Abstract
Background:
The REHAB-HF trial showed that an early, multi-domain rehabilitation intervention improved physical function, frailty, quality-of-life, and depression in older patients hospitalized with acute decompensated heart failure (ADHF).
Objectives:
Assess for treatment interactions by ejection fraction (EF) subgroup [≥45% (HFpEF) versus <45% (HFrEF)].
Methods:
Three-month outcomes were: Short Physical Performance Battery (SPPB); 6-minute walk distance (6-MWD); Kansas City Cardiomyopathy Questionnaire (KCCQ). Six-month endpoints included all-cause rehospitalization and death, and a global rank of death, all-cause rehospitalization, and SPPB. Pre-specified significance level for interaction was p≤0.1.
Results:
Among 349 total participants, 185 (53%) had HFpEF and 164 (47%) had HFrEF. Compared with HFrEF, HFpEF participants were more often women (61% versus 43%) and had significantly worse baseline physical function, frailty, quality-of-life and depression. Although interaction p-values for 3-month outcomes were not significant, effect sizes were larger for HFpEF versus HFrEF: SPPB +1.9 (1.1–2.6) versus +1.1 (0.3–1.9); 6-MWD +40 (9–72) versus +27 (−6–59) meters; KCCQ +9 (2–16) versus +6 (−2–14). All-cause rehospitalization rate was nominally lower with intervention in HFpEF but not HFrEF [effect size 0.83 (0.64–1.09) versus 0.99 (0.74–1.33); interaction p=0.40]. There were significantly greater treatment benefits in HFpEF versus HFrEF for all-cause death [interaction p=0.08; intervention rate ratio 0.63 (0.25–1.61) versus 2.21 (0.78–6.25)], and the global rank endpoint (interaction p=0.098) with benefit seen in HFpEF [probability index 0.59 (0.50–0.68)] but not HFrEF.
Conclusions:
Among older patients hospitalized with ADHF, compared with HFrEF, those with HFpEF had significantly worse impairments at baseline, and may derive greater benefit from the intervention.
Keywords: heart failure, HFpEF, physical function, rehabilitation, aging
INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF) is the dominant form of heart failure in the older population (1). In contrast to HF with reduced EF (HFrEF), the pathophysiology of HFpEF is not well understood, there have been few proven treatments, and outcomes have been worsening rather than improving over time (2,3). The health and economic impact of HFpEF is at least as great as that of HFrEF, with similar severity of functional limitations, acute hospitalization rates, and substantial mortality (4,5).
Several recent studies have shown that exercise training in patients with HFpEF is a safe and effective intervention to improve exercise capacity (6,7). However, studies of exercise training in HF have focused almost exclusively on chronic, stable HF patients. Physical function limitations are worse in the setting of acute decompensated HF (ADHF) compared with chronic HF(8) and the application of exercise training intervention data from chronic HF to patients with acute HF is uncertain. The prior literature regarding the safety and efficacy of exercise training that specifically targets patients hospitalized with HF is limited to observational data and one small, randomized trial, both of which showed benefit (9,10).
The recently reported multicenter, randomized, attention-controlled REHAB-HF trial showed that an innovative, early, transitional, tailored, progressive, multi-domain (balance, strength, mobility, endurance) physical rehabilitation intervention produced large, statistically significant improvements in physical function (SPPB, 6-MWD), frailty, quality of life (QOL), and depression in older patients hospitalized with ADHF (11,12). Although underpowered, there was a non-significant 10% lower rate of all-cause hospitalizations in the intervention group at 6 months. Randomization was stratified by heart failure phenotype (HFpEF versus HFrEF) to enable subgroup analyses. Thus, we aimed to assess for differential treatment effects with the REHAB-HF intervention versus attention control based on EF phenotype. Compared with HFrEF patients, we hypothesized that patients with HFpEF may derive a larger benefit from the intervention given the more significant baseline deficits in physical function, frailty, depression and QOL and lack of other therapies (13).
METHODS
The trial design (14), details of intervention fidelity (15), cross-sectional baseline characteristics of a subset of the patients by EF phenotype (13) and primary trial results (11,12) have been published. In brief, REHAB-HF was a 7-site, 349-patient, randomized, attention-controlled trial of a novel, 12-week, tailored, progressive, multi-domain physical rehabilitation intervention in patients ≥60 years hospitalized with ADHF regardless of EF. Participants had to have been independent and ambulatory prior to admission, and were expected to be discharged home. Key exclusion criteria were end-stage HF (e.g., continuous inotropes after discharge or ventricular assist device anticipated within the next 6 months) or kidney disease requiring dialysis, active participation in formal, facility-based cardiac rehabilitation and inability to participate in the study due to dementia, stroke or other disorder. The protocol was approved by institutional review boards at each site.
The innovative intervention was an early, transitional, tailored, progressive, one-on-one, multi-domain physical rehabilitation program (focused on strength, balance, mobility, and endurance) designed to address the broad range of deficits observed in this older ADHF population (14,15). It started as soon as possible following hospital admission with the transition to the outpatient facility early post-discharge. Outpatient sessions were 60 minutes, 3 days/week for 12 weeks or 36 sessions, which were complemented by home exercise (low-intensity walking and strengthening exercises, gradually increasing toward a goal of 30 minutes daily) on non-program days initiated following a study staff visit to evaluate the participant’s home environment. At the 3-month visit, participants were transitioned to the independent maintenance phase for months 4–6 with individualized exercise prescriptions and follow-up phone calls. The attention-controlled group received usual care plus telephone calls every 2 weeks and had an in-person clinical visit at months 1 and 3 post-discharge from index.
Physical and cognitive function outcome measures were assessed by personnel who were blinded to randomization assignment and EF subtype. The primary outcome was the Short Physical Performance Battery (SPPB) at 3-month follow-up and the secondary outcome was all-cause rehospitalizations during 6 months follow-up. The SPPB is a validated measure of global physical function in frail older persons that predicts a wide range of clinical outcomes (16,17). It has 3 components (standing balance, gait speed, and strength), which each scored 0–4 for a total score ranging from 0–12 where lower scores indicate more severe physical dysfunction. Additional efficacy parameters at 3-months included: 6-minute walk distance (6-MWD), QOL by the Kansas City Cardiomyopathy Questionnaire (KCCQ) and EuroQoL Visual Analogue Scale (VAS), depression by the Geriatric Depression Scale-15 (GDS-15), frailty by modified Fried criteria, and cognition via the Montreal Cognitive Assessment (MoCA). Clinical events were assessed at 6-months and included: all-cause rehospitalization (secondary outcome) and death, HF-specific rehospitalizations, death, and falls. A global rank endpoint, as has been previously reviewed (18), of death + all-cause rehospitalization + 3-month SPPB score was also prespecified. In the overall trial, the intervention significantly improved SPPB, 6-MWD, KCCQ, Fried frailty scores and GDS-15 (11,12).
Randomization was stratified by EF phenotype; EF phenotype was a key pre-planned subgroup analysis. In the trial protocol, HFpEF was defined as EF ≥45% and HFrEF was defined as <45%. The study aimed to enroll at least 30% of patients within the HFpEF strata. EF was assessed during the course of clinical care via echocardiogram, magnetic resonance imaging, cardiac catheterization, or nuclear medicine scan and was abstracted from the medical record with the use of the most recent measurement. Further details on EF ascertainment were previously described in the baseline manuscript (13) with the majority of patients having documentation during the index hospitalization. All patients provided written informed consent and the study was approved by the Institutional Review Board of all of the participating sites.
Statistical methods.
Participant characteristics were reported as means±SD, median (IQR) or frequency (%). For baseline characteristics, t-tests were used to compare continuous variables and Chi-square tests were used for categorical variables. Effects of the intervention on the 3-month outcomes (including primary outcome) were analyzed using analysis of covariance, adjusting for baseline measure, clinical site, age, and sex—the consistent covariate list used in REHAB-HF analyses. We additionally adjusted for baseline diabetes status due to a significant imbalance between treatment arms, and BMI due to significant imbalance between HFpEF and HFrEF arms. Least square means were used to estimate intervention effects. Effect sizes were reported with 95% confidence intervals (CIs). Effects of the intervention on 6-month clinical events based on counts or days (e.g., all-cause rehospitalizations, HF rehospitalizations, and deaths) were estimated and analyzed using Poisson regression, adjusting for clinical site, age, and sex. The secondary outcome, all-cause rehospitalization, was also adjusted for baseline SPPB score as pre-specified. Effects of the intervention on proportion-based outcomes (e.g., patients with hospitalizations, patients with falls) were analyzed using logistic regression adjusted for clinical site, age, sex, diabetes, and BMI.
The global rank composite outcome combined non-commensurate endpoints including death, number of all-cause rehospitalizations, and 3-month SPPB into a single non-parametric outcome in a hierarchical manner with death being ranked first and SPPB being ranked last. The participants were ranked from the most adverse response (lowest rank) to the most favorable response (highest rank). A non-parametric two-way analysis of variance(19) was used to estimate the mean global ranks for the treatment groups and test the treatment by EF interaction. The effect size was presented as probability index(20) which represents the probability that a randomly selected patient from the treatment group has a favorable response to a randomly selected patient from the control group.
The LVEF of 45% cut-off was used for analysis given that this was the standard at the time the trial was designed, randomization was stratified by this variable, and this was the pre-specified analytic plan in the original protocol. However, in the context of contemporary classification, we performed additional exploratory analyses on select outcomes with EF cut-points of <40% and ≥50%. A p-value <0.05 was used for determining statistical significance for overall comparisons. The interaction between EF category and treatment group was pre-specified as significant if p<0.10. Given the hypothesis-generating nature of this analysis, we pre-specified a plan to report the effect sizes in the EF subgroups regardless of whether interaction p-values were significant.
RESULTS
The REHAB-HF trial of 349 patients included 185 (53%) with HFpEF (93 intervention, 92 control) and 164 (47%) HFrEF (82 in each arm). Table 1 presents the baseline characteristics by EF as well as treatment arm. In general, the treatment groups were well-balanced within the EF strata. In the HFpEF cohort, the intervention arm had significantly more patients with DM than the attention-control arm (67% vs. 45%, p=0.003). For the HFrEF cohort, there were relatively fewer patients with peripheral vascular disease, yet the prevalence was higher in the treatment group (15% vs. 2%, p=0.005). Compared with patients with HFrEF, patients with HFpEF were more often women (61% vs. 43%) and with higher body mass index (BMI) (mean 35.0 vs. 30.5 kg/m2) and systolic blood pressure (mean 131 vs. 121 mmHg). Patients with HFpEF had a higher burden of comorbidities including atrial fibrillation, arthritis, sleep disordered breathing and depression. ACE-inhibitor and beta-blocker use were higher in patients with HFrEF. Table 2 presents the baseline functional status and QOL measures in the EF strata. Both groups had marked impairments in physical function and QOL with high rates of frailty and impaired cognition. Following adjustment for sex, race, BMI and comorbidity number, the HFpEF group had significantly worse baseline scores for SPPB, 6-MWD, gait speed, KCCQ, frailty and depression.
Table 1.
Baseline Characteristics of HFpEF and HFrEF Groups by Treatment Arm.
| HFpEF | HFrEF | HFpEF/HFrEF p-value | |||||
|---|---|---|---|---|---|---|---|
| Characteristics | All (N=185) | Rehabilitation Intervention (N=93) | Attention Control (N=92) | All (N=164) | Rehabilitation Intervention (N=82) | Attention Control (N=82) | |
| Age, years | 72.6±8.1 | 72.7±8.5 | 72.4±7.8 | 72.8±8.1 | 73.5±8.5 | 72.0±7.5 | 0.83 |
| Women | 112 (61%) | 54 (58%) | 58 (63%) | 71 (43%) | 31 (38%) | 40 (49%) | 0.001 |
| Non-white | 83 (45%) | 41 (44%) | 42 (46%) | 89 (54%) | 40 (49%) | 49 (60%) | 0.08 |
| BMI (kg/m2) | 35.0±8.8 | 35.4±8.3 | 34.7±9.3 | 30.5±7.4 | 29.9±7.0 | 31.1±8.0 | <0.001 |
| Systolic blood pressure (mmHg) | 131±23 | 130±22 | 132±24 | 121±19 | 121±20 | 121±19 | <0.001 |
| NYHA Class | |||||||
| II | 34 (18%) | 17 (18%) | 17 (18%) | 33 (20%) | 17 (21%) | 16 (20%) | 0.71 |
| III | 97 (52%) | 54 (58%) | 43 (47%) | 93 (57%) | 46 (56%) | 47 (57%) | |
| IV | 41 (22%) | 18 (19%) | 23 (25%) | 27 (16%) | 14 (17%) | 13 (16%) | |
| Unknown | 13 (7%) | 4 (4%) | 9 (10%) | 11 (7%) | 5 (6%) | 6 (7%) | |
| B-type natriuretic peptide, pg/mL (n=113), median (IQR) | 409 (178–673) | 463 (208–684) | 330 (160–650) | 1041 (608–1661) | 1048 (641–1586) | 985 (591–1661) | <0.001 |
| N-terminal proBNP, pg/mL (n=56), median (IQR) | 2378 (1152–4314) | 2094 (1353–3761) | 2738 (791–4547) | 4725 (2180–9511) | 6812 (3131–11851) | 3065 (1510–5851) | <0.001 |
| Index hospital length of stay, days | 4 (3–7) | 4 (3–6) | 5 (3–7) | 3 (5–7) | 5 (3–7) | 5 (3–7) | 0.25 |
| Prior hospitalization last 6 months | 85 (46%) | 40 (43%) | 45 (49%) | 71 (43%) | 36 (44%) | 35 (43%) | 0.62 |
| Comorbidities | |||||||
| Hypertension | 175 (95%) | 89 (96%) | 86 (93%) | 146 (89%) | 70 (85%) | 76 (93%) | 0.06 |
| History of myocardial infarction | 25 (14%) | 12 (13%) | 13 (14%) | 38 (23%) | 19 (23%) | 19 (23%) | 0.019 |
| History of coronary revascularization | 52 (28%) | 27 (29%) | 25 (27%) | 50 (30%) | 28 (34%) | 22 (27%) | 0.63 |
| Atrial fibrillation | 103 (56%) | 52 (56%) | 51 (55%) | 73 (45%) | 37 (45%) | 35 (44%) | 0.037 |
| Diabetes mellitus | 103 (56%) | 62 (67%) | 41 (45%) | 79 (48%) | 39 (48%) | 40 (49%) | 0.16 |
| Hyperlipidemia | 124 (67%) | 60 (65%) | 64 (70%) | 106 (65%) | 50 (61%) | 56 (68%) | 0.64 |
| Chronic obstructive pulmonary disease | 59 (32%) | 36 (39%) | 23 (25%) | 39 (24%) | 18 (22%) | 21 (26%) | 0.09 |
| Chronic kidney disease | 61 (33%) | 29 (31%) | 32 (35%) | 56 (34%) | 30 (37%) | 26 (32%) | 0.82 |
| Stroke | 30 (16%) | 14 (15%) | 16 (17%) | 22 (13%) | 12 (15%) | 10 (12%) | 0.46 |
| Peripheral vascular disease | 26 (14%) | 15 (16%) | 11 (12%) | 14 (9%) | 12 (15%) | 2 (2%) | 0.11 |
| Arthritis, muscle/joint pain, or connective tissue disease | 93 (50%) | 53 (57%) | 40 (43%) | 61 (37%) | 31 (38%) | 30 (37%) | 0.014 |
| History of Cancer | 36 (19%) | 18 (19%) | 18 (20%) | 39 (24%) | 24 (29%) | 15 (18%) | 0.33 |
| Sleep disordered breathing | 89 (48%) | 49 (53%) | 40 (43%) | 36 (22%) | 19 (23%) | 17 (21%) | <0.001 |
| Depression | 43 (23%) | 20 (22%) | 23 (25%) | 19 (12%) | 9 (11%) | 10 (12%) | 0.005 |
| Dementia or cognitive impairment | 4 (2%) | 3 (3%) | 1 (1%) | 6 (4%) | 3 (4%) | 3 (4%) | 0.40 |
| Urinary incontinence* | 22 (14%) | 12 (15%) | 10 (13%) | 18 (14%) | 7 (11%) | 11 (17%) | 0.87 |
| Patients with falls in last 3 months† | 24 (15%) | 13 (16%) | 11 (14%) | 20 (15%) | 11 (16%) | 9 (14%) | 0.93 |
| Heart Failure Therapies (at discharge) | |||||||
| Loop diuretic | 172 (94%) | 86 (92%) | 86 (95%) | 154 (94%) | 76 (93%) | 78 (95%) | 0.87 |
| Beta-blocker | 130 (71%) | 65 (70%) | 65 (71%) | 146 (89%) | 73 (89%) | 73 (89%) | <0.001 |
| Angiotensin-converting enzyme inhibitors | 59 (32%) | 29 (31%) | 30 (33%) | 72 (44%) | 36 (44%) | 36 (44%) | 0.023 |
| Angiotensin II receptor blockers | 32 (17%) | 19 (20%) | 13 (14%) | 43 (26%) | 19 (23%) | 24 (29%) | 0.046 |
| Aldosterone antagonist | 27 (15%) | 13 (14%) | 14 (15%) | 36 (22%) | 16 (20%) | 20 (24%) | 0.08 |
| Digoxin | 8 (4%) | 4 (4%) | 4 (4%) | 11 (7%) | 4 (5%) | 7 (9%) | 0.33 |
| Insulin | 62 (34%) | 35 (38%) | 27 (30%) | 37 (23%) | 19 (23%) | 18 (22%) | 0.022 |
| Oral Diabetic Agents | 51 (28%) | 36 (39%) | 18 (20%) | 34 (21%) | 16 (20%) | 19 (22%) | 0.13 |
| Implantable cardioverter-defibrillator | 4 (2%) | 1 (1%) | 3 (3%) | 57 (35%) | 32 (39%) | 25 (30%) | <0.001 |
| Biventricular pacemaker | 5 (3%) | 3 (3%) | 2 (2%) | 20 (12%) | 9 (11%) | 11 (13%) | <0.001 |
Values shown as N (%), mean±SD or median (IQR). Abbreviations: BMI: body mass index; NYHA: New York Heart Association; BNP: B-type natriuretic peptide; HF: heart failure.
Data collection in AC=76, RI=78.
Data collection in AC=77, RI=79
Table 2.
Adjusted and unadjusted differences (reference = HFrEF) in baseline physical function, quality of life, depression, and cognition in patients ≥60 years hospitalized with ADHF by heart failure phenotype.
| Unadjusted Analysis | Adjusted Analysis | ||||||
|---|---|---|---|---|---|---|---|
| HFpEF (n=185) | HFrEF (n=164) | P-value | HFpEF (n=185) | HFrEF (n=164) | Group Difference (95% CI) | P-value | |
| SPPB Score | 5.7±2.7 | 6.5±2.7 | 0.007 | 5.8±0.2 | 6.4±0.2 | −0.6 (−1.2, −0.1) | 0.024 |
| Balance Score | 2.5±2.4 | 2.7±2.5 | 0.29 | 2.5±0.1 | 2.7±0.1 | −0.2 (−0.4, 0.1) | 0.24 |
| 4-meter Walk Score | 2.1±1.1 | 2.5±1.0 | 0.001 | 2.2±0.1 | 2.4±0.1 | −0.3 (−0.5, 0.0) | 0.016 |
| Chair Rise Score | 1.0±1.1 | 2.3±1.2 | 0.035 | 1.0±0.1 | 1.3±0.1 | −0.2 (−0.5, 0.0) | 0.10 |
| 6 Minute Walk Distance (m) | 174±103 | 214±105 | <0.001 | 179±7 | 209±8 | −30 (−52, −8) | 0.007 |
| Gait Speed (m/s) | 0.57±0.22 | 0.64±0.22 | 0.002 | 0.58±0.02 | 0.63±0.02 | −0.05 (−0.10, −0.01) | 0.026 |
| Modified Fried Frailty Criteria* | 2.5±1.1 | 2.2±1.0 | 0.009 | 2.4±0.1 | 2.2±0.1 | 0.2 (0.0, 0.5) | 0.05 |
| KCCQ Overall | 37±20 | 45±21 | <0.001 | 38±2 | 45±2 | −7 (−12, −3) | 0.002 |
| KCCQ Clinical | 37±21 | 45±21 | <0.001 | 37±2 | 45±2 | −8 (−12, −3) | 0.001 |
| EuroQol Visual Analogue Scale | 56±21 | 61±23 | 0.037 | 56±2 | 61±2 | −4 (−9, 0) | 0.07 |
| Cognition (MoCA Score) | 22.1±4.1 | 21.6±4.7 | 0.27 | 22.1±0.3 | 21.6±0.3 | 0.5 (−0.4, 1.4) | 0.27 |
| Depression (GDS-15 Score) | 5.3±3.5 | 4.0±3.0 | <0.001 | 5.3±0.2 | 4.0±0.3 | 1.3 (0.5, 2.0) | <0.001 |
Unadjusted values shown as mean±SD or frequency (%). Adjusted values shown as LSmean±SE adjusted for sex, race, and BMI and total number of comorbidities. KCCQ scores range 0–100, with higher score meaning better health status. MoCA score ranges 0–30 with higher score meaning better cognitive function. GDS-15 score ranges 0–15 with higher score meaning worse depressive symptoms.
Abbreviations: KCCQ: Kansas City Cardiomyopathy Questionnaire; PCS: physical composite score; MCS: mental composite score; MoCA: Montreal Cognitive Assessment.
Adjusted for sex, race, and BMI only.
Intervention Retention and Adherence.
Of the 175 participants randomized to Rehabilitation Intervention, 12 died prior to completing the intervention (9 HFrEF, 3 HFpEF), 14 dropped from the study (8 HFrEF, 6 HFpEF), and 16 discontinued intervention participation (7 HFrEF, 9 HFpEF) but provided the primary and secondary outcomes. Overall intervention retention was 82%, excluding participants who died. Excluding participants who died or dropped but including participants who discontinued the intervention, participants with HFrEF and HFpEF completed 26.2±1.7 and 26.1±1.3 outpatient intervention sessions, respectively, for intervention adherence rates of 73±4% and 73±3%, respectively. After adjusting for sessions that had to be missed due to medical appointments and illness, intervention adherence rates for the outpatient facility-based sessions were 78±4% and 81±3% in patients with HFrEF and HFpEF, respectively.
Table 3 presents the analysis of 3-month outcomes by HF phenotype. Although interaction p-values were not statistically significant (all >0.2), intervention effect sizes appeared nominally larger in patients with HFpEF vs. HFrEF for SPPB, 6-MWD, KCCQ and EuroQoL; GDS-15 and frailty score improvements were similar between the HFpEF and HFrEF groups. The effect sizes by HF phenotype are displayed in Central Illustration.
Table 3.
Analysis of 3-month Functional Outcomes and 6-month Clinical Outcomes by Heart Failure Phenotype: HFpEF vs. HFrEF.
| HFpEF | HFrEF | P-value for interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 3-month Functional Outcomes | Rehabilitation Intervention | Attention Control | Effect Size | p-value | Rehabilitation Intervention | Attention Control | Effect Size | p-value | |
| SPPB Score | 8.3±0.3 | 6.4±0.3 | 1.9 (1.1, 2.6) | <0.001 | 8.4±0.3 | 7.3±0.3 | 1.1 (0.3, 1.9) | 0.009 | 0.20 |
| Balance Score | 3.2±0.1 | 2.6±0.1 | 0.6 (0.3, 1.0) | <0.001 | 3.3±0.1 | 3.1±0.1 | 0.1 (−0.2, 0.5) | 0.49 | 0.06 |
| 4-meter Walk Score | 2.9±0.1 | 2.4±0.1 | 0.5 (0.2, 0.8) | 0.002 | 3.1±0.1 | 2.6±0.1 | 0.5 (0.2, 0.8) | 0.003 | 0.95 |
| Chair Rise Score | 2.1±0.1 | 1.4±0.1 | 0.8 (0.4, 1.1) | <0.001 | 2.1±0.1 | 1.7±0.1 | 0.4 (0.1, 0.8) | 0.021 | 0.20 |
| 6 Minute Walk Distance (m) | 295±11 | 255±12 | 40 (9, 72) | 0.012 | 291±12 | 264±11 | 27 (−6, 59) | 0.11 | 0.54 |
| Gait Speed (m/s) | 0.79±0.02 | 0.68±0.02 | 0.11 (0.05, 0.18) | <0.001 | 0.81±0.02 | 0.69±0.02 | 0.12 (0.06, 0.18) | <0.001 | 0.93 |
| Modified Fried Frailty Criteria* | 1.5±0.1 | 1.7±0.1 | −0.3 (−0.6, 0.1) | 0.11 | 1.2±0.1 | 1.5±0.1 | −0.3 (−0.7, 0.1) | 0.10 | 0.93 |
| KCCQ Overall | 68±2 | 59±3 | 9 (2, 16) | 0.014 | 70±3 | 64±3 | 6 (−2, 14) | 0.13 | 0.59 |
| KCCQ Clinical | 67±2 | 61±3 | 7 (−0, 14) | 0.06 | 70±3 | 66±3 | 5 (−3, 12) | 0.24 | 0.67 |
| EuroQol Visual Analogue Scale | 72±2 | 63±2 | 9 (3, 16) | 0.004 | 71±3 | 66±3 | 4 (−3, 11) | 0.23 | 0.29 |
| Cognition (MoCA Score) | 22.6±0.4 | 22.8±0.4 | −0.2 (−1.3, 1.0) | 0.77 | 21.8±0.4 | 22.1±0.4 | −0.4 (−1.6, 0.8) | 0.56 | 0.81 |
| Depression (GDS-15 Score) | 3.3±0.3 | 4.1±0.3 | −0.9 (−1.7, −0.0) | 0.043 | 3.4±0.3 | 4.1±0.3 | −0.7 (−1.6, 0.3) | 0.16 | 0.76 |
| 6-month Clinical Outcomes | |||||||||
| All-cause rehospitalizations, rate | 1.20 (0.98, 1.48) | 1.44 (1.19, 1.75) | 0.83 (0.64, 1.09) | 0.18 | 1.22 (0.97, 1.54) | 1.23 (0.98, 1.54) | 0.99 (0.74, 1.33) | 0.95 | 0.40 |
| Heart failure rehospitalizations, rate | 0.51 (0.37, 0.70) | 0.65 (0.48, 0.87) | 0.79 (0.53, 1.17) | 0.24 | 0.66 (0.48, 0.91) | 0.72 (0.53, 0.98) | 0.92 (0.62, 1.35) | 0.66 | 0.60 |
| All-Cause Rehospitalization and Death, rate | 1.31 (1.07, 1.60) | 1.59 (1.32, 1.90) | 0.82 (0.64, 1.06) | 0.13 | 1.38 (1.11, 1.72) | 1.31 (1.05, 1.63) | 1.06 (0.80, 1.40) | 0.70 | 0.20 |
| Deaths, rate | 0.08 (0.04, 0.17) | 0.12 (0.06, 0.23) | 0.63 (0.25, 1.61) | 0.34 | 0.13 (0.06, 0.25) | 0.06 (0.02, 0.15) | 2.21 (0.78, 6.25) | 0.14 | 0.08 |
| Patients with ≥2 all-cause rehospitalizations, proportion | 0.33 (0.21, 0.54) | 0.63 (0.41, 0.97) | 0.53 (0.28, 1.01) | 0.06 | 0.38 (0.23, 0.63) | 0.41 (0.25, 0.68) | 0.92 (0.46, 1.85) | 0.82 | 0.26 |
| Patients with ≥2 HF rehospitalizations, proportion | 0.08 (0.04, 0.18) | 0.13 (0.07, 0.26) | 0.63 (0.24, 1.66) | 0.35 | 0.16 (0.09, 0.31) | 0.18 (0.10, 0.34) | 0.89 (0.38, 2.1) | 0.78 | 0.61 |
| All-cause rehospitalization days | 7.5 (4.9, 11.3) | 8.4 (5.5. 12.8) | 0.89 (0.50, 1.59) | 0.70 | 8.7 (5.5, 13.5) | 8.1 (5.3, 12.5) | 1.06 (0.58, 1.94) | 0.84 | 0.68 |
| Patients with ≥1 fall, proportion | 0.37 (0.23, 0.59) | 0.53 (0.34, 0.82) | 0.70 (0.37, 1.33) | 0.28 | 0.34 (0.20, 0.57) | 0.61 (0.38, 0.97) | 0.56 (0.28, 1.12) | 0.10 | 0.65 |
| Patients with ≥1 injurious fall, proportion | 0.07 (0.03, 0.17) | 0.10 (0.05, 0.21) | 0.75 (0.25, 2.20) | 0.60 | 0.05 (0.02, 0.14) | 0.09 (0.04, 0.20) | 0.61 (0.18, 2.05) | 0.43 | 0.81 |
3-month follow-up data presented as LS means±SE adjusted for baseline value, clinical site, age, sex, diabetes, and BMI. 6-month clinical event data presented as LS means (95% CI) of rate for count-based data or proportion of patients for frequency data, adjusted for clinical site, age, sex, diabetes, and BMI. All-cause rehospitalization at 6 months also adjusted for baseline SPPB score. Effect sizes shown with 95% CIs. For clinical outcomes based on counts, effect sizes shown as rate ratios. For clinical outcomes based on proportions, effect sizes shown as odds ratios.
KCCQ scores range 0–100, with higher score meaning better health status. MoCA score ranges 0–30 with higher score meaning better cognitive function. GDS-15 score ranges 0–15 with higher score meaning worse depressive symptoms.
Abbreviations: SPPB: short physical performance battery; KCCQ: Kansas City Cardiomyopathy Questionnaire; MoCA: Montreal Cognitive Assessment; GDS: Geriatric Depression Scale.
Modified Fried Frailty Criteria excludes weight loss criteria due to difficulty in ascertaining weight changes due to fluid status.
Central Figure.
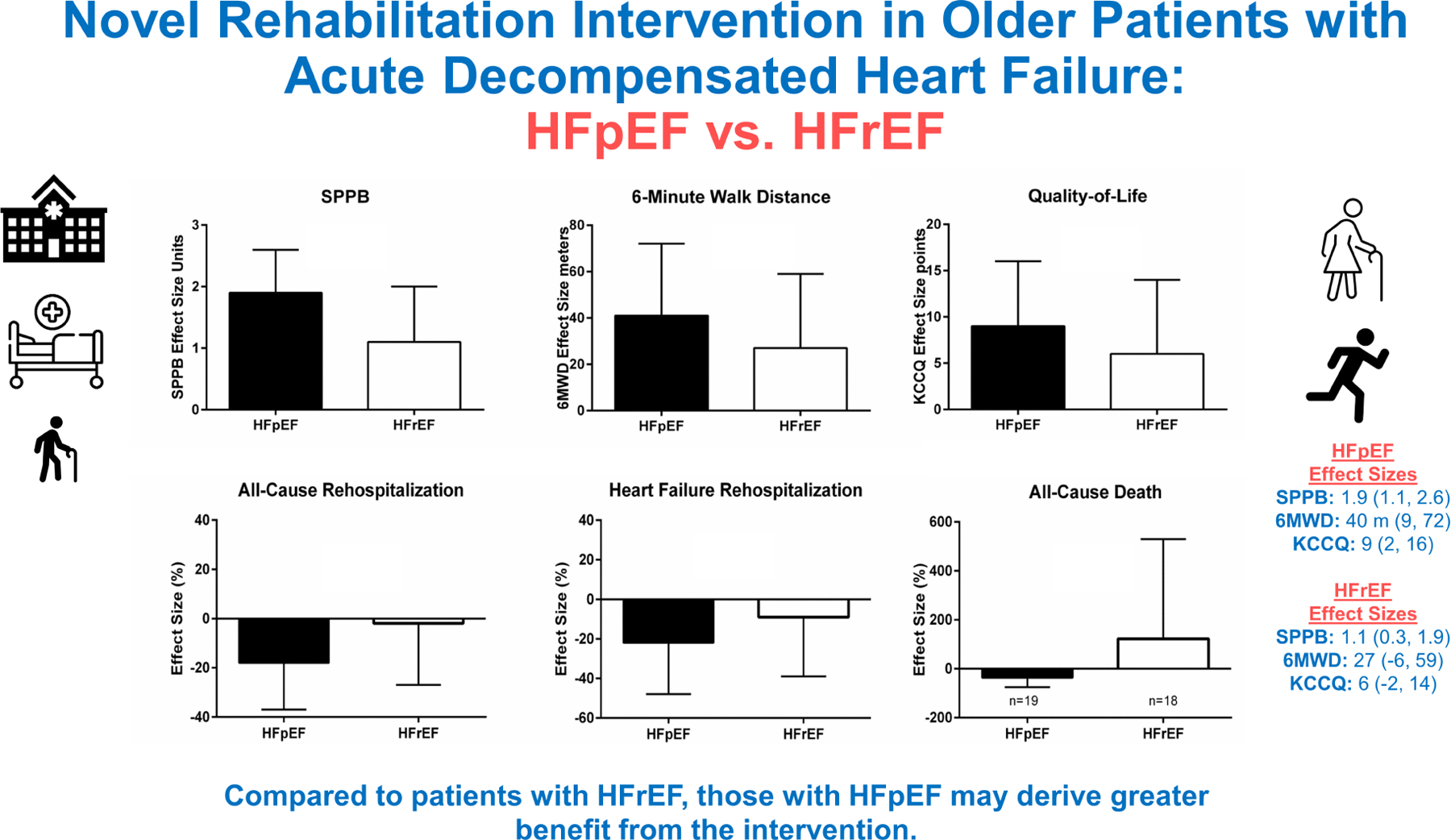
Effect of the novel REHAB-HF intervention in patients admitted with acute decompensated heart failure on outcomes at 3-months (SPPB, 6-minute walk distance, and Quality of life) and at 6-months (All-cause rehospitalization, Heart Failure Rehospitalization, All-cause Death) in participants with HFpEF (shown in black) compared to HFrEF (shown in white).
Abbreviations: SPPB indicates short physical performance battery; 6MWD indicates 6-minute walk distance; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; QOL; indicates quality of life via Kansas City Cardiomyopathy Questionnaire (KCCQ).
Table 3 presents the analysis of 6-month clinical events by HF phenotype. For the secondary endpoint of all-cause rehospitalization rate, although there was insufficient evidence to suggest significant heterogeneity by HF phenotype (interaction p=0.40), the point estimate for the effect size for all-cause rehospitalization showed a nominally larger benefit in HFpEF [0.83 (0.64, 1.09) vs. 0.99 (0.74, 1.33); a similar pattern was observed for HF rehospitalization. The effect sizes for 6-month clinical events by HF phenotype are displayed in Central Illustration. There was evidence of a significant treatment by EF group interaction for death. In the HFpEF group, the intervention was associated with a nominally lower death rate compared to the HFrEF group [0.63 (0.25, 1.61) vs. 2.21 (0.78, 6.25); interaction p=0.08]; however, the event counts were small (19 deaths overall in HFpEF vs. 18 deaths overall in HFrEF). Supplemental Tables 1 and 2 provide the primary cause of rehospitalization and death by HF phenotype. The primary cause of death for HFrEF was cardiovascular and specifically HF (15 cardiovascular deaths [83%] out of 18 total deaths with 12 HF deaths [67%]). In contrast, for HFpEF, <50% of deaths were due to cardiovascular (8 [42%] out of 19 deaths) and only 4 (21%) were HF deaths. An exploratory analysis with LVEF cutoffs of <40% and ≥50% for HFrEF and HFpEF, respectively, demonstrated overall consistent results (Supplemental Table 3).
Table 4 presents the pre-specified global rank endpoint data. There was significant heterogeneity of treatment effect by EF for the global rank endpoint of death + all-cause rehospitalization + SPPB (interaction p=0.098) with treatment benefit seen in HFpEF [probability index 0.59 (0.50, 0.68)] but not HFrEF. Specifically, the within-group treatment effects were statistically significant in the HFpEF group (P=0.04) but not the HFrEF group (P=0.69). There was similar evidence of treatment heterogeneity by EF group for the other exploratory global rank endpoints including composites with 6-MWD change and KCCQ change (Table 4).
Table 4.
Global Rank Endpoints by Heart Failure Phenotype: HFpEF vs. HFrEF.
| Outcome | Rehabilitation Intervention Mean Rank | Attention Control Mean Rank | p-value | p-value for interaction |
|---|---|---|---|---|
| Death + All-cause rehospitalizations + SPPB | ||||
| HFpEF | 179.5 | 149.0 | 0.04 | 0.098 |
| HFrEF | 168.8 | 175.6 | 0.69 | |
| Death + All-cause rehospitalizations + 10 Percent Change 6MWD | ||||
| HFpEF | 178.3 | 147.1 | 0.03 | 0.063 |
| HFrEF | 163.1 | 171.6 | 0.59 | |
| Death + All-cause rehospitalizations + 5-Point Change KCCQ | ||||
| HFpEF | 176.0 | 150.1 | 0.07 | 0.078 |
| HFrEF | 161.0 | 172.6 | 0.46 |
The interaction between EF category and treatment group was pre-specified as significant if p≤0.10. Abbreviations: SPPB: short physical performance battery; 6MWD: six-minute walk distance; KCCQ: Kansas City Cardiomyopathy Questionnaire
DISCUSSION
In this pre-specified analysis of the multi-center REHAB-HF trial, an innovative, early, transitional, tailored, multi-domain physical rehabilitation intervention in older adults with ADHF provided large, broad improvements in physical function and QOL for both HFpEF and HFrEF. Compared with HFrEF patients, HFpEF patients had significantly worse baseline physical function, frailty, QOL, and depression. Although the interaction p-values did not reach statistical significance, the magnitude of the benefits associated with the intervention were nominally larger in HFpEF patients for a number of physical function (SPPB and 6-MWD) and QOL (KCCQ and EuroQoL) outcomes. These improvements were achieved despite lower baseline function and QOL exhibited by participants with HFpEF. In addition, the intervention had a trend toward reduction of 6-month all-cause hospitalizations in HFpEF patients (rate ratio of 0.83, p=0.18) with no apparent benefit in the HFrEF group (rate of 0.99, p=0.95). Moreover, there was statistically significant heterogeneity in the treatment effect by EF group for all-cause death (interaction p=0.08) with a rate ratio of 0.63 in HFpEF vs. 2.21 in HFrEF (albeit with a modest number of events). There was also statistically significant heterogeneity of treatment effect by EF for the pre-specified global rank endpoints including death + all-cause hospitalization + SPPB (interaction p=0.098) with treatment benefit seen in HFpEF [probability index 0.59 (0.50, 0.68)] but not HFrEF. Similar results were seen for global rank endpoints that included 6MWD or KCCQ. Taken together, these data suggest that in patients with HFpEF, the intervention provides improvements in physical function and QOL, and may reduce both mortality and rehospitalizations. However, the intervention appears to have relatively less benefit and certainty regarding safety in patients with HFrEF.
For all outcomes, including physical function, frailty, QOL, depression, rehospitalization, and death, there appeared to potentially be greater benefits from the intervention in older patients with HFpEF than HFrEF. For example, the effect size for the SPPB was 1.1 units in HFrEF but was a much larger 1.9 units in HFpEF; 6MWD and KCCQ score effect sizes where 50% larger for HFpEF than for HFrEF. The differences between HFpEF and HFrEF response to the rehabilitation intervention were larger for the 6-month clinical event outcomes. For example, for the trial’s secondary endpoint of all-cause rehospitalization, there was a non-significant 18% reduction in the rate for HFpEF versus no measurable benefit in HFrEF. Similarly, the proportion of patients with two or more hospitalizations was 20% lower in the intervention group for HFpEF versus essentially unchanged for HFrEF. There appeared to be an increase in deaths in HFrEF, whereas there was a non-significant 37% decrease in death in the HFpEF group (interaction p-value 0.08). The finding of reduced death in HFpEF but paradoxically increased death in HFrEF should be interpreted with caution given the modest number of events. However, the interaction p-value was statistically significant. This suggests that while the intervention is safe and may reduce morbidity and mortality in HFpEF, there is considerably less certainty regarding HFrEF.
The current trial results regarding potentially greater relative benefit of exercise intervention for HFpEF compared to HFrEF are supported by results of prior trials. In the large NIH-funded HF-ACTION trial in 2,133 patients (mean age 60 years) with HFrEF, with 2 years of traditional endurance exercise training, there was a modest (6%) improvement in physical function (peak exercise oxygen consumption) and borderline improvement in clinical events (21,22). In the primary outcome analysis of HF-ACTION, there was no significant improvement in the benefit of exercise training for all-cause mortality in HFrEF patients over a median follow-up of 2.5 years (HR 0.96; 95% CI 0.79–1.17). After protocol-specified adjustments there were modest reductions in the composite endpoints of all-cause mortality + all-cause hospitalization and cardiovascular mortality + HF hospitalization. REHAB-HF suggests the possibility of a clinical outcome benefit with the intervention in HFpEF patients over the relatively modest follow-up period of 6 months. Furthermore, in the only head-to-head, blinded, controlled trial comparing exercise training in HFpEF versus HFrEF specifically in older (mean age 72 years) patients with chronic stable HF, there was a robust, 22% improvement in exercise capacity (peak oxygen consumption) in the patients with HFpEF but no change in the patients with HFrEF (23).
Multiple other lines of evidence support the credibility of benefit from the novel physical rehabilitation intervention in the patients with HFpEF. HFpEF is a true geriatric syndrome(1), likely initiated and promoted by systemic inflammation, involves multiple organs including severely impaired skeletal muscle function, and events in HFpEF appear driven by frailty and multiple comorbidities(24). Aging and the aforementioned comorbidities may initiate and/or aggravate chronic systemic inflammation that may affect myocardial remodeling and dysfunction in HFpEF through a signaling cascade, which begins with coronary microvascular endothelial dysfunction (25). Each of these factors would be expected to be positively influenced by the physical rehabilitation intervention which is pleiotropic and can improve multiple organs and outcomes, including skeletal muscle dysfunction (26). In addition, the pleiotropic stimulus of exercise training allows for mechanistic considerations regarding the beneficial effect of exercise training in HFpEF including anti-inflammation. Furthermore, prior studies suggested that the majority of exercise training-related improvement in exercise capacity in HFpEF may be related to microvascular and/or skeletal muscle adaptations that increase diffusive oxygen transport and/or utilization by the active muscles (27–29).
The potential promise of the REHAB-HF intervention for the care of the large and increasing population of older patients with HFpEF and its uncertainty for HFrEF aligns with the differing needs of these two groups of patients. As highlighted in a recent NIH workshop (30), HFpEF is the dominant form of HF in the older population. Unfortunately, due in part to the poor understanding of the pathophysiology of the disease, there have been limited drug treatments, none of which have been shown to improve physical function (31) and clinical outcomes are worsening. This is in stark contrast to patients with HFrEF, who tend to be younger and for whom many proven pharmacological treatments are available. Furthermore, in its 2014 bulletin approving traditional cardiac rehabilitation for patients with chronic, stable HFrEF (32), CMS declined to approve coverage for patients with HFpEF, citing the lack of adequate trial data. Thus, there is a critical need for effective strategies to improve outcomes specifically in patients with HFpEF.
Although the results suggesting a potentially larger benefit of the intervention for older ADHF patients with HFpEF are intriguing, they provide an insufficient basis for changing clinical care guidelines because: (1) the subgroup analysis, although pre-specified, was designated in the protocol as exploratory; (2) the sample size of the HFpEF subgroup was relatively modest (n=185); (3) there was no adjustment for multiple comparisons; and (4) the number of sites was modest (n=7) and not geographically well-dispersed.
These phase 2 trial results support a potential future phase 3 trial to definitely determine whether the innovative early REHAB-HF intervention will improve clinical outcomes for the highest risk HFpEF patients – those hospitalized for ADHF. The robust data and experience gained from the present, successful phase 2 trial will significantly enhance the efficiency and likelihood of success of a phase 3 trial. If positive, the phase 3 trial could alter treatment paradigms, change treatment guidelines and CMS reimbursement policy, and potentially reduce health care costs, while also improving physical function, frailty, and QOL in this large, under-served older population.
Clinical Application
The REHAB-HF intervention integrates principles from physical therapy and cardiac rehabilitation, but differs from both in several important ways (14,15,21). Although it borrows some principles and techniques from physical therapy and is conducted 1:1. However, it utilizes the REHAB-HF 16-cell stratification grid to precisely assess an individual patient’s status across 4 domains. In addition, the sessions are far greater in number, tend to be more intense, and are sustained over a longer period of time. Furthermore, a key goal of REHAB-HF intervention is increasing endurance. The REHAB-HF intervention is also distinct from traditional cardiac rehabilitation. It begins right away during index hospitalization, when deficits are the most severe and the patient is most vulnerable. It assesses and corrects balance, mobility, and strength before promoting endurance. It uses a stratification grid with 4 performance levels for each of the 4 domains of physical function to individualize the program to accommodate all levels of disability and there is a home assessment to ensure safe independent exercise. Traditional cardiac rehab is based primarily on endurance exercise with some strength training. Typical patients seen in cardiac rehab are at much higher function levels and it occurs in a group setting. Subjecting a frail older patient immediately to endurance training without first correcting balance, mobility, and strength deficits can limit efficacy and result in falls and injuries. Finally, CMS policy excludes recently hospitalized patients with HFrEF from cardiac rehab until at least 6 weeks of stability have been achieved; and HFpEF is currently not a covered indication for cardiac rehabilitation by CMS (32). Thus, cardiac rehab is not available to these patients. If one uses HF-ACTION as the prototype of cardiac rehab in chronic stable HF, the relative improvements in physical function in these ADHF patients who underwent the REHAB-HF intervention, as discussed above, were proportionally much greater. Suggesting that both efficacy and safety may be better. Importantly, the REHAB-HF strategy applied the intervention to a broad population of older ADHF patients.
Limitations
The present study has several strengths, including: a diverse group of older frail ADHF patients with high comorbidity burdens, high rates of frailty, severe baseline physical dysfunction, and poor QOL, and both community and tertiary care sites, enhancing generalizability of results; a carefully designed and conducted innovative physical rehabilitation intervention; and stratification of randomization by EF group with pre-specified subgroup analyses. The present study also has some potential limitations. EF was assessed during the course of clinical care and the most recent measurement was abstracted from the medical record. The sample size was likely insufficient power for clinical events, including rehospitalizations. Although staff assessing the primary outcome were blinded to group assignment and EF subgroup, it was not possible to blind participants. Despite randomization, there were imbalances in some baseline characteristics, however, overall results were not changed significantly in models adjusting for these variables.
CONCLUSION
An innovative, early, transitional, tailored, multi-domain physical rehabilitation intervention provided large improvements in physical function and QOL in older patients with both HFpEF and HFrEF, but the observed effect sizes appeared potentially larger in patients with HFpEF than HFrEF. There was a statistically significant interaction indicating greater benefit of the intervention for all-cause mortality and for the pre-specified global rank endpoint in the HFpEF group. While this phase 2 trial was underpowered for clinical outcomes, we present hypothesis-generating data supporting a potential future phase 3 clinical trial to assess whether the intervention improves clinical outcomes in HFpEF patients with ADHF, a large and growing population of high-risk patients for whom limited evidence-based treatments are available. In conclusion, HFpEF patients had significantly worse baseline functional status, QOL, and depression and appeared to potentially respond more favorably to the intervention compared with HFrEF.
Supplementary Material
CLINICAL PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
Older patients hospitalized for ADHF have severe physical dysfunction, impaired QOL, high rates of frailty, depression, and cognitive dysfunction, and these appear more severe in patients with HFpEF. A novel, early, transitional, tailored, progressive, multi-domain intervention improves physical function, frailty, and depression in both HFpEF and HFrEF, patients with HFpEF appear to potentially be more responsive to the intervention, and had significantly greater benefit for all-cause death and global rank endpoint.
TRANSLATIONAL OUTLOOK:
These data support a potential future phase 3 clinical trial to assess whether the REHAB-HF intervention improves clinical outcomes in HFpEF patients with ADHF, a large and growing population of high-risk patients for whom limited evidence-based treatments are available.
Funding Statement:
This study was supported in part by the following research grants from the National Institutes of Health: R01AG045551; R01AG18915; P30AG021332; P30AG028716; U24AG059624. Also supported in part by the Kermit Glenn Phillips II Chair in Cardiovascular Medicine and by the Oristano Family Fund at Wake Forest School of Medicine.
Disclosures
RJM received research support and honoraria from Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Medtronic, Merck, Novartis, Roche, Sanofi and Vifor.
SDR received research support from Abbott, AstraZeneca, Janssen Research and Development, Lundbeck, Monteris, and Merck, and consulting relationships with Minomic International, SVC Systems and Regeneron Pharmaceuticals.
Dr. Upadhya: received research support from Novartis and Corvia.
DJW received research support and consulting fees from Amgen, CVRx, Cytokinetics, Fibrogen, Novartis, NovoNordisk.
Dr. Kitzman reported receiving honoraria outside the present study as a consultant for Bayer, Merck, Medtronic, Relypsa, Merck, Corvia Medical, Boehringer-Ingelheim, NovoNordisk, Astra Zeneca, and Novartis, and grant funding outside the present study from Novartis, Bayer, NovoNordisk, and Astra Zeneca, and has stock ownership in Gilead Sciences
ABBREVIATIONS
- ADHF
Acute Decompensated Heart Failure
- EF
ejection fraction
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- SPPB
short physical performance battery
- 6MWD
six-minute walk distance
- QOL
quality of life
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- EuroQol VAS
European quality of life Visual Analogue Scale
- GDS-15
Geriatric Depression Scale-15
- MoCA
Montreal Cognitive Assessment
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The remaining authors report no relevant conflicts of interest.
Clinical Trial Registration:Clinicaltrials.gov identifier, NCT02196038
REFERENCES
- 1.Upadhya B, Pisani B, Kitzman DW. Evolution of a Geriatric Syndrome: Pathophysiology and Treatment of Heart Failure with Preserved Ejection Fraction. Journal of the American Geriatrics Society 2017;65:2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah SJ, Borlaug BA, Kitzman DW et al. Research Priorities for Heart Failure With Preserved Ejection Fraction: National Heart, Lung, and Blood Institute Working Group Summary. Circulation 2020;141:1001–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah KS, Xu H, Matsouaka RA et al. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. Journal of the American College of Cardiology 2017;70:2476–2486. [DOI] [PubMed] [Google Scholar]
- 4.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. The New England Journal of Medicine 2006;355:251–9. [DOI] [PubMed] [Google Scholar]
- 5.Dunlay SM, Redfield MM, Weston SA et al. Hospitalizations after heart failure diagnosis a community perspective. Journal of the American College of Cardiology 2009;54:1695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitzman DW, Brubaker P, Morgan T et al. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. Jama 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitzman DW, Brubaker PH, Herrington DM et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Journal of the American College of Cardiology 2013;62:584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reeves GR, Whellan DJ, Patel MJ et al. Comparison of Frequency of Frailty and Severely Impaired Physical Function in Patients ≥60 Years Hospitalized With Acute Decompensated Heart Failure Versus Chronic Stable Heart Failure With Reduced and Preserved Left Ventricular Ejection Fraction. The American journal of cardiology 2016;117:1953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scrutinio D, Passantino A, Catanzaro R et al. Inpatient cardiac rehabilitation soon after hospitalization for acute decompensated heart failure: a propensity score study. Journal of Cardiopulmonary Rehabilitation and Prevention 2012;32:71–7. [DOI] [PubMed] [Google Scholar]
- 10.Babu AS, Maiya AG, George MM, Padmakumar R, Guddattu V. Effects of Combined Early In-Patient Cardiac Rehabilitation and Structured Home-based Program on Function among Patients with Congestive Heart Failure: A Randomized Controlled Trial. Heart Views 2011;12:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitzman D, Whellan D, Duncan P et al. Physical Rehabilitation for Older Patients with Acute Decompensated Heart Failure. New Eng J Med 2021;In Press, May 16, 2021. [Google Scholar]
- 12.Kitzman D, Whellan D, Duncan P et al. A Novel Physical Rehabilitation Intervention for Older Patients with Acute Decompensated Heart Failure: The REHAB-HF Trial. Late Breaking Clinical Trial Abstract Presentation at 2021 ACC Annual Scientific Meeting. JACC 2021;In Press. [Google Scholar]
- 13.Warraich HJ, Kitzman DW, Whellan DJ et al. Physical Function, Frailty, Cognition, Depression, and Quality of Life in Hospitalized Adults ≥60 Years With Acute Decompensated Heart Failure With Preserved Versus Reduced Ejection Fraction. Circulation Heart failure 2018;11:e005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reeves GR, Whellan DJ, Duncan P et al. Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial: Design and rationale. American heart journal 2017;185:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pastva AM, Duncan PW, Reeves GR et al. Strategies for supporting intervention fidelity in the rehabilitation therapy in older acute heart failure patients (REHAB-HF) trial. Contemporary Clinical Trials 2018;64:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavasini R, Guralnik J, Brown J et al. Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med 2016;14: 10.1186/s12916-016-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soubra R, Aly C, Novella J. A Systematic Review of Thirty-One Assessment Tests to Evaluate Mobility in Older Adults. Biomed Res Int 2019;Epub:10.1155/2019/1354362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felker GM, Maisel AS. A global rank end point for clinical trials in acute heart failure. Circulation Heart Failure 2010;3:643–6. [DOI] [PubMed] [Google Scholar]
- 19.Shah DA, Madden LV. Nonparametric analysis of ordinal data in designed factorial experiments. Phytopathology 2004;94:33–43. [DOI] [PubMed] [Google Scholar]
- 20.Brown PM, Ezekowitz JA. Composite End Points in Clinical Trials of Heart Failure Therapy: How Do We Measure the Effect Size? Circulation Heart Failure 2017;10. [DOI] [PubMed] [Google Scholar]
- 21.O’Connor CM, Whellan DJ, Lee KL et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flynn KE, Piña IL, Whellan DJ et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandey A, Kitzman DW, Brubaker P et al. Response to Endurance Exercise Training in Older Adults with Heart Failure with Preserved or Reduced Ejection Fraction. Journal of the American Geriatrics Society 2017;65:1698–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murad K, Kitzman DW. Frailty and multiple comorbidities in the elderly patient with heart failure: implications for management. Heart Failure Reviews 2012;17:581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. Journal of the American College of Cardiology 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- 26.Kitzman DW, Haykowsky MJ, Tomczak CR. Making the Case for Skeletal Muscle Myopathy and Its Contribution to Exercise Intolerance in Heart Failure With Preserved Ejection Fraction. Circulation Heart Failure 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esposito F, Reese V, Shabetai R, Wagner PD, Richardson RS. Isolated quadriceps training increases maximal exercise capacity in chronic heart failure: the role of skeletal muscle convective and diffusive oxygen transport. Journal of the American College of Cardiology 2011;58:1353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. Journal of the American College of Cardiology 2012;60:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitzman DW, Nicklas B, Kraus WE et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. American Journal of Physiology Heart and Circulatory Physiology 2014;306:H1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pandey A, Shah SJ, Butler J et al. A Gero-centric Approach to Exercise Intolerance and Heart Failure with Preserved Ejection Fraction (HFpEF) in Older Adults: Elucidating and Targeting Extra-cardiac Contributors. JACC 2021;In Review. [Google Scholar]
- 31.Pieske B Angiotensin receptor neprilysin inhibition compared with individualized medical therapy for comorbidities in patients with heart failure and preserved ejection fraction - the PARALLAX trial. European Society of Cardiology Virtual Congress, Aug 30, 2020. [Google Scholar]
- 32.CMS. Centers for Medicare and Medicaid Services. Decision Memo for Cardiac Rehabilitation (CR) Programs - Chronic Heart Failure (CAG-00437N). February 18, 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


