Abstract
The insect renal (Malpighian) tubules are functionally homologous to the mammalian kidney. Accumulating evidence indicates that renal tubule crystals form in a manner similar to mammalian kidney stones. In Drosophila melanogaster, crystals can be induced by diet, toxic substances, or genetic mutations that reflect circumstances influencing or eliciting kidney stones in mammals. Incredibly, many mammalian proteins have distinct homologs in Drosophila, and the function of most homologs have been demonstrated to recapitulate their mammalian and human counterparts. Here, we discuss the present literature establishing Drosophila as a nephrolithiasis model. This insect model may be used to investigate and understand the etiology of kidney stone diseases, especially with regard to calcium oxalate, calcium phosphate and xanthine or urate crystallization.
Introduction
The Malpighian tubules (MTs) are the renal organ for insects. These tubules provide osmoregulation, electrolyte balance, and waste-elimination in a manner analogous to the mammalian kidney. These functional similarities allow the insect to serve as a dynamic in vivo model to experimentally probe many translational scenarios. Experimental studies have focused on the Drosophila melanogaster model because (a) ~70% of their genes have human homologs; (b) the entire genome has been sequenced and annotated for >20 years [1•]; (c) FlyBase curates Drosophila databases, education, resources and tools; and (d) several international consortia (e.g. VDRC, Bloomington, Berkeley) maintain mutants and RNAi fly lines for every expressed gene in the genome. Additionally, insect experimental models can be maintained with minimal costs and produced in rapid generation timelines (e.g. two weeks for F1 adult). With these numerous benefits, the Drosophila model is a valuable tool for elucidating renal function and renal diseases which are typically investigated in mammals.
Emerging evidence has revealed that insects produce renal crystals with remarkable similarity to mammalian kidney stones [2,3••,4••,5••]. Nephrolithiasis (i.e. kidney stones) affects approximately 10% of the United States population [6]. Additionally, nephrolithiasis is a care and longevity concern for companion animals [7,8•]. Presently, experiments on insect renal crystals have provided productive and translatable evidence for conditions already known to cause nephrolithiasis. Here, we review recent developments on renal crystals in Drosophila and reflect on potential avenues of research.
Malpighian tubule crystals
Nephrolithiasis describes the presence of stones and calcifications that appear specifically in the mammalian kidney; however, the term does not apply to crystals in insect tubules. As insect and mammalian renal organs differ, one cannot refer to insect renal tubules as nephrons or kidneys, nor MTs crystals as kidney stones. Thus, crystallization in insect MTs are noted as tubulolithiasis, and the resulting crystals will be termed tubuloliths.
In the Drosophila model of nephrolithiasis, tubuloliths appear within the MT lumen. Drosophila have anterior and posterior MT pairs with each pair meeting and emptying into the gut through a common ureter [9,10]. All tubules consist of a single layer epithelium made up of two cell types [2,11]: principal and stellate cells. Principal cells are the most abundant cell type within MTs and transport ions though proton gradients created by H+-ATPase on the apical and basolateral surfaces [12,13•]. The basolateral surface of principal cells also allows ion movement via Na+- and K+-ATPases and the Na+-dependent Cl−/HCO3− exchanger, NDAE1 [14•,15•]. Stellate cells are dispersed amongst the principal cells. The stellate cells express apical and basolateral transporters which allow for net salt movement from hemolymph to tubule lumen [13•,16••,17•]: aquaporins (Drip and Prip, respectively) and chloride transporters (secCl and Clc-a, respectively). Together the principal and stellate cells transport ions and water into the lumen which, under certain conditions, can promote solute precipitation and crystal, that is, tubulolith, formation.
Crystal accumulation within MTs is readily identified by various microscopic techniques. Calcium oxalate tubuloliths form as organized crystal lattice structures with birefringent properties making the crystals within dissected MTs easily visualized by polarized light microscopy [3••,4••,16••] (Figure 1a,b). In addition to light microscopy, crystal identification and quantification may be accomplished by scanning electron microscopy and energy dispersive X-ray spectroscopy [3••,4••,5••,16••]. Imaging of crystals in whole flies was shown using micro-computed tomography [4••]. The many analytical strategies and the simplicity for ex vivo assessments offer notable benefits of the Drosophila tubulolithiasis model.
Figure 1. Calcium oxalate (CaOx) crystallization in Drosophila renal tubules.
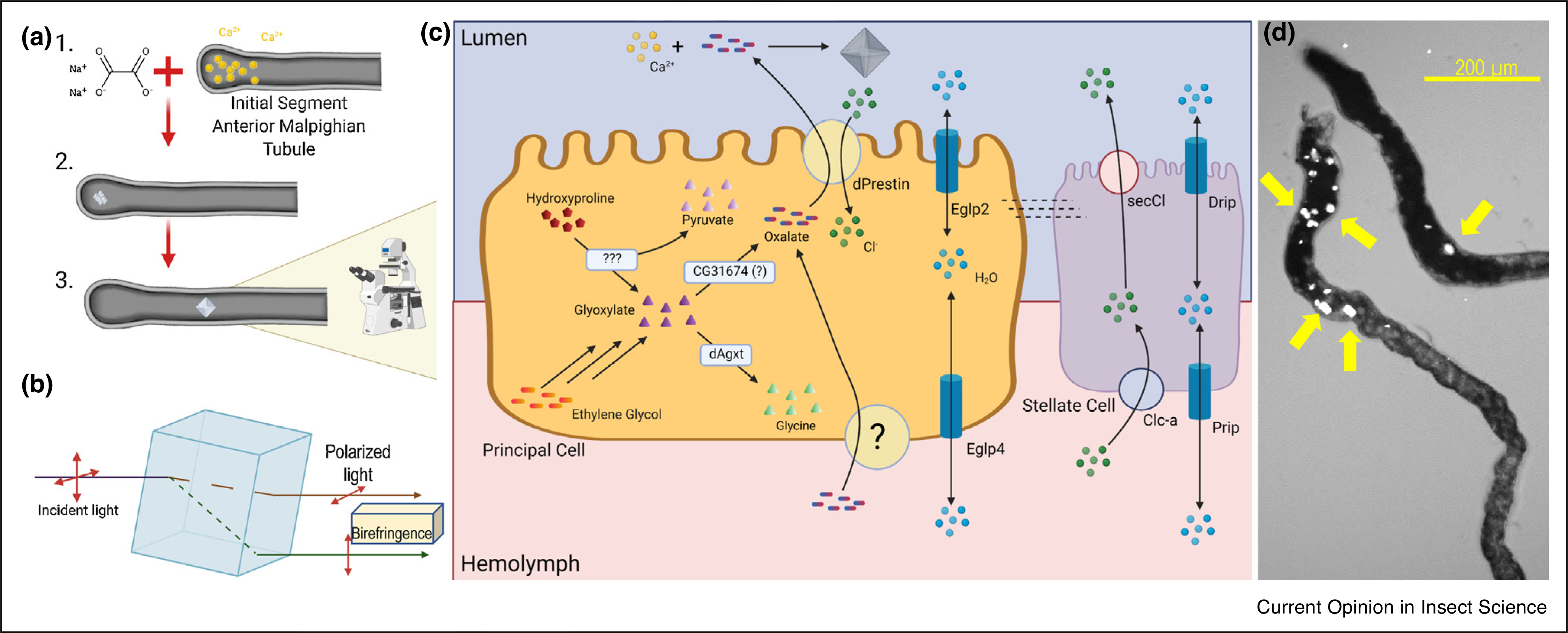
Crystals in Drosophila Malpighian tubules are birefringent and easily visualized under polarized light microscopy: (a) Calcium ions that are secreted into the lumen may form precipitates that evolve into crystals. (b) Diagram depicting how polarized light passes through molecular crystal lattice. (c) Model of CaOx crystal formation. Oxalate can be transported into the principal cells from the hemolymph and/or produced from hydroxyproline or ethylene glycol precursors. Both hydroxyproline and ethylene glycol are degraded into glyoxylates in multistep pathways with hydroxyproline degradation also producing pyruvate. Glyoxylate is converted to oxalate by a proposed CG31676 protein or diverted to glycine by dAgxt. Oxalate is transported across the principal cell, apical membrane by dPrestin Cl− exchange. Cl− ions that drive oxalate export are supplied from stellate cell transporters, Clc-a (basolateral) and secCl (apical). Oxalate in the MT lumen precipitates with Ca2+ (secreted from the blind ends of the tubule) to form CaOx crystals. Water transport by Prip, Drip, Eglp2, and Eglp4 may influence solute saturation and precipitation. (d) CaOx tubuloliths in anterior MT exhibiting birefringence due to polarized light. Yellow arrows indicate crystals, scale bar = 200 μm.
Composition of tubuloliths is primarily determined by experimental conditions or genetic phenotypes. Analysis of crystal composition has been done by various methods including energy-dispersive X-ray spectroscopy [3••,18], X-ray diffraction [4••], high performance liquid chromatography-mass spectrometry, micro-X-ray fluorescence and inductively coupled plasma optical emission spectroscopy [5••]. Kidney stones are classified based on composition and, thus far, only three classes have been exploited for studies in the Drosophila model: calcium oxalate (CaOx), calcium phosphate, and xanthine crystals.
Calcium oxalate crystals (Figure 1)
Oxalate is absorbed by the gut (e.g. from the diet) and generated by metabolism. Dietary intake and degradation of hydroxyproline or uptake and oxidation of a toxic precursor, for example, ethylene glycol, increases mammalian blood or insect hemolymph oxalate. Oxalate transported from hemolymph into the MT lumen is readily precipitated with luminal Ca2+, particularly at alkaline pH, that is, due to exceeding CaOx solubility. The resulting CaOx crystals form rectangular prisms shapes that are easy to identify and quantify using birefringence (Figure 1b).
Xanthine and urate crystals (Figure 2)
Figure 2. Xanthine and Urate Crystallization in Drosophila renal tubules.
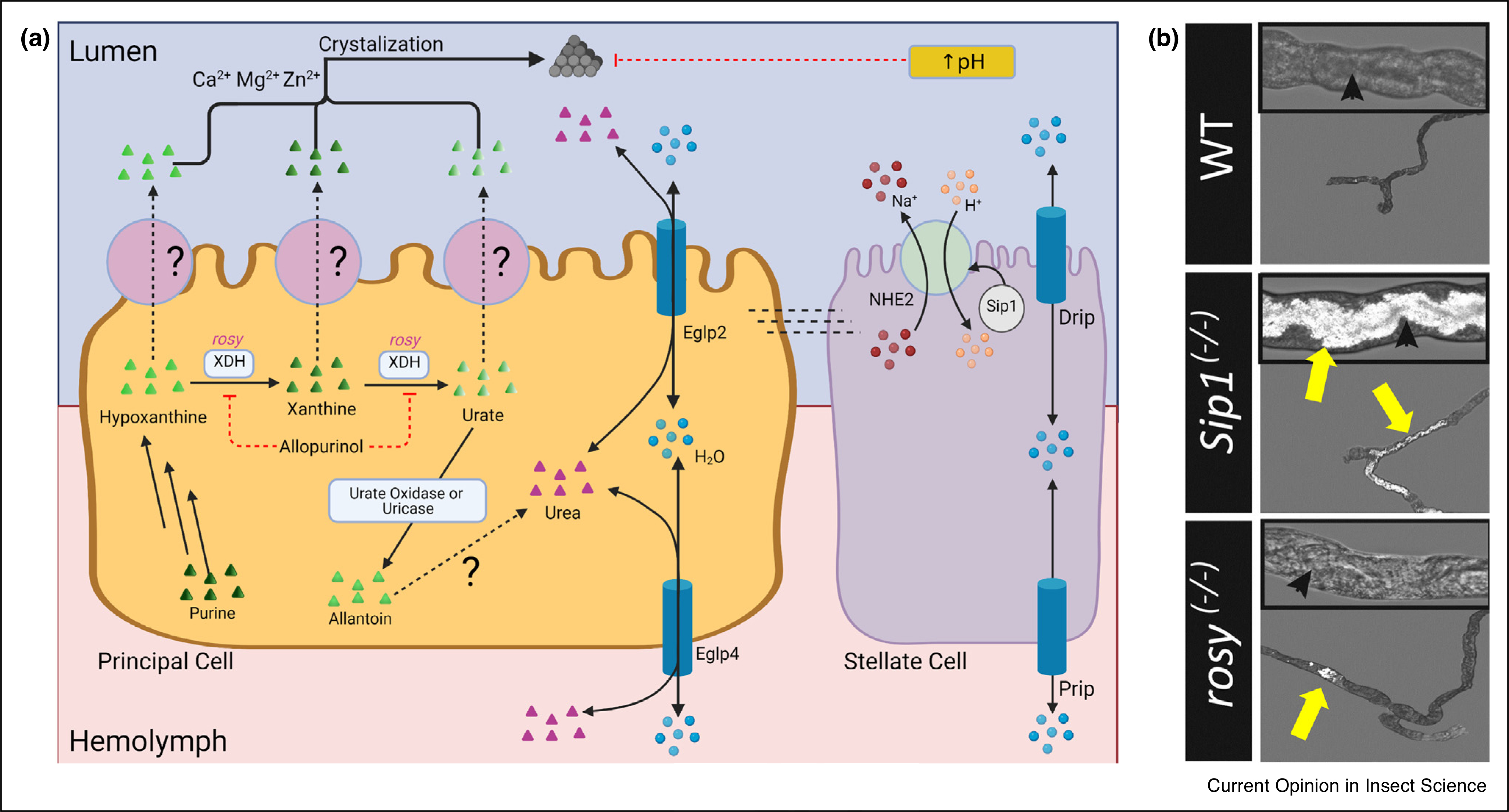
(a) Model of xanthine and urate crystal formation. Purine degradation leads to the production of hypoxanthine. Xanthinuria dehydrogenase/oxidase (Xdh) converts hypoxanthine to xanthine, and then to urate. Urate is then oxidized by urate oxidase (i.e. uricase) to allantoin. Hypoxanthine, xanthine, urate, and allantoin are transported into the lumen by unknown transporter(s) on the apical membrane of principal cells. Allantoin is converted to urea in A. aegypti but this pathway has not yet been identified in other insects. Crystals can form from hypoxanthine, xanthine, or urate with the presence of Zn2+, Ca2+ or Mg2+. Xdh is inhibited by Allopurinol. Crystal formation is inhibited by increased pH from NHE2 and/or Sip-1. Water transport by Prip, Drip, Eglp2, and Eglp4 may influence solute saturation and precipitation. (b) Urate tubuloliths in anterior MT of Sip1(−/−) mutants and xanthine tubuloliths in rosy(−/−) mutants exhibit birefringence due to polarized light (reproduced with permission from Ref. [42•]). Yellow arrows indicate the tubuloliths; black arrowheads mark MT lumen.
Purine degradation occurs via metabolism of hypoxanthine (to xanthine, then to urate) and ultimately yields allantoin. Each of these intermediates can precipitate with luminal metals (e.g. Ca2+, Zn2+, and Mg2+). There is increased crystallization at low pH.
Calcium phosphate crystals (Figure 3)
Figure 3. Calcium phosphate crystallization in Drosophila renal tubules.
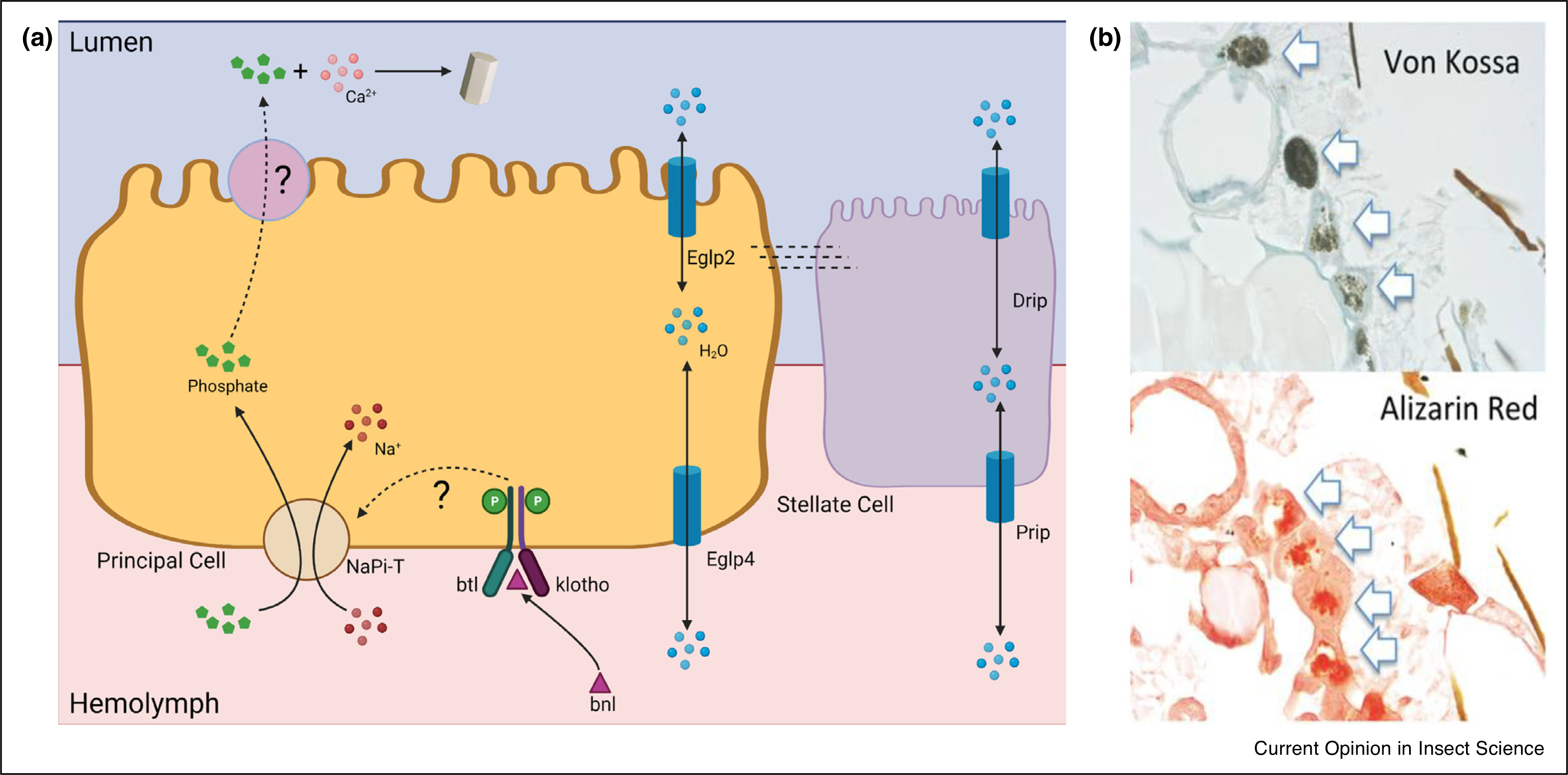
(a) Model of calcium phosphate crystal formation. Phosphate is transported from the hemolymph to the tubule lumen by a basolateral NaPi-T and an unknown apical phosphate transporter. Phosphate transport may be regulated by binding of fibroblast growth factor, breathless (btl), to a receptor complex, such as the branchless (bnl) + klotho dimer. Water transport by Prip, Drip, Eglp2, and Eglp4 may influence solute saturation and precipitation. (b) Paraffin sections of phosphate-fed Drosophila are stained with Von Kossa stain (phosphate, black precipitate; top) or Alizarin Red stain (calcium, red stain; bottom). Arrows indicate positive staining tubuloliths in anterior MT (reproduced with permission from Ref. [31••]).
Drosophila manage excess inorganic phosphate from the diet by MT secretion. This secreted phosphate can readily precipitate with Ca2+ to form hydroxyapatite4 MT tubuloliths.
Nutritional aspects of tubule crystals
Dietary composition and ultimately absorption influences whether or not Ca2+ will precipitate in the MT lumen. Medical advice for the prevention of nephrolithiasis most often directs humans and companion animals toward diets low in oxalates or purines, high in citrate, and high in fluids to minimize precipitation [19,20]. Accumulating evidence finds that insects are just as likely to succumb to tubulolithiasis from dietary factors as are mammals.
The majority of kidney stones (70–85%) [21] are composed of calcium oxalate. If a fly ingests ~0.1 μg of Ca2+ per day (0.1–0.5 mg/kg of body weight), this is comparable to 14 mg/kg intake recommended for adult humans (i.e. 1000 mg/day for 70 kg body weight) [22–24]. Approximately 30% of Ca2+ in the fly is temporarily retained in lipid droplets at the blind ends of anterior MTs. This stored Ca2+ does not normally result in crystallization, but once secreted into the lumen, it may precipitate with other substrates to initiate stone formation (Figure 1a) [22,25]. By consuming mostly fruit and vegetation, the fly’s diet likely includes oxalate that is particularly insoluble with Ca2+ [16••]. Naturally occurring oxalate is prominent in cocoa, tree nuts, and green leafy vegetables (e.g. spinach) with concentrations of 0.1% (dark chocolate) and up to 2% (fresh spinach leaves) [26,27]. While oxalate-induced tubulolithiasis in Drosophila is nutritionally possible, is more useful as model to study the etiology of kidney stones. Indeed, when wildtype Drosophila are fed a formula diet containing as little as 0.01% sodium oxalate (NaOx), the luminal Ca2+ will precipitate with the oxalate to produce CaOx crystals in the MT lumen within three days (Figure 1a) [3••,4••,28•,29]. Feeding the amino acid precursors to oxalate, for example, hydroxyproline, can elicit CaOx tubuloliths in flies similar to inducing nephroliths in rats [3••,30].
Other nutritional aspects of nephrolithiasis have also been studied in Drosophila. Calcium phosphate crystals accumulate in adult MTs when flies are fed a diet high in inorganic phosphate [31••]. Purine-induced tubulolithiasis results from interruptions in the purine degradation pathway, leading to accumulations of xanthine in secretions and the appearance of xanthine crystals [5••] (Figure 2). Xanthine crystal accumulation is exacerbated by high dietary zinc (Zn2+) [5••]. CaOx precipitation and stone prevention strategies, such as citrate, have been tested in Drosophila [29,32•]. These studies revealed that pharmaceutical potassium citrate [29], but not citrate from commercial juices [32•], lessens CaOx crystals by 20%−50% compared to ethylene glycol-induced controls and by 50% compared to oxalate-induced controls [3••].
Fluid intake and secretion are hypothesized to exacerbate tubulolithiasis similar to urinary supersaturation in mammals. MTs secrete water to maintain osmotic balance in the hemolymph analogous to osmoregulation by the vertebrate nephron using similar water channels [33]. Osmoregulation in insects is managed by the aquaporins Prip (basolateral) and Drip (apical) in stellate cells, and by the aquaglyceroporins Eglp4 (basolateral) and Eglp2 (apical) in the principal cells [17•] (Figures 1–3). The principal cell aquaglyceroporins also transport small water-soluble molecules such as glycerol and urea (Figure 2). Knockdown of just one of the aquaporins or aquaglyceroporins can significantly decrease the fluid secretion rate through the MTs [17•,21]. Conversely, increasing fluid intake and water secretion would dilute primary urine and could prevent MT precipitation events.
Detoxification and waste metabolism
Metabolized waste products are eliminated via the insect MT, similar to the mammalian nephron, and can initiate lithogenesis in the renal tubule. A notable, recent history example of a lithogenic toxin was due to the misuse of melamine. In 2007 and 2008, some international nutritional companies replaced protein with melamine in dog foods and infant formula. Melamine is nitrogen-rich and falsely increases protein assessments by the Kjeldahl protein assay. Despite inadequate protein content, these food products appeared to meet dietary nutrition standards. Subsequently, melamine was found to cause renal damage and nephrolithiasis to both dogs [34•] and infants [35] who had consumed the melamine-laced foods. Chen and colleagues later tested the toxic affect in Drosophila and found that the flies were also susceptible to melamine toxicity and CaOx crystal effects [18].
Historically, CaOx formation is known to be caused by ingestion of other non-nutritive components, for example, ethylene glycol, pharmaceuticals, and vitamins. Ethylene glycol is an additive for brake fluid and radiator fluid to lower the freezing point during cold weather. It is metabolized to oxalate for detoxification (liver) and elimination (kidney and intestine). Ethylene glycol fed to flies also induces CaOx tubuloliths [3••]. Pharmaceuticals such as N-acetyl-hydroxyproline and Baclofen5 both inhibit the conversion of proline to hydroxyproline (via hydroxyproline dehydrogenase 2) and, in Drosophila were shown to decrease tubuloliths [36]. Pyridoxine (vitamin B6) is needed to convert oxalate to glycine. It is used to treat primary hyperoxaluria type 1 (PH1) by promoting conversion of oxalate to glycine and thus prevent CaOx crystallization. In Drosophila, feeding pyridoxine lessoned CaOx crystal burden but only at high doses [36]. Together, these studies illustrate that several different causes of mammalian nephrolithiasis are closely mirrored by Drosophila tubulolithiasis.
Genetic predisposition for tubule crystals
Genome-wide association studies (GWAS) in humans and other mammals have revealed protein mutations that cause nephrolithiasis [37]. Interestingly, many of the mutated proteins identified in mammalian nephrolithiasis share structural and functional homology with insect proteins (Table 1). Mutations of these homolog proteins can occur in the fly by natural genetic variation, by knocking-down the gene of interest, or by transgenic expression.
Table 1.
Conserved homologs of human kidney stone diseases in Drosophila melanogaster
| Diseases | Human Mutation | Fly Ortholog | References |
|---|---|---|---|
|
| |||
| PH1/PH2 | Slc26a6 | dPrestin | [4••,28•] |
| Xanthinuria | Xdh | Xdh (rosy) | [5••,40,41,42•] |
| Uric Acid Stones | NHERF-1 | Sip1 | [42•] |
| Primary hyperoxaluria type 1 | AGXT | dAgxt | [4••,36] |
| PH2 | GRHPR | CG31674a, unconfirmed | X |
| PH3 | HOGA1 | Unknown | X |
| Dent Disease Type 1 | ClC-5 | Clc-c, unconfirmed | X |
| Dent Disease Type 2 | OCRL | OCRL, unconfirmed | x |
CG31674 is most often predicted as a GRHPR-homolog but this is unconfirmed. Some algorithms do designate CG31674 as a HOGA1- homolog.
One of the first examples of a naturally occurring mutation is rosy, which affects purine degradation. Adenine and guanine degradation follow a common pathway in animals and some plants. Purines are oxidized to hypoxanthine and xanthine and then to uric acid for final secretion and elimination (Figure 2). Insects, including Drosophila, along with birds express a uricase that catabolizes urate to allantoin. Aedes aegypti have enzymes that further metabolize allantoin to urea and glyoxylic acid [38], but this pathway does not appear to exist in Drosophila as the catalytic histidines are mutated [39•]. In humans, mutations in the xanthine dehydrogenase/oxidase (Xdh) gene cause xanthinuria type 1 evidenced by elevations in urinary xanthine and xanthine stones. Drosophila stood out as a promising model for studying this pathway when the homologous Xdh enzyme, rosy, was mutated. These rosy flies have decreased urate secretion, increased hypoxanthine and xanthine secretion, and MT xanthine / urate tubuloliths [5••,40,41,42•]. Drosophila are now being used as a model to further understand xanthinuria and uric acid transport. Allopurinol is a Xdh inhibitor and is used to minimize urate crystals in synovial fluids in patients with gout. In wildtype Drosophila, allopurinol inhibits Xdh and causes tubulolithiasis similar to rosy [5••,42•]. The same study found that xanthine crystals contain a mixture of divalent metal ions (e.g. Ca2+, Zn2+, Mg2+) and that Zn2+ secretion promotes xanthine crystallization [5••]. Urate is downstream of xanthine metabolism and can form crystals as well. In humans and mice, mutations of the Na+/H+ exchange regulatory factor 1 (NHERF-1) increases uric acid excretion and the potential for urate crystals [43]. In Drosophila, mutations in the NHERF-1 homologue, Sip-1, not only increased uric acid excretion, but also increased urate crystallization by perturbing MT lumen pH [42•].
Another major benefit of using the Drosophila tubulolithiasis model is that investigators may use the simple genetics of the Gal4/UAS system [44••] for tissue-specific RNAi knockdown or transgenic expression of target lithogenic genes. This Gal4/UAS system, imported from yeast, allows crossing the promoter-driven, Gal4-expressing flies with flies that carry the upstream activating sequence (UAS) controlling the construct of interest (RNAi or transgenic protein) [44••]. Furthermore, tissue-specific regulatory elements can be placed before the Gal4 to target expression for testing a tissue-specific function of a protein. By directing expression, the UAS/Gal4 system efficiently isolates the genetic manipulation without causing systemic impairments [45].
The Gal4/UAS system has been vital to understanding oxalate transport and CaOx crystal formation (Figure 1). One example is the Slc26a6 Cl−/Ox2− exchanger being associated with human hyperoxaluria [46•] and mouse bladder stones [47•]. Drosophila have a homologous protein, dPrestin (Slc26a6), that is highly expressed in the principal cells of the MT [4••]. In vivo studies with knockdown of dPrestin confirmed that it too transports oxalate into the MT lumen. With the knockdown isolated to only the MT, dPrestin lessened the occurrence of CaOx crystals as expected [4••,28•]. In humans, PH1 is caused by mutations in alanine glyoxylate aminotransferase [48]. Again, Drosophila have a homologous transporter, dAGXT, that prevents CaOx tubulolith formation by diverting glyoxylate degradation to glycine rather than oxalate. As expected, dAGXT knock-down increased CaOx crystal proliferation [36]. Human PH also includes type 2 (Glyoxylate Reductase, GRHPR) and type 3, (4-hydroxy-2-oxoglutarate aldolase, HOGA1) [49]. PH2 has a potential homolog in Drosophila (Table 1). Dent Disease is another hereditary, kidney stone disease caused by mutations in either ClC-5, an endosomal 2Cl−/H+ exchanger, or OCRL, an inositol phosphatase enzyme [50•,51]. Both Dent disease proteins have potential homologs in Drosophila (Table 1). Additional studies of these homologs are needed to understand the link between oxalate precursors and luminal crystallization.
Calcium phosphate crystals are the second most common crystal type in mammalian nephrolithiasis [21]. Emerging data in Drosophila reveals that a sodium-dependent phosphate transporter, NaPi-T, plays a major role in secreting excess phosphate from the hemolymph into the MTs [31••] (Figure 3). NaPi-T is thought to be on the basolateral membrane of the principal cells. Similar to control of mammalian phosphate transport, NaPi-T activity may be regulated by the fibroblast growth factor, branchless (bnl) and the receptor dimer, breathless (btl) + klotho [31••]. Further investigation will be needed to identify the apical phosphate transporters and verify the hormonal regulation of phosphate transporters.
Bacterial infection and diversity in the kidney has been associated with kidney stones. Bacterial extracellular proteins may influence this lithogenic effect. Gram negative bacteria (e.g. E. coli), have been cultured from human-derived kidney stones and are suspected to influence stone formation or growth [52•]. The Drosophila protein, subdued, is a member of the TMEM16 protein family expressed in the MTs and was found to exhibit defense against the gram-negative bacteria Serratia marcescens. Further evidence showed that when subdued was knocked down, the fly was more susceptible to infection [53•]. The ability to alter bacterial defense can give a unique window into the relationship between bacterial infections and kidney stone formation.
Summary
Malpighian tubulolithiasis has evolved into a realistic model for nephrolithiasis of the mammalian kidney. Numerous studies with Drosophila have confirmed nutritional, toxic, and genetic situations that promote crystals and correspond to mammalian kidney stone etiologies. Known genetic mutations that cause hereditary kidney stone diseases often have homologs in Drosophila. These homologs can be exploited for mechanistic evaluation of stone formation. As more crystallization mutations are identified or suspected, we believe that the Drosophila tubulolithiasis model will be vital for fast and efficient evaluation of those candidates and possible therapeutic targets.
Acknowledgements
The authors are especially grateful to the technical support of Heather L. Holmes. We thank Drs Julian Dow and Shireen Davies and their laboratories for more than a decade of help, instruction and collaboration for our Drosophila work. We also thank past members of the Romero lab who contributed to the original establishment and use of this model. Cartoon images in figures were prepared using BioRender.
Funding
Work presented in this review has been funded by the Oxalosis & Hyperoxaluria Foundation and the N.I.H.: U54-DK100227 (MFR), R01-DK092408 (MFR), R25-DK101405 (MFR, DRT), T32-DK007013 (CJR).
Footnotes
Conflict of interest statement
Nothing declared.
Hydroxyapatite refers to a group of calcium and phosphate compounds: brushite (CaHPO4*2H2O), monetite (CaHPO4), octa-calcium phosphate CaH(PO4)3*2½ H2O), and whitlockite Ca3(PO4)2).
β-(4-chlorophenyl)-γ-aminobutyric acid (β-(4-chlorophenyl)-GABA.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1. •. Myers EW, Sutton GG, Delcher AL et al. : A whole-genome assembly of Drosophila. Science 2000, 287:2196–2204. Original sequenciing of the Drosophila genome.
- 2.Dow JAT, Romero MF: Drosophila provides rapid modelling of renal development, function and disease. Am J Physiol Renal Physiol 2010, 299:F1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ••. Chen YH, Liu HP, Chen HY et al. : Ethylene glycol induces calcium oxalate crystal deposition in Malpighian tubules: a Drosophila model for nephrolithiasis/urolithiasis. Kidney Int 2011, 80:369–377. One of two original papers demonstrating Drosophila as a model of calcium oxalate crystalization. This work elicited crystals with ethylene glycol and hydroxyproline.
- 4. ••. Hirata T, Cabrero P, Bondeson DP et al. : In vivo Drosophila model for calcium oxalate nephrolithiasis. Am J Physiol Renal Physiol 2012, 303:F1555–1562. Second original paper demonstrating Drosophila as a model of calcium oxalate crystalization. This work demonstrated the involvement of dPrestin (Slc26a5/a6) as the oxalate transporter similar to the mammalian proximal tubule.
- 5. ••. Chi T, Kim MS, Lang S et al. : A Drosophila model identifies a critical role for zinc in mineralization for kidney stone disease. PLoS One 2015, 10:e0124150. This works illustrates involvement of ZnT transporters in urate crystal formation.
- 6.U.S. Department of Health and Human Services: Urinary tract stones. In Urologic Diseases in America. Edited by Litwin MS, Saigal CS. 2012:313–345www.niddk.nih.gov:.
- 7.Abufaraj M, Xu T, Cao C et al. : Prevalence and trends in kidney stone among adults in the USA: analyses of national health and nutrition examination survey 2007–2018 data. Eur Urol Focus 2020. [DOI] [PubMed] [Google Scholar]
- 8. •. O’Kell AL, Grant DC, Khan SR: Pathogenesis of calcium oxalate urinary stone disease: species comparison of humans, dogs, and cats. Urolithiasis 2017, 45:329–336. Work compares calcium oxalate stone disease in several mammalian species.
- 9.Dow JT, Davies SA: Integrative physiology and functional genomics of epithelial function in a genetic model organism. Physiol Rev 2003, 83:687–729. [DOI] [PubMed] [Google Scholar]
- 10.Chintapalli VR, Terhzaz S, Wang J et al. : Functional correlates of positional and gender-specific renal asymmetry in Drosophila. PLoS One 2012, 7:e32577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyenbach KW, Skaer H, Dow JA: The developmental, molecular, and transport biology of Malpighian tubules. Ann Rev Entomol 2010, 55:351–374. [DOI] [PubMed] [Google Scholar]
- 12.Coast GM, Webster SG, Schegg KM et al. : The Drosophila melanogaster homologue of an insect calcitonin-like diuretic peptide stimulates V-ATPase activity in fruit fly Malpighian tubules. J Exp Biol 2001, 204:1795–1804. [DOI] [PubMed] [Google Scholar]
- 13. •. Cabrero P, Terhzaz S, Davies SA et al. : Chloride channels in stellate cells are essential for uniquely high secretion rates in neuropeptidestimulated Drosophila diuresis. Proc Natl Acad SciUSA 2014, 111:14301–14306. This work shows novel chloride transport system (Clc-a) in stelate cells of Malpighian tubules. The study also illustrates that Clc-a is critical for fluid secretion by the Malpighian tubule.
- 14. •. Romero MF, Henry D, Nelson S et al. : Cloning and characterization of a Na+ driven anion exchanger (NDAE1): a new bicarbonate transporter. J Biol Chem 2000, 275:2455224559. This study is the first molecular characterization of a basolateral bicarbonate transporter (i.e. a Slc4 protein) in Drosophila. This was also the first demonstration that Na+-dependent Cl−-HCO3− exchange is encoded by a single protein in any species.
- 15. •. Sciortino CM, Fletcher BR, Shrode LD et al. : Localization of endogenous and recombinant Na+-driven anion exchanger protein, NDAE1, from Drosophila melanogaster. Am J Physiol Cell Physiol 2001, 281 :C449–463. Immunolocalization of NDAE1 to the basolateral membrane of epitheliaof the Malpighian tubule and the gut.
- 16. ••. Teigler DJ, Arnott HJ: Crystal development in the malpighian tubules of Bombyx mori (L.). Tissue Cell 1972, 4:173–185. Historically, this is the first demonstration that crystals can occur in Malpighian tubules of an insect.
- 17. •. Cabrero P, Terhzaz S, Dornan AJ et al. : Specialized stellate cells offer a privileged route for rapid water flux in Drosophila renal tubule. Proc Natl Acad Sci USA 2020, 117:1779–1787. This recent study defines Drip and Prip as stellate cell aquaporins and Eglp2 and Eglp4 as principal cell aquaglyceroporins.
- 18.Chen WC, Lin WY, Chen HY et al. : Melamine-induced urolithiasis in a Drosophila model. J Agric Food Chem 2012, 60:2753–2757. [DOI] [PubMed] [Google Scholar]
- 19.Massey LK, Roman-Smith H, Sutton RA: Effect of dietary oxalate and calcium on urinary oxalate and risk of formation of calcium oxalate kidney stones. J Am Diet Assoc 1993, 93:901–906. [DOI] [PubMed] [Google Scholar]
- 20.Holmes RP, Assimos DG: The impact of dietary oxalate on kidney stone formation. Urol Res 2004, 32:311–316. [DOI] [PubMed] [Google Scholar]
- 21.Shoag J, Tasian GE, Goldfarb DS, Eisner BH: The new epidemiology of nephrolithiasis. Adv Chronic Kidney Dis 2015, 22:273–278. [DOI] [PubMed] [Google Scholar]
- 22.Browne A, O’Donnell MJ: Segment-specific Ca(2+) transport by isolated Malpighian tubules of Drosophila melanogaster: a comparison of larval and adult stages. J Insect Physiol 2016, 87:1–11. [DOI] [PubMed] [Google Scholar]
- 23.Jumbo-Lucioni P, Ayroles JF, Chambers MM et al. : Systems genetics analysis of body weight and energy metabolism traits in Drosophila melanogaster. BMC Genomics 2010, 11:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross AC, Manson JE, Abrams SA et al. : The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011, 96:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dube K, McDonald DG, O’Donnell MJ: Calcium transport by isolated anterior and posterior Malpighian tubules of Drosophila melanogaster. roles of sequestration and secretion. J Insect Physiol 2000, 46:1449–1460. [DOI] [PubMed] [Google Scholar]
- 26.Merusi C, Corradini C, Cavazza A et al. : Determination of nitrates, nitrites and oxalates in food products by capillary electrophoresis with pH-dependent electroosmotic flow reversal. Food Chem 2010, 120:615–620. [Google Scholar]
- 27.Massey LK: Food oxalate: Factors affecting measurement, biological variation, and bioavailability. J Am Diet Assoc 2007, 107:1191–1194. [DOI] [PubMed] [Google Scholar]
- 28. •. Landry GM, Hirata T, Anderson JB et al. : Sulfate and thiosulfate inhibit oxalate transport via a dPrestin (mSlc26a6)-dependent mechanism in an insect model of calcium oxalate nephrolithiasis. Am J Physiol Renal Physiol 2016, 310:F152–159. This study illustrates that manipulation of dPrestin/Slc26a5/a6 substrates, calcium oxalate crystalization in Malpighian tubules may be controlled.
- 29.Han S, Zhao C, Pokhrel G et al. : Hydroxycitric acid tripotassium inhibits calcium oxalate crystal formation in the Drosophila melanogaster model of hyperoxaluria. Med Sci Monit 2019, 25:3662–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan SR, Glenton PA, Byer KJ: Dietary oxalate and calcium oxalate nephrolithiasis. J Urol 2007, 178:2191–2196. [DOI] [PubMed] [Google Scholar]
- 31. ••. Rose E, Lee D, Xiao E et al. : Endocrine regulation of MFS2 by branchless controls phosphate excretion and stone formation in Drosophila renal tubules. Sci Rep 2019, 9:8798. This recent study demonstrated that hydroxyappatite crystals form in Malpighian tubules with alterations in dietary phosphate. The study further shows a similar FGF-signaling pathway to control phosphate as is found in the mammalian proximal tubule.
- 32. •. Ho CY, Chen YH, Wu PY et al. : Effects of commercial citrate-containing juices on urolithiasis in a Drosophila model. Kaohsiung J Med Sci 2013, 29:488–493. This study demonstrates that the source of citrate matters to interfer with calcium oxalate crystallization.
- 33.Su W, Cao R, Zhang X-Y, Guan Y: Aquaporins in the kidney: physiology and pathophysiology. Am J Physiol Renal Physiol 2020, 318:F193–F203. [DOI] [PubMed] [Google Scholar]
- 34. •. Brown CA, Jeong K-S, Poppenga RH et al. : Outbreaks of renal failure associated with melamine and cyanuric acid in dogs and cats in 2004 and 2007. J Vet Diagn Invest 2007, 19:525–531. This study highlights the mechanism of renal failure associated with melamine added to the diet.
- 35.Gossner CM, Schlundt J, Ben Embarek P et al. : The melamine incident: implications for international food and feed safety. Environ Health Perspect 2009, 117:1803–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang H, Male M, Li Y et al. : Efficacy of Hydroxy-L-proline (HYP) analogs in the treatment of primary hyperoxaluria in Drosophila melanogaster. BMC Nephrol 2018, 19:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sima C, lordache P, Poenaru E et al. : Genome-wide association study of nephrolithiasis in an Eastern European population. Int Urol Nephrol 2021, 53:309–313. [DOI] [PubMed] [Google Scholar]
- 38.Scaraffia PY, Tan G, Isoe J et al. : Discovery of an alternate metabolic pathway for urea synthesis in adult Aedes aegypti mosquitoes. Proc Natl Acad Sci USA 2008, 105:518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. •. Gaines PJ, Tang L, Wisnewski N: Insect allantoinase: cDNA cloning, purification, and characterization of the native protein from the cat flea, Ctenocephalides felis. Insect Biochem Mol Biol 2004, 34:203–214. Provides sequence of Drosophila allantoinase. However, this enzyme is mutated at the critical histidines.
- 40.Mitchell HK, Glassman E: Hypoxanthine in rosy and maroon-like mutants of Drosophila melanogaster. Science 1959, 129:268. [DOI] [PubMed] [Google Scholar]
- 41.Hilliker AJ, Duyf B, Evans D, Phillips JP: Urate-null rosy mutants of Drosophila melanogaster are hypersensitive to oxygen stress. Proc Natl Acad Sci USA 1992, 89:4343–4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. •. Ghimire S, Terhzaz S, Cabrero P et al. : Targeted renal knockdown of Na+/H+ exchanger regulatory factor Sip1 produces uric acid nephrolithiasis in Drosophila. Am J Physiol Renal Physiol 2019, 317:F930–F940. This study illustrates the roles of NHE, Sip1 and rosy in urate crystallization.
- 43.Cunningham R, Brazie M, Kanumuru S et al. : Sodium-hydrogen exchanger regulatory factor-1 interacts with mouse urate transporter 1 to regulate renal proximal tubule uric acid transport. J Am Soc Nephrol 2007, 18:1419–1425. [DOI] [PubMed] [Google Scholar]
- 44. ••. Brand AH, Perrimon N: Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118:401–415. This is the key study demonstrating the building and use of the Gal4/UAS system in Drosophila.
- 45.Klueg KM, Alvarado D, Muskavitch MA, Duffy JB: Creation of a GAL4/UAS-coupled inducible gene expression system for use in Drosophila cultured cell lines. Genesis 2002, 34:119–122. [DOI] [PubMed] [Google Scholar]
- 46. •. Monico CG, Weinstein A, Jiang Z et al. : Phenotypic and functional analysis of human SLC26A6 variants in patients with familial hyperoxaluria and calcium oxalate nephrolithiasis. Am J Kidney Dis 2008, 52:1096–1103. This study identifies a SLC62A6 mutation that paradoxically has a minor effect on renal oxalate transport.
- 47. •. Jiang Z, Asplin JR, Evan AP et al. : Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 2006, 38:474–478. This study with Slc26a6(−/−) mice demonstrates that calcium oxalate bladder stones form.
- 48.Williams E, Rumsby G: Selected exonic sequencing of the AGXT gene provides a genetic diagnosis in 50% of patients with primary hyperoxaluria type 1. Clin Chem 2007, 53:1216–1221. [DOI] [PubMed] [Google Scholar]
- 49.Monico CG, Rossetti S, Belostotsky R et al. : Primary hyperoxaluria type III gene HOGA1 (formerly DHDPSL) as a possible risk factor for idiopathic calcium oxalate urolithiasis. Clin J Am Soc Nephrol 2011, 6:2289–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. •. Lloyd SE, Gunther W, Pearce SH et al. : Characterisation of renal chloride channel, CLCN5, mutations in hypercalciuric nephrolithiasis (kidney stones) disorders. Hum Mol Genet 1997, 6:1233–1239. Dent disease 1 mutations in CLCN5 are associated with urinary calcium increase and kidney stones.
- 51.Hoopes RR Jr, Raja KM, Koich A et al. : Evidence for genetic heterogeneity in Dent’s disease. Kidney Int 2004, 65:1615–1620. [DOI] [PubMed] [Google Scholar]
- 52. •. Schwaderer AL, Wolfe AJ: The association between bacteria and urinary stones. Ann Transl Med 2017, 5:32. This work demonstrates a more direct association of urogenital track, bacterial infection and kidney stones
- 53. •. Wong XM, Younger S, Peters CJ et al. : Subdued, a TMEM16 family Ca2+-activated Clchannel in Drosophila melanogaster with an unexpected role in host defense. eLife 2013, 2:e00862. This study demonstrates that Drosophila subdued is a Ca2+-activated Cl-channel, a membrane scramblase and protects against gram-negative bacterial infection in flies.


