Abstract
Wound healing in the oral and maxillofacial region is a complicated and interactive process. Severe mucosal or skeletal muscle injury by trauma or surgery induces worse healing conditions, including delayed wound closure and repair with excessive scar tissue. These complications lead to persistent functional impairment, such as digestive behavior or suppression of maxillofacial growth in infancy. Osteopontin (OPN), expressed in a variety of cells, is multifunctional and comprises a number of functional domains. Seven amino acids sequence, SVVYGLR (SV peptide), exposed by thrombin cleavage of OPN, has angiogenic activity and promotes fibroblast differentiation into myofibroblasts and increased expression of collagen type III. Additionally, synthetic SV peptide shows faster dermal and oral mucosal wound closure by facilitating cell motility and migratory activities in dermal- or mucosal-derived keratinocytes and fibroblasts. Moreover, cell motility and differentiation in myogenic cell populations are accelerated by SV peptide, which contributes to the facilitation of matured myofibers and scarless healing and favorable functional regeneration after skeletal muscle injury. SV peptide has high affinity with TGF-β, with potential involvement of the TGF-β/Smad signaling pathway. Clinical application of single-dose SV peptide could be a powerful alternative treatment option for excessive oral and maxillofacial wound care to prevent disadvantageous events.
Keywords: Wound healing, Skeletal muscle regeneration, Osteopontin, SVVYGLR
1. Introduction
Oral and maxillofacial regions participate in producing many rhythmically coordinated functional activities, including chewing, sucking, swallowing, and articulation. Above all, skeletal muscles, which are involved in the construction of the orofacial structure, mainly function to form balanced jaw movements and to finely adjust the force of movements in those activities. Skeletal muscles, by themselves, have high self-renewal potency and can be healed without issue from damage caused by exercise or minor injury. However, serious damage by injury or surgery and progressive diseases such as oral cancer may lead to muscle necrosis and the formation of intramuscular scar tissue, which often result in insufficient muscular regeneration and long-lasting oral functional impairment [[1], [2], [3]]. In addition, excessive wounds of the oral mucosa and orofacial skin can cause dysregulation or delay in the healing process, leading to discomfort with rough texture or oral functional impairment, primarily because of the development of hypertrophic scarring. Furthermore, as occasionally seen in primary cleft palate repair for infants with cleft palate, open mucosal wounds with denuded bone in the growth period have often been associated with disadvantageous effects of maxillary growth because of the excess production of fibrosis in the raw surface region, which results in malocclusion and midfacial deformity [4].
To obtain a natural wound appearance and prevent functional impairment, adequate tissue regeneration with regression of scarring is necessary [5]. As a current standard treatment option for skeletal muscle injury, surgical intervention including suture repair of lacerated mucosa/skin and muscle tissue or autologous tissue reconstruction for the complex volumetric soft-tissue defects by free vascularized flap transplantation are commonly applied. In addition, the combined use of physical therapy such as exercise and various forms of rehabilitation are believed to enhance angiogenesis and accelerate muscle fiber formation, especially in surgically transplanted cases to some extent. For superficial or deep widespread dermal or mucosal defects, dermal graft or dermal fat grafting are frequently used, which compromise rapid wound healing; however, the transposed skin often results in discomfort and oral dysfunction [6]. Alternative options are the use of in vitro–engineered mucosal cell sheets or an ex vivo–produced oral mucosa equivalent, as they demonstrate rapid oral wound healing with less fibrosis [7,8]. Nevertheless, these treatment options have some limitations, such as the need for a certain period of time and dedicated facilities to prepare a transplant sheet, the risk of onset of unknown side effects due to transplantation of cultured cells, and the issue of medical costs.
To improve mucosal or muscle regeneration, biological scaffolds have been investigated in preclinical injured models. Collagen-gelatin scaffolds retaining basic fibroblast growth factor, thymosin β4, and leptin have demonstrated accelerating mucosal wound repair through enhancement of angiogenesis in the earlier phase of healing process [9].
Recently, studies have reported the use of cell-based therapies using induced pluripotent stem cell–derived cells, a local injection of a mixture containing muscle-specific miRNAs, or the delivery of growth factors including basic fibroblast growth factor, insulin growth factor, nerve growth factor, transforming growth factor beta–1 (TGF-β1), and platelet-derived growth factor to accelerate muscle regeneration in animal models [[10], [11], [12], [13], [14], [15]]. However, these treatment options have not yet reached clinical application because of emerging problems, such as the spontaneous, malignant transformation of stem cells [16,17]; restriction of the route of administration; instability of effective concentration owing to their short biologic half-life in vivo [11,18]; resistance to degradation; the immune response; off-target effect by therapeutic miRNA [19]; and high-cost issues in manufacturing and quality control.
2. Osteopontin and osteopontin-derived SVVYGLR sequence
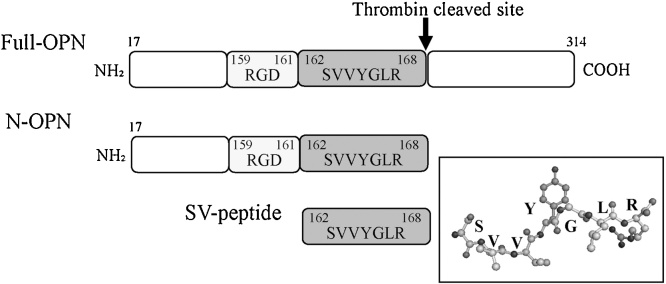
Extracellular matrix (ECM) constituents are critical components of tissue regeneration during the course of wound healing via the production of scaffolding, creation of a temporary matrix, and regulation of cellular function, such as the adhesion or migration of cells during the healing process [20,21]. Osteopontin (OPN) is one of the constituents of the ECM and is present in a wide variety of cells [22,23]. OPN is upregulated at sites of inflammation and tissue repair and is expressed in the skin during the fibrotic processes in wound healing [[23], [24], [25]]. A previous study reported that OPN plays a key role in the activation, adhesion, and migration of myogenic cells as well as in the process of fibrosis after muscle degeneration and inflammation, similarly to other compounds in the ECM, such as collagen and fibronectin [24,26]. OPN consists of a number of functional domains [27] that interact potently with various kinds of cells, including endothelial cells and smooth muscle cells. The tripeptide arginyl-glycyl-aspartic acid (RGD) sequence is involved, as in many other ECMs, such as fibronectin, collagen, and laminin, and it is well known to mediate interactions via integrins composed of αvβ1, αvβ1, αvβ3, αvβ5, αvβ6, α8β1, and α5β1 [28]. In addition, upon thrombin cleavage, Ser-Val-Val-Tyr-Gly-Leu-Arg (SVVYGLR: SV peptide) sequence (SLAYGLR in mice) consecutive to the C-terminal side of the RGD could be exposed at the C-terminus of the N-terminal fragmented OPN (Fig. 1) [29]. The SVVYGLR motif has also been revealed to mediate the interactions with integrins composed of other subunits, α9β1, α4β1, and α4β7 [30]. Upon dermal/mucosal wound or skeletal muscle injury, there is an increase in acute induction of OPN and the expression of thrombin associated with inflammatory responses; thus, fragmented OPN exposing the SV motif on the C-terminus is believed to be accumulated during the early phase of the wound-healing process. Likewise, thrombin-cleaved N-terminal OPN induces enhanced adhesive properties and is thought to have a different function as compared with full-length OPN [29,31,32]. Those characteristics were considered to be, in part, due to the exposed seven-amino-acid sequence. Because of the biological potency of this short peptide, the SV peptide, it should be useful and beneficial for tissue engineering and regeneration, as it can be easily metabolized, and the decomposition products might be identifiable with lower molecular weight than growth factors. To investigate the possible bioactivity and usefulness of the SV peptide for tissue engineering and regeneration, researchers have conducted a series of in vitro and in vivo studies using the synthetic peptide SVVYGLR [[33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]]. The salient features of the obtained findings thus far are put forth in the following text.
Fig. 1.
Schematic diagram of the structure of osteopontin (OPN).
Full OPN has a site of proteolytic cleavage by thrombin. Thrombin-cleaved N-terminal OPN (N-OPN) fragment exposes seven amino acids sequence, SVVYGLR, at the C-terminus. Inset shows the three-dimensional structure of seven amino acids.
3. Novel functional properties of the synthetic SV peptide
3.1. Angiogenic activity of the synthetic SV peptide
Angiogenesis is critically involved in a variety of pathologic processes, including cancer progression, rheumatoid arthritis, and arteriosclerosis [45,46]. Fast and functional vascularization is important during the early phase of epithelial or muscle wound repair to supply nutrients and oxygen to the wound area. In general, vascular endothelial growth factor (VEGF), TGF-β1, and fibroblast growth factor are well-known potent proangiogenic agents that exert angiogenic activity [47,48]. Herein, wound angiogenesis is considered to be regulated by interactions among endothelial cells, growth factors, and ECM constituents, including OPN. Previous studies have revealed the potency of angiogenesis in OPN [49]. Interestingly, OPN-derived synthetic SV peptide activates adhesion to endothelial cells and potentiates endothelial cell migration and can induce tube formation comparable with that of VEGF, one of the strongest angiogenic factors, indicating that SV peptide can appropriately differentiate endothelial cells in an in vitro angiogenesis assay [33]. Further investigation using an in vivo dorsal air sac assay consistently revealed comparable angiogenic responses between VEGF and SV peptide [34]. Carbonate substituted apatite-collagen sponges combined with SV peptide also enable their function as a proper environment to recruit vascular endothelial cells and facilitate proliferation of bone marrow stromal cells in the graft [35].
3.2. Induction of smooth-muscle actin and collagen type III by SV peptide
During the proliferation phase of the wound-healing process, fibroblasts migrate into the wound area and produce granulation tissue and then differentiate into myofibroblasts or produce ECM components [50,51]. These events finally result in the wound contraction or the production of scar tissue [51]. Myofibroblasts, which were first described in 1971 [52], are well known to share morphological features with fibroblasts and smooth muscle cells, and they are characterized by the expression of alpha-smooth-muscle actin (α-SMA) [53]. A previous study revealed that the expression of OPN is highly increased during fibroblast differentiation into myofibroblasts, suggesting a possible role of OPN in the function of myofibroblasts in tissue repair [54]. Supporting this idea, the expression of α-SMA is increased by the addition of SV peptide into cardiac fibroblasts [36]. Conversely, nonfunctional SV (GYRVLSV: random SV) peptide, which has the same molecular weight but a different sequence from that of SV peptide, does not change the expression of α-SMA in cardiac fibroblasts [36]. Consistent with these findings, transplantation of an SV-expressing myoblast sheet or SV peptide-release collagen gels into the myocardium in an infarcted or dilated rat model demonstrates a significant increase in the expression of SMA-positive cells and smooth muscle myosin heavy chain type 2–positive cells, indicating cardiac fibroblasts in the infarcted area are more likely to differentiate into the myofibroblasts [[36], [37], [38]]. The capillary density in the infarcted region is increased, suggesting that the SV peptide promotes angiogenesis, as seen in the endothelial cells [38].
In addition, the expression level of collagen type III (Col III) and the Col III/collagen type I (Col I) ratio in the transplanted tissue is significantly increased by SV peptide [37,38,40]. Herein, as compared with undifferentiated cardiac fibroblasts, myofibroblasts have great contractile capability and participate, in part, in maintaining the structural integrity of healing scars. Moreover, the amount and distribution of Col I and Col III contribute to the mechanical properties of the heart [55], in which Col III provides elasticity to the ECM, whereas Col I causes tensile force by stiff fibrillar protein [56]. Upon cardiac injury, Col III is increased as the initial collagen, which is replaced by Col I and consequently causes left ventricular systolic dysfunction [37]. SV peptide-induced histological changes thus improve the cardiac contractile property and inhibit the dilation of the left ventricular chamber by facilitating fibroblasts to SMA-positive myofibroblasts and the increased expression of Col III [38]. Consistent with those findings, Col III–secretory fibroblast sheets and recombinant thrombin-cleaved N-terminal osteopontin (N-OPN) fragments demonstrate substantially equal effects. N-OPN and other many ECM proteins are composed of the RGD sequence, which is well known to influence cell motility, migration, proliferation, and differentiation via its interaction with integrin [40]. Interestingly, RGD sequence-removed N-OPN also prevents cardiac dysfunction, whereas SVVYGLR sequence-removed N-OPN and thrombin-cleaved C-terminal OPN have demonstrated no effectiveness on cardiac functional properties, indicating the functional significance of the SV peptide [40].
3.3. SV peptide facilitates dermal and oral mucosal wound healing
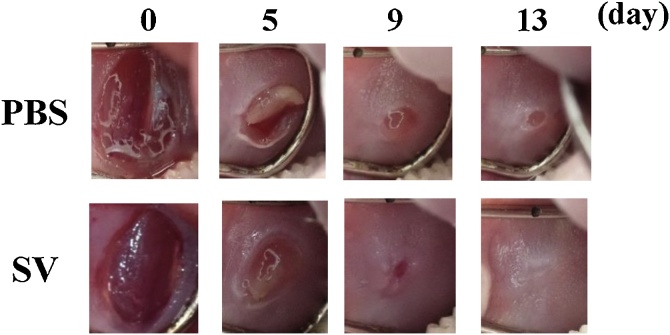
The same healing pathway is involved in dermal and oral mucosal injury, including keratinocytes and fibroblasts in the earlier phase and wound contraction promoted by myofibroblasts in the later phase, respectively [57,58]. In particular, resident and migrated fibroblasts are believed to play a critical role in the deposition and remodeling of the provisional ECM [59]. Based on previous findings that SV peptide promotes angiogenesis and differentiation of cardiac fibroblasts into active myofibroblasts, this peptide is likely to have some effects on the dermal or oral mucosal wound-healing process as well. Consistent with those findings, the SV peptide facilitates cell motility and migratory activities of dermal fibroblasts and keratinocytes [39]. In general, oral fibroblasts and keratinocytes differ in terms of phenotype and intrinsic characteristics, such as the expression of fibroblast-derived growth factors in response to inflammatory stimuli upon the injury, which might contribute to faster and scarless healing of oral wounds as compared with that in dermal wounds [5,57,60]. A recent study demonstrated that the SV peptide also increases the cell migration ability and the cell motility of both oral-derived cell populations [42]. In vivo experiments using a rat skin wound model and oral mucosal wound model showed faster wound healing accompanied by an increased rate of wound contraction (Fig. 2) [39,42]. Both fibroblasts and keratinocytes are believed to participate in wound healing by adhesion to the ECM and cytoplasmic elongation and contraction [61,62]. Taken together, the SV peptide-induced facilitation of migration of these cells should contribute to an accelerated wound closure. In addition, an immunohistochemical study found that treatment of dermal or oral mucosal wounds with SV peptide increases the number of heat shock protein-47 positive fibroblasts, α-SMA-positive myofibroblasts, and von Willebrand factor–positive new capillaries in the prepared region in an earlier period (days 1–3 after injury) in both animal models [39,42]. These results suggest that SV peptide-induced angiogenesis also, in part, stimulates and accelerates the wound-healing process [42,63].
Fig. 2.
SVVYGLR (SV peptide) facilitates mucosal wound healing in vivo.
Photographs taken during the healing process of the punch wound prepared at the buccal mucosa show the acceleration of wound closure in the SV peptide group compared with the PBS group [42].
3.4. SV peptide promotes cell motility of myogenic cells and facilitates differentiation
Skeletal muscle has self-renewal capabilities upon damage induced by exercise or minor injury and can be healed without issue. Herein, some myogenic cellular populations participate in the regenerative process. Satellite cells, which are precursor cells normally present in a dormant state, are activated upon injury, proliferate, and partly differentiate into myoblasts and embryonic progenitor cells. Myoblasts undergo terminal differentiation into multinucleated myotubes by fusing with other myoblasts; myotubes then form new muscle fibers to regenerate [3,13,[41], [42], [43],64].
Recent studies have evaluated human-derived skeletal muscle satellite cells (HSkMSC) and myoblasts (HSMM) for the effects of the SV peptide on their biological properties [43]. Both HSkMSC and HSMM showed no significant difference in the pattern of proliferation or quantification of cell adhesion to fibronectin in the condition treated by SV peptide as compared with treatment with random SV or phosphate buffered saline (PBS) [43]. Conversely, the SV peptide significantly increased the number of migrated cells and cell motility in both cell groups as compared with random SV or PBS [43]. During muscle regeneration, activated satellite cells migrate from a distant undamaged region, and a portion of them differentiate into myoblasts, which further migrate into the injured region and fuse with each other to form myofibers. The migration of myogenic cells is thus critically important, and the SV peptide-induced elevation of migration activities and cell motilities in HSkMSC and HSMM suggests the facilitation of differentiation for myogenesis
The expression of myogenic regulatory transcription factors is critical for maintaining normal muscle activity and repairing damaged muscle [65]. MyoD and myogenin are types of transcription factors, and MyoD functions to promote the proliferation of satellite cells and the transition of cells into differentiation and later has a role in myogenin induction, whereas myogenin participates in the terminal determination and fusion of myoblasts and their differentiation into myotubes, respectively [66,67]. As expected, the expression of myogenin-positive nuclei is significantly increased in HSMM treated with the SV peptide, as compared with those of immunoreactivity treated with random SV or PBS. Histological evaluation also revealed more immunoreactive cells MyoD and myogenin in the regenerated tissue treated by the SV peptide as compared with random SV or PBS [43], which provides support for the idea that the SV peptide modulates the biological properties of myogenic cells by increasing the expression of myogenic transcription factors.
Myosin heavy chain (MHC) is a late-stage differentiation marker of myogenesis in myotube and mature fiber formation. A recent immunohistochemical study demonstrated that the number of MHC-positive nascent myotubes with multinucleated cells was highly promoted from cultured HSMM treated by the SV peptide in comparison with other cultured conditions treated by random SV or PBS [44]. Taken together, during skeletal muscle regeneration, the SV peptide facilitates myogenic cell differentiation and preferably increases the efficiency of myotube formation.
3.5. SV peptide has potent utility in oral functional recovery via facilitation of matured skeletal muscle regeneration and scarless healing
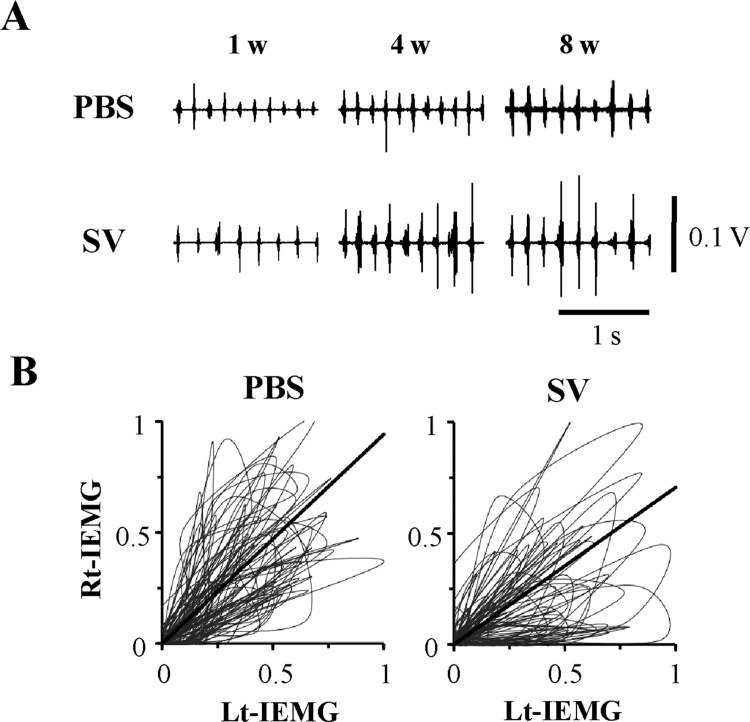
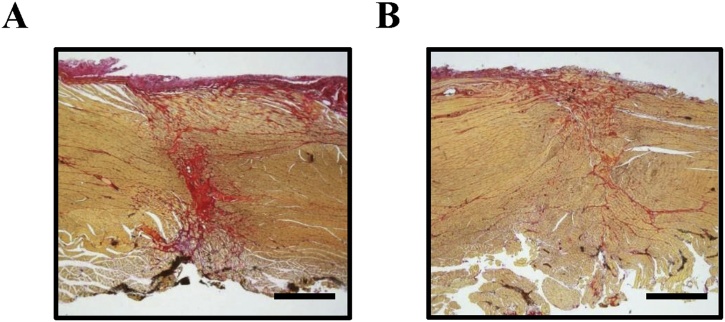
Based on the findings obtained from the SV peptide-induced functions on the biological properties of myogenic cell populations [43,44], this novel synthetic peptide should also have some beneficial effects on functional regeneration after extensive skeletal muscle injury. Volumetric muscle loss (VML) models, which impede muscle regeneration because of denervation, protein degradation, and proliferation of scar tissue, leading to motor dysfunctions, are typically used to assess muscle regeneration, particularly in the lower extremities [68,69]. To evaluate the effect of the SV peptide on the VML model with oral and maxillofacial dysfunction, a rat model with volumetric excision of the bilateral masseter muscles has been used [41]. A single dose of the SV peptide or PBS was locally injected into the cut end of the muscles immediately after injury, and electromyogram (EMG) activities were recorded from the injured muscles during the chewing phase, which demonstrated greater recovery of the maximum amplitude and duration of each burst in the SV peptide group than in the PBS group. These effects gradually increased and persisted for a long period of time after injury (Fig. 3A) [41]. The Lissajous figure, obtained by synthesizing two simple vibrations that are orthogonal to each other, is useful for analyzing the coordination of bilateral masticatory muscle activities by tracing the chewing cycles [70,71]. In the above VML model, wherein a single dose of the SV peptide was injected into only one side, the Lissajous figures synthesized from the bilateral masseter muscle activities showed a significant change in the slope of the regression line, reflecting a substantial increase in the magnitude of the muscle activity by the SV peptide (Fig. 3B) [41]. The total feeding time tended to decrease in the SV peptide group, resulting in an increase in the calculated feeding rate over time, supporting the results of the EMG and Lissajous figure analyses. Microscopic findings revealed the suppression of the formation of granulation tissue in the earlier period of the wound-healing process in the group treated with SV peptide, thereby subsequently producing less fibrotic scar tissue in the later period as compared with those treated by PBS (Fig. 4) [41]. In general, the diameter of the myofiber could be influenced by pathological conditions, and VML tends to cause atrophic changes with a decreased diameter by denervation and protein degradation [72]. Interestingly, the local administration of the SV peptide clearly shows a significant increase in the average diameter of the regenerated muscle fiber in comparison with the PBS and the untreated VML groups. Those results suggest that a single dose of the SV peptide hastens injured muscle functional recovery accompanied by facilitative effects on protein synthesis related to myofiber regeneration, which potentially contributes to the sustained suppressive effects on fibrotic changes observed during the healing process after excessive loss of muscle over the long-term period, unlike other agents [11].
Fig. 3.
SV peptide accelerates the functional recovery of impaired skeletal muscle activities in vivo.
(A) Representative raw electromyography (EMG) traces recorded at 1, 4, and 8 weeks after the masseter muscle injury treated by SV peptide show higher EMG amplitude post-treatment as compared to that treated by PBS [41]. (B) Lissajous figures synthesized from bilateral masseter muscle activities in the VML model recorded 8 weeks after PBS or SV peptide treatment. Each graph presents tracing of the voltage trajectory, with each peak voltage value normalized by the maximum value within 50 bursts recorded from the injected (left) side on the X-axis and the non-injected (right) side on the Y axis. The slope of the fitted line, obtained by regression analysis, shows a significant decrease in the SV peptide group compared with the PBS group [41].
Fig. 4.
SV peptide facilitates the regenerative process of skeletal muscles in the VML model.
Sirius red staining, 8 weeks after the injury was treated with PBS (A) or SV peptide (B), respectively (×200 magnification; scale bars = 1 mm) [41].
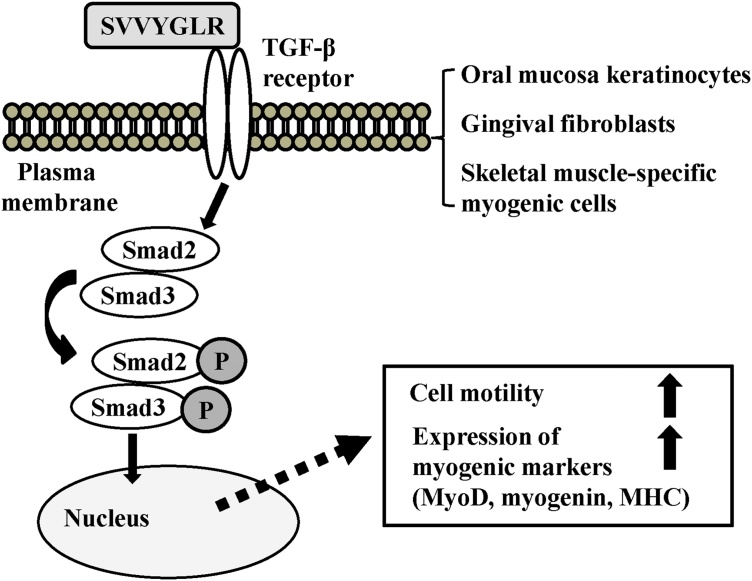
3.6. Involvement of the TGF-β1/Smad signaling pathway underlying the SV peptide-induced effects on tissue regeneration
During the wound-healing process, fibroblasts migrate into the wound space and synthesize and remodel the ECM [59]. Herein, the activation of the TGF-β/Smad signaling pathway is believed to be associated with the differentiation of fibroblasts into α-SMA-positive myofibroblasts, such as the SV peptide or thrombin-cleaved N-OPN–exposed SVVYGLR motif [73]. TGF-β also regulates angiogenesis via the activation of vascular endothelial and smooth muscle cells [73,74]. OPN, by itself, has a potency for angiogenesis, as described above [49] and is also required for the differentiation of myofibroblasts [54]. Consistent with those findings, a previous study revealed that the SV peptide, but not random SV, has a great affinity for TGF-β receptor II [36]. Phosphorylation of TGF-β receptor 1, Smad2, and Smad3 are induced in cardiac fibroblasts exposed to the SV peptide or TGF-β1 to the same degree, suggesting the same mechanism could be involved in SV peptide-induced myocardium-protecting actions through promoting angiogenesis, differentiation of fibroblasts into α-SMA-positive myofibroblasts, and overexpression of Col III [37]. In support of this idea, the TGF-β1 receptor inhibitors, SB431542 or SB505124, substantially suppress the SV peptide-induced migration activities of gingival fibroblasts and oral mucosa keratinocytes [42]. Upon skeletal muscle injury, TGF-β interacts with other diffusible signaling proteins, such as growth factors or environmental elements, and regulates the biological properties of myogenic cells, influencing muscle repair. The TGF-β1 receptor inhibitor substantially suppresses the SV peptide-induced migration activities of HSkMSC and HSMM [44]. The expression of phosphorylation of Smad3 is consistently increased in both myogenic cells treated by the SV peptide in comparison with random SV and PBS [44]. Although TGF-β signaling is considered to be involved in the SV peptide-induced facilitative effects on mucosal wound healing and muscle regeneration (Fig. 5), TGF-β1 also participates in fibrotic change in the later period of the healing process by promoting ECM preservation [4,75,76]. In fact, TGF-β1 levels in skeletal muscle cells increase by age and are believed to contribute to aging-associated fibrosis. With regard to TGF-β–related conflicting actions, previous studies described the application of a single dose of SV peptide to the VML animal model or oral mucosal wound animal model [41,42], in which this short-length peptide was rapidly degenerated and lost its potency to activate TGF-β receptors during the later phase of the healing process, resulting to less scar formation and beneficial effects on tissue regeneration and functional recovery.
Fig. 5.
Schematic for the upregulation of cell motility and expression of myogenic markers by SV peptide based on our previous reports [37,[41], [42], [43], [44]].
Synthetic SVVYGLR binds to the TGF-β receptor, which results in the phosphorylation of Smad2 and Smad3. The phosphorylated Smad2/3 translocates to the nucleus to regulate gene expression and upregulates migratory activities in mucosal keratinocytes, gingival fibroblasts, satellite cells, and myoblasts accompanied by the increased expression of myogenic markers such as MyoD, myogenin, and MHC. As a result, SV peptide accelerates mucosal wound healing or skeletal muscle regeneration upon injury.
The SVVYGLR motif by thrombin’s proteolytic cleavage of full-length OPN has the capability of binding to integrin α4β1, α9β1, and α4β7 in a non–RGD-dependent manner [29]. Human-derived myoblasts express integrin αvβ3, which is believed to regulate the proliferation and differentiation of mouse-derived satellite cells [77]. In addition, the β1 subunit might be related to the proliferation, differentiation, and fusion of myoblasts [78]. As a general concern, integrins directly regulate the activation of TGF-β or influence TGF-β–induced signal transduction. Fibrosis during the wound-healing process is considered to be regulated by integrin–TGF-β crosstalk; that is, TGF-β induces the expression of integrins such as α1β1 or α2β1 and mediates remodeling of collagen and contraction of myofibroblasts [79]. The possibility that integrin–TGF-β crosstalk is involved in the SV peptide-induced facilitative effects on cell motility, migration activities, and cell differentiation should be informative and warrants further investigation.
4. Conclusion: therapeutic implication of SV peptide
The induction of OPN expression after injury or surgical invasion functions to mediate diverse biological functions such as inflammatory responses [21], and a deficiency of OPN has been reported to result in delayed inflammatory responses and muscle regeneration [80]. Increased expression of thrombin, as one of the proinflammatory features, causes cleavage of resident full-length OPN and promotes N-terminal fragment, which gains multifunctional effects including angiogenesis and the increased expression of Col III via the SVVYGLR motif on its C-terminus, as described in this study [[33], [34], [35], [36], [37], [38], [39], [40]]. Additional local administration of synthetic SV peptide augments the local expression level of SVVYGLR motif in the injured site, leading to an acceleration of subsequent cellular reactions related to tissue regeneration, including cell motility, migration, and differentiation in myogenic and epidermal cell populations. In most cases of wound healing in extensive tissue defects by injury or surgery, complete recovery via morphological reconstruction by the original structural components is compromised because of secondary developed fibrosis [71]. The overexpression of OPN has been reported to reduce the regenerative response to injury or decreased muscle strength in aged muscle [81,82]. The application of a single dose of synthetic SV peptide into an open wound immediately after surgery, such as in cleft palate repair, might facilitate wound closure and produce more mature myofibers via stimulation of biological properties of epidermal or myogenic cells in the earlier phase of the healing process. As a novel feature of this short peptide, small molecules with fast metabolism will prevent excessive production of myofibroblasts with a contractile property via the TGF-β receptor activation in the later period of the healing process. Consequently, the SV peptide favorably improves injured skeletal muscle functions with less production of scar tissue, which might also help to avoid the secondary impairment of bone growth in pediatric surgery.
The use of the alanine scan assay has revealed that the fourth amino acid Y (tyrosine) in this short-length peptide plays a key role in the emergence of angiogenesis and that both terminal amino acids S (serin) and R (arginine) are not required for functional expression. In addition to faster metabolism, lower risk of immune response, and lower cost owing to low molecular weight, these characteristics provide another advantage: the possibility of developing more effective mutant peptides by substituting Y for other amino acids or the possibility that terminal amino acids can be used as conjugating sites with carrier proteins or scaffold materials (Table 1) [33,34]. Further investigations to determine the optimal administration in clinical applications should be beneficial and informative.
Table 1.
Comparison of therapeutic approaches to skeletal muscle repair and healing.
| Cell-based therapy (iPS-derived cell/stem cell sheet) | Gene therapy (microRNA) | Growth factor therapy (bFGF, IGF, NGF, PDGF, TGF-β) | Peptide therapy (SVVYGLR) | |
|---|---|---|---|---|
| Route of administration | Requires many cells | Direct administration | Direct administration | Direct administration |
| Scaffolding material required | (Scaffold material required) | (Scaffold material required) | (Scaffold material not required) | |
| (Thoracotomy required for heart) | Excellent operability by use of viral vector and lipid nanoparticles | Short biologic half-life in vivo/instability of effective concentration | Low molecular weight and applicable to various dosage forms (emulsion, ointment, etc) | |
| (Need for multiple factors) | ||||
| Safety/toxicity/metabolism | Risk of malignant transformation | Risk of immune response and gene mutation | Risk of immune response and adverse effects | Small molecules with fast metabolism/lower risk of immune response |
| Risk of bacterial or viral infections and transmission | Off-target effects | |||
| Synthesis/cost | High cost in terms of productivity and quality control, time-consuming process (culturing and adjusting transplanted cells in a sterilized environment) | Manufacturing cost is relatively low | High-cost issue | Possible to synthesize in large quantities with lower cost (highly efficient synthesis methods have been established) |
| High-cost issue in terms of quality control | (Time restriction from dispensing to administration to avoid denaturation and decreased activity) |
Conflicts of interest
None.
Acknowledgments
This research was supported by the Translational Research Program-Strategic Promotion for the Practical Application of Innovative Medical Technology (TRSPRINT, A83 to S.T.) grant from the Japan Agency for Medical Research and Development, AMED and a Grant-in-Aid for Challenging Exploratory Research (16K15821 to M.K.), a Grant-in-Aid for Scientific Research (C) (19K10265 to S.T.) from the Japan Society for the Promotion of Science.
References
- 1.Bjordal K., Ahlner-Elmqvist M., Hammerlid E., Boysen M., Evensen J.F., Biörklund A. A prospective study of quality of life in head and neck cancer patients. Part II: longitudinal data. Laryngoscope. 2001;111:1440–1452. doi: 10.1097/00005537-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Corona B.T., Rivera J.C., Owens J.G., Wenke J.C., Rathbone C.R. Volumetric muscle loss leads to permanent disability following extremity trauma. J Rehabil Res Dev. 2015;52:785–792. doi: 10.1682/JRRD.2014.07.0165. [DOI] [PubMed] [Google Scholar]
- 3.Liu J., Saul D., Böker K.O., Ernst J., Lehman W., Schilling A.F. Current methods for skeletal muscle tissue repair andregeneration. Biomed Res Int. 2018;16 doi: 10.1155/2018/1984879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Beurden H.E., Von den Hoff J.W., Torensma R., Maltha J.C., Kuijpers-Jagtman A.M. Myofibroblasts in palatal wound healing: prospects for the reduction of wound contraction after cleft palate repair. J Dent Res. 2005;84:871–880. doi: 10.1177/154405910508401002. [DOI] [PubMed] [Google Scholar]
- 5.Larjava H., Wiebe C., Gallant-Behm C., Hart D.A., Heino J., Häkkinen L. Exploring scarless healing of oral soft tissues. J Can Dent Assoc. 2011;77:b18. [PubMed] [Google Scholar]
- 6.Szpaderska A.M., Zuckerman J.D., DiPietro L.A. Differential injury responses in oral mucosal and cutaneous wounds. J Dent Res. 2003;82:621–626. doi: 10.1177/154405910308200810. [DOI] [PubMed] [Google Scholar]
- 7.Roh J.L., Jang H., Lee J., Kim E.H., Shin D. Promotion of oral surgical wound healing using autologous mucosal cell sheets. Oral Oncol. 2017;69:84–91. doi: 10.1016/j.oraloncology.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Izumi K., Feinberg S.E., Iida A., Yoshizawa M. Intraoral grafting of an ex vivo produced oral mucosa equivalent: a preliminary report. Int J Oral Maxillofac Surg. 2003;32:188–197. doi: 10.1054/ijom.2002.0365. [DOI] [PubMed] [Google Scholar]
- 9.Zhu T., Park H.C., Son K.M., Kwon J.H., Park J.C., Yang H.C. Effects of thymosin β4 on wound healing of rat palatal mucosa. Int J Mol Med. 2014;34:816–821. doi: 10.3892/ijmm.2014.1832. [DOI] [PubMed] [Google Scholar]
- 10.Quattrocelli M., Cassano M., Crippa S., Perini I., Sampaolesi M. Cell therapy strategies and improvements for muscular dystrophy. Cell Death Differ. 2010;17:1222–1229. doi: 10.1038/cdd.2009.160. [DOI] [PubMed] [Google Scholar]
- 11.Borselli C., Storrie H., Benesch-Lee F., Shvartsman D., Cezar C., Lichtman J.W. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci U S A. 2010;107:3287–3292. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyagawa S., Sawa Y. Building a new strategy for treating heart failure using Induced Pluripotent Stem Cells. J Cardiol. 2018;72:445–448. doi: 10.1016/j.jjcc.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Baoge L., Van Den Steen E., Rimbaut S., Philips N., Witvrouw E., Almqvist K.F. Treatment of skeletal muscle injury: a review. ISRN Orthop. 2012;2021 doi: 10.5402/2012/689012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori S., Hatori N., Kawaguchi N., Hamada Y., Shih T.C., Wu C.Y. The integrin-binding defective FGF2 mutants potently suppress FGF2 signalling and angiogenesis. Biosci Rep. 2017;37 doi: 10.1042/BSR20170173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakasa T., Ishikawa M., Shi M., Shibuya H., Adachi N., Ochi M. Acceleration of muscle regeneration by local injection of muscle-specific microRNAs in rat skeletal muscle injury model. J Cell Mol Med. 2010;14:2495–2505. doi: 10.1111/j.1582-4934.2009.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rybalko V.Y., Pham C.B., Hsieh P.L., Hammers D.W., Merscham-Banda M., Suggs L.J. Controlled delivery of SDF-1α and IGF-1: CXCR4(+) cell recruitment and functional skeletal muscle recovery. Biomater Sci. 2015;3:1475–1486. doi: 10.1039/c5bm00233h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Røsland G.V., Svendsen A., Torsvik A., Sobala E., McCormack E., Immervoll H. Long-term cultures of bone marrow derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell C.A., McGeachie J.K., Grounds M.D. The exogenous administration of basic fibroblast growth factor to regenerating skeletal muscle in mice does not enhance the process of regeneration. Growth Factors. 1996;13:37–55. doi: 10.3109/08977199609034565. [DOI] [PubMed] [Google Scholar]
- 19.Sen C.K., Ghatak S. MiRNA control of tissue repair and regeneration. Am J Pathol. 2015;185:2629–2640. doi: 10.1016/j.ajpath.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori R., Shaw T.J., Martin P. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J Exp Med. 2008;205:43–51. doi: 10.1084/jem.20071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pagel C.N., Wasgewatte Wijesinghe D.K., Taghavi Esfandouni N., Mackie E.J. Osteopontin, inflammation and myogenesis: influencing regeneration, fibrosis and size of skeletal muscle. J Cell Commun Signal. 2014;8:95–103. doi: 10.1007/s12079-013-0217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Brien E.R., Garvin M.R., Stewart D.K., Hinohara T., Simpson J.B., Schwartz S.M. Osteopontin is synthesized by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arterioscler Thromb. 1994;14:1648–1656. doi: 10.1161/01.atv.14.10.1648. [DOI] [PubMed] [Google Scholar]
- 23.Sodek J., Ganss B., Mckee M.D. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- 24.Uaesoontrachoon K., Yoo H.J., Tudor E.M., Pike R.N., Mackie E.J., Pagel C.N. Osteopontin and skeletal muscle myoblasts: association with muscle regeneration and regulation of myoblast function in vitro. Int J Biochem Cell Biol. 2008;40:2303–2314. doi: 10.1016/j.biocel.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Denhardt D.T., Noda M., O’Regan A.W., Pavlin D., Berman J.S. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukherjee B.B., Nemir M., Beninati S., Cordella-Miele E., Singh K., Chackalaparampil I. Interaction of osteopontin with fibronectin and other extracellular matrix molecules. Ann N Y Acad Sci. 1995;760:201–212. doi: 10.1111/j.1749-6632.1995.tb44631.x. [DOI] [PubMed] [Google Scholar]
- 27.Kazanecki C.C., Uzwiak D.J., Denhardt D.T. Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J Cell Biochem. 2007;102:912–924. doi: 10.1002/jcb.21558. [DOI] [PubMed] [Google Scholar]
- 28.Barry S.T., Ludbrook S.B., Murrison E., Horgan C.M. A regulated interaction between alpha5beta1 integrin and osteopontin. Biochem Biophys Res Commun. 2000;267:764–769. doi: 10.1006/bbrc.1999.2032. [DOI] [PubMed] [Google Scholar]
- 29.Yokosaki Y., Matsuura N., Sasaki T., Murakami I., Schneider H., Higashiyama S. The integrin a9b1 bind to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J Biol Chem. 1999;274:36328–36334. doi: 10.1074/jbc.274.51.36328. [DOI] [PubMed] [Google Scholar]
- 30.Ito K., Kon S., Nakayama Y., Kurotaki D., Saito Y., Kanayama M. The differential amino acid requirement within osteopontin in alpha4 and alpha9 integrin-mediated cell binding and migration. Matrix Biol. 2009;28:11–19. doi: 10.1016/j.matbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 31.O’Regan A., Berman J.S. Osteopontin: a key cytokine in cell-mediated and granulomatous inflammation. Int J Exp Pathol. 2000;81:373–390. doi: 10.1046/j.1365-2613.2000.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith L.L., Cheung H.K., Ling L.E., Chen J., Sheppard D., Pytela R. Osteopontin N-terminal domain contains a cryptic adhesive sequence recognized by alpha9beta1 integrin. J Biol Chem. 1996;271:28485–28491. [PubMed] [Google Scholar]
- 33.Hamada Y., Nokihara K., Okazaki M., Fujitani W., Matsumoto T., Matsuo M. Angiogenic activity of osteopontin-derived peptide SVVYGLR. Biochem Biophys Res Commun. 2003;310:153–157. doi: 10.1016/j.bbrc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Hamada Y., Yuki K., Okazaki M., Fujitani W., Matsumoto T., Hashida M.K. Osteopontin derived peptide SVVYGLR induces angiogenesis in vivo. Dent Mater J. 2004;23:650–655. doi: 10.4012/dmj.23.650. [DOI] [PubMed] [Google Scholar]
- 35.Hamada Y., Egusa H., Kaneda Y., Hirata I., Kawaguchi N., Hirao T. Synthetic osteopontin-derived peptide SVVYGLR can induce neovascularization in artificial bone marrow scaffold biomaterials. Dent Mater J. 2007;26:487–492. doi: 10.4012/dmj.26.487. [DOI] [PubMed] [Google Scholar]
- 36.Uchinaka A., Kawaguchi N., Hamada Y., Mori S., Miyagawa S., Saito A. Transplantation of myoblast sheets that secrete the novel peptide SVVYGLR improves cardiac function in failing hearts. Cardiovasc Res. 2013;99:102–110. doi: 10.1093/cvr/cvt088. [DOI] [PubMed] [Google Scholar]
- 37.Uchinaka A., Hamada Y., Mori S., Miyagawa S., Saito A., Sawa Y. SVVYGLR motif of the thrombin-cleaved N-terminal osteopontin fragment enhances the synthesis of collagen type III in myocardial fibrosis. Mol Cell Biochem. 2015;408:191–203. doi: 10.1007/s11010-015-2495-y. [DOI] [PubMed] [Google Scholar]
- 38.Mizuno Y., Uchinaka A., Horii Y., Mori S., Hamada Y., Miyagawa S. Improvement of cardiac function after implanting the osteopontin-derived peptide SVVYGLR in a hamster model of dilated cardiomyopathy. Interact Cardiovasc Thorac Surg. 2015;21:506–514. doi: 10.1093/icvts/ivv197. [DOI] [PubMed] [Google Scholar]
- 39.Uchinaka A., Kawaguchi N., Ban T., Hamada Y., Mori S., Maeno Y. Evaluation of dermal wound healing activity of synthetic peptide SVVYGLR. Biochem Biophys Res Commun. 2017;491:714–720. doi: 10.1016/j.bbrc.2017.07.124. [DOI] [PubMed] [Google Scholar]
- 40.Uchinaka A., Yoshida M., Tanaka K., Hamada Y., Mori S., Maeno Y. Overexpression of collagen type III in injured myocardium prevents cardiac systolic dysfunction by changing the balance of collagen distribution. J Thorac Cardiovasc Surg. 2018;156:217–226. doi: 10.1016/j.jtcvs.2018.01.097. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka S., Matsushita Y., Hamada Y., Kawaguchi N., Usuki T., Yokoyama Y. Osteopontin-derived synthetic peptide SVVYGLR has potent utility in the functional regeneration of oral and maxillofacial skeletal muscles. Peptides. 2019;116:8–15. doi: 10.1016/j.peptides.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka S., Yasuda T., Hamada Y., Kawaguchi N., Fujishita Y., Mori S. Synthetic peptide SVVYGLR upregulates cell 2motility and facilitates oral mucosal wound healing. Peptides. 2020;134 doi: 10.1016/j.peptides.2020.170405. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka S., Fujishita Y., Kawaguchi N., Usuki T., Yokoyama Y., Wu X. The synthetic peptide SVVYGLR promotes cell motility of myogenic cells and facilitates differentiation in skeletal muscle regeneration. Dent Mater J. 2021;40:766–771. doi: 10.4012/dmj.2020-317. [DOI] [PubMed] [Google Scholar]
- 44.Hamada Y., Tanaka S., Fujishita Y., Cho J.S., Usuki T., Yokoyama Y. The synthetic peptide SVVYGLR promotes myogenic cell motility via the TGF-β1/Smad signaling pathway and facilitates skeletal myogenic differentiation in vitro. Dent Mater J. 2021;40:957–963. doi: 10.4012/dmj.2020-354. [DOI] [PubMed] [Google Scholar]
- 45.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other diseases. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 46.Colville-Nash P.R., Willoughby D.A. Growth factors in angiogenesis: current interest and therapeutic potential. Mol Med Today. 1997;3:14–23. doi: 10.1016/S1357-4310(96)10048-4. [DOI] [PubMed] [Google Scholar]
- 47.Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 48.Facchiano A., Russo K., Facchiano A.M., De Marchis F., Facchiano F., Ribatti D. Identification of a novel domain of fibroblast growth factor 2 controlling its angiogenic properties. J Biol Chem. 2003;278:8751–8760. doi: 10.1074/jbc.M209936200. [DOI] [PubMed] [Google Scholar]
- 49.Takano S., Tsuboi K., Tomono Y., Mitsui Y., Nose T. Tissue factor, osteopontin, alphavbeta3 integrin expression in microvasculature of gliomas associated with vascular endothelial growth factor expression. Br J Cancer. 2000;82:1967–1973. doi: 10.1054/bjoc.2000.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Badid C., Mounier N., Costa A.M., Desmoulière A. Role of myofibroblasts during normal tissue repair and excessive scarring: interest of their assessment in nephropathies. Histol Histopathol. 2000;15:269–280. doi: 10.14670/HH-15.269. [DOI] [PubMed] [Google Scholar]
- 51.Clark R.A. In: The molecular and cellular biology of wound repair. Clark R.A., editor. Plenum Press; New York: 1996. Wound repair-overview and general considerations; pp. 3–50. [Google Scholar]
- 52.Gabbiani G., Ryan G.B., Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 53.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 54.Pereira R.O., Carvalho S.N., Stumbo A.C., Rodrigues C.A., Porto L.C., Moura A.S. Osteopontin expression in coculture of differentiating rat fetal skeletal fibroblasts and myoblasts. In Vitro Cell Dev Biol Anim. 2006;42:4–7. doi: 10.1007/s11626-006-0003-0. [DOI] [PubMed] [Google Scholar]
- 55.Lapiere C.M., Nusgens B., Pierard G.E. Interaction between collagen type I and type III in conditioning bundles organization. Connect Tissue Res. 1977;5:21–29. doi: 10.3109/03008207709152608. [DOI] [PubMed] [Google Scholar]
- 56.Pauschinger M., Knopf D., Petschauer S., Doerner A., Poller W., Schwimmbeck P.L. Dilated cardiomyopathy is associated with significant changes in collagen type I/III ratio. Circulation. 1999;99:2750–2756. doi: 10.1161/01.cir.99.21.2750. [DOI] [PubMed] [Google Scholar]
- 57.Turabelidze A., Guo S., Chung A.Y., Chen L., Dai Y., Marucha P.T. Intrinsic differences between oral and skin keratinocytes. PLoS One. 2014;9 doi: 10.1371/journal.pone.0101480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 59.Werner S., Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 60.Lee H.G., Eun H.C. Differences between fibroblasts cultured from oral mucosa and normal skin: implication to wound healing. J Dermatol Sci. 1999;21:176–182. doi: 10.1016/s0923-1811(99)00037-7. [DOI] [PubMed] [Google Scholar]
- 61.Dale P.D., Sherratt J.A., Maini P.K. A mathematical model for collagen fiber formation during fetal and adult dermal wound healing. Proc Biol Sci. 1996;263:653–660. doi: 10.1098/rspb.1996.0098. [DOI] [PubMed] [Google Scholar]
- 62.Werner S., Krieg T., Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 63.Tonnesen M.G., Feng X., Clark R.A. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5:40–46. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 64.Pannérec A., Marazzi G., Sassoon D. Stem cells in the hood: the skeletal muscle niche. Trends Mol Med. 2012;18:599–606. doi: 10.1016/j.molmed.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 65.Baghdadi M.B., Tajbakhsh S. Regulation and phylogeny of skeletal muscle regeneration. Dev Biol. 2018;433:200–209. doi: 10.1016/j.ydbio.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 66.Dedkov E.I., Kostrominova T.Y., Borisov A.B., Carlson B.M. MyoD and myogenin protein expression in skeletal muscles of senile rats. Cell Tissue Res. 2003;311:401–416. doi: 10.1007/s00441-002-0686-9. [DOI] [PubMed] [Google Scholar]
- 67.Megeney L.A., Rudnicki M.A. Determination versus differentiation and the MyoD family of transcription factors. Biochem Cell Biol. 1995;73:723–732. doi: 10.1139/o95-080. [DOI] [PubMed] [Google Scholar]
- 68.Ma J., Baker A.R., Calabro A., Derwin K.A. Exploratory study on the effect of osteoactivin on muscle regeneration in a rat volumetric muscle loss model. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu X., Corona B.T., Chen X., Walters T.J. A standardized rat model of volumetric muscle loss injury for the development of tissue engineering therapies. Biores Open Access. 2012;6:280–290. doi: 10.1089/biores.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deguchi T., Garetto L.P., Sato Y., Potter R.H., Roberts W.E. Statistical analysis of differential lissajous EMG from normal occlusion and Class III malocclusion. Angle Orthod. 1995;65:151–160. doi: 10.1043/0003-3219(1995)065<0151:SAODLE>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 71.Kumai T. Lissajous figures of differential electromyograms of the paired temporal and paired masseter muscles in human mastication. Arch Oral Biol. 1988;33:851–854. doi: 10.1016/0003-9969(88)90112-4. [DOI] [PubMed] [Google Scholar]
- 72.Schiaffino S., Dyar K.A., Ciciliot S., Blaauw B., Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280:4294–4314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- 73.Lenga Y., Koh A., Perera A.S., McCulloch C.A., Sodek J., Zohar R. Osteopontin expression isrequired for myofibroblast differentiation. Circ Res. 2008;102:319–327. doi: 10.1161/CIRCRESAHA.107.160408. [DOI] [PubMed] [Google Scholar]
- 74.Orlova V.V., Liu Z., Goumans M.J., ten Dijke P. Controlling angiogenesis by two unique TGF-β type I receptor signaling pathways. Histol Histopathol. 2011;26:1219–1230. doi: 10.14670/HH-26.1219. [DOI] [PubMed] [Google Scholar]
- 75.Darby I.A., Laverdet B., Bonté F., Desmoulière A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. 2014;7:301–311. doi: 10.2147/CCID.S50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coolen N.A., Schouten K.C., Boekema B.K., Middelkoop E., Ulrich M.M. Wound healing in a fetal, adult, and scar tissue model: a comparative study. Wound Repair Regen. 2010;18:291–301. doi: 10.1111/j.1524-475X.2010.00585.x. [DOI] [PubMed] [Google Scholar]
- 77.Liu H., Niu A., Chen S.E., Li Y.P. Beta3-integrin mediates satellite cell differentiation in regenerating mouse muscle. FASEB J. 2011;25:1914–1921. doi: 10.1096/fj.10-170449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schwander M., Leu M., Stumm M., Dorchies O.M., Ruegg U.T., Schittny J. Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev Cell. 2003;4:673–685. doi: 10.1016/s1534-5807(03)00118-7. [DOI] [PubMed] [Google Scholar]
- 79.Margadant C., Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11:97–105. doi: 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uaesoontrachoon K., Wasgewatte Wijesinghe D.K., Mackie E.J., Pagel C.N. Osteopontin deficiency delays inflammatory infiltration and the onset of muscle regeneration in a mouse model of muscle injury. Dis Model Mech. 2013;6:197–205. doi: 10.1242/dmm.009993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paliwal P., Pishesha N., Wijaya D., Conboy I.M. Age dependent increase in the levels of osteopontin inhibits skeletal muscle regeneration. Aging (Albany, NY) 2012;4:553–566. doi: 10.18632/aging.100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vetrone S.A., Montecino-Rodriguez E., Kudryashova E., Kramerova I., Hoffman E.P., Liu S.D. Osteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-beta. J Clin Invest. 2009;119:1583–1594. doi: 10.1172/JCI37662. [DOI] [PMC free article] [PubMed] [Google Scholar]