Abstract
Background:
Posttraumatic stress disorder (PTSD) has been associated with increased cardiovascular risk, however, underlying mechanisms have not been fully specified. PTSD is associated with stress-related hormones, including dysregulated glucocorticoid activity. Dysregulation of aldosterone, a mineralocorticoid activated by psychological stress and implicated in cardiovascular damage, may be a relevant pathway linking PTSD and cardiovascular risk. Few studies to date have evaluated the association between PTSD and aldosterone, none with repeated measures of aldosterone. We examined if trauma and PTSD were associated with altered aldosterone levels relative to women unexposed to trauma.
Methods:
The association of trauma exposure and chronic PTSD with plasma aldosterone levels was investigated in 521 middle-aged women in the Nurses’ Health Study II. Aldosterone was assessed at two time points, 10-16 years apart, and trauma exposure and PTSD were also ascertained for both time points. Regarding exposure assessment, women were characterized based on a structured diagnostic interview as: having chronic PTSD (PTSD at both time points; n=174); being trauma-exposed (trauma exposure at first time point but no PTSD; n=174); and being unexposed (no trauma exposure at either time point; reference group for all analyses; n=173). Linear mixed models examined associations of trauma and PTSD status with log-transformed aldosterone levels, adjusting for covariates and health-related variables that may confound or lie on the pathway between PTSD and altered aldosterone levels.
Results:
Across the sample, mean aldosterone concentration decreased over time. Adjusting for covariates, women with chronic PTSD had significantly lower aldosterone levels averaged over time, compared to women unexposed to trauma (β=−0.08, p=0.04). Interactions between trauma/PTSD group and time were not significant, indicating change in aldosterone over time did not differ by trauma/PTSD status. Post-hoc exploratory analyses suggested that menopausal status partially mediated the relationship between chronic PTSD status and aldosterone level, such that postmenopausal status explained 7% of the effect of PTSD on aldosterone.
Conclusions:
These findings indicate that PTSD is associated with lower levels of aldosterone. Further work is needed to understand implications of this type of dysregulation in a key biological stress system for cardiovascular and other health outcomes previously linked with PTSD.
Keywords: posttraumatic stress disorder, aldosterone, trauma, women
1. Introduction
Posttraumatic stress disorder (PTSD) is linked to higher cardiovascular disease (CVD) risk, relative to both trauma-exposed individuals without PTSD and unexposed controls (Brudey et al., 2015). Further, trauma exposure itself has been associated with CVD risk (Le Carolyn et al., 2013). However, mechanisms underlying the relationships between trauma, PTSD, and CVD are not yet clear. A biological pathway potentially connecting PTSD to cardiovascular injury is the renin-angiotensin-aldosterone system (Ressler et al.) (Ressler et al.), a neuro-hormonal system that involves a biological cascade of renin from the kidneys, angiotensin in blood and tissues, and ultimately aldosterone secreted from the adrenal cortex.
Aldosterone activates type I glucocorticoid receptor (GR) (mineralocorticoid receptor), which regulates renal sodium and potassium handling and is involved in blood pressure regulation (Murck et al., 2014). Excess type I GR activity has pro-inflammatory effects that lead to tissue damage throughout the cardiovascular system (He and Anderson, 2013; Xanthakis and Vasan, 2013). Consistent evidence links altered aldosterone or aldosterone-renin ratios (generally higher levels) with increased risk of hypertension, congestive heart failure, and stroke (Gaddam et al., 2009; He and Anderson, 2013; Xanthakis and Vasan, 2013). Aldosterone is also associated with stress responses and mood regulation. Key biological systems, including the sympathetic nervous system (SNS), are activated by stress and SNS in turn activates the renin-angiotensin-aldosterone system (Ressler et al.) and the hypothalamic–pituitary–adrenal (HPA) axis. Consistent with these processes, acute stress responses have been linked to increases in circulating aldosterone levels (Kubzansky and Adler, 2010) and RAAS activation more broadly (Groeschel and Braam, 2011), as recently demonstrated in an experimental study of acute stress and RAAS parameter activity (Gideon et al., 2020). However, additional studies suggest that chronic stressors (e.g., living alone) and psychological distress (e.g., depressive symptoms, suicidality) are linked to RAAS activation in complex ways. For example, in one study living alone while experiencing chronic distress was associated with elevated aldosterone levels (Häfer et al., 2012), while in another study individuals with major depressive disorder who had attempted suicide demonstrated blunted aldosterone levels (Hallberg et al., 2011). These inter-relationships suggest RAAS functioning broadly, and circulating aldosterone specifically, may serve as a biological mechanism linking chronic stress related to PTSD with CVD outcomes, although the direction of dysregulation in aldosterone may vary.
Much of the research regarding psychopathology and stress-related glucocorticoids has focused on cortisol, a glucocorticoid released following activation of the HPA axis. Prior work has demonstrated acute stressors activate the HPA axis and trigger a biological cascade leading to higher levels of circulating cortisol among other substrates. In stress, corticotropin-releasing factor (CRF) is released from the hypothalamus, triggering adrenocorticotropic hormone (ACTH) release from the anterior pituitary, stimulating cortisol release from the adrenal cortex (Miller et al., 2007). This process is regulated through a negative feedback loop, whereby activation of GRs by cortisol inhibits CRF and ACTH release. While research has generally found higher distress is associated with dysregulated cortisol, the direction of effects can vary depending on the measure of distress. For example, research has found higher distress as measured by common mental disorders and perceived stress are associated with higher cortisol levels, while findings with PTSD have followed a somewhat different pattern. This research generally finds plasma cortisol levels are significantly lower among individuals with PTSD compared to trauma-unexposed individuals (Klaassens et al., 2012; Morris et al., 2012). Investigators speculate chronic PTSD and ongoing distress may cause stress-related immunological systems to lose their capacity to respond appropriately to stimuli as a result of repeated and prolonged activation, leading to inadequate cortisol secretion (Meewisse et al., 2007; Miller et al., 2007). This process is believed to involve dysregulation of normative negative feedback loops via increased sensitivity of GRs or higher expression of GRs throughout the body (Miller et al., 2007; Yehuda et al., 2004), manifesting as a blunting of circulating cortisol.
Thus, psychological stress has been linked with both higher and lower levels of HPA axis hormones suggesting a general dysregulation in stress response that may depend on the nature of the stressor (Tsigos and Chrousos, 2002). Drawing on prior work linking dysregulated HPA axis activity and blunted cortisol levels with PTSD, we hypothesize PTSD will be similarly associated with dysregulation of aldosterone, which is regulated by the CRF-ACTH axis and RAAS. Psychological stress stimulates ACTH secretion. ACTH, in turn, stimulates adrenal production of both cortisol and aldosterone (Kubzansky and Adler, 2010) leading to stimulation of type II GR, which is activated by cortisol, and type I GR, which is activated by aldosterone and, when 11β-hydroxysteroid dehydrogenase type II is absent or insufficient, by cortisol (Murck et al., 2014). ACTH increases activity of P450 side chain cleavage enzyme, leading to increases in pregnenolone, which is converted through multiple enzymatic steps to cortisol in the zona fasciculata and to aldosterone in the zona glomerulosa. The final enzymatic steps of aldosterone biosynthesis are catalyzed by aldosterone synthase which converts 11-deoxycorticosterone to aldosterone (Stowasser and Gordon, 2016). Considering the relationships between these biological processes, plasma concentrations of aldosterone may follow similar patterns to cortisol in prolonged stress. In PTSD, the HPA axis is characterized by increased sensitivity of the negative feedback mechanisms regulating the HPA axis, resulting in lower circulating cortisol levels (Yehuda, 2009). We hypothesize that PTSD will similarly result in increased activity of type I GRs, resulting in volume expansion and lowering of aldosterone levels.
Despite plausibility for a biological relationship between stress-related psychopathology and aldosterone, there has been relatively little research on aldosterone with trauma and emotional distress. Most research linking aldosterone with psychological factors has been with depression and has found serum aldosterone concentrations are generally elevated when comparing individuals with versus without depression (Hafner et al., 2013; Hallberg et al., 2011; Murck et al., 2014). Two small cross-sectional studies found that patients with hyperaldosteronism, characterized by high circulating aldosterone levels, had higher prevalence of generalized anxiety disorder compared to healthy controls (Sonino et al., 2006; Sonino et al., 2011). To our knowledge, only two studies using the same community-based sample of adults have directly assessed the relationship between PTSD identified by clinical interview and plasma aldosterone levels (Terock et al., 2019a; Terock et al., 2019b). These analyses identified either no association between PTSD and aldosterone levels (Terock et al., 2019a) or modestly elevated aldosterone among individuals with PTSD (Terock et al., 2019b). However, prior studies were cross-sectional, and thus could not determine the direction of effects or assess whether PTSD led to an increase in aldosterone concentration. Beyond examining circulating aldosterone levels in PTSD, studies assessing genotypes associated with angiotensin-converting enzymes have indicated a relationship between RAAS-related activity and PTSD (Holman, 2012; Nylocks et al., 2015). Angio-receptor blocker and angiotensin-converting enzyme inhibitor use have been associated with lower PTSD symptoms (Khoury et al., 2012), further suggesting links between RAAS alterations and PTSD pathogenesis.
To evaluate the association between trauma, PTSD, and aldosterone, in the present study, we investigated plasma aldosterone levels across two time points, 10-16 years apart, within a sample of middle-aged women. We considered PTSD in relation to the pooled average of aldosterone across both time points, as well as to change in aldosterone over time. We hypothesized aldosterone levels would be lower in women with chronic PTSD, relative to those with no trauma exposure. We additionally evaluated associations in women who experienced trauma exposure but no PTSD. Given evidence that trauma exposure may impact physiological stress response systems even absent posttraumatic stress symptomology (Morris et al., 2012), we hypothesized women with trauma exposure would have lower aldosterone levels relative to women with no trauma. We adjusted for potential confounding factors, including age and race/ethnicity, that may be associated with trauma and PTSD and also impact aldosterone levels (Brown et al., 2014; Rifkin et al., 2014). We also considered variables potentially on the causal pathway from trauma exposure and PTSD to plasma aldosterone levels. Pathway variables included behavior-related factors, namely average sodium intake and body mass index (BMI) which are related to aldosterone levels and regulation (Bentley-Lewis et al., 2007; Graudal et al., 2012), and PTSD is linked to both unhealthy diets and increased weight gain (Kim et al., 2019; Kubzansky et al., 2014). We further considered other relevant physiological-related factors that can influence aldosterone levels and may also be pathway variables, including statin and anti-hypertensive medication use (Baudrand et al., 2015; Haas et al., 2020; Sarzani et al., 2010) and menopausal status (Kathiresan et al., 2005; Solanki et al., 2020). Circulating aldosterone is lower post-menopause compared to pre-menopause (Solanki et al., 2020) potentially via estrogen deficiency impacting RAAS components (O'Donnell et al., 2014), and women with PTSD are more likely to have surgeries that cause cessation of menses (Katon et al., 2020). Therefore, women with chronic PTSD may have earlier menopause which in turn may result in lower aldosterone levels. The current study extends prior evidence by using repeated measures of plasma aldosterone, allowing for assessment of change in aldosterone levels, and using a gold standard interview to assess PTSD, providing accurate diagnosis of PTSD compared to self-reported screening measures.
2. Material and Methods
2.1. Participants and Study Procedure
Participants for the current study included women from the Nurses’ Health Study II (NHS II), a longitudinal cohort of 116,429 female nurses enrolled in 1989 (aged 25-42) and followed every two years, at which time they completed questionnaires assessing health related factors. Across follow-up, women are invited to complete additional questionnaires, including one focusing on experiences of violence (2001) and another one including screening for PTSD (2008), and a subset of women were invited to participate in a PTSD substudy in 2009. Blood samples from a subset of the full cohort of women were collected at two time points: draw 1 was between 1996-1999 (at ages 32-52 years) and draw 2 was between 2008-2012 (at ages 46-65 years). A total of 92,888 women who had completed the 1995 biennial questionnaire and had no previous diagnoses of cancer were invited to participate in the first blood draw, 29,611 (31.9%) of whom participated. All women who completed the first blood draw were invited to participate in the second blood draw, 16,424 (55.5 %) of whom participated. For both draws, women were mailed collection kits with all necessary supplies and blood sample-related questionnaires. A majority of women were in a fasting state (63.1% at draw 1 and 76.0% at draw 2; fasting status was unassociated with aldosterone level at each draw, p>.05) and obtained their samples in the morning (77.7% at draw 1 and 87.1% at draw 2 sampled between 6 and 10am; sampling time of day was unassociated with aldosterone level at each draw, p>.05). Samples were returned to the NHS II laboratory via overnight courier and were processed by the lab, separated into plasma, red blood cell, and white blood cell components and stored in liquid nitrogen freezers (Sumner et al., 2017). Prior studies with these data suggest that women who participated in the blood sample collection were similar to the whole NHS II cohort (Sumner et al., 2017). Mailed return of the questionnaires implied consent. This study was approved by the Institutional Review Boards of the Brigham and Women's Hospital and the Harvard T.H. Chan School of Public Health.
2.2. Measures
2.2.1. Trauma exposure and PTSD assessment
Women’s trauma exposure was characterized according to the violence-related questionnaire in 2001, the trauma exposure and PTSD screening questionnaire in 2008, and the PTSD diagnostic interview in 2009. In 2001, women reported on their childhood experiences of physical, emotional or sexual abuse (Rich-Edwards et al., 2012). In 2008, a stress and trauma questionnaire was mailed to 60,804 women who completed the 2001 violence-related questionnaire and also the 2007 biennial questionnaire. 54,224 (89% response rate) women returned the questionnaire where they reported any lifetime exposure to 26 potentially traumatic events (modified Brief Trauma Questionnaire) (Morgan et al., 2003) and their experience of seven PTSD symptoms related to their worst trauma (if one was reported) (Short Screening Scale for DSM-IV PTSD) (Breslau et al., 1999). Those reporting any trauma exposure (defined as any lifetime trauma exposure queried in 2008 or any childhood abuse queried in 2001) were then invited to participate in a PTSD diagnostic interview administered via phone in 2009. Among those who agreed to be interviewed, 2,112 probable cases of PTSD based on the screening questionnaire and 2,001 trauma-exposed matched controls were identified. A total of 3,013 (73% of those selected) of these women completed the diagnostic interview. PTSD case status was determined based on a structured diagnostic interview for diagnosis of DSM-IV PTSD by trained interviewers, considered the gold standard assessment of PTSD status (Koenen et al., 2009). Details on the study protocol are reported elsewhere (Koenen et al., 2009).
Trauma/PTSD exposure categories included three groups of women: 1) no trauma exposure, 2) trauma exposure with no PTSD, including women who reported their worst trauma exposure prior to the first blood draw but had low or no PTSD symptoms in the diagnostic interview, and 3) chronic PTSD, including women who reported their worst trauma exposure prior to the first blood draw and met diagnostic criteria for PTSD. As women’s worst trauma was before blood draw 1 (1996-1999) which led to PTSD that persisted and was diagnosed at the interview in 2009, these women were considered to have “chronic PTSD”. We randomly selected 175 women from each of these three exposure groups who did not have history of any cardiovascular disease (i.e., stroke, myocardial infarction) as of the first blood draw for inclusion in the current study. This resulted in the following groups: no trauma exposure (reference group) (n=175 out of 1,174 eligible), trauma exposure prior to the first blood draw but with no PTSD symptoms at either blood draw (n=175 out of 187 eligible), and chronic PTSD (n=174 out of 220 eligible). As the groups were based on retrospective reporting of timing of trauma exposure and PTSD symptoms in the 2009 interview, we purposefully defined groups assuming that group membership was stable across blood draws (i.e., no trauma ever reported by 2009; trauma exposure prior to blood draw 1 but no clinically-elevated PTSD symptoms at any point by 2009; trauma exposure prior to blood draw 1 and elevated PTSD symptoms beginning at the trauma exposure and continuing to 2009); see Figure 1 for measurement timing and exposure group definition. More detailed criteria for selection of groups has been described elsewhere (Sumner et al., 2017). Three women did not have aldosterone assays at the second blood draw; therefore, the final analytic sample size was 521 comprising three groups: no trauma (n=173), trauma/no PTSD (n=174), and chronic PTSD (n=174). Among the analytic sample, all blood draw 1 samples were collected in 1996-1999, while all blood draw 2 samples were collected in 2010 or 2011. In primary analyses, we considered trauma/PTSD status according to these three groups. In addition, we created a count of traumatic events ever experienced as of 2009 (potential range 0-26; no trauma group=0) and a measure of PTSD symptom severity as the sum of 17 interview items reflecting DSM-IV PTSD criteria (rated from 1=not at all to 5=extremely, potential range 17-85; no trauma group mean=0) (Koenen et al., 2009) for consideration in descriptive and secondary analyses.
Figure 1.
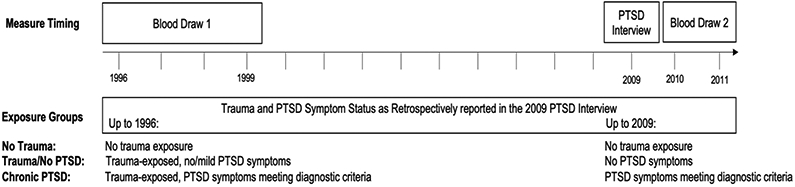
Timeline of study measurements and derivation of trauma/PTSD exposure groups PTSD interview included lifetime trauma assessment, structured diagnostic interviews for PTSD according to DSM-IV, and timing of trauma exposure and PTSD symptoms (used to derive trauma and PTSD status as of 1996 (before blood draw 1) and as of 2009 (before blood draw 2) to derive exposure groups.
2.2.2. Aldosterone
The Clinical and Epidemiologic Research Lab at Children’s Hospital Boston conducted the assays to determine plasma aldosterone concentration (in pg/mL). Each individual had two samples, one from each blood draw. Assays were conducted in two batches (with individual’s first and second blood draw samples included in the same batch): mean intra-assay coefficients of variation (CV, within-plate consistency) were 20.1% and 8.4%, and mean inter-assay CVs (between-plate consistency) were 12.7% and 5.5%. While the biorepository standard of CVs is <15% and some of the CVs were slightly elevated, given the novel nature of this research question we determined analyses were still warranted. None of the calculated values were beyond the limit of detection.
2.2.3. Covariates
Covariates included age and race/ethnicity, which constituted potential confounders, and statin and anti-hypertensive medication use were considered relevant covariates due to known associations with aldosterone. Age and race/ethnicity were time-invariant and were assessed at draw 1 through self-report questionnaire at the time of blood draw (i.e., age in years) or the closest preceding biennial NHS II questionnaire (i.e., race/ethnicity: white versus non-white). Statin use was time-updated by including self-reported use of any current statin or other cholesterol-lowering drug in the past two years (i.e., use versus no use) at the closest preceding biennial NHS II questionnaire to each blood draw. Similarly, time-updated anti-hypertensive medication use was defined with self-reported current use of thiazide diuretic or any antihypertensives in the past two years at the closest preceding biennial NHS II questionnaire to each blood draw. We also examined variables that may be on the pathway linking PTSD status to aldosterone levels including menopausal status, BMI, sodium intake, and physical activity. Menopausal status and BMI were time-updated and assessed at draws 1 and 2 through self-reported questionnaires at the time of blood draw. Menopausal status was determined with a single item assessing whether women were pre- versus post-menopausal. Self-reported menopausal status has been validated in the Nurses’ Health Study, NHS II’s sister study, and was found to be largely accurate (Colditz et al., 1987). BMI was derived via self-reported height and weight (i.e., kg/m2); a validation study in a subset of NHS participants suggested that self-reported weight was highly correlated with measured weight (r=.96) (Willett et al., 1983). Sodium intake, a known regulator of aldosterone and therefore a relevant covariate and potential pathway mediator, was also time-updated and assessed at the closest preceding biennial NHS II questionnaire, with average daily sodium intake in mg/day estimated from a self-reported food frequency questionnaire (Willett et al., 1985). Self-reported average past month physical activity was time-updated from the biennial NHS II questionnaires (<1 time/week, 1 time/week, 2-3 times/week, 4+ times/week), as physical activity has been associated with PTSD (Zen et al., 2012) and may impact aldosterone levels (Kirby and Convertino, 1986). As depression is often comorbid with PTSD and may also be associated with aldosterone levels (Emanuele et al., 2005; Murck et al., 2014), we also considered self-reported depressive symptoms closest to each blood draw. Depressive symptoms at the first blood draw were reported in 1997 using the 5-item Mental Health Inventory (Berwick et al., 1991) and in 2008 using the 10-item Center for Epidemiologic Studies-Depression scale (Andresen et al., 1994); standardized symptom scores (coded so higher scores indicate greater symptoms) for both time points were used in analytic models. Self-reported past 6-month use of hormone replacement therapy assessed by questionnaire at the time of blood draw. As prior studies have suggested that post-menopausal hormone replacement therapy is associated with higher aldosterone levels (Kathiresan et al., 2005), any hormone replacement therapy use was additionally included as a potential covariate in sensitivity analyses.
2.3. Statistical Analyses
We compared the distribution of relevant covariates at draw 1 across trauma/PTSD groups using analysis of variance and Chi-square tests or Fischer’s exact tests. We calculated mean levels of aldosterone for each draw (draws 1 and 2) and also mean change over time (draw 2 – draw 1) for each trauma/PTSD group. Due to positive skew in aldosterone concentrations (draw 1 skewness=2.12, kurtosis=6.52, draw 2 skewness=2.30, kurtosis=7.98), values were log-transformed for all subsequent analyses. We descriptively examined mean raw aldosterone levels across the three exposure groups, as well as correlations between trauma count and PTSD symptom severity with aldosterone levels at draws 1 and 2.
Linear mixed models were used to determine associations between trauma/PTSD status and aldosterone levels (measured as a continuous variable) considering both the pooled average across time (time in years from baseline [draw 1, set as 0 years] to draw 2) and rate of change, using women without trauma exposure as the reference group. A series of models were conducted predicting log transformed aldosterone level as follows: Model 1 included age, time between blood draws, and trauma/PTSD group with a random effect for intercept and time (averaging the trauma/PTSD effect across the two time points); Model 2 expanded upon Model 1 by including an interaction term between each trauma/PTSD group and time to determine if rate of change in aldosterone across time varied by trauma/PTSD status (interaction model); Model 3 expanded upon Model 1 to include covariates (i.e., race/ethnicity, statin use, anti-hypertensive use); Model 4 expanded upon Model 3 to include potential pathway variables (i.e., menopausal status, BMI, sodium consumption); and Model 5 expanded on Model 4 to include depressive symptoms. Continuous variables (i.e., age at draw 1, BMI, sodium consumption) were grand mean centered for all analyses, while depression symptoms were standardized (mean=0, SD=1). As aldosterone was log-transformed in all models, parameter estimates were transformed (100*(exp(β)–1) for interpretation as percentage difference in mean aldosterone between the trauma/PTSD group with the reference group of no trauma exposure. The primary models were also conducted with continuous PTSD symptoms as the primary predictor, as well as an indicator variable for trauma exposure (any trauma exposure versus no trauma exposure).
A post-hoc exploratory analysis was conducted following identification of menopausal status as a potential mediating factor between PTSD and aldosterone levels. Linear mixed models similar to the primary models were conducted to examine the relation between PTSD and aldosterone with and without adjustment for menopausal status, to assess mediation. The percent of relation between PTSD status and aldosterone levels accounted for by menopausal status was estimated as (1- (β for exposure, adjusted/ β for exposure, unadjusted))* 100 (Lin et al., 1997). Lastly, we re-ran the primary models additionally adjusting for any hormone therapy use.
3. Results
3.1. Descriptive statistics
The analytic sample was largely white (96.7% white) with a mean age 43.8 (SD=4.8) years at the first blood draw and mean=12.9 (SD=0.9) years between blood draws. Some differences in covariates across trauma/PTSD groups were evident (Table 1). The majority of the sample was pre-menopausal at the first blood draw (73.3%), and reported menopausal status marginally differed between trauma/PTSD groups, with a higher proportion of women with chronic PTSD being post-menopausal at draw 1 after adjusting for age, relative to other groups (p=0.05). Trauma/PTSD groups significantly differed at baseline by age (women with no trauma were younger), BMI (women with chronic PTSD had higher BMI), daily sodium intake (women with chronic PTSD had higher sodium intake), and depressive symptoms (women with chronic PTSD had higher depressive symptoms; all p’s<0.05).
Table 1.
Distribution of covariates among the full sample and by Trauma/PTSD group status at first blood draw (1996-1999)
| Full Sample (n=521) |
No trauma (n=173) |
Trauma/no PTSD (n=174) |
Chronic PTSD (n=174) |
||
|---|---|---|---|---|---|
| Mean (SD) or N (%) |
Mean (SD) or N (%) |
Mean (SD) or N (%) |
Mean (SD) or N (%) |
p-value a | |
| Age, years | 43.8 (4.8) | 42.9 (4.8) | 44.6 (4.7) | 44.0 (4.6) | 0.002 |
| Years between blood draws | 12.9 (0.9) | 13.0 (0.9) | 13.0 (0.8) | 12.8 (0.9) | 0.06 |
| White race, N (%) | 504 (96.7) | 167 (96.8) | 170 (97.5) | 167 (96.1) | 0.94 |
| Statin use, N (%) | 24 (4.6) | 5 (2.9) | 10 (5.7) | 8 (4.7) | 0.18 |
| Anti-hypertensive use, N (%) | 297 (57.0) | 93 (53.5) | 101 (58.0) | 105 (60.4) | 0.14 |
| Post-menopausal, N (%) | 139 (26.7) | 43 (24.9) | 40 (23.2) | 55 (31.3) | 0.05 |
| BMI, kg/m2 | 25.7 (5.6) | 25.1 (5.5) | 25.3 (4.9) | 27.1 (6.6) | 0.01 |
| Daily Sodium, mg | 2,223.6 (741.0) | 2,201.8 (694.1) | 2,131.5 (655.7) | 2,362.3 (912.6) | 0.03 |
| Physical Activity Frequency | |||||
| <1 times/week | 184 (35.6) | 53 (30.6) | 73 (41.9) | 55 (32.0) | 0.35 |
| 1 time/week | 113 (21.9) | 36 (20.8) | 31 (18.0) | 48 (28.1) | |
| 2-3 times/week | 155 (30.0) | 61 (35.7) | 46 (26.4) | 47 (27.2) | |
| 4+ times/week | 65 (12.6) | 22 (12.9) | 24 (13.7) | 22 (12.7) | |
| Depressive symptoms | 0.00 (1.00) | −0.07(1.0) | −0.37 (0.7) | 0.52 (1.1) | <0001 |
Note. PTSD=posttraumatic stress disorder.
Values are means (SD) or percentages and are standardized to the age distribution of the study population; as values are standardized, some frequencies across PTSD/trauma groups will not sum to those in the full sample. Depressive symptoms are standardized (mean=0, SD=1).
p-values correspond to omnibus tests (ANOVA for continuous variables; Chi-square or Fischer’s exact test when cell sizes are small for categorical variables) comparing proportion or means of covariates by PTSD group status, adjusting for age at baseline
Across the full analytic sample, raw aldosterone levels decreased over time between the two blood draws (draw 1 mean=180.8 [SD=86.4] and draw 2 mean=169.9 [SD=93.7]) and were negatively correlated with age (r=−0.13, p=0.002; between age and aldosterone at draw 1; r=−0.11, p=0.009; between age and aldosterone at draw 2).
3.2. PTSD and aldosterone levels
At draw 1, mean raw aldosterone was 184.7 (SD=74.4) for the no trauma group, 180.7 (SD=86.2) for the trauma/no PTSD group, and 177.0 (SD=97.3) for the chronic PTSD group. Aldosterone levels decreased by draw 2, largely for the trauma-exposed groups: no trauma mean=183.0 (SD=107.2), trauma/no PTSD mean=163.0 (SD=81.5), and chronic PTSD mean=163.9 (90.0). Trauma count as of blood draw 2 (mean=4.9, SD=4.7) was significantly correlated with log aldosterone at draw 2 (r=−0.12, p=0.01), but not at draw 1 (r=−0.07, p=0.12). PTSD symptom severity (mean=23.0, SD=22.0) was significantly negatively correlated with log aldosterone at both draw 1 (r=−0.10, p=0.02) and draw 2 (r=−0.11, p=0.01).
Adjusting for age at first draw and time between blood draws, women with chronic PTSD had significantly lower average aldosterone levels pooled across time compared to those with no trauma exposure (β=−0.08, SE=0.04, p=0.03; Model 1). This corresponds to 7.8% lower mean aldosterone levels. Women with trauma exposure but no PTSD also had slightly lower aldosterone levels compared to the no trauma controls, but the effect was not statistically significant (β=−0.04, SE=0.04, p=0.26). None of the interaction terms between trauma/PTSD groups and time between blood draws were statistically significant (all p>0.10; Model 2), indicating no differences in the rate of change in aldosterone levels over time between the trauma/PTSD groups. As this suggested no longitudinal effects of trauma/PTSD group on aldosterone over time, remaining models considered aldosterone levels pooled across time only and did not include interaction terms. After adjusting for race/ethnicity, statin and anti-hypertensive medication use, the association of chronic PTSD relative to no trauma exposure with aldosterone level was still evident (β=−0.08, SE=0.04, p=0.03; Model 3). Including potential pathway variables of menopausal status, BMI and sodium intake largely did not impact associations between chronic PTSD with aldosterone levels relative to trauma-unexposed women (β=−0.09, SE=0.04, p=0.02; Model 4). Lastly, associations between chronic PTSD and aldosterone levels were not altered by further adjustment for depressive symptoms (β=−0.09, SE=0.04, p=0.03; Model 5), and depressive symptoms were unassociated with aldosterone levels (β=0.003, SE=0.02, p=0.84). See Table 2 for detailed results.
Table 2.
Trauma/PTSD status regression coefficients and standard errors from linear mixed models predicting log aldosterone (n=521)
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| Time, Age, and Trauma/PTSD Fixed Effects |
Time, Age, and Trauma/PTSD × Time Fixed Effects |
Time, Age, and Trauma/PTSD with Covariates |
Time, Age, and Trauma/PTSD with Covariates and Pathway Variables |
Time, Age, and Trauma/PTSD with Covariates, Pathway Variables and Depression |
|
| β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | |
| Fixed Effects | |||||
| Time | −0.01 (0.002)*** | −0.01 (0.003)+ | −0.01 (0.002)*** | −0.004 (0.003) | −0.004 (0.003) |
| Age at draw 1 Trauma/PTSD | −0.01 (0.003)*** | −0.01 (0.003)*** | −0.01 (0.003)** | −0.01 (0.004)* | −0.01 (0.004)* |
| No trauma | Reference | Reference | Reference | Reference | Reference |
| Trauma/no PTSD | −0.04 (0.04) | −0.02 (0.04) | −0.04 (0.04) | −0.04 (0.04) | −0.04 (0.04) |
| Chronic PTSD | −0.08 (0.04)* | −0.07 (0.04)+ | −0.08 (0.04)* | −0.09 (0.04)* | −0.09 (0.04)* |
| Trauma/PTSD x Time | |||||
| No trauma x Time | Reference | ||||
| Trauma/no PTSD x Time | −0.004 (0.004) | ||||
| Chronic PTSD x Time | −0.001 (0.004) | ||||
| Non-white | −0.05 (0.09) | −0.08 (0.09) | −0.08 (0.09) | ||
| Statin use | −0.01 (0.04) | −0.02 (0.04) | −0.02 (0.04) | ||
| Anti-hypertensive use | −0.05 (0.03) | −0.04 (0.03) | −0.04 (0.03) | ||
| Post-menopausal | −0.08 (0.04)* | −0.08 (0.04)* | |||
| BMI | 0.002 (0.003) | 0.002 (0.003) | |||
| Sodium | 0.00003 (0.00002)+ | 0.00003 (0.00002)+ | |||
| Physical activity <1/wk | Reference | Reference | |||
| Physical activity 1/wk | 0.09 (0.04)* | 0.09 (0.04)* | |||
| Physical activity 2-3/wk | 0.01 (0.03) | 0.01 (0.03) | |||
| Physical activity 4+/wk | 0.03 (0.04) | 0.03 (0.04) | |||
| Depressive symptoms | 0.003 (0.02) | ||||
| Error Variance | |||||
| Level-1 | 0.10 (0.01)*** | 0.10 (0.01)*** | 0.10 (0.01)*** | 0.10 (0.01)*** | 0.10 (0.01)*** |
| Intercept | 0.06 (0.01)*** | 0.06 (0.01)*** | 0.06 (0.01)*** | 0.06 (0.01)*** | 0.06 (0.01)*** |
| Time | 0.0003 (0.0001)** | 0.0003 (0.0001)** | 0.0003 (0.0001)** | 0.0003 (0.0001)** | 0.0003 (0.0001)** |
| Model Fit | |||||
| BIC | 1196.0 | 1213.6 | 1205.8 | 1241.6 | 1248.1 |
Notes. PTSD=posttraumatic stress disorder. Aldosterone was log-transformed pg/mL. Time=years since draw 1. All models adjust for age. Covariates are race/ethnicity (non-white versus white [reference]), statin use (versus non-use [reference]), and anti-hypertensive use (versus non-use [reference]). Pathway variables are post-menopausal status (versus pre-menopausal [reference]), BMI, sodium intake, and physical activity. Age, BMI, and sodium intake were grand-mean centered; depressive symptoms were standardized (mean=0, SD=1). Covariates (besides race/ethnicity), pathway variables and depression were time-updated to reflect values at draws 1 and 2. BIC= Bayesian information criterion. Smaller BIC indicates better model fit.
p<.10
p<.05
p<.01
p<.0001
Of note, menopausal status was significantly associated with aldosterone levels pooled across time in the adjusted model, with post- versus pre-menopausal status being associated with significantly lower aldosterone (β=−0.08, SE=0.04, p=0.04). Women who were post-versus pre-menopausal had 8.1% lower mean aldosterone, after adjusting for PTSD status, age, race/ethnicity, statin use and anti-hypertensive medication use, BMI, sodium intake, and physical activity.
3.3. Additional Analyses
Consistent with the categorical effects identified, continuous PTSD symptoms were associated with lower levels of aldosterone on average across follow-up, adjusting for all potential confounders, pathway variables, and depression (effect on average aldosterone levels for every one increase in PTSD symptoms: β=−0.002, SE=0.001, p=0.04).
Because menopausal status was associated with both PTSD (see Table 1) and aldosterone, we did a closer evaluation of the relationships to explore if menopausal status might mediate the relationship between chronic PTSD and decreased aldosterone levels. In exploratory post hoc analyses examining mediation by menopausal status, menopausal status was responsible for 7.2% (95% CI 1.9-23.9%; p=0.04) of the effect of trauma/PTSD status on aldosterone level, adjusting for age, race/ethnicity, statin use, anti-hypertensive medication use, BMI, sodium intake, and physical activity. Finally, hormone replacement therapy was not associated with aldosterone levels, and adding hormone replacement therapy to models did not change the primary results (results not shown).
4. Discussion
In this novel examination of PTSD in relation to repeated measures of aldosterone, we found chronic PTSD among middle-aged women was significantly associated with lower aldosterone levels, compared to trauma-unexposed women. The association of chronic PTSD with aldosterone was slightly attenuated but remained significant when adjusting for relevant covariates (i.e., race/ethnicity, statin use, anti-hypertensive medication use) as well as potential pathway variables (i.e., menopausal status, BMI and sodium intake). Our descriptive findings suggest that higher trauma count was negatively correlated with aldosterone levels at blood draw 2, consistent with our primary findings that PTSD was associated with lower aldosterone. With exploratory analyses we found menopausal status appeared to partially mediate the effect of PTSD status on aldosterone levels, whereby ~6.9% of the association of PTSD with lower aldosterone levels was accounted for by pre- versus post-menopausal status. No significant interaction effects with time were found, suggesting that rate of change in aldosterone levels across the time period of study did not differ by trauma/PTSD status. Despite previous evidence of associations between depression and elevated plasma aldosterone (Emanuele et al., 2005) and the strong association between PTSD and depressive symptoms in our sample, depressive symptoms were unassociated with aldosterone levels and adding depressive symptoms to the model did not impact the associations between PTSD and aldosterone.
Although it may seem contradictory, lower levels of circulating aldosterone associated with chronic PTSD status is biologically feasible. As noted earlier, prior work has consistently identified lower plasma cortisol levels among individuals with PTSD relative to peers without trauma exposure, with particularly strong effects among women (Meewisse et al., 2007). It is possible that lower aldosterone levels indicate increased type I GR activity and sensitivity throughout the body as a consequence of chronic activation of the stress response and mobilization of adrenocorticosteroids (Murck et al., 2014). Increased type I GR sensitivity would lead to sodium and water retention, volume expansion, and decreases in renin leading to overall lower levels of circulating aldosterone. Further, as excess type I GR activity is pro-inflammatory, this may contribute to systematic low-grade inflammation in PTSD (Kim et al., 2018). Excess type I GR activation is associated with elevated CVD risk (Bauersachs et al., 2015), via its pathophysiological roles in volume expansion, hypertension, and other adverse cardiac, renal and vascular effects. While elevated aldosterone levels can lead to excess type I GR activation, there may be other mechanisms leading to excess activation. Specifically, chronic PTSD may influence RAAS functioning by leading to chronically elevated type I GR activity (Gesing et al., 2001). The concept that PTSD is associated with chronic GR activation leading to volume expansion is consistent with prior work demonstrating that PTSD is associated with increased risk of hypertension (Sumner et al., 2016). Future studies should examine potential mechanisms relating to chronic PTSD, RAAS, and factors influencing type I GR activity, including potential mechanisms that could lead to activation of both GR types in PTSD.
As noted, few studies have assessed PTSD in relation to the RAAS or aldosterone. Our current findings are not consistent with those found in two cross-sectional studies conducted within the same sample, that identified either no differences or elevated plasma aldosterone levels in relation to PTSD status (Terock et al., 2019a; Terock et al., 2019b). Among this population-based sample of 3,092 adults in the Study of Health in Pomerania, no significant differences in plasma aldosterone levels were identified between those without trauma exposure, those with trauma exposure and no PTSD, and those with PTSD (n=65) in unadjusted and adjusted models (Terock et al., 2019a). However, in a separate study using a smaller subset of this base population (n=2,016), individuals with PTSD (n=33) had modestly elevated levels of plasma aldosterone (aldosterone in ng/l β=0.23, p=0.04) relative to those without PTSD, in models adjusted for the same biological, behavioral, and psychological factors (Terock et al., 2019b). The authors did not explain the discrepancy between these two findings; it is unclear whether the smaller sample was nested within the larger or included separate individuals, and the focus of the former study was the effect of any lifetime trauma and PTSD, while the focus of the latter was the effect of childhood trauma on aldosterone and renin levels. Additionally, there were few individuals with PTSD in both studies; thus, even when a statistically significant association was identified, the authors suggested PTSD was “only barely” associated with aldosterone. Relative to our current findings of PTSD and lower aldosterone concentrations, possible explanations for the discrepancy between studies include differences in the sample population, as the prior studies’ sample included men and was slightly older (mean age of PTSD group was 56.0 years) relative to ours, included fewer individuals with PTSD, and included different covariates in the models. In particular, these prior studies additionally adjusted for serum creatinine and potassium concentrations when assessing differences by PTSD status (Terock et al., 2019a; Terock et al., 2019b). Further, our focus was lifetime trauma and PTSD therefore we did not distinguishing timing of trauma exposure (e.g., childhood versus adulthood), which could further explain inconsistencies with prior findings.
Despite these inconsistencies, there is evidence that PTSD is associated with activity of the RAAS more generally. In the study of the larger sample from the Study of Health in Pomerania study, PTSD was associated with elevated plasma renin levels, relative to those without trauma exposure or those with trauma exposure but no PTSD (Terock et al., 2019a). Another study assessed an RAAS-related gene, a single nucleotide polymorphism (SNP; rs4291) in the angiotensin converting enzyme (ACE) gene, among individuals exposed to the acute stress of the 9/11 terrorist attack (Holman, 2012). The genotype associated with higher serum ACE activity (rs4291 TT) was associated with both increased vulnerability to acute stress and increased risk of CVD three years prospectively. Lastly, another study of an ACE gene SNP and PTSD among a community sample of 3,803 individuals found that T carriers of the rs4311 SNP had more PTSD symptoms and greater likelihood of diagnosis (Nylocks et al., 2015). These findings indicate a connection between psychological stress responses, RAAS and serum ACE activity, and cardiovascular-related outcomes, potentially suggesting RAAS activity as a biological mechanism linking psychological stress and cardiovascular disease. However, further longitudinal research will be necessary to determine whether chronic PTSD induces physiological changes in RAAS, leading to cardiovascular risk, or if dysregulated RAAS activity predisposes both PTSD and cardiovascular disease.
Consistent with previous research, aldosterone levels were negatively associated with age (Bauer, 1993). However, we did not find significant associations between BMI levels, statin or anti-hypertensive medication use with aldosterone in adjusted models despite evidence for these associations in the literature (Baudrand et al., 2015; Sarzani et al., 2010). Our findings with menopausal status were somewhat unexpected. First, we found post- versus pre-menopausal status was associated with significantly lower aldosterone levels, in models adjusting for age, PTSD, race/ethnicity, statin use, BMI and sodium intake. To our knowledge, only a few other mostly cross-sectional studies have assessed the relationship between aldosterone and menopausal status. Their findings were generally consistent with ours, indicating that post-menopausal women not on hormone replacement therapy tended to have lower circulating aldosterone levels compared to pre-menopausal women, while those on hormone replacement therapy had higher levels (Kathiresan et al., 2005; Solanki et al., 2020). Of note, hormone replacement therapy did not impact our results when included in sensitivity analyses. Perhaps more intriguing was our finding that within our analytic sample, women with chronic PTSD were more likely to be post-menopausal compared to trauma exposed or unexposed women. This raises the question of whether chronic PTSD might lead to earlier menopausal transition. There is little published evidence regarding the association between PTSD and early menopausal transition, however this relationship is biologically consistent with a broader area of work where PTSD is associated with accelerated biological aging (Miller and Sadeh, 2014).
The current findings must be interpreted in light of several limitations. Aldosterone was measured from plasma collected at two separate time periods, from 10 to 16 years apart, and assayed together in 2015 after four to 19 years of frozen storage. Although prior work suggests steroids in general are stable in prolonged frozen storage (Handelsman et al., 2020) and none of the aldosterone assay values were below the limit of detection, it is possible that aldosterone levels identified from our plasma samples were impacted by the varying time period in which samples were collected and overall length of storage. Information on trauma exposure, PTSD symptoms, and the timing of these experiences were based on retrospective self-report, however the diagnostic interview is considered the gold standard assessment of PTSD and age at onset was determined to be highly reliably reported. As depressive symptoms were assessed with two different measures capturing distress symptomology immediately prior to each blood draw, our adjustment for concurrent depression may have been limited. We included women whose PTSD onset prior to the first blood draw and was maintained, therefore we were not able to determine whether changes in aldosterone levels occurred before or after PTSD onset, which would help make a stronger case that PTSD is causally related to altered aldosterone levels. Moreover, we did not see an effect of chronic PTSD on rate of change in aldosterone levels over time. However, repeated assessment of aldosterone at two time points may provide a stronger measure of this outcome, permitting better capacity to detect associations with chronic PTSD as compared with having a single measure of the outcome at one time point. We were unable to assess concentrations of other components of the renin-angiotensin-aldosterone cascade, which may provide further insight to the functioning of the full system beyond that of plasma aldosterone levels. Our criteria excluding women with any cardiovascular disease potentially selected a healthier sample of women who did not have elevated aldosterone. Lastly, generalizability of the current findings to more diverse populations is limited due to our relatively homogeneous sample of white, professional women.
4.1. Conclusions
Despite these limitations, these findings represent an important investigation of a largely understudied biological pathway potentially implicated in the disease processes associated with PTSD. In particular, the current findings used two repeated assessments of plasma aldosterone across middle adulthood in women, suggesting a sustained pattern of lower aldosterone among women with chronic PTSD relative to women unexposed to trauma. Interestingly, this is contradictory to the proposed relationship whereby elevated aldosterone serves as a mediator between chronic PTSD and cardiovascular damage (Kubzansky and Adler, 2010). Although mechanisms are not clear, it is possible that repeated, chronic activation of the biological stress response leading to increased type I GR activity and ultimately blunted circulating aldosterone may represent a complex process explaining some of the elevated risk for cardiovascular disease associated with chronic PTSD. Future research should determine prospective associations between PTSD and circulating aldosterone levels in larger and diverse samples and explore potential dysregulation in the RAAS and aldosterone related to chronic PTSD.
Highlights.
Posttraumatic stress disorder (PTSD) was associated with aldosterone levels in women
The effect of PTSD on aldosterone was partially mediated through menopausal status
Trauma and PTSD may be linked to dysregulated renin-angiotensin-aldosterone functioning
Acknowledgments
The authors would like to acknowledge the Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital, and Harvard Medical School for managing the NHS II. This study was supported by National Institute of Health Grant U01 CA176726 (for NHS II infrastructure) and National Institute of Mental Health Grant R01MH101269 (to KCK and LDK) and National Heart Lung and Blood Institute K24HL103845 (to GKA). Kristen Nishimi is supported by NIH grant T32 MH 017119-33, the Lee Kum Sheung Center for Health and Happiness Dissertation Research Award, and the VA Data Science Fellowship at the San Francisco VA Healthcare System. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andresen EM, Malmgren JA, Carter WB, Patrick DL, 1994. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). American Journal of Preventive Medicine 10, 77–84. doi: 10.1016/S0749-3797(18)30622-6. [DOI] [PubMed] [Google Scholar]
- Baudrand R, Pojoga LH, Vaidya A, Garza AE, Vohringer PA, Jeunemaitre X, Hopkins PN, Yao TM, Williams J, Adler GK, Williams GH, 2015. Statin Use and Adrenal Aldosterone Production in Hypertensive and Diabetic Subjects. Circulation 132, 1825–1833. doi: 10.1161/CIRCULATIONAHA.115.016759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer JH, 1993. Age-related changes in the renin-aldosterone system. Physiological effects and clinical implications. Drugs Aging 3, 238–245. doi: 10.2165/00002512-199303030-00005. [DOI] [PubMed] [Google Scholar]
- Bauersachs J, Jaisser F, Toto R, 2015. Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension 65, 257–263. [DOI] [PubMed] [Google Scholar]
- Bentley-Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH, Garg R, 2007. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab 92, 4472–4475. doi: 10.1210/jc.2007-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick DM, Murphy JM, Goldman PA, Ware JE Jr., Barsky AJ, Weinstein MC, 1991. Performance of a five-item mental health screening test. Med Care 29, 169–176. doi: 10.1097/00005650-199102000-00008. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL, Kessler RC, Schultz LR, 1999. Short screening scale for DSM-IV posttraumatic stress disorder. American Journal of Psychiatry 156, 908–911. doi: 10.1176/ajp.156.6.908. [DOI] [PubMed] [Google Scholar]
- Brown JM, Underwood PC, Ferri C, Hopkins PN, Williams GH, Adler GK, Vaidya A, 2014. Aldosterone dysregulation with aging predicts renal vascular function and cardiovascular risk. Hypertension 63, 1205–1211. doi: 10.1161/HYPERTENSIONAHA.114.03231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudey C, Park J, Wiaderkiewicz J, Kobayashi I, Mellman TA, Marvar PJ, 2015. Autonomic and inflammatory consequences of posttraumatic stress disorder and the link to cardiovascular disease. American journal of physiology. Regulatory, integrative and comparative physiology 309, R315–321. doi: 10.1152/ajpregu.00343.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz GA, Stampfer MJ, Willett WC, Stason WB, Rosner B, Hennekens CH, Speizer FE, 1987. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. American Journal of Epidemiology 126, 319–325. doi: 10.1093/aje/126.2.319. [DOI] [PubMed] [Google Scholar]
- Emanuele E, Geroldi D, Minoretti P, Coen E, Politi P, 2005. Increased plasma aldosterone in patients with clinical depression. Arch Med Res 36, 544–548. doi: 10.1016/j.arcmed.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Gaddam KK, Pimenta E, Husain S, Calhoun DA, 2009. Aldosterone and cardiovascular disease. Curr Probl Cardiol 34, 51–84. doi: 10.1016/j.cpcardiol.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Gesing A, Bilang-Bleuel A, Droste SK, Linthorst AC, Holsboer F, Reul JM, 2001. Psychological stress increases hippocampal mineralocorticoid receptor levels: involvement of corticotropin-releasing hormone. Journal of Neuroscience 21, 4822–4829. doi: 10.1523/JNEUROSCI.21-13-04822.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gideon A, Sauter C, Fieres J, Berger T, Renner B, Wirtz PH, 2020. Kinetics and interrelations of the renin aldosterone response to acute psychosocial stress: a neglected stress system. The Journal of Clinical Endocrinology & Metabolism 105, e762–e773. doi: 10.1210/clinem/dgz190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graudal NA, Hubeck-Graudal T, Jürgens G, 2012. Effects of low-sodium diet vs. high-sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Cochrane Review). American journal of hypertension 25, 1–15. doi: 10.1038/ajh.2011.210. [DOI] [PubMed] [Google Scholar]
- Groeschel M, Braam B, 2011. Connecting chronic and recurrent stress to vascular dysfunction: no relaxed role for the renin-angiotensin system. American Journal of Physiology-Renal Physiology 300, F1–F10. [DOI] [PubMed] [Google Scholar]
- Haas AV, Baudrand R, Easly RM, Murray GR, Touyz RM, Pojoga LH, Jeunemaitre X, Hopkins PN, Rosner B, Williams JS, 2020. Interplay Between Statins, Cav1 (Caveolin-1), and Aldosterone. Hypertension 76, 962–967. doi: 10.1161/HYPERTENSIONAHA.120.14777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häfner S, Baumert J, Emeny R, Lacruz M, Bidlingmaier M, Reincke M, Kuenzel H, Holle R, Rupprecht R, Ladwig K, 2012. To live alone and to be depressed, an alarming combination for the renin–angiotensin–aldosterone-system (RAAS). Psychoneuroendocrinology 37, 230–237. [DOI] [PubMed] [Google Scholar]
- Hafner S, Baumert J, Emeny RT, Lacruz ME, Bidlingmaier M, Reincke M, Ladwig KH, 2013. Hypertension and depressed symptomatology: a cluster related to the activation of the renin-angiotensin-aldosterone system (RAAS). Findings from population based KORA F4 study. Psychoneuroendocrinology 38, 2065–2074. doi: 10.1016/j.psyneuen.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Hallberg L, Westrin A, Isaksson A, Janelidze S, Traskman-Bendz L, Brundin L, 2011. Decreased aldosterone in the plasma of suicide attempters with major depressive disorder. Psychiatry Res 187, 135–139. doi: 10.1016/j.psychres.2010.07.038. [DOI] [PubMed] [Google Scholar]
- Handelsman D, Desai R, Seibel M, Le Couteur D, Cumming R, 2020. Circulating sex steroid measurements of men by mass spectrometry are highly reproducible after prolonged frozen storage. The Journal of steroid biochemistry and molecular biology 197, 105528. [DOI] [PubMed] [Google Scholar]
- He BJ, Anderson ME, 2013. Aldosterone and cardiovascular disease: the heart of the matter. Trends Endocrinol Metab 24, 21–30. doi: 10.1016/j.tem.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman EA, 2012. Acute stress and cardiovascular health: is there an ACE gene connection? Journal of traumatic stress 25, 592–597. doi: 10.1002/jts.21746. [DOI] [PubMed] [Google Scholar]
- Kathiresan S, Larson MG, Benjamin EJ, Corey D, Murabito JM, Fox CS, Wilson PW, Rifai N, Meigs JB, Ricken G, Lifton RP, Levy D, Vasan RS, 2005. Clinical and genetic correlates of serum aldosterone in the community: the Framingham Heart Study. Am J Hypertens 18, 657–665. doi: 10.1016/j.amjhyper.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Katon JG, Callegari LS, Bossick AS, Fortney J, Gerber MR, Lehavot K, Lynch KE, Ma E, Smith R, Tartaglione E, 2020. Association of depression and post-traumatic stress disorder with receipt of minimally invasive hysterectomy for uterine fibroids: findings from the US department of veterans affairs. Women's Health Issues 30, 359–365. doi: 10.1016/j.whi.2020.06.005. [DOI] [PubMed] [Google Scholar]
- Khoury NM, Marvar PJ, Gillespie CF, Wingo A, Schwartz A, Bradley B, Kramer M, Ressler KJ, 2012. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. The Journal of clinical psychiatry 73, 849–855. doi: 10.4088/JCP.11m07316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Roberts AL, Rimm EB, Chibnik LB, Tworoger SS, Nishimi K, Sumner JA, Koenen KC, Kubzansky LD, 2019. Posttraumatic stress disorder and changes in diet quality over 20 years among U.S. women. Psychol Med, 1–10. doi: 10.1017/S0033291719003246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-K, Amidfar M, Won E, 2018. A review on inflammatory cytokine-induced alterations of the brain as potential neural biomarkers in post-traumatic stress disorder. Progress in NeuroPsychopharmacology and Biological Psychiatry. doi: 10.1016/j.pnpbp.2018.06.008. [DOI] [PubMed] [Google Scholar]
- Kirby CR, Convertino V, 1986. Plasma aldosterone and sweat sodium concentrations after exercise and heat acclimation. Journal of Applied Physiology 61, 967–970. doi: 10.1152/jappl.1986.61.3.967. [DOI] [PubMed] [Google Scholar]
- Klaassens ER, Giltay EJ, Cuijpers P, van Veen T, Zitman FG, 2012. Adulthood trauma and HPA-axis functioning in healthy subjects and PTSD patients: a meta-analysis. Psychoneuroendocrinology 37, 317–331. doi: 10.1016/j.psyneuen.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Koenen KC, De Vivo I, Rich-Edwards J, Smoller JW, Wright RJ, Purcell SM, 2009. Protocol for investigating genetic determinants of posttraumatic stress disorder in women from the Nurses' Health Study II. BMC Psychiatry 9, 29. doi: 10.1186/1471-244X-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Adler GK, 2010. Aldosterone: A forgotten mediator of the relationship between psychological stress and heart disease. Neuroscience & Biobehavioral Reviews 34, 80–86. doi: 10.1016/j.neubiorev.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Bordelois P, Jun HJ, Roberts AL, Cerda M, Bluestone N, Koenen KC, 2014. The weight of traumatic stress: a prospective study of posttraumatic stress disorder symptoms and weight status in women. JAMA Psychiatry 71, 44–51. doi: 10.1001/jamapsychiatry.2013.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Carolyn MH, Neylan TC, Na B, Regan M, Zhang Q, Cohen BE, 2013. Lifetime trauma exposure and prospective cardiovascular events and all-cause mortality: findings from the Heart and Soul Study. Psychosomatic medicine 75, 849–855. doi: 10.1097/PSY.0b013e3182a88846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DY, Fleming TR, De Gruttola V, 1997. Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med 16, 1515–1527. doi:. [DOI] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M, 2007. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry 191, 387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES, 2007. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull 133, 25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Miller MW, Sadeh N, 2014. Traumatic stress, oxidative stress and post-traumatic stress disorder: neurodegeneration and the accelerated-aging hypothesis. Mol Psychiatry 19, 1156–1162. doi: 10.1038/mp.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CA, Rasmusson AM, Winters B, Hauger RL, Morgan J, Hazlett G, Southwick S, 2003. Trauma exposure rather than posttraumatic stress disorder is associated with reduced baseline plasma neuropeptide-Y levels. Biol. Psychiatry 54, 1087–1091. doi: 10.1016/s0006-3223(03)00433-5. [DOI] [PubMed] [Google Scholar]
- Morris MC, Compas BE, Garber J, 2012. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev 32, 301–315. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murck H, Buttner M, Kircher T, Konrad C, 2014. Genetic, molecular and clinical determinants for the involvement of aldosterone and its receptors in major depression. Nephron Physiol 128, 17–25. doi: 10.1159/000368265. [DOI] [PubMed] [Google Scholar]
- Nylocks K, Michopoulos V, Rothbaum AO, Almli L, Gillespie C, Wingo A, Schwartz A, Habib L, Gamwell K, Marvar P, 2015. An angiotensin - converting enzyme (ACE) polymorphism may mitigate the effects of angiotensin - pathway medications on posttraumatic stress symptoms. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 168, 307–315. doi: 10.1002/ajmg.b.32313. [DOI] [PubMed] [Google Scholar]
- O'Donnell E, Floras JS, Harvey PJ, 2014. Estrogen status and the renin angiotensin aldosterone system. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 307, R498–R500. doi: 10.1152/ajpregu.00182.2014. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V, 2011. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470, 492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Edwards JW, Mason S, Rexrode K, Spiegelman D, Hibert E, Kawachi I, Jun HJ, Wright RJ, 2012. Physical and sexual abuse in childhood as predictors of early-onset cardiovascular events in women. Circulation 126, 920–927. doi: 10.1161/CIRCULATIONAHA.111.076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin DE, Khaki AR, Jenny NS, McClelland RL, Budoff M, Watson K, Ix JH, Allison MA, 2014. Association of renin and aldosterone with ethnicity and blood pressure: the Multi-Ethnic Study of Atherosclerosis. Am J Hypertens 27, 801–810. doi: 10.1093/ajh/hpt276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarzani R, Guerra F, Mancinelli L, Roberti L, Bordicchia M, Rappelli A, 2010. Body mass index as a predictor of plasma aldosterone levels in overweight/obese hypertensive patients in chronic anti-hypertensive treatment: PP. 34.368. Journal of Hypertension 28, e561. doi: 10.1097/01.hjh.0000379906.50630.5c. [DOI] [Google Scholar]
- Solanki P, Gwini SM, Doery JC, Choy KW, Shen J, Young MJ, Fuller PJ, Yang J, 2020. Age - and sex - specific reference ranges are needed for the aldosterone/renin ratio. Clinical endocrinology 93, 221–228. doi: 10.1111/cen.14199. [DOI] [PubMed] [Google Scholar]
- Sonino N, Fallo F, Fava GA, 2006. Psychological aspects of primary aldosteronism. Psychother Psychosom 75, 327–330. doi: 10.1159/000093956. [DOI] [PubMed] [Google Scholar]
- Sonino N, Tomba E, Genesia ML, Bertello C, Mulatero P, Veglio F, Fava GA, Fallo F, 2011. Psychological assessment of primary aldosteronism: a controlled study. J Clin Endocrinol Metab 96, E878–883. doi: 10.1210/jc.2010-2723. [DOI] [PubMed] [Google Scholar]
- Stowasser M, Gordon RD, 2016. Primary aldosteronism: changing definitions and new concepts of physiology and pathophysiology both inside and outside the kidney. Physiological reviews 96, 1327–1384. [DOI] [PubMed] [Google Scholar]
- Sumner JA, Chen Q, Roberts AL, Winning A, Rimm EB, Gilsanz P, Glymour MM, Tworoger SS, Koenen KC, Kubzansky LD, 2017. Cross-Sectional and Longitudinal Associations of Chronic Posttraumatic Stress Disorder With Inflammatory and Endothelial Function Markers in Women. Biol Psychiatry 82, 875–884. doi: 10.1016/j.biopsych.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Kubzansky LD, Roberts AL, Gilsanz P, Chen Q, Winning A, Forman JP, Rimm EB, Koenen KC, 2016. Post-traumatic stress disorder symptoms and risk of hypertension over 22 years in a large cohort of younger and middle-aged women. Psychol Med 46, 3105–3116. doi: 10.1017/S0033291716001914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terock J, Hannemann A, Janowitz D, Freyberger HJ, Felix SB, Dorr M, Nauck M, Volzke H, Grabe HJ, 2019a. Associations of trauma exposure and post-traumatic stress disorder with the activity of the renin-angiotensin-aldosterone-system in the general population. Psychol Med 49, 843–851. doi: 10.1017/S0033291718001496. [DOI] [PubMed] [Google Scholar]
- Terock J, Hannemann A, Janowitz D, Van der Auwera S, Bahls M, Volzke H, Grabe HJ, 2019b. Differential activation of the renin-angiotensin-aldosterone-system in response to childhood and adulthood trauma. Psychoneuroendocrinology 107, 232–240. doi: 10.1016/j.psyneuen.2019.05.026. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP, 2002. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. Journal of psychosomatic research 53, 865–871. [DOI] [PubMed] [Google Scholar]
- Willett W, Stampfer MJ, Bain C, Lipnick R, Speizer FE, Rosner B, Cramer D, Hennekens CH, 1983. Cigarette smoking, relative weight, and menopause. American journal of epidemiology 117, 651–658. doi: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE, 1985. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 122, 51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- Xanthakis V, Vasan RS, 2013. Aldosterone and the risk of hypertension. Curr Hypertens Rep 15, 102–107. doi: 10.1007/s11906-013-0330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, 2009. Status of glucocorticoid alterations in post-traumatic stress disorder. Ann N Y Acad Sci 1179, 56–69. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Halligan SL, Meaney M, Bierer LM, 2004. The ACTH response to dexamethasone in PTSD. American Journal of Psychiatry 161, 1397–1403. doi: 10.1176/appi.ajp.161.8.1397. [DOI] [PubMed] [Google Scholar]
- Zen AL, Whooley MA, Zhao S, Cohen BE, 2012. Post-traumatic stress disorder is associated with poor health behaviors: findings from the heart and soul study. Health Psychology 31, 194. doi: 10.1037/a0025989. [DOI] [PMC free article] [PubMed] [Google Scholar]


