Abstract
Parasite dilution occurs in varied systems, via multiple potential mechanisms. We used laboratory manipulation and field survey to test for invader-induced parasite dilution via two specific mechanisms: host-host competition and encounter reduction. In the laboratory, single Aedes triseriatus larvae were exposed to one of 8 combinations of: +/− parasitic Ascogregarina barretti, +/−1 cohabiting Aedes albopictus larva during parasite exposure, and +/−1 cohabiting A. albopictus larva after infectious parasite removal. Larval infection intensity (predicted to decrease via dilution by encounter reduction) was not significantly affected by A. albopictus. Adult infection prevalence and intensity (predicted to decrease via dilution by host-host competition) were significantly greater with A. albopictus, suggesting parasite amplification by interspecific competition, an effect potentially mediated by competition increasing A. triseriatus development time. In the field, we tested for effects of potential dilution host abundances on prevalence and abundance of A. barretti in A. triseriatus larvae. Piece-wise path analysis yielded no evidence of host-host competition impacting parasitism in the field, but instead indicated a significant direct negative effect of Aedes spp. abundance on parasite abundance in A. triseriatus, which is consistent with dilution via encounter reduction in the field, but only in treeholes, not in man-made containers. Our results are consistent with the hypothesis that a non-competent invader can alter the native host/parasite relationship, but our laboratory and field data yield differing results. This difference is likely due to laboratory experiment testing for per capita effects of dilution hosts on parasitism, but the field analysis testing for effects of dilution host abundance on parasitism. Individually, host-host competition with the invader amplifies, rather than dilutes, parasite success. In contrast, our path analysis is consistent with the hypothesis that dilution of parasitism results from increased abundance of noncompetent hosts in the field.
Keywords: Aedes, dilution, amplification, Ascogregarina, invasive species, parasite
Introduction
Biological invasions can have strong impacts on the invaded community, including on established native host-parasite relationships. Host-parasite pairings are postulated to have a reciprocal evolutionary relationship that can influence how each party interacts with other members of the community (Price et al. 1986, Lord et al. 2014). Additionally, diversity of potential host species within a community can either reduce or enhance infection (Civitello et al. 2015, Rohr et al. 2020). Accordingly, invasive species can drive changes in the invaded ecological community (Telfer & Bown 2012) by acting as an additional host within the invaded community. In some cases, adding invasive species to a coevolved host/parasite system can cause a dilution effect (Young et al. 2017, Loxton et al. 2017, Westby et al. 2019a, Tierney et al. 2020).
The Dilution Effect hypothesis predicts a negative relationship between host diversity and pathogen prevalence or abundance (Ostfeld & Keesing 2000a, b). Biodiversity effects on disease prevalence have been debated due to disagreements on the importance of community composition and host competence (the ability of a host to harbor and to transmit a parasite) as opposed to biodiversity per se, (Ostfeld & Keesing 2000b, 2012, Randolph & Dobson 2012, Wood & Lafferty 2013, Civitello et al. 2015, Rohr et al. 2020), the spatial scale at which dilution may be most common (Rohr et al. 2020), the likelihood that greater diversity produces dilution, amplification (i.e., increased parasite prevalence), or neither (Civitello et al. 2015, Rohr et al. 2020), and the importance of different mechanisms that may produce a Dilution Effect (Strauss et al. 2016, 2018). These issues focus on functional variation of species as potential hosts, postulating that dilution occurs when some host species exhibit greater competence compared to others, and that increases in biodiversity increase the frequency of low-competence hosts (Ostfeld & Keesing 2012, Wood & Lafferty 2013).
Our focus in this paper is testing the relative importance of two causal mechanisms for dilution: encounter reduction (decreases in parasite contact with highly competent hosts with increased biodiversity), and host-host competition (reduction of fitness of highly competent hosts through competition from less-competent hosts, which in turn results in reduced parasite success) (Keesing et al. 2006, Randolph & Dobson 2012, Civitello et al. 2015, Strauss et al. 2016, 2018). Complicating matters is the possibility for conflicting effects of alternative mechanisms by which biodiversity impacts parasitism (Luis et al. 2018, Rohr et al. 2020). Host-host interspecific competition, in particular, can have complex effects on parasites (Keesing et al. 2006). Host-host competition could reduce populations of competent hosts, and thus reduce parasite abundance (host regulation; Keesing et al. 2006). Host-host interspecific competition may cause dilution even if it does not affect host population, if it reduces energy budgets of individual hosts via resource shortage resulting in a reduced load of parasites that can complete development in individual hosts (transmission reduction; Keesing et al. 2006; Strauss et al. 2015). Reduced host energy budgets from interspecific competition may, however, produce the opposite of dilution, if competitively-stressed hosts are less able to mount immune defenses against parasites. In that case competition may increase parasite load in competent hosts, thus resulting in amplification of parasite abundance (Rohr et al. 2020). Incomplete understanding of the Dilution Effect is likely due to a shortage of manipulative investigations of potential causes of dilution, as theoretical models and observational studies lack the specificity to assess relative importance and generalizability of mechanisms potentially contributing to dilution (Keesing et al. 2006, Telfer & Bown 2012, Civitello et al. 2015, Strauss et al. 2016, 2018, Luis et al. 2018, Roberts & Heesterbeek 2018). Thus, we need detailed investigations of mechanisms that may modify existing host-parasite interactions, and the repercussions of those modifications on host and parasite populations.
To pursue these questions, we used the mosquito Aedes triseriatus and its species-specific parasite Ascogregarina barretti. The mosquito larva feeds on bacteria and detritus in the aquatic habitat and during feeding the larva ingests “free-living” A. barretti oocysts (Beier & Craig 1985). The oocysts release sporozoites that develop into trophozoites within the larval midgut (Beier & Craig 1985, Chen 1999). During host pupation, parasite sexual reproduction results in formation of gametocysts (Chen & Yang 1996, Chen 1999). Hundreds of oocysts develop within the gametocyst and are then deposited via defecation or adult death back into the larval habitat by the adult host (Beier & Craig 1985, Chen 1999). Parasitism by A. barretti prolongs host development time, and reduces adult size and fecundity, all of which can impact host population growth (e.g., Siegel et al. 1992, Van Rhein et al. 2000, Soghigian & Livdahl 2017). Parasites with free-living infectious stages, like Ascogregarina, are postulated to be likely to be affected by biodiversity of their community (Rohr et al. 2020), and the species specificity of A. barretti seems likely to result in dilution by encounter reduction via ingestion by non-competent hosts (Johnson et al. 2010, Venesky et al. 2014).
Invasive species are a source of ecological change that can alter the interactions of coadapted pairs, like A. triseriatus and A. barretti, via novel relationships among invasive and native hosts and parasites (e.g., Poulin 2017, Llopis-Belenguer et al. 2020). Invaders may escape from their coevolved predators and parasites as the invasion proceeds, which may enhance the invader’s competitive impact on the native species (Aliabadi & Juliano 2002, Torchin et al. 2003, Liu & Stirling 2006). Alternatively, if the invader acts as a dilution host, it could reduce parasite pressure on the native host and thus increase native host competitive ability (Gendron & Marcogliese 2016, Searle et al. 2016, Westby et al. 2019a).
The mosquito Aedes albopictus is a prominent invader in the native range of A. triseriatus. The two often coexist in the same larval habitat, wherein strong interspecific competition for nutritional resources can occur (reviewed by Juliano 2009). Aedes albopictus is generally considered the better competitor in many ecological contexts (e.g., Livdahl & Willey 1991, Aliabadi & Juliano 2002, Bevins 2007, Yee et al. 2007, Smith et al. 2013). Aedes albopictus are likely to ingest A. barretti oocysts during foraging; however, A. barretti is unable to complete its life cycle in A. albopictus, rendering it a noncompetent host (Tseng 2007). Host-host competition reduces survivorship of A. triseriatus to adulthood (Livdahl & Willey 1991) and this interaction can impact the parasite. Thus, A. albopictus, A. triseriatus, and A. barretti are a system wherein arrival of A. albopictus doubles species richness in the system, but does so by adding a noncompetent host with the potential to produce a Dilution Effect via encounter reduction, via host-host competition, or both.
Based on this, we tested experimentally the hypothesis that the presence of an invading host will alter the native host/parasite interaction through host-host competition or per capita encounter reduction (as opposed to encounter reduction dependent on density of the invading host), contributing to a Dilution Effect. Because native A. triseriatus fitness decreases in the presence of invading A. albopictus due to competition for nutritional resources, our hypothesis predicts that parasite burden in the native host will decrease in the presence of the invader through increased mortality or decreased growth and development of competition-stressed infected native hosts, resulting in reduced parasite success within competition-stressed native hosts. Our hypothesis also predicts that infection intensity and prevalence of A. barretti within larvae of A. triseriatus will decrease in the presence of invasive A. albopictus due to encounter reduction caused by ingestion of the parasites by the invader, resulting in unproductive infections that remove a portion of the infectious population of A. barretti.
Whichever per capita mechanisms may contribute to dilution, assemblages of potential hosts and competitors, such as the one including A. triseriatus and A. barretti, vary with season and location. If the dilution effect is important in this system, we expect that abundances of A. albopictus and other potential dilution hosts should be negatively associated with parasitism of A. triseriatus in the field. We therefore sought to link our mechanistic experiment to field sampling (Venesky et al. 2014) by surveying aquatic communities inhabited by host, parasite, and potential dilution hosts to test for effects of richness and abundance of non-competent host species, particularly other Aedes species, on A. barretti infection prevalence and intensity in the native host.
Methods
Laboratory Manipulations
Origin and maintenance of colonies of mosquitoes and A. barretti are described in Appendix S1. Laboratory trial 1 focused on collection of host fitness and trophozoite and gametocyst parasite life-stage data, whereas trial 2 focused on collection oocyst parasite life-stage data.
Trial 1
Newly hatched individual A. triseriatus larvae (N=400) were placed individually into 16mL glass vials with 10mL reverse osmosis (RO) purified water and randomly assigned to one of 8 combinations of three treatment factors (Appendix S1: Table S1): Early cohabitant (+ or −), Late cohabitant (+ or −), and Parasite (+ or −). Our Early cohabitant treatment was designed to test ability of invasive A. albopictus to dilute infection via parasite removal causing encounter reduction. Our Late cohabitant treatment was implemented after removing infectious parasite oocysts and infection was no longer possible, so that any dilution effect could be produced only by host-host competition lowering the success of the parasite (Appendix S1: Table S1). While young instar A. albopictus could compete for resources with A. triseriatus, we tried to minimize the effect of resource competition by providing ample food during the early phase (Appendix S1). Beginning on Day 1, 1st instar A. triseriatus larvae were exposed to either a single first instar A. albopictus larva (Early cohabitant+) or no other larvae (Early cohabitant−) for 72 hours. Also, on Day 1, 1000 A. barretti oocysts were added to the vials housing A. triseriatus larvae assigned to Parasite+ treatment. This dose was chosen as a moderate-low dosage (Fellous & Koella 2009). Larvae assigned to Parasite− treatments received parasite free control water for 72 hours. On Day 4, the A. triseriatus larvae were transferred to a new parasite-free vial either alone (Late cohabitant−) or with an A. albopictus larva (Late cohabitant+) until pupation.
Trophozoite infection of larvae:
A random sample of 5 4th instar larvae from each treatment group was dissected to quantify infection prevalence (proportion infected) and infection intensity (trophozoite abundance /infected individual) (Sulaiman 1992). Infection prevalence is a measure of the infectivity of the parasite population to the host population, which is a component of parasite fitness. Infection intensity is both a component of parasite fitness and a measure of host qualitative resistance (Restiff & Koella 2004, Kutzer & Armitage 2016).
Gametocyst infection of adults:
Adult A. triseriatus (53 male, 76 female) from parasite+ were dissected for gametocyst infection prevalence (proportion infected by the sexually-produced stage of the parasite, Chen 1999) and infection intensity (gametocyst production / infected adult).
Host fitness:
Relative fitness of hosts was quantified by survivorship to adulthood, development time (days from hatch to emergence, a component of per capita population growth; Livdahl & Sugihara 1984, Chmielewski et al. 2010), and adult size (female wing length, a predictor of fecundity; e.g., Livdahl 1984, Aspbury & Juliano 1999).
Trial 2
A second laboratory trial was conducted with only the 4 parasite+ treatments indicated above (Appendix S1: Table S1), 30 replicates per treatment, and larvae infected, housed, and fed as in the first trial. The specific goal of this trial was to quantify oocyst production, rather than gametocyst production as we did in trial 1, because oocysts, as infectious propagules, are a direct measure of the reproductive success of the parasites in the host. A random sample of 5 4th instar A. triseriatus larvae were dissected to assay infection intensity and prevalence, as in Trial 1. All other individuals were reared to pupation, removed from the habitat vial, rinsed, and transferred to a 1.5mL microcentrifuge tube with 0.5mL water. At eclosion adults and any pupae that died prior to eclosion, were crushed into their pupal water.
Oocyst infection of adults and pupae:
Oocyst prevalence and abundance in the water were quantified (Fellous & Koella 2009, McIntire & Juliano 2021) yielding our measures of oocyst prevalence (proportion of adults and deceased pupae bearing oocysts) and oocyst infection intensity (oocysts per infected adult or deceased pupa). Oocyst production for individuals that died as pupae was included in this analysis, as late pupal death can result in successful completion of the parasite lifecycle (Chen 1999).
Parasite Fitness:
The abundance of oocysts produced per exposed A. triseriatus was taken as measures of parasite fitness, quantifying offspring of the next parasite generation arising from the cohort of oocysts given to a single host (mean dose of 1000 oocysts/A. triseriatus larva). This fitness metric includes negative impacts of infection and of the competing host on mortality of host larvae, because such mortality necessarily results in failure of the parasite to produce the next generation of oocysts.
Laboratory Manipulation Analysis
All statistical analyses for effects of treatment factors and interactions (Appendix S1: Table S1) were conducted with SAS v9.4. Larval infection prevalence was not analyzed, as the Parasite+ groups were all infected, and the Parasite− groups were all uninfected. Therefore, all infection analyses were done on Parasite+ treatments only, using generalized linear models (PROC GLIMMIX) testing for effects of early cohabitant, late cohabitant, and interaction, with Trial as a random block effect. (See Appendix S1 for distribution assumptions).
Host development time (log transformed days to adulthood) for each sex and female size were analyzed by general linear model (PROC GLM). Host survivorship was analyzed using a binary distribution (died before eclosion/successful eclosion; PROC GLIMMIX).
Larval Community Survey
We sampled seven wooded sites at ~40°N and altitude of 150-190 MASL in Illinois and Indiana, USA. Plastic buckets (22.7 L) were set out at each site in April 2019, stocked with 13.6 L of water and leaf detritus, and left undisturbed for colonization until mid-May. At the same time, we located, marked, and measured for volume naturally occurring treeholes (numbers of treeholes and buckets, and sampling methods, given in Appendix S1: Table S2). We sampled fortnightly from mid-May through September (9 sample periods, capturing seasonal variation in abundances of larval A. triseriatus, which decrease, and other Aedes species, which increase, as the season progresses (Westby et al. 2015). All mosquito larvae were identified to species or genus. Up to 10 A. triseriatus 3rd or 4th instar larvae were dissected from each container to obtain A. barretti parasite count (trophozoites per larva) and infection prevalence (Sulaiman 1992).
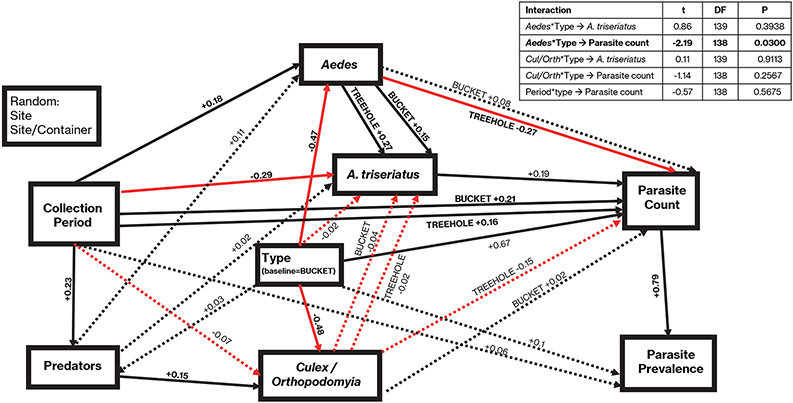
A path diagram (Figure 3) depicts the hypothesized relationships among groups within the resulting dataset, focusing on direct and indirect effects of larval mosquito community members’ abundances on A. barretti parasite count and prevalence in A. triseriatus. Our model contains 9 manifest variables: Aedes abundance (all non A. triseriatus Aedes species: A. albopictus, A. japonicus, and A. hendersoni), Culex/Orthopodomyia abundances (grouped as these species spend less time foraging at the bottom and more time filtering and at the surface (Zavortink 1968, Yee et al. 2004), Predator abundance (Toxorhynchites and Anopheles species), A. triseriatus abundance, container Type (Bucket or Treehole), Collection Period (as repeated measures), Infection Prevalence, Parasite Count, and Site (a random effect). Parasite Count (=mean number of parasites in a sample of hosts) was used as a measure of infection intensity so that our path analysis could include samples with no parasitized hosts. Traditional calculation of infection intensity (Blackmore et al. 1995) excludes uninfected individuals, which would have excluded samples with mean infection prevalence of zero from our analysis. All quantitative variables except Collection Period and Infection Prevalence were log transformed to meet the assumption of multivariate normality. Three interaction terms were included to test whether effects differ for long-standing treeholes vs. newly-placed buckets: Type X Aedes, Type X Culex/Orthopodomyia, and Type X Collection Period.
Figure 3.
pSEM model (C-stat12=8.718, p=0.727 AICc=177.119) testing the hypothesis that other Aedes cause dilution of A. barretti infection in A. triseriatus. Boxes represent abundances of species groups and A. barretti infection rates in A. triseriatus in community survey samples. Black and red lines show positive and negative direct path coefficients, respectively; solid and dashed lines of both colors show direct path coefficients significantly and not significantly different from 0, respectively, at α=0.05. Bivariate plots of abundances and parasite counts are provided in Appendix S2: Figures S1 and S2.
Our model represented the postulated causal relationships predicted by our hypothesis of dilution of infection. Our tests of hypotheses for dilution of infection focus on paths from Aedes and Culex/Orthopodomyia to A. triseriatus, where negative path coefficients are predicted by the hypothesis of dilution by Host-Host Competition on A. triseriatus, and direct paths from Aedes and Culex/Orthopodomyia to Parasite Count, where negative path coefficients are predicted by the hypothesis of dilution by Encounter Reduction. Our parasite measure quantifies an early developmental stage of the parasite lifecycle, so reductions in this early stage represent reductions in host encounters with the parasite. Paths from A. triseriatus to other mosquito groups were excluded because our analysis method requires models to be acyclic (Lefcheck 2016) and effects of Aedes and Culex/Orthopodomyia groups on A. triseriatus are critical for testing our hypotheses. We analyzed these data using the piecewiseSEM package V2.1.0 (Shipley 2009, 2013, Lefcheck 2016, 2019, R version 4.0.3; details given in Appendix S1).
Results
Laboratory Manipulations
Trophozoite infection of larvae:
There were no significant effects of Early (F1,35=0.18, P=0.6730) or Late (F1,35=0.68, P=0.4163) cohabitant treatments, or of the interaction (F1,35=0.32, P=0.5781), on larval infection intensity (Appendix S1: Table S3). These results are not consistent with our prediction for dilution by either mechanism.
Gametocyst infection of adults:
Neither gametocyst prevalence nor gametocyst infection intensity in males was impacted by Early or Late cohabitant treatments (Appendix S1: Table S4). Gametocyst prevalence in females was significantly greater in females exposed to a Late cohabitant (Appendix S1: Table S4, Figure 1a). Gametocyst infection intensity was increased by an Early cohabitant in female hosts (Appendix S1: Table S4, Figure 1b). The results of female gametocyst infection are contrary to those predicted for dilution by either mechanism.
Figure 1.

A. Gametocyst prevalence for females showing greater prevalence in females exposed to Late cohabitant. B. Mean gametocysts/infected female, showing greater intensity in hosts exposed to Early cohabitant. C. Oocyst prevalence showing greater prevalence with Late cohabitant. D. Oocyst intensity showing decreased intensity in individuals exposed to Early cohabitation, though non-significant at α=0.05 (Appendix S1, Table S4). Oocyst values combined for males and females. All LSMeans±SE. Expanded in Appendix S2: Figure S3.
Oocyst infection of adults and pupae:
Late cohabitant significantly increased oocyst prevalence (Appendix S1: Table S4, Figure 1c), which is not consistent with dilution of infection by competition. Early cohabitant tended to result in fewer oocysts in host individuals, but the effect was not significant (Appendix S1: Table S4, Figure 1d).
Parasite Fitness:
Parasite Fitness (oocysts per exposed host) was not significantly affected by Early cohabitants (F1,116=0.63, P=0.4281), Late cohabitants (F1,116=0.00, P=0.9510), or interaction (F1,116=0.32, P=0.5705).
Host fitness:
Early+ and Early− had similar, low mortality during the first 72 hours (4/200 and 5/200 individuals, respectively), consistent with limited competitive effect of the Early cohabitant. There were significant interactions of parasite and Late cohabitant and of parasite and Early cohabitant affecting host survivorship to adulthood (Appendix S1: Table S5). The interaction of parasite and Early cohabitant arose because of reduced survivorship in individuals exposed to the parasite, but only in the absence of the early cohabitant (Figure 2a). The interaction of parasite and Late cohabitant indicated reduced survivorship in individuals exposed to the parasite when experiencing Late cohabitant (Figure 2b). Host development time was analyzed by sex. Both Late cohabitant and Early cohabitant, but not parasite, increased development time of males (Appendix S1: Table S5, Figure 2c, d). Early cohabitant increased female development time but only in the absence of parasites (Appendix S1: Table S5, Figure 2e). The interaction of Late cohabitant and Parasite was also significant for female development time, with Late cohabitant increasing development time for Parasite+ females (Appendix S1: Table S5, Figure 2f). All of these increases in development time with Early or Late cohabitant are consistent with Host-Host competition but not with dilution. Analysis of female size yielded no significant effects for all main effects and interactions (Appendix S1: Table S5).
Figure 2.

LSMeans±SE for significant effects for survivorship and days from hatch to emergence. Panel A: interaction of Early cohabitant * Parasite showing lesser survivorship with Early cohabitant in parasite+ individuals. Panel B: interaction of Late cohabitant * Parasite showing lesser survivorship with Late cohabitant, when exposed to the parasite. For Parasite−, the addition of Late cohabitant increased survival. Panel C & D: increased development time for males with Early and Late cohabitant, respectively. E: interaction of Early cohabitation and Parasite for Females; Early cohabitants significantly increased development time only in the absence of the parasite. F: interaction of Late cohabitant and Parasite for Females; Late cohabitant increased development time, only in parasite+. Expanded in Appendix S2: Figure S4.
Community Survey
Field sampling yielded 179 observations (wet container-date combinations with A. triseriatus) that could be used in piecewise SEM. The C statistic yielded acceptable fit results (Figure 3). In our model, A. triseriatus abundance had a significant positive relationship to A. barretti parasite count (directly) and was positively related to prevalence (indirectly) (Figure 3). As this path was positive, a significant negative effect of our other mosquito groups on A. triseriatus would multiplicatively indicate the potential for an indirect negative effect of these groups on A. barretti infection in A. triseriatus. However, neither Aedes nor Culex/Orthopodomyia group abundances had significant direct negative effects on abundance of A. triseriatus larvae, thus giving no evidence for dilution of infection via indirect effects of competition on A. triseriatus abundance (Figure 3). There was no significant direct effect of Culex/Orthopodamyia abundance on Parasite count (Figure 3). The interaction of Aedes X Type was significant, with a significant, negative effect of Aedes abundance on Parasite Count only in Treeholes (Figure 3). No other interaction had a significant effect on Parasite Count (Figure 3). Our model provides support for dilution via encounter reduction by Aedes only in treeholes, and no support for dilution via host-host competition in either habitat type.
Discussion
Our results show how a non-competent invader can alter a native host/parasite relationship, but our laboratory and field data yield different conclusions about mechanisms, likely because laboratory and field designs differ in their ability to detect different dilution mechanisms. In the laboratory, we found no evidence for dilution by encounter reduction, and individual, host-host competition amplified parasite success. In contrast, our field data support the hypothesis of parasite dilution with increased abundance of the invader.
Infection prevalence, for both gametocysts in females and oocysts, increased in the presence of the Late cohabitant (Figure 1a,c, Appendix Sl: Table S4). As no difference in the trophozoite stage was detected, this indicates an increase in parasite success caused by the per capita effect of a noncompetent, competing host. Trophozoite failure in the native host is often due to unsuccessful migration to the Malpighian tubules during pupation (Chen 1999, 2013), which may be alleviated by prolonged larval development due to interspecific competition. In this system, parasite migration is cued not by parasite development, but by the timing of host hormonal cues, with short duration of the larval stage of the host equating to decreased time for parasite development (Chen 1999). Thus, increased parasite success may have been facilitated by extended development time due to the competitor.
Survivorship of A. triseriatus to adulthood was reduced for infected individuals; paradoxically, this effect was negated by the early presence of a single individual of the competitor (Figure 2a). This positive effect of a heterospecific larva early, when A. triseriatus was exposed to oocysts, is not, however, consistent with dilution via encounter reduction because larval infection intensity was unaffected by an Early cohabitant. If that Early cohabitant caused encounter reduction via its foraging, infections of A. triseriatus larvae should have been less prevalent or less intense. This effect of an early cohabitant on survivorship is also not consistent with host-host competition. A late cohabitant reduced survivorship of parasitized A. triseriatus larvae (Figure 2b), indicating that competition could contribute to dilution if that greater mortality of parasitized larvae reduced parasite oocyst production, which it did not (Figure 1a, c).
Our laboratory data show that the invader can alter the host/parasite interaction, but also indicate that the per capita Dilution Effect of a young larva is weak, to the point of undetectable. Our measure of oocyst intensity suggests that the abundance of oocysts may be slightly, but not significantly, reduced by the presence of the competitor during the early larval stage (Appendix S1: Table S1, Figure 1d), but such a reduction also was not detected in our analysis of Parasite Fitness. The failure to detect any effect of early competition on infection intensity in larvae suggests that any effect on oocyst production is not a product of encounter reduction, but rather results from negative effects of the early competitor on successful completion of development of the parasite within the host, once infected. In contrast, the early competitor significantly increased the number of gametocysts produced (Appendix S1: Table S1, Figure 1b), which is inconsistent with dilution by competition. Overall, these laboratory results are not consistent with a per capita Dilution Effect of the parasite by the invader by either proposed mechanism. Competition from the invader, particularly late in development, appears more likely to increase parasite success once it infects the native host, suggesting that the per capita competitive effect of the invader synergistically modifies the host-parasite interaction, resulting in amplification of parasite success. This is inconsistent with dilution, and with outcomes of interactions between A. triseriatus / A. barretti and another invasive mosquito, A. japonicus (Westby et al. 2019a).
The larval habitat of A. triseriatus was occupied by a variable assemblage of mosquitoes. Seasonal variation in host assemblages may play a role in the dynamics of the host-parasite relationship, via variation in competence of cohabiting species and effects on parasite-competent host encounter rates (Telfer & Bown 2012). Larvae of A. japonicus and A. albopictus, nonnative mosquitoes often found in A. triseriatus’ larval habitat, can be efficient at removal of parasite oocysts from the environment but have minimal competence as hosts (Westby et al. 2019, McIntire unpublished data). Both species commonly (~43% of A. triseriatus-containing samples) shared the larval habitat with A. triseriatus in our survey. We found a strong positive effect of sample period on their occurrence (Figure 3), with A. japonicus more prevalent in mid-season and A. albopictus more prevalent in the last third of the season. We found no significant negative impacts of our Aedes group on A. triseriatus abundance. Direct effects of abundance of Aedes on A. triseriatus in our path model were positive (Figure 3), suggesting shared responses to unmeasured environmental variables (e.g., resource availability).
In contrast to our laboratory data, our field data and analysis provide relatively strong support for the hypothesis of dilution by encounter reduction, but only for Aedes as a group, and only in the environmental context of natural treeholes. The negative direct impact of our Aedes group abundance on A. barretti Parasite Count in A. triseriatus in treeholes (Figure 3) suggests a direct effect of the Aedes group on Parasite Count, and not an indirect effect via competition with A. triseriatus, which was not evident in our field data. Our field data cannot rule out such indirect effects in other ecological contexts; however, our laboratory results suggest that competitive effects of other Aedes on A. triseriatus are more likely to produce parasite amplification, possibly via prolonged A. triseriatus development or reduced immune defenses, in competitively stressed larvae. Interspecific competition with A. japonicus can decrease A. triseriatus larval development time compared to intraspecific competition only (Hardstone & Andreadis 2012). Altered development timing due to temperature can reduce Ascogregarina reproduction in hosts developing quickly in warm conditions (Chen & Yang 1996), and such a competitive effect of A. japonicus on timing could contribute to parasite dilution. Any impact of such decreased development time on parasite abundance would not be identifiable in our sampling as we could not track larval development time in the field, and our assessment of Parasite Count was for a pre-reproductive parasite life stage. Although such reduced development time could be a mechanism by which competing Aedes group reduces parasitism of A. triseriatus by A. barretti in our field samples, our laboratory data show that the per capita effect of competition with A. albopictus is more likely to prolong development of A. triseriatus (Figure 2c-f) and in any case often results is parasite amplification (Figure 1a,c). The significant direct negative path coefficient for impact of Aedes abundance on parasite count in larval A. triseriatus thus is best explained by encounter reduction caused by Aedes abundance in the field. This result, coupled with the absence of any evidence for per capita encounter reduction in the laboratory, suggests that dilution in this system is highly dependent on abundance of the dilution hosts.
Our field data raise the question: What is different between treeholes, where we detect a significant effect of abundance of Aedes reducing A. barretti parasitism, and buckets, where we detect no effect of Aedes abundance (Figure 3)? Among the obvious differences between the two habitats are that the treeholes have a longer water-holding history (years) compared to the buckets that were placed in the sites in the year of the investigation. This longer history may have contributed to a greater buildup of A. barretti oocysts, an effect found for treehole vs. human-made containers (Van Rhein et al. 2000) and in older vs. younger containers (Aliabadi & Juliano 2002; K. Medley, personal communication). Our path analysis showed that prevalence and abundance of A. barretti parasitism were greater in treeholes than in buckets (Figure 3, significant positive direct Type effect on parasite abundance, and indirect effect on prevalence), with a mean burden in treeholes 4 to 14 times greater than that in buckets at comparable sampling dates (see below). This greater abundance of the parasite may allow for a greater scope for dilution of parasitism by encounter reduction. Likely of equal importance is the smaller volume of treeholes vs. buckets (initial volumes 0.045-5.0 L vs. 13.6 L, respectively) which would increase the potential impact of larvae on the environment.
No sample period effect was found for Culex/Orthopodomyia, which occurred in ~45% of our A. triseriatus-containing samples. We found no significant direct effect of Culex/Orthopodomyia abundance on parasite abundance in either habitat, as we expected based on their habit of foraging near the surface (Zavortink 1968, Yee et al. 2004). The small estimated effect in treeholes was negative (Figure 3). We found no significant competitive effect of Culex/Orthopodomyia on A. triseriatus abundance, which is consistent with previous studies of competition between Aedes sp. and Culex (e.g., Costanzo et al. 2005, Murrell and Juliano 2012, Costanzo et al. 2014, Santana-Martinez et al. 2017, but see Müller at al. 2018) or A. triseriatus and O. signifera (Livdahl 1984, Chambers 1985).
Comparison of parasite burden in the field vs. our laboratory experiment indicates comparable parasite burdens in both venues. Parasite burden in the field was significantly affected by day, container type, and interaction using a repeated measures, generalized linear model with site random and a negative binomial distribution (Appendix S1). Mean±SE trophozoite burden per sample day in treeholes and buckets ranged from 14.8±6.4 to 98.4±47.6 and 0.9±0.6 to 23.6±9.2, respectively, and increased over time in both habitats. Mean±SE trophozoite burdens in the laboratory experiment were similar: Trial 1 - 85.6±13.8, Trial 2 - 33.9±5.9. We find evidence for dilution by encounter reduction in treeholes in the field, where burden is greatest, suggesting that doses that would produce the burdens we see in the laboratory would still allow for dilution by encounter reduction, if there were sufficient numbers of dilution hosts. We interpret these results to indicate that lack of encounter reduction in the lab is not a result an excessive dose of oocysts, but rather of the inability of 1 small larva to produce the effect. The per capita effect is small at least for a single young larva, and it is abundance of dilution hosts that yields the effect.
Synthesizing our laboratory and field studies involves two issues: Why is host-host competition undetected in the field? And what do we expect for a per capita effect of a cohabiting competitor? Our field study quantifies abundances of A. triseriatus and its competitors, and thus will only detect host-host competition effects if it reduces A. triseriatus abundance. Reduced survivorship is suggested by the negative effect of late competition on parasitized hosts in the laboratory (Figure 2b). Sublethal effects of competition (e.g., slowed development and growth) would not be detected in the field. We see evidence in the field for dilution at the trophozoite stage suggesting encounter reduction. Future field studies should quantify parasite later stages (gametocysts, oocysts) that could provide information about these sublethal effects of a competitor on parasite success. Our laboratory experiment provides an answer for the second question of the expected effect of host-host competition on this parasite. Competition is expected to reduce the resources (food) available to an infected host, which could reduce parasite success in a host because the parasite also has reduced resources, which predicts dilution by host-host competition. Alternatively, low resources could reduce the host’s ability to mount effective immune defenses, if defenses are costly (which is widely assumed), which predicts amplification by host-host competition. If both effects occur, the net result will depend on their relative impacts on the parasite. Our laboratory experiment showed that presence of the late competitor, after infectious oocysts were removed, amplified later gametocyst and oocyst stages (Figure 1a,c), slowed host development, and for parasitized females reduced host survival to adulthood (Figure 2b,d,f), suggesting that host debilitation and reduced defenses result in a stronger amplification effect of host-host competition. Westby et al. (2019b) found effects of host food availability on Ascogregarina, but did not test for parasite development to reproduction.
Our results demonstrate that the invasive host impacts not only A. triseriatus development time and mortality, but also A. barretti infection dynamics. This work highlights the difficulty in predicting impacts of invasive species in natural communities that include host-parasite interactions, as it is difficult to predict the importance of interactions and interaction modification involving parasites. Thus, it may be common in diverse systems for invasive species to produce a dilution effect (e.g., Gendron & Marcogliese 2016, Westby et al. 2019a, Tierney et al. 2020), but the mechanisms behind that effect may vary and alternative impacts of invasion are possible (Loxton et al. 2017, Luis et al. 2018). Thus, modification of host-parasite interactions by invasive species is an important topic for investigations of community ecology and epidemiology.
Supplementary Material
Acknowledgments
We thank J.A. Farrell, R.D. Farrell, J.A. McIntire, G.A. McIntire, and M. Gonzalez for aid in the laboratory and the field; V.A. Borowicz, A. Strauss, and an anonymous referee for helpful comments; NIAID grants to SAJ (1R15AI094322-01A1 and 1R15AI124005-01); R.D. Weigel grants from the Beta Lambda chapter of Phi Sigma Biological Honor Society and a Dr. Robert H. Gray Ecology/Biology Scholarship to KMM.
Data Availability:
The data that support the findings of this study are openly available at https://figshare.com in the project https://figshare.com/projects/McIntire_Chappell_Juliano_2021_Ecology_-_Dilution/111554
References
- Aliabadi BW and Juliano SA. 2002. Escape from gregarine parasites affects the competitive interactions of an invasive mosquito. Biol. Invasions 1985, 283–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspbury AS & Juliano SA 1998. Negative effects of drying and prior exploitation on the detritus resource in an ephemeral aquatic habitat. Oecologia 115:137–148. [DOI] [PubMed] [Google Scholar]
- Beier JC, and Craig GB. 1985. Integrated mosquito control methodologies Vol 2. Academic Press. [Google Scholar]
- Bevins SN 2007. Timing of resource input and larval competition between invasive and native container inhabiting mosquitoes (Diptera: Culicidae). J. Vector Ecol 32:252–62 [DOI] [PubMed] [Google Scholar]
- Blackmore MS, Scoles GA, and Craig GB Jr. 1995. Parasitism of Aedes aegypti and Ae. albopictus (Diptera: Culicidae) by Ascogregarina spp. (Apicomplexa: Lecudinidae) in Florida. J. Med. Entomol 32(6):847–852. [DOI] [PubMed] [Google Scholar]
- Chambers RC 1985. Competition and predation among larvae of three species of treehole breeding mosquitoes. pp. 25–54 in Lounibos LP, Rey JR, Frank JH, eds. 1985. Ecology of Mosquitoes: Proceedings of a Workshop. Vero Beach: Fla. Med. Entomol. Lab. [Google Scholar]
- Chen WJ, Huang CG, Fan-Chiang MH, Liu YH, and Lee YF. 2013. Apoptosis of Ascogregarina taiwanensis (Apicomplexa: Lecudinidae), which failed to migrate within its natural host. J of Exper. Biol, 216:230–235. [DOI] [PubMed] [Google Scholar]
- Chen WJ, 1999. The life cycle of Ascogregarina taiwanensis (Apicomplexa:Lecudinidae). Parasitol. today 15(4):153–156. [DOI] [PubMed] [Google Scholar]
- Chen WJ and Yang CH. 1996. Developmental synchrony of Ascogregarina taiwanensis (Apicomplex: Lecunidinidae) in Aedes albopictus (Diptera: Culicidae). J Med. Entomol 33(2):212–215. [DOI] [PubMed] [Google Scholar]
- Chmielewski MW, Khatchikian C, and Livdahl T. 2010. Estimating the per capita rate of population change: How well do life-history surrogates perform? Ann. Entomol Soc. Am 103(5):734–741 [Google Scholar]
- Civitello DJ, Cohen J, Fatima H, Halstead NT, Liriano J, McMahon TA, Ortega CN, Sauer EL, Sehgal T, Young S, and Rohr JR. 2015. Biodiversity inhibits parasites: Broad evidence for the dilution effect. Proc. Natl. Acad. Sci. U.S.A, 112(28):8667–8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo KS, Mormann K, and Juliano SA. 2005. Asymmetrical competition and patterns of abundance of Aedes albopictus and Culex pipiens (Diptera: Culicidae). J Med. Entomol 42:559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo KS, Muturi EJ, Lampman RL, and Alto RW. 2014. The effect of resource type and ratio on competition with Aedes albopictus and Culex pipiens (Diptera: Culicidae) J Med Entomol. 48(1):29–38. [DOI] [PubMed] [Google Scholar]
- Fellous S, and Koella JC. 2009. Infectious dose affects the outcome of the within-host competition between parasites. The Am. Nat 173:177–184 [DOI] [PubMed] [Google Scholar]
- Gendron AD and Marcogliese DJ. 2016. Reduced survival of a native parasite in the invasive round goby: Evidence for the dilution hypothesis? Aquat. Invasions 11(2):189–198. [Google Scholar]
- Hardstone MC and Andreadis TG. 2012. Weak larval competition between the invasive mosquito Aedes japonicus japonicus (Diptera:Culicidae) and three resident container-inhabiting mosquitoes in the laboratory. J Med. Entomol 49(2):277–285. [DOI] [PubMed] [Google Scholar]
- Johnson P, Dobson AP, Lafferty K, Marcogliese DJ, Memmott J, Orlofske SA, Poulin R, Thieltges DW. 2010. When parasites become prey: Ecological and epidemiological significance of eating parasites. Trends Ecol Evol. 25(6):362–371. [DOI] [PubMed] [Google Scholar]
- Juliano SA 2009. Species interactions among larval mosquitoes: Context dependence across habitat gradients. Annu Rev. Entomol 54:37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing F, Holt RD, and Ostfeld RS. 2006. Effects of species diversity on disease risk. Ecol. Lett 9:485–498. [DOI] [PubMed] [Google Scholar]
- Kutzer MA and Armitage SA. 2016. Maximizing fitness in the face of parasites: a review of host tolerance. Zoology 119(4):281–289. [DOI] [PubMed] [Google Scholar]
- Lefcheck JS 2016. piecewiseSEM: Piecewise structural equation modeling in R for ecology, evolution, and systematics. Methods Eco Evol. 7(5): 573–579. [Google Scholar]
- Lefcheck J 2019. Structural equation modeling in R for Ecology and Evolution. https://jslefche.github.io/sem_book/Accessed: 11/17/2020 [Google Scholar]
- Liu H and Stirling P. 2006. Testing the enemy release hypothesis: a review and meta-analysis. Biol. Invasions 8:1535–1545. [Google Scholar]
- Livdahl TP 1984. Interspecific interactions and the r-K continuum: laboratory comparisons of geographic strains of Aedes triseriatus. Oikos 42:193–202 [Google Scholar]
- Livdahl TP and Sugihara G. 1984. Non-linear interactions of populations and the importance of estimating per capita rates of change. J Anim. Ecol 53(2):573–580 [Google Scholar]
- Livdahl TP and Willey MS. 1991. Prospects for an invasion: competition between Aedes albopictus and native Aedes triseriatus. Science 253, 189–191. [DOI] [PubMed] [Google Scholar]
- Llopis-Belenguer C, Blasco-Costa I, Balbuena JA, Sarabeev V and Stouffer DB. 2020. Native and invasive hosts play different roles in host–parasite networks. Ecography 43: 559–568. doi: 10.1111/ecog.04963 [DOI] [Google Scholar]
- Lord CC, Alto BW, Anderson SL, Connelly CR, Day J, Richards SL, Smart CT, and Tabachnick WJ. 2014. Can horton hear the whos? The importance of scale in mosquito-borne disease. J. Med. Entomol 51(2), 297–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loxton K, Lawton C, Stafford P, and Holland C. 2017. Parasite dynamics in an invaded ecosystem: Helminth communities of native wood mice are impacted by the invasive bank vole. Parasitology, 144(11): 1476–1489. doi: 10.1017/S0031182017000981 [DOI] [PubMed] [Google Scholar]
- Luis AD, Kuenzi AJ, and Mills JN. 2018. Species diversity concurrently dilutes and amplifies transmission in a zoonotic host-pathogen system through competing mechanisms. Proc. Natl. Acad. Sci. U.S.A, 115(31):7979–7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire KM and Juliano SA 2021. Detrimental effects of a failed infection by a co-invasive parasite on a native congeneric parasite and its native host. Biol. Invasions DOI: 10.1007/s10530-021-02464-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R, Knautz T, Vollroth S, Berger R, Kreß A, Reuss F, Groneberg DA, and Kuch U. 2018. Larval superiority of Culex pipiens to Aedes albopictus in a replacement series experiment: prospects for coexistence in Germany. Parasites & Vectors 11:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell EG and Juliano SA. 2012. Competitive abilities in experimental microcosms are accurately predicted by a demographic index for R*. PLoS ONE 7(9): e43458. doi: 10.1371/journal.pone.0043458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostfeld R and Keesing F. 2000a. Biodiversity and disease risk: The case of Lyme Disease. Conservation Biol. 14(3):722–728. [Google Scholar]
- Ostfeld RS and Keesing F. 2000b. Biodiversity series: The function of biodiversity in the ecology of vector-borne zoonotic diseases. Can. J. of Zool 78(12):2061–2078. [Google Scholar]
- Ostfeld RS and Keesing F. 2012. Effects of host diversity on infectious disease. Annu. Rev. Ecol. Evol. Syst 43:157–182. [Google Scholar]
- Poulin R (2017) Invasion ecology meets parasitology: Advances and challenges. Int J Parasitol: Parasit Wildl 6(3):361–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price PW, Westoby M, Rice B, Atsatt PR, Fritz RS, Thompson JN, and Mobley K. 1986. Parasite mediation in ecological interactions. An. Rev. Ecol. Syst 17:487–505. [Google Scholar]
- Randolph SE and Dobson ADM. 2012. Pangloss revisited: a critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology, 139(7):847–863. [DOI] [PubMed] [Google Scholar]
- Restif O and Koella JC. 2004. Concurrent evolution of resistance and tolerance to pathogens. Am. Nat 164(4):E90–E102. [DOI] [PubMed] [Google Scholar]
- Roberts MG and Heesterbeek JAP. 2018. Quantifying the dilution effect for models in ecological epidemiology. J Royal Society interface. 15(140):20170791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Civitello DJ, Halliday FW, Hudson PJ, Lafferty KD, Wood CL, and Mordecai EA. 2020. Towards common ground in the biodiversity–disease debate. Nature Ecology and Evolution 4:24–33. 10.1038/s41559-019-1060-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana-Martinez JC, Molina J, and Dussán J. 2017. Asymmetrical competition between Aedes aegypti and Culex quinquefasciatus (Diptera:Culicidae) coexisting in breeding sites. Insects 8(4):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle CL, Cortez MH, Hunsberger KK, Grippi DC, Olesky IA, Shaw CL, de la Serna SB, Lash CL, Dhir KL, and Duffy MA. 2016. Population density, not host competence, drives patterns of disease in an invaded community. Am Nat. 188(5):554–566 [DOI] [PubMed] [Google Scholar]
- Shipley B 2009. Confirmatory path analysis in a generalized multilevel context. Ecology, 90(2), 363–368. [DOI] [PubMed] [Google Scholar]
- Shipley B 2013. The AIC model selection method applied to path analytic models compared using a d-separation test. Ecology 94(3): 560–564. [DOI] [PubMed] [Google Scholar]
- Siegel JP, Novak RJ, and Maddox JV. 1992. Effects of Ascogregarina barretti (Eugregarinida: Lecudinidae) Infection on Aedes triseriatus (Diptera: Culicidae) in Illinois. J Med. Entomol 29, 968–973. [DOI] [PubMed] [Google Scholar]
- Smith C, Baldwin AH, Sullivan J, and Leisnham PT. 2013. Effects of elevated atmospheric CO2 on competition between the mosquitoes Aedes albopictus and Ae. triseriatus via changes in litter quality and production. J. Med. Entomol 50(3): 521–532 [DOI] [PubMed] [Google Scholar]
- Soghigian J and Livdahl T. 2017. Differential response to mosquito host sex and parasite dosage suggest mixed dispersal strategies in the parasite Ascogregarina taiwanensis. PlosOne, 12(9):e0184573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss AT, Civitello DJ, Cáceres CE and Hall SR, S.R. 2015. Success, failure and ambiguity of the dilution effect among competitors. Ecol Lett, 18: 916–926. 10.1111/ele.12468 [DOI] [PubMed] [Google Scholar]
- Strauss AT, Schocket MS, Civitello DJ, Hite JK, Penczykowski RM, Duffy MA, Cáceres CE, and Hall SR. 2016. Habitat, predators, and hosts regulate disease in Daphnia through direct and indirect pathways. Ecological Monographs. 86:393–411. [Google Scholar]
- Strauss AT, Bowling AM, Duffy MA, Cáceres CE, and Hall SR. 2018. Linking host traits, interactions with competitors and disease: Mechanistic foundations for disease dilution. Functional Ecology. 32:1271–1279. [Google Scholar]
- Sulaiman I, 1992. Infectivity and pathogenicity of Ascogregarina culicis (Eugregarinida: Lecudinidae) to Aedes aegypti (Diptera: Culicidae). J Med. Entomol, 29:1–4. [DOI] [PubMed] [Google Scholar]
- Telfer S and Bown K. 2012. The effects of invasion on parasite dynamics and communities. Funct. Ecol 26(6): 1288–1299. [Google Scholar]
- Tierney PA, Caffrey JM, Vogel S, Matthews SM, Costantini E, and Holland CV. (2020). Invasive freshwater fish (Leuciscus leuciscus) acts as a sink for a parasite of native brown trout Salmo trutta. Biol. Invasions 22:2235–2250 [Google Scholar]
- Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, and Kuris AM. 2003. Introduced species and their missing parasites. Nature. 421:628–630. [DOI] [PubMed] [Google Scholar]
- Tseng M, 2007. Ascogregarine parasites as possible biocontrol agents. J Am. Mosquito Control Assoc, 23:30–34. [DOI] [PubMed] [Google Scholar]
- Van Rhein SL, Flanary BE, and Juliano SA. 2000. Effects of habitat type and drying on Ascogregarina barretti (Eugregarinida: Lecudinidae) infection in Aedes triseritatus (Diptera: Culicidae). J. Med. Entomol 37:950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venesky MD, Liu X, Sauer EL, and Rohr JR. 2014. Linking manipulative experiments to field data to test the dilution effect. J. Anim. Ecol 83, 557–565. [DOI] [PubMed] [Google Scholar]
- Westby KM, Fritzen C, Paulsen D, Poindexter S, and Moncayo AC. 2015. La Cross Encephalitis Virus infection in field-collected Aedes albopictus, Aedes japonicus, and Aedes triseriatus in Tennessee. J. Am. Mosquito Control Assoc 31(3):233–241. [DOI] [PubMed] [Google Scholar]
- Westby KM, Sweetman BM, Adalsteinsson SA, Biro EG, and Medley KA. 2019b. Host food quality and quantity differentially affect Ascogregarina barretti parasite burden, development and within-host competition in the mosquito Aedes triseriatus. Parasitology 146:1665–1672. 10.1017/S0031182019000994 [DOI] [PubMed] [Google Scholar]
- Westby KM, Sweetman BM, Van Horn TR, Biro EG, and Medley KA. 2019a. Invasive species reduces parasite prevalence and neutralizes negative environmental effects on parasitism in a native mosquito. J Anim. Ecol 88(8):1512–1225. [DOI] [PubMed] [Google Scholar]
- Wood CL and Lafferty KD. 2013. Biodiversity and disease: A synthesis of ecological perspectives on Lyme disease transmission. Trends Ecol. Evol 28:239–247. [DOI] [PubMed] [Google Scholar]
- Yee DA, Kaufman MG, and Juliano SA. 2007. The significance of ratios of detritus types and microorganism productivity to competitive interactions between aquatic insect detritivores. J. Anim. Ecol 76:1105–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Kesavaraju B, and Juliano SA. 2004. Larval feeding behavior of three co-occurring species of container mosquitoes. J Vector Ecol. 29(2): 315–322. [PMC free article] [PubMed] [Google Scholar]
- Young H, Parker I, Gilbert G, Guerra A, and Nunn C. 2017. Introduced species disease ecology, and biodiversity- disease relationships. Trends Ecol. Evol 32:41–54. [DOI] [PubMed] [Google Scholar]
- Zavortink TJ 1968. Mosquito studies (Diptera, Culicidae). VIII. A prodrome of the genus Orthopodomyia. Contributions of the American Entomological Institute. 3(2):1–221. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available at https://figshare.com in the project https://figshare.com/projects/McIntire_Chappell_Juliano_2021_Ecology_-_Dilution/111554