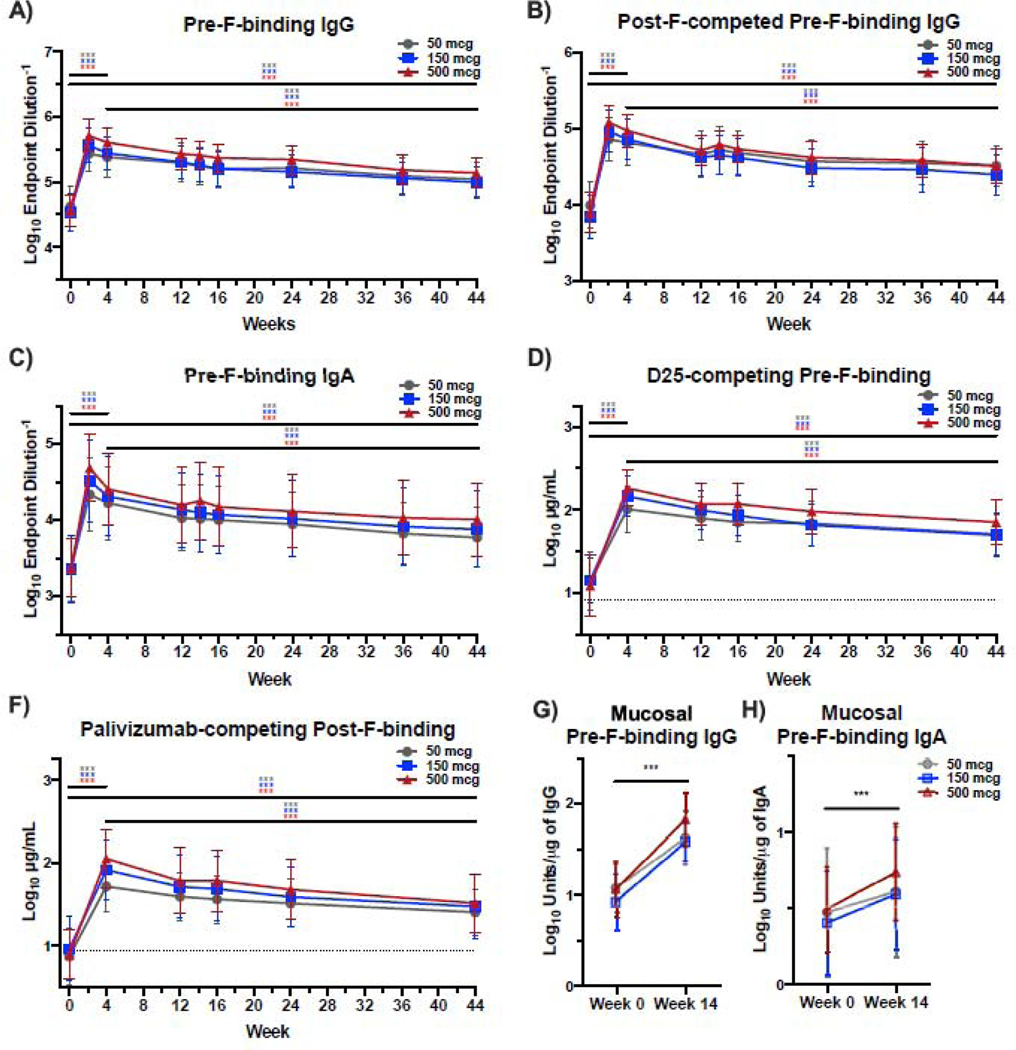
Mirroring neutralizing activity, the first immunization with DS-Cav1 elicited a robust boost in serum IgG and IgA binding to subtype A pre-F (p<0·001, figure 3A, C). A significant increase in antibodies targeting pre-F-exclusive epitopes was confirmed by measurement of post-F-competed pre-F-binding IgG (figure 3B). Pre-F-binding IgG waned between W4 and W44 but remained significantly above baseline at W44 (p=0·0001, 3·2-fold at 50 mcg, 3·4-fold at 150 mcg, and 4·0-fold at 500 mcg). The 500 mcg dose group had significantly higher pre-F-binding IgG at W4 than the 50 mcg group (p<0·001), but there was no significant difference by W44 suggesting only a transient dose effect (figure S2C). Compared to baseline, pre-F-binding serum IgA remained 4·1-fold (50 mcg), 4·3-fold (150 mcg) and 4·8-fold (500 mcg) higher at W44 (p<0·001). Vaccine dose had no effect on pre-F-binding IgA at either W4 or W44 (figure S2D). To further characterize serum antibody, we quantified D25-competing antibodies that bind the apex of pre-F, and antibodies targeting surfaces shared by both conformations of F (palivizumab-competing, side-binding).11 Apex- and side-binding antibodies were strongly induced by W4 after vaccination (p<0·001) and remained significantly above baseline at W44 (p<0·001) (figure 3C, D), demonstrating that DS-Cav1 vaccination elicited robust and durable antibody responses targeting both the apex and the side of the F protein.
Figure 3.
DS-Cav1 elicits robust and durable pre-F-binding antibodies that recognize the apex and side of the F protein.
Pre-F-binding IgG (Panel A) and IgA (Panel C) measured by ELISA and pre-F binding IgG in the presence of excess post-F (Panel B) which competes for post-F exclusive and dual-binding antibodies, demonstrating that binding antibodies are directed to the pre-F-exclusive and shared surfaces of pre-F and post-F. Panel E shows the level of pre-F-binding antibodies that compete with binding of the D25 antibody (apex-binding) and Panel F shows the level post-F-binding antibodies that competed with palivizumab antibody (side-binding). Panels G and H show levels of pre-F-binding IgG (Panel G) and IgA (Panel H) in mucosal samples. Statistical differences comparing weeks 0, 4, and 44 are determined by linear regression with no adjustment for multiple comparisons. Significance indicated as ***p<0·001.
A second DS-Cav1 immunization mitigated antibody waning between W12 and W16 as measured by pre-F binding IgG (p<0·001) and IgA (p=0·004) and levels of D25- or palivizumab-competing antibody (p<0·001) (figure S3A-D). However, the difference in these measures was abrogated between W12 and W44 among subjects receiving one or two immunizations, suggesting that a second immunization of DS-Cav1 did not have an impact on long-term antibody levels (figure S3A-D).
We performed a correlates analysis to identify assays that could serve as a surrogate for neutralization in large, advanced stage clinical trials. At W4 and W44, RSV A neutralization was highly correlated with post-F-competed RSV A neutralization (R=0·81 at W4, R=0·84 at W44) and RSV B neutralization (R=0·79 at W4, R=0·73 at W44) (all p<0·001) (figure S4A). Pre-F-binding IgG, post-F-competed pre-F-binding IgG, and D25-competing antibody measures were highly correlated with RSV A neutralization at W4 (R>0·7, p<0·001) (figure S4A). At W44, D25-competing antibody remained highly correlated with neutralizing activity (R=0·76, p<0·001) (figure S4B). Post-F-binding IgG and palivizumab-competing antibody were less correlated with RSV A neutralization (R=0·54 at W4 and R=0·51 at W44) (figure S4A, B), suggesting that these readouts may not serve as reliable surrogates for neutralization.
As mucosal surfaces are the first line of defense against RSV, and mucosal F-specific antibodies have been correlated with protection from infection,12–16 we quantified pre-F-specific IgG and IgA in nasopharyngeal fluid obtained at W0 and W14 (two weeks after the second immunization). Pre-F-specific IgG was detectable in NP samples from 65 subjects at both timepoints, and pre-F-specific IgA was detectable from 64 subjects at both timepoints. At W14, DS-Cav1 vaccination significantly boosted mucosal pre-F-specific IgG (p<0·001), with a more modest increase in mucosal pre-F-specific IgA (p<0·001) (figure 3G, H, S5A, B). The fold-change in mucosal pre-F-specific IgG was similar to the fold-change in pre-F-specific IgG in the serum (figure S5C). However, the fold-change in pre-F-specific IgA was greater in the serum than in the mucosa (figure S5D). In summary, intramuscular immunization with DS-Cav1 not only increased serum F-specific antibody and neutralizing activity, but also significantly increased pre-F-binding IgG and IgA at the site of RSV infection.
Discussion
Analysis of all 95 subjects in the VRC 317 Phase 1 study through 44 weeks post-vaccination determined that the DS-Cav1 subunit vaccine was safe and well-tolerated up to doses as high as 500 mcg. One vaccination with subtype A protein was capable of eliciting a robust increase in neutralizing activity against both RSV A and B subtypes that exceeds the increase seen after RSV infection of people in a similar age category.12 A modest, transient advantage of increasing antigen dose or providing a second vaccination at 12 weeks was observed, but the impact of each of these on long-term neutralizing activity was marginal and unlikely to be clinically or biologically meaningful in an efficacy trial. Importantly, the impact of a single dose on neutralizing activity proved durable through completion of the trial at 44 weeks, suggesting the possibility that protective immunity could be improved for more than one RSV season.
In contrast to other trials comparing adjuvanted to unadjuvanted RSV F subunit protein in the post-F conformation, we observed no significant impact of alum adjuvant on immunogenicity.17-19 This could be the result of age differences of vaccinated subjects (60 and older in previous studies vs. 18–50 in this study) or the relatively small number of subjects in each dose group, but it is more likely due to the overall higher immunogenicity of pre-F, which preserves and elicits potent antibody to pre-F exclusive sites not present on previous antigens. It is possible that a different adjuvant may impact neutralizing activity.20 As evidenced by the similar induction and maintenance of antibodies to both the apex and side of the pre-F, antibodies are generated to pre-F exclusive and shared antigenic sites, and we did not observe a preferential elicitation or decay of antibodies of either specificity. The boost in polyclonal antibody to all neutralization-sensitive epitopes offers an advantage over post-F, which presents fewer epitopes, and approaches such as prophylactic monoclonal antibody, for which efficacy may be susceptible to minor antigenic drift in selected RSV F epitopes.8,21–23
The greatest limitation of our study is that it is a relatively small phase 1 study, where responses to vaccine were tested only in 15–20 healthy adults per study arm. The major target populations for a pre-F subunit protein vaccine are pregnant women and the elderly. For maternal immunization, the goal of vaccination is to extend the window in which placentally-transferred antibody confers protection. Together with studies demonstrating a near 1:1 mother to child transfer ratio for RSV-specific IgG, these data suggest that a single vaccination during the second or third trimester could result in neutralizing activity in infants at birth that exceeds typical levels by 10-fold.24 Maternal immunization with an unstabilized F vaccine that boosted neutralizing activity two- to three-fold afforded partial protection of infants up to 90 days of life.25 While our study did not involve pregnant women, a 10-fold rise in neutralizing activity in infants could extend protection through the first six months of life, when an increase in airway size and maturation of the immune response improve clinical outcomes of RSV infection.25–29 The robust antibody response in the absence of adjuvant is promising for maternal populations where adjuvant use may be contraindicated. While there was no advantage for alum adjuvant in the young, healthy VRC 317 cohort, our study did not involve any older adults, and does not offer insight into whether adjuvant effects could counteract the waning immunity and comorbidities that increase risk of severe disease in the elderly. Vectored vaccine platforms are also being explored to deliver pre-F for this high risk group.30
Mucosal IgA and IgG have been associated with protection from RSV infection and severe disease.12–16 Intramuscular immunization with a pre-F vaccine elicited a similar fold-increase in mucosal IgG in the nasopharynx/upper respiratory tract as in the sera. We would anticipate similar levels in the lower respiratory tract, where IgG could prevent the manifestation of severe disease.24,26,31,32 There was also a modest increase of IgA in the mucosa which was not proportional to the more dramatic increase in serum IgA. This is not surprising, since intramuscular immunization is not expected to induce anti-RSV secretory IgA compared to intranasal antigen delivery.12
In summary, DS-Cav1 offers a marked improvement over previous RSV vaccines based on post-F or structurally undefined F antigens. A single dose of unadjuvanted pre-F was able to induce robust and durable neutralizing activity that exceeded that of RSV infection. While we cannot evaluate efficacy in this small phase 1 trial in a low-risk population, a sustained increase in neutralizing activity in high-risk cohorts following DS-Cav1 immunization or perinatal antibody transfer from vaccinated mothers may protect against severe lower respiratory tract disease caused by RSV infection, and consequently, possible long-term sequelae on lung development and function. Various vaccine approaches based on stabilized RSV pre-F antigens have advanced into clinical evaluation, and efficacy studies in both maternal and elderly populations are ongoing. After decades of RSV vaccine development efforts, a structure-guided approach to precision antigen design has yielded an effective immunogen and engendered hope that an RSV vaccine is on the near horizon. Furthermore, these findings have implications for vaccines against other enveloped viruses, such as the SARS-CoV-2 spike protein as a COVID-19 vaccine, demonstrating the value of structure-based vaccine design and advantages of stabilizing immunogens based on trimeric class I fusion proteins in the prefusion conformation.
Supplementary Material
Research in Context.
Evidence before this study
Respiratory syncytial virus (RSV) remains a significant cause of global morbidity and mortality, and there is currently no licensed vaccine. Several vaccines based on the concept of stabilizing the F protein in the prefusion conformation (pre-F) to retain the most neutralization-sensitive antigenic sites are currently in clinical evaluation. The pre-F subunit vaccine DS-Cav1 potently boosted neutralizing activity in healthy adults at doses of 50 and 150 mcg with and without alum adjuvant, yet the benefits of further increasing antigen dose and inclusion of alum adjuvant are unknown. More critically, the durability of DS-Cav1-boosted immunity and the impact of the dose and adjuvant on longitudinal RSV neutralizing activity have not been determined. We searched Pubmed and Google Scholar on January 2, 2021 for clinical trial reports addressing the durability of immune responses to stabilized pre-F subunit vaccines using the following search strategy: RSV AND clinical trial AND Pre-F subunit AND durability. We found no primary data reporting the durability of neutralizing activity elicited by a structurally-defined, stabilized pre-F subunit vaccine in humans.
Added value of this study
In this study, we delineate the immunogenicity of the DS-Cav1 subunit vaccine at doses up to 500 mcg, as well as the acute and longitudinal impact of altering dose, inclusion of the alum adjuvant, and administration of a second immunization at week 12. We demonstrate sustained neutralizing activity following DS-Cav1 vaccination and determine that, while increases in dose or a second immunization have a modest transient effect, they do not further improve the maintenance of serum neutralizing activity. Finally, we show increased levels of F-specific antibody in the mucosa of the upper respiratory tract after DS-Cav1 immunization, indicating more RSV-specific immunity at the site of infection.
Implications of all the available evidence
We provide timely results anticipating the outcome of ongoing efficacy trials in the rapidly evolving field of RSV vaccines. Particularly for the vaccination of healthy pregnant women, our findings predict that the use of higher doses of antigen and adjuvant are not necessary to provide sustained neutralizing activity conferred by optimized, pre-F stabilized immunogens.
Acknowledgements
We thank the vaccine trial participants for their contribution and commitment to vaccine research. We thank Clare Whittaker, Christopher Moore, John Rathmann, and Giune Padilla for assay setup. The following reagent was obtained through BEI Resources, NIAID, NIH: Panel of Human Antiserum and Immune Globulin to Respiratory Syncytial Virus, NR-32832. We also thank our colleagues at the US National Institutes of Health Clinical Center and National Institute of Allergy and Infectious Diseases (NIAID) for their contributions, including the NIAID Institutional Review Board, the Emmes Corporation, and colleagues at the NIAID Vaccine Research Centre, including the Vaccine Clinical Materials Program and VRC Pilot Plant. This work was supported with intramural funding from the National Institutes of Allergy and Infectious Diseases.
Footnotes
Declaration of Interests
M.C. and B.S.G. are inventors on patents for the stabilization of the RSV F protein. The other authors declared no conflicts of interest.
Data Sharing
Qualified researchers can request access to the raw data and clinical study protocol with amendments. Study documents will be redacted to protect the privacy of trial participants. For access, please contact the corresponding authors. Approvals are at the discretion of the authors.
References
- 1.Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390(10098): 946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352(17): 1749–59. [DOI] [PubMed] [Google Scholar]
- 3.Shi T, Denouel A, Tietjen AK, et al. Global Disease Burden Estimates of Respiratory Syncytial Virus-Associated Acute Respiratory Infection in Older Adults in 2015: A Systematic Review and Meta-Analysis. J Infect Dis 2019. [DOI] [PubMed] [Google Scholar]
- 4.Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89(4): 422–34. [DOI] [PubMed] [Google Scholar]
- 5.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol 1969; 89(4): 449–63. [DOI] [PubMed] [Google Scholar]
- 6.Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol 1969; 89(4): 435–48. [DOI] [PubMed] [Google Scholar]
- 7.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 1969; 89(4): 405–21. [DOI] [PubMed] [Google Scholar]
- 8.Mazur NI, Higgins D, Nunes MC, et al. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis 2018; 18(10): e295–e311. [DOI] [PubMed] [Google Scholar]
- 9.McLellan JS, Chen M, Joyce MG, et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 2013; 342(6158): 592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crank MC, Ruckwardt TJ, Chen M, et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science 2019; 365(6452): 505–9. [DOI] [PubMed] [Google Scholar]
- 11.Phung E, Chang LA, Morabito KM, et al. Epitope-Specific Serological Assays for RSV: Conformation Matters. Vaccines (Basel) 2019; 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Habibi MS, Jozwik A, Makris S, et al. Impaired Antibody-mediated Protection and Defective IgA B-Cell Memory in Experimental Infection of Adults with Respiratory Syncytial Virus. Am J Respir Crit Care Med 2015; 191(9): 1040–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vissers M, Ahout IML, de Jonge MI, Ferwerda G. Mucosal IgG Levels Correlate Better with Respiratory Syncytial Virus Load and Inflammation than Plasma IgG Levels. Clin Vaccine Immunol 2015; 23(3): 243–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagga B, Cehelsky JE, Vaishnaw A, et al. Effect of Preexisting Serum and Mucosal Antibody on Experimental Respiratory Syncytial Virus (RSV) Challenge and Infection of Adults. J Infect Dis 2015; 212(11): 1719–25. [DOI] [PubMed] [Google Scholar]
- 15.Jt Mills, Van Kirk JE Wright PF, Chanock RM. Experimental respiratory syncytial virus infection of adults. Possible mechanisms of resistance to infection and illness. J Immunol 1971; 107(1): 123–30. [PubMed] [Google Scholar]
- 16.Walsh EE, Falsey AR. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis 2004; 190(2): 373–8. [DOI] [PubMed] [Google Scholar]
- 17.Falloon J, Yu J, Esser MT, et al. An Adjuvanted, Postfusion F Protein-Based Vaccine Did Not Prevent Respiratory Syncytial Virus Illness in Older Adults. J Infect Dis 2017; 216(11): 1362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falloon J, Ji F, Curtis C, et al. A phase 1a, first-in-human, randomized study of a respiratory syncytial virus F protein vaccine with and without a toll-like receptor-4 agonist and stable emulsion adjuvant. Vaccine 2016; 34(25): 2847–54. [DOI] [PubMed] [Google Scholar]
- 19.Leroux-Roels G, De Boever F, Maes C, Nguyen TL, Baker S, Gonzalez Lopez A. Safety and immunogenicity of a respiratory syncytial virus fusion glycoprotein F subunit vaccine in healthy adults: Results of a phase 1, randomized, observer-blind, controlled, dosage-escalation study. Vaccine 2019; 37(20): 2694–703. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham AL, Lal H, Kovac M, et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N Engl J Med 2016; 375(11): 1019–32. [DOI] [PubMed] [Google Scholar]
- 21.Ruckwardt TJ, Morabito KM, Graham BS. Immunological Lessons from Respiratory Syncytial Virus Vaccine Development. Immunity 2019; 51(3): 429–42. [DOI] [PubMed] [Google Scholar]
- 22.Ruzin A, Pastula ST, Levin-Sparenberg E, et al. Characterization of circulating RSV strains among subjects in the OUTSMART-RSV surveillance program during the 2016–17 winter viral season in the United States. PLoS One 2018; 13(7): e0200319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin MP, Yuan Y, Takas T, et al. Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants. N Engl J Med 2020; 383(5): 415–25. [DOI] [PubMed] [Google Scholar]
- 24.Chu HY, Steinhoff MC, Magaret A, et al. Respiratory syncytial virus transplacental antibody transfer and kinetics in mother-infant pairs in Bangladesh. J Infect Dis 2014; 210(10): 1582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madhi SA, Polack FP, Piedra PA, et al. Respiratory Syncytial Virus Vaccination during Pregnancy and Effects in Infants. N Engl J Med 2020; 383(5): 426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochola R, Sande C, Fegan G, et al. The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLoS One 2009; 4(12): e8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruckwardt TJ, Morabito KM, Graham BS. Determinants of early life immune responses to RSV infection. Curr Opin Virol 2016; 16: 151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambert L, Sagfors AM, Openshaw PJM, Culley FJ. Immunity to RSV in Early-Life. Front Immunol 2014; 5: 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375(9725): 1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadoff J, De Paepe E, Haazen W, et al. Safety and Immunogenicity of the Ad26.RSV.preF Investigational Vaccine Coadministered With an Influenza Vaccine in Older Adults. J Infect Dis 2020. [DOI] [PubMed] [Google Scholar]
- 31.Lamprecht CL, Krause HE, Mufson MA. Role of maternal antibody in pneumonia and bronchiolitis due to respiratory syncytial virus. J Infect Dis 1976; 134(3): 211–7. [DOI] [PubMed] [Google Scholar]
- 32.Ogilvie MM, Vathenen AS, Radford M, Codd J, Key S. Maternal antibody and respiratory syncytial virus infection in infancy. J Med Virol 1981; 7(4): 263–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.