Abstract
Background:
Some patients with heart failure (HF) progress to advanced HF, characterized by debilitating HF symptoms refractory to therapy. There are limited data on the epidemiology and outcomes of patients with advanced HF.
Objectives:
To evaluate the prevalence, characteristics, and outcomes of patients with advanced HF in a geographically defined population.
Methods:
This was a population-based cohort study of all Olmsted County, Minnesota adults with and without HF 2007–2017. The 2018 ESC advanced HF diagnostic criteria were operationalized and applied to all patients with HF. Hospitalization and mortality in advanced HF, overall and by EF type (reduced EF <40% [HFrEF], mid-range EF 40–49% [HFmrEF], preserved EF ≥50% [HFpEF]) were examined using Andersen-Gill and Cox models.
Results:
Of 6836 adults with HF, 936 (13.7%) met criteria for advanced HF. The prevalence of advanced HF increased with age and was higher in men. At advanced HF diagnosis, 396 (42.3%) patients had HFrEF, 134 (14.3%) had HFmrEF, and 406 (43.4%) had HFpEF. The median (IQR) time from advanced HF diagnosis to death was 12.2 (3.7–29.9) months. The mean rate of hospitalization was 2.91 (95% CI 2.78–3.06) per person-year in the first year after advanced HF diagnosis. There were no differences in risks of all-cause mortality or hospitalization by EF. Patients with advanced HFpEF were at lower risk for cardiovascular mortality compared with advanced HFrEF (HR 0.79, 95% CI 0.65–0.97).
Conclusions:
In this population-based study, over half of patients with advanced HF had mid-range or preserved EF, and survival was poor regardless of EF.
Keywords: advanced heart failure, outcomes, mortality, hospitalization, epidemiology, population health
INTRODUCTION
More than 6 million Americans are currently living with heart failure (HF)(1), which is a chronic, progressive condition. However, many patients’ HF symptoms respond well to guideline-directed medical therapy, and their hospitalizations and deaths are driven by the presence of comorbid conditions.(2,3) Some patients with HF go on to develop advanced HF (also known as American College of Cardiology/ American Heart Association Stage D HF),(4) characterized by persistent and severe symptoms of HF despite optimized medical, surgical, and device therapy.(5) Patients with advanced HF may be eligible for life-prolonging therapies such as heart transplant and left ventricular assist device (LVAD), as well as other important interventions to alleviate symptom burden and ease suffering.
Despite the importance of advanced HF, our understanding of it is truncated due to challenges in identifying advanced HF in populations.(6,7) No single criterion for advanced HF exists. Diagnosing advanced HF requires severe cardiac dysfunction; episodes of congestion, low output or malignant arrhythmias; severe impairment of exercise; and severe and persistent symptoms of HF despite attempts to optimize clinical status.(8) Therefore, it is challenging to establish a case definition of advanced HF that can be applied to diverse populations without a thorough medical record review.(9)
As a result, we don’t know the prevalence of advanced HF or the population characteristics. Epidemiologic studies of HF have often not separated advanced HF from other patients with HF.(10–12) A single population-based study to parse out advanced HF only identified 5 patients with advanced HF.(13) Most of our knowledge of advanced HF comes from LVAD clinical trials and referral populations.(14–19) However, those patients may not be representative of the true advanced HF population, as cardiologist and HF specialist referral varies by patient characteristics,(20,21) resulting in a highly selected population of young, predominantly male, patients with advanced HF with reduced ejection fraction (EF, HFrEF).(19) We have very limited knowledge of the characteristics of patients with advanced HF with preserved EF (HFpEF) or mid-range EF (HFmrEF), populations which may have different risks of outcomes. To address these gaps in knowledge, we operationalized and systematically applied the 2018 European Society of Cardiology (ESC) advanced HF case definition(8) to a geographically defined population. In this manuscript, we report the characteristics and outcomes of patients with advanced HF in the community.
METHODS
Study Design and Setting.
This is a retrospective cohort study of adult residents of Olmsted County, Minnesota. Population-based research is possible because there are few healthcare providers; the largest of which is Mayo Clinic. Medical records from all sources of care are linked via the Rochester Epidemiology Project,(22,23) enabling longitudinal capture of care for the county’s residents. Patients were excluded from analysis if they declined to provide Minnesota Research Authorization (1.2% of potential patients excluded). This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Prevalent HF Cohort.
We developed a cohort of adults with prevalent HF from January 1, 2007 through December 31, 2017. We identified all patients with a billing code for HF (ICD-9 code 428 or ICD-10 code I50) in the inpatient or outpatient setting from 1998 forward. Medical records were reviewed to determine if the patient had a confirmed diagnosis of HF by a clinician and signs or symptoms of HF; the first date that occurred was documented. For those with billing codes prior to 2007, records were reviewed if the patient was still alive as of January 1, 2007.
Identifying Advanced HF.
We next applied the ESC definition of advanced HF(8) (Supplemental Table1) to the cohort to identify all patients with advanced HF in the study period. The ESC criteria include: 1) episodes of congestion, low output or malignant arrhythmias, 2) evidence of severe cardiac dysfunction, including severely reduced EF, isolated right ventricular failure, non-operable severe valvular heart disease, non-operable severe congenital heart disease, or severe diastolic dysfunction or LV structural abnormalities and preserved or mid-range EF, 3) severe impairment of exercise, and 4) severe and persistent symptoms of HF. To have advanced HF, a patient must meet all 4 criteria, despite attempts to optimize medical, surgical and device therapy.
This definition was operationalized to facilitate application. For criterion 1, we used billing codes to identify individuals in the prevalent HF cohort who had one or more hospitalizations or emergency department (ED) visits for HF or ventricular arrhythmias from initial HF diagnosis until death or last follow-up through December 31, 2018. Each hospitalization or ED visit was treated as a potential advanced HF index date. Next, each potential advanced HF index date was reviewed to determine if the patient met the other advanced HF criteria within 12 months. To apply criterion 2, transthoracic echocardiograms were reviewed to determine if there was evidence of severe cardiac dysfunction (see Supplemental Table 1 for definitions). Next, the medical records of patients with at least one event meeting the first two criteria were reviewed by an advanced HF cardiologist (SMD). Events were considered to meet criteria 3 (severe exercise impairment) if there was one or more of the following: 1) a low peak VO2 (≤14 mL/kg/min or ≤50% predicted for age and sex) on a cardiopulmonary exercise test, 2) short 6 minute walk test distance (≤300m), or patient-reported significant exercise limitations due to HF as documented in the medical record. Finally, all events meeting criteria 1–3 were reviewed to determine if the patient had evidence of severe and persistent symptoms of HF (New York Heart Association functional class IIIb or IV, Criteria 4), despite attempts to optimize. The first event that met all four criteria was the advanced HF index date.
Patient Characteristics.
Patient characteristics were obtained from the electronic medical record. For laboratory data, the value closest to the date of advanced HF was used within one year. Echocardiographic characteristics at the time of advanced HF were obtained from the transthoracic echocardiogram closest to the advanced HF index date and within one year in all cases. Echocardiograms were performed at Mayo Clinic and Olmsted Medical Center and read by cardiologists with expertise in echocardiography. Patients were classified as advanced HFrEF (EF<40%), HFmrEF (EF 40–49%) or HFpEF (EF≥50%)(24) based on the EF at advanced HF. EF at the time of first diagnosis of HF (where known) was obtained from the echocardiogram closest to that date.
Study Outcomes.
Data on all-cause hospitalizations in Olmsted County or at other Mayo Clinic sites were obtained using Rochester Epidemiology Project resources. For patients hospitalized at advanced HF index date, only subsequent hospitalizations were analyzed. In-hospital or inter-hospital transfers were considered a single hospitalization. Surveillance for death occurs via several methods. The Mayo Clinic registration office records deaths noted in clinical care and local obituaries. Death data are also obtained quarterly from the State of Minnesota Department of Vital and Health Statistics. Cardiovascular (CV) mortality was defined by death certificate cause of death ICD-10 codes I00-I99.
Statistical Analysis.
Baseline characteristics were summarized using mean (standard deviation, SD), median (interquartile range, IQR), and N (%) where appropriate. Differences by EF group were compared using ANOVA for continuous variables and chi square tests for binary variables. Annual period prevalence was calculated for advanced HF: 1) among patients with HF, and 2) among adults (≥18 years old) in the community. All advanced HF cases diagnosed within a given year in addition to cases diagnosed prior to that year and alive as of January 1st of that year were included in the numerator. For prevalence among patients with HF, the denominator was all HF cases still alive in the given year, including patients with newly diagnosed HF that year as well as those diagnosed with HF in prior years and alive as of January 1st. For prevalence among community adults, the denominator was adult Olmsted County residents alive that year. Poisson models were used to model the change in prevalence of advanced HF in the community in the study period; the unit of observation was advanced HF counts for each calendar year with the natural log of the number of adult Olmsted County residents as the offset. Poisson models were adjusted for age (linear and nonlinear terms) and sex; there was an interaction of year*age that was retained in the models.
Cox proportional hazards models were used to model survival after advanced HF diagnosis by EF. Andersen-Gill models(2,25), which analyze repeated events, were used to model risk of hospitalization (all-cause and HF-specific) by EF. Models were adjusted for age, sex, and Charlson comorbidity index.(26) Patients were censored from analysis at the time of LVAD, heart transplant, last follow-up, or death. All analyses were performed using SAS version 9.4. A P<0.05 was used as the level of significance.
RESULTS
Of 6836 adult residents of Olmsted County, Minnesota with HF in the study period, 936 (13.7%) had advanced HF (Figure 1). The annual prevalence (proportion of patients with) of advanced HF among all patients with HF ranged from 4.6 to 6.7% per year (median 5.3%, Figure 2). The proportion of community men (p=0.034) and women (p<0.001) whose HF was advanced increased over time (Figure 2A). The annual prevalence was lower than the overall prevalence due to the short survival once patients developed advanced HF, as discussed below. The annual age-adjusted prevalence of advanced HF among all adults in the county was higher in men than women and increased over time in both sexes (Figure 3A). The prevalence of advanced HF in the community was much higher in those 80 years and older compared with younger age groups (Figure 3B). Comparing 70 year olds across the study period, the prevalence of advanced HF increased by 9% per year (risk ratio [RR] 1.09, 95% CI 1.07–1.11); the prevalence increased by 8% per year (RR 1.08, 95% CI 1.06–1.09) for 80 year olds.
Figure 1. Study Flow Diagram.
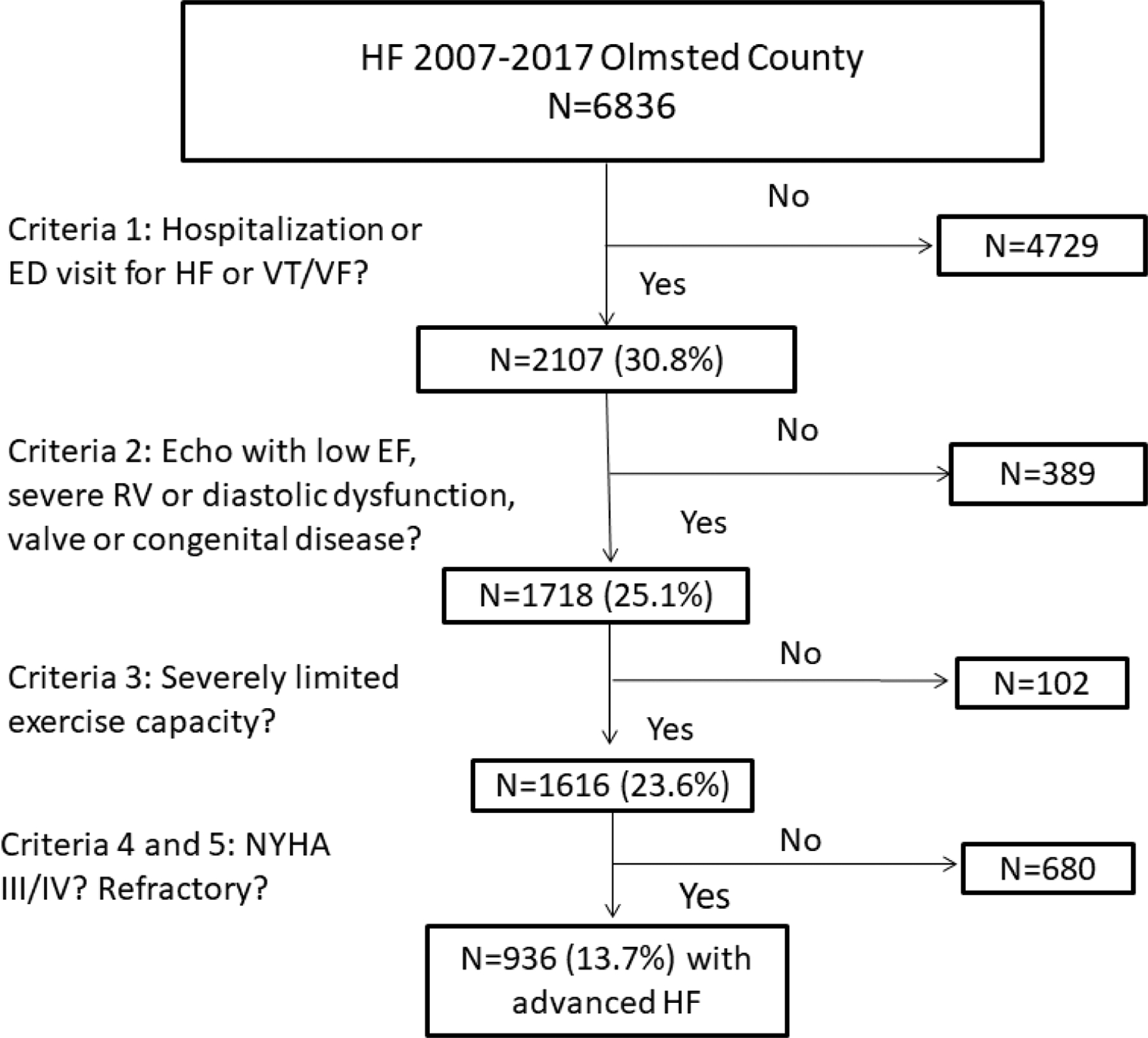
The number of patients meeting each of the European Society of Cardiology criteria for advanced heart failure is shown. ED=emergency department; EF= ejection fraction, HF= heart failure, NYHA= New York Heart Association, RV= right ventricular, VT/VF= ventricular tachycardia/ ventricular fibrillation
Figure 2. Prevalence of Advanced Heart Failure in Patients with Heart Failure.
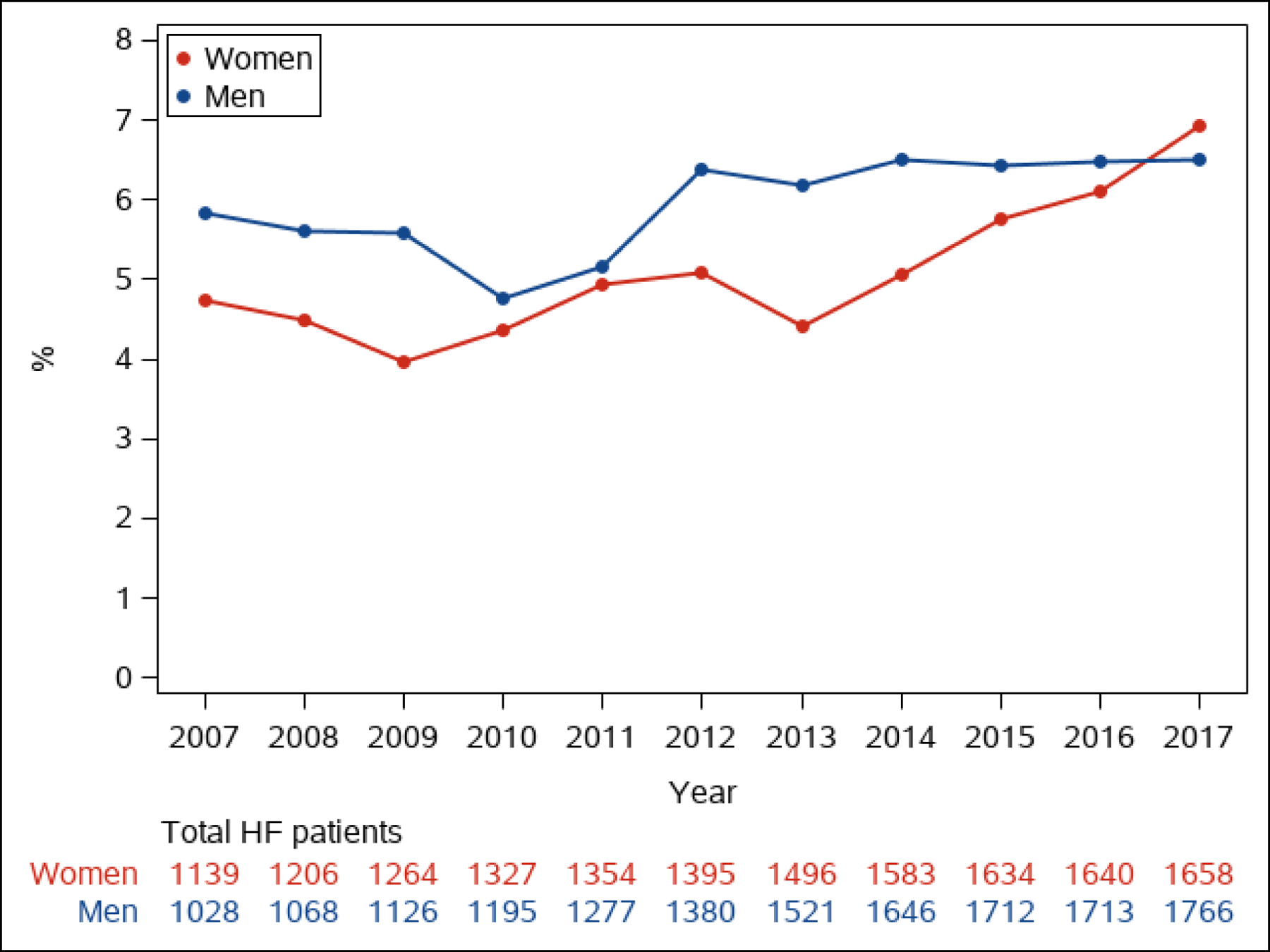
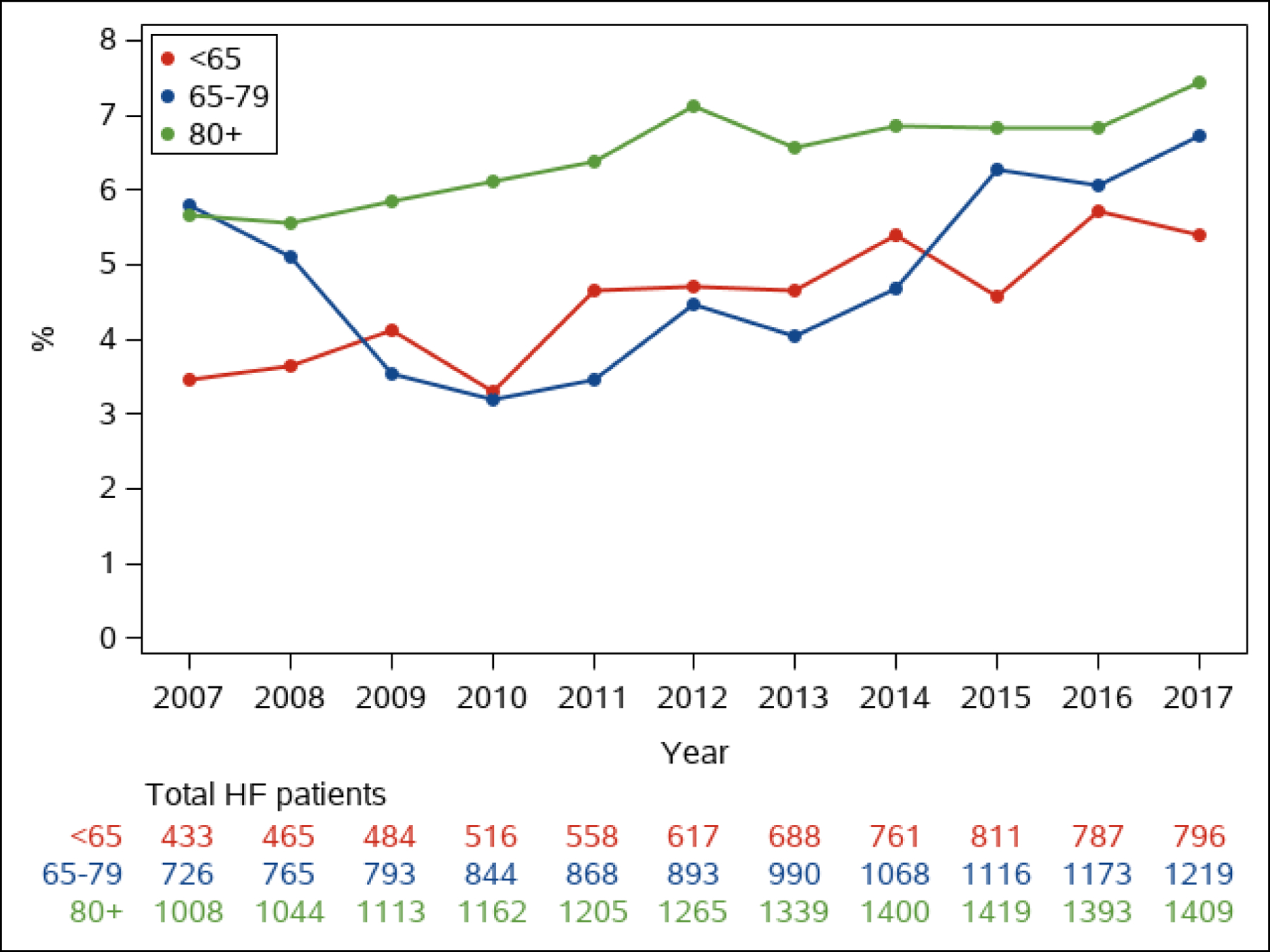
The annual proportion of patients whose HF is advanced is shown in men and women (Figure 2A) and by age group (<65, 65–79, 80+ years old, Figure 2B)
Figure 3. Prevalence Rates of Advanced Heart Failure Among Adults in the Community.
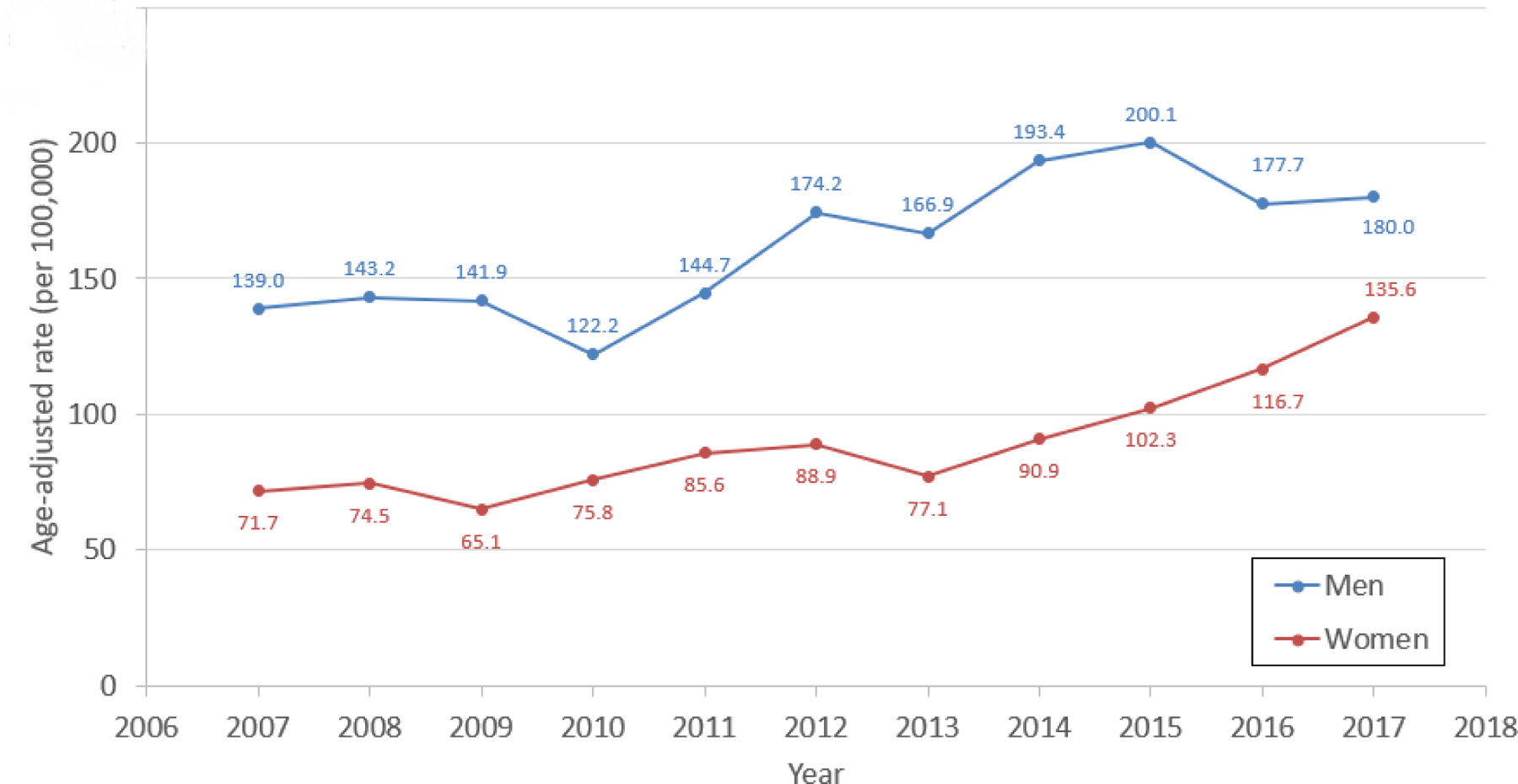
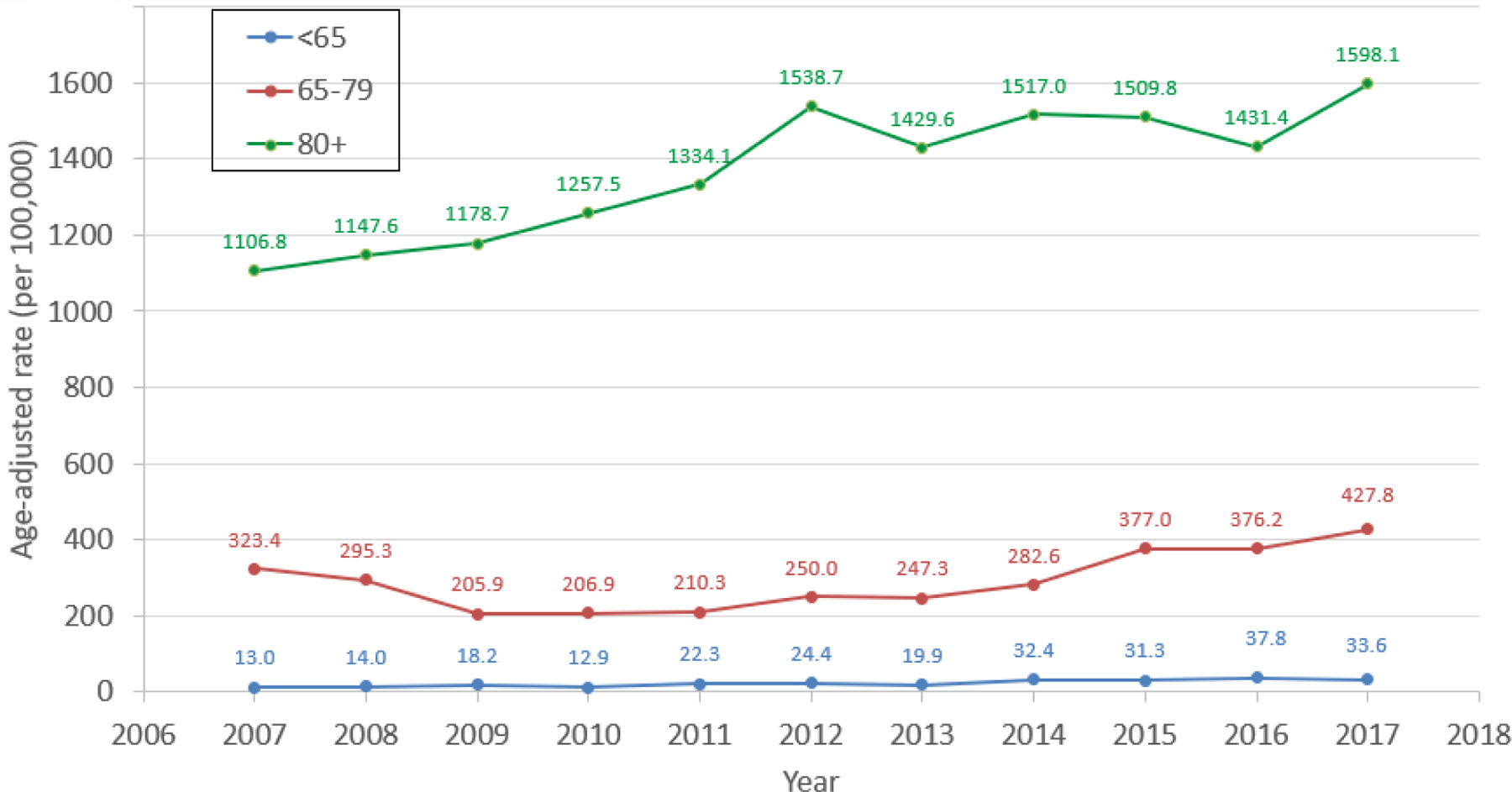
The annual rate of advanced HF among Olmsted County adults in the study period is shown. Figure 3A shows the age-adjusted prevalence rate of advanced HF in men and women, while Figure 3B shows the prevalence rate of advanced HF by age group (<65, 65–79, 80+ years old).
Advanced HF Patient Characteristics.
On average, patients with advanced HF were 77 years old, over half were men (55.5%), and comorbid conditions were common (Table 1). At advanced HF diagnosis, 396 (42.3%) patients had HFrEF, 134 (14.3%) had HFmrEF, and 406 (43.4%) had HFpEF. Most patients with advanced HFrEF also had low EF (<40%) at initial HF diagnosis (67.7%); similarly, most patients with advanced HFpEF had preserved EF (≥50%) at initial HF diagnosis. However, only 38.3% of those with advanced HFmrEF had mid-range EF (40–49%) at initial HF diagnosis, while 22.4% had EF<40%, and 39.3% had EF≥50%. Patients with advanced HFrEF were younger (mean age 73.9 vs. 78.6 years), more often male (72.2% vs. 38.4%), less often obese (39.0% vs. 47.3%), more often had CAD (77.8% vs. 59.9%), and less often had chronic obstructive pulmonary disease (50.3% vs. 66.0%) than those with advanced HFpEF. Patients with HFmrEF were the oldest subgroup (mean 80.4 years), and had the numerically highest prevalence of all forms of vascular disease. All three EF subgroups had about one-quarter of patients with severely reduced renal function (estimated glomerular filtration rate <30 mL/min).
Table 1.
Characteristics of Patients with Advanced Heart Failure
| Overall Advanced HF (N=936) | Advanced HFrEF (N=396) | Advanced HFmrEF (N=134) | Advanced HFpEF (N=406) | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||
| Age, mean (SD) | 76.9 (14.6) | 73.9 (15.9) | 80.4 (10.9) | 78.6 (13.8) | <0.001 | ||||
| Male, n (%) | 519 (55.5) | 286 (72.2) | 77 (57.5) | 156 (38.4) | <0.001 | ||||
| White Race, n (%) | 876 (93.7) | 367 (92.9) | 132 (98.5) | 377 (92.9) | 0.46 | ||||
| Hispanic ethnicity, n (%) | 15 (1.6) | 4 (1.0) | 4 (3.0) | 7 (1.7) | 0.28 | ||||
| Married/partner, n (%) | 438 (50.8) | 217 (60.1) | 60 (47.2) | 161 (42.9) | <0.001 | ||||
| Comorbidities a | |||||||||
| Obese (≥30), n (%) | 401 (43.3) | 152 (39.0) | 58 (43.6) | 191 (47.3) | |||||
| Hypertension, n (%) | 829 (88.6) | 326 (82.3) | 127 (94.8) | 376 (92.6) | <0.001 | ||||
| Hyperlipidemia, n (%) | 684 (73.1) | 280 (70.7) | 107 (79.9) | 297 (73.2) | 0.12 | ||||
| CAD, n (%) | 661 (70.6) | 308 (77.8) | 110 (82.1) | 243 (59.9) | <0.001 | ||||
| Diabetes, n (%) | 415 (44.3) | 164 (41.4) | 66 (49.3) | 185 (45.6) | 0.23 | ||||
| COPD, n (%) | 537 (57.4) | 199 (50.3) | 70 (52.2) | 268 (66.0) | <0.001 | ||||
| PVD, n (%) | 487 (52.0) | 191 (48.2) | 79 (59.0) | 217 (53.5) | 0.08 | ||||
| CVD, n (%) | 217 (23.2) | 82 (20.7) | 37 (27.6) | 98 (24.1) | 0.22 | ||||
| Dementia, n (%) | 65 (6.9) | 23 (5.8) | 10 (7.5) | 32 (7.9) | 0.50 | ||||
| Depression, n (%) | 257 (27.5) | 91 (23.0) | 43 (32.1) | 123 (30.3) | 0.03 | ||||
| Charlson index, mean (SD) | 5.0 (2.5) | 4.8 (2.5) | 5.5 (2.4) | 5.0 (2.5) | 0.02 | ||||
| Medications at Advanced HF | |||||||||
| ACE/ARB/ARNI | 576 (61.5) | 264 (66.7) | 84 (62.7) | 228 (56.2) | 0.036 | ||||
| Beta blocker | 699 (74.7) | 294 (74.2) | 112 (82.6) | 293 (72.3) | 0.030 | ||||
| MRA | 146 (15.6) | 75 (18.9) | 20 (14.9) | 51 (12.6) | 0.044 | ||||
| Labs | |||||||||
| <134, n (%) | 163 (17.4) | 61 (15.4) | 27 (20.2) | 75 (18.5) | |||||
| Creatinine, mean (SD) | 1.59 (0.97) | 1.64 (1.05) | 1.62 (0.98) | 1.53 (0.89) | 0.21 | ||||
| ≥60, n (%) | 263 (28.1) | 123 (31.1) | 37 (27.6) | 103 (25.4) | |||||
| B-type natriuretic peptide, median (IQR)b | 1011 (532, 1671) | 1099 (625, 1783) | 1510 (763, 2110) | 743 (309, 1227) | <0.001 | ||||
| NT-pro-BNP, median (IQR)b | 5699 (2841, 12740) | 8334 (3942, 15917) | 5610 (2964, 15028) | 4258 (2166, 8714) | <0.001 | ||||
| Anemia, n (%) | 664 (70.9) | 266 (67.2) | 103 (76.9) | 295 (72.7) | 0.06 | ||||
| >1.5, n (%) | 82 (10.2) | 52 (14.4) | 8 (7.1) | 22 (6.7) | |||||
| <3.3, n (%) | 248 (32.0) | 97 (29.4) | 50 (42.7) | 101 (30.7) | |||||
| Echo Criteria for Advanced HF | |||||||||
| Moderate or more RV dysfunction, n (%) | 294 (31.4) | 174 (43.9) | 32 (23.9) | 88 (21.7) | <0.001 | ||||
| Severe valvular stenosis/ regurgitation, n(%) | 227 (24.3) | 97 (24.5) | 26 (19.4) | 104 (25.6) | 0.34 | ||||
| Severe diastolic dysfunction criteriac, n(%) | 706 (75.4) | 276 (69.7) | 117 (87.3) | 313 (77.1) | <0.001 | ||||
| Severe congenital heart disease, n (%) | 14 (1.5) | 5 (1.3) | 3 (2.2) | 6 (1.5) | 0.72 | ||||
| ≥50%, n (%) | 387 (52.4) | 50 (16.8) | 42 (39.3) | 295 (88.3) | |||||
ACE/ARB/ARNI= angiotensin converting enzyme inhibitor/ angiotensin receptor blocker/ angiotensin receptor neprilysin inhibitor, BMI= body mass index, CAD= coronary artery disease, COPD= chronic obstructive pulmonary disease, CVD= cerebrovascular disease, eGFR= estimated glomerular filtration rate, HFmrEF= heart failure with mid-range ejection fraction, HFrEF= heart failure with reduced ejection fraction, HFpEF= heart failure with preserved ejection fraction, IQR= interquartile range, PVD= peripheral vascular disease, MRA= mineralocorticoid receptor antagonist, RV= right ventricular, SD= standard deviation
Comorbidities are defined as having 2 codes >30 days apart during the 5 year prior to advanced HF diagnosis. Hypertension, hyperlipidemia, CAD, and depression are based on DHHS code sets; diabetes, COPD, PVD, CVD, and dementia are based on Charlson code sets.
Missing data: 9 patients were missing BMI, 133 bilirubin, 160 albumin, 194 NTproBNP, 715 BNP, 126 missing EF at incident HF
See supplemental Table 1 for severe diastolic dysfunction criteria used to define advanced HF
Echocardiographic Data in Advanced HF.
Echocardiographic data at the time of advanced HF is shown in Supplemental Table 2. Moderate and severe right ventricular dysfunction were more common in advanced HFrEF than the other two EF subtypes. Approximately one-quarter of patients had at least moderate mitral regurgitation (26.0%) and nearly half had at least moderate tricuspid regurgitation (41.2%). Significant mitral regurgitation was more common in advanced HFrEF (34.1%) compared with HFmrEF (23.1%) and HFpEF (19.0%).
LVAD and Heart Transplantation.
A total of 31 (3.3%) patients received an LVAD and 27 (2.9%) patients underwent heart transplantation during follow-up. The mean (SD) age at LVAD implantation was 53.4 (16.7) years and 24 (77.4%) were men. Patients undergoing heart transplant were slightly younger (mean [SD] age 47.2 [15.2]) and 25 (92.6%) were men.
Survival After Advanced HF Diagnosis.
Excluding those who received heart transplant or LVAD, 86% of patients with advanced HF died during follow-up. Mortality was very high in patients who met all ESC criteria for advanced HF (1-year mortality 49.9%, 95% CI 46.5%–53.0%). By comparison, 1-year mortality in patients only meeting the first criteria (hospitalizations or ED visits for HF or ventricular arrhythmias was 25.3% (95% CI 23.4–27.2%), and was similar in the subset who also had echocardiographic evidence of severe cardiac dysfunction (25.0%, criteria 2), and severe exercise limitation (25.2%, criteria 3). The large step-up in mortality occurred in patients whose symptoms were refractory to attempts to optimize. The Kaplan Meier estimated median (IQR) time from advanced HF diagnosis until death was 12.2 (3.7–29.9) months. The median (IQR) survival was 12.0 (3.5–31.4) months in advanced HFrEF, 9.5 (3.0–24.7) months in advanced HFmrEF, and 13.4 (3.9–29.7) months in advanced HFpEF (log rank P=0.34, Central Illustration A). There were no significant differences in survival by EF subtype (adjusted HR 1.00, 95% CI 0.81–1.24 for HFmrEF, HR 0.99, 95% CI 0.84–1.16 for HFpEF, compared with HFrEF, P=0.99, Supplemental Table 3). However, patients with advanced HFpEF were at lower risk for CV mortality compared with those with advanced HFrEF (adjusted HR 0.79, 95% CI 0.65–0.97).
Central Illustration. Survival and Hospitalizations Following Advanced Heart Failure.
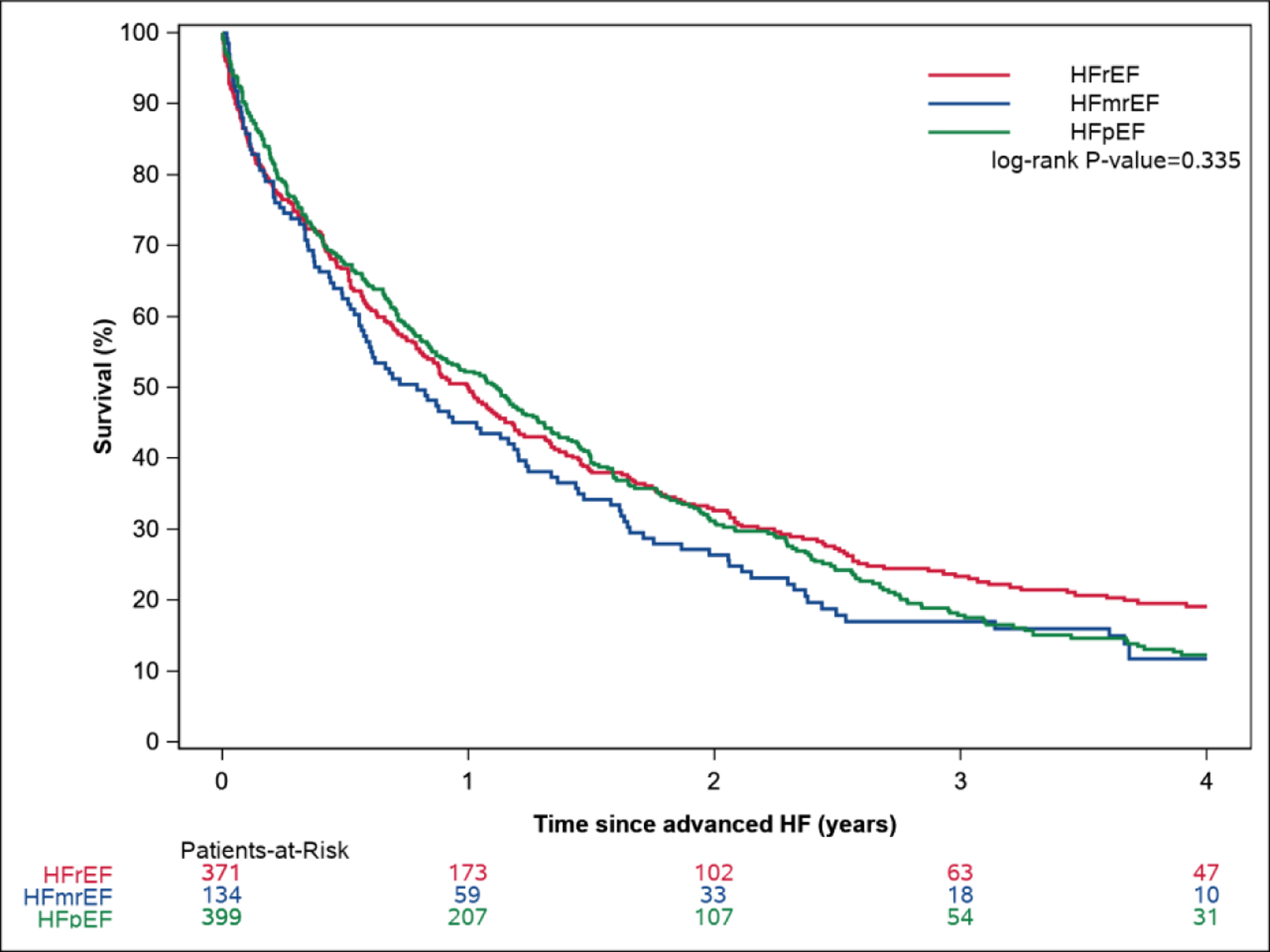
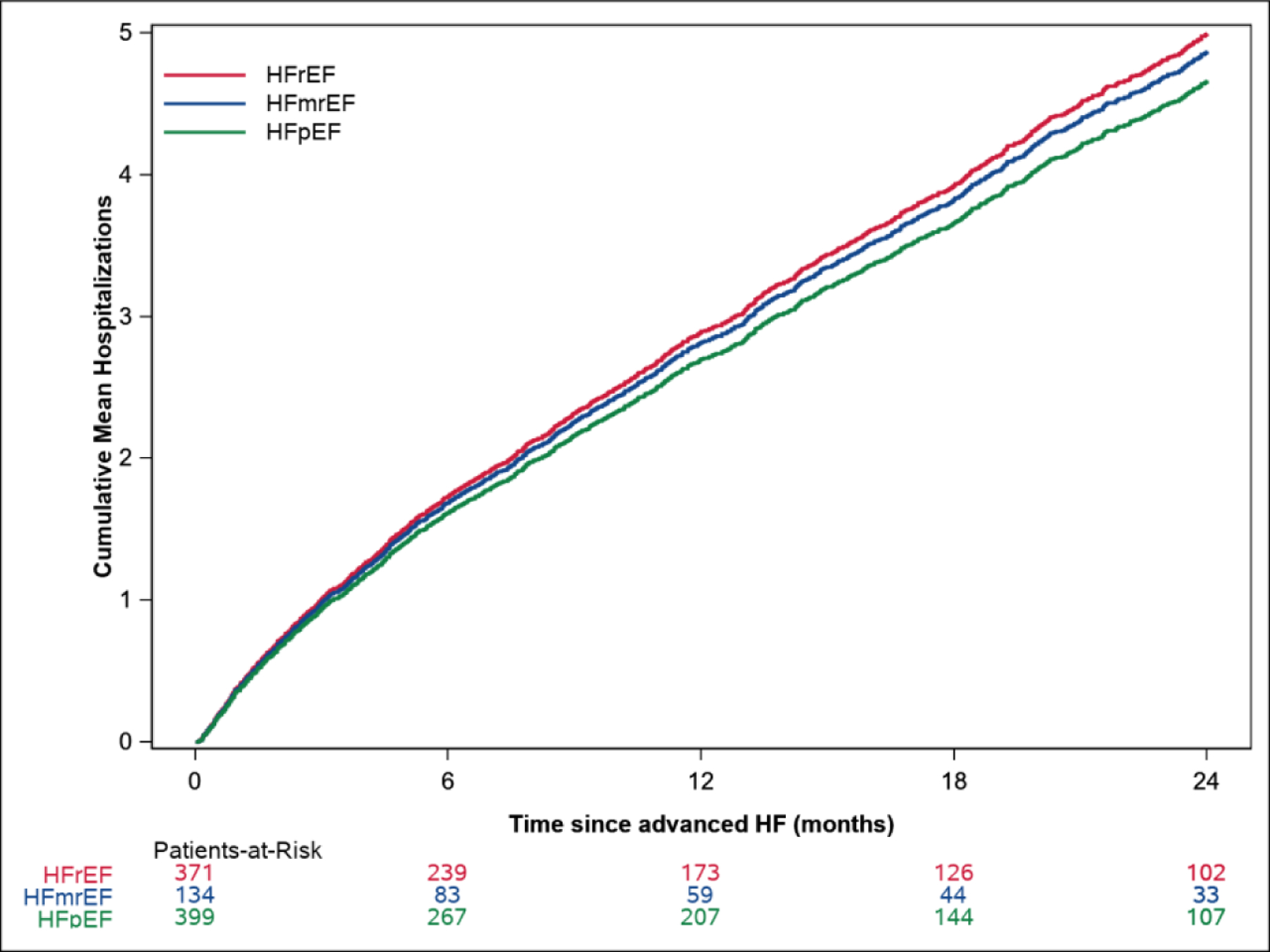
The Kaplan Meier survival curves by EF are shown in Central Illustration A. Mean cumulative hospitalizations after advanced HF by EF are shown in Central Illustration B.
Hospitalizations After Advanced HF Diagnosis.
Following advanced HF diagnosis, a total of 3138 all-cause hospitalizations, including 1008 (32.1%) with a primary diagnosis of HF, were observed. Other than HF, the most common causes of hospitalization were pneumonia/bronchitis, respiratory failure, sepsis, gastrointestinal issues, arrhythmia, coronary artery disease and acute kidney injury (Supplemental Table 4). Cumulative all-cause hospitalizations in advanced HF by EF are shown in Central Illustration B. The mean rate of all-cause hospitalization was 2.91 (95% CI 2.78–3.06) per person-year of follow-up in the first year after advanced HF diagnosis. There were no significant differences in the risks of all-cause or HF hospitalization by EF subtype (adjusted HR for all-cause hospitalization 0.98, 95% CI 0.77–1.23 for HFmrEF, HR 0.97, 95% CI 0.82–1.15 for HFpEF, compared with HFrEF, P=0.94, Supplemental Table 3).
DISCUSSION
In this comprehensive, large community cohort with longitudinal follow-up, we found that 13.7% of patients with HF had advanced HF at some point in the study period, and advanced HF was most common in the elderly. There were key differences in demographics, comorbid conditions, and cardiac structural and functional characteristics in patients with advanced HFrEF, HFmrEF, and HFpEF. However, despite these differences, mortality and hospitalization risks were similar. Half of patients with advanced HF died within one year. Survivors usually required multiple hospital admissions per year, and one-third of hospitalizations were for HF.
It has been speculated that anywhere from 5–25% of patients with HF have advanced HF,(6,9) though the true prevalence was unknown. In a 2004 screening study of a random sample of adults 45 years and older, 244 of 2029 (12.0%) participants had symptomatic HF, and of those, only 5 (2.0% of those with HF and 0.25% of the overall sample) were categorized as advanced HF.(13) This study was not powered to identify advanced HF, which was defined using responses to an exercise questionnaire. In a HF registry, 3.9% of patients were identified to have refractory symptoms on oral medical therapy and recent hospitalizations for HF.(19) In patients with symptomatic HF cared for in a heart failure clinic, 4.5% progressed to advanced HF annually.(18) After rigorously applying the ESC advanced HF definition,(8) we found that, in a given year, 5.1–7.7% of patients with HF had advanced HF. Furthermore, the prevalence of advanced HF among adults in the community was approximately 125 per 100,000 (0.125%) in women and 200 per 100,000 (0.2%) in men in recent years. Extrapolating these data to the U.S. adult (209 million) and HF (6.2 million(1)) populations, we would expect that approximately 300,000–400,000 adult Americans have advanced HF. The prevalence of advanced HF is particularly high in those 80 years and older, where more than 1000 of every 100,000 in the community (>1%) have advanced HF.
There are limited data on the prevalence or characteristics of patients with advanced HFmrEF and advanced HFpEF. In a registry of patients with advanced HF from 2003–2006, the vast majority of patients had reduced EF; only 25% had an EF>38%.(19) As advanced HF research has primarily focused on the HFrEF population, it is important to note that we found advanced HFpEF (43% of patients) to be just as common as advanced HFrEF (42% of patients). The EF distribution in our study closely resembles that reported in Medicare beneficiaries hospitalized with HF.(27) We also found that the vast majority of those with advanced HFpEF had preserved EF since incident HF diagnosis, whereas advanced HFmrEF included a substantial proportion of patients that started out with preserved EF. This aligns with our prior observation that, on average, EF declines over time in HFpEF.(28) While demographic features varied by EF, including that advanced HFrEF patients were younger and more often male compared with HFmrEF and HFpEF, in many ways the three populations were similar. The prevalence of some comorbidities such as renal dysfunction and diabetes were comparable in all EF groups. While most advanced HF therapies have been best studied in patients with HFrEF,(4,5) our finding that advanced HFmrEF and HFpEF comprise over half of patients with advanced HF underscores the need for further investigation of effective therapies for these patients.
In the absence of use of life-prolonging therapies such as LVAD and heart transplant, outcomes in advanced HF have been poor. Only a small proportion of patients with advanced HF received LVAD and heart transplant in our study. This may reflect the underlying demographics (mean age 77 years) and comorbidity burden, but also underscores the reality that in the United States each year, of the more than 6 million individuals with HF, only around 2000 patients receive heart transplants (0.03% of those with HF) with slightly more receiving LVADs (n=2639 in 2019(29)). In a landmark LVAD trial, only 25% of patients with advanced HF and severely reduced EF (<25%) who were randomized to medical therapy survived one year.(16) More recent data from patients treated with intravenous inotropes as palliation has suggested that survival is over 50% at one year.(30) However, these studies included selected patients with HFrEF treated with advanced therapies. In our study including patients with a wide range of EF, we found that half of patients with advanced HF died within one year and only about one-quarter survived more than two years. No difference in risks of hospitalization or death by EF subtype was observed. These findings confirm that patients with advanced HF identified using the ESC criteria are very high risk for adverse outcomes, regardless of EF. Further work is needed to develop electronic algorithms to identify patients with advanced HF that can be more easily applied to populations. In addition, given the very poor survival after the onset of advanced HF, optimizing use of GDMT early in the HF trajectory is paramount to preventing the development of advanced HF and improving outcomes. Trials that compare the effectiveness of novel approaches to optimize use of GDMT in patients with newly diagnosed HF are needed.
Limitations and Strengths.
There are limitations to acknowledge to aid in interpretation of these data. First, application of the advanced HF definition relied upon testing and documentation available in the medical record, which could lead to misclassification. However, the geographic isolation of the population and REP infrastructure enabled comprehensive capture of testing and care that occurred. Second, the characteristics and outcomes of advanced HF may differ in populations of varying racial and ethnic diversity. The prevalence of certain etiologies of advanced HF, including cardiac amyloidosis, require further evaluation. Finally, while other definitions of advanced HF exist,(4,5) their criteria overlap with the ESC definition,(8) which is the most contemporary and relies on use of objective and routinely collected information. However, there are several notable strengths. In comparison to some national HF registries,(31) which are largely cross-sectional, we followed individuals longitudinally and obtained detailed phenotypic and outcomes data. Furthermore, we were able to identify the subset of individuals with advanced HF by operationalizing and rigorously applying an advanced HF definition to a geographically defined population.
Conclusions
These results provide essential knowledge of the epidemiology and outcomes of patients with advanced HF, including describing differences by EF. Our findings indicate that advanced HF often occurs in patients with HFmrEF and HFpEF, and is associated with poor prognosis, regardless of EF.
Supplementary Material
PERSPECTIVES:
Competency in Medical Knowledge:
Advanced heart failure often occurs in patients with mid-range and preserved ejection fraction. Half of patients with advanced heart failure die within one year.
Translational Outlook 1:
Once patients develop advanced heart failure, outcomes are poor. Future studies to compare the effectiveness of novel approaches to optimize use of guideline-directed medical therapy in patients with newly diagnosed heart failure are needed to prevent the onset of advanced heart failure.
Translational Outlook 2:
Identification of patients with advanced heart failure has been hindered by complex definitions that are difficult to apply to populations. Further work is needed to develop electronic algorithms to identify patients with advanced heart failure who may benefit from advanced therapies more easily.
Acknowledgments:
We would like to thank Ellen Koepsell RN, Janet Gatzke RN, and Christina Stenzel for their assistance with data collection.
Funding Sources:
This study was funded by the National Institutes of Health (R01 HL144529, PI: Dunlay) and made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health (R01 AG034676).
ABBREVIATIONS:
- CI
confidence interval
- ED
emergency department
- ESC
European Society of Cardiology
- HF
heart failure
- HFmrEF
heart failure with mid-range ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HR
hazard ratio
- LVAD
left ventricular assist device
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no relationships with industry to disclose.
REFERENCES
- 1.Virani SS, Alonso A, Benjamin EJ et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 2.Dunlay SM, Redfield MM, Weston SA et al. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol 2009;54:1695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail 2008;1:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 5.Fang JC, Ewald GA, Allen LA et al. Advanced (stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee. J Card Fail 2015;21:519–34. [DOI] [PubMed] [Google Scholar]
- 6.Abouezzeddine OF, Redfield MM. Who has advanced heart failure?: definition and epidemiology. Congest Heart Fail 2011;17:160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Truby LK, Rogers JG. Advanced Heart Failure: Epidemiology, Diagnosis, and Therapeutic Approaches. JACC Heart Fail 2020;8:523–536. [DOI] [PubMed] [Google Scholar]
- 8.Crespo-Leiro MG, Metra M, Lund LH et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018;20:1505–1535. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg B, Fang J, Mehra M, Stevenson LW. Advanced heart failure: Trans-Atlantic perspectives on the Heart Failure Association of the European Society of Cardiology position statement. Eur J Heart Fail 2018;20:1536–1539. [DOI] [PubMed] [Google Scholar]
- 10.Shah AM, Claggett B, Loehr LR et al. Heart Failure Stages Among Older Adults in the Community: The Atherosclerosis Risk in Communities Study. Circulation 2017;135:224–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xanthakis V, Enserro DM, Larson MG et al. Prevalence, Neurohormonal Correlates, and Prognosis of Heart Failure Stages in the Community. JACC Heart Fail 2016;4:808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gidding SS, Lloyd-Jones D, Lima J et al. Prevalence of American Heart Association Heart Failure Stages in Black and White Young and Middle-Aged Adults: The CARDIA Study. Circ Heart Fail 2019;12:e005730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ammar KA, Jacobsen SJ, Mahoney DW et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007;115:1563–70. [DOI] [PubMed] [Google Scholar]
- 14.Nieminen MS, Cleland JG, Eha J et al. Oral levosimendan in patients with severe chronic heart failure --the PERSIST study. Eur J Heart Fail 2008;10:1246–54. [DOI] [PubMed] [Google Scholar]
- 15.Packer M, Carver JR, Rodeheffer RJ et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med 1991;325:1468–75. [DOI] [PubMed] [Google Scholar]
- 16.Rose EA, Gelijns AC, Moskowitz AJ et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435–43. [DOI] [PubMed] [Google Scholar]
- 17.Yancy CW, Krum H, Massie BM et al. The Second Follow-up Serial Infusions of Nesiritide (FUSION II) trial for advanced heart failure: study rationale and design. Am Heart J 2007;153:478–84. [DOI] [PubMed] [Google Scholar]
- 18.Kalogeropoulos AP, Samman-Tahhan A, Hedley JS et al. Progression to Stage D Heart Failure Among Outpatients With Stage C Heart Failure and Reduced Ejection Fraction. JACC Heart Fail 2017;5:528–537. [DOI] [PubMed] [Google Scholar]
- 19.Costanzo MR, Mills RM, Wynne J. Characteristics of “Stage D” heart failure: insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM). Am Heart J 2008;155:339–47. [DOI] [PubMed] [Google Scholar]
- 20.Ansari M, Alexander M, Tutar A, Bello D, Massie BM. Cardiology participation improves outcomes in patients with new-onset heart failure in the outpatient setting. J Am Coll Cardiol 2003;41:62–8. [DOI] [PubMed] [Google Scholar]
- 21.Philbin EF, Jenkins PL. Differences between patients with heart failure treated by cardiologists, internists, family physicians, and other physicians: analysis of a large, statewide database. Am Heart J 2000;139:491–6. [DOI] [PubMed] [Google Scholar]
- 22.Melton LJ 3rd. History of the Rochester Epidemiology Project. Mayo Clinic proceedings 1996;71:266–74. [DOI] [PubMed] [Google Scholar]
- 23.Rocca WA, Peterson BJ, McDonnell SK et al. The Mayo Clinic family study of Parkinson’s disease: study design, instruments, and sample characteristics. Neuroepidemiology 2005;24:151–67. [DOI] [PubMed] [Google Scholar]
- 24.Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 25.Andersen PK, Gill RD. Cox’s regression model for counting processes: a large sample study. Ann Stat 1982;10:1100–1120. [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 27.Gupta A, Allen LA, Bhatt DL et al. Association of the Hospital Readmissions Reduction Program Implementation With Readmission and Mortality Outcomes in Heart Failure. JAMA Cardiol 2018;3:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail 2012;5:720–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teuteberg JJ, Cleveland JC Jr., Cowger J et al. The Society of Thoracic Surgeons Intermacs 2019 Annual Report: The Changing Landscape of Devices and Indications. Ann Thorac Surg 2020;109:649–660. [DOI] [PubMed] [Google Scholar]
- 30.Mody BP, Khan MH, Zaid S et al. Survival With Continuous Outpatient Intravenous Inotrope Therapy in the Modern Era. Am J Ther 2020. [DOI] [PubMed] [Google Scholar]
- 31.Fonarow GC. Improving quality of care and outcomes for heart failure. -Role of registries. Circ J 2011;75:1783–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


