Abstract
Purpose:
To examine the association between Post-Concussion Symptom Scale (PCSS) scores, Convergence Insufficiency Symptom Survey (CISS) scores, and oculomotor deficits post-concussion.
Methods:
Records of adolescent patients examined in a multidisciplinary concussion clinic between July 2014 and May 2019 were reviewed. PCSS and CISS scores, results of eye examination and oculomotor assessment, concussion history, and demographics were abstracted.
Results:
140 patient records (median age, 15.3 years; 52 males, presented 109 days (median) from their most recent concussion) met inclusion criteria. Mean total scores on PCSS and CISS were 46.67 ± 25.89 and 27.13 ± 13.22, respectively, and were moderately correlated with each other (r = 0.53, p < 0.001). Oculomotor deficits were observed in 123 (88%) patients. Step-wise linear regression identified increased PCSS total score to be significantly associated with decreased amplitude of accommodation (p < 0.001) and increased CISS total score to be significantly associated with receded near point of convergence, poor developmental eye movement test error scores, and cause of concussion.
Conclusion:
High PCSS scores may indicate an accommodation deficit and thus prompt an oculomotor assessment in patients following a concussion. Using the CISS and a detailed oculomotor assessment may reveal underlying oculomotor deficits, which may benefit from treatment.
Keywords: Concussion, Symptom Surveys, Vision, Adolescent
Traumatic brain injury (TBI) results from an impact to the head or elsewhere on the body with an impulsive force transmitted to the brain that disrupts normal functioning (1). Concussion is a form of mild TBI defined as a traumatically induced alteration of mental status that can cause disturbances to many cortical and subcortical pathways in the brain (2). Following a concussion, patients may experience a wide variety of symptoms, such as headache, dizziness, and nausea (3, 4). In addition to cognitive impairments (loss of memory, delayed reaction times and processing speeds) (5), patients may also experience vestibular and visual impairments (6–10).
Common vision complaints after a concussion include blurred or double vision, eye fatigue, light sensitivity, difficulty focusing, appearance of words moving on the page, loss of place when reading, and difficulty sustaining attention on a visual task. These symptoms have been reported in the adult population, and they have been shown to interfere with reading and other tasks of daily living (8, 11, 12). Similar vision-related symptoms are also typically reported by patients with oculomotor deficits. A high prevalence of oculomotor deficits (vergence dysfunction in 24–64% and accommodative dysfunction in 20–71%) have been reported in military personnel/veterans and in civilians following concussion (8, 12–16). Master et al. (2016) reported accommodative disorders in 51%, convergence insufficiency in 49%, and saccadic dysfunction in 29% of adolescents (aged 11 to 17 years) post-concussion. In their patient cohort, 46% had more than one vision-related diagnosis (17). In comparison, prevalence of naturally occurring convergence insufficiency (CI) and accommodation deficit (AD) reported in the literature is between 9%- 33% (18–22).
Symptom inventories are typically used during post-concussion evaluation to determine the presence and severity of symptoms that inform a treatment plan. The Post-Concussion Symptom Scale (PCSS) is widely used and is recommended by the International Conference on Concussion in Sport, used as part of the Sport Concussion Assessment Tool, Version 5 (23). The PCSS has adequate reliability and validity for assessing and monitoring common concussion-related symptoms (4). Although the PCSS assesses a wide variety of symptoms experienced by patients after a concussion, only two questions specifically relate to vision problems, namely “blurred vision” and “sensitivity to light.” It is unclear whether these two items fully represent the gamut of vision-related symptoms experienced by patients following a concussion, especially since many aspects of visual and oculomotor deficit are reported in patients with concussion.
Symptoms related to oculomotor deficits, particularly convergence insufficiency, have been reliably assessed using the Convergence Insufficiency Symptom Survey (CISS) (24). A CISS total score of ≥16 was found to reliably differentiate symptomatic children with naturally occurring convergence insufficiency (i.e., no history of concussion or head injury) from those with normal binocular vision (24). Although the CISS assesses symptoms that relate specifically to vision, it is not typically administered outside of optometry and ophthalmology clinics.
Despite the PCSS having only two ambiguous questions related to vision, Pearce et al., (2015) showed that patients who had a receded near point of convergence (NPC) reported a greater number of total symptoms on the PCSS compared with those who did not have a receded NPC (25). This suggests that the PCSS questionnaire might also be useful in identifying patients with oculomotor deficits post-concussion. However, Master et al. (2016) reported that only a small proportion (29%) of patients with concussion who had a vision/oculomotor diagnosis reported vision-related symptoms on their PCSS questionnaire. Additionally, the CISS total score was significantly higher in patients with a vision/oculomotor diagnosis compared with patients without a vision/oculomotor diagnosis after a concussion (17).
Based on the findings by Pearce et al. (2015) and Master et al. (2016), it is unclear if the PCSS is a useful tool to identify patients with accommodation, vergence, and saccadic deficits following a concussion. Understanding the association between symptoms reported on the PCSS (a more widely used symptom survey following concussion) and oculomotor impairments following concussion is the first step in evaluating the usefulness of PCSS to facilitate referrals to appropriate eye care specialists for further evaluation. Additionally, assessing the relationship between symptom scores reported on the PCSS and CISS would further aid in evaluating the ability of the PCSS to detect vision related symptoms. Early diagnosis and management of associated vision impairments—using adequate correction of refractive error, reading glasses for deficits in accommodation, and eye exercises to improve visual efficiency skills—might be beneficial in alleviating oculomotor deficits related symptoms in patients with concussion (26–29).
In this study, we evaluate the association of symptoms reported in the PCSS questionnaire with accommodation, vergence, and visual tracking deficits post-concussion. We also determine the relationship between symptoms reported using the PCSS, not a vision-specific symptom questionnaire, to the CISS, a vision-specific questionnaire.
METHODS
This is a retrospective study of medical records of patients with persistent post-concussion symptoms examined in the multidisciplinary concussion clinic (MDCC) at Boston Children’s Hospital (Boston, Massachusetts) from July 2014 to May 2019. The diagnosis of concussion was made by the treating physician in accordance with the Consensus Statement on Concussion in Sport from their 5th international conference, held in Berlin in 2016 (23). Patients who sustained a traumatic, rapid acceleration of the head followed by the signs and symptoms characteristic of concussion were included. Patients with multisystem post-concussion symptoms persisting beyond 4 weeks were seen in the MDCC by specialists from physical therapy, otolaryngology, sports medicine or neurology (clinic sessions alternate between one of these two specialists), psychology, optometry, and ophthalmology. Demographic and concussion-related details were recorded during MDCC visit. Inclusion criteria were completion of the PCSS and CISS surveys at the visit, 20/30 or better best-corrected visual acuity in each eye for distance and near, appropriate refractive error correction present for testing (described below), and completion of a comprehensive eye examination with an oculomotor assessment (defined below). Exclusion criteria were age >21 years, strabismus, amblyopia, ocular disease, previous vision therapy, incomplete visual function examination, patient not evaluated by optometry/ophthalmology, and whether the first vision evaluation was not completed through the MDCC.
The PCSS questionnaire measures symptom severity post-concussion on a 0 to 6 point Likert-like scale, with 0 indicating no symptoms and 6 indicating severe symptoms. Symptoms included in the questionnaire were grouped into somatic (headache, pressure in head, neck pain, nausea or vomiting, sensitivity to light, sensitivity to noise); vestibular (balance problems or dizziness, hearing problems/ringing, vision problems); emotional (more emotional than usual, irritability, sadness, nervous or anxious); cognitive (confusion, feeling “like in a fog,” difficulty concentrating, difficulty remembering, feeling slowed down); and sleep (drowsiness, fatigue or low energy, trouble falling asleep) categories, as described previously by Howell et al. (30, 31). The version of the PCSS used in this study did not include the following symptoms: “don’t feel right,” “feeling dinged or slowed,” and “sleeping more than usual.” Grouping of symptoms into different domains reduces the dimensionality of the information obtained from the questionnaire and aids in identifying whether specific symptom domains are associated with prolonged recovery post-concussion (32, 33). When analyzing symptom responses, in addition to the previously described symptom categories we also designated a separate category called “vision-related symptoms.” Responses to the questions on headache, sensitivity to light, vision problems, feeling like in a fog, difficulty concentrating, and difficulty remembering were grouped under the “vision-related symptoms” category. Some questions in the vision category also belonged to other categories. Other than sensitivity to light and vision problems, the rest of the questions are similar to items represented in the CISS.
Patients also completed the CISS, which is a vision-specific questionnaire validated to screen for patients with naturally occurring convergence insufficiency (24). Severity of symptoms were graded on a 5-point scale with descriptors: never, infrequently, sometimes, fairly often, and always. In this study, due to an error in administration, 29 patients filled out a CISS questionnaire with only the first 14 questions instead of 15 questions. Therefore, only responses from the first 14 questions were included for analysis.
The optometric and ophthalmic examinations consisted of a comprehensive eye evaluation including visual acuity, confrontation visual fields, pupillary examination, oculomotor evaluation, anterior segment evaluation, dilated fundoscopic examination, and refraction (myopia defined as spherical equivalent refractive error ≤ −0.25 diopter (D); hyperopia defined as spherical equivalent refractive error ≥ +0.25 D). Cycloplegic refraction data were available for 127 of the 140 patients; in the remaining 13 patients, dry refraction was used. All patients were examined by one optometrist (AR) and one ophthalmologist (AS), both of whom followed a standard clinical protocol for all patients. All patients with hyperopia ≥ +1.50 D, myopia ≤ −0.75 D, astigmatism > 0.75 D, and anisometropia > 0.75 D wore habitual refractive error correction during assessment.
Oculomotor assessment included evaluation of vergence, accommodation, and visual tracking. The following tests were performed to evaluate vergence: eye alignment at distance and near (unilateral and prism alternating cover test); near point of convergence (NPC); vergence amplitudes, including convergence (Positive Fusional Vergence) and divergence (Negative Fusional Vergence) at near (40 cm); and near vergence facility using a prism wedge of 3Δ base in (BI) and 12Δ base out (BO). The following tests were used to evaluate the accommodation system: amplitude of accommodation using the push-up method, monocular and binocular accommodative facility using ± 2.00 D flippers. The developmental eye movement (DEM) test was used to evaluate visual tracking ability. A detailed description of the tests can be found in earlier publications (27, 34). Results of oculomotor testing were used to provide vergence, accommodation, and visual tracking deficit diagnoses to each patient, based on criteria described in Table 1 (17, 27).
Table 1.
Diagnostic Criteria for Vergence, Accommodation, and Saccadic Dysfunction
| Clinical Diagnosis & Findings | Diagnostic Criteria |
|---|---|
| Convergence Insufficiency | First criterion plus one other must be met |
|
| |
| Exophoria at near | 4Δ greater exophoria than at distance |
| Near point of convergence | ≥ 7 cm break |
| Positive fusional vergence | ≤ 15 Δ break or Sheard’sa criterion not met |
| Near vergence facility (3∆ BI/12∆ BO) | ≤ 9 cpm with difficulty fusing BO |
|
| |
| Convergence Deficit b | First criterion plus one other must be met |
|
| |
| Near point of convergence | ≥ 7 cm break |
| Positive fusional vergence | ≤ 15Δ break or Sheard’sa criterion not met |
| Near vergence facility (3Δ BI/12Δ BO) | ≤ 9 cpm with difficulty fusing BO |
|
| |
| Convergence Excess | First criterion plus one other must be met |
|
| |
| Esophoria at near | ≥ 3Δ |
| Negative fusional vergence | < 8Δ or fails Sheard’sa criterion |
| Near vergence facility (3Δ BI/12Δ BO) | ≤ 9 cpm; difficulty fusing BI prism |
|
| |
| Divergence Deficit | Both criteria must be met |
|
| |
| Negative fusional vergence | < 8Δ or fails Sheard’sa criterion |
| Near vergence facility (3Δ BI/12Δ BO) | ≤ 9 cpm; difficulty fusing BI prism |
|
| |
| Vergence Dysfunction | Either criterion is met |
|
| |
| Vergence amplitudes | Positive fusional vergence ≤ 15Δ break or Sheard’s criteriona not met, and negative fusional vergence < 8Δ or Sheard’sa criterion not met |
| Near vergence facility (3Δ BI/12Δ BO) | ≤ 9 cpm with difficulty fusing BO and BI |
|
| |
| Accommodative Insufficiency | First criterion or second criterion must be met |
|
| |
| Accommodative amplitude | 2D less than 15–1/4 age at presentation |
| Accommodative facility | ≤ 6 cpm (difficulty with −2D lens) |
|
| |
| Accommodative Infacility | Criteria must be met |
|
| |
| Accommodative facility | ≤ 6 cpm (difficulty with both +2D and −2D lenses) |
|
| |
| Accommodative Excess | Criteria must be met |
|
| |
| Accommodative facility | ≤ 6 cpm (difficulty with +2D lens) |
|
| |
| Accommodative Dysfunction | Both criteria must be met |
|
| |
| Accommodative amplitude | 2D less than 15 - 1/4 age at presentation |
| Accommodative facility | ≤ 6 cpm (difficulty with +2D) |
|
| |
| Visual Tracking Deficit | First criterion or second criterion must be met |
|
| |
| Developmental Eye Movement Ratio Score | 1 SD or more below the mean |
| Developmental Eye Movement Accuracy Score | 1 SD or more below the mean |
Compensating vergence amplitudes (positive or negative fusional vergence) of at least twice the magnitude of the near phoria
participants did not have exophoria greater at near than at far
Δ = prism diopter
BI = base-in
BO = base-out
cpm = cycles per minute
D = diopters
SD = standard deviation
Information abstracted from the patient files included age, gender, number of self-reported concussions, date of most recent concussion, cause of the concussion for which patient was seen at the MDCC, spherical equivalent refractive error (SRE) determined by cycloplegic or noncycloplegic refraction, eye alignment at distance and near, NPC (break and recovery values), divergence and convergence amplitudes for near (blur, break, and recovery values), vergence facility, monocular accommodative amplitude (blur and clear distance values), monocular and binocular accommodative facility, and time taken to complete the DEM test. For monocular measures that were obtained from both eyes, values from the right eye were used for further analysis. In addition, PCSS and CISS scores were obtained from the respective questionnaire responses.
Statistical Analysis
Mean ± 1 standard deviation (SD) or median (interquartile range (IQR)) were reported for continuous variables. Our main outcome variables, correlation between symptom scores, were reported as Pearson’s correlation coefficient.
To determine if subset(s) of the oculomotor measures assessed in this study were associated with the PCSS symptoms, a stepwise linear regression was performed. This analysis helps identify which oculomotor measures might account for the variance observed in the symptoms reported on the PCSS. To minimize effects of multicollinearity among the independent variables, highly correlated variables (r > 0.7) were removed from the stepwise regression analysis. Stepwise linear regression analysis was performed for the PCSS total score as the dependent variable (y in the regression model) and the time since last concussion; cause of concussion; number of concussions; gender; and clinical eye exam measures (SRE, distance and near phoria, near point of convergence, vergence facility, positive and negative vergence amplitudes, accommodation amplitude, and monocular and binocular accommodation facility as independent variables. In the stepwise regression analysis we used the distance of recovery to fusion measured for near point of convergence and the estimate based on push-away method (blur to recovery distance) for amplitude of accommodation, as these measures attributed to greater variance in the model. Statistical test results with a p-value of less than 0.01 was used to address type 1 errors due to multiple comparisons. Similarly, PCSS subset scores and CISS total scores were also used as dependent variables in separate stepwise linear regressions with the same set of independent variables. All statistical analyses were performed using the SPSS software (IBM SPSS Statistics 19 for Windows, SPSS Inc., IBM Corporation, Armonk, NY).
RESULTS
Between July 2014 and May 2019, 183 patients were seen at the MDCC clinic, of whom 140 met inclusion criteria. Table 2 details patient characteristics and concussion demographics from included-patient’s records. In total 43 patients were excluded due to poor visual acuity (n=10), strabismus or amblyopia (n=14), incomplete recording of PCSS scores (n=3), incomplete oculomotor examination (n=6), not seen by Optometry/Ophthalmology (n=4), seen by Optometry/Ophthalmology prior to the MDCC clinic visit (n=4), and older than 21 years of age (n=2).
Table 2.
Demographics and Patient Characteristics
| Variable | N = 140 |
|---|---|
| Age (years; median, IQR) | 15.3 (13.6 – 17.3) |
|
| |
| Sex | |
|
| |
| Female | 88 (62.8%) |
|
| |
| Concussion | |
|
| |
| Time since concussion to assessment (days; median, IQR) | 109 (64 - 192) |
| Number of concussions | |
|
| |
| First lifetime concussion | 77 (54.5%) |
| Second lifetime concussion | 27 (19.6%) |
| Third lifetime concussion | 20 (14.3%) |
| More than 4 lifetime concussions | 16 (11.4%) |
| Mechanism of concussion | |
|
| |
| Sports related | 67 (47.9%) |
| Other (slips, trips, falls) | 54 (38.6%) |
| Motor vehicle accident | 19 (13.6%) |
Data are presented as frequency (percentage) for categorical data and median (interquartile range (IQR)) for continuous data
Symptom Responses
The mean total symptom score reported on the PCSS was 46.67 ± 25.89. Further itemized analyses of the responses to questions on the PCSS were used to better understand the symptom profile in this post-concussion cohort. Figure 1A reports the mean scores of responses to each of the questions on the PCSS. The severity of most symptoms reported were low to moderate, with highest ratings for “difficulty concentrating” and “headache” (Figure 1A). In particular, one of the questions within the vestibular symptom category pertains to the presence and severity of vision problems. For that particular question, the median response by patients was 1 (IQR: 3) on a scale from 0 to 6.
Figure 1:
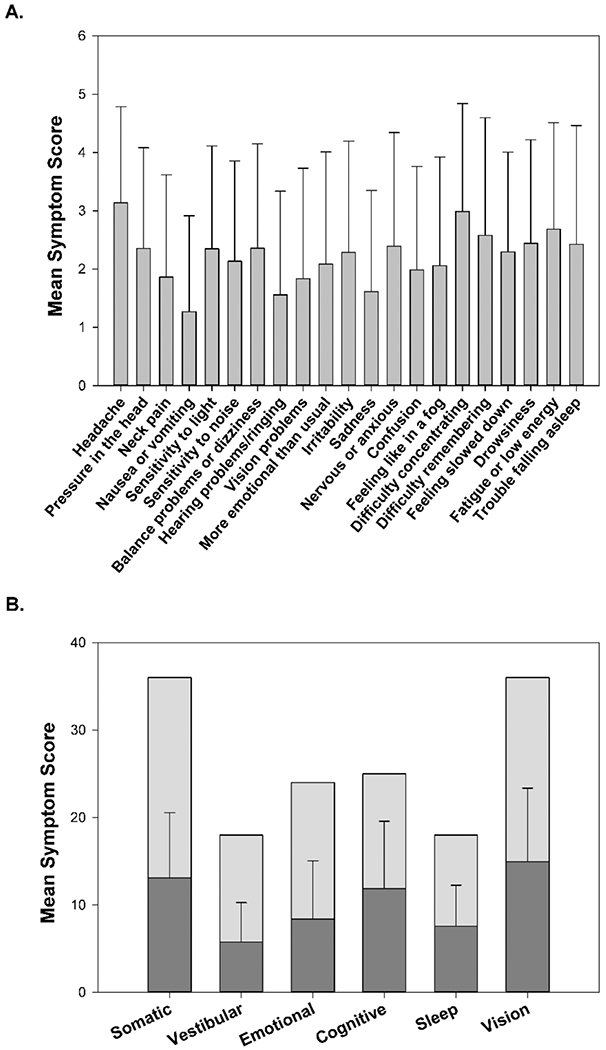
Post Concussion Symptom Scale (PCSS) responses. (A) Mean and standard deviation (SD) for the responses for individual questions on the PCSS questionnaire. (B) Mean and SD for the categories are represented by the dark grey bars. The light grey bar indicates the maximum possible score within each category. The maximum possible score varies between categories, as the number of questions grouped within each category is different.
Total score for each symptom category on the PCSS was also calculated to determine whether higher scores were reported for any of the categories. Figure 1B shows the mean total score for each of the symptom categories in the PCSS: somatic, vestibular, emotional, cognitive, sleep, and vision.
The mean CISS total score was 27.13 ± 13.22. Figure 2 reports the mean scores of responses to each of the questions on the CISS. As shown in Figure 3, a moderate correlation was observed between total score for the PCSS and CISS (r = 0.53, p < 0.001). In addition, correlations between CISS total score and the total scores in each of the PCSS categories were calculated. CISS total score was moderately correlated with symptom ratings for somatic (r = 0.48, p < 0.001); vestibular (r = 0.48, p < 0.001); emotional (r = 0.30, p < 0.001); cognitive (r = 0.47, p < 0.001); sleep (r = 0.48, p < 0.001); and vision (r = 0.53, p < 0.001) categories (Figure 4).
Figure 2:

Convergence Insufficiency Symptom Survey (CISS) responses. Mean and standard deviation (SD) for the responses for individual questions on the CISS questionnaire.
Figure 3:

Scatter plot between the Convergence Insufficiency Symptom Survey (CISS) and Post-Concussion Symptom Scale (PCSS) total scores.
Figure 4:

Scatter plots between Convergence Insufficiency Symptom Survey (CISS) total score and subtotal scores for each of the categories of symptoms in Post Concussion Symptom Scale (PCSS): Somatic (A), Vestibular (B), Emotional (C), Cognitive (D), Sleep (E), and Vision related (F).
Results of refraction
Eighty-two patients were hyperopic (median = +0.75D, IQR = +0.50D), 40 were myopic (median = −1.19 D, IQR = 1.94D), and 18 were emmetropic.
Oculomotor Assessment
Eye alignment testing at distance and near showed that all patients were orthophoric (0Δ misalignment) at distance and 97 patients (69%) were orthophoric at near. Only 31 patients (22%) had a near exophoria of 4Δ or greater. Of the 140 patients, 123 patients (88%) had abnormal oculomotor findings and 17 patients had normal oculomotor function. As shown in Figure 5A, among the patients who had oculomotor deficits, 10 (7%) had vergence-only deficits; 34 (24%) had accommodation-only deficits; 8 (6%) had visual tracking-only deficits; 19 (14%) had both vergence and accommodation deficits; 7 (5%) had both vergence and visual tracking deficits; 22 (16%) had both accommodation and visual tracking deficits; and 23 (16%) had vergence, accommodation, and visual tracking deficits. Of the 59 (42.1% of total) patients with abnormal vergence findings, 25 (42%) had convergence insufficiency (CI), 20 (34%) had convergence deficits (CD), 5 (9%) had convergence excess (CE), 2 (3%) had divergence deficit (DD), and 7 (12%) had nonspecific vergence dysfunction (Figure 5B). Of the 98 (70% of total) patients with accommodation deficits, 29 (30%) had accommodative insufficiency (AI), 35 (36%) had accommodative excess (AE), 23 (23%) had accommodative dysfunction (AD), 5 (5%) had accommodative infacility (AIF), and 6 (6%) had both AI and AIF (Figure 5C). Visual tracking deficits, as measured using the DEM, were seen in 60 (42.9% of the total) patients. Details of the abnormal findings for measures of vergence, accommodation, and DEM are given in Table 3.
Figure 5:

(A) Venn diagram of oculomotor deficits observed post-concussion in the adolescent population (n=123). (B) and (C) Subtypes of deficits observed postconcussion in the adolescent population for the vergence and accommodation categories, respectively. CI: Convergence Insufficiency; CD: Convergence Deficit; CE: Convergence Excess; DD: Divergence Deficit; VD: non-specific Vergence Dysfunction; AI: Accommodative Insufficiency; AD: Accommodative Dysfunction; AE: Accommodative Excess; AIF: Accommodative Infacility
Table 3.
Oculomotor Assessments: Mean Scores and Number of Patients who Failed Assessment (based on the criteria listed in Table 1)
| Oculomotor Assessments | Mean ± 1 SD [Median, Range] | Number of Patients Who Failed Criteria (n =140) |
|---|---|---|
| Near point of convergence | 9.1 cm ± 5.0 [7.6 cm, 2.0 to 33.0] | 76 (54.2%) |
|
| ||
| Near vergence facility | 13.6 cpm ± 6.1 [14.3 cpm, 0 to 28.0] | 36 (25.7%) |
|
| ||
| Positive fusional vergence (at near) | 21.7Δ ± 8.9 [20.0Δ, 4.0 to 40.0] | 39 (27.8%) |
|
| ||
| Negative fusional vergence (at near) | 10.9Δ ± 3.6 [10.0Δ, 4 to 25.0] | 23 (16.4%) |
|
| ||
| Monocular amplitude of accommodation | 9.9 D ± 2.6 [10.0 D, 2.5 to 20.00] | 55 (39.3%) |
|
| ||
| Monocular accommodative facility | 6.0 cpm ± 3.6 [6.0 cpm, 0.5 to 15.5] | 78 (55.7%) |
|
| ||
| Binocular accommodative facility | 7.1 cpm ± 3.7 [6.0 cpm, 0 to 15] | 23 (16.4%) |
|
| ||
| DEM – Vertical scanning time (standard score) | 99.42 ± 20.18 [104, 30 to 136] | 29 (20.7%) |
|
| ||
| DEM – Horizontal scanning time (standard score) | 90.85 ± 30.9 [97, −100 to 133] | 42 (30.0%) |
|
| ||
| DEM – Ratio score (Horizontal/Vertical; standard score) | 88.19 ± 26.8 [92, −20 to 151] | 54 (38.6%) |
|
| ||
| DEM – Error score (standard score) | 99.54 ± 32.86 [111, −142 to 115] | 15 (10.7%) |
Measures of vergence and accommodation (mean ± one standard deviation (SD), median, and range) and the number of patients who failed the test based on the criteria listed for the respective tests in Table 1. Monocular measures were recorded from the right eye of the patient. DEM = developmental eye movement test. Δ = prism diopter; cpm = cycles per minute; D = diopters
Regression analysis: symptoms versus oculomotor measures
A stepwise linear regression for PCSS total score was significant (F = 27.13, p < 0.001, r2 = 0.16) with monocular amplitude of accommodation being the only significant predictor of the PCSS total score (coefficient (β) = −0.405, p < 0.001). A separate stepwise regression analysis was done for each of the PCSS symptom category total score as a dependent variable.
Each of the separate regression models for the somatic (p < 0.001, F = 14.07, r2 = 0.17); vestibular (p < 0.001, F = 16.15, r2 = 0.19);emotional (p = 0.002, F = 10.00 r2 = 0.09); cognitive (p < 0.001, F = 13.19, r2 = 0.092); and sleep (p < 0.001, F = 17.65, r2 = 0.15) symptom categories were statistically significant. For all these models, the only significant variable was monocular amplitude of accommodation with β of −0.397 (p < 0.001); −0.374 (p < 0.001); −0.260 (p = 0.002); −0.303 (p = 0.001); and −0.387 (p = 0.001) for the somatic, vestibular, emotional, cognitive, and sleep regression models, respectively. Similarly for the vision symptom category (which denotes the total score for a subset of questions within the PCSS related to vision and those similarly worded to the questions on the CISS), the regression model was significant at p < 0.001 (F = 13.47, r2 = 0.16). The significant variable was monocular amplitude of accommodation (β = −0.319, p < 0.001).
A stepwise regression for CISS total score was also significant (F = 10.5, p < 0.001, r2 = 0.24), with NPC (β = 0.391, p < 0.001), cause of concussion (β = 0.217, p = 0.006), and DEM error scores (β = −0.213, p = 0.006) all being significant variables.
DISCUSSION
In this study we examined the association between post-concussion symptoms reported on the PCSS and CISS as well as oculomotor measures to evaluate the potential usefulness of the PCSS in identifying post-concussion adolescent patients with vision-related (accommodation, vergence, and visual tracking) impairments. Results of the stepwise regression analysis show that the magnitude of the oculomotor measures were associated, at least in part, with the symptoms reported on the PCSS. Particularly, reduced monocular accommodation amplitude is a significant factor associated with symptoms reported on the total PCSS score and specifically in the somatic, vestibular, sleep, and vision categories. In a recent retrospective study by Master et. al. (2018), accommodative amplitude deficits were reported in 22% of patients, and the presence of an acute (<14 days) accommodative amplitude deficit predicted a longer recovery time for patients (35). Our results align with these findings and show a significant association of accommodative deficit and PCSS in our persistently symptomatic patient cohort.
In our analysis of PCSS categories, the low amplitude of accommodation accounting for significant variance in the somatic, vestibular, and sleep domain’s PCSS scores could be an outcome of impairment in the autonomic nervous system, as reported in recent concussion literature (36–38). Accommodation and pupillary responses are controlled by the autonomic nervous system (39), hence deficits in amplitude of accommodation could be suggestive of top-down control of this system.
It is not surprising that monocular amplitude of accommodation can only account for some of the variance in PCSS total score and subset scores (16-19%), as concussion affects many systems that can also contribute to symptoms reported on the PCSS. It is important to note that the comprehensive eye examination with a detailed oculomotor evaluation is only part of a multidisciplinary evaluation of these patients. It is common for patients to have more than one post-concussion diagnosis that can contribute to reported symptoms (40). Those nonvisual diagnoses were not considered in this analysis and are beyond the scope of this retrospective study.
As expected, NPC was significantly associated with CISS scores, a questionnaire primarily developed to monitor symptoms in convergence insufficiency. Stepwise regression also identified cause of concussion and more errors on the DEM test as being associated with higher CISS scores. Also, the CISS total score was moderately correlated with PCSS total score and the scores from the PCSS symptom categories (vision, cognitive, vestibular, sleep, and somatic). These correlations suggest a possible commonality to the mechanism contributing to the reported symptoms. Although the CISS total score in this study is not directly equivalent to the total scores reported in the literature for CISS responses, its use for correlational analysis is still a valid measure within the context of this study and likely underestimates the correlation (with only 14 rather than 15 questions used in a typical CISS).
The CISS is widely used and validated to assess symptoms associated with convergence insufficiency (24), but it is not commonly used in the routine evaluation of concussion. The PCSS, on the other hand, was not designed to specifically assess many symptoms that may relate to vision or oculomotor deficits. While “vision problems” is one item rated within this symptom inventory, such vague language may not be able to fully encompass the various vision-specific problems that an individual experiences after a concussion. However, results from this study suggest that responses to other items, in addition to the “vision problems” item in the PCSS, might also reflect underlying deficits in the visual system. In contrast, the CISS probes many different areas of visual function and may be an additional clinical tool, in conjunction with the PCSS, to identify vision problems and make appropriate referral decisions. Further research with prospective study designs incorporating an age-matched control group without concussion and concussion patients without oculomotor deficit will be needed to determine an appropriate cut-off for the PCSS total score or the PCSS vision category score that can be used to refer patients for comprehensive vision evaluations, including oculomotor assessments.
In this study, adolescent patients at least 21 days post-injury with persistent concussion symptoms had a high occurrence of oculomotor deficits. We found that 88% of these patients had one or more oculomotor deficits. About 42% of the patients had abnormal vergence findings and 70% of the patients had accommodation deficits. In a similar adolescent cohort following concussion, Master et al. (2016) reported 69% with one or more oculomotor deficits, of which 49% had convergence insufficiency and 51% had accommodative dysfunction (17). Differences in the type and magnitude of oculomotor deficits observed in our study and that of Master et al. (2016) may be due to differences in the diagnostic criteria, more detailed subclassification of vergence and accommodation deficits, or differences in the time between concussion and evaluation. More than half of the patients in the Master et al. (2016) study were evaluated within 90 days of having a concussion compared with our study, in which only 1/3rd of the patients were evaluated within 90 days of their concussion. Studies on adolescent post-concussion patients have shown clinical findings and oculomotor deficits to be more common in the acute phase (i.e., within 4 weeks of having a concussion). However, our study shows that many of the patients continue to have oculomotor deficits at or after 12 weeks post-concussion (the median time between concussion and evaluation in our study cohort) (17, 27, 41).
Additionally, the most commonly failed oculomotor test in this study was the NPC (76/140, 54%), but only 44 patients of the 76 who had receded NPC met all of the classical diagnostic criteria for convergence insufficiency or convergence deficit diagnoses. In another retrospective study of 275 pediatric patients examined about 16 days (median time of evaluation) after a concussion, only 24% had a receded NPC (41). Our study cohort was from the MDCC, which typically examines patients with persistent concussion symptoms, and this may have contributed to the higher incidence of receded NPC. We also showed in another retrospective study (subset of patients represented in this study cohort) of 83 patients (mean age = 15.3 years; examined 28 days after concussion) that, although 89% of participants had receded NPC, only 44% of them met the diagnostic criteria of having convergence insufficiency or convergence deficit (27). This study further illustrates that, in patients with persistent concussion symptoms, the incidence of receded NPC is high, even though not all meet the criteria for having convergence insufficiency or a convergence deficit.
Our findings should be interpreted in light of the study’s limitations. The sample of individuals included was from a single geographic region and consisted of chronically symptomatic patients who reported to a specialty concussion clinic at a tertiary-care, academic medical facility. Thus, the generalizability of the data to populations outside of this setting is limited. Further research involving a prospective study design with a well-balanced study population and a larger control group (consisting of patients who have not had a concussion or those who are earlier in their post-concussion recovery) could be beneficial in further understanding the association between symptoms reported on PCSS and oculomotor deficits. This work could help develop much needed threshold criteria for patient referrals to optometric or ophthalmological evaluations following concussion.
In summary, deficits in accommodation, vergence, and visual tracking were common after concussion in our sample of adolescents with persistent post-concussion symptoms seen at a specialty care multidisciplinary concussion clinic. Our results indicate that underlying oculomotor deficits, specifically amplitude of accommodation, might be associated, at least in part, with many of the symptoms reported by patients on the PCSS. Additionally, when the CISS is administered in addition to the PCSS, it can further identify the presence of underlying oculomotor deficits. Considering the high prevalence of oculomotor deficits post-concussion, it is vital to diagnose and manage these disorders at an early stage to minimize symptoms and possibly shorten recovery time. Prospective studies are needed with an adequate age-matched control group to determine cut-off scores for referral for a comprehensive vision examination. Given the broad nature of the PCSS, its relevance to the assessment of oculomotor deficit-related symptoms is not apparent, as the PCSS is designed to identify the diverse set of clinical symptoms that may arise following a concussion. However, the PCSS questionnaire along with the CISS questionnaire might better identify patients needing a vision evaluation.
ACKNOWLEDGEMENT
We thank Jane Patrick for editing the manuscript and Alicia Wang for helping with data retrieval, managing and updating the multidisciplinary concussion database.
Funding:
Children’s Hospital Ophthalmology Foundation Award (AR); American Academy of Optometry Career Starter Grant (AR); Research to Prevent Blindness award (Stanford University-TLR); and National Eye Institute P30-EY026877 (Stanford University-TLR)
Conflicts of Interest:
Dr. O’Brien receives royalties from (1) Springer International for the book Head and Neck Injuries in Young Athletes and (2) Wolters Kluwer Publishing for working as an author for UpToDate Online.
Dr. Meehan receives royalties from (1) ABC-Clio publishing for the sale of his books, Kids, Sports, and Concussion: A guide for coaches and parents and Concussions; (2) Springer International for the book Head and Neck Injuries in Young Athletes and (3) Wolters Kluwer for working as an author for UpToDate. His research is funded, in part, by philanthropic support from the National Hockey League Alumni Association through the Corey C. Griffin Pro-Am Tournament and a grant from the National Football League.
Drs. Gowrisankaran, Shah, Roberts, Wiecek, Hawash, Howell, and Raghuram have no conflict of interest to report.
Footnotes
Financial Disclosures: The authors have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Sussman ES, Pendharkar AV, Ho AL, Ghajar J. Mild traumatic brain injury and concussion: terminology and classification. Handb Clin Neurol. 2018;158:21–4. Epub 2018/11/30. doi: 10.1016/B978-0-444-63954-7.00003-3. [DOI] [PubMed] [Google Scholar]
- 2.Broglio SP, Cantu RC, Gioia GA, Guskiewicz KM, Kutcher J, Palm M, et al. National Athletic Trainers’ Association position statement: management of sport concussion. J Athl Train. 2014;49(2):245–65. Epub 2014/03/08. doi: 10.4085/1062-6050-49.1.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303–12. Epub 2012/04/28. doi: 10.1177/0363546512444554. [DOI] [PubMed] [Google Scholar]
- 4.Lovell MR, Iverson GL, Collins MW, Podell K, Johnston KM, Pardini D, et al. Measurement of symptoms following sports-related concussion: reliability and normative data for the post-concussion scale. Appl Neuropsychol. 2006;13(3):166–74. Epub 2007/03/17. doi: 10.1207/s15324826an1303_4. [DOI] [PubMed] [Google Scholar]
- 5.Barr WB, McCrea M. Sensitivity and specificity of standardized neurocognitive testing immediately following sports concussion. J Int Neuropsychol Soc. 2001;7(6):693–702. Epub 2001/09/29. [DOI] [PubMed] [Google Scholar]
- 6.Corwin DJ, Wiebe DJ, Zonfrillo MR, Grady MF, Robinson RL, Goodman AM, et al. Vestibular Deficits following Youth Concussion. J Pediatr. 2015;166(5):1221–5. Epub 2015/03/10. doi: 10.1016/j.jpeds.2015.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mucha A, Collins MW, Elbin RJ, Furman JM, Troutman-Enseki C, DeWolf RM, et al. A Brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. 2014;42(10):2479–86. Epub 2014/08/12. doi: 10.1177/0363546514543775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciuffreda KJ, Kapoor N, Rutner D, Suchoff IB, Han ME, Craig S. Occurrence of oculomotor dysfunctions in acquired brain injury: a retrospective analysis. Optometry. 2007;78(4):155–61. Epub 2007/04/03. doi: 10.1016/j.optm.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Ciuffreda KJ, Ludlam D, Thiagarajan P. Oculomotor diagnostic protocol for the mTBI population. Optometry. 2011;82(2):61–3. Epub 2011/02/01. doi: 10.1016/j.optm.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Stelmack JA, Frith T, Van Koevering D, Rinne S, Stelmack TR. Visual function in patients followed at a Veterans Affairs polytrauma network site: an electronic medical record review. Optometry. 2009;80(8):419–24. Epub 2009/07/29. doi: 10.1016/j.optm.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Capo-Aponte JE, Urosevich TG, Temme LA, Tarbett AK, Sanghera NK. Visual dysfunctions and symptoms during the subacute stage of blast-induced mild traumatic brain injury. Mil Med. 2012;177(7):804–13. Epub 2012/07/20. [DOI] [PubMed] [Google Scholar]
- 12.Goodrich GL, Kirby J, Cockerham G, Ingalla SP, Lew HL. Visual function in patients of a polytrauma rehabilitation center: A descriptive study. J Rehabil Res Dev. 2007;44(7):929–36. Epub 2007/12/14. [DOI] [PubMed] [Google Scholar]
- 13.Brahm KD, Wilgenburg HM, Kirby J, Ingalla S, Chang CY, Goodrich GL. Visual impairment and dysfunction in combat-injured servicemembers with traumatic brain injury. Optom Vis Sci. 2009;86(7):817–25. Epub 2009/06/13. doi: 10.1097/OPX.0b013e3181adff2d. [DOI] [PubMed] [Google Scholar]
- 14.Green W, Ciuffreda KJ, Thiagarajan P, Szymanowicz D, Ludlam DP, Kapoor N. Static and dynamic aspects of accommodation in mild traumatic brain injury: a review. Optometry. 2010;81(3):129–36. Epub 2010/03/10. doi: 10.1016/j.optm.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Szymanowicz D, Ciuffreda KJ, Thiagarajan P, Ludlam DP, Green W, Kapoor N. Vergence in mild traumatic brain injury: a pilot study. J Rehabil Res Dev. 2012;49(7):1083–100. Epub 2013/01/24. [DOI] [PubMed] [Google Scholar]
- 16.Thiagarajan P, Ciuffreda KJ, Ludlam DP. Vergence dysfunction in mild traumatic brain injury (mTBI): a review. Ophthalmic Physiol Opt. 2011;31(5):456–68. Epub 2011/03/18. doi: 10.1111/j.1475-1313.2011.00831.x. [DOI] [PubMed] [Google Scholar]
- 17.Master CL, Scheiman M, Gallaway M, Goodman A, Robinson RL, Master SR, et al. Vision Diagnoses Are Common After Concussion in Adolescents. Clin Pediatr (Phila). 2016;55(3):260–7. Epub 2015/07/15. doi: 10.1177/0009922815594367. [DOI] [PubMed] [Google Scholar]
- 18.Atowa UC, Wajuihian SO, Hansraj R. Vergence Profile and Prevalance of Non-Strabismic Vergence Anomalies Among School Children in Abia State, Nigeria. Ophthalmic Epidemiol. 2019;26(2):121–31. Epub 2018/10/12. doi: 10.1080/09286586.2018.1532523. [DOI] [PubMed] [Google Scholar]
- 19.Hussaindeen JR, Rakshit A, Singh NK, George R, Swaminathan M, Kapur S, et al. Prevalence of non-strabismic anomalies of binocular vision in Tamil Nadu: report 2 of BAND study. Clin Exp Optom. 2017;100(6):642–8. Epub 2016/11/20. doi: 10.1111/cxo.12496. [DOI] [PubMed] [Google Scholar]
- 20.Rouse MW, Borsting E, Hyman L, Hussein M, Cotter SA, Flynn M, et al. Frequency of convergence insufficiency among fifth and sixth graders. The Convergence Insufficiency and Reading Study (CIRS) group. Optom Vis Sci. 1999;76(9):643–9. Epub 1999/09/25. doi: 10.1097/00006324-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Rouse MW, Hyman L, Hussein M, Solan H. Frequency of convergence insufficiency in optometry clinic settings. Convergence Insufficiency and Reading Study (CIRS) Group. Optom Vis Sci. 1998;75(2):88–96. Epub 1998/03/21. doi: 10.1097/00006324-199802000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Wajuihian SO, Hansraj R. Vergence anomalies in a sample of high school students in South Africa. J Optom. 2016;9(4):246–57. Epub 2016/01/12. doi: 10.1016/j.optom.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCrory P, Meeuwisse W, Dvorak J, Aubry M, Bailes J, Broglio S, et al. Consensus statement on concussion in sport-the 5(th) international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838–47. Epub 2017/04/28. doi: 10.1136/bjsports-2017-097699. [DOI] [PubMed] [Google Scholar]
- 24.Borsting EJ, Rouse MW, Mitchell GL, Scheiman M, Cotter SA, Cooper J, et al. Validity and reliability of the revised convergence insufficiency symptom survey in children aged 9 to 18 years. Optom Vis Sci. 2003;80(12):832–8. Epub 2003/12/23. [DOI] [PubMed] [Google Scholar]
- 25.Pearce KL, Sufrinko A, Lau BC, Henry L, Collins MW, Kontos AP. Near Point of Convergence After a Sport-Related Concussion: Measurement Reliability and Relationship to Neurocognitive Impairment and Symptoms. Am J Sports Med. 2015;43(12):3055–61. Epub 2015/10/11. doi: 10.1177/0363546515606430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallaway M, Scheiman M, Mitchell GL. Vision Therapy for Post-Concussion Vision Disorders. Optom Vis Sci. 2017;94(1):68–73. Epub 2016/08/10. doi: 10.1097/OPX.0000000000000935. [DOI] [PubMed] [Google Scholar]
- 27.Raghuram A, Cotter SA, Gowrisankaran S, Kanji J, Howell DR, Meehan WP 3rd, et al. Postconcussion: Receded Near Point of Convergence is not Diagnostic of Convergence Insufficiency. Am J Ophthalmol. 2019;206:235–44. Epub 2019/04/21. doi: 10.1016/j.ajo.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Scheiman MM, Talasan H, Mitchell GL, Alvarez TL. Objective Assessment of Vergence after Treatment of Concussion-Related CI: A Pilot Study. Optom Vis Sci. 2017;94(1):74–88. Epub 2016/07/29. doi: 10.1097/OPX.0000000000000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thiagarajan P, Ciuffreda KJ, Capo-Aponte JE, Ludlam DP, Kapoor N. Oculomotor neurorehabilitation for reading in mild traumatic brain injury (mTBI): an integrative approach. NeuroRehabilitation. 2014;34(1):129–46. Epub 2013/11/29. doi: 10.3233/NRE-131025. [DOI] [PubMed] [Google Scholar]
- 30.Howell DR, Kriz P, Mannix RC, Kirchberg T, Master CL, Meehan WP 3rd. Concussion Symptom Profiles Among Child, Adolescent, and Young Adult Athletes. Clin J Sport Med. 2019;29(5):391–7. Epub 2018/06/23. doi: 10.1097/JSM.0000000000000629. [DOI] [PubMed] [Google Scholar]
- 31.Howell DR, O’Brien MJ, Beasley MA, Mannix RC, Meehan WP 3rd. Initial somatic symptoms are associated with prolonged symptom duration following concussion in adolescents. Acta Paediatr. 2016;105(9):e426–32. Epub 2016/05/28. doi: 10.1111/apa.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pardini J, Stump J, Lovell MR, Collins MW, Moritz K, Fu F. The Post Concussion Symptom Scale (PCSS): a factor analysis Br J Sports Med. 2004;38:661–2. [Google Scholar]
- 33.Lau BC, Collins MW, Lovell MR. Sensitivity and specificity of subacute computerized neurocognitive testing and symptom evaluation in predicting outcomes after sports-related concussion. Am J Sports Med. 2011;39(6):1209–16. Epub 2011/02/03. doi: 10.1177/0363546510392016. [DOI] [PubMed] [Google Scholar]
- 34.Raghuram A, Gowrisankaran S, Swanson E, Zurakowski D, Hunter DG, Waber DP. Frequency of Visual Deficits in Children With Developmental Dyslexia. JAMA Ophthalmol. 2018;136(10):1089–95. Epub 2018/07/22. doi: 10.1001/jamaophthalmol.2018.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Master CL, Master SR, Wiebe DJ, Storey EP, Lockyer JE, Podolak OE, et al. Vision and Vestibular System Dysfunction Predicts Prolonged Concussion Recovery in Children. Clin J Sport Med. 2018;28(2):139–45. Epub 2017/10/25. doi: 10.1097/JSM.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 36.Esterov D, Greenwald BD. Autonomic Dysfunction after Mild Traumatic Brain Injury. Brain Sci. 2017;7(8). Epub 2017/08/12. doi: 10.3390/brainsci7080100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leddy JJ, Kozlowski K, Fung M, Pendergast DR, Willer B. Regulatory and autoregulatory physiological dysfunction as a primary characteristic of post concussion syndrome: implications for treatment. NeuroRehabilitation. 2007;22(3):199–205. Epub 2007/10/06. [PubMed] [Google Scholar]
- 38.Pertab JL, Merkley TL, Cramond AJ, Cramond K, Paxton H, Wu T. Concussion and the autonomic nervous system: An introduction to the field and the results of a systematic review. NeuroRehabilitation. 2018;42(4):397–427. Epub 2018/04/18. doi: 10.3233/NRE-172298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDougal DH, Gamlin PD. Autonomic control of the eye. Compr Physiol. 2015;5(1):439–73. Epub 2015/01/16. doi: 10.1002/cphy.c140014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah AS, Raghuram A, Kaur K, Lispon S, Shoshany T, Stevens R, et al. Specialty-specific Diagnoses in Pediatric Patients with Post-Concussion Syndrome: Experience from a Multi-disciplinary Concussion Clinic. Accepted for publication.CJSM. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Storey EP, Master SR, Lockyer JE, Podolak OE, Grady MF, Master CL. Near Point of Convergence after Concussion in Children. Optom Vis Sci. 2017;94(1):96–100. Epub 2016/07/09. doi: 10.1097/OPX.0000000000000910. [DOI] [PubMed] [Google Scholar]


