1. Introduction
In the last two decades, glucagon-like peptide-1 (GLP-1) receptor agonists have emerged as promising therapeutics for diabetes mellitus, a disease with increasing global burden – 34 million in the US, 422 million worldwide [1]. GLP-1 is part of a family of neuroendocrine peptide hormones known as incretins that are produced by intestinal L-cells as well as certain neurons within the brainstem in response to nutrient ingestion [2]. GLP-1 helps maintain euglycemia by inducing endogenous insulin secretion from pancreatic beta cells and suppressing glucagon secretion. GLP-1 also delays gastric emptying and causes increased satiety, which can lead to intentional weight loss when utilized in a supraphysiologic manner. The appeal of GLP-1 receptor (GLP-1R) agonists as an antiglycemic agent is that they act to decrease blood sugar in a glucose-dependent manner, thereby decreasing risk of hypoglycemic episodes and mitigating weight gain. Currently, GLP-1R agonists are approved by the US Food and Drug Administration (FDA) to treat type 2 diabetes mellitus (T2D) and weight loss in obesity (Table 1).
Table 1.
Current FDA-approved GLP-1R agonists and dosing
| GLP-1R Agonist | Route | Dosing |
|---|---|---|
| Short-acting | ||
| Exenatide | SC | Twice daily |
| Lixisenatide | SC | Once daily |
| Long-acting | ||
| Exenatide extended release | SC | Once weekly |
| Liraglutide* | SC | Once daily |
| Dulaglutide | SC | Once weekly |
| Semaglutide* | SC or PO | Once weekly SC or once daily PO |
All GLP-1R agonists are currently approved for use in T2D with the exception of liraglutide and semaglutide, which are also approved for chronic weight management and weight loss in obesity
GLP-1R, glucagon-like peptide-1 receptor; T2D, type 2 diabetes mellitus; SC, subcutaneous; PO, oral
2. Beyond diabetes
Beyond the pancreas, GLP-1R is expressed abundantly in human lung, heart, brain, kidney, and GI tract [3]. Interestingly, the expression of GLP-1 seems to be substantially higher in the lung compared to other organs, prompting further efforts to understand the role of GLP-1R in the lung [4]. Subsequent studies have demonstrated potential anti-inflammatory effects of GLP-1R agonists in both acute and chronic pulmonary disease [5,6]. In mice, liraglutide attenuated bleomycin-induced pulmonary fibrosis, ovalbumin (OVA)-induced chronic airway inflammation, and lipopolysaccharide (LPS)-induced acute lung injury (ALI) [7–9]. In a meta-analysis of large clinical trials investigating the cardiorenal benefits of GLP-1R agonists in T2D, there was a trend towards reduction of reported pulmonary adverse events in the GLP-1R agonist treatment groups [10]. While overall low incidence of respiratory disorders in these studies resulted in low statistical power, outcomes suggested benefits of GLP1-R agonist use in respiratory disease beyond that of glycemic control. These results have led to significant interest in the potential role for GLP-1R agonists in asthma, the most common chronic inflammatory pulmonary disease worldwide and often comorbid with type 2 diabetes and obesity.
It is estimated that over 25 million people in the US (330 million globally) currently suffer from asthma [11]. Classically, asthma is considered a predominantly allergic disease, mediated by type 2 inflammatory immune cells such as CD4+ T-helper type 2 (Th2) cells, mast cells, eosinophils, basophils, and group 2 innate lymphoid cells (ILC2s). In this chronic lung disease, airway hyperresponsiveness leads to bronchospasm, mucus production, and reversible obstruction, though over time, airway remodeling can result in airway smooth muscle hypertrophy, mucus metaplasia, and collagen deposition. As part of the innate immune response to certain environmental antigens, interleukin-33 (IL-33) is secreted predominantly by airway epithelial cells and macrophages. IL-33 potentiates a type 2 allergic inflammatory response, activating immune cells including ILC2s, Th2 cells, mast cells, and basophils [12,13]. These cells then, in turn, secrete numerous cytokines including IL-4, IL-5, IL-9, and IL-13, which stimulate structural cells in the lung such as goblet cells and smooth muscle cells to cause airway remodeling. Furthermore, these type 2 cytokines can act back on leukocytes such as CD4+ T-cells, eosinophils, basophils, mast cells, and B-cells to further amplify allergic responses. Of note, IL-4 and IL-13 can induce B-cells to isotype switch to produce IgE [14].
In vivo studies in mice reveal how far upstream GLP-1R agonists work in dampening allergen-induced airway inflammation. Treatment with liraglutide decreased IL-33 release into the airway one hour after challenge with an extract of the fungal allergen Alternaria alternata in a mouse model of allergic airway inflammation that mimics asthma. Furthermore, liraglutide inhibited the type 2 inflammatory response as defined by decreased ILC2 proliferation, IL-5 and IL-13 production from these cells, and lung eosinophilia [15]. These results suggest that GLP-1R signaling inhibits the early innate allergic immune response in the lung. The inhibitory effect of GLP-1R signaling on Th2 inflammation was reproduced outside of the setting of atopic airway inflammation. In mice challenged with respiratory syncytial virus (RSV), liraglutide administration decreased RSV infection-induced IL-13 secretion, lung IL-33 protein expression, and the total number of lung ILC2, CD4+ T cells, and basophils [16]. In these RSV-challenged, liraglutide-treated mice, there was also decrease in methacholine-induced airway responsiveness and airway mucus scores, further demonstrating that GLP1-R signaling attenuates IL-13 mediated physiology.
Subsequently, a recent retrospective cohort study demonstrated a reduction in number of asthma exacerbations in patients with T2D on GLP-1R agonists compared to other antiglycemic agents (comparative incidence rate ratio ranged 1.83–2.98) [17]. Findings were robust even when accounting for changes in hemoglobin A1c and BMI, concurrent metformin use, and exposures to other antiglycemic agents. In subgroup analysis, this effect was particularly prominent in those with moderate/severe asthma.
3. Proposed Mechanisms
The roles of GLP-1R agonists in reducing airway inflammation in asthma are not completely defined, though various mechanisms have been suggested (Figure 1).
Figure 1.
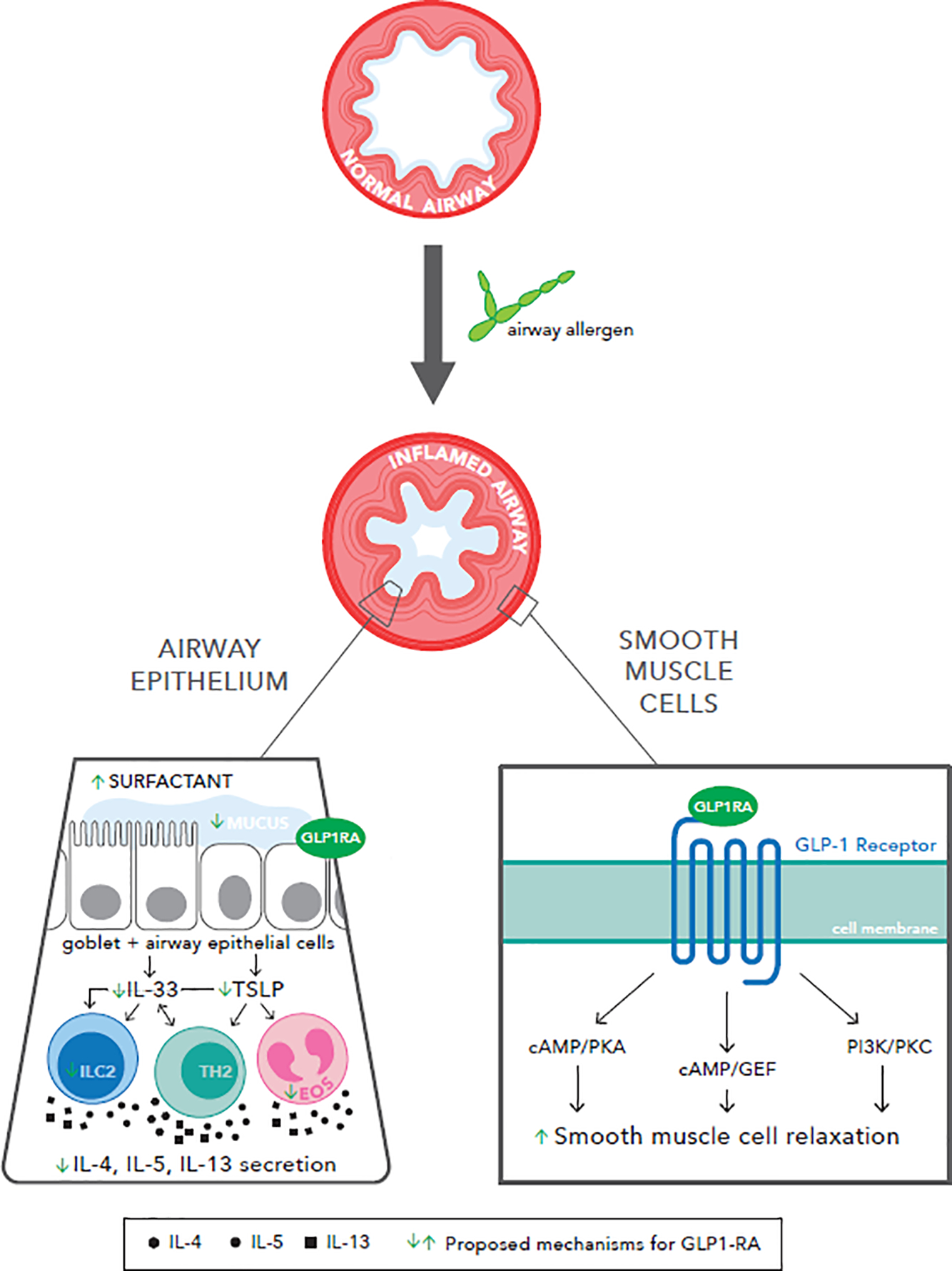
Various mechanisms have been proposed for the role of GLP-1R agonists in reducing inflammatory airway disease, including airway modification with increased surfactant production and decreased mucus secretion, decreased Type 2 inflammatory signaling, and increased smooth muscle relaxation.
3.1. Smooth muscle relaxation
GLP-1R belongs to a family of proteins known as Gs-protein coupled receptors, which activate downstream pathways such as cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA), cAMP/guanine-nucleotide exchange factor, and phosphatidylinositol-3 kinase/Protein Kinase C pathways. Activation of cAMP is the mechanism by which β2 adrenergic agonists, prostaglandin E2, and phosphodiesterase inhibitors cause relaxation of airway smooth muscle [18].
These potential shared downstream pathways offer several mechanisms by which GLP-1R agonists reduce obstruction and inflammation. In a mouse model of asthma, liraglutide and exenatide inhibited OVA-induced airway inflammation via decrease in airway mucus hypersecretion and putative PKA-dependent inactivation of nuclear factor-kB [8], whereas, in another study, use of a PKA-inhibitor abrogated the bronchodilatory effects of exendin-4 (also known as exenatide) [19]. In another study investigating the role of dendritic cells in allergic asthma, decreased cAMP promoted Th2 differentiation and yielded a prominent allergic phenotype, whereas cAMP stimulation inhibited these responses [20].
There remains some discrepancy between human and murine GLP-1R expression and some ambiguity about exactly which cell types express GLP-1R. One study found GLP-1R to be expressed in smooth muscle cells of arteries and arterioles of human lung, while this receptor was more ubiquitously expressed throughout the murine lung [21]. Another study reported this receptor to be localized in the pulmonary vasculature, airway smooth muscle cells, airway epithelial cells, and type II alveolar cells [19]. A major problem in the field has been the relative non-specificity of the commercially available anti-GLP-1R antibodies for immunohistochemistry and flow cytometry, thus the true cellular expression of the receptor has been difficult to ascertain using these techniques.
3.2. Structural modification
GLP-1R agonists may serve an independent pulmonary-protective role even in individuals without lung disease, as evidenced by relative increase in average forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) in individuals on metformin and GLP-1R agonists compared to metformin alone or metformin and insulin [22]. This suggests that GLP-1R agonists may augment their anti-inflammatory effects by improving the mechanics of airflow. That said, it remains unclear whether GLP-1R agonists improve lung compliance. Various studies have reported contradictory findings regarding the effect of GLP-1R agonists on decreasing mucus or increasing surfactant production [4,15,23,24]. In mouse models of ALI, GLP-1R agonists blunted the reduction of surfactant in response to LPS administration [25–27]. It is possible the response to GLP-1R agonists may also be related to the underlying pulmonary disease process rather than a direct effect of the treatment itself [28].
3.3. Connection with hyperglycemia, obesity, and metabolic syndrome
Asthma is increasingly characterized as a heterogenous disease with at least two prominent phenotypes – “Th2-high” or eosinophilic and “Th2-low” or neutrophilic. The former is considered the classic, steroid-responsive phenotype, while the latter is considered more refractory to traditional therapies [29,30]. In particular, adults with comorbid obesity and asthma tend towards the Th2-low phenotype [31]. GLP-1R signaling has been investigated in a polygenic model of obesity using TALLYHO mice. TALLYHO mice had greater allergen-induced airway neutrophilia and lung protein expression of IL-5, IL-13, CCL11, CXCL1, and CXCL5, in addition to ICAM-1 expression on lung epithelial cells, compared with lean mice, and all of these endpoints were reduced by GLP-1R agonist treatment. Allergen challenge increased bronchoalveolar lavage fluid (BALF) IL-33 in both TALLYHO and SWR mice compared to saline challenge, but there was no difference in the BALF IL-33 levels between the TALLYHO and lean control strains. However, TALLYHO, but not lean, mice had significantly higher airway thymic stromal lymphopoietin (TSLP) in BALF following allergen challenge compared to saline challenge. GLP-1R agonist treatment significantly decreased the allergen-induced TSLP and IL-33 release in TALLYHO mice. Interestingly, TSLP or ST2 inhibition with a neutralizing antibody decreased airway eosinophils, but did not reduce airway neutrophils in TALLYHO mice, suggesting a novel mechanism by which GLP-1R signaling is reducing allergen-induced airway inflammation [32].
While the association between obesity, metabolic syndrome, and asthma is well-recognized, it is not well-understood. One postulated mechanism considers dysregulated arginine metabolism and nitric oxide (NO) production in obesity as a potential link to respiratory disease [32]. GLP-1R agonists are thought to inhibit this dysregulated arginine gene product, known as asymmetric dimethylarginine, a competitive inhibitor of NO synthase, thereby allowing for increased levels of NO [33].
In another obese mouse model of ALI, liraglutide mitigated LPS-induced injury by inhibiting NLRP3 inflammasome and IL-1β [5]. This was also demonstrated in a lean mouse model of asthma, suggesting a possible common pathway that may link allergic and non-allergic asthma [25].
4. Conclusions
GLP-1 signaling remains an exciting novel target for treatment of chronic airway inflammation in asthma. Given its potential for multi-system anti-inflammatory effects, GLP-1 agonists may be an attractive therapeutic agent for those with diabetes and comorbid asthma and obesity; however, further studies are needed to better characterize the endogenous function of GLP-1 signaling in the lungs where GLP-1R is ubiquitously expressed. The aforementioned studies highlight the need for further comparison between lean and obese asthma models, as well as prospective studies of GLP-1R agonists in well-phenotyped asthma populations. In doing this, we may elucidate mechanisms for lung protection in both acute and chronic pulmonary disease beyond that of weight loss and modulation of hyperglycemia.
Funding:
This study was supported by the Department of Veterans Affairs via grant BX004299 (to RS Peebles Jr); and the National Institutes of Health via grants R38HL143619-03 (to AY Wu), AI 095227 (to RS Peebles Jr), AI 111820 (to RS Peebles Jr), AI 124456 (to RS Peebles Jr), AI 145265 (to RS Peebles Jr), and AI 145397 (to RS Peebles Jr).
Abbreviations used in this paper:
- ALI
acute lung injury
- BALF
bronchoalveolar lavage fluid
- cAMP
cyclic adenosine monophosphate
- COPD
chronic obstructive pulmonary disease
- FDA
US Food and Drug Administration
- FEV1
forced expiratory volume in one second
- FVC
forced vital capacity
- GLP-1
glucagon-like peptide-1
- GLP-1R
glucagon-like peptide-1 receptor
- ILC2
group 2 innate lymphoid cells
- IL
interleukin
- LPS
lipopolysaccharide
- NO
nitric oxide
- OVA
ovalbumin
- PKA
protein kinase A
- RSV
respiratory syncytial virus
- T2D
type 2 diabetes mellitus
- Th2
T-helper type 2
- TSLP
thymic stromal lymphopoietin
Footnotes
Declaration of Interest:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed
Reviewer Disclosures:
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References:
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- [1].Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services. 2020. [Google Scholar]
- [2].Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. [DOI] [PubMed] [Google Scholar]
- [3].Pyke C, Heller RS, Kirk RK, et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155:1280–1290. [DOI] [PubMed] [Google Scholar]
- [4].Viby N-E, Isidor MS, Buggeskov KB, et al. Glucagon-like peptide-1 (GLP-1) reduces mortality and improves lung function in a model of experimental obstructive lung disease in female mice. Endocrinology. 2013;154:4503–4511. [DOI] [PubMed] [Google Scholar]
- [5].Hur J, Kang JY, Kim YK, et al. Glucagon-like peptide 1 receptor (GLP-1R) agonist relieved asthmatic airway inflammation via suppression of NLRP3 inflammasome activation in obese asthma mice model. Pulm Pharmacol Ther. 2021;67:102003. [DOI] [PubMed] [Google Scholar]
- [6].Nguyen D-V, Linderholm A, Haczku A, et al. Glucagon-like peptide 1: A potential anti-inflammatory pathway in obesity-related asthma. Pharmacol Ther. 2017;180:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gou S, Zhu T, Wang W, et al. Glucagon like peptide-1 attenuates bleomycin-induced pulmonary fibrosis, involving the inactivation of NF-κB in mice. Int Immunopharmacol. 2014;22:498–504. [DOI] [PubMed] [Google Scholar]
- [8].Zhu T, Wu X-L, Zhang W, et al. Glucagon Like Peptide-1 (GLP-1) Modulates OVA-Induced Airway Inflammation and Mucus Secretion Involving a Protein Kinase A (PKA)-Dependent Nuclear Factor-κB (NF-κB) Signaling Pathway in Mice. Int J Mol Sci. 2015;16:20195–20211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhu T, Li C, Zhang X, et al. GLP-1 Analogue Liraglutide Enhances SP-A Expression in LPS-Induced Acute Lung Injury through the TTF-1 Signaling Pathway. Mediators Inflamm. 2018;2018:3601454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Wei J-P, Yang C-L, Leng W-H, et al. Use of GLP1RAs and occurrence of respiratory disorders: A meta-analysis of large randomized trials of GLP1RAs. Clin Respir J. 2021; * Meta-analysis of large clinical trials demonstrating trend towards reduction of pulmonary adverse events in GLP-1RA treatment groups.
- [11].Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016. Geneva, World Health Organization. 2018. [Google Scholar]
- [12].Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. [DOI] [PubMed] [Google Scholar]
- [13].Pecaric-Petkovic T, Didichenko SA, Kaempfer S, et al. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chan BCL, Lam CWK, Tam L-S, et al. IL33: Roles in Allergic Inflammation and Therapeutic Perspectives. Front Immunol. 2019;10:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Toki S, Goleniewska K, Reiss S, et al. Glucagon-like peptide 1 signaling inhibits allergen-induced lung IL-33 release and reduces group 2 innate lymphoid cell cytokine production in vivo. J Allergy Clin Immunol. 2018;142:1515–1528.e8. * Liraglutide inhibits ILC2 proliferation, IL5 and IL13 production, and lung eosinophilia in a mouse model of allergic airway inflammation.
- [16]. Bloodworth MH, Rusznak M, Pfister CC, et al. Glucagon-like peptide 1 receptor signaling attenuates respiratory syncytial virus-induced type 2 responses and immunopathology. J Allergy Clin Immunol. 2018;142:683–687.e12. * GLP-1R signaling inhibits RSV-induced IL-13 expression and IL-33 secretion.
- [17]. Foer D, Beeler PE, Cui J, et al. Asthma Exacerbations in Patients with Type 2 Diabetes and Asthma on Glucagon-like Peptide-1 Receptor Agonists. Am J Respir Crit Care Med. 2021;203:831–840. ** Retrospective cohort study demonstrates reduced number of asthma exacerbations in patients with T2D on GLP-1R agonists.
- [18].Billington CK, Ojo OO, Penn RB, et al. cAMP regulation of airway smooth muscle function. Pulm Pharmacol Ther. 2013;26:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rogliani P, Calzetta L, Capuani B, et al. Glucagon-Like Peptide 1 Receptor: A Novel Pharmacological Target for Treating Human Bronchial Hyperresponsiveness. Am J Respir Cell Mol Biol. 2016;55:804–814. [DOI] [PubMed] [Google Scholar]
- [20].Lee J, Kim TH, Murray F, et al. Cyclic AMP concentrations in dendritic cells induce and regulate Th2 immunity and allergic asthma. Proc Natl Acad Sci U S A. 2015;112:1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Körner M, Stöckli M, Waser B, et al. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med Off Publ Soc Nucl Med. 2007;48:736–743. [DOI] [PubMed] [Google Scholar]
- [22].Rogliani P, Matera MG, Calzetta L, et al. Long-term observational study on the impact of GLP-1R agonists on lung function in diabetic patients. Respir Med. 2019;154:86–92. [DOI] [PubMed] [Google Scholar]
- [23].Nohara H, Nakashima R, Kamei S, et al. Intratracheal GLP-1 receptor agonist treatment up-regulates mucin via p38 and exacerbates emphysematous phenotype in mucus hypersecretory obstructive lung diseases. Biochem Biophys Res Commun. 2020;524:332–339. [DOI] [PubMed] [Google Scholar]
- [24].Vara E, Arias-Díaz J, Garcia C, et al. Glucagon-like peptide-1(7–36) amide stimulates surfactant secretion in human type II pneumocytes. Am J Respir Crit Care Med. 2001;163:840–846. [DOI] [PubMed] [Google Scholar]
- [25].Xu J, Wei G, Wang J, et al. Glucagon-like peptide-1 receptor activation alleviates lipopolysaccharide-induced acute lung injury in mice via maintenance of endothelial barrier function. Lab Investig J Tech Methods Pathol. 2019;99:577–587. [DOI] [PubMed] [Google Scholar]
- [26].Zhou F, Zhang Y, Chen J, et al. Liraglutide attenuates lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol. 2016;791:735–740. [DOI] [PubMed] [Google Scholar]
- [27].Suzuki T, Tada Y, Gladson S, et al. Vildagliptin ameliorates pulmonary fibrosis in lipopolysaccharide-induced lung injury by inhibiting endothelial-to-mesenchymal transition. Respir Res. 2017;18:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sato T, Shimizu T, Fujita H, et al. GLP-1 Receptor Signaling Differentially Modifies the Outcomes of Sterile vs Viral Pulmonary Inflammation in Male Mice. Endocrinology. 2020;161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Telenga ED, Tideman SW, Kerstjens H a. M, et al. Obesity in asthma: more neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy. 2012;67:1060–1068. [DOI] [PubMed] [Google Scholar]
- [30].Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].McCravy M, Ingram JL, Que LG. Dysregulated Metabolism in the Pathophysiology of Non-Allergic Obese Asthma. J Asthma Allergy. 2021;14:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Toki S, Newcomb DC, Printz RL, et al. Glucagon-like peptide-1 receptor agonist inhibits aeroallergen-induced activation of ILC2 and neutrophilic airway inflammation in obese mice. Allergy. 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Singh VP, Aggarwal R, Singh S, et al. Metabolic Syndrome Is Associated with Increased Oxo-Nitrative Stress and Asthma-Like Changes in Lungs. PloS One. 2015;10:e0129850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ojima A, Ishibashi Y, Matsui T, et al. Glucagon-like peptide-1 receptor agonist inhibits asymmetric dimethylarginine generation in the kidney of streptozotocin-induced diabetic rats by blocking advanced glycation end product-induced protein arginine methyltranferase-1 expression. Am J Pathol. 2013;182:132–141. [DOI] [PubMed] [Google Scholar]


